Abstract
Use of nitrogen (N) fertilizer is underway to increase in Sub-Saharan Africa (SSA). The effect of increasing N rates on ammonia (NH3) volatilization—a main pathway of applied-N loss in cropping systems—has not been evaluated in this region. In two soils (Alfisols, ALF; and Andisols, AND) with maize crop in the East African highlands, we measured NH3 volatilization following urea broadcast at six rates (0–150 kg N ha−1) for 17 days, using a semi-open static chamber method. Immediate irrigation and urea deep placement were tested as mitigation treatments. The underlying mechanism was assessed by monitoring soil pH and mineral N (NH4+ and NO3−) concentrations. More cumulative NH3-N was volatilized in ALF than in AND at the same urea-N rate. Generally, higher urea-N rates increased proportional NH3-N loss (percent of applied N loss as NH3-N). Based on well-fitted sigmoid models, simple surface urea application is not recommended for ALF, while up to 60 kg N ha−1 could be adopted for AND soils. The susceptibility of ALF to NH3 loss mainly resulted from its low pH buffering capacity, low cation exchange capacity, and high urease activity. Both mitigation treatments were effective. The inhibited rise of soil pH but not NH4+ concentration was the main reason for the mitigated NH3-N losses, although nitrification in the irrigation treatment might also have contributed. Our results showed that in acidic soils common to SSA croplands, proportional NH3-N loss can be substantial even at a low urea-N rate; and that the design of mitigation treatments should consider the soil’s inherent capacity to buffer NH3 loss.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Globally, ammonia (NH3) volatilization from the application of synthetic nitrogen (N) fertilizers accounts for about 14% of annual NH3-N emissions (Bouwman et al. 2002). This N loss as emitted NH3 causes a no-win situation between resource utilization and eco-environmental conservation. Ammonia loss from applied N results in low fertilizer-N use efficiency, posing a substantial financial cost to farmers (Pan et al. 2016). Furthermore, this N resource loss from agricultural systems turns into pollutants in the atmosphere and causes cascading effects (Galloway et al. 2003) including soil acidification, eutrophication, and declining biodiversity (e.g., Scudlark et al. 2005).
Increased use of fertilizer (especially N) is unequivocally a critical step in offsetting soil nutrient depletion and securing food production in Sub-Saharan Africa (SSA). Use of fertilizer-N in SSA is extremely limited, typically less than 10 kg N ha−1 year−1 (Vitousek et al. 2009), which is the main cause of stagnantly low yields of cereal production in past decades. Regional and national efforts are underway to increase fertilizer use six-fold to reach an average of 50 kg mineral fertilizer ha−1 year−1 (AGRA 2009). In trial sites of the Millennium Villages Project, the recommended application rate of N for maize (Zea mays L.) cultivation varied regionally up to 129 kg N ha−1 based on national research and extension services (Nziguheba et al. 2010). Considering the low N input in current African croplands, Hickman et al. (2015) pointed out that at least 6 Tg N year−1 would be required just to reach an average application rate of 75 kg N ha−1 year−1 for cereal production on existing agricultural land. This unprecedented amount of N input will be subject to a large amount of NH3-N loss if not properly managed, provided that urea heavily dominates N fertilizer consumption (over 50%; IFA 2017) and topdressing of urea is the most common N management practice across SSA croplands.
Surprisingly, field measurements of NH3 volatilization from applied fertilizer in SSA croplands are rare, and to our knowledge, the way that NH3 loss responds to increasing rates of N application has not yet been evaluated in this region. Understanding the relationship between NH3 volatilization and increasing N application rates is urgently needed to develop proper guidance on N management practices for local farmers. Field measurements in SSA are also essential to reducing uncertainties in global synthetic analysis of NH3 emissions (Pan et al. 2016; Jiang et al. 2017).
Urea is the most extensively used N fertilizer in tropical agroecosystems (IFA 2017). Its hydrolysis is known as a key process in inducing NH3 volatilization by producing highly concentrated NH4+ with sharply raised pH (Black et al. 1987b). In acidic soils common to SSA croplands, pH rises during urea hydrolysis owing to proton consumption by hydrolyzed CO32− and HCO3− (Ferguson et al. 1984). As the pH increases, NH3 volatilization occurs owing to the reaction between OH− and NH4+. Therefore, mitigation strategies have been developed based mainly on two goals: reducing NH4+ concentration and inhibiting pH rise. The former includes retarding urea hydrolysis with urease inhibitors (Cantu et al. 2017) and physical absorption of NH4+ by applied biochar (Subedi et al. 2015). The latter includes amendments with acidifying effects, e.g., pyrite with copper sulphate (Reddy and Sharma 2000). Acceleration of the nitrification process by activating nitrifying bacteria can reduce both soil NH4+ concentration and pH (Fleisher and Hagin 1981).
The soil’s inherent capacity to buffer NH3 loss after urea application, however, is rarely involved in the design of mitigation strategy. Soils with high cation exchange capacity (CEC) and pH buffering capacity (PBC) are likely to have low NH3 emissions even when the added amount of urea is considerable (Haden et al. 2011). Soil properties like CEC and PBC can vary extensively across soils (Haden et al. 2011), resulting in variable inherent capacity of the soil to buffer NH3 loss. Therefore, soil-specific assessment is essential to developing a practical mitigation strategy. Some farming practices like irrigation and deep placement of urea are also well-established strategies for mitigating NH3 volatilization (Holcomb et al. 2011; Rochette et al. 2013a); nevertheless, their performance should be tested under practical situations.
Increasing the urea application rate can be expected to change the soil NH4+ concentration and pH range, which is expected to affect NH3 volatilization. Interestingly, previous studies have reported inconsistent results on the effect of urea-N rate on proportional NH3-N loss (percent of applied N loss as NH3-N; or simply the emission factor), even within the category of acidic soil. Black et al. (1987a) reported a positive effect, Tian et al. (2001) and Rimski-Korsakov et al. (2012) indicated a negative effect, and Watson and Kilpatrick (1991) showed no clear correlation between urea application rate and proportional NH3-N loss. As pointed out by Bouwman et al. (2002), different factors (e.g., soil and environmental) and processes (e.g., urea hydrolysis and nitrification) interact. This is supported by a 7-year site study by Ma et al. (2010), where tremendous variation in NH3 volatilization was observed both across years within the same soil type and across soil types within the same year.
