Abstract
A soil column experiment was conducted to examine the effects of fertilizer N source and depth of placement on soil profile N2O accumulation and surface emissions at 44% and 77% water-filled pore space (WFPS). The used N fertilizers were polymer-coated urea, stabilized urea with urease and nitrification inhibitors, and conventional granular urea. Conventional urea and stabilized urea were applied either uniformly at 0–65 cm or deeply at a 40- to 65-cm depth of 65 cm repacked soil columns, whereas polymer-coated urea was subsurface banded at a 10-cm depth to reflect fertilizer application practices at a field scale. Profile N2O concentrations at 5, 15, 30, and 60 cm and surface flux were monitored over 3 months. Compared to conventional urea, stabilized urea and polymer-coated urea generally reduced N2O accumulation in the column, but not cumulative emissions. Across fertilizer sources, compared with uniform addition, deep placement reduced column N2O accumulation at 44% but not at 77% WFPS. Deep placement also reduced emissions 56–71% than for uniform placement. Column N2O accumulation doubled at 77% than 44% WFPS, whereas cumulative emissions and applied N–based emission factors were lower at the former WFPS value. Cumulative N2O emissions increased exponentially with total accumulation at 44% but not 77% WFPS. Reduced N2O emissions at high WFPS were likely due to consumption and low diffusivity of the gas in the soil profile, rather than low production by denitrification. These results suggest fertilizer N leached down the profile is less prone to N2O loss while emission reductions by using more efficient fertilizers may be limited.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
As an important greenhouse gas in the atmosphere, nitrous oxide (N2O) contributes to both global warming and stratospheric ozone destruction (Ravishankara et al. 2009). The atmospheric N2O concentration has increased considerably from 270 ppbv in the pre-industrial era to 324 ppbv at present as a consequence of human activities (IPCC 2013). Agricultural soils are a major source of atmospheric N2O, contributing 60–80% of global anthropogenic N2O emissions, primarily due to nitrogen (N) addition, including both synthetic and organic fertilizers (Bouwman et al. 2002). Therefore, it is of great importance to develop N management practices to reduce N2O emissions from the agricultural system.
In soil, N2O exists in soil atmosphere and soil solution, with the partition between phases determined mainly by its solubility and soil conditions such as aeration. Emissions of N2O from soil surface are the result of N2O production, consumption, and transport processes within soil profiles and affected by environmental and management factors. Several studies have linked N2O accumulation in the soil to emissions. For example, Nan et al. (2016) reported fertilizer-induced N2O emissions from a cornfield on the Loess Plateau of China were associated with N2O accumulation in the surface layer (0–15 cm) of soil. Yao et al. (2018) reported N2O emissions from soils cropped with or without corn plants were most related with N2O effluxes at 10-cm depth, which were stimulated by concentration gradients along soil profile. Similarly, our study in Manitoba, Canada, confirmed N2O emission with N fertilization was most related to N2O accumulation near to the surface rather than at depth (Gao et al. 2014). Together, these results suggest that the deep placement of fertilizer could be a potential strategy to reduce N2O emissions. While these studies have provided some insights into the effect of N fertilizer application on the relationship between N2O accumulation and surface emissions, there are no reports linking N2O accumulation gradients to emissions for placement of different sources of N fertilizers.
Nitrous oxide is produced through the microbiological processes of nitrification and denitrification, affected by soil factors such as temperature, moisture, and availability of mineral N and soil organic C (Beauchamp 1997). Long-term (> 15 years) fertilizer N application increased soil NO3− accumulation and N2O emission, which were attributed to the decreased N2O reductase activity and an increased ratio of N2O/(N2 + N2O) (Qin et al. 2017). Soil water content is one of the dominant factors regulating N2O emission from soils. Increasing water content due to soil thawing or rainfall can stimulate N2O production and emissions from croplands in a temperate area (Gao et al. 2014). Soil water-filled pore space (WFPS) is highly correlated with nitrification and denitrification processes as it regulates substrate and oxygen availability for microbial activities (Liu et al. 2017; Stark and Firestone 1995). Under soil WFPS levels favoring nitrification, Wang et al. (2016) reported N2O production in the NH4+-amended arable soils was determined by nitrification through ammonia-oxidizing bacteria rather than archaea. Granli and Bockman (1994) proposed that increasing soil moisture up to 60% WFPS can enhance both nitrification and denitrification. Huang et al. (2014) found the highest N2O emissions occurred at 70% WFPS for a calcareous fluvo-aquic soil, with emissions contributed from both nitrification (35–53%) and denitrification (44–58%) pathways. Following application of 15N-labeled fertilizer, Bateman and Baggs (2005) demonstrated nitrification dominated N2O emissions from a silt loam soil at 35–60% WFPS, whereas denitrification was the sole source of N2O emissions at 70% WFPS. While these studies have focused on quantifying the biological processes source of N2O production, limited information is available on how soil water content affects N2O concentrations in the soil profile and its linkage to surface emissions.
