Abstract
The conversion of natural forests to tree plantations alters the quality and decreases the quantity of litter inputs into the soil, but how the alteration of litter inputs affect soil organic matter (SOM) decomposition remain unclear. We examined SOM decomposition by adding 13C-labeled leaf-litter of Chinese fir (Cunninghamia lanceolata (Lamb.) Hook) to soils from a natural evergreen broad-leaved forest and an adjacent Chinese fir plantation converted from a natural evergreen broad-leaved forest 42 years ago. Over 195 days, we monitored CO2 efflux and its δ13C, microbial biomass, and the composition of microbial groups by phospholipid fatty acids (PLFAs). To distinguish priming mechanisms, partitioning of C sources in CO2 and microbial biomass was determined based on δ13C. Leaf-litter addition to natural forest increased microbial biomass and induced up to 14% faster SOM decomposition (positive priming) than that in soil without litter. In contrast, negative priming in soils under plantation indicated preferential use of added leaf-litter rather than recalcitrant SOM. This preferential use of leaf-litter was supported by an increased fungal to bacterial ratio and litter-derived (13C) microbial biomass, reflecting increased substrate recalcitrance, the respective changes in microbial substrate utilization and increased C use efficiency. The magnitude and direction of priming effects depend on microbial preferential utilization of new litter or SOM. Concluding, the impact of coniferous leaf-litter inputs on the SOM priming is divergent in natural evergreen broad-leaved forests and plantations, an important consideration in understanding long-term C dynamics and cycling in natural and plantation forest ecosystems.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Identifying land-use and management practices that maintain and increase soil carbon (C) storage is important for C removal from the atmosphere and improving ecosystem services (Herrero et al. 2016). There is debate about the amount and duration of additional C that can be stored annually in soil following changes in land use or management (Smith 2014). Land-use changes from natural to artificial ecosystems, e.g., plantations, in tropical and subtropical areas contribute 12–15% of the global anthropogenic CO2 emissions (Van der Werf et al. 2009; Pan et al. 2011). The tropics and subtropics have the largest area of plantation forests worldwide (Brockerhoff et al. 2008; Huang et al. 2013).
Approximately 65% of the Chinese forests and associated C stocks are located in the southeastern subtropical provinces of Fujian, Hunan, and Zhejiang (Piao et al. 2009). More than 32% of these forests are plantations with the dominant species being Chinese fir (Cunninghamia lanceolata) (see Fig. S1; FAO 2006). Most of these plantations were converted from natural evergreen broad-leaved forests over the past decades (SFA 2009; Lu et al. 2015). Such ecosystem conversion from diverse broad-leaved forests to conifer monocultures has resulted in decreased litter quality and quantity that influences soil C storage, decomposition, and dynamics (Chen et al. 2005; Yang et al. 2009). The litter from Chinese fir has higher ratios of C to nitrogen (N) and lignin to N than those of the broad-leaved tree species (Lin et al. 2011), and therefore, a slower decomposition rate (Yu et al. 2015).
In this context, the priming effect (PE) is defined as the acceleration or retardation of SOM decomposition, i.e., strong short-term changes in soil organic matter (SOM) decomposition caused by fresh C inputs (Kuzyakov et al. 2000; Kuzyakov 2010), which can accelerate SOM decomposition (i.e., positive PE) up to 380% or decrease SOM decomposition (i.e., negative PE) up to 50% (Kuzyakov et al. 2000; Kuzyakov 2002; Cheng et al. 2014). Current views suggest that PE mainly depends on changes in microbial activities and/or strategies after fresh substrate inputs, which provide easy-to-use nutrient and/or more energy for microbial growth (Fontaine et al. 2003; Blagodatskaya and Kuzyakov 2008). However, the extent to which priming changes SOM dynamics could be leveled out by microbial adaptation to substrate decomposition. This suggests that microorganisms adapt to the litter, which decomposes faster when placed in its habitat of origin than in other environments (Ayres et al. 2009; Veen et al. 2015; Yu et al. 2015). Consequently, any changes in the response of soil-C decomposition to the litter addition could be ascribed to the adaptation of the microbial community (Cheng 1999; Meidute et al. 2008; Lü et al. 2015; Don et al. 2017). Negative PE occurs when microorganisms utilize the newly added substrate more than the existing SOM. Positive PE may occur when the added substrate induces microbial growth and activity including extracellular enzyme production (Blagodatskaya et al. 2014), and therefore, microorganisms utilize SOM more intensively (Kuzyakov et al. 2000). The above mechanisms might be important determinants of the SOM fate when tree species dominance changes, and especially after conversion from a natural forest to monoculture plantation.
