Abstract
Implantable cardioverter-defibrillator (ICD) is effective to prevent sudden death in HCM patients. We reviewed ICD records to analyze the relation between life-threatening arrhythmia and late gadolinium enhancement (LGE) on cardiovascular magnetic resonance (CMR) in Japanese hypertrophic cardiomyopathy (HCM) patients. In 102 consecutive patients (median age 63 years, 63 males) implanted with an ICD after CMR with gadolinium enhancement (median follow-up 2.8 years), the outcome of life-threatening arrhythmic events (appropriate ICD interventions for ventricular tachycardia or ventricular fibrillation) was examined. Appropriate interventions rate were 10.3% per year for secondary prevention and 7.4% per year for primary prevention. The annualized ICD-related complication rate was 3.7%. 43/91 patients (47%) implanted ICD for primary prevention had maximum wall thickness ≥20 mm plus LGE in ≥4 of 17 left ventricular segments (cut-off value obtained from ROC curve); the appropriate ICD intervention rate was significantly higher in this group than in other patients group (annualized event rate, 11.1 vs. 4.6%; log-rank P = 0.038). A combination of myocardial hypertrophy and LGE is a useful outcome predictive factor for life-threatening ventricular arrhythmia in Japanese HCM patients.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Life-threatening arrhythmias comprising ventricular tachycardia (VT) and ventricular fibrillation (VF) are important causes of sudden death in patients with hypertrophic cardiomyopathy (HCM). In these patients, implantable cardioverter-defibrillator (ICD) is considered to be useful for the prevention of sudden death [1,2,3,4,5,6,7]. According to the Japanese Circulation Society Guidelines for Diagnosis and Treatment of Patients with Hypertrophic Cardiomyopathy, the indication of ICD implantation for secondary prevention is recommended as Class I [8]. On the other hand, the indication for primary prevention defined as the presence of at least one of the five conventional risk factors of sudden death [family history of sudden death; syncope; left ventricular wall thickness ≥30 mm; nonsustained ventricular tachycardia (NSVT); and abnormal blood pressure response during exercise] is recommended as Class IIa. However, studies have shown no significant difference in the rate of appropriate ICD intervention regardless of the total number of risk factors identified among the five above-mentioned factors [3, 4, 6]. In addition, ICD-related complications including inappropriate ICD intervention and device trouble are important issues after implantation. Therefore, establishing clear criteria for ICD implantation in the Japanese Circulation Society Guidelines is important for patients with HCM which affect many young adults.
In recent years, many reports have demonstrated a relationship between late gadolinium enhancement (LGE) on cardiovascular magnetic resonance imaging (CMR) and the prognosis of sudden death from America and Europe, and the usefulness of LGE in predicting sudden death has been anticipated [9,10,11]. However, there are few reports on the relationship between CMR findings and ICD intervention rate only in HCM patients with implanted ICD [12].
The purpose of the present study was to examine the usefulness of CMR in predicting the prognosis of life-threatening arrhythmia in a relatively large number of Japanese HCM patients implanted with ICD.
Methods
Patient selection
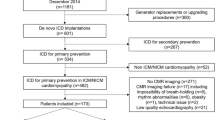
We retrospectively studied 102 HCM patients with ICD implantation within 1 year after gadolinium enhancement CMR at Sakakibara Heart Institute between January 2006 and Aug 2013. Use of their clinical data for the retrospective study was explained to the patients, and consent in writing was obtained. HCM was diagnosed in the case of left ventricular (LV) wall thickness ≥15 mm on CMR in the absence of other cardiac or systemic diseases that could account for the hypertrophy. Given that the aim of this study was to investigate LGE in HCM, patients with congenital heart disease and those with a history of coronary bypass surgery, old myocardial infarction, heart valve surgery, or percutaneous transluminal septal myocardial ablation or myectomy before CMR were excluded. Patients with renal dysfunction (estimated glomerular filtration rate <30 ml/min/1.73 m2) were also excluded. The clinical records of all 102 patients were reviewed, and the clinical findings, medications and echocardiographic characteristics at the time of CMR were extracted. Among the 5 conventional risk factors (family history of sudden death; syncope; LV wall thickness ≥30 mm; NSVT; and abnormal blood pressure response during exercise) for sudden death in the Japanese Circulation Society HCM Guideline, the abnormal blood pressure response during exercise was not included in the analysis because the exercise test was performed in very few study patients. NSVT was defined as heart rate ≥120 beats/min for ≥3 consecutive beats. Subsequent device interrogations were routinely performed at every 3 months after ICD implantation until September 2014. Life-threatening arrhythmic events were defined as appropriate ICD interventions for VT or VF.