Maize is the staple food for SSA people. Highlands in East Africa are generally densely populated and intensively cultivated for production, and are known as the “bread basket.” For example, in Tanzania, about 46% of maize production is contributed by the southern highlands, which make up only 28% of the mainland area of this country (Bisanda et al. 1998).
The aim of this study was therefore to evaluate the effect of urea application rate on NH3 volatilization in SSA croplands with different soil properties. The specific objectives were to (1) quantify the amount of NH3 volatilization and emission factors as affected by urea application rate and soil properties; (2) determine the soil’s inherent capacity to buffer NH3 loss, above which large NH3 losses occur; and (3) figure out effective strategies to mitigate NH3 losses and assess the underlying mechanism in maize fields in the East African highlands.
Materials and methods
Site description
Field experiments were conducted at two maize fields with different soil properties in the East African highlands. One site is located in the village of Mangalali in the Iringa region of Tanzania (07° 46′ S, 35° 34′ E), which has an elevation of 1480 m. Maize has been cultivated by local farmers for more than 5 years. The soil is classified as coarse-loamy, isohyperthermic, Kanhaplic Haplustalfs (ALF). The other site is located in the town of Uyole in the Mbeya region of Tanzania (08° 55′ S, 33° 31′ E), which has an elevation of 1780 m. It is used as experimental maize plots inside the Mbeya Agricultural Training Institute. The soil was classified as clay-loam, isothermic, Dystric Vitric Haplustands (AND). Soil classification was performed based on the USDA system (Soil Survey Staff 2006). Detailed properties of ALF and AND are presented in Table 1.
Experimental design and treatments
At each site, maize (Zea mays L.) was grown in a field (~ 700 m2) with a spacing of 0.7 m × 0.3 m, giving a population of ~ 48,000 plants ha−1. To facilitate experimental setup and sampling process, we cleared a small area (6 m × 8 m) from the field before the start of the experiment. Maize had finished tasselling when being removed. Ammonia volatilization was measured on this area without maize cultivation. Maize removal was not expected to affect the experiment because we focused on the NH3 volatilization from soils applied with urea. Further, volatilized NH3 was measured with enclosures (see the semi-open static chamber system below), which largely excluded the interaction between crop and the fertilizer inside the enclosure, leading to negligible difference between field conditions of maize removal and maize under cultivation. Enclosures are much used in the field experiments allowing many treatments to be evaluated in the same field (Sommer et al. 2004).
For the measurement of NH3 volatilization, a randomized complete block design was adopted for eight treatments: six urea application rates with surface broadcast (0, 30, 50, 70, 100, and 150 kg N ha−1, denoted as 0N, 30N, 50N, 70N, 100N, and 150N, respectively) and two mitigation treatments with an application rate of 100 kg N ha−1 (irrigation of 10 mm water immediately after urea application, denoted as 100N + W; and deep placement of urea at 5 cm depth, 100N + DP). Three replicates were measured for each treatment, resulting in a total of 24 plots. Each plot was 0.5 m × 0.5 m in size. Plots were separated by a 0.5 m buffer. The plot size was designed based on the size of chamber for NH3 volatilization measurement (12 cm diameter cylinder; see below). According to local practices, urea is always applied after rainfall during the rainy season. We therefore applied irrigation equivalent to 5 mm rainfall to the ALF plots 1 day before starting the experiment. In the AND plots, the experiment was started 1 day after a rainfall event (22 mm).
Adjacent to the plots for NH3 volatilization measurement, nine plots (0.5 m × 0.5 m size; separated by a 0.5 m buffer) receiving three treatments (100N, 100N + W, and 100N + DP; with three replicates) in a randomized complete block design were set up for soil sampling.
NH3 volatilization measurement
A semi-open static chamber system was used for NH3 volatilization measurement. A polyvinyl chloride cylinder of 12 cm diameter and 30 cm height was inserted about 10 cm into the soil at each plot. Two foam disks with a density of 0.026 g cm−3 and a thickness of 2 cm were placed horizontally inside each chamber at 10 and 20 cm above the soil surface, respectively. The lower disk trapped NH3 volatilized from the soil, while the higher one prevented contamination from atmospheric NH3. The diameter of the foam disks was made slightly larger than that of the chamber so that they would remain in place when the foam expanded against the sides of the chamber. These foam disks were soaked with acid reagents (1 M H3PO4 + 4% v/v glycerol) before use. A volume of 20 ml of acid reagent was verified to be sufficient to saturate the foam disk evenly but not drip from the foam or leach down the sides of the chamber. During the experimental period, the chamber was sheltered from the direct effects of rainfall and sunshine with a round PVC plate (42.5 cm in diameter) supported by four wood sticks driven into the soil. The plate was slightly inclined by adjusting the heights of the wood sticks and placed about 10 cm above the top of the chamber to allow air flow.
The foam disks were collected and replaced with freshly soaked foams 1, 3, 5, 7, 9, 12, and 17 days after urea application. Foam disks were sealed in plastic bags during transport. The trapped NH4+ was extracted by three sequential extractions with 100, 100, and 50 ml of 1 M KCl. Each time after adding KCl solution, the foam disk was squeezed ten times by hand and the extract was then transferred to a 500 ml volumetric flask. The final volume of the KCl extract was then brought to 500 ml by adding 1 M KCl. To verify the reliability of this extraction method, several fourth extractions were conducted for samples from the 150N treatment, and these confirmed that the amounts of NH4+ remaining were negligible. The KCl extract was filtered (No. 6 filter paper, Adventec, Japan) and determined colorimetrically using a flow injection auto-analyzer (Flow Analysis Method, JIS K-0170, AQLA-700 Flow Injection Analyzer, Aqualab Inc., Japan).