Here, we present a case study based on a lab incubation of soils from two adjacent stands (one natural forest and one converted to a Chinese fir plantation), to understand the consequences of the input of Chinese fir litter to SOM decomposition. By using the same litter, any changes in the response of soil-C decomposition to the litter addition are directly or indirectly linked to the nutrient status (e.g., N) and microbial community. We hypothesized that (1) the addition of leaf-litter from the Chinese fir plantation would stimulate microbial-mediated SOM decomposition in natural forest soils (i.e., positive PE) because these microorganisms are not adapted for this specific litter, whereas (2) similar inputs of leaf-litter to plantation soil would facilitate the decomposition of litter-derived C instead of SOM mineralization (i.e., negative PE) owing to the microbial adaptation of litter decomposition and consequently, faster and more complete litter processing. We base this second hypothesis on the fact that the coniferous plantation soils have higher fungal abundance compared with natural forest soils (Guo et al. 2016).
Materials and methods
Soil sampling
In March 2015, soils were sampled from two forests: a mature natural forest dominated by Castanopsis kawakamii Hayata (Fagaceae) and an adjacent 42-year-old first-rotation Chinese fir plantation (converted from the above natural forest in 1973; hereinafter referred to as plantation), located at the Forest Ecosystem and Global Change Research Station (FEGCRS) (26°09′24″ N, 117°28’03″ E, 300 a.s.l.), Sanming (Lyu et al. 2017; Liu et al. 2017). Four replicate plots (20 × 20 m) were randomly selected in each forest type. Each plot was separated by buffer zones of more than 20 m. In each plot, 20 points were randomly selected to collect soil samples (top 10 cm) with an auger (3.5-cm diameter). The 20 soil cores were then pooled as a single representative sample from each plot. In total, four composite soil samples were collected from each forest type. The soil is classified as latosol soil in the Chinese soil classification, equivalent to Oxisol in the USDA Soil Taxonomy (State Soil Survey Service of China 1998; Guo et al. 2016). General soil characteristics are described in Table 1. As this study is unreplicated at the stand level, it is best interpreted as a case study of stand differences which result from the conversion to plantation 42 years ago, though no treatment data or replicate stands are available.
Pulse labeling of Chinese fir seedlings
Pulse labeling was performed in three-quarters of the Chinese fir seedlings from July 18, 2014, to January 26, 2015, when the seedlings were about 1 m high. The remaining unlabeled seedlings were used to measure natural 13C abundance. Each seeding was moved into a chamber (2 × 1.8 × 1.5 m) for 13CO2 pulse labeling twice monthly (Fig. S2). The 13CO2 pulse was started from 8:00 to 12:00 a.m. by slowly injecting 20 ml 0.1 M HCl with a syringe into a bottle containing dissolved 13C-sodium carbonate (99% 13C, Cambridge Isotope Laboratories Inc., Andover, MA, USA). A total quantity of 5 g NaH13CO3 was used every time for each chamber. The released 13CO2 was pumped into the chamber, which had a fan to homogenize the internal air. A pulse was given once per hour for 4 h. Seedlings were immediately removed from the chamber after being labeled. Following labeling (denoted as day 0), the Chinese fir leaf-litter was cut with scissors in both unlabeled and labeled plots.
Laboratory incubation
After removing roots and stones, each soil sample was sieved (< 2 mm) while still field-moist. The sieved soil samples were stored at 4 °C until the incubation experiment started (about 2 weeks). Each replicate of each composite soil sample was incubated with either the addition of 13C-labeled (≈ + 150‰) Chinese fir leaf-litter (litter addition) or no addition (control). The litter C and N content and C/N ratio were 45.2%, 0.83%, and 54.5, respectively.
Forty grams (oven-dry equivalent) of soil were weighed into 50-ml polyvinylchloride cups, which were placed into a 1000-ml Mason jar and pre-incubated for 7 days at 20 °C with a constant soil moisture at 50% of the field water-holding capacity (determined on a separate sample). After the pre-incubation, 13C-labeled litter was mixed with soil homogeneously at a rate of 675 mg C of 13C-labeled litter per kilogram soil (calculated based on the annual litter fall of Chinese fir plantations and the bulk density of the top 10 cm of soils). Soil moisture was adjusted with deionized water and constantly maintained at 60% of the water-holding capacity for the whole incubation period. This soil moisture was close to average field conditions and ensured that microbial processes were not water limited.
