Abstract
To investigate the effects of shrubification—the global phenomena of an increase of shrubs in grasslands—on C, N, and P cycles, the changes in soil organic C, N, and P mineralization in an alpine meadow were compared across five plant communities: grasses and four shrub species–dominated patches. The nutrient content and stoichiometry (C:N:P) of leaves, litter, microbial biomass, and soil organic matter (SOM) were analyzed during vegetation season. Net rates of N and P mineralization were measured in situ in the top 20 cm of soil throughout the growing season, and organic C mineralization was determined under controlled conditions. Microbial C:N, C:P, and N:P ratios in the top 20 cm generally decreased with increasing plant size (height combined with crown diameter), associated with greater input of litter with lower C:nutrient ratios under shrubs. The net N and P mineralization rates in soil under shrubs were about 3- to sevenfold and 4- to 15-fold faster, respectively, compared with those under grasses. The increase in organic C mineralization under shrubs compared with that under grasses was much smaller than the increase of N or P mineralization under shrubs. This indicates faster turnover of nutrients than C leading to decoupling of organic C and nutrient mineralization across plant communities by shrubification. The C:N, C:P, and N:P ratios of organic pools mineralized in soil decreased with increasing plant size, but increased with respective microbial C:N, C:P, and N:P ratios across plant communities. This indicates that specific SOM pools were mineralized depending on plant communities and microbial stoichiometry in soil. Consequently, the decoupling of organic C and nutrient mineralization across plant communities is driven by microbial stoichiometry and increases by shrubification.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
A patchy structure of vegetation is very common globally: in subtropical savannas; arid, semiarid, and temperate grasslands; and tundra (Eldridge et al. 2011; Howison et al. 2017). This striking convergence in vegetation community structure can take two forms at least: woody patches interspersed in grassland (Eldridge et al. 2011) and a patchy distribution of herbaceous species (Howison et al. 2017; Zhao et al. 2021). The vegetation pattern consisting of woody and herbaceous patches is currently increasing—a phenomenon named “shrubification”—due to overgrazing, fire, the increase in CO2 concentration in the atmosphere, N deposition, and climate warming (Eldridge and Ding 2020; Brandt et al. 2013; Carboni et al. 2017). The patchy distribution of herbaceous species in grassland is explained by selective grazing by herbivores, heterogeneities in hydrothermal conditions, erosion intensities, and salinity levels caused by microtopography, and soil disturbance caused by burrowing mammals (HilleRisLambers et al. 2001; Howison et al. 2017). In either case, vegetation patchiness, in turn, induces heterogeneity in soil biochemical properties (Ettema and Wardle 2002; Hernandez et al. 2015). The availability of nutrients is usually higher under shrubs than in grassy areas (He and Li 2016; Baer et al. 2006; Eldridge et al. 2011; Gao et al. 2019). Soil organic C mineralization and N cycling vary spatially among vegetation patches in herbaceous plant-only structured grasslands (Zhao et al. 2021). However, how organic C mineralization changes with alterations of nutrient mineralization has never been investigated across vegetation mosaics in such spatially heterogeneous ecosystems.
Microorganisms must meet their growth requirements for energy and nutrients. According to the consumer-driven nutrient recycling theory, microorganisms mineralize organically bound N and P when the nutrient content in these substrates is high compared with the microbial growth requirements (Sterner and Elser 2002). When a nutrient is limited in relation to C in the substrate, more organic C is mineralized by microorganisms for mining the nutrient (Fang et al. 2020; Ge et al. 2020; Hicks et al. 2021). These findings suggest that organic C and nutrient mineralization in soil are regulated by the stoichiometry (elemental ratios) of both substrates and decomposer communities (Elser and Urabe 1999).
The control of organic C and nutrient mineralization by substrate stoichiometry is well known (Enríquez et al. 1993; Mooshammer et al. 2012; Liu et al. 2021; Schleuss et al. 2021). However, the regulation of consumer stoichiometry on an element release from soil organic matter (SOM) has not been proved experimentally. Due to variations in their physiology and life forms, plants produce litter of different qualities. Litter stoichiometry changes the microbial stoichiometry at organism and community levels (Sistla and Schimel 2012; Zechmeister-Boltenstern et al. 2015; Song et al. 2020; Camenzind et al. 2021). The microbial stoichiometric C:nutrient ratios reflect the nutrient demand by microorganisms for growth relative to C, or C and energy demand relative to nutrients (Clayton et al. 2021). Consequently, the microbial C and/or nutrient demand determines which pools to decompose. As such, changes in the stoichiometry of decomposer communities can affect organic C and nutrient mineralization across vegetation mosaics (Elser and Urabe 1999).
Plant inputs into soil determine the microbial stoichiometry (Binkley et al. 2000; Zhang et al. 2019; Wei et al. 2020). Shrubs growing in grassland increase the soil C:nutrient ratios compared with grasses (Zhou et al. 2018). These imply that the stoichiometric imbalances between microbial nutrient demand and content in SOM under shrubs will increase during shrubification. With increasing nutrient limitation (i.e., increasing stoichiometric imbalances), microorganisms will selectively decompose pools with high nutrient content from the bulk SOM to meet their nutrient demand (Ehtesham and Bengtson 2017; Murphy et al. 2015; Rousk et al. 2016). This will lead to more nutrients being released from substrates per unit of C mineralization under shrubs than under grasses. Consequently, nutrient mineralization under shrubs can increase greater compared with organic C mineralization, and this decoupling raises with SOM accumulation by shrubification.
The Qinghai–Tibet Plateau is a huge C reservoir due to high SOM content, vast area (Yang et al. 2008), and very slow mineralization rates. Ecosystems in this region are extremely sensitive to climate and land use changes as a result of harsh environmental conditions and very slow vegetation recovery. A significant change in grassland ecosystems in the past decades is the expansion of shrubs (Brandt et al. 2013; Lu et al. 2021). The effects of shrubification on organic C stocks, pools, accumulation, and decomposition as well as the C:N:P ratios have not been evaluated. The change in the organic C mineralization by shrubification is directly related to C storage, whereas N and P mineralization is linked with the availability of nutrients to plants and thus productivity. Therefore, an integrated investigation of organic C and nutrient mineralization is important for better understanding of the effects of the shrub expansion on C sequestration in grassland ecosystems.
Our objectives were (i) to investigate the effects of the shift of plant community composition from grasses to shrubs (four shrub species–dominated patch types) induced by shrubification on organic C, N, and P mineralization and (ii) to compare the patterns of organic C and nutrient mineralization across vegetation mosaics in a grazed alpine meadow on the Qinghai–Tibet Plateau. We tested following two hypotheses:
-
H1: The stoichiometric C:N, C:P, and N:P ratios of mineralized pools increase with respective elemental ratios of soil microorganisms in response to the chemical characteristics of plant litter inputs;
-
H2: Soil organic C, N, and P mineralizations are not coupled to each other across plant communities, and such decoupling increases with shrub size.