Environmental monitoring
At each site, in the plots used for NH3 volatilization measurement, four soil moisture probes (ECH2O TE, Decagon Devices, Inc., USA) were connected to a digital data logger (Em50, Decagon Devices, Inc., USA) to monitor soil moisture and temperature at a 5 cm depth, at a frequency of every minute. One of these four sensors was inserted into the ambient field, while the other three were used for soils inside the chambers receiving the 100N, 100N + W, and 100N + DP treatments, respectively. Soil moisture expressed as volumetric water content was separately calibrated with soils sampled from each field (R2 = 0.96 for the calibration function with n = 8 in ALF and R2 = 0.97 with n = 5 in AND). Rainfall at each site was recorded every 10 min by a TE525MM rain gauge connected to a CR1000 data logger (Campbell Scientific, Inc., USA).
Soil sampling and analysis
In the 24 plots used for NH3 volatilization measurement, soils were sampled from the top layer (0–10 cm) before urea application to evaluate the initial soil characteristics. Soils were air-dried and sieved through 2-mm mesh before being transported to Japan for analysis of total C (TC), total N (TN), pH (initial pH), major exchangeable cations (Ca2+, Mg2+, Na+, K+), CEC, PBC, and urease activity. Air-drying was verified to have negligible influence on the soil urease activity in this study (see Fig. S1 in the supplementary material).
The contents of TC and TN were determined with a CN analyzer (Vario Max CN, Elementar, Germany). Major exchangeable cations were extracted with 1 M ammonium acetate at pH 7.0. Exchangeable Ca2+ and Mg2+ were determined by atomic absorption spectroscopy and Na+ and K+ by flame emission spectroscopy (AA-660 instrument, Shimadzu, Japan). To determine CEC, we washed the residual soil with ethanol after ammonium acetate extraction and then extracted the remaining NH4+ with 10% sodium chloride. Extracted NH4+ was determined by steam distillation and titration. To determine PBC, titratable acidity was measured following Sakurai et al. (1989) with a potentiometric automatic titrator (COM-1600, Hiranuma Sangyo Co., Ltd., Japan). Consumption of OH− at pH 8.3 was used to represent PBC, which is thus expressed as mmol OH− kg−1 soil. Soil urease activity was determined as the release of NH4+-N after 2-h incubation following the procedure by Kandeler and Gerber (1988). Urease activity was determined at 25 °C (without pH buffer) and 37 °C (with and without pH buffer), and expressed as mg NH4+-N kg−1 soil h−1.
In the nine plots specially set up for soil sampling, chambers were also installed to maintain a similar condition to the plots for NH3 measurement, but with a larger diameter (35 cm). The larger area covered allowed two subsamples to be taken from the chamber during each sampling activity. Two subsamples were mixed to reduce uncertainties caused by sampling error. Soil samplings were conducted at 0, 1, 3, 5, 7, 9, 12, and 17 days after urea application, and were analyzed for soil moisture, pH, and mineral N (NH4+ and NO3−) concentrations. To detect whether immediate irrigation can move dissolved urea and NH4+ down to deeper soil and thereby reduce the surface NH4+ concentration, we sampled only the upper 3–4 cm soil from the 100N and 100N + W treatments. This is because the vertical diffusion of urea-N and NH4+ are often limited to within 3 cm after surface urea application (Black et al. 1987b). For the 100N + DP treatment, in order to capture the effect of deep placement of urea on pH change, we sampled soil from top 7 cm covering the placement depth.
Field moist soils (around 50 g for each sample) were immediately transported to the local laboratory for oven drying at 60 °C after recording the total moist weight. The dry weight of each sample was then recorded before sieving through 2-mm mesh for subsequent analysis at the laboratory in Japan. The difference in soil weight before and after oven drying, together with bulk density, was used to calculate the volumetric water content. The soil pH (1:5 soil:water ratio) was measured with a glass electrode (pH/ion meter LUQUA F-74BW, Horiba Ltd., Japan). Mineral N was extracted from 10.0 g soil (dry base) with 30.0 ml of 1 M KCl for 30 min on a reciprocating shaker, and the suspension was centrifuged (2000×g, 10 min) and filtered through filter paper (No. 6, Advantec, Japan). Extracted NH4+ and NO3− were determined colorimetrically using the same flow injection auto-analyzer (Flow Analysis Method, JIS K-0170, AQLA-700 Flow Injection Analyzer, Aqualab Inc., Japan).
Statistical analysis
An independent two-sample t test was used to examine whether initial soil characteristics differed between the two sites. Two-way analysis of variance (ANOVA) was used to examine the effects of site and urea-N rate on cumulative NH3-N loss, with the interaction site × block included as a between-subjects factor to reduce experimental error from source of variation. Residuals were plotted with fitted values to check the model assumptions of independence and common variance. Normality of the residuals was checked by the Shapiro-Wilk test. Levene’s test was applied to statistically check equal variance across treatments. The statistically significant differences were identified as P < 0.05 unless stated otherwise. For three treatments (100N, 100N + W, and 100N + DP), Pearson correlation was conducted between NH3-N loss and the soil variables of mineral N concentration, pH, and moisture content, respectively.
A linear function was fitted to cumulative NH3-N loss with urea-N rate for ALF, while a piecewise function was fitted for AND. The breakpoint in the piecewise function was statistically estimated using the segmented package for the R software version 3.3.3 (http://www.r-project.org). Sigmoid (three and four parameters), exponential (growth and rise to maximum), and quadratic curves were fitted to proportional NH3-N loss with urea-N rate at each site using the non-linear least square method (the nls function in R). Model comparison was conducted using the Bayesian Information Criterion (BIC) together with “pseudo R2,” which was calculated as 1—(residual sum of squares/total sum of squares). All statistical analyses were carried out with R (version 3.3.3).
Results
Soil properties and environmental factors
Significant differences (P < 0.05) in initial soil characteristics were observed between ALF and AND, as shown in Table 1. ALF was lower in TC, TN, initial pH, CEC, major cations, PBC, and water holding capacity as compared with AND, but higher in urease activity measured under all conditions (25 °C without pH buffer, 37 °C with and without pH buffer).