Measurement of CO2 fluxes and isotope ratios
We used the alkali absorption method to trap evolved CO2 from soils. A glass vial containing 20 mL NaOH solution (0.5 M) was placed in each Mason jar to trap CO2 released from the incubating soil. Jars were kept sealed. Three Mason jars without soil were used to trap CO2 from the ambient air trapped in each jar. The CO2 captured by the NaOH solution was measured after 1, 4, 8, 14, 22, 31, 51, 74, 103, 133, and 195 days by titration with 0.25 M HCl after adding 5 mL 0.5 M BaCl2 to precipitate all carbonates. The solution was filtered to separate the BaCO3 precipitate, which was then heated at 50 °C for 24 h. Soil CO2 production was calculated from the difference between the values of evolved CO2 in the Mason jars minus the mean of those without soil. The δ13C was measured using a GasBench II coupled to an isotope ratio mass spectrometer (Finnigan MAT-253, Thermo Electron). The δ13C of soil samples was measured with a continuous flow isotope ratio mass spectrometer. The standard deviation of δ13C of ten repeated samples was < 0.3‰.
Analyses of soil properties, microbial biomass, and main microbial groups
Soil C and N concentrations were determined using a Vario MAX CN elemental analyzer (Elementar Vario EL III, Germany). Soil texture was measured using a Mastersizer 2000 particle-sizing instrument (Malvern Instruments, Worcestershire, UK). Soil pH was measured with a pH meter, in a 1:2.5 mass/volume soil and water suspension. Soil availability N was extracted with 2 M KCl to determine the exchangeable NH4+-N and NO3−-N concentrations, using a SKALAR San++ Analyzer (Skalar, Breda, The Netherlands).
Destructive samplings of soils from four replicates per treatment were performed at 0, 30, 133, and 195 days to determine the concentrations of dissolved organic C (DOC) and microbial biomass C (MBC), which was measured by the chloroform fumigation extraction method (Vance et al. 1987). At the end of incubation, the composition of microbial functional groups was evaluated using phospholipid fatty acid (PLFA) analysis (Tunlid et al. 1989; Lü et al. 2015; Gunina et al. 2014).
Partitioning of C sources in CO2 and microbial biomass and calculation of priming effects
The 13C labeling of Chinese fir leaf-litter allowed the separation of soil (Rs) and leaf-litter-derived C (Rc) in released CO2 using the mass balance approach. Priming effect (μg C g−1) of leaf-litter addition on native SOM mineralization was defined by the following equations, using the symbols proposed by Fontaine et al. (2011):
where As13 is the 13C abundance of soil C, Ac13 is the 13C abundance of leaf-litter, Rt is the total CO2 emitted from soil with leaf-litter, and At13 is 13C abundance measured in the CO2 trap.
The PE induced by the addition of litter was calculated as:
where Rs control soil is the CO2 emitted by the control soil and Rs soil with litter is the CO2 evolved from the soil treated with litter.
Plitter is the proportion of soil CO2 efflux derived from litter C. This equation was also used to partition the contributions of litter- and SOM-derived C to organic C recovered in the K2SO4 extracts from fumigated (F) and non-fumigated (NF) soils. The MBC derived from litter (and by difference from SOM) was calculated as in Paterson and Sim (2013):
The relationship between the specific priming effect (PE) and incubation time was best explained and fitted by two different models (growth and peak functions) for the natural forest and coniferous plantation.
Statistics
A repeated ANOVA was performed to analyze the temporal effects of leaf-litter addition on SOM mineralization (released CO2) and PE. A t test was used to test the effects of litter addition on the contents of DOC, MBC, PLFAs, etc. at a significant level of p < 0.05 (SPSS version 21, SPSS Inc., Chicago, IL, USA). Changes in the composition of main microbial groups were analyzed using principal component analysis (PCA; proportional mol% of PLFA) in PRIMER (v6.1.15, Primer-E Ltd., Plymouth UK). Analysis of similarities (ANOSIM) based on Bray-Curtis distances was used to test the differences in microbial communities between the litter addition and control treatments (Oksanen et al. 2007).
Results
Soil organic matter and litter mineralization
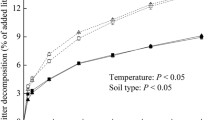
Leaf-litter addition increased the total CO2 efflux (determined as cumulative CO2 produced per gram SOC) by 44 ± 3% and 37 ± 1% in natural forest and plantation soil, respectively (p < 0.05, Fig. 1). The total amount of SOM-mineralized C in the natural forest soil (20.5 g C kg−1 SOC) was higher than that under plantation (16.2 g C kg−1 SOC) over 195 days (Fig. 1).
Litter addition decreased the amount of soil-derived C in MBC (qCO2) of natural forest, but not for the plantation soil (Table 2). Cumulative litter mineralization (normalized per gram microbial biomass C, i.e., qCO2) in soils from the natural forest was higher (26%, p < 0.01) than under the plantation after 195 days. The natural forest and plantation had comparable CUE (similar qCO2) within control soil, whereas natural forest had higher (up to 10%) soil CUE (lower qCO2) than plantation within soil with litter addition (Table 2).