CMR protocols and image analysis
Cardiovascular magnetic resonance was performed with a 1.5-T MR scanner (Magnetom Sonata; Siemens Medical Solutions, Erlangen, Germany) using a 6-channel phased-array body and spine coil. All images were acquired using the ECG-gated breath-hold technique. First, steady-state free-precession cine images were acquired in long-axis (2-, 3-, and 4-chamber) and short-axis views covering the LV from base to apex (TR, 56.8 ms; TE, 1.2 ms). Subsequently, LGE images were acquired 10 min after i.v. injection of 0.1 mmol/kg gadodiamide hydrate (Omniscan; Daiichi Sankyo, Tokyo, Japan) in accordance with the standardized CMR protocols, using the inversion recovery technique in identical views (TR, 600 ms; TE, 1.26 ms; TI was individually optimized to null normal myocardial signal using a TI-scout sequence). Two cardiologists and 1 radiologist evaluated late enhancement images for the presence of LGE areas within the LV myocardium for each patient, and the results were obtained via consensus using a previously reported method [13]. The LGE was determined by visual inspection, and if the LGE presence and area were obscure and difficult to determine visually, the aforementioned measurement of signal intensity was applied. The mean signal intensity (and SD) of the normal myocardium was calculated, and a threshold ≥5 SD exceeding the mean was used to define areas of LGE. The extent of LGE was assessed using the LV 17-segment model [14]. The extent of LGE was scored on a scale of 0–17, as the sum of the segments in the 17-segment model with LGE. Maximum LV wall thickness was determined by measuring the minimum thickness of the thickest LV myocardium in the cine image at end-diastole.
Echocardiography
Two-dimensional, Doppler, and M-mode echocardiography was performed at rest using standard methods. LV obstruction was defined as peak resting gradient ≥30 mmHg. LV volumes and LV ejection fraction (LVEF) were calculated using the biplane Simpson’s rule from the apical 2-chamber and 4-chamber views.
Statistical analysis
Data are given as median for continuous variables and as percentage for categorical variables. Differences between means were tested using Mann–Whitney U test. Categorical variables were compared using Chi-squared test or Fisher’s exact test, as appropriate. Event-free rates from life-threatening arrhythmia were calculated according to the Kaplan–Meier method and were compared using the log-rank test. The univariate Cox proportional hazards regression analysis was performed using the clinical, echocardiography, and CMR variables considered possibly related to life-threatening arrhythmic events. For multivariate analysis, those variables with P < 0.1 on univariate analysis were entered into the model. Furthermore, the multivariable model was constructed using a stepwise selection method with an entrance and stay criteria of P < 0.10. P < 0.05 was considered to be statistically significant. Analysis was performed using SPSS ver. 20.0.
Results
ICD-related complications
Implantable cardioverter-defibrillator-related complications were classified into device-related complications and inappropriate ICD interventions. The annual ICD-related complication rate was 3.7% per year (device-related complications, 1.5%/year; inappropriate ICD interventions, 2.4%/year) in all the study patients. In contrast, the annual life-threatening arrhythmic event rate was 7.7% per year in all the study patients. During the follow-up period, 14/102 (13.7%) patients had ICD-related complications. Device-related complications occurred in 6 patients, in which one required a generator and lead removal due to generator pocket infection and in which 5 required a lead revision. Inappropriate ICD interventions occurred in 9 patients due to sinus tachycardia in 5 patients, atrial fibrillation/tachycardia in 3, and device malfunction in 1. In those patients, both a device-related complication (i.e., lead revision) and an inappropriate ICD intervention occurred in only one patient.