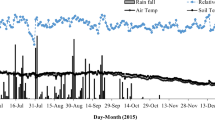
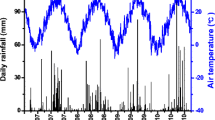
Rainfall distribution, temporal variation of soil moisture, and temperature at 5 cm depth in the field and inside the chambers under three treatments (100N, 100N + W, and 100 N + DP) are presented in Fig. 1. After the experiment started, several rainfall events were recorded in ALF but not in AND plots. Large difference of soil moisture between the 100N treatment and the field was observed in ALF but not AND (Fig. 1). In ALF, lower soil moisture in the 100N treatment compared with that in the field was attributed to the shelters above the chamber, which prevented direct water supply from rainfall. In AND, no difference of soil moisture between the 100N treatment and the field was expected as no rainfall events occurred after starting the experiment. At both ALF and AND, soil moisture was higher in 100N + W, followed by 100N, and lower in 100N + DP. The practice of deep placement probably reduced the soil bulk density and resulted in lower volumetric water content. Averaged daily soil temperature inside the chamber was higher for ALF (22.3 °C) than for AND (17.3 °C) and was negligibly affected by the treatments. Daily maximum soil temperature was lower inside the chambers compared with that in fields in both ALF and AND.
Environmental factors including a, b soil moisture and c, d temperatures of three treatments (100N, 100N + W, and 100N + DP) and the field as well as rainfall monitored during the study period in ALF and AND, respectively. 100N surface application of urea with 100 kg N ha−1, 100N + W irrigation of 10 mm water immediately after urea application with 100 kg N ha−1, 100N + DP deep placement of urea (100 kg N ha−1) at 5 cm depth
NH3 volatilization under different urea-N rates at two croplands
NH3-N loss between sampling dates differed by urea-N rate and sampling time during the first week after urea application at both ALF and AND (Fig. 2). NH3-N loss from the 0N treatment remained low and constant across the study period at both sites. In urea-applied plots, the peak of NH3-N loss between sampling dates occurred on sampling day 3, with the peak in ALF much higher than that in AND under the same urea-N rate (Fig. 2). On sampling day 3, NH3-N loss between sampling dates contributed 73–82 and 42–55% of the cumulative NH3-N loss in ALF and AND, respectively. NH3-N loss between sampling dates dropped progressively to a low level after sampling day 3 in ALF, while it extended to sampling day 7 in AND.
Cumulative NH3-N loss was significantly (P < 0.001) affected by urea-N rate, and the significant interaction (P < 0.001) of urea-N rate × site resulted in consistently higher—more than double—cumulative NH3-N loss from ALF than from AND at the same urea-N rate (Table 2, S1). In urea-applied plots, cumulative NH3-N loss ranged from 11.1 to 77.6 kg N ha−1 and from 1.9 to 32.7 kg N ha−1 for ALF and AND, respectively, corresponding to the range of proportional NH3-N loss from 36.4 to 51.6% and from 5.2 to 21.6%, respectively (Table S1). In ALF, the first 3 days contributed to more than 85% of cumulative NH3-N losses in all urea-applied plots, while it took 7 days to reach a similar contribution in AND.
The response of cumulative NH3-N loss to increasing urea-N rates varied across sites (Fig. 3a, b). A linear pattern (R2 = 0.996, P < 0.001) with a relatively high slope (0.527) was observed in ALF (Fig. 3a). The intercept with urea-N rate showed that only about 5 kg N ha−1 could be applied without subjecting to NH3-N loss. In AND, a piecewise pattern (R2 = 0.96, P = 0.02) was found, with a breaking point occurring at a urea-N rate of 59 kg N ha−1 (Fig. 3b), indicating a threshold of cumulative NH3-N loss at much higher urea-N rate.
In describing the response of proportional NH3-N loss to urea-N rate, a sigmoid model with three parameters (BIC = 14.8) was equally well fitted as an exponential rise to maximum model (BIC = 12.7) for ALF (Table 3). A sigmoid model with four parameters (BIC = 2.2) provided a clearly better fit than any other models for AND (Table 3). Both well-fitted models for ALF showed a sharp increase in proportional N loss and reached a ceiling level (51%, as indicated by the parameter a in both models) with increasing urea-N rate (Fig. 3c; Table 3). In the sigmoid curve for AND, a “lag phase” of low proportional N loss was observed before the fast “growing phase” and the final “maximum phase” (Fig. 3d). The “lag phase” representing the inherent capacity of the soil to buffer NH3-N loss was missing in ALF, while a range of urea-N rate from 0 to 60 kg N ha−1 could be safely adopted in AND. As indicated by the parameter b in sigmoid models for ALF and AND (Table 3), the maximum increasing rate of proportional NH3-N loss occurred at a much lower rate of urea-N in ALF (14 kg N ha−1) than in AND (84 kg N ha−1).
Performance of NH3-N loss mitigation treatments
At the two sites, both mitigation treatments (100N + W and 100N + DP) effectively reduced the cumulative NH3-N loss (Fig. 4) to near-background level (the 0N treatment). At each site, cumulative NH3-N loss from 100N + W (0.7 and 1.6 kg N ha−1 in ALF and AND, respectively) was slightly higher than that from 100N + DP (0.2 and 0.3 kg N ha−1 in ALF and AND, respectively), but not significantly different (P > 0.9 and P > 0.3 in ALF and AND, respectively).