To test above hypotheses, the nutrient content and stoichiometry (C:N:P) of leaves, litter, microbial biomass, and soil were analyzed regularly. Net rates of N and P mineralization were measured in situ in the top 20 cm of soil throughout the growing season. Organic C mineralization was determined under controlled conditions.
Materials and methods
Site description
The study site was located in Maqu County, Gannan Tibetan Autonomous Prefecture, Gansu Province (34° 30′ N, 101° 45′ E; 3432 m above sea level) on the eastern Qinghai–Tibet Plateau. A mean annual temperature of 2.2 °C and a mean annual rainfall of 672 mm have been recorded at a nearby weather station at Lanzhou University Research Station of Alpine Meadow and Wetland Ecosystems. In June 2019, we defined a field (100 m × 60 m) with a mosaic distribution of shrub and herbaceous species (Fig. S1). The terrain of the field was gently sloping (< 3°). The vegetation in the field was roughly divided into five areas (labeled A1, A2, A3, A4, and A5, respectively) (Fig. S1). Grassy vegetation (A1) covered about 22% of the field, consisting of species from the genera Kobresia, Leontopodium, Anemone, Melissitus, Elymus, Stipa, Carex, Poa, and Saussurea (Fig. S1). Shrubby patches included four types: (i) dominated by Caragana brevifolia (a leguminous plant, mainly distributed in A2), (ii) Potentilla fruticosa (A3), (iii) Spiraea alpina (A4), and (iv) Salix oritrepha (A5) (Fig. S1). The shrub height increased in the following order: P. fruticosa (0.3–0.4 m) < C. brevifolia (0.5–0.6 m) < S. alpina (0.9–1.2 m) < S. oritrepha (1.2–2.2 m) (Table 1). The crown diameter was about 0.4–0.6 m for P. fruticosa, 0.3–0.5 m for C. brevifolia, 0.5–0.8 m for S. alpina, and 1–2 m for S. oritrepha. Because C. brevifolia was slimmer than P. fruticosa, the size (height combined with crown diameter) of shrubs generally increased according to the following order: C. brevifolia < P. fruticosa < S. alpina < S. oritrepha. The four shrubby patches of C. brevifolia, P. fruticosa, S. alpina, and S. oritrepha covered around 20%, 8%, 23%, and 27% of the field, respectively. Locals informed that all shrub species in the field were native, but their covers increased over the past two decades.
The soil is classified as shrubby meadow soil according to the Chinese soil classification system (similar to Cryaquolls in the US Soil Taxonomy), having loamy texture (clay: 8–13%; silt: 30–64%; sand: 40–62%) in the top 20 cm across the field. Soil pH was lower in shrubby (ranging 6.3–6.6) than in grassy (6.8) areas. Soil organic C, total N, and total P contents were all higher in shrubby (organic C ranging from 39.4 to 71.8 g kg−1; total N 3.1–5.6 g kg−1; total P 679–819 mg kg−1) than in grassy (18.7 g kg−1, 1.6 g kg−1; 474 mg kg−1) areas. The field was used for grazing yak (Bos grunniens) and sheep (Ovis aries) in the winter season (late October to early June) each year.
Analysis of soil N and P mineralization
The net N and P mineralization in soil was measured from 17 May to 18 October 2020 (153 days), by consecutively incubating buried samples, similar to the method used by Clark (1990) and Gao et al. (2019). Each incubation period lasted for 1 month. Four sampling plots (each with a diameter of 4–5 m) were defined for each shrub-dominated (C. brevifolia, P. fruticosa, S. alpina, or S. oritrepha) or grass patch as four replicates (Fig. S1). Each plot was situated at a distance of more than 10 m from the other plots. In total, 20 sampling plots (5 vegetation patch types × 4 replicates) were established within the field (Fig. S1). Within each plot, after removal of herbaceous plants and litter, two PVC tubes (each with an inner diameter of 4.5 cm and a height of 22 cm) were driven down to a depth of 20 cm at two randomly selected points to sample the soil. All tubes were designed with 15 evenly distributed holes (0.1 cm in diameter) on their walls to drain water if the soil became saturated. Each tube containing soil was tightly capped at the bottom and was then placed vertically back in the 0–20-cm layer at its original location to allow in situ incubation. After incubation for 1 month, the tubes were taken out. The soil from the tubes was then pooled to form a composite sample for each plot. This composite soil sample was used for extraction of the inorganic N and P content after the period of incubation. At the beginning of each incubation period, soils were also sampled at two points (close to the two tubes, respectively) using an auger (inner diameter 3.8 cm) down to the 20 cm. The two subsamples were pooled to form a composite soil sample, which was then used to measure the initial inorganic N and P content before incubation, as well as the microbial biomass C, N, and P; urease and acid phosphatase activity; pH; organic C; total N; and total P.
The non-incubated and incubated soil samples were transported on ice to the laboratory. In the lab, we immediately passed all the soil samples through a sieve with the 2-mm aperture. A portion of each non-incubated soil sample was stored at 4 °C for measurements of inorganic N and inorganic P content, enzyme activities, and microbial biomass C, N, and P. The remaining portion of each non-incubated soil sample was air-dried at room temperature for measurements of pH, organic C, total N, and total P content. The whole of each incubated soil sample was stored at 4 °C for measurement of inorganic N and P content only. Inorganic N and P and microbial C, N, and P were extracted within 1 day after sampling, and enzyme activities were determined within 1 week after sampling.
Inorganic N was extracted by dispersing 20 g of fresh soil at field moisture in 80 mL of 0.5 M potassium sulfate (K2SO4) and then shaking the solution for 1 h at 200 rev min−1 with a horizontal shaker. The ammonium (NH4+) and nitrate (NO3−) content of the filtrates was determined using a San++ Automated Wet Chemistry Analyzer (Skalar, Breda, Netherlands). Inorganic P was extracted by shaking 1 g of fresh soil in 50 mL of 0.5 M sulfuric acid (H2SO4) for 16 h (Bunemann et al. 2016). Inorganic P in the filtrates was measured using the ammonium molybdate spectrophotometric method at 700 nm (UV-1800; Mapada, Shanghai, China). The net amount of N or P mineralized during the incubation period was calculated as the difference in inorganic N (the sum of N in NH4+ and NO3−) or inorganic P in the soils between the beginning and the end of the incubation period. Phosphorus mineralization is usually difficult to detect owing to strong sorption of the released inorganic P onto solid surfaces. In our case, however, measuring in situ soil P mineralization according to the above protocol was feasible because of the low content of iron and aluminum oxides, as well as negligible carbonates versus the high organic P content in soil (Achat et al. 2010; Gao et al. 2019; Mou et al. 2020).