Clinical characteristics
Table 1 summarizes the clinical characteristics of the 102 HCM patients. Median (Q1–Q3) age was 63 (52–71) years old. Regarding the conventional risk factors, family history of sudden death was identified in 22 patients (21.6%), syncope in 35 patients (34.3%), VT in 48 patients (47.1%), and maximum wall thickness ≥30 mm in 13 patients (12.7%). Atrial fibrillation was identified in 18 patients (17.6%). Median (Q1–Q3) LVEF was 63.7 (58.9–68.1) %. Intraventricular pressure gradient ≥30 mmHg, indicating obstructive HCM, was present in 51 patients (50.0%). 91 patients (89.2%) were implanted with ICD for primary prevention. We prescribed 100 patients (98.0%) β-blocker and 41 patients (40.2%) class I antiarrhythmic dugs. LGE was present in the 86 patients (84.3%). Median (Q1–Q3) LGE score was 5 (3–8). On analysis of the 4 conventional risk factors (excluding abnormal blood pressure response during exercise from the 5 conventional factors) in primary prevention patients, 19/91 patients (21%) had no risk factors; 51/91 patients (56%) had 1 risk factor; and 21/91 patients (23%) had ≥2 risk factors. Each patient without risk factors was implanted with ICD based on consensus by several expert cardiologists for the presence of one or more of the following reasons: bradyarrhythmia, LV aneurysm, DDD pacing for reduction of outflow obstruction, easily induced sustained VT/VF in electrophysiological study, and massive LGE.
We divided 91 patients implanted with ICD for primary prevention into the life-threatening arrhythmic events positive and the negative group, and compared clinical and imaging characteristics, shown in Table 2. Compared with the events negative group, the events positive group had a higher prevalence of male gender (19/23, 82.6% vs. 37/68, 54.4%, P = 0.016) and maximum LV wall thickness ≥20 mm plus LGE score ≥4 (15/23, 65.2% vs. 28/68, 41.2%, P = 0.046). Compared with the events negative group, the events positive group had a significantly lower prevalence of class I antiarrhythmic drug use (5/23, 21.7% vs. 33/68, 48.5%, P = 0.024). Maximum LV wall thickness, presence of LGE and LGE score did not significantly differ between the two groups. Maximum LV wall thickness and LGE score were analyzed using receiver operating characteristic curve to determine an optimal cut-off level. The cut-off level of maximum wall thickness was 20 mm, that of LGE score was 4 point.
Figure 1 shows CMR images of cine and LGE in two patients (A, B). The cine mode images (upper panel) and LGE images (lower panel) are shown at the identical slice in the same view for each patient. Patient A with left ventricular hypertrophy but without LGE did not experience the appropriate ICD intervention. Patient B with left ventricular hypertrophy and with marked LGE experienced the appropriate ICD intervention during follow-up period.
Follow-up results
We examined life-threatening arrhythmic events until September 2014 [median (Q1–Q3) follow-up 2.8 (1.6–5.1) years]. During the follow-up period, life-threatening arrhythmic events occurred in 27/102 study patients (sudden death due to VF regardless of appropriate ICD interventions in 1, alive with appropriate ICD interventions in 26). The annual event rate was 7.7% per year in all study patients. Figure 2a shows Kaplan–Meier survival curves for life-threatening arrhythmic events in the primary and secondary prevention group. The annual event rate was not significantly different in the two groups (primary 7.4%/year; secondary 10.3%/year, P = 0.582). Figure 2b shows Kaplan–Meier survival curves for life-threatening arrhythmic events in primary prevention patients with regard to the number of risk factors. The annual event rate was not significantly different in the no risk factor, 1 risk factor, and ≥2 risk factors groups (7.3 vs. 6.4 vs. 10.5%/year, P = 0.673). Figure 3 shows Kaplan–Meier survival curves for life-threatening arrhythmic events in the group with maximum LV wall thickness ≥20 mm plus LGE score ≥4 and in the other group without. The former group had a significantly higher event rate than in the latter group (11.1 vs. 4.6%/year, P = 0.038). No significant difference in the event rate was found between the groups with and without maximum LV wall thickness ≥20 mm (P = 0.097, not posted as figure) or between the groups with and without LGE score ≥4 (P = 0.082; not posted as figure).
