Abstract
An analysis of soil chemical properties and microbial community compositions in spring, summer, and autumn over a growing season was conducted at three tea farms managed using the same cultivation method on Jeju Island, Korea. The contents of SOM (soil organic matter), TC (total C), TN (total N), TS (total S), potassium (K+), and lithium (Li+) increased from spring to autumn, and significant differences were observed among tea farms. Across all tea farms, the dominant bacterial phyla were Gammaproteobacteria (25.84 ± 1.66%), Alphaproteobacteria (17.99 ± 0.51%), Actinobacteria (18.38 ± 1.29%), and Acidobacteria (14.49 ± 0.79%), and the dominant fungal phyla were Ascomycota (45.16 ± 1.57%), Basidiomycota (26.76 ± 1.79%), and Mortierellomycota (23.59 ± 2.43%). We found distinct differences in the composition of the bacterial community among tea farms, whereas strong seasonal variations were observed in the composition of the fungal community. Important factors in determination of the bacterial relative abundance included water content, SOM, soil pH, EC (electrical conductivity), and contents of DOC (dissolved organic C), ammonium (NH4+), calcium (Ca2+), K+, and magnesium (Mg2+); however, only EC, DOC, and nitrate (NO3−) were important factors in the fungal relative abundance. The differences in soil chemical properties and microbial community compositions among tea farms could be attributed to the differences in environmental factors depending on the geographic location of tea farms. Seasonal variations in the contents of chemical components of tea leaves, such as catechins, total amino acids, theanine, and caffeine, were greater than the differences among the farms. The quality parameters of tea showed significant correlation with soil fungal diversity indices, indicating the possibility for use of soil fungal diversity as a biological indicator of tea quality.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Tea is one of the most popular beverages worldwide. The tea industry has shown continuous growth (Gunathilaka and Tularam 2016) with an annual increase in global tea consumption of 4.5% over the last decade. According to the FAO (2018), 5.5 million tons of tea were consumed in 2016. Among various types of teas, green tea, with its complex flavor and cultural significance (Ho et al. 2015), as well as the health benefits for patients with various diseases including cancer and cardiovascular diseases (Cabrera et al. 2006; Chacko et al. 2010), has received significant attention. In addition to the benefits of green tea, the safety and health benefits of Lactobacillus plantarum strains isolated from green tea leaves have been reported (Arellano et al. 2020; Park et al. 2020).
Among the numerous chemicals in green tea, catechins, amino acids, and caffeine are regarded as key quality indicators (Yao et al. 2005; Ho et al. 2015; Han et al. 2017). Catechins contribute to the overall flavor and astringency of green tea, whereas several free amino acids, particularly theanine, impart the umami and sweet taste (Kaneko et al. 2006; Han et al. 2017), while caffeine provides a bitter taste (Mozumder et al. 2020). Therefore, the complex balance of those constituents is considered to contribute to the overall flavor of the tea and determine the unique characteristics of green tea (Khokhar and Magnusdottir 2002; Wang et al. 2011). Geographic characteristics and environmental factors including soil moisture, nutrient availability, soil properties, altitude, seasons, and climate of tea farms have an effect on the contents of catechins, theanine, and caffeine (Fernandez et al. 2002; Kodama et al. 2007; Duncan et al. 2016; Han et al. 2017; Zhao et al. 2017). Thus, the combination of these characteristics determines the unique sensory properties of green tea in a particular region (Chan 2012; Han et al. 2017). Despite these climatic studies, few addressing the importance of tea farm soils, particularly soil microorganisms, have been reported. The function of soil microorganisms is critical in terrestrial ecosystem processes, including nutrient cycling, decomposition of organic matter, and primary production (Wagg et al. 2014; Kim et al. 2021), thereby contributing to the maintenance of soil fertility (Fierer 2017). Tea trees are perennial crops with a productive life span of more than 100 years (Mozumder et al. 2020), and soil chemistry and soil microorganisms interact closely with tea trees; therefore, the importance of soil microorganisms in tea cultivation should not be disregarded.
The direct effects of soil microorganisms on plants occur through root-associated organisms that form mutualistic or pathogenic relationships with plants, and indirect effects occur through the action of free-living microorganisms that increase the availability of essential nutrients and produce a variety of bioactive chemicals that protect the plants from pathogens (van der Heijden et al. 2008; Singh et al. 2011). Thus, soil microorganisms affect the growth and health of plants (Chaparro et al. 2012; Cai et al. 2017) and contribute to the quality and other characteristics of crops (Veloso et al. 2020). Interactions between soil microorganisms and plants have been reported in a variety of crops including coffee (Evizal et al. 2012; Jurburg et al. 2020) and grapes (Belda et al. 2017; Gupta et al. 2019); however, few studies have reported on the relationship of soil microorganisms in tea cultivation.
Previous studies examining mainly microbial biomass, community composition, or diversity among soils from tea farms focused on the periods of tea cultivation. For example, Xue et al. (2006) reported higher microbial biomass and activity in a 50-year-old tea orchard compared with 8- and 90-year-old orchards. Zhao et al. (2012) reported a decrease in the Shannon diversity index in tea orchards of different ages in the following order: 45 years > 25 years > 7 years > 70 years. Other studies investigating the community compositions of bacteria (Li et al. 2016) and fungi (Li et al. 2020) according to the age of tea farms found that long-term cultivation had resulted in significant change in the compositions of the bacterial and fungal community. Han et al. (2007) also reported that the microbial composition and biomass were strongly influenced by the intensity and duration of tea cultivation and that soil pH was an important factor. These previous studies have demonstrated that soil nutrient contents are diminished by long-term tea cultivation (Dang 2005; Li et al. 2016) and soil pH is significantly reduced due to the accumulation of fertilizers, leaf litter, antimicrobial substances, and root exudates, resulting in significant changes in soil microbial compositions (Tokuda and Hayatsu 2002; Xue et al. 2006). However, these previous studies only compared changes in soil chemical properties and microbial communities according to the tea cultivation period. These studies are limited to one-season observation and lack of understanding of spatiotemporal changes in soil chemical properties and microbial communities. Temporal and spatial observations are essential in the effort to attain a complete understanding of soil-microbial interactions on tea farms (Pandey and Palni 2002; Chen et al. 2021).
Depending on the geographic location of the tea farm, environmental characteristics such as slope, altitude, and microclimate are different. Because soil properties and microbial communities are sensitive to these environmental factors, they would be expected to exhibit not only seasonal but also spatial variations. In addition, as soil microorganisms affect the health and quality of crops (Singh et al. 2011; Evizal et al. 2012; Veloso et al. 2020), elucidating the relationship between soil microbial communities and tea characteristics is important. Given the importance of plant-soil-microorganism interactions in the rhizosphere, efforts to link tea cultivation and the knowledge of soil microorganisms at tea farms could be helpful in development of more effective soil management strategies for sustaining plant growth and improve the quality of tea under long-term cultivation (Dutta et al. 2015; Bhattacharyya and Sarmah 2018; Pineda et al. 2020).