Performance of mitigation treatments in reducing cumulative NH3-N loss after urea application at a ALF and b AND. 100N surface application of urea with 100 kg N ha−1, 100N + W irrigation of 10 mm water immediately after urea application with 100 kg N ha−1, 100N + DP deep placement of urea (100 kg N ha−1) at 5 cm depth. Error bars represent standard errors of the means (n = 3)
Variation in soil mineral N, pH, and moisture
Soil NH4+ concentrations varied in response to treatment (100N, 100N + W, and 100N + DP) and sampling time at both ALF and AND (Fig. 5a, b). At each site, soil NH4+ concentrations in the 100N treatment peaked after urea application and were consistently higher than those in the other two treatments (100N + W and 100N + DP; Fig. 5a, b; Table S2). Soil NH4+ concentrations in the 100N treatment ranged from 1.5 to 140 mg N kg−1 in ALF, and from 44 to 428 mg N kg−1 in AND. At each site, the 100N + W and 100N + DP treatments resulted in similar soil NH4+ concentrations, ranging from 1.5 to 79 mg N kg−1 in ALF and from 44 to 200 mg N kg−1 in AND, respectively. At each site, soil NO3− concentrations were generally higher in the 100N + W treatment after urea application, and the variations in the other two treatments were similar (Fig. 5c, d). In ALF, soil NO3− concentrations gradually increased from 1.6 mg N kg−1 to a similar level (ca. 72 mg N kg−1) among the three treatments (Fig. 5c). In AND, the range of soil NO3− concentrations was much larger in the 100N + W treatment (5.8–219 mg N kg−1) than in the other two treatments with a similar range (5.8–130 mg N kg−1; Fig. 5d).
Soil factors including a, b NH4+ concentration, c, d NO3− concentration, e, f pH, and g, h moisture content for three treatments (100N, 100N + W, and 100N + DP) monitored in ALF and AND, respectively. 100N surface application of urea with 100 kg N ha−1, 100N + W irrigation of 10 mm water immediately after urea application with 100 kg N ha−1, 100N + DP deep placement of urea (100 kg N ha−1) at 5 cm depth. Note that in the 100N and 100N + W treatments, soils were sampled from 0 to 3 cm, whereas in the 100N + DP treatment, soils were sampled from 0 to 7 cm. Error bars represent standard errors of the means (n = 3)
Soil pH followed very similar variation patterns to soil NH4+ concentrations in ALF and AND (Fig. 5e, f; Table S2). In ALF, pH in the 100N treatment peaked at 8.4, which was much higher than the peak in the 100N + W treatment (7.1) or 100N + DP treatment (6.9). In AND, the highest pH peak was also found in the 100N treatment (7.3), followed by those in the 100N + DP treatment (6.8) and 100N + W treatment (6.7). Soil moisture at both sites was generally higher in the 100N + W treatment and lower in the 100N + DP treatment, with soil moisture in the 100N treatment varying in between across sampling times (Fig. 5g, h; Table S2).
Soil pH and NO3− concentrations were most frequently correlated with NH3-N loss between sampling dates (Table 4). Soil NO3− concentrations were negatively correlated with NH3-N loss, with the exception of the weak correlation found in the 100N + DP treatments at both sites. Soil pH was significantly (P < 0.1) and positively correlated with NH3-N loss in all the treatments that showed relatively high cumulative NH3-N loss (the 100N treatment in ALF and 100N and 100N + W treatments in AND). Significant correlation between soil NH4+ concentration and NH3-N loss was only found in the 100N treatment in AND. Soil moisture was significantly (P < 0.05) correlated with NH3-N loss in the 100N treatment in ALF and in the 100N + W treatment in AND.
Discussion
NH3 volatilization in response to urea application across two croplands
ALF was much more susceptible than AND to NH3-N loss after urea application (Figs. 2 and 3; Table S1), as the lowest proportional NH3-N loss from the 30N treatment in ALF (36.4%) was about 15% higher than the highest loss from the 150N treatment in AND (21.6%). This could be attributed to the different soil properties (PBC, CEC, and urease activity) and environmental factor (soil temperature) (Table 1; Fig. 1). Enhancement of the native soil PBC (i.e., by adding hydroxyl-Al polymer or acid cation exchange resin) has been shown to reduce soil surface pH and thus cumulative NH3-N loss after urea application (Ferguson et al. 1984). A close and negative correlation between proportional NH3-N loss and CEC (R = − 0.846) was reported for eight arable soils applied with cattle urine (Whitehead and Raistrick 1993). Higher soil urease activity stimulates urea hydrolysis rate, raising soil pH and NH4+ concentrations more sharply and leading to higher NH3-N loss (Soares et al. 2012). Furthermore, at the same study site receiving surface urea application, lower proportional N loss was recorded in the winter than in the summer (Elliot and Fox 2014), possibly because the low temperatures depressed urease activity. All the findings mentioned above suggested that ALF would be more susceptible to NH3-N loss, as it was lower in PBC and CEC, and higher in urease activity and soil temperature. Lower initial soil pH is expected to contribute to the reduction of NH3-N loss (He et al. 1999), yet in ALF, the weak PBC outweighed its low initial pH in reducing NH3-N loss (Table 1).
The sigmoid model was found to be helpful in describing the relationship between urea-N rate and proportional NH3-N loss in the present study. Sigmoidal curves are commonly fitted to cumulative NH3-N loss with time (e.g., Soares et al. 2012; Subedi et al. 2015), but are seldom considered for proportional N loss with urea-N rate. This is partly due to the limited numbers of urea-N rates tested in previous studies, most of which tested two or three rates in addition to the control, as summarized in a study by Rochette et al. (2013b). Furthermore, the resolution of the lower range of urea-N rates was too low (mostly one rate under 100 kg N ha−1) to be fitted with a sigmoidal curve to capture the inherent capacity of the soil to buffer NH3-N loss. It is, however, very important for small farm holders in SSA to adopt a relatively low urea-N rate and achieve high urea-N use efficiency. The full sigmoid curve would also have been drawn for ALF provided that a lower urea-N rate (i.e., < 20 kg N ha−1) had been included in the measurement.
Parameter y 0 in the sigmoid model for AND (Fig. 3d; Table 3) could be explained by the high local soil pH and NH4+ concentration exceeding the buffering capacity of the limited soil in contact with each urea granule (Black et al. 1987b). At lower urea-N rates, most urea diffusions from adjacent granules did not overlap, which might explain the constant proportional NH3-N loss of the “lag phase” in AND. Such a “lag phase” might also result from CEC with pH-dependent charges. Before being saturated, more NH4+ would be retained by greater CEC at increasing urea-N rates, keeping the loss of NH4+ through NH3 emissions proportional.