Priming effect
In natural forest, the CO2 production from SOM mineralization after litter addition increased by 13.6 ± 0.04% (i.e., positive PE), but decreased by 4.1 ± 0.17% in plantation soil (i.e., negative PE, Fig. 2). Remarkably, a slow positive PE (1.1 μg C-CO2 g−1 SOC) in natural forest soil was observed in the initial stage of ca. 50 days (Fig. 2). However, in plantation soil, the cumulative PE (2.0 μg C-CO2 g−1 SOC) remained negative during the initial stage (Fig. 2). This demonstrated that during the initial intensive phase, litter decomposition exceeded the negative PE. After a period of 50 days, the direction of PE in plantation soil switched rapidly from negative to positive. However, during the slow phase of litter decomposition, the PE under plantation increased drastically. The magnitude of PE in natural forest soil was similar to that of plantation soil in the later stage (i.e., after 50 days; Fig. 2). The cumulative positive PE in plantation soil during the later stage balanced out the negative PE in the initial stage, indicating similar effects on SOM storage after litter addition.
Priming effects after addition of 13C labeled Chinese fir litter during a 195-day incubation period in two subtropical forest soils, explained by the main mechanism of microbial substrate adaptation. The priming dynamics were estimated by two models (growth and peak functions) for the natural and plantation forests, respectively. The peak function for plantation forest that represents the shifts of priming effect from negative to positive. There are two stages, i.e., microbial substrate adaptation (initial stage) and rapid priming of SOM decomposition (later stage). Results are means ± standard errors (n = 4). NS non-significant difference. * p < 0.05; ** p < 0.01; *** p < 0.001
Microbial biomass
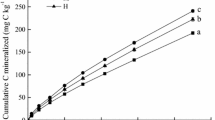
Litter addition increased MBC in natural forest soil, but not under plantation (Table 3). The MBC under natural forest was higher than that under plantation in soil with and without litter addition (Table 3). In natural forest, litter addition increased soil-derived MBC compared to the control (by up to 31%, p < 0.05), whereas MBC decreased by 13% under plantation (Fig. 3, p < 0.05). The MBC derived from litter in natural forest soil (24 ± 4.6 mg C kg−1 soil) was similar to that in plantation soil (27 ± 3.2 mg C kg−1 soil; Fig. 3). The percentage of litter-derived MBC to the total MBC in plantation was twice that in natural forest (Fig. 3).
Effects of litter addition on composition of main soil microbial groups
In plantation soil, litter addition affected both microbial biomass (Fig. 3) and the composition of main microbial groups (Fig. 4), with proportional increase of fungi and gram-positive bacteria compared to soil without litter. Litter addition increased ratio of gram-positive to gram-negative bacteria (G+/G−) and fungal to bacterial ratio (F/B) in the plantation soil. This result suggests changes occurred in microbial substrate utilization under plantation, but remained stable under the natural forest (Fig. 4). The main separation of the natural and plantation forest soils according to the PLFA composition along PC1 (explained by 62.8%) indicates that there were strong differences between microbial communities in two forest soils (Fig. 5). The PCA separation of the forests between soils with and without litter addition based on the PLFA composition was much less expressed for the natural forest soil compared to the plantation soil (Fig. 5).
The ratios of gram-positive bacteria to gram-negative bacteria (G+/G− ratio) and fungi to bacteria (F/B ratio) after a 195-day incubation period in two subtropical forest soils. Results are means ± standard errors (n = 4). *Significant difference between control and litter addition treatments at p < 0.05
Discussion
High specific C mineralization (expressed as CO2 per gram SOC) indicates lower SOM recalcitrance (Mikan et al. 2002; Fierer et al. 2003; Salomé et al. 2010). Specific C mineralization in control soil under natural forest was up to 27% greater (p < 0.05) than under plantation forest (Fig. 1). Therefore, SOM in natural forest is less recalcitrant than under plantation. Litter addition triggered up to 1.4 times higher mineralization of total CO2 efflux than that in soil without litter addition, which caused an increase (up to 14%) in SOM decomposition under natural forest. In contrast, litter addition increased the total CO2 efflux by 37% in plantation soil, whereas SOM decomposition slightly decreased by 4.1%. Consequently, litter addition triggered positive PE under natural forest but negative PE under plantation. This confirmed a high susceptibility of SOM to decomposition in the presence of litter under natural forest. Thus, our first hypothesis that litter addition would stimulate microbial-mediated decomposition of SOM in natural forest soils was confirmed.