The aim of this study was to identify seasonal variations in soil chemical properties, microbial community compositions, and tea leaf compositions among tea farms with different geographic locations within a close proximity and to determine the relationship between them. We hypothesized that differences in season and geographic location of tea farms determine the differences in soil chemical conditions, leading to differences in the soil microbial community compositions among tea farms, and that these variations may be associated with the quality of tea. The significance of this study is that it is the first study to report spatial and temporal variations in the soil chemical properties and microbial community compositions of tea farms.
Materials and methods
Study site and sampling
The study sites were Seogwang (SG), Dolsongyi (DS), and Hannam (HN) tea farms on Jeju Island, South Korea (Fig. S1). Detailed information on each sampling site is provided in Table 1. The period of tea cultivation is over 40 years at all three farms, which belong to the AmorePacific Group (Seoul, South Korea) and are managed with identical cultivation practices and fertilization schemes using the same tea species (Camellia sinensis). Detailed information on the fertilizer applied to each tea farm is provided in the supplementary material (Table S1).
Soil samples were collected at the time of leaf harvest over the growing season (April, June, and September 2020). Before soil sampling, litter, living vegetation, and plant debris were carefully removed using a knife and a shovel, and soil samples were collected to a depth of 10 cm from the soil surface with 4 replicates. All samples were immediately transported to the lab on ice and maintained at 4 °C for chemical analysis or stored at –80 °C until microbial analysis. Weather information over the sampling periods is provided in the supplementary material (Fig. S2).
Soil properties
Soil water content was measured by weight loss after drying at 105 °C for 24 h in an oven, and soil organic matter (SOM) was measured via the loss-on-ignition at 450 °C for 4 h in an oven. Dissolved organic C (DOC) was extracted with distilled water, filtered through a 0.45-μm membrane filter, and measured using a TOC analyzer (Shimadzu, Kyoto, Japan). Soil pH and electrical conductivity (EC) were measured in a 1:5 (soil/water) suspension using a pH meter (Thermo Scientific, Waltham, MA, USA). Total C (TC), total N (TN), and total S (TS) were measured in milled samples (Mixer Mill MM 400, Retsch) using an elemental analyzer (Vario MICRO Cube, Langenselbold, Germany). Analysis of ions, including ammonium (NH4+), nitrate (NO3−), potassium (K+), calcium (Ca2+), sodium (Na+), magnesium (Mg2+), and lithium (Li+), was performed at the National Instrumentation Center for Environmental Management (NICEM, Seoul, South Korea).
Jeju is a volcanic island; thus, the soil of our study sites is classified as Andisol (volcanic ash soils). Detailed information on soil texture was provided by AmorePacific Group (Seoul, South Korea) and presented in the supplementary material (Table S2).
Leaf composition
Fresh tea leaf samples were collected from each study site in June and September 2020. Thirty grams of fresh tea leaves was collected and composited from a radius of 2 m from the soil sampling site. Three replicate samples were then prepared for each sample occasion. Tea leaves were dried and then milled in a grinder (Cyclotec 1093, Foss Pacific Pty Ltd, Hillerød, Denmark). For analysis of leaf composition, 3 g of leaf powder was used. Near-infrared spectra were obtained using a spectrometer (XDS, Foss Pacific Pty Ltd) at a wavelength range of 400–2500 nm.
For determination of the catechin content, 50 mL of 50% methanol was added to 0.5 g of leaf powder. Ultrasonic extraction was performed for 30 min, and the supernatant was filtered through a 0.45-μm PTFE syringe filter. A UPLC (Waters, Milford, MA, USA) with a photodiode array detector was used for quantification of catechin content. An Agilent Zorbax Eclipse XDB C18 (2.1 × 100 mm, 1.8 μm) column with mobile phases of solvents A and B (A, 0.05% trifluoroacetic acid; B, 70% methanol and 30% acetonitrile) was used for chromatographic separation. The injection volume was 20 μL, and the temperature of the column oven was 40 °C. Elution was performed at a flow rate of 1 mL/min. The detection wavelength was set at 280 nm.
DNA extraction and sequencing
A DNeasy PowerSoil Kit (Qiagen, Hilden, Germany) was used according to the manufacturer’s instructions for extraction of microbial DNA from 0.25 g soil. A NanoDrop ND-1000 Spectrophotometer (NanoDrop Technologies Inc., Wilmington, DE, USA) was used for assessment of the quality and quantity of DNA.
For all of the DNA samples, we amplified the V3–V4 regions of the bacterial 16S rRNA gene with the primer set of 341F (5′-CCTACGGGNGGCWGCAG-3′) and 805R (5′-GACTACHVGGGTATCTAATCC-3′) (Klindworth et al. 2012) and the ITS1 region of the fungi with the primer set of ITS1-F (5′-CTTGGTCATTTAGAGGAAGTAA-3′) (Gardes and Bruns 1993) and ITS2 (5′-GCTGCGTTCTTCATCGATGC-3′) (White et al. 1990). Sequencing was performed at Macrogen, Inc. (Seoul, South Korea) using the Illumina Miseq platform (Illumina, Inc., San Diego, California, USA).
Sequencing data processing
QIIME2 (Bolyen et al. 2019) was used for analysis of paired-end reads. Reads were demultiplexed and trimmed according to sample-specific indexes and were filtered, denoised, and merged with DADA2 (Callahan et al. 2016). Merged reads were collapsed to representative sequences or ASVs, and taxonomic assignments were assigned to 99% sequence identity based on the Silva 132 database for bacteria (Quast et al. 2013) and UNITE database for fungi (Xue et al. 2019). Alpha diversity metrics (Shannon diversity and observed species) were calculated to obtain a comprehensive description of the within-sample bacterial community. Although next-generation sequencing is useful for evaluating the composition and diversity of soil microorganisms, it does not indicate whether these genes are expressed (Nannipieri et al. 2019).
Statistical analysis
A repeated-measures analysis of variance (repeated-measures ANOVA) followed by Bonferroni post-hoc test was performed in R (v. 4.0.2) for determination of differences in sample properties. Non-metric multidimensional scaling (NMDS) based on Bray–Curtis distance matrices was used for visual identification of differences between microbial community compositions. The significance of NMDS results was tested using permutational multivariate analysis of variance (PERMANOVA). PERMANOVA was also used to determine the differences in the tea composition between locations or seasons. Pearson’s correlation analysis was performed to determine the correlation between the soil chemical properties and the microbial relative abundance or between the microbial relative abundance and characteristics of tea leaves. Canonical correspondence analysis (CCA) was used to measure the soil chemical properties that have the most significant influence on microbial community compositions.
Results
The soil chemical properties of tea farm soils
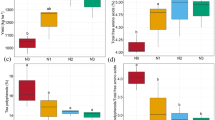
No significant differences in soil water content or soil pH were observed among the tea farms; however, all soil pH were under 5 (Fig. 1). In general, higher nutrient concentrations were observed at Seogwang (SG) tea farm compared with the other sites. Higher contents of SOM, TC, and TN were observed at Seogwang (SG) tea farm compared with Dolsongyi (DS) and Hannam (HN) tea farms (p < 0.05). Higher content of DOC was observed in June compared with other months (p < 0.05). The highest concentrations of K+ and Li+ were observed at Dolsongyi (DS) tea farm (p < 0.05). Significantly higher contents of SOM, TC, TN, and K+ were observed in September compared with other months. Information on soil chemical properties is provided in Table S3.