Analysis of soil microbial C, N, and P content and enzyme activity
Soil microbial C and N content was measured using a chloroform fumigation extraction method (Brookes et al. 1985; Vance et al. 1987). Soil microbial P was also determined using a chloroform fumigation (0.03 M ammonium fluoride (NH4F) + 0.025 M hydrogen chloride (HCl)) extraction method (Morel et al. 1996), with the potential effects of P adsorption corrected (Khan and Joergensen 2012). Urease activity was measured by determining the amount of NH4+ released from fresh soil after incubation for 2 h with 0.2 M urea in Tris-hydroxymethyl-aminomethane buffer at 37 ℃ (Tabatabai and Bremner 1972). Acid phosphatase activity was assayed by colorimetric determination of p-nitrophenol released from fresh soil after incubation for 1 h with p-nitrophenyl phosphate at a pH value of 6.5 and temperature of 37 °C (Hopkins et al. 2008). Activities of urease and acid phosphatase are related to hydrolysis rates of urea and phosphomonoesters in soil, respectively (Nannipieri et al. 2018). We defined the inorganic P extracted using 0.03 M NH4F + 0.025 M HCl from these soils with pH values < 7.0 as available P (Lu 2000).
Analysis of soil organic C, total N, total P content, and pH
Soil organic C, total N, and total P were analyzed in air-dried soil samples (ground to pass the 0.15-mm aperture). Soil organic C was measured by the Walkley and Black dichromate oxidation method (Nelson and Sommers 1982), and a factor of 1.33 was applied to adjust for the recovery of organic C (Bao 2000). Total N was measured by the Kjeldahl method after soil had been digested in concentrated H2SO4 in the presence of catalysts (Lu 2000). Total P was determined colorimetrically after digestion in a mixture of perchloric acid (HClO4) and H2SO4 (Lu 2000). On the basis of a modified Hedley P fractionation procedure, Mou et al. (2020) reported that organic P accounts for about 50% of total soil P in this area. Therefore, in the present study, we estimated soil organic P content by multiplying the total P by a factor of 0.5. Soil pH was determined for air-dried samples using a pH meter (PHS-3C; INESA Scientific Instrument Co., Ltd., Hangzhou, China) (soil:water = 1:2.5).
Analysis of C and nutrients in leaves and litter
In the growing season of 2019, leaves (aboveground biomass for grasses at community level; leaves and twigs for shrubs at species level) were sampled four times. Litter was collected from three random 0.25-m2 quadrats under each type of vegetation in late June, early August, and mid August. After leaf and litter were oven-dried to a constant weight at 65 °C, they were weighed and then ground to a powder with a ball mill. The C and N content of leaves and litter was measured using an elemental analyzer (vario MACRO cube; Elementar, Langenselbold, Germany). The total P content was measured colorimetrically after samples had been digested in a hydrogen peroxide (H2SO4–H2O2) mixture (Lu 2000).
Determination of soil organic C mineralization potential
It is difficult to accurately measure soil organic C mineralization in situ. As an alternative, we measured soil organic C mineralization in the laboratory under controlled conditions. Replicate composite soil samples were taken in late summer from the top 20-cm layer of soil under each of the five vegetation types. Each 30 g air-dried soil sample (passing the 2-mm aperture) was incubated for 76 days at 15 °C (equivalent to the mean daily soil temperature in the field in the growing season), after the water content had been adjusted to 70% of the water-holding capacity. The CO2 released during incubation was trapped in sodium hydroxide (NaOH) solution and quantified by titrating the surplus NaOH using standard H2SO4 solution in the presence of excess BaCl2 (barium chloride). On days 1, 3, 6, 13, 22, 28, 42, and 60, the traps were replaced with new ones. Due to the spatial variation in organic C content within each vegetation type, the measured organic C mineralization data were normalized by the soil organic C content at specific locations, where N and P mineralizations were concurrently measured during the growing season. This was achieved by multiplying the measured mean C mineralization per mass of soil organic C of replicate samples under a vegetation type (C mineralization in lab) by the organic C content of a specific location (plot) where N and P mineralizations were measured (in situ soil organic C content, the average over sampling dates in the season). That is, the in situ C mineralization was calculated as.
\(\mathrm{In\:situ\:C\:mineralization}=\mathrm{In\:situ\:soil\:organic\:C\:content }\left(\mathrm{g }{\mathrm{kg}}^{-1}\mathrm{ soil}\right)\times \mathrm{C\:mineralization\:in\:lab\:}\left(\mathrm{mg\:C\:}{\mathrm{g}}^{-1}\mathrm{\:soil\:organic\:C}\right)\)
Despite differences in incubation conditions between nutrient and organic C mineralization, the results of N and P or organic C mineralization were comparable among vegetation types.
Statistical analysis
The elemental content in soil was based on the dry mass at 105 °C, and in leaves or litter on the dry mass at 65 °C. The elemental ratios in all data pools were expressed as molar ratios. Ratios of SOM C:N, C:P, and N:P were calculated using soil organic C, total N, and organic P content. We used two sets of analysis of variance (ANOVA) to assess the experimental data:
-
1.
ANOVA 1: Repeated measures ANOVA with sampling dates as repeated measures and vegetation type as a fixed factor was performed on all regularly measured parameters, including elemental contents and ratios in leaves, microbial elemental contents and ratios in soil, mineralization rates of soil N and P, soil enzyme activity, and soil inorganic N and available P content. Prior to statistical analysis, all data pools were checked for normality by the Kolmogorov–Smirnov test. Leaf P content and N:P ratio, soil microbial C:P and N:P, and urease and acid phosphatase activity did not follow the normal distribution. Therefore, a logarithmic transformation was made for leaf P content and N:P ratio, and reciprocal transformation for soil microbial C:P and N:P ratio and urease and acid phosphatase activity. The least significance difference was used to identify differences between means at p < 0.05.
-
2.
ANOVA 2: We did not expect that the effects of vegetation type on regularly determined litter accumulation, elemental contents of litter, soil pH, organic C, total N, and total P content to change with sampling date. Therefore, regularly measured values of these variables in a sampling plot under each vegetation type were averaged across sampling dates, and one-way ANOVA was then conducted to assess the effects of vegetation on these variables (including elemental ratios) at p < 0.05. In ANOVA 2, all data pools followed the normal distribution.