Predictors for life-threatening arrhythmic events
Table 3 shows the results of the univariate and multivariate Cox proportional hazards regression analysis. Male gender (HR 2.545, 95% CI 0.8652–7.519, P = 0.090), class I antiarrhythmic use (HR 0.405, 95% CI 0.149–1.096, P = 0.075), LGE score ≥4 (HR 2.242, 95% CI 0.880–5.714, P = 0.090), and maximum LV wall thickness ≥20 mm plus LGE score ≥4 (HR 2.415, 95% CI 1.022–5.714, P = 0.044) were variables with P value <0.1 on univariate analysis. Furthermore, 3 variables (male gender, class I antiarrhythmic use, and maximum LV wall thickness ≥20 mm plus LGE score ≥4) were included in the multivariate Cox proportional hazards regression analysis using a stepwise selection method. Finally, only maximum LV wall thickness ≥20 mm plus LGE score ≥4 (HR 2.415, 95% CI 1.022–5.714, P = 0.044) was identified as independent predictor for life-threatening arrhythmic events.
Discussion
In the present study, the appropriate ICD intervention for the life-threatening arrhythmia and ICD-related complications was analyzed in Japanese patients with HCM. The annual appropriate intervention rate was 7.4% per year for the primary prevention and 10.3% per year for the secondary prevention. The annual ICD-related complications rate was 3.7% per year. Furthermore, a combination of maximum LV wall thickness ≥20 mm and LGE score ≥4 was a useful outcome predictive factor for life-threatening arrhythmia.
Previous studies reported that appropriate ICD intervention rates were 7–11% per year when ICD was implanted for secondary prevention [1, 3, 4, 6, 7]. In the present study, the appropriate intervention rate was 10.3% per year for secondary prevention, similar to those reported previously.
On the other hand, appropriate ICD intervention rates of 2–5% per year have been reported when ICD was implanted for primary prevention [1, 3,4,5,6,7]. Conventionally, indication of ICD implantation for primary prevention is decided based on the evaluation of five risk factors for sudden death in HCM patients: family history of sudden death; syncope; LV wall thickness ≥30 mm; NSVT; and abnormal blood pressure response during exercise [15, 16]. In general, patients who possess at least one of these risk factors are considered for implantation [8, 17]. However, many previous studies found no significant difference in the appropriate ICD intervention rate even when the number of risk factors increased [3, 4, 6]. A retrospective cohort study reported that the incidence of sudden death did not differ in patients with 0 or 1 risk factor, but the risk of sudden death increased with 2 or above risk factors [18].
As in previous reports, we also found no significant difference in appropriate ICD intervention rate for life-threatening arrhythmia even when the total number of risk factors increased. Another issue is that when evaluating the five conventional risk factors, there is uncertainty in evaluating the four factors (syncope; family history of sudden death; NSVT; and abnormal blood pressure response during exercise). For syncope, while unexplained syncope is an important risk factor, the risk of neurally mediated syncope is not different from that in patients without syncope [19]. However, it is often difficult for patients to remember the exact situation of syncope. The same applies to detailed memory of a family history of sudden death. For NSVT, the frequency and interval of Holter ECG recordings differ depending on the doctor’s enthusiasm. As for the evaluation of abnormal blood pressure response during exercise, since many HCM patients have the obstructive type, there is often hesitation in performing exercise test in these patients due to exercise-related risk.
Interestingly, the fact that a high appropriate intervention rate was also observed in patients with no conventional risk factor in this study further supports the notion that it is difficult to decide indication of ICD placement by evaluating the five conventional risk factors alone. Spirito et al. reported that risk of sudden death was not negligible in HCM patients without risk factors, and mentioned the importance of risk evaluation other than the five conventional risk factors [20]. Recently, a new clinical risk prediction model for HCM patients has been proposed at the European Society of Cardiology [21]. However, this model is inadequate in its present form [22].
In this our study, the appropriate intervention rate for patients with primary prevention indication was 7.4% per year, which was higher than previous reports [1, 3,4,5,6,7]. LGE was detected in more LV segments for HCM patients for primary prevention in the present study than for HCM patients in our previous large cohort study (4.7 ± 3.4 vs. 3.5 ± 3.1 segments per patient) [13]. We also reported that the arrhythmia event rate was higher in the group with LGE score ≥4 than in that with LGE score ≤3 [9]. Therefore, a possible reason for the higher intervention rate in this study is that we considered the finding of LGE on CMR for deciding indication of ICD placement.