The soil chemical properties of three tea farms. The data represent means ± standard error (n = 4). Letters denote statistically significant differences; uppercase letters are the difference between seasons, and lowercase letters are the difference between tea farms (p < 0.05). DOC; dissolved organic C
Microbial community compositions and diversity
At the phylum level, Proteobacteria (47.11 ± 1.33%), Actinobacteria (18.38 ± 1.29%), Acidobacteria (14.49 ± 0.79%), Verrucomicrobia (4.11 ± 0.33%), Chloroflexi (3.90 ± 0.44%), Bacteroidetes (3.64 ± 0.32%), Firmicutes (2.34 ± 0.63%), Planctomycetes (2.72 ± 0.19%), Gemmatimonadetes (1.58 ± 0.23%), and Nitrospirae (0.80 ± 0.08%) were dominant in the bacterial communities across all tea farms; together, these phyla accounted for more than 99% of the total bacterial abundance (Fig. 2a). Significantly greater abundance of Actinobacteria at Seogwang (SG) tea farm and Firmicutes at Hannam (HN) tea farm was observed throughout all seasons (p < 0.05). Among Proteobacteria, the most abundant class was Gammaproteobacteria (25.84 ± 1.66%), followed by Alphaproteobacteria (17.99 ± 0.51%) and Betaproteobacteria (2.20 ± 0.31%). Ascomycota (45.16 ± 1.57%), Basidiomycota (26.76 ± 1.79%), and Mortierellomycota (23.59 ± 2.43%) were the dominant fungal phyla across all tea farms (Fig. 2c). Unlike bacterial communities, significant seasonal changes were observed in fungal communities (p < 0.05). In April, Ascomycota was dominant at all sites (41.47 ± 3.13%), followed by Mortierellomycota (37.82 ± 4.62%); however, Basidiomycota accounted for only 14.06 ± 1.93%. Over the sampling periods, the relative abundance of Mortierellomycota showed a significant decrease, and that of Basidiomycota showed a gradual increase at all sites. In September, Basidiomycota accounted for more than 36.97 ± 1.50% of the total abundance, and Mortierellomycota only accounted for 15.04 ± 1.80% (Fig. 2c). In NMDS plots (Fig. 2 b and d), distinct spatial variations were observed in the composition of the bacterial community; however, distinct seasonal variations were observed in the composition of the fungal community. PERMANOVA indicated significant differences in bacterial composition among tea farms (F-value = 14.5349, R2 = 0.4077, p = 0.001) and in fungal composition among seasons (F-value = 23.5807, R2 = 0.4710, p = 0.001).
The relative abundance at the phylum level and non-metric multidimensional scaling (NMDS) of microbial communities. a The bacterial community composition. b The fungal community composition. c The bacterial community grouped by tea farms (p = 0.001). d The fungal community separated by month (p = 0.001)
At the genus level, 749 bacterial genera and 465 fungal genera were detected (Fig. S3). Spatial variations were clearly observed in the bacterial communities at the genus level clearly, with Chujaibacter (Proteobacteria) having the greatest abundance at Seogwang (SG) tea farm, and Thioalbus (Proteobacteria) was the most abundant at Dolsongyi (DS) tea farm. On the other hand, seasonal variations were clearly observed in the fungal communities at the genus level. Across all seasons, Chaetomium (Ascomycota) and Mortierella (Mortierellomycota) showed a decrease, while Humicola (Ascomycota), Tausonia (Basidiomycota), Holtermanniella (Basidiomycota), Papiliotrema (Basidiomycota), Solicoccozyma (Basidiomycota), and Trichosporon (Basidiomycota) showed an increase.
Bacterial and fungal diversities were estimated using the Shannon index and observed species (Fig. 3), and seasonal variations tended to be more pronounced than those among tea farms. Bacterial diversity did not show significant spatiotemporal variations; however, fungal diversity was constant from April to June and then decreased from June to September.
Relationship between soil chemical properties and microbial relative abundance
Prior to analysis of the relationship between soil chemical properties and microbial relative abundance, a correlation analysis was performed to confirm the autocorrelation among soil chemical properties. A positive correlation was observed among SOM, TC, TN, and TS (data not shown). To avoid the use of redundant variables, only SOM was used for the relative abundance of microbial phyla that showed correlation with SOM, TC, TN, and TS. According to the results of correlation analysis, the relative abundance of Acidobacteria showed a significantly negative correlation with SOM, whereas those of Actinobacteria and Alphaproteobacteria showed significantly positive correlations with SOM and DOC (Table 2). However, the relative abundance of Gammaproteobacteria showed no correlation with soil nutrients. In the fungal community, the relative abundance of Ascomycota showed negative correlation with TC and TN which showed positive correlation with that of Basidiomycota (Table 2).
CCA was performed to determine the effects of soil chemical properties on the microbial community compositions; significant correlations were found between them (Fig. 4). The length of the soil chemical factor arrows indicated the strength of the soil properties in affecting the microbial community compositions. In the bacterial community, the first two axes explained 42.46% of the total variation; water content, SOM, soil pH, EC, DOC, NH4+, Ca2+, K+, and Mg2+ were the significant factors. In the fungal community, the first two axes explained 30.40% of the total variation; EC, DOC, and NO3− were the significant factors.
Canonical correspondence analysis (CCA) biplot of microbial community composition with environmental variables (WC, water content; SOM, soil organic matter; DOC, dissolved organic C; EC, electrical conductivity). a The bacterial community grouped by tea farms (p = 0.001). b The fungal community separated by month (p = 0.001). The arrows denote the relationship of significant soil properties with the samples. The length of the arrows indicates the strength of the relationship between the samples and soil properties
Composition of tea leaves
Spatiotemporal factors affected the chemical composition of tea leaves (PERMANOVA F-value = 81.14, R2 = 0.1776, p = 0.001) where seasonal differences were greater (PERMANOVA F-value = 472.68, R2 = 0.5174, p = 0.001) than spatial differences (PERMANOVA F-value = 133.32, R2 = 0.2919, p = 0.001) (Fig. 5). A higher concentration of catechins was found in June compared with that in September (p < 0.05), while higher concentrations of total amino acids and theanine were found in September compared with those in June (p < 0.05), except at Hannam (HN). Caffeine content showed no significant seasonal difference. Among various types of catechins, the highest levels of epigallocatechin-3-gallate (EGCG), epicatechin gallate (ECG), and gallocatechin gallate (GCG) were observed in June, while the highest levels of epigallocatechin (EGC) and epicatechin (EC) were observed in September (p < 0.05) (Figs. 5 and S4).
The major composition of tea leaves. The data represent means ± standard error (n = 3). Letters denote statistically significant differences; uppercase letters indicate the difference between seasons, and lowercase letters indicate the difference between tea farms (p < 0.05). EGCG, epigallocatechin-3-gallate; EGC, epigallocatechin; ECG, epicatechin-3-gallate; GCG, gallocatechin gallate
Relationship between soil microbial diversity and tea quality
A correlation analysis between the soil microbial diversity and major components of tea leaves was performed. Significant correlations were found between soil fungal diversity and major components of tea leaves (Table 3). For example, catechin showed positive correlation with fungal diversity, whereas total amino acids and theanine showed negative correlation with fungal diversity (Table 3). The ratios of theanine/catechin and theanine/caffeine were calculated as indices of tea quality (Krahe et al. 2018), followed by analysis of the relationship between these indices and soil microbial diversity (Table 3). Tea quality indices showed a strong negative correlation with the Shannon diversity of the fungal community. A relatively moderate negative correlation was observed between these quality indices and species number of fungal community (Table 3).