The proportional NH3-N loss generally increased with urea-N rate in the present study (Fig. 3b), yet patterns with various effects of urea-N rate have been reported for acidic soils receiving surface application, including higher, similar, and lower proportional NH3-N losses at higher urea-N rates (Black et al. 1987b; Watson and Kilpatrick 1991; Tian et al. 2001). With an increased urea-N rate, more urea granules were hydrolyzed on the same area of soil, causing higher local soil pH and NH4+ concentrations (Black et al. 1987b). Urea hydrolysis rate could also be stimulated by an increased substrate (urea) concentration within a certain range (Singh and Nye 1984). Both could contribute to a greater proportional NH3-N loss at the higher urea-N rate. For studies reporting no effect of urea-N rate, a maximum level to which soil pH could rise might prevent further increase in proportional NH3-N loss at higher urea-N rate (Watson and Kilpatrick 1991). This seems to explain the “maximum phase” in ALF (Fig. 3c), which started at a relatively low urea-N rate. Saturation of urease activity at higher urea-N rate (Dalal 1975), however, might explain the “maximum phase” in AND (Fig. 3d), as much lower urease activity (Table 1) and peak of pH were found in the 100N treatment (Fig. 5f). The relationship between urea-N rate and proportional NH3-N loss in the present study can be described by combining the above-mentioned two patterns—greater proportional N loss and leveling out with further increasing urea-N rate—with AND exhibiting a considerable inherent capacity to buffer NH3-N loss, which formed the “lag phase.”
The only study, to our knowledge, reporting lower proportional NH3-N loss at higher N rates (acidic soil receiving surface urea application; Tian et al. 2001) was conducted on wheat crops in the winter season. A corresponding explanation, however, was not provided. Temperature is a controlling factor for microbial activity. At low temperatures (10 °C), nitrification activity can still be high (Avrahami et al. 2003), while urease activity is likely to be depressed (Sahrawat 1984). Together, these can lead to two consequences: easier saturated urease activity, meaning comparable hydrolysis rate among different urea-N rates; and extended duration of NH3 volatilization (Elliot and Fox 2014), which allows the nitrification process to be activated to stimulate the reduction of NH3-N losses (Fleisher and Hagin, 1981). However, the absence of in-situ measurements of soil pH and mineral N concentrations prevents a full explanation of the result.
Mitigation of NH3 loss
Both the 100N + W and 100N + DP mitigation treatments performed well in our study to reduce NH3-N loss (Fig. 4). In order to assess the underlying mechanism of such reductions, soil mineral N, pH, and moisture were monitored for three treatments (100N, 100N + W, and 100N + DP) during the study period.
The effective inhibition of soil pH increase following urea hydrolysis is likely the main reason for the good performance of both mitigation treatments. The timing of depressed peaks in soil pH coincided with dropped peaks of NH3-N loss in both mitigation treatments (Figs. 2 and 5e, f), and positive correlations (P < 0.1) were found for those treatments with relatively large cumulative NH3-N losses (Table 4). Mitigation treatments did reduce soil NH4+ concentrations after urea application (Fig. 5a, b; Table S2); nonetheless, NH3-N loss in the 100N treatments simply dropped with decreased soil pH while NH4+ concentrations remained relatively high (i.e., after day 3; Figs. 2 and 5a, b). Reduced correlations between soil NH4+ concentration and NH3-N loss by mitigation treatment were found in AND but not in ALF (Table 4). Therefore, there may not be a response of NH3-N loss to high NH4+ concentrations in the absence of favorable soil pH (i.e., pH > 7.4 in ALF and > 6.8 in AND). Rochette et al. (2013b) also reported that soil pH raised above 7 was the main factor explaining the exponentially increased NH3-N loss. Our result is further supported by the low NH3-N loss reported in studies that NH4+-N fertilizer was added without inducing a rise in soil pH (Sommer et al. 2004; Zaman et al. 2008).
The nitrification process seemed to be affected by the 100N + W treatment (Fig. 5c, d; Table S2), and possibly contributed to NH3-N loss reduction in ALF but not in AND. Close inspection of Fig. 5c reveals that in ALF, the 100N + W treatment resulted in a higher NO3− concentration in the early period of this study. During this period, substantial NH3-N losses occurred, and therefore active nitrification might have contributed to NH3-N loss reduction. By contrast, a higher NO3− concentration in AND was observed in the later period of the study (Fig. 5d), during which soil pH had already dropped because of NH3 volatilization and nitrification could only further acidify the soil. Different activation timing of the nitrification process in the 100N + W treatment between the two sites may result from the different initial soil moisture status. Nevertheless, activating the nitrification process before or during urea hydrolysis could help reduce NH3-N loss (Fleisher and Hagin 1981), whereas inhibited or delayed nitrification may increase NH3-N loss from soil after urea application (Soares et al. 2012).
Soil moisture is unlikely to explain the reduction of NH3-N loss in our mitigation study, although it responded to the different treatments (Table S2; Fig. 5g, h). As expected, soil moisture was higher in the 100N + W treatment and generally lower in the 100N + DP treatment (Fig. 5g, h), while the 100N treatment, which volatilized a substantial amount of NH3-N (Fig. 4), had intermediate soil moisture content. A change in soil moisture may influence NH3-N loss in two ways: from initially dry to adequately moist condition, it stimulates urea hydrolysis and thus increases NH3-N loss; and from adequately moist to saturated condition, it induces downward movement of urea and NH4+ solution and thus reduces NH3-N loss (Black et al. 1987a; Kissel et al. 2004). Local farming practices in which urea application is carried out when the soil is wet (after rainfall) actually increase the risk of NH3-N loss, as Black et al. (1987a) and Sigunga et al. (2002) reported that NH3-N loss increased with wetter soil (starting from the permanent wilting point) and reached a maximum with soil at field capacity.