The organic C mineralization rate was calculated by dividing the total cumulative C released as CO2 in days 29–76 by the total number of 48 days (Fig. S2). In the two-way ANOVA, we found the effects of vegetation type on microbial C:N, C:P, and N:P ratios and mineralization rates of soil N and P did not change with sampling date or duration. For this reason, as well as for matching the structure of organic C mineralization data, we recalculated the mineralization rates of soil N and P by dividing the total cumulative N or P released over five in situ incubation periods by the total number of days (153) of the five periods. Based on the C mineralization rate and recalculated mineralization rates of soil N and P, we then computed the mineralization ratios of C:N, C:P, and N:P. We also recalculated the elemental ratios of soil microbial biomass by averaging these ratios over five sampling dates within each of the replicate plots under every vegetation type. Afterwards, one-way ANOVA was used to assess the effects of vegetation on mineralization rates and ratios of soil C, N, and P, and elemental ratios of soil microbial biomass.
All ANOVAs were performed using SPSS version 19.0 (SPSS Inc., Chicago, IL, USA). Regression relationships between parameters were plotted using Origin 2018 (Origin Lab, USA).
Results
Carbon and nutrient content and their stoichiometric ratios in leaves and litter
The effects of vegetation on leaf C and N content changed with sampling time (significant interactions at p = 0.005 and p = 0.002, respectively; Fig. 1a, b), but vegetation independently affected the leaf P content (main effect p < 0.001; Fig. 1c) (Table S1). On every sampling date, the leaf C content in all shrubs was generally higher than in grasses (Fig. 1a). Averaged over dates, the leaf N content in non-leguminous shrubs was higher by 18–36% and that in leguminous shrubs by 71%, compared with that in grasses (Fig. 1b). The leaf P content in all shrubs was 15–33% higher than in grasses (Fig. 1c).
Dynamics of C, N, and P content (g C, N, or P kg−1) (a–c) and their molar ratios in leaves (d–f) under five vegetation types, namely grasses (at community level) and four shrubs (species level): Caragana brevifolia, Potentilla fruticosa, Spiraea alpina, and Salix oritrepha. Bars represent ± 1 standard error (n = 3). The time scale shows the sampling dates. Results of repeated measures analysis of variance are shown in Table S1
The effects of vegetation on leaf C:N and N:P ratios changed with sampling date (both with significant interaction effect at p < 0.001; Fig. 1d, f), but vegetation independently affected the leaf C:P ratio (main effect p < 0.001; Fig. 1e) (Table S1). On each sampling date, the C:N and C:P ratios of leaves of all shrubs were lower than those of grasses (Fig. 1d, e). The leaf N:P ratio throughout the growing season was higher in leguminous C. brevifolia than in grasses and non-leguminous shrubs (Fig. 1f).
The aboveground litter accumulation was negligible in grassy areas and increased with the size of shrubs (Table 1). The litter N and P content under all shrub species were 225–315% and 67–124% higher than those under grasses, respectively (Table 1). As a result, litter under all shrub species had lower C:N and C:P ratios but a higher N:P ratio than litter under grasses (Table 1).
Contents of soil organic C and nutrients and stoichiometric ratios of soil organic matter
The soil pH under all shrubs was lower by at least 0.2 unit than in grassy areas (Table 1). The soil organic C content under shrubs was higher by 111–284%, total N by 94–250%, and total and organic P by 43–73%, than under grasses, all increasing with plant size (Table 1). The C:N ratio of SOM under shrubs generally increased, and both the C:P and N:P ratios rose markedly in the following order: grasses < C. brevifolia ≤ P. fruticosa < S. alpina ≤ S. oritrepha (Table 1).
Microbial C, N, and P content and their stoichiometric ratios
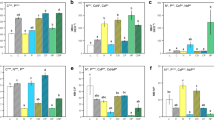
The effects of vegetation on the microbial C, N, and P content in soil depended on the sampling time (interactions at p = 0.018, < 0.001, and < 0.001, respectively; Fig. 2a-c) (Table S1). The microbial C, N, and P contents in soil on each sampling date were always highest under S. oritrepha (607–1086 mg kg−1, 157–251 mg kg−1, and 44–85 mg kg−1, respectively, across dates); intermediate under C. brevifolia, P. fruticosa, and S. alpina (339–697 mg kg−1, 87–152 mg kg−1, and 27–56 mg kg−1, respectively, across dates and plant communities); and lowest under grasses (194–395, 50–75, and 12–19 mg kg−1, respectively, across dates) (Fig. 2a, c). Averaged across dates and compared with grasses, the microbial biomass C, N, and P contents were 59–160%, 80–225%, and 106–331% higher, respectively, under the four types of shrub patches (Fig. 2a-c). Across sampling dates, smaller proportions of soil organic C existed as soil microbial C under shrubs compared with grasses (Fig. 2d, Table S1). The proportion of total N existing as microbial N was not different between shrubs and grasses (Fig. 2e, Table S1). However, more organic P was present as microbial P under shrubs than under grasses (Fig. 2f, Table S1).
Dynamics of microbial C, N, and P content expressed as per unit of soil (a–c) or as per unit of soil organic C (SOC), total N (TN), and organic P (OP) (d–f), respectively, and molar ratios of elements in microbial biomass (g–i) in the top 20 cm of soil depending on five vegetation types, namely the grasses and four shrubberies dominated by Caragana brevifolia, Potentilla fruticosa, Spiraea alpina, and Salix oritrepha, respectively. Bars represent ± 1 standard error (n = 4). The time scale shows the sampling dates during the growing season in 2020. Results of repeated measures analysis of variance are shown in Table S1
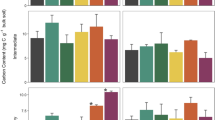
Vegetation independently affected the microbial C:N (main effect, p = 0.011), C:P (p < 0.001), and N:P (p < 0.001) ratios (Fig. 2g-i, Table S1). Averaged over sampling dates, the microbial C:N ratio was lower under all shrubs than that under grasses (Fig. 3a). The microbial C:P and N:P ratios were generally lowest under S. oritrepha; intermediate under C. brevifolia, P. fruticosa, and S. alpina; and highest under grasses (Fig. 3b, c). Soil microbial C:N and C:P ratios were generally correlated with respective C:N and C:P ratios of plant leaves or aboveground litter (Fig. 4a-d).