Discussion
Variations in soil chemical properties among tea farms
In this study, soils from the three tea farms that we evaluated were acidic (pH < 5), which is in accordance with findings of previous studies (Li et al. 2016; 2020). In general, tea cultivation results in soil acidification due to high rates of N fertilizer, low N use efficiency of plants, and accumulation of leaf litter or root exudates (Zhao et al. 2012; Karak et al. 2015; Yan et al. 2020). Thus, in general, the soil pH of tea farms decreases with tea cultivation periods (Li et al. 2020; Yan et al. 2020).
In our study, the content of SOM was 44.31%. Considering that approximately 58% of SOM is composed of soil organic C (SOC) (Bianchi et al. 2008), a higher SOC content would be found at our sites compared with those reported at tea farms in China (Zhao et al. 2012; Li et al. 2016; Chen et al. 2021) and Japan (Tokuda and Hayatsu 2002). SOC not only serves as an energy source for soil microorganisms to increase enzyme activity but also acts as nutrient storage, contributes to soil aeration, reduces soil compaction, improves infiltration, and increases water holding capacity; therefore, it is closely related to soil fertility (Chang et al. 2007; Munson et al. 2012; Karak et al. 2015). Thus, tea cultivation could be positively affected by high levels of SOM (Lal 2006; Kamau et al. 2008).
The contents of TC, TN, K+, and Li+ showed seasonality and increased significantly in September (Fig. 1). Tea trees show rapid growth in summer when they are actively absorbing nutrients from soil (Xu et al. 2012). However, the growth rate of tea trees gradually declined over the summer, and nutrient consumption decreased; thus, it appeared that the nutrients accumulated in the soil in autumn (Fig. 1). In addition, the chemical contents of the soil also showed spatial variation, which was highest in Seogwang (SG) (Fig. 1 and Table S1). The differences among tea farms may be attributed to differences in environmental factors such as temperature, precipitation, solar radiation, and altitude, depending on the spatial characteristics of the tea farms (Fig. S2) (Corneo et al. 2013; Zhou et al. 2016; Ren et al. 2018; Pieristѐ et al. 2020). These differences can also lead to differences among soil microbial community compositions among tea farms (Ren et al. 2018; Yang et al. 2020). Furthermore, decomposition of organic fertilizers or leaf litter could occur through different mechanisms due to the activities of distinct soil microbes at each tea farm, which could also result in differences in soil chemical concentrations (Sun et al. 2016; Kim et al. 2021). Therefore, even if the three farms are located relatively close to each other and are under identical cultivation practices with the same tea tree species, the chemical properties of the soil could also depend considerably on the spatial characteristics of tea farms.
Differences in the relative abundance of soil microbial community
Consistent with previous reports, Gammaproteobacteria, Alphaproteobacteria, Actinobacteria, and Acidobacteria were the dominant phyla across all tea farms (Li et al. 2016; Wang et al. 2019); however, the relative abundance of phyla was different. In our study, the relative abundances of Gammaproteobacteria and Alphaproteobacteria were higher, while that of Acidobacteria was lower (Fig. 2a) than those reported by Li et al. (2016) and Wang et al. (2019). These results may be due to the higher contents of SOM, TC, and TN in our soils compared with those of other studies. In the results of correlation analysis between soil chemical contents and these phyla, the relative abundance of Acidobacteria showed a negative correlation with soil chemical contents (Table 2), whereas that of Alphaproteobacteria showed a positive correlation with soil chemical contents (Table 2). Previous studies have demonstrated that the relative abundance of Acidobacteria is higher in soils with low resource availability but relatively lower in soils with high organic C concentrations (Fierer et al. 2007; Li et al. 2016), whereas the relative abundances of Gammaproteobacteria and Alphaproteobacteria are higher in soils with large amounts of available nutrients (Li et al. 2016). However, no relationship was found between the relative abundance of Gammaproteobacteria and the chemical contents of the soil (Table 2). Our results also showed that Ascomycota accounted for 41.47–50.73%, which is relatively lower than a previous report of higher relative abundance (53.4–90.8%) of Ascomycota throughout tea cultivation (Li et al. 2020). This difference may be because Ascomycota is an oligotrophic phylum that prefers nutrient-poor environments (Sterkenburg et al. 2015; Chen et al. 2017); thus, Ascomycota could have low dominance at our sites with high nutrient availability (Table 2). Another possibility could be differences in primers used between two studies.
Distinctive differences in the relative abundances of bacterial phyla were observed for tea soils from different farms. At Seogwang (SG) tea farm, Actinobacteria was the most abundant phylum throughout all seasons (Fig. 2a). This phylum is known to be dominant in organic matter-rich soils (Barka et al. 2016) as observed in this study (Table 2). In particular, higher organic matter content was observed for Seogwang (SG) tea farm compared with other sites. Actinobacteria plays an important role in decomposition of soil organic matter, thereby making a major contribution to turnover of organic matter and the C cycle, which are critical in replenishment of soil nutrients (Anandan et al. 2016; Zhang et al. 2020). This property of Actinobacteria may have resulted in a higher concentration of soil nutrients in soils from Seogwang (SG) tea farm than those in soils at the other tea farms.
Ascomycota, Basidiomycota, and Mortierellomycota are the most abundant fungal phyla observed in the composition of fungal community on the global scale (Tedersoo et al. 2014). Likewise, they accounted for 95.50% of the total fungal abundance in our study. Significant seasonal changes were observed in the composition of fungal community. At both the phylum and genus levels, the relative abundance of Mortierellomycota showed a gradual decrease from spring to autumn, while that of Basidiomycota showed a gradual increase (Figs. 2c and S3b). Significant seasonal changes were observed in the genus level for Ascomycota (Fig. S3b). These trends of the fungal community could be caused by increased amounts of plant litter and organic matter in soil supplied by tea cultivation practices such as leaf plucking and pruning since April. Soil with high organic matter content may be associated with an increase in the relative abundance of Basidiomycota (Table 2). In addition, release of nutrients from litter and organic matter can lead to an increase in the relative abundance of Basidiomycota, which is positively correlated with the concentrations of NO3−, K+, and Na+ (data not shown), and are known to be more abundant in soils with high nutrient availability (Zumsteg et al. 2012; Sterkenburg et al. 2015).