In addition, recent studies from Western countries have proposed to include LGE on CMR as additional risk factor of sudden death in HCM patients. Maron et al. have proposed the addition of LGE greater than 15% of left ventricular mass to the five conventional risk factors as major risk markers [23].
Although many reports have demonstrated a relationship between LGE and sudden cardiac death, it is difficult to accurately determine whether a sudden cardiac death is due to life-threatening ventricular arrhythmia. At the present time, ICD can sensitively detect arrhythmia. In previous large HCM studies [10, 11], ICD was implanted only in a part of the study patients, and the outcome event was often defined as the occurrence of sudden cardiac death, aborted cardiac arrest, or appropriate ICD therapy. In the large number of study with 20% HCM patients implanted ICD, Chan et al. noted that the outcome event occurred in 37 patients: 14 died suddenly, 6 survived an aborted cardiac arrest, and 17 had appropriate ICD therapy for VT/VF [10].
While many studies have examined the ICD intervention rates in HCM patients, there are few reports on the relationship between CMR findings and ICD intervention rate only in HCM patients implanted with ICD. Prinz et al. reported the correlation of the incidence of ICD interventions with LGE findings in 87 HCM patients implanted with ICD [12]. They noted that severe myocardial fibrosis (LGE in ≥2 of 17 left ventricular segments) was independently associated with VT/VF events on multiple linear regression analysis. On the other hand, the five conventional risk factors were not significantly associated with VT/VF events. In order to clearly evaluate the relationship between life-threatening arrhythmia and LGE, we also focused on HCM patients implanted with ICD. In our study of HCM patients implanted with ICD, the degree of hypertrophy and the extent of LGE individually were not identified as independent prognostic factors of life-threatening arrhythmic events. Although LGE is more likely to be found at sites with more severe hypertrophy in HCM [24], variation in the extent of LGE among patients with the same wall thickness is often observed in the clinical setting. Furthermore, even in the same patient, progression of LGE over time has been reported [25, 26]. To address this issue, we examined a composite factor consisting of degree of hypertrophy and extent of LGE, and we found that a combination of myocardial hypertrophy and LGE was an independent prognostic factor for life-threatening arrhythmic events.
Given these circumstances, risk evaluation using CMR as shown in the present study is an objective risk evaluation method. We expect that combining this wall thickness factor with LGE would improve the prognostic value of the risk factors.
Limitations
This study has several limitations. First, the study population of 102 patients was not enough. Next, because of the single-center retrospective study design, the indications of ICD implantation were not consistent. Furthermore, for analysis of patient background, one of the five conventional risk factors could not be evaluated. Finally, the evaluation of LGE on CMR was not quantitative, but based on visual inspection. In the future, a multicenter prospective study should be considered, which enrolls a large number of patients, evaluates all relevant risk factors and measures LGE quantitatively.
Conclusions
In the present single-center study of 102 Japanese HCM patients implanted with ICD, a combination of myocardial hypertrophy and LGE is a useful outcome predictive factor for life-threatening ventricular arrhythmia.