Effects of five vegetation types, namely grasses and shrubby patches dominated by Caragana brevifolia, Potentilla fruticosa, Spiraea alpina, and Salix oritrepha, on molar ratios of soil microbial C:N (a), C:P (b), and N:P (c); mineralization rates (mg C, N, or P kg−1 soil day−1) of soil organic C (d), N (e), and P (f); and mineralization molar ratios of C:N (g), C:P (h), and N:P (i), in the top 20 cm. Bars represent ± 1 standard error (n = 4). Lowercase letters indicate that means are different between vegetation types at p < 0.05
Dependences of soil microbial stoichiometric C:N (a, c) and C:P (b, d) ratios on respective C:N and C:P ratios of plant leaves (a, b) or aboveground litter (c, d). All data are means over sampling dates and replicated plots. Bars are ± 1 standard error (n = 20 for microbial biomass; n = 12 for leaves; n = 9 for litter). All regression lines are significant at p < 0.05. Note that the points representing the stoichiometric ratios of/under grasses are always outside of those of the shrubs. The black arrows show the increase in plant size
Mineralization rates and ratios of C and nutrients
The soil organic C mineralization rate under the four shrub species was 62–95% faster compared with that under grasses (Fig. 3d). Vegetation independently affected the mineralization rates of soil N (main effect, p < 0.001) and P (p < 0.001) (Fig. 5a, b; Table S1). The N and P mineralization rates in soil under shrubs over the season were about 3- to sevenfold and 4- to 15-fold greater, respectively, compared with those under grasses (Fig. 3e, f). Soil urease and acid phosphatase activities on every sampling date were greater under all shrubs than under grasses (Fig. 5c, d; Table S1). Responding to rapid nutrient mineralization under shrubs, soil N availability was higher under all shrubs and P availability was higher under shrubs with the exception of C. brevifolia, compared with grasses (Fig. 5e, f; Table S1).
Dynamics of soil N and P mineralization rates (a, b), urease and acid phosphatase activities (c, d), and mineral N and available P contents (e, f) in the top 20 cm of soil depending on five vegetation patch types, namely the grasses and four shrubberies dominated by Caragana brevifolia, Potentilla fruticosa, Spiraea alpina, and Salix oritrepha, respectively. Bars represent ± 1 standard error (n = 4). The time scale shows the sampling dates or incubation period during the growing season in 2020. Results of repeated measures analysis of variance are shown in Table S1
The C:N, C:P, and N:P ratios of mineralized pools over the season all decreased with increasing plant size (Fig. 3g-i). Across vegetation types, the C:N, C:P, and N:P ratios of mineralized pools increased with the C:N, C:P, and N:P ratios of soil microorganisms, respectively, but decreased with the C:N, C:P, and N:P ratios of SOM, respectively (Fig. 6a-f).
Correlations of the stoichiometric elemental ratios (mole:mole) between the mineralized pool and microbial biomass (a–c) and between the mineralized pool and bulk soil organic matter (SOM) (d–f) in the top 20 cm across grasses and shrubby patches dominated by Caragana brevifolia, Potentilla fruticosa, Spiraea alpina, and Salix oritrepha. All regression lines are significant at p < 0.05. The thin dashed lines in the left-hand plots denote the 1:10 or 1:1 ratios
Discussion
Stoichiometry of soil organic matter and microbial biomass
There were contrasting changes in the stoichiometry between microbial biomass and SOM across vegetation types (Fig. 3a-c, Table 1). This indicates increasing stoichiometric imbalances between nutrient demand by microorganisms and content in SOM with plant size. The higher organic C, total N, and organic P in soil under shrubs compared with those under grasses were attributed to the greater input of shrub litter (Table 1). Shrubs also have much larger root systems compared with grasses (He and Li 2016; Gao et al. 2019), resulting in a larger and longer-lasting belowground C input through root turnover and rhizodeposition (Hicks et al. 2020; Stock et al. 2019). In shrubby patches, however, the increases in N and P in SOM were smaller than that in C, and the increase in organic P was, in turn, smaller than that in N, resulting in increased SOM C:N, C:P, and N:P ratios with increasing shrub size (Table 1).
The higher levels of plant litter input under shrubs also fed larger sizes of soil microbial biomass in shrubby relative to grassy areas. However, the increase in the microbial C under shrubs was smaller than that in the organic C, microbial N under shrubs increased as a similar magnitude as the total N, but microbial P in shrubby patches increased more than the organic P. This is indicated by the decreased soil organic C–normalized microbial C under shrubs, unchanged total N–normalized microbial N under shrubs, but increased organic P–normalized microbial P under shrubs compared with grasses (Fig. 2d-f). Differential accruals of microbial C, N, and P relative to respective C, N, and P in SOM under shrubs implied that the microbial turnover played a more important role in the cycling or accumulation of nutrients than C by shrubification.
Changes in the microbial stoichiometry depend on the stoichiometry of substrates derived from plant inputs (Binkley et al. 2000; Zhang et al. 2019; Wei et al. 2020). The lower microbial C:N and C:P ratios under shrubs (Fig. 2g, h and Fig. 3a, b) were consistent with the lower C:N and C:P ratios of the leaves (Fig. 1d, e) or litter (Table 1) under all shrubs compared with those under grasses. Regression analysis indicated that microbial C:N and C:P ratios generally increased with respective leaf or litter stoichiometric C:N and C:P ratios across plant communities (Fig. 4a-d). The withered leaves accumulated on the soil surface undergo physical fragmentation and leaching by rain. Recurrent input of leachates from litter with nutrients (Berg and McClaugherty 2008; Wang et al. 2021a) leads to changes in microbial community composition (Chandregowda et al. 2018; Schroeder et al. 2020; Wei et al. 2020; Liu et al. 2021). The nutrient content of microbial groups depends on their taxonomy and life strategies (Elser et al. 2003; Fierer et al. 2007; Ren et al. 2017). In addition, microorganisms can adjust their stoichiometry by altering the allometric patterns and protoplasm chemistry at the individual cell level (Sistla and Schimel 2012; Chen et al. 2019; Camenzind et al. 2021).
Microbial stoichiometric regulation of soil organic C and nutrient mineralization
The C:N and C:P ratios of mineralized pools were clearly lower under shrubs than those under grasses (Fig. 3g, h), indicating that elemental composition of decomposing substrates differs between grasses and shrubs. Concurrently, soil microbial stoichiometric C:N and C:P ratios were lower in shrub communities (Fig. 3a, b). The C:N, C:P, and N:P ratios of mineralized pools increased with increasing respective C:N, C:P, and N:P ratios of microbial biomass across plant communities (Fig. 6a-c). Therefore, our first hypothesis (H1) that the stoichiometric C:N, C:P, and N:P ratios of mineralized pools increase with respective elemental ratios of soil microorganisms in response to the chemical characteristics of plant litter inputs was confirmed. As such, decomposing pools were defined by microbial stoichiometry. We calculated the stoichiometric elemental ratios of the mineralized soil pools using net N and P mineralization. In future studies, the gross N and P mineralization should be measured and the conditions should be the same as for C mineralization to further confirm the microbial stoichiometric regulation of C, N, and P mineralization across plant communities.
The smaller microbial C:nutrient ratios in soil under shrubs relative to those under grasses implied an increase in the nutrient demand by microorganisms relative to C (or a decrease in the C demand relative to nutrients) in shrubby areas. To meet their stoichiometric demand for growth, microbial communities under shrubs mobilized certain specific SOM pools having low C:nutrient ratios (Ehtesham and Bengtson 2017; Murphy et al. 2015; Rousk et al. 2016). Due to selective mineralization of high-nutrient-content compounds, more nutrients relative to C under shrubs were continuously removed from SOM, or more C relative to nutrients was preserved. This increased SOM C:N and C:P ratios with the plant size (Table 1). Consequently, the stoichiometric ratios of mineralized pools were negatively correlated with respective C:N, C:P, and N:P ratios of the bulk SOM (Fig. 6d-f).