Effects of environmental factors on soil microbial community compositions
More pronounced spatial variations than seasonal variations were observed for the soil bacterial community; however, more pronounced seasonal variations were observed for the fungal community (Fig. 2). These trends appeared to be related to the chemical properties of the soil, particularly soil pH. In CCA analysis, soil pH was an important soil chemical factor in the bacterial community, but not in the fungal community (Fig. 4). Low soil pH imposes significant restrictions on the composition of the bacterial community because bacteria can grow in a narrow range of pH (Rousk et al. 2010; Hou et al. 2020). In contrast, fungi can survive within a wide range of pH, and soil pH imposes little restriction on the composition of the fungal community (Rousk et al. 2010). Therefore, low pH of soil observed in our study may have had a significant effect on the composition of the bacterial community, which may offset seasonal variations in the bacterial community. On the other hand, the distinct seasonal variations of the fungal community imply that, unlike bacteria, they are more affected by climate factors than by the chemical properties of the soil. In CCA, nine out of 15 soil chemical factors were significant in the bacterial community, whereas only three factors were significant in the fungal community (Fig. 4). This result is consistent with findings from global studies that reported only a minor influence of soil chemical properties on fungi and that climate parameters are the most important environmental factors predicting the composition of a fungal community (Tedersoo et al. 2014; Egidi et al. 2019). For the same reason, significant seasonal variation was observed with regard to fungal diversity. Bacterial diversity did not show significant spatiotemporal variation, whereas in general lower fungal diversity was observed in autumn than in the other seasons. An increase in precipitation might explain the lower fungal diversity in autumn (Hawkes et al. 2011).
Seasonality of tea composition
Climatic factors have a significant influence on the contents of catechins, free amino acids, and theanine in tea plants (Lee et al. 2010; Ahmed et al. 2014; Dai et al. 2015). The effects of climate on these tea components were evident in our study, but seasonal changes were more pronounced than variations among the locations of tea farms (Figs. 5 and S4). Consistent with previous studies, that found lower catechin contents were found in tea harvested in cooler months compared with tea harvested in warmer months (Caffin et al. 2004), higher catechin concentrations, including EGCG and ECG, which are important quality indicators of green tea (Horie and Kohata 1998), were found in tea leaves harvested in June compared with those harvested in September (Fig. 5). Gong et al. (2020) also reported higher concentration of catechin in summer harvest than in spring or autumn harvest. In contrast, higher theanine concentration was observed in September compared with June (Fig. 5). More intense sunlight and longer days in June might explain these results. Theanine is a precursor of catechin (Kito et al. 1968), and intense sunlight is known to accelerate biosynthesis of catechin from theanine (Yao et al. 2005; Lee et al. 2013; Too et al. 2015). Therefore, it may be that higher catechin content with lower theanine were observed in June due to long daytime and higher solar altitude, while lower catechin contents with higher theanine may have been observed in September for a similar reason.
Relationship between soil microbial diversity and tea quality
One of the aims of our study was to relate soil microbial diversity of the tea farms to the quality of tea assessed as leaf composition. Tea quality is dependent on several parameters, including nutritional, health, and sensory attributes as well as organoleptic properties (e.g., color, appearance, texture, aroma, and taste) (Liang et al. 2008; Ahmed et al. 2019). Among the various constituents of tea, the balance between theanine, catechin, and caffeine determines the overall taste of tea, contributing to the quality and commercial value of tea (Krahe et al. 2018). The theanine/catechin ratio affects the balance between sweetness (theanine) and astringent taste (catechin); a higher ratio is more favorable and corresponds to greater quality and commercial value. The theanine/caffeine ratio affects the balance between sweetness (theanine) and bitterness (caffeine), and a higher ratio results in higher quality and commercial value. We found a moderate positive relationship between the fungal diversity of tea soil and the quality indices of tea (Table 3); this is due to the seasonal variations between them. The spatiotemporal characteristics of tea farms and the resulting differences in environmental factors play an important role in determining not only the composition of tea (Fernandez et al. 2002; Han et al. 2017; Zhao et al. 2017; Gong et al. 2020), but also the community composition, abundance, and activity of soil microorganisms (Corneo et al. 2013; Cao et al. 2016; Ren et al. 2018; Pieristѐ et al. 2020). Soil microbial diversity showed a sensitive response to changes in environmental factors and can be used as a representative indicator of environmental factors on tea farms (Shi et al. 2014). Therefore, we suggest the potential for use of soil microbial diversity as a biological indicator of tea quality in tea soil.
Conclusion
We analyzed the soil chemical properties and microbial community compositions of three tea farms with different geographic locations within a close proximity according to seasonal changes. The sampling locations of the three tea farms were based on the identical soil classification and have been exposed to the same fertilization and management schemes but exhibited dissimilar soil chemical properties and microbial community compositions. It is believed that these differences are influenced by microclimate, such as temperature, precipitation, and sunlight. The correlation between soil microbial diversity and tea quality were also identified. Our results demonstrated the potential for use of soil fungal diversity as a biological indicator of tea quality which was not considered in previous studies on the ecosystems of tea farms. The significance of this study is that it is the first study to report spatial and temporal variations in soil chemical properties and microbial community compositions in tea farms. Although this study analyzed microbial community compositions and diversity at the DNA level, the detection of genes does not necessarily indicate that those genes were expressed. Therefore, future studies linking microbial community compositions and microbial activity should be considered to understand the microbial functions of tea. For example, mRNA-based methods could overcome those limitations.
Data availability
Sequence data is available from the authors upon request. The dataset reported in this study has been deposited in the NCBI Sequence Read Archive (SRA) database (Accession number [PRJNA759091] and [PRJNA759080]).
Code availability
Not applicable.
References
Ahmed S, Griffin TS, Kraner D, Schaffner MK, Sharma D, Hazel M, Leitch AL, Orians CM, Han W, Stepp JR, Robbat A, Matyas C, Long C, Xue D, Houser RF, Cash SB (2019) Environmental factors variably impact tea secondary metabolites in the context of climate change. Front Plant Sci 10:939
Ahmed S, Stepp JR, Orians C, Griffin T, Matyas C, Robbat A, Cash S, Xue Y, Long C, Unachukwu U, Buckley S, Small D, Kennelly E (2014) Effects of extreme climate events on tea (Camellia sinensis) functional quality validate indigenous farmer knowledge and sensory preferences in tropical China. PLoS ONE 9:e109126.
Anandan R, Dharumadurai D, Manogaran GP (2016) An introduction to actinobacteria. In: Dhanasekaran D, Jiang Y (Eds) Actinobacteria: basics and biotechnology applications. Intechopen, London, UK, pp 1–37.
Arellano K, Vazquez J, Park H, Lim J, Ji Y, Kang HJ, Cho D, Jeong HW, Holzapfel WH (2020) Safety evaluation and whole-genome annotation of Lactobacillus plantarum strains from different sources with special focus on isolates from green tea. Probiotics Antimicrob Proteins 12:1057–1070
Barka EA, Vatsa P, Sanchez L, Gaveau-Vaillant N, Jacquard C, Meier-Kolthoff JP, Klenk HP, Clément C, Ouhdouch Y, van Wezel GP (2016) Taxonomy, physiology, and natural products of Actinobacteria. Microbiol Mol Biol Rev 80:1–43
Belda I, Zarraonaindia I, Perisin M, Palacios A, Acedo A (2017) From vineyard soil to wine fermentation: microbiome approximations to explain the “terroir” concept. Front Mcrobiol 8:821
Bhattacharyya P, Sarmah S (2018) The role of microbes in tea cultivation. In: Sharma VS, Gunasekare MTK (Eds) Global tea science: current status and future needs. Burleigh Dodds science publishing, Cambridge, UK, pp 155-188.