The differences in the direction of the PE depend on N availability, specifically exchangeable NH4+ (Chen et al. 2014; Li et al. 2017). In general, positive PE owing to the “N mining” occurs when N availability is a limiting factor for microorganisms (Kuzyakov 2002; Fontaine et al. 2003; Dijkstra et al. 2013; Chen et al. 2014), whereas the “preferential substrate utilization” of litter instead of SOM induces negative PE (Kuzyakov 2002; Fontaine et al. 2004, 2011). However, N availability in the natural broad-leaved forest soil is higher than that in plantation soil (Table 1), so the positive PE is opposite to the proposed mechanisms.
Microorganisms in natural forest soil are usually adapted to utilize high-quality litter (low C/N, high N availability, diverse litter sources). These microorganisms need to mine more SOM to meet their demand for N when supplied with low-quality leaf litter. Microorganisms in natural forest mainly utilize litter-derived C as an energy source rather than for microbial growth to mineralize SOM, resulting in positive PE in natural forest (Schimel and Weintraub 2003). This can be supported by the higher litter-derived qCO2 in natural forest than that in plantation (Table 2 and Fig. 3). Higher qCO2 in soil under natural forest indicates that microorganisms have lower C use efficiency (CUE) than that in plantation soil. The higher CUE (lower qCO2) and more SOM-derived microbial biomass after litter addition relative to that of the control under natural forest promoted the growth of microorganisms better adapted to degrade SOM. Consequently, microorganisms in natural forest prefer to decompose SOM rather than added recalcitrant litter, thereby driving the positive PE (Creamer et al. 2015).
In contrast, microorganisms in plantation soil prefer to utilize the litter rather than SOM, as they have adapted over 42 years to Chinese fir litter and thus decompose them faster than that in natural forest soil (Veen et al. 2015; Yu et al. 2015). Microorganisms in plantation soil dominated by fungi, which favor utilization of litter-derived C (Guo et al. 2016; Kramer et al. 2012) to meet their growth demands and thus incorporate more litter-derived C into microbial biomass (Fig. 3). Litter addition decreased soil CUE (higher qCO2) relative to the control soil under plantation, but microorganisms in plantation soil had higher litter CUE (lower qCO2) than natural forest. Consequently, litter addition stimulated the growth of microorganisms less specialized to degrade SOM, resulting in negative PE. Thus, our second hypothesis was also confirmed.
Despite the differences in the direction and magnitude of priming between two soils immediately after litter addition, positive priming consistently increased in later stages (Fig. 2). These differences in SOM decomposition after litter addition between the two soils are related to microbial community composition (Creamer et al. 2015). Soil priming reflects the activity of certain microbial groups (particularly fungi) more than others (De Graaff et al. 2010; Fontaine et al. 2011; Garcia-Pausas and Paterson 2011; Paterson and Sim 2013; Lü et al. 2015). Priming in natural forest soil could not arise from changes in microbial community composition, as microbial community composition remained stable after litter addition (Figs. 4 and 5). Instead, the high CUE and SOM-derived microbial biomass suggested that these microorganisms—mainly K-strategists (Blagodatskaya et al. 2014)—are favored by SOM utilization. The priming intensity in natural forest soil might be driven by microbial communities and their activities rather than community composition. However, the composition of main soil microbial groups changed after litter addition in plantation. Microbial community composition is associated with shifts in microbial substrate utilization (Collins et al. 2016) and can underpin the PE direction (Blagodatskaya and Kuzyakov 2008; Dungait et al. 2011). Litter addition increased the F/B ratio in the plantation soil, which implied changes in microbial substrate utilization (Shihan et al. 2017; Lin et al. 2018), whereas there were no changes of F/B ratio under natural forest. Consequently, during the intensive litter mineralization stage (initial stage ca. 50 days), negative PE in plantation soil is mainly attributed to the preferential substrate utilization (Blagodatskaya and Kuzyakov 2008; Garcia-Pausas and Paterson 2011). At later stages, the PE strongly exceeded litter decomposition and was accompanied by decreased incorporation of SOM-derived C into microbial biomass. This indicated that the PE were possible due to microbial shifts, i.e., from fast- to slow-growing (e.g., fungi) litter feeding populations. This also suggested a new mechanism of real PE, primarily from the litter-feeding microorganisms regulating PE (Miltner et al. 2009, 2012; Shahbaz et al. 2017).
Overall, the microbial community in natural forest soil continued to decompose SOM (positive PE) and produced more microbial biomass from SOM. The increased SOM-derived microbial populations accelerated SOM decomposition which depended on activity and biomass of microbial community rather than changes in the composition of main soil microbial groups. However, microorganisms in plantation soil switched from SOM mineralization to incorporation of litter C into microbial biomass, resulting in negative PE and soil C accumulation.