The ratio range of N:P mineralization was similar to that of microbial N:P (Fig. 6c), suggesting that specific (high-nutrient-content) pools for decomposition included also microbial biomass (Díaz-Raviña et al. 1995; Blagodatsky and Richter 1998; Cui et al. 2020). The smaller microbial C:nutrient ratios in soils in shrubby areas could reflect an increasing abundance of fast-growing populations of microorganisms compared with grassy areas. Microbial populations with faster growth rates also generally have higher death rates (Blagodatsky and Richter 1998; Kuzyakov and Mason-Jones 2018; Chen et al. 2019), and thus more rapid turnover of nutrients within their biomass. In addition, microorganisms produce extracellular enzymes to hydrolyze high-nutrient-content polymers (e.g., proteins, chitin, nucleic acids) with small C:N and C:P ratios to meet their stoichiometric requirements for N and P (Mooshammer et al. 2014). The activities of urease and acid phosphatase were higher under shrubs than under grasses (Fig. 5c, d), and the ratio of urease to acid phosphatase activity was correlated with the N:P ratio in the microbial biomass or in the mineralized pool (Fig. S3a-b). Soil enzyme stoichiometry is tightly linked to microbial community composition and is nutrient-dependent across plant communities (Zhang et al. 2019; Wang et al. 2021b, c). The C:N and C:P mineralization ratios showed an approximately tenfold wider range than the microbial C:N and C:P ratios, respectively (Fig. 6a, b). This is because the released N and P were partly reutilized by microorganisms (Chen et al. 2019; Cui et al. 2020).
Decoupling of soil organic C, N, and P mineralization
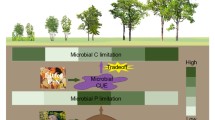
The rates of net soil N and P mineralization increased under shrubs relative to grasses much more than that of organic C mineralization (Fig. 3d-f). Phosphorus mineralization increased more than N mineralization under shrubs (Fig. 3e, f). Because microbial decomposers specifically target nutrient-rich pools, the decoupling of C and nutrient mineralization can occur (Ehtesham and Bengtson 2017; Murphy et al. 2015; Rousk et al. 2016; Cui et al. 2020). Therefore, our second hypothesis (H2) that soil C, N, and P mineralizations are not coupled to each other across vegetation patches and such decoupling increases with plant size was also confirmed. The more rapid nutrient mineralization in relation to C mineralization from the bulk SOM having high C:nutrient ratios under shrubs (Fig. 7) demonstrates that microorganisms can efficiently (re)cycle N and P in nutrient-limited ecosystems. This mechanism might have facilitated the expansion of shrubs in grasslands, helping overcome their nutrient constraints. The slower organic C mineralization compared with that of N and P under shrubs indicates great potential for C sequestration by shrubification, against increasing stoichiometric imbalance between nutrient demand by microorganisms and content in SOM.
Decoupling of organic C, N, and P mineralizations in soil by microbial stoichiometry in shrubby grasslands. Shrubs growing in grasslands increase C and nutrient inputs to soil and maintain larger and faster-growing microbial communities (i.e., low C:nutrient ratios) compared to grasses. To meet their high nutrient demands, microbial decomposers under shrubs increase the nutrient mineralization from specific pools (with high nutrient content) of the bulk soil organic matter to a greater extent compared to C mineralization. Consequently, N, P, and C mineralizations are decoupled by succession from grasses to big shrubs, and C, N, and P are mineralized proportionately to their ratios in the microbial biomass. Note that the C, N, and P mineralization rates (y-axis) are presented as ratios under shrubs relative to those under grasses
Conclusions
We demonstrated that the patchy distribution of vegetation in shrubby grasslands changes the litter inputs and differentially affects C and nutrient cycling associated with changed microbial stoichiometry in the soil (Fig. 7). The greater input of litter with high nutrient content under shrubs induced a larger microbial biomass with lower stoichiometric C:nutrient ratios in the soil as compared with grasses. To meet their greater nutrient requirements under shrubs, microorganisms under shrubs accelerated nutrient cycling by efficient selection of specific SOM pools with higher nutrient content in shrubby compared with grassy areas. Additionally, P mineralization under shrubs was stimulated by the greater microbial requirement for P compared with N. The increase in soil organic C mineralization under shrubs compared with that under grasses was much smaller than that of N or P mineralization (Fig. 7). These results indicate decoupling of C, N, and P mineralization from each other across the plant communities. We suggest that the decoupling of C, N, and P mineralization across plant communities in shrubby grasslands is associated with changed microbial stoichiometry. Shrubification benefits from soil N and P mineralization to overcome nutrient limitations, and less-stimulated mineralization of C than N and P constitutes a great potential for C sequestration under shrubs.
Data availability
Data presented in this paper can be obtained by sending a written request to the corresponding author.