Bianchi SR, Miyazawa M, de Oliveira EL, Pavan MA (2008) Relationship between the mass of organic matter and carbon in soil. Braz Arch Biol Technol 51:263–269
Bolyen E, Rideout JR, Dillon MR, Bokulich N, Abnet CC, Al-Ghalith GA, Alexander H, Alm EJ, Arumugam M, Asnicar F, Bai Y, Bisanz JE, Bittinger K, Brejnrod A, Brislawn CJ, Brown CT, Callahan BJ, Caraballo-Rodriguez AM, Chase J, Cope EK, Da Silva R, Diener C, Dorrestein PC, Douglas GM, Durall DM, Duvallet C, Edwardson CF, Ernst M, Estaki M, Fouquier J, Gauglitz JM, Gibbons SM, Gibson DL, Gonzalez A, Gorlick K, Guo JR, Hillmann B, Holmes S, Holste H, Huttenhower C, Huttley GA, Janssen S, Jarmusch AK, Jiang LJ, Kaehler BD, Bin Kang K, Keefe CR, Keim P, Kelley ST, Knights D, Koester I, Kosciolek T, Kreps J, Langille MGI, Lee J, Ley R, Liu YX, Loftfield E, Lozupone C, Maher M, Marotz C, Martin BD, McDonald D, McIver LJ, Melnik AV, Metcalf JL, Morgan SC, Morton JT, Naimey AT, Navas-Molina JA, Nothias LF, Orchanian SB, Pearson T, Peoples SL, Petras D, Preuss ML, Pruesse E, Rasmussen LB, Rivers A, Robeson MS, Rosenthal P, Segata N, Shaffer M, Shiffer A, Sinha R, Song SJ, Spear JR, Swafford AD, Thompson LR, Torres PJ, Trinh P, Tripathi A, Turnbaugh PJ, Ul-Hasan S, vanderHooft Vargas Vazquez-BaezaVogtmann von Hippel Walters Wan Wang Warren Weber Williamson Willis XuZaneveld Zhang Zhu KnightCaporaso JJJFYEMWYMJKCCHDADZZJRYQRJG (2019) Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat Biotechnol 37:852–857
Cabrera C, Artacho R, Giménez R (2006) Beneficial effects of green tea-a review. J Am Coll Nutr 25:79–99
Caffin N, D’Arcy B, Yao L, Rintoul G (2004) Developing an index of quality for Australian tea. RIRDC Publication No. 04/033, Project No. UQ-88A, Rural Industries Research and Development Corporation Publication, Queensland, Australia, pp. 1–192.
Cai F, Pang G, Li R, Li R, Gu X, Shen Q, Chen W (2017) Bioorganic fertilizer maintains a more stable soil microbiome than chemical fertilizer for monocropping. Biol Fertil Soils 53:861–872
Callahan BJ, McMurdie PJ, Rosen MJ, Han AW, Johnson AJA, Holmes SP (2016) DADA2: high-resolution sample inference from Illumina amplicon data. Nat Methods 13:581–583
Cao H, Chen R, Wang L, Jiang L, Yang F, Zheng S, Wang G, Lin X (2016) Soil pH, total phosphorus, climate and distance are the major factors influencing microbial activity at a regional spatial scale. Sci Rep 6:25815
Chacko SM, Thambi PT, Kuttan T, Nishigaki I (2010) Beneficial effects of green tea: a literature review. Chin Med 5:1–9
Chan SC (2012) Terroir and green tea in China: the case of Meijiawu dragon well (Longjing) tea. In: Augustin-Jean L, Ilbert H, Saavedra-Rivano N (eds) Geographical indications and international agricultural trade: the challenge for Asia. Palgrave Macmillan, London, UK, pp 226–238
Chang EH, Chung RS, Tsai YH (2007) Effect of different application rates of organic fertilizer on soil enzyme activity and microbial population. Soil Sci Plant Nutr 53:132–140
Chaparro JM, Sheflin AM, Manter DK, Vivanco JM (2012) Manipulating the soil microbiome to increase soil health and plant fertility. Biol Fertil Soils 48:489–499
Chen YL, Xu TL, Veresoglou SD, Hu HW, Hao ZP, Hu YJ, Liu L, Deng Y, Rillig MC, Chen BD (2017) Plant diversity represents the prevalent determinant of soil fungal community structure across temperate grasslands in northern China. Soil Biol Biochem 110:12–21
Chen WC, Ko CH, Su YS, Lai WA, Shen FT (2021) Metabolic potential and community structure of bacteria in an organic tea plantation. Appl Soil Ecol 157:103762
Corneo PE, Pellegrini A, Cappellin L, Roncador M, Chierici M, Gessler C, Pertot I (2013) Microbial community structure in vineyard soils across altitudinal gradients and in different seasons. FEMS Microbiol Ecol 84:588–602
Dai W, Qi D, Yang T, Lv H, Guo L, Zhang Y, Zhu Y, Peng Q, Xie D, Tan J, Lin Z (2015) Nontargeted analysis using ultraperformance liquid chromatography-quadrupole time-of-flight mass spectrometry uncovers the effects of harvest season on the metabolites and taste quality of tea (Camellia sinensis L.). J Agric Food Chem 63:9869–9878
Dang MV (2005) Soil-plant nutrient balance of tea crops in the northern mountainous region. Vietnam Agric Ecosyst Environ 105:413–418
Duncan JMA, Saikia SD, Gupta N, Biggs EM (2016) Observing climate impacts on tea yield in Assam, India. Appl Geogr 77:64–71
Dutta J, Handique PJ, Thakur D (2015) Assessment of culturable tea rhizobacteria isolated from tea estates of Assam, India for growth promotion in commercial tea cultivars. Front Microbiol 6:1252
Egidi E, Delgado-Baquerizo M, Plett JM, Wang J, Eldridge DJ, Bardgett DB, Maestre FT, Singh BK (2019) A few Ascomycota taxa dominate soil fungal communities worldwide. Nat Commun 10:2369
Evizal R, Tohari T, Prijambada ID, Widada J, Widianto D (2012) Soil bacterial diversity and productivity of coffee-shade tree agro-ecosystems. J Trop Soils 17:181–187
FAO (2018) CCP Intergovernmental group on tea, Current market situation and medium term outlook. CCP:TE 18/CRS1 pp. 1–16.