Conclusions
Two PE mechanisms (Fig. 6) were proposed in soils under subtropical natural forest and 42-year-old coniferous plantation forest. The selective microbial decomposition of Chinese fir leaf-litter leads to positive PE in natural forest soil, but negative PE under plantation. This suggests that the input of Chinese fir litter following conversion of natural broad-leaved forests to plantations may accelerate SOM decomposition but would have subsequently been followed by negative priming with stand development due to microbial adaptation to plantation litter. Consequently, the priming-related soil C losses after subtropical forest conversion may ultimately have little or no long-term impact on soil C stocks. Therefore, the conversion of natural forests to plantations should consider divergent impacts of leaf-litter inputs on SOM priming. Further research efforts should focus on examining the effects of reciprocal litter transplant, i.e., home-field advantage of litter decomposition on PE under contrasting litter quality to assess how forest conversion with different tree species impacts on soil C dynamics.
Schematic diagram illustrating positive and negative priming effects caused by additions of 13C-labeled litter (from the plantation) into the soils of natural and plantation forests. The red lines show processes for microbial decomposition and assimulation of soil organic matter (SOM); the blue lines identify processes for added labeled leaf-litter. The thickness of the line indicates the magnitude of the process
Change history
13 October 2018
The author regret that a typographical error was present in the Fig. 2 of the published original version of this article; the text “Natual” in the image should have been “Natural”. The correct Fig. 2 is now presented correctly in this article.
References
Ayres E, Steltzer H, Berg S, Wall DH (2009) Soil biota accelerate decomposition in high-elevation forests by specializing in the breakdown of litter produced by the plant species above them. J Ecol 97:901–912
Blagodatskaya E, Kuzyakov Y (2008) Mechanisms of real and apparent priming effects and their dependence on soil microbial biomass and community structure: critical review. Biol Fertil Soils 45:115–131
Blagodatskaya E, Khomyakov N, Myachina O, Bogomolova I, Blagodatsky S, Kuzyakov Y (2014) Microbial interactions affect sources of priming induced by cellulose. Soil Biol Biochem 74:39–49
Brockerhoff EG, Jactel H, Parrotta JA, Quine CP, Sayer J (2008) Plantation forests and biodiversity: oxymoron or opportunity? Biodivers Conserv 17:925–951
Chen GS, Yang YS, Xie JS, Guo JF, Gao R, Qian W (2005) Conversion of a natural broad-leafed evergreen forest into pure plantation forests in a subtropical area: effects on carbon storage. Ann Forest Sci 62:659–668
Chen R, Senbayram M, Blagodatsky S, Myachina O, Dittert K, Lin X, Blagodatskaya E, Kuzyakov Y (2014) Soil C and N availability determine the priming effect: microbial N mining and stoichiometric decomposition theories. Glob Chang Biol 20:2356–2367
Cheng WX (1999) Rhizosphere feedbacks in elevated CO2. Tree Physiol 19:313–320
Cheng WX, Parton WJ, Gonzalez-Meler MA, Phillips R, Asao S, McNickle GG, Brzostek E, Jastrow JD (2014) Tansley review: synthesis and modeling perspectives of rhizosphere priming. New Phytol 201:31–44
Collins CG, Carey CJ, Aronson EL, Kopp CW, Diez JM (2016) Direct and indirect effects of native range expansion on soil microbial community structure and function. J Ecol 104:1271–1283
Creamer CA, Menezes ABD, Krull ES, Sanderman J, Newton-Walters R, Farrell M (2015) Microbial community structure mediates response of soil C decomposition to litter addition and warming. Soil Biol Biochem 80:175–188
De Graaff MA, Classen AT, Castro HF, Schadt CW (2010) Labile soil carbon inputs mediate the soil microbial community composition and plant residue decomposition rates. New Phytol 188:1055–1064
Don A, Böhme IH, Dohrmann AB, Poeplau C, Tebbe CC (2017) Microbial community composition affects soil organic carbon turnover in mineral soils. Biol Fertil Soils 53:445–456
Dijkstra FA, Carrillo Y, Pendall E, Morgan JA (2013) Rhizosphere priming: a nutrient perspective. Front Microbiol 4:216
Dungait JAJ, Kemmitt SJ, Michallon L, Guo S, Wen Q, Brookes PC, Evershed RP (2011) Variable responses of the soil microbial biomass to trace concentrations of 13C-labelled glucose, using 13C-PLFA analysis. Eur J Soil Sci 62:117–126