References
Maron BJ, Shen WK, Link MS, Epstein AE, Almquist AK, Daubert JP, Bardy GH, Favale S, Rea RF, Boriani G, Estes NA 3rd, Spirito P (2000) Efficacy of implantable cardioverter-defibrillators for the prevention of sudden death in patients with hypertrophic cardiomyopathy. N Engl J Med 342:365–373
Begley DA, Mohiddin SA, Tripodi D, Winkler JB, Fananapazir L (2003) Efficacy of implantable cardioverter defibrillator therapy for primary and secondary prevention of sudden cardiac death in hypertrophic cardiomyopathy. Pacing Clin Electrophysiol 26:1887–1896
Maron BJ, Spirito P, Shen WK, Haas TS, Formisano F, Link MS, Epstein AE, Almquist AK, Daubert JP, Lawrenz T, Boriani G, Estes NA 3rd, Favale S, Piccininno M, Winters SL, Santini M, Betocchi S, Arribas F, Sherrid MV, Buja G, Semsarian C, Bruzzi P (2007) Implantable cardioverter-defibrillators and prevention of sudden cardiac death in hypertrophic cardiomyopathy. JAMA 298:405–412
Syska P, Przybylski A, Chojnowska L, Lewandowski M, Sterlinski M, Maciag A, Gepner K, Pytkowski M, Kowalik I, Maczynska-Mazuruk R, Ruzyllo W, Szwed H (2010) Implantable cardioverter-defibrillator in patients with hypertrophic cardiomyopathy: efficacy and complications of the therapy in long-term follow-up. J Cardiovasc Electrophysiol 21:883–889
Vriesendorp PA, Schinkel AF, Van Cleemput J, Willems R, Jordaens LJ, Theuns DA, van Slegtenhorst MA, de Ravel TJ, ten Cate FJ, Michels M (2013) Implantable cardioverter-defibrillators in hypertrophic cardiomyopathy: patient outcomes, rate of appropriate and inappropriate interventions, and complications. Am Heart J 166:496–502
Thavikulwat AC, Tomson TT, Knight BP, Bonow RO, Choudhury L (2016) Appropriate implantable defibrillator therapy in adults with hypertrophic cardiomyopathy. J Cardiovasc Electrophysiol 27:953–960
Magnusson P, Gadler F, Liv P, Morner S (2016) Risk markers and appropriate implantable defibrillator therapy in hypertrophic cardiomyopathy. Pacing Clin Electrophysiol 39:291–301
JCS Joint Working Group (2016) Guidelines for diagnosis and treatment of patients with hypertrophic cardiomyopathy (JCS 2012) - digest version. Circ J 80(3):753–774. doi:10.1253/circj.CJ-66-0122
Hen Y, Iguchi N, Utanohara Y, Takada K, Machida H, Takayama M, Sumiyoshi T (2014) Prognostic value of late gadolinium enhancement on cardiac magnetic resonance imaging in Japanese hypertrophic cardiomyopathy patients. Circ J 78:929–937
Chan RH, Maron BJ, Olivotto I, Pencina MJ, Assenza GE, Haas T, Lesser JR, Gruner C, Crean AM, Rakowski H, Udelson JE, Rowin E, Lombardi M, Cecchi F, Tomberli B, Spirito P, Formisano F, Biagini E, Rapezzi C, De Cecco CN, Autore C, Cook EF, Hong SN, Gibson CM, Manning WJ, Appelbaum E, Maron MS (2014) Prognostic value of quantitative contrast-enhanced cardiovascular magnetic resonance for the evaluation of sudden death risk in patients with hypertrophic cardiomyopathy. Circulation 130:484–495
Briasoulis A, Mallikethi-Reddy S, Palla M, Alesh I, Afonso L (2015) Myocardial fibrosis on cardiac magnetic resonance and cardiac outcomes in hypertrophic cardiomyopathy: a meta-analysis. Heart 101:1406–1411
Prinz C, Schwarz M, Ilic I, Laser KT, Lehmann R, Prinz EM, Bitter T, Vogt J, van Buuren F, Bogunovic N, Horstkotte D, Faber L (2013) Myocardial fibrosis severity on cardiac magnetic resonance imaging predicts sustained arrhythmic events in hypertrophic cardiomyopathy. Can J Cardiol 29:358–363
Hen Y, Iguchi N, Utanohara Y, Takada K, Machida H, Takara A, Teraoka K, Sumiyoshi T, Takamisawa I, Takayama M, Yoshikawa T (2016) Extent of late gadolinium enhancement on cardiac magnetic resonance imaging in Japanese hypertrophic cardiomyopathy patients. Circ J 80:950–957
Cerqueira MD, Weissman NJ, Dilsizian V, Jacobs AK, Kaul S, Laskey WK, Pennell DJ, Rumberger JA, Ryan T, Verani MS (2002) Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart. A statement for healthcare professionals from the cardiac imaging committee of the council on clinical cardiology of the American Heart Association. Circulation 105:539–542