Fernandez PL, Pablos F, Martin MJ, Gonzalez AG (2002) Study of catechin and xanthine tea profiles as geographical tracers. J Agric Food Chem 50:1833–1839
Fierer N (2017) Embracing the unknown: disentangling the complexities of the soil microbiome. Nat Rev Microbiol 15:579–590
Fierer N, Bradford MA, Jackson RB (2007) Toward an ecological classification of soil bacteria. Ecology 88:1354–1364
Gardes M, Bruns TD (1993) ITS primers with enhanced specificity for basidiomycetes -application to the identification of mycorrhizae and rusts. Mol Ecol 2:113–118
Gong AD, Lian SB, Wu NN, Zhou YJ, Zhao SQ, Zhang LM, Cheng L, Yuan HY (2020) Integrated transcriptomics and metabolomics analysis of catechins, caffeine and theanine biosynthesis in tea plant (Camellia sinensis) over the course of seasons. BMC Plant Biol 20:294
Gunathilaka RPD, Tularam GA (2016) The tea industry and a review of its price modelling in major tea producing countries. J Manag Strategy 7:34–39
Gupta VRGV, Bramley RGV, Greenfield P, Yu J, Herderich MJ (2019) Vineyard soil microbiome composition related to rotundone concentration in Australian cool climate ‘Peppery’ shiraz grapes. Front Microbiol 10:1607
Han W, Kemmitt SJ, Brookes PC (2007) Soil microbial biomass and activity in Chinese tea gardens of varying stand age and productivity. Soil Biol Biochem 39:1468–1478
Han WY, Huang JG, Li X, Li ZX, Ahammed GJ, Yan P, Stepp JR (2017) Altitudinal effects on the quality of green tea in east China: a climate change perspective. Eur Food Res Technol 243:323–330
Hawkes CV, Kivlin SN, Rocca JC, Huguet V, Thomsen MA, Suttle KB (2011) Fungal community responses to precipitation. Glob Change Biol 17:1637–1645
Ho CT, Zheng X, Li S (2015) Tea aroma formation. Food Sci Human Wellness 4:9–27
Horie H, Kohata K (1998) Application of capillary electrophoresis to tea quality estimation. J Chromatogr A 802:219–223
Hou J, Wu L, Liu W, Ge Y, Mu T, Zhou T, Li Z, Zhou J, Sun X, Luo Y, Christie P (2020) Biogeography and diversity patterns of abundant and rare bacterial communities in rice paddy soils across China. Sci Total Environ 730:139116
Jurburg SD, Shek KL, McGuire K (2020) Soil microbial composition varies in response to coffee agroecosystem management. FEMS Microbiol Ecol 96:1–11
Kamau DM, Spiertz JHJ, Oenema O (2008) Carbon and nutrient stocks of tea plantations differing in age, genotype and plant population density. Plant Soil 307:29–39
Kaneko S, Kumazawa K, Masuda H, Henze A, Hofmann T (2006) Molecular and sensory studies on the umami taste of Japanese green tea. J Agric Food Chem 54:2688–2694
Karak T, Paul RK, Boruah RK, Sonar I, Bordoloi B, Dutta AK, Borkotoky B (2015) Major soil chemical properties of the major tea-growing areas in India. Pedosphere 25:316–328
Khokhar S, Magnusdottir SGM (2002) Total phenol, catechin, and caffeine contents of teas commonly consumed in the United Kingdom. J Agric Food Chem 50:565–570
Kim J, Lee J, Yang Y, Yun J, Ding W, Yuan J, Khim JS, Kwon BO, Kang H (2021) Microbial decomposition of soil organic matter determined by edaphic characteristics of mangrove forests in East Asia. Sci Total Environ 763:142972
Kito M, Kokura H, Izaki J, Sasaoka K (1968) Theanine, a precursor of the phloroglucinol nucleus of catechins in tea plants. Phytochemistry 7:599–603
Klindworth A, Pruesse E, Schweer T, Peplies J, Quast C, Horn M, Glöckner FO (2012) Evaluation of general 16S ribosomal RNA gene PCR primers for classical and next-generation sequencing-based diversity studies. Nucleic Acids Res 41:e1.
Kodama S, Ito Y, Nagase H, Yamashita T, Kemmei T, Yamamoto A, Hayakawa K (2007) Usefulness of catechins and caffeine profiles to determine growing areas of green tea leaves of a single variety, Yabukita, in Japan. J Health Sci 53:491–495
Krahe JC, Krahe MA, Roach PD (2018) Development of an objective measure of quality and commercial value of Japanese-styled green tea (Camellia L. sinensis): the quality index tool. J Food Sci Technol 55:2926–2934
Lal R (2006) Enhancing crop yields in the developing countries through restoration of the soil organic carbon pool in agricultural lands. Land Degrad Dev 17:197–209
Lee JE, Lee BJ, Chung JO, Hwang JA, Lee SJ, Lee Ch, Hong YS (2010) Geographical and climatic dependencies of green tea (Camellia sinensis) metabolites: a 1H NMR-based metabolomics study. J Agric Food Chem 58:10582–10589
Lee LS, Choi JH, Son N, Kim SH, Park JD, Jang DJ, Jeong Y, Kim HJ (2013) Metabolomic analysis of the effect of shade treatment on the nutritional and sensory qualities of green tea. J Agric Food Chem 61:332–338
Li YC, Li Z, Li ZW, Jiang YH, Weng BQ, Lin WX (2016) Variations of rhizosphere bacterial communities in tea (Camellia sinensis L.) continuous cropping soil by high-throughput pyrosequencing approach. J Appl Microbiol 121:787–799
Li YC, Li Z, Arafat Y, Lin WX (2020) Studies on fungal communities and functional guilds shift in tea continuous cropping soils by high-throughput sequencing. Ann Microbiol 70:7
Liang YR, Ye Q, Jin J, Liang H, Lu JL, Du YY, Dong JJ (2008) Chemical and instrumental assessment of green tea sensory preference. Int J Food Prop 11:258–272
Mozumder NHMR, Lee YR, Hwang KH, Lee MS, Kim EH, Hong YS (2020) Characterization of tea leaf metabolites dependent on tea (Camellia sinensis) plant age through 1H NMR-based metabolomics. Appl Biol Chem 63:10
Munson SM, Lauenroth WK, Burke IC (2012) Soil carbon and nitrogen recovery on semiarid Conservation Reserve Program lands. J Arid Environ 79:25–31
Nannipieri P, Penton CR, Purahong W, Schloter M, van Elsas JD (2019) Recommendations for soil microbiome analyses. Biol Fertil Soils 55:765–766
Pandey A, Palni LMS (2002) Tea rhizosphere: characteristics features, microbial diversity and applications. Int J Tea Sci 1:10–24
Park H, Cho D, Huang E, Seo J, Kim W, Todorov SD, Ji Y, Holzapfel WH (2020) Amelioration of alcohol induced gastric ulcers through the administration of Lactobacillus plantarum APSulloc 331261 isolated from green tea. Front Microbiol 11:420
Pieristѐ M, Forey E, Sahraoui AL, Meglouli H, Laruelle F, Delporte P, Robson M, Chauvat M (2020) Spectral composition of sunlight affects the microbial functional structure of beech leaf litter during the initial phase of decomposition. Plant Soil 451:515–530
Pineda A, Kaplan I, Hannula SE, Ghanem W, Bezemer TM (2020) Conditioning the soil microbiome through plant-soil feedbacks suppresses an aboveground insect pest. New Phytol 226:595–608