FAO (2006) Global forest resources assessment 2005: progress towards sustainable forest management. Food and Agriculture Organization of the United Nations, Rome
Fierer N, Allen AS, Schimel JP, Holden PA (2003) Controls on microbial CO2 production: a comparison of surface and subsurface soil horizons. Glob Chang Biol 9:1322–1332
Fontaine S, Bardoux G, Abbadie L, Mariotti A (2004) Carbon input to soil may decrease soil carbon content. Ecol Lett 7:314–320
Fontaine S, Henault C, Aamor A, Bdioui N, Bloor JMG, Maire V, Mary B, Revaillot S, Maron PA (2011) Fungi mediate long term sequestration of carbon and nitrogen in soil through their priming effect. Soil Biol Biochem 43:86–96
Fontaine S, Mariotti A, Abbadie L (2003) The priming effect of organic matter: a question of microbial competition? Soil Biol Biochem 35:837–843
Garcia-Pausas J, Paterson E (2011) Microbial community abundance and structure are determinants of soil organic matter mineralisation in the presence of labile carbon. Soil Biol Biochem 43:1705–1713
Gunina A, Dippold M, Glaser B, Kuzyakov Y (2014) Fate of low molecular weight organic substances in an arable soil: from microbial uptake to utilisation and stabilisation. Soil Biol Biochem 77:304–313
Guo J, Yang Z, Lin C, Liu X, Chen G, Yang Y (2016) Conversion of a natural evergreen broadleaved forest into coniferous plantations in a subtropical area: effects on composition of soil microbial communities and soil respiration. Biol Fertil Soils 52:799–809
Herrero M, Henderson B, Havlík P, Thornton PK, Conant RT, Smith P, Wirsenius S, Hristov AN, Gerber PJ, Gill M, Butterbach-Bahl K, Valin H, Garnett T, Stehfest E (2016) Greenhouse gas mitigation potentials in the livestock sector. Nat Clim Chang 6:452–461
Huang ZQ, He ZM, Wan XH, Hu ZH, Fan SH, Yang YS (2013) Harvest residue management effects on tree growth and ecosystem carbon in a Chinese fir plantation in subtropical China. Plant Soil 364:303–314
Kramer S, Marhan S, Ruess L, Armbruster W, Butenschoen O, Haslwimmer H, Kuzyakov Y, Pausch J, Scheunemann N, Schoene J, Schmalwasser A, Totsche KU, Walker F, Scheu S, Kandeler E (2012) Carbon flow into microbial and fungal biomass as basis for the belowground food web of agroecosystems. Pedobiologia 55:111–119
Kuzyakov Y (2002) Review: factors affecting rhizosphere priming effects. J Plant Nutr Soil Sci 165:382–396
Kuzyakov Y (2010) Priming effects: interactions between living and dead organic matter. Soil Biol Biochem 42:1363–1371
Kuzyakov Y, Friedel JK, Stahr K (2000) Review of mechanisms and quantification of priming effects. Soil Biol Biochem 32:1485–1498
Li QR, Tian YQ, Zhang XY, Xu XL, Wang HM, Kuzyakov Y (2017) Labile carbon and nitrogen additions affect soil organic matter decomposition more strongly than temperature. Appl Soil Ecol 114:152–160
Lin C, Yang Y, Guo J, Chen G, Xie J (2011) Fine root decomposition of evergreen broadleaved and coniferous tree species in midsubtropical China: dynamics of dry mass, nutrient and organic fractions. Plant Soil 338:311–327
Lin Z, Li Y, Tang C, Luo Y, Fu W, Cai X, Li Y, Yue T, Jiang P, Hu S, Chang SX (2018) Converting natural evergreen broadleaf forests to intensively managed moso bamboo plantations affects the pool size and stability of soil organic carbon and enzyme activities. Biol Fertil Soils 54:467–480
Liu XF, Lin TC, Yang ZJ, Vadeboncoeur MA, Lin CF, Xiong DC, Lin WS, Chen GS, Xie JS, Li YQ, Yang YS (2017) Increased litter in subtropical forests boosts soil respiration in natural forests but not plantations of Castanopsis carlesii. Plant Soil 418:141–151
Lü MK, Xie JS, Wang C, Guo JF, Wang MH, Liu XF, Chen YM, Chen GS, Yang YS (2015) Forest conversion stimulated deep soil C losses and decreased C recalcitrance through priming effect in subtropical China. Biol Fertil Soils 51:857–867
Lu Y, Coops NC, Wang T, Wang G (2015) A process-based approach to estimate Chinese fir (Cunninghamia lanceolata) distribution and productivity in southern China under climate change. Forests 6:360–379
Lyu MK, Xie JS, Ukonmaanaho L, Jiang MH, Li YQ, Chen YM, Yang ZJ, Zhou YX, Lin WS, Yang YS (2017) Land-use change exerts a strong impact on deep soil C stabilization in subtropical forests. J Soils Sediments 17:2305–2317
Meidute S, Demoling F, Bååth E (2008) Antagonistic and synergistic effects of fungal and bacterial growth in soil after adding different carbon and nitrogen sources. Soil Biol Biochem 40:2334–2344