References
Achat DL, Bakker MR, Zeller B, Pellerin S, Bienaimé S, Morel C (2010) Long-term organic phosphorus mineralization in Spodosols under forests and its relation to carbon and nitrogen mineralization. Soil Biol Biochem 42:1479–1490
Baer SG, Church JM, Williard KWJ, Groninger JW (2006) Changes in intrasystem N cycling from N2-fixing shrub encroachment in grassland: multiple positive feedbacks. Agric Ecosyst Environ 115:174–182
Bao S (2000) Soil agrochemical analysis. China Agricultural Press, Beijing
Berg B, McClaugherty C (2008) Plant litter: decomposition, humus formation, carbon sequestration, 2nd edn. Springer-Verlag, Berlin Heidelberg
Binkley D, Giardina C, Bashkin MA (2000) Soil phosphorus pools and supply under the influence of Eucalyptus saligna and nitrogen-fixing Albizia facaltaria. For Ecol Manag 128:241–247
Blagodatsky A, Richter O (1998) Microbial growth in soil and nitrogen turnover: a theoretical model considering the activity state of microorganisms. Soil Biol Biochem 30:1743–1755
Brandt JS, Haynes MA, Kuemmerle T, Waller DM, Radeloff VC (2013) Regime shift on the roof of the world: Alpine meadows converting to shrublands in the southern Himalayas. Biol Conserv 158:116–127
Brookes PC, Landman A, Pruden G, Jenkinson DS (1985) Chloroform fumigation and the release of soil-nitrogen–a rapid direct extraction method to measure microbial biomass nitrogen in soil. Soil Biol Biochem 17:837–842
Bunemann EK, Augstburger S, Frossard E (2016) Dominance of either physicochemical or biological phosphorus cycling processes in temperate forest soils of contrasting phosphate availability. Soil Biol Biochem 101:85–95
Camenzind T, Grenz KP, Lehmann J, Rillig MC (2021) Soil fungal mycelia have unexpectedly flexible stoichiometric C: N and C: P ratios. Ecol Lett 24:208–218
Carboni M, Gueguen M, Barros C, Georges D, Boulangeat I, Douzet R, Dullinger S, Klonner G, van Kleunen M, Essl F (2017) Simulating plant invasion dynamics in mountain ecosystems under global change scenarios. Glob Change Biol 24:e289–e302
Chandregowda MH, Murthy K, Bagchi S (2018) Woody shrubs increase soil microbial functions and multifunctionality in a tropical semi-arid grazing ecosystem. J Arid Environ 155:65–72
Chen J, Seven J, Zilla T, Dippold MA, Blagodatskaya E, Kuzyakov Y (2019) Microbial C:N: P stoichiometry and turnover depend on nutrients availability in soil: A 14C, 15N and 33P triple labelling study. Soil Biol Biochem 131:206–216
Clark JS (1990) Landscape interactions among nitrogen mineralization, species composition, and long-term fire frequency. Biogeochemistry 11:1–22
Clayton J, Lemanski K, Bonkowski M (2021) Shifts in soil microbial stoichiometry and metabolic quotient provide evidence for a critical tipping point at 1% soil organic carbon in an agricultural post-mining chronosequence. Biol Fertil Soils 57:435–446
Cui J, Zhu ZK, Xu XL, Liu SL, Jones DL, Kuzyakov Y, Shibistova O, Wu JS, Ge TD (2020) Carbon and nitrogen recycling from microbial necromass to cope with C: N stoichiometric imbalance by priming. Soil Biol Biochem 142:107720
Díaz-Raviña M, Acea MJ, Carballas T (1995) Seasonal changes in microbial biomass and nutrient flush in forest soils. Biol Fertil Soils 19:220–226
Ehtesham E, Bengtson P (2017) Decoupling of soil carbon and nitrogen turnover partly explains increased net ecosystem production in response to nitrogen fertilization. Sci Rep 7:46286
Eldridge DJ, Ding J (2020) Remove or retain: ecosystem effects of woody encroachment and removal are linked to plant structural and functional traits. New Phytol 229:2637–2646
Eldridge DJ, Bowker MA, Maestre FT, Roger E, Reynolds JF, Whitford WG (2011) Impacts of shrub encroachment on ecosystem structure and functioning: towards a global synthesis. Ecol Lett 14:709–722
Elser JJ, Urabe J (1999) The stoichiometry of consumer-driven nutrient recycling: theory, observation, and consequences. Ecology 80:735–751
Elser JJ, Acharya K, Kyle M, Cotner J, Makino W, Markow T, Watts T, Hobbie S, Fagan W, Schade J (2003) Growth rate-stoichiometry couplings in diverse biota. Ecol Lett 6:936–943
Enríquez S, Duarte CM, Sand-Jensen K (1993) Patterns in decomposition rates among photosynthetic organisms: the importance of detritus C:N: P content. Oecologia 94:457–471
Ettema CH, Wardle DA (2002) Spatial soil ecology. Trends Ecol Evol 17:177–183
Fang Y, Singh BP, Collins D, Armstrong R, Zwieten LV, Tavakkoli E (2020) Nutrient stoichiometry and labile carbon content of organic amendments control microbial biomass and carbon-use efficiency in a poorly structured sodic-subsoil. Biol Fertil Soils 56:219–233
Fierer N, Bradford MA, Jackson RB (2007) Toward an ecological classification of soil bacteria. Ecology 88:1354–1364
Gao XL, Li XG, Zhao L, Kuzyakov Y (2019) Regulation of soil phosphorus cycling in grasslands by shrubs. Soil Biol Biochem 133:1–11
Ge T, Luo Y, Singh BP (2020) Resource stoichiometric and fertility in soil. Biol Fertil Soils 56:1091–1092
Hernandez RR, Debenport SJ, Leewis MCCE, Ndoye F, Nkenmogne KIE, Soumare A, Thuita M, Gueye M, Miambi E, Chapuis-Lardy L, Diedhiou I, Dick RP (2015) The native shrub, Piliostigma reticulatum, as an ecological “resource island” for mango trees in the Sahel. Agric Ecosyst Environ 204:51–61
He JL, Li XG (2016) Potentilla fruticosa has a greater capacity to translocate phosphorus from the lower to upper soils than herbaceous grasses in an alpine meadow. Agric Ecosyst Environ 228:19–29
Hicks LC, Lajtha K, Rousk J (2021) Nutrient limitation may induce microbial mining for resources from persistent soil organic matter. Ecology 102:e03328
Hicks LC, Leizeagal A, Rousk K, Michelsen A, Rousk J (2020) Simulated rhizosphere deposits induce microbial N-mining that may accelerate shrubification in the subarctic. Ecology 101:e03094
HilleRisLambers R, Rietkerk M, van den Bosch F, Prins HHT, de Kroon H (2001) Vegetation pattern formation in semi-arid grazing systems. Ecology 82:50–61
Hopkins DW, Sparrow AD, Shillam LL, English LC, Dennis PG, Novis P, Elberling B, Gregorich EG, Greenfield LG (2008) Enzymatic activities and microbial communities in an Antarctic dry valley soil: responses to C and N supplementation. Soil Biol Biochem 40:2130–2136
Howison RA, Olff H, van de Koppel J, Smit C (2017) Biotically driven vegetation mosaics in grazing ecosystems: the battle between bioturbation and biocompaction. Ecol Monogr 87:363–378
Khan KS, Joergensen RG (2012) Relationships between P fractions and the microbial biomass in soils under different land use management. Geoderma 173:274–281
Kuzyakov Y, Mason-Jones K (2018) Viruses in soil: nano-scale undead drivers of microbial life, biogeochemical turnover and ecosystem functions. Soil Biol Biochem 127:305–317