Quast C, Pruesse E, Yilmaz P, Gerken J, Schweer T, Yarza P, Peplies J, Glöckner FO (2013) The SILVA ribosomal RNA gene database project: improved data processing and web-based tools. Nucleic Acids Res 41:D590–D596
Ren C, Chen J, Lu X, Doughty R, Zhao F, Zhong Z, Han X, Yang G, Feng Y, Ren G (2018) Responses of soil total microbial biomass and community compositions to rainfall reductions. Soil Biol Biochem 116:4–10
Rousk J, Bååth E, Brookes PC, Lauber CL, Lozupone C, Caporaso JG, Knight R, Fierer N (2010) Soil bacterial and fungal communities across a pH gradient in an arable soil. ISME J 4:1340–1351
Shi L, Mortimer PE, Slik JWF, Zou X, Xu J, Feng W, Qiao L (2014) Variation in forest soil fungal diversity along a latitudinal gradient. Fungal Divers 64:305–315
Singh JS, Pandey VC, Singh DP (2011) Efficient soil microorganisms: a new dimension for sustainable agriculture and environmental development. Agric Ecosyst Environ 140:339–353
Sterkenburg E, Bahr A, Durling MB, Clemmensen KE, Lindahl BD (2015) Changes in fungal communities along a boreal forest soil fertility gradient. New Phytol 207:1145–1158
Sun L, Xun W, Huang T, Zhang G, Gao J, Ran W, Li D, Shen Q, Zhang R (2016) Alteration of the soil bacterial community during parent material maturation driven by different fertilization treatments. Soil Biol Biochem 96:207–215
Tedersoo L, Bahram M, Põlme S, Kõljalg U, Yorou NS, Wijesundera R, Ruiz LV, Vasco-Palacios AM, Thu PQ, Suija A, Smith ME, Sharp C, Saluveer E, Saitta A, Rosas M, Riit T, Ratkowsky D, Pritsch K, Põldmaa K, Piepenbring M, Phosri C, Peterson M, Parts K, Pärtel K, Otsing E, Nouhra E, Njouonkou AL, Nilsson RH, Morgado LN, Mayor J, May TW, Majuakim L, Lodge DJ, Lee SS, Larsson KH, Kohout P, Hosaka K, Hiiesalu I, Henkel TW, Harend H, Guo LD, Greslebin A, Grelet G, Geml J, Gates G, Dunstan W, Dunk C, Drenkhan R, Dearnaley J, Kesel AD, Dang T, Chen X, Buegger F, Brearley FQ, Bonito G, Anslan S, Abell S, Abarenkov K (2014) Global diversity and geography of soil fungi. Science 346:1256688
Tokuda SI, Hayatsu M (2002) Soil microbial biomass and fluorescein diacetate hydrolytic activity in Japanese acidic tea field soils. Soil Sci Plant Nutr 48:865–869
Too JC, Kinyanjui T, Wanyoko JK, Wachira FN (2015) Effect of sunlight exposure and different withering durations on theanine levels in tea (Camellia sinensis). Food Nutr Sci 6:1014
van der Heijden MGA, Bardgett RD, van Straalen NM (2008) The unseen majority: soil microbes as drivers of plant diversity and productivity in terrestrial ecosystems. Ecol Lett 11:296–310
Veloso TGR, da Silva MdCS, Cardoso WS, Guarconi RC, Kasuya MCM, Pereira LL (2020) Effects of environmental factors on microbiota of fruits and soil of Coffea arabica in Brazil. Sci Rep 10:14692
Wagg C, Bender FB, Widmer F, van der Heijden MGA (2014) Soil biodiversity and soil community composition determine ecosystem multifunctionality. Proc Natl Acad Sci U S A 111:5266–5270
Wang LY, Wei K, Jiang YW, Cheng H, Zhou J, He W, Zhang CC (2011) Seasonal climate effects on flavanols and purine alkaloids of tea (Camellia sinensis L.). Eur Food Res Technol 233:1049–1055
Wang S, Li T, Zheng Z, Chen HYH (2019) Soil aggregate-associated bacterial metabolic activity and community structure in different aged tea plantations. Sci Total Environ 654:1023–1032
White TJ, Bruns T, Lee S, Taylor J (1990) Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sinsky JJ, White TJ (Eds) PCR protocols: a guide to methods and applications. Academic Press, San Diego, CA, pp 315-322.
Xu W, Song Q, Li D, Wan X (2012) Discrimination of the production season of Chinese green tea by chemical analysis in combination with supervised pattern recognition. J Agric Food Chem 60:7064–7070
Xue D, Yao H, Huang C (2006) Microbial biomass, N mineralization and nitrification, enzyme activities, and microbial community diversity in tea orchard soils. Plant Soil 288:319–331
Xue C, Hao Y, Pu X, Penton C, Wang Q, Zhao M, Zhang B, Ran W, Huang Q, Shen Q, Tiedje J (2019) Effect of LSU and ITS Genetic Markers and Reference Databases on Analyses of Fungal Communities. Biol Fertil Soils 55:79–88
Yan P, Wu L, Wang D, Fu J, Shen C, Li X, Zhang L, Zhang L, Fan L, Wenyan H (2020) Soil acidification in Chinese tea plantations. Sci Total Environ 715:136963
Yang Y, Lee SH, Jang I, Kang H (2020) Soil bacterial community structures across biomes in artificial ecosystems. Ecol Eng 158:106067
Yao L, Caffin N, D’Arcy B, Jiang Y, Shi J, Singanusong R, Liu X, Datta N, Kakuda Y, Xu Y (2005) Seasonal variations of phenolic compounds in Australia-grown tea (Camellia sinensis). J Agric Food Chem 53:6477–6483
Zhang S, Sun L, Wang Y, Fan K, Xu Q, Li Y, Ma Q, Wang J, Ren W, Ding Z (2020) Cow manure application effectively regulates the soil bacterial community in tea plantation. BMC Microbiol 20:190
Zhao J, Wu X, Nie C, Wu T, Dai W, Liu H, Yang R (2012) Analysis of unculturable bacterial communities in tea orchard soils based on nested PCR-DGGE. World J Microbiol Biotechnol 28:1967–1979
Zhao H, Yu C, Li M (2017) Effects of geographical origin, variety, season and their interactions on minerals in tea for traceability. J Food Compos Anal 63:15–20
Zhou J, Deng Y, Shen L, Wen C, Yan Q, Ning D, Qin Y, Xue K, Wu L, He Z, Voordeckers JW, Van Nostrand JD, Buzzard V, Michaletz ST, Enquist BJ, Weiser MD, Kaspari M, Waide R, Yang Y, Brown JH (2016) Temperature mediates continental-scale diversity of microbes in forest soils. Nat Commun 7:12083
Zumsteg A, Luster J, Göransson H, Smittenberg RH, Brunner I, Bernasconi SM, Zeyer J, Frey B (2012) Bacterial, archaeal and fungal succession in the forefield of a receding glacier. Microb Ecol 63:552–564
Funding
This research has been supported by funds from the AMOREPACIFIC CORPORATION and the Ministry of Education of Korea (2020R1I1A2072824). JK was supported by the fellowship funded by the Ministry of Education of Korea (2019R1A6A3A01091184).
Author information
Authors and Affiliations
Contributions
YY, JK, and HK designed the research; YY and JK conducted the experiments; JOC and DC assisted in sampling; JHR, YDH, and WKK contributed to data collection; YY wrote the manuscript; JK, JOC, DC, and HK revised the manuscript.
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Yang, Y., Kim, J., Chung, JO. et al. Variations in the composition of tea leaves and soil microbial community. Biol Fertil Soils 58, 167–179 (2022). https://doi.org/10.1007/s00374-021-01615-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00374-021-01615-8