Enhanced efficiency N fertilizers are formulated to match crop N needs while reducing losses to the environment (Akiyama et al. 2010; Halvorson et al. 2014). Two typical products are polymer-coated urea and stabilized urea, the latter containing a urease inhibitor and/or a nitrification inhibitor. Many studies have reported their benefits on mitigation of N2O emissions from cropping systems (Asgedom et al. 2014; Gao et al. 2015; Halvorson et al. 2014; Sanz-Cobena et al. 2016). The double use of a urease inhibitor (N-(n-butyl) thiophosphoric triamide [NBPT]) and a nitrification inhibitor (dicyandiamide (DCD)) with urea reduced N2O emissions from drip-irrigated cotton in an arid area of China (Ma et al. 2018). In the irrigated agricultural system, N fertilizers are subject to downward movement with water and thus may cause the redistribution of NO3−-N and NH4+-N in the soil profile (Cook and Sanders 1991; Deare et al. 1995). Chilundo et al. (2018) recently reported that fertilizers with drip irrigation increased concentrations of NO3−-N and NH4+-N up to 90 cm in the soil profile, increasing the risk of N loss. Behera and Panda (2009) found that fertilizer N was more likely subject to downward movement under drip irrigation than conventional furrow irrigation. For drip-irrigated corn, Fanish and Muthukrishnan (2013) reported fertilizer N was almost evenly distributed up to 25 cm below the water emitters. These studies suggest fertilizers applied through drip irrigation greatly affect vertical distribution of NO3−-N and NH4+-N along the soil profile. However, it remains unknown if inorganic N transported/leached deeper into the soil profile with drip irrigation contributes to surface N2O emissions.
A laboratory soil column experiment was done to investigate the effect of fertilizer source (urea, polymer-coated urea, and stabilized urea) on profile N2O accumulation and surface emission, under well (44% WFPS) and poor (77% WFPS) aeration conditions. To better simulate the uniform distribution or leached N due to drip fertigation, urea and stabilized urea were applied either uniformly or at 40- to 65-cm depth of 65-cm repacked soil columns, whereas polymer-coated urea was subsurface banded at 10-cm depth. Conventional urea and stabilized urea are commonly split-applied with drip irrigation water (drip-fertigated) and likely move downward and accumulate in the deep soil layer under field conditions. In contrast, polymer-coated urea must be banded in the soil to be effective as it is buoyant and may float in water during irrigation. Therefore, different placement strategies were used for urea sources to reflect fertilizer application practices at the field scale. We hypothesized (1) the use of polymer-coated urea and stabilized urea can reduce N2O production and emission than urea due to slow NO3− release, (2) fertilizers in deep layers are less prone to N2O emission loss, and 3() soil moisture reduces N2O accumulation and emission due to denitrification.
Materials and methods
Study site and soil properties
The study site is at the National Grey Desert Soil Station (43° 56′ N, 87° 28′ E) of Xinjiang Academy of Agricultural Sciences, Northwestern China. The soil is classified as gray desert soil according to the Chinese soil classification and as Typic Argigypsids in the USDA-NRCS system. In April 2016, soil samples at depths of 0–10 cm, 10–20 cm, 20–40 cm, and 40–65 cm were collected. The soil was passed through a 2-mm sieve to remove residue, then air-dried and stored at room temperature prior to use. The surface soil (0–20 cm) is sandy loam texture (clay 27, silt 343, and sand 630 g kg−1) as determined using the pipette method (Loveland and Whalley 1991).
Soil properties at each depth are given in Table 1. The bulk densities were 1.13, 1.25, 1.48, and 1.59 Mg m−3 for soils at 0–10, 10–20, 20–40, and 40–65 cm deep, respectively. Soil pH ranged between 8.02 and 8.24, with values greater at lower depths. Soil electrical conductivity increased from 0.22 to 0.40 mS cm−1 with soil depths increasing from 0 to 10 to 40–65 cm. Soil NO3−-N concentrations increased with soil depth, being 27.0, 36.9, 54.4, and 65.0 mg kg−1, for soils at 0–10, 10–20, 20–40, and 40–65 cm deep, respectively. Soil exchangeable NH4+-N concentrations were 12.3, 9.6, 9.8, and 9.4 mg kg−1, for soils at 0–10, 10–20, 20–40, and 40–65 cm deep, respectively. Soil organic C content ranged between 6.2 and 10.0 g C kg−1, with values lower at 40–65 cm deep. Total N content was also lower at 40–65 cm than other depths, being 0.98, 0.98, 0.98, and 0.79 g N kg−1 for soils at 0–10, 10–20, 20–40, and 40–65 cm deep, respectively. Analysis on soil physical and chemical properties followed Carter (1993). Soil bulk density was determined using the soil core method. Soil pH and electrical conductivity were determined on a 1:5 soil/water suspension. Soil NO3− and exchangeable NH4+ concentrations were measured using a continuous flow analyzer (AA3, SEAL Analytical, Norderstedt, Germany) with 0.01 M CaCl2 extracts (1:10, w:v). Organic matter was determined by wet oxidation. The total Kjeldahl N was determined after microwave digestion with sulfuric acid and salicylic acid.