Liu XJA, Hayer M, Mau RL, Schwartz E, Dijkstra P, Hungate BA (2021) Substrate stoichiometric regulation of microbial respiration and community dynamics across four different ecosystems. Soil Biol Biochem 163:108458
Lu RK (2000) Soil and agrochemical analysis methods. China Agricultural Science & Technology Press, Beijing
Lu X, Liang E, Camarero JJ, Ellison AM (2021) An unusually high shrubline on the Tibetan Plateau. Ecology 102:e03310
Mooshammer M, Wanek W, Schnecker J, Wild B, Leitner S, Hofhansl F, Blochl A, Hammerle I, Frank AH, Fuchslueger L (2012) Stoichiometric controls of nitrogen and phosphorus cycling in decomposing beech leaf litter. Ecology 93:770–782
Mooshammer M, Wanek W, Zechmeister-Boltenstern S, Richter A (2014) Stoichiometric imbalances between terrestrial decomposer communities and their resources: mechanisms and implications of microbial adaptations to their resources. Front Microbiol 5:22
Morel C, Tiessen H, Stewart JWB (1996) Correction for P-sorption in the measurement of soil microbial biomass P by CHCl3 fumigation. Soil Biol Biochem 28:1699–1706
Mou XM, Wu YN, Niu ZQ, Jia B, Guan ZH, Chen J, Li H, Cui HY, Kuzyakov Y, Li XG (2020) Soil phosphorus accumulation changes with decreasing temperature along a 2300 m altitude gradient. Agric Ecosyst Environ 301:107050
Murphy CJ, Baggs EM, Morley N, Wall DP, Paterson E (2015) Rhizosphere priming can promote mobilisation of N-rich compounds from soil organic matter. Soil Biol Biochem 81:236–243
Nannipieri P, Trasar-Cepeda C, Dick RP (2018) Soil enzyme activity: a brief history and biochemistry as a basis for appropriate interpretations and meta-analysis. Biol Fertil Soils 54:11–19
Nelson DW, Sommers LE (1982) Total carbon, organic carbon and organic matter, second ed. In: Page AL, Miller RH, Keeney DR (eds) Methods of soil analysis, part 2, chemical and microbiological properties agronomy monograph, vol 9. American Society of Agronomy Inc, Madison, pp 539–579
Ren C, Chen J, Deng J, Zhao FZ, Han XH, Yang GH, Tong XG, Feng YZ, Shelton S, Ren GX (2017) Response of microbial diversity to C:N: P stoichiometry in fine root and microbial biomass following afforestation. Biol Fertil Soils 53:457–468
Rousk K, Michelsen A, Rousk J (2016) Microbial control of soil organic matter mineralization responses to labile carbon in subarctic climate change treatments. Glob Change Biol 22:4150–4161
Schleuss PM, Widdig M, Biederman LA, Borer ET, Crawley MJ, Kirkman KP, Seabloom EW, Wragg PD, Spohn M (2021) Microbial substrate stoichiometry governs nutrient effects on nitrogen cycling in grassland soils. Soil Biol Biochem 155:108168
Schroeder J, Jannoura R, Beuschel R, Pfeiffer B, Dyckmans J, Murugan R, Chavannavar S, Wachendorf C, Joergensen RG (2020) Carbon use efficiency and microbial functional diversity in a temperate Luvisol and a tropical Nitisol after millet litter and N addition. Biol Fertil Soils 56:1139–1150
Sistla AA, Schimel JP (2012) Stoichiometric flexibility as a regulator of carbon and nutrient cycling in terrestrial ecosystems under change. New Phytol 196:68–78
Song M, Yu L, Fu S, Korpelainen H, Li C (2020) Stoichiometric flexibility and soil bacterial communities respond to nitrogen fertilization and neighbor competition at the early stage of primary succession. Biol Fertil Soils 56:1121–1135
Sterner RW, Elser JJ (2002) Ecological stoichiometry: the biology of elements from molecules to the biosphere. Princeton University Press, Princeton
Stock S, Köster M, Dippold MA, Najera F, Merino-Guzman C, Matus F, Boy J, Merino C, Spielvogel S, Gorbushina A (2019) Environmental drivers and stoichiometric constraints on enzyme activities in soils from rhizosphere to continental scales. Geoderma 337:973–982
Tabatabai MA, Bremner JM (1972) Assay of urease activity in soils. Soil Biol Biochem 4:479–487
Vance ED, Brookes PC, Jenkinson DS (1987) An extraction method for measuring soil microbial biomass-C. Soil Biol Biochem 19:703–707
Wang L, Chen Y, Zhou Y, Xu Z, Tan B, You C, Zhang L, Li H, Zheng H, Guo L, Wang L, Huang Y, Zhang J, Liu Y (2021a) Environmental conditions and litter nutrients are key determinants of soluble C, N, and P release during litter mixture decomposition. Soil till Res 209:104928
Wang G, Gao Q, Yang Y, Hobbie SE, Reich PB, Zhou J (2021b) Soil enzymes as indicators of soil function: a step toward greater realism in microbial ecological modeling. Glob Change Biol 28:1935–1950
Wang J, Wu Y, Li J, He Q, Bing H (2021c) Soil enzyme stoichiometry is tightly linked to microbial community composition in successional ecosystems after glacier retreat. Soil Biol Biochem 162:108429
Wei X, Zhu Z, Liu Y, Luo Y, Deng Y, Xu X, Liu S, Richter A, Shibistova O, Guggenberger G, Wu J, Ge T (2020) C:N: P stoichiometry regulates soil organic carbon mineralization and concomitant shifts in microbial community composition in paddy soil. Biol Fertil Soils 56:1093–1107
Yang YH, Fang JY, Tang YH, Ji CJ, Zheng CY, He JS, Zhu B (2008) Storage, patterns and controls of soil organic carbon in the Tibetan grasslands. Glob Chang Biol 14:1592–1599
Zechmeister-Boltenstern S, Keiblinger KM, Mooshammer M, Peňuelas J, Richter A, Sardans J, Wanek W (2015) The application of ecological stoichiometry to plant–microbial–soil organic matter transformations. Ecol Monogr 85:133–155
Zhang W, Xu Y, Gao D, Wang X, Liu W, Deng J, Han X, Yang G, Feng Y, Ren G (2019) Ecoenzymatic stoichiometry and nutrient dynamics along a revegetation chronosequence in the soils of abandoned land and Robinia pseudoacacia plantation on the Loess Plateau, China. Soil Biol Biochem 134:1–14
Zhao YN, Mou XM, Wei M, Li XG (2021) Effect of vegetation mosaic on spatial heterogeneity of soil organic carbon mineralization and nitrification in an alpine meadow. Appl Soil Ecol 165:104007
Zhou Y, Boutton TW, Wu XB (2018) Soil phosphorus does not keep pace with soil carbon and nitrogen accumulation following woody encroachment. Glob Change Biol 24:1992–2007
Acknowledgements
The authors thank the Lanzhou University Research Station of Alpine Meadow and Wetland Ecosystems for supporting the field sampling and measurements. YK is grateful for the support provided by the RUDN University Strategic Academic Leadership Program. The authors highly appreciate the constructive comments from the anonymous reviewers and Editor Paolo Nannipieri, which have greatly helped improve the quality of this paper.
Funding
This work was financed by the National Natural Science Foundation of China (41571279).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chen, J., Jia, B., Gang, S. et al. Decoupling of soil organic carbon and nutrient mineralization across plant communities as affected by microbial stoichiometry. Biol Fertil Soils 58, 693–706 (2022). https://doi.org/10.1007/s00374-022-01655-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00374-022-01655-8