Mikan CJ, Schimel JP, Doyle AP (2002) Temperature controls of microbial respiration in arctic tundra soils above and below freezing. Soil Biol Biochem 34:1785–1795
Miltner A, Kindler R, Knicker H, Richnow HH, Kästner M (2009) Fate of microbial biomass-derived amino acids in soil and their contribution to soil organic matter. Org Geochem 40:978–985
Miltner A, Bombach P, Schmidt-Brücken B, Kästner M (2012) SOM genesis: microbial biomass as a significant source. Biogeochemistry 111:41–55
Oksanen J, Kindt R, Legendre P, O’Hara B, Stevens MHH, Oksanen MJ, Suggests M (2007) The vegan package. Commun Ecol Package 10
Pan Y, Birdsey RA, Fang J, Houghton R, Kauppi PE, Kurz Werner A, Phillips OL, Shvidenko A, Lewis SL, Canadell JG, Ciais P, Jackson RB, Pacala S, McGuire AD, Piao S, Rautiainen A, Sitch S, Hayes D (2011) A large and persistent carbon sink in the world’s forests. Science 333:988–993
Paterson E, Sim A (2013) Soil-specific response functions of organic matter mineralization to the availability of labile carbon. Glob Chang Biol 19:1562–1571
Piao S, Fang J, Ciais P, Peylin P, Huang Y, Sitch S, Wang T (2009) The carbon balance of terrestrial ecosystems in China. Nature 458:1009–1013
Salomé C, Nunan N, Pouteau V, Lerch TZ, Chenu C (2010) Carbon dynamics in topsoil and in subsoil may be controlled by different regulatory mechanisms. Glob Chang Biol 16:416–426
Schimel JP, Weintraub MN (2003) The implications of exoenzyme activity on microbial carbon and nitrogen limitation in soil: a theoretical model. Soil Biol Biochem 35:549–563
SFA (2009) China’s forestry 2004–2008. China Forestry Publishing House, Beijing
Shihan A, Hättenschwiler S, Milcu A, Joly FX, Santonja M, Fromin N (2017) Changes in soil microbial substrate utilization in response to altered litter diversity and precipitation in a Mediterranean shrubland. Biol Fertil Soils 53:171–185
Shahbaz M, Kuzyakov Y, Sanaullah M, Heitkamp F, Zelenev V, Kumar A, Blagodatskaya E (2017) Microbial decomposition of soil organic matter is mediated by quality and quantity of crop residues: mechanisms and thresholds. Biol Fertil Soils 53:287–301
Smith P (2014) Do grasslands act as a perpetual sink for carbon? Glob Chang Biol 20:2708–2711
State Soil Survey Service of China (1998) China soil. China Agriculture Press, Beijing
Tunlid A, Hoitink HAJ, Low C, White DC (1989) Characterization of bacteria that suppress rhizoctonia damping-off in bark compost media by analysis of fatty-acid biomarkers. Appl Environ Microbiol 55:1368–1374
Van der Werf GR, Morton DC, DeFries RS, Olivier JG, Kasibhatla PS, Jackson RB, Collatz GJ, Randerson JT (2009) CO2 emissions from forest loss. Nat Geosci 2:737–738
Vance ED, Brookes PC, Jenkinson DS (1987) An extraction method for measuring soil microbial biomass C. Soil Biol Biochem 19:703–707
Veen GFC, Freschet GT, Ordonez A, Wardle DA (2015) Litter quality and environmental controls of home-field advantage effects on litter decomposition. Oikos 124:187–197
Yang Y, Guo J, Chen G, Yin Y, Gao R, Lin C (2009) Effects of forest conversion on soil labile organic carbon fractions and aggregate stability in subtropical China. Plant Soil 323:153–162
Yu ZP, Huang ZQ, Wang MH, Liu RQ, Zheng LJ, Wan XH, Hu ZH, Davis MR, Lin TC (2015) Nitrogen addition enhances home-field advantage during litter decomposition in subtropical forest plantations. Soil Biol Biochem 90:188–196
Acknowledgements
We thank Soil Science Consulting (https://soilscicon.wordpress.com) for help in the preparation of the manuscript.
Funding
The research was funded by the National Key Research and Development Program (No. 2016YFD0600204), the National Natural Science Foundation of China (Nos. U1405231, U1505233, and 31470501), and the National “973” Program of China (No. 2014CB954003). The publication was supported by the Government Program of Competitive Growth of Kazan Federal University and with the support of the “RUDN University program 5-100.”
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
ESM 1
(DOCX 979 kb)
Rights and permissions
About this article
Cite this article
Lyu, M., Xie, J., Vadeboncoeur, M.A. et al. Simulated leaf litter addition causes opposite priming effects on natural forest and plantation soils. Biol Fertil Soils 54, 925–934 (2018). https://doi.org/10.1007/s00374-018-1314-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00374-018-1314-5