Implications for managing NH3 loss in SSA cropland
Approaches to mitigating NH3-N loss have been extensively investigated (Sommer et al. 2004; Holcomb et al. 2011), including utilization of urease inhibitor, slow-release urea, and neutral or acidic N fertilizer produced at a higher cost. However, the availability of many approaches to small-farm holders in rain-fed SSA agriculture is largely limited, owing to their limited accessibility to resources and low-income levels. Knowing the soil’s inherent capacity to buffer NH3-N loss and involving it in the design of mitigation strategy are therefore critical. For instance, soils with similar properties and climatic conditions to AND in our study are likely to buffer NH3-N loss inherently when a considerable amount of urea-N is applied. A single application of up to 60 kg N ha−1 urea is quite sufficient to improve the yield. In contrast, soils with similar properties and climatic conditions to ALF in our study should avoid surface application of urea, even at a low rate, such as 30 kg N ha−1.
The rain-fed cropping system is dominant in SSA agriculture, and irrigation is rare owing to the lack of water resources and accessible facilities. In soils with a small inherent capacity to buffer NH3-N loss, we recommend that local farmers determine the timing of urea application based on weather forecast or personal experience, ensuring that rain falls soon after urea application or even applying the urea during a rainfall event. To our knowledge, however, local farmers prefer to apply urea after rainfall, which actually increases the risk of NH3-N loss, as previously discussed.
Deep placement of urea could require high labor costs in SSA croplands where only manpower is usually available. Dripping pipes (drilled with equally distributed holes) can be buried at around 5 cm soil depth and connected to a bucket at a relatively higher elevation. Urea can then be dissolved in the bucket before application. Such a simple drip system is easy to construct and is recommended to achieve the same performance as deep placement.
Conclusions
To our knowledge, this is the first study to report the effect of urea-N rate on the proportional NH3-N loss in SSA croplands. In two soils (ALF and AND) cropped to maize in the East African highlands, ALF was found to be much more susceptible than AND to NH3 loss after surface urea application, mainly owing to the different soil properties (PBC, CEC, and urease activity) and environmental factor (soil temperature). ALF had no inherent capacity to buffer NH3 loss, so surface urea application is not recommended, while up to 60 kg N ha−1 could be applied in AND without inducing substantial proportional NH3-N loss. Mitigation of NH3 loss through irrigation and urea deep placement all performed well, mainly owing to their effective inhibition of soil pH rise following urea hydrolysis; the contribution from the nitrification process in the irrigation treatment could also be a factor. Suitable strategies (i.e., rain forecast-based urea application and simple drip system) are recommended based on the results of our mitigation treatments. Our results highlight that in acidic soils common to SSA croplands, proportional NH3-N loss can be substantial even at a low urea-N rate, and that soil’s inherent capacity to buffer NH3 loss should be involved in forming N management practices. Future research needs to better understand the underlying mechanisms of NH3 volatilization from applied N fertilizer for designing effective mitigation strategies targeting different agro-ecological zones.
References
AGRA (2009) Building on the new momentum in African agriculture: AGRA in 2008. Alliance for a Green Revolution in Africa, Nairobi
Avrahami S, Liesack W, Conrad R (2003) Effects of temperature and fertilizer on activity and community structure of soil ammonia oxidizers. Environ Microbiol 5:691–705
Bisanda S, Mwangi W, Verkuijl H, Moshi AJ, Anandajayasekeram P (1998) Adoption of maize production technologies in the Southern Highlands of Tanzania. International maize and wheat improvement center (CIMMYT), the United Republic of Tanzania, and the Southern Africa Centre for Cooperation in agricultural research (SACCAR), Mexico, DF
Black AS, Sherlock RR, Smith NP (1987a) Effect of timing of simulated rainfall on ammonia volatilization from urea, applied to soil of varying moisture content. J Soil Sci 38:679–687
Black AS, Sherlock RR, Smith NP (1987b) Effect of urea granule size on ammonia volatilization from surafce-applied urea. Fert Res 11:87–96
Bouwman AF, Boumans LJM, Batjes NH (2002) Estimation of global NH3 volatilization loss from synthetic fertilizers and animal manure applied to arable lands and grasslands. Global Biogeochem Cy 16:8–1-8-14
Cantu RR, Aita C, Doneda A, Giacomini DA, Dessbesell A, Arenhardt M, De Bastiani GG, Pujol SB, Rochette P, Chantigny MH, Giacomini SJ (2017) Alternatives to regular urea for abating N losses in lettuce production under sub-tropical climate. Biol Fert Soils 53:589–599
Dalal RC (1975) Urease activity in some Trinidad soils. Soil Biol Biochem 7:5–8
Elliot JR, Fox TR (2014) Ammonia volatilization following fertilization with urea or ureaform in a thinned loblolly pine plantation. Soil Sci Soc Am J 78:1469–1473
Ferguson RB, Kissel DE, Koelliker JK, Basel W (1984) Ammonia volatilization from surface-applied urea—effect of hydrogen-ion buffering capacity. Soil Sci Soc Am J 48:578–582
Fleisher Z, Hagin J (1981) Lowering ammonia volatilization losses from urea application by activation of nitrification process. Fert Res 2:101–107
Galloway JN, Aber JD, Erisman JW, Seitzinger SP, Howarth RW, Cowling EB, Cosby BJ (2003) The nitrogen cascade. Bioscience 53:341–356
Haden VR, Xiang J, Peng S, Ketterings QM, Hobbs P, Duxbury JM (2011) Ammonia toxicity in aerobic rice: use of soil properties to predict ammonia volatilization following urea application and the adverse effects on germination. Eur J Soil Sci 62:551–559