Elliott PM, Poloniecki J, Dickie S, Sharma S, Monserrat L, Varnava A, Mahon NG, McKenna WJ (2000) Sudden death in hypertrophic cardiomyopathy: identification of high risk patients. J Am Coll Cardiol 36:2212–2218
Christiaans I, van Engelen K, van Langen IM, Birnie E, Bonsel GJ, Elliott PM, Wilde AA (2010) Risk stratification for sudden cardiac death in hypertrophic cardiomyopathy: systematic review of clinical risk markers. Europace 12:313–321
Gersh BJ, Maron BJ, Bonow RO, Dearani JA, Fifer MA, Link MS, Naidu SS, Nishimura RA, Ommen SR, Rakowski H, Seidman CE, Towbin JA, Udelson JE, Yancy CW (2011) 2011 ACCF/AHA guideline for the diagnosis and treatment of hypertrophic cardiomyopathy: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation 124:2761–2796
O’Mahony C, Tome-Esteban M, Lambiase PD, Pantazis A, Dickie S, McKenna WJ, Elliott PM (2013) A validation study of the 2003 American College of Cardiology/European Society of Cardiology and 2011 American College of Cardiology Foundation/American Heart Association risk stratification and treatment algorithms for sudden cardiac death in patients with hypertrophic cardiomyopathy. Heart 99:534–541
Spirito P, Autore C, Rapezzi C, Bernabo P, Badagliacca R, Maron MS, Bongioanni S, Coccolo F, Estes NA, Barilla CS, Biagini E, Quarta G, Conte MR, Bruzzi P, Maron BJ (2009) Syncope and risk of sudden death in hypertrophic cardiomyopathy. Circulation 119:1703–1710
Spirito P, Autore C, Formisano F, Assenza GE, Biagini E, Haas TS, Bongioanni S, Semsarian C, Devoto E, Musumeci B, Lai F, Yeates L, Conte MR, Rapezzi C, Boni L, Maron BJ (2014) Risk of sudden death and outcome in patients with hypertrophic cardiomyopathy with benign presentation and without risk factors. Am J Cardiol 113:1550–1555
O’Mahony C, Jichi F, Pavlou M, Monserrat L, Anastasakis A, Rapezzi C, Biagini E, Gimeno JR, Limongelli G, McKenna WJ, Omar RZ, Elliott PM (2014) A novel clinical risk prediction model for sudden cardiac death in hypertrophic cardiomyopathy (HCM risk-SCD). Eur Heart J 35:2010–2020
Maron BJ, Casey SA, Chan RH, Garberich RF, Rowin EJ, Maron MS (2015) Independent assessment of the European society of cardiology sudden death risk model for hypertrophic cardiomyopathy. Am J Cardiol 116:757–764
Maron BJ, Maron MS (2016) Contemporary strategies for risk stratification and prevention of sudden death with the implantable defibrillator in hypertrophic cardiomyopathy. Heart Rhythm 13:1155–1165
Choudhury L, Mahrholdt H, Wagner A, Choi KM, Elliott MD, Klocke FJ, Bonow RO, Judd RM, Kim RJ (2002) Myocardial scarring in asymptomatic or mildly symptomatic patients with hypertrophic cardiomyopathy. J Am Coll Cardiol 40:2156–2164
Todiere G, Aquaro GD, Piaggi P, Formisano F, Barison A, Masci PG, Strata E, Bacigalupo L, Marzilli M, Pingitore A, Lombardi M (2012) Progression of myocardial fibrosis assessed with cardiac magnetic resonance in hypertrophic cardiomyopathy. J Am Coll Cardiol 60:922–929
Choi HM, Kim KH, Lee JM, Yoon YE, Lee SP, Park EA, Lee W, Kim YJ, Cho GY, Sohn DW, Kim HK (2015) Myocardial fibrosis progression on cardiac magnetic resonance in hypertrophic cardiomyopathy. Heart 101:870–876
Acknowledgements
This study was supported by the Sakakibara Clinical Research Grant for Promotion of Science, 2016. We thank Mr. Naokazu Mizuno and Mr. Jun Matsuda of the staff at Sakakibara Heart Institute for technical assistance.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Hen, Y., Tsugu-Yagawa, M., Iguchi, N. et al. Prognostic value of cardiovascular magnetic resonance imaging for life-threatening arrhythmia detected by implantable cardioverter-defibrillator in Japanese patients with hypertrophic cardiomyopathy. Heart Vessels 33, 49–57 (2018). https://doi.org/10.1007/s00380-017-1030-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00380-017-1030-3