He ZL, Alva AK, Calvert DV, Banks DJ (1999) Ammonia volatilization from different fertilizer sources and effects of temperature and soil pH. Soil Sci 164:750–758
Hickman JE, Tully KL, Groffman PM, Diru W, Palm CA (2015) A potential tipping point in tropical agriculture: avoiding rapid increases in nitrous oxide fluxes from agricultural intensification in Kenya. J Geophys Res-Biogeo 120:938–951
Holcomb JC, Sullivan DM, Horneck DA, Clough GH (2011) Effect of irrigation rate on ammonia volatilization. Soil Sci Soc Am J 75:2341–2347
IFA (2017) International Fertilizer Association Database: http://ifadata.fertilizer.org/ucSearch.aspx. Accessed 20 June 2017
Jiang Y, Deng A, Bloszies S, Huang S, Zhang W (2017) Nonlinear response of soil ammonia emissions to fertilizer nitrogen. Biol Fert Soils 53:269–274
Kandeler E, Gerber H (1988) Short-term assay of soil urease activity using colorimetric determination of ammonium. Biol Fert Soils 6:68–72
Kissel D, Cabrera ML, Vaio N, Craig JR, Rema JA, Morris LA (2004) Rainfall timing and ammonia loss from urea in a loblolly pine plantation. Soil Sci Soc Am J 68:1744–1750
Ma BL, Wu TY, Tremblay N, Deen W, McLaughlin NB, Morrison MJ, Stewart G (2010) On-farm assessment of the amount and timing of nitrogen fertilizer on ammonia volatilization. Agron J 102:134–144
Nziguheba G, Palm CA, Berhe T, Denning G, Dicko A, Diouf O, Diru W, Flor R, Frimpong F, Harawa R, Kaya B, Manumbu E, McArthur J, Mutuo P, Ndiaye M, Niang A, Nkhoma P, Nyadzi G, Sachs J, Sullivan C, Teklu G, Tobe L, Sanchez PA (2010) The African green revolution: results from the millennium villages project. In: Donald LS (ed) Advances in Agronomy, vol 109 Academic Press, London, pp 75–115
Pan B, Lam SK, Mosier A, Luo Y, Chen D (2016) Ammonia volatilization from synthetic fertilizers and its mitigation strategies: a global synthesis. Agri Ecosyst Environ 232:283–289
Reddy DD, Sharma KL (2000) Effect of amending urea fertilizer with chemical additives on ammonia volatilization loss and nitrogen-use efficiency. Biol Fert Soils 32:24–27
Rimski-Korsakov H, Rubio G, Lavado RS (2012) Fate of the nitrogen from fertilizers in field-grown maize. Nutr Cycl Agroecosys 93:253–263
Rochette P, Angers DA, Chantigny MH, Gasser M-O, MacDonald JD, Pelster DE, Bertrand N (2013a) Ammonia volatilization and nitrogen retention: how deep to incorporate urea? J Environ Qual 42:1635–1642
Rochette P, Angers DA, Chantigny MH, Gasser M-O, MacDonald JD, Pelster DE, Bertrand N (2013b) NH3 volatilization, soil concentration and soil pH following subsurface banding of urea at increasing rates. Can J Soil Sci 93:261–268
Sahrawat KL (1984) Effects of temperature and moisture on urease activity in semi-arid tropical soils. Plant Soil 78:401–408
Sakurai K, Ohdate Y, Kyuma K (1989) Potentiometric automatic titration (PAT) method to evaluate zero point of charge (ZPC) of variable charge soils. Soil Sci Plant Nutr 35:89–100
Scudlark JR, Jennings JA, Roadman MJ, Savidge KB, Ullman WJ (2005) Atmospheric nitrogen inputs to the Delaware inland bays: the role of ammonia. Environ Pollut 135:433–443
Sigunga DO, Janssen BH, Oenema O (2002) Ammonia volatilization from vertisols. Eur J Soil Sci 53:195–202
Singh R, Nye PH (1984) The effect of soil pH and high urea concentrations on urease activity in soil. J Soil Sci 35:519–527
Soares JR, Cantarella H, Menegale MLC (2012) Ammonia volatilization losses from surface-applied urea with urease and nitrification inhibitors. Soil Biol Biochem 52:82–89
Sommer SG, Schjoerring JK, Denmead OT (2004) Ammonia emission from mineral fertilizers and fertilized crops. In: Donald LS (ed) Advances in Agronomy, vol 82 Academic Press, London, pp 557–622
Soil Survey Staff (2006) Keys to soil taxonomy, 10th edn. United States Department of Agriculture Natural Resources Conservation Service, Washington, DC
Subedi R, Kammann C, Pelissetti S, Taupe N, Bertora C, Monaco S, Grignani C (2015) Does soil amended with biochar and hydrochar reduce ammonia emissions following the application of pig slurry? Eur J Soil Sci 66:1044–1053
Tian G, Cai Z, Cao J, Li X (2001) Factors affecting ammonia volatilisation from a rice–wheat rotation system. Chemosphere 42:123–129
Vitousek PM, Naylor R, Crews T, David MB, Drinkwater LE, Holland E, Johnes PJ Katzenberger J, Martinelli LA, Matson PA, Nziguheba G, Ojima D, Palm CA, Robertson GP, Sanchez PA, Townsend AR, Zhang FS (2009) Nutrient imbalances in agricultural development. Science 324:1519–1520
Watson CJ, Kilpatrick DJ (1991) The effect of urea pellet size and rate of application on ammonia volatilization and soil-nitrogen dynamics. Fert Res 28:163–172
Whitehead DC, Raistrick N (1993) The volatilization of ammonia from cattle urine applied to soils as influenced by soil properties. Plant Soil 148:43–51
Zaman M, Nguyen ML, Blennerhassett JD, Quin BF (2008) Reducing NH3, N2O and NO3 −-N losses from a pasture soil with urease or nitrification inhibitors and elemental S-amended nitrogenous fertilizers. Biol Fert. Soils 44:693–705
Acknowledgements
The authors thank the Mbeya Agriculture Training Institute at Uyole in Tanzania for providing one of the research sites, and the assistance from the Uyole Agriculture Research Institute in Tanzania for its establishment. The authors also thank Mr. Boniface from the village of Mangalali in Iringa, Tanzania for his coordination in managing the other site. This research is financially supported by the Inter-Graduate School Program for Sustainable Development and Survivable Societies at Kyoto University, and by the Japan Society for the Promotion of Science KAKENHI (Grant No. 24228007).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
ESM 1
(DOCX 219 kb)
Rights and permissions
About this article
Cite this article
Zheng, J., Kilasara, M.M., Mmari, W.N. et al. Ammonia volatilization following urea application at maize fields in the East African highlands with different soil properties. Biol Fertil Soils 54, 411–422 (2018). https://doi.org/10.1007/s00374-018-1270-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00374-018-1270-0