Experimental design and preparation of soil column
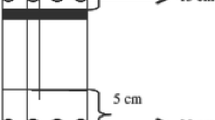
Soils of each depth were packed into polyvinyl chloride (PVC) cylinders (68.0 cm in height, 19.2 cm in inner diameter, and 20.0 cm in outer diameter, Fig. 1) to simulate bulk density under field conditions. In each cylinder, 3.2, 3.6, 8.5, and 11.6 kg air-dried soil was packed at 0–10 cm, 10–20 cm, 20–40 cm, and 40–65 cm deep, respectively. Deionized water was uniformly added to the surface of each soil layer during packing to achieve volumetric water content (VWC) of 20% and 35%, respectively. Each soil column was then maintained at the designed VMC levels by spraying deionized water onto the soil daily. Only a small volume (< 2 mL) of water was added daily due to low evaporation under room temperature condition. The volumetric water content of 20 and 35% corresponded to 44% and 77% WFPS, respectively. The WFPS was calculated as VWC ∕ [1 − (ρb ∕ PD)] × 100, where ρb is the average bulk density (Mg m−3) across soil profile and PD is the particle density assumed to be 2.65 Mg m−3.
A 2 × 6 factorial laboratory experiment was established involving two soil moisture (44% and 77% WFPS) and six fertilizer treatments. The fertilizer treatments were (1) a control without N addition and applications of 240 kg N ha−1 equivalent using (2) deep placement of granular urea at the 40- to 65-cm depth; (3) uniform application of granular urea through the 0- to 65-cm column; (4) deep placement of stabilized urea at 40- to 65-cm depth; (5) uniform application of stabilized urea through 0- to 65-cm column; and (6) subsurface banded polymer-coated urea at 10-cm depth. A rate of 240 kg N ha−1 is typical for local crop production. The stabilized urea was SuperU (46% N, KOCH Agronomic Services LLC, Wichita, USA), which contains urease (NBPT) and nitrification (DCD) inhibitors that are uniformly distributed through the granule during the manufacturing process. The polymer-coated urea was Environmentally Smart Nitrogen (44% N, formerly Agrium Inc., now Nutrien Ltd., Saskatoon, Canada), which consists of urea granules coated with a polymer permeable to water that gradually releases urea over the growing season. For treatments of deep placement, the fertilizers were well-mixed with the 40- to 65-cm soils before packing. For treatments of uniform placement, appropriate amounts of fertilizers were mixed with soils at each depth before packing. For treatment of polymer-coated urea, the fertilizer was subsurface banded at 10 cm under the soil surface after packing all of the soils. Columns were left at room temperature (25 °C) over the experimental period of 3 months.
The 44% and 77% WFPS treatments were chosen based on field observations that average and highest soil moisture levels close to the tape under drip fertigation were approximately 40% and 70% over the growing season of cotton at the research station. Treatments were replicated four times in a randomized complete block design, comprising of totally 48 soil columns.
Soil profile gas sampling, analysis, and calculations
A soil profile gas sampler was installed in each column at the time of packing. The samples were 65 cm long and constructed from polythene (PE) tubes (40.8 mm i.d. and 50.0 mm o.d.). Each sampler had four 5.0-cm long silicone tubes, allowing gases but not water to pass, for the collection of gas samples at 5, 15, 30, and 60 cm beneath the soil surface, respectively (Fig. 1). Each silicone tube (36.8 mm i.d., 40.0 mm o.d., and volume 49 mL) was plugged at both ends and covered with a 5-cm PE tube (40.8 mm i.d. and 50.0 mm o.d.) that had 12 holes in the wall for soil gas equilibration from surrounding soil layer. One end of the silicone tube was connected to a stainless steel tube (0.6 mm i.d.) with the sample port fitted with a rubber septum. Gas samples were taken from each sector through the rubber septum using a 35-mL airtight syringe. Gas samples were collected on 1, 2, 4, 7, 10, 16, 20, 27, 39, 52, 60, 72, 83, and 95 days after treatment, for a total of 14 occasions. Concentrations of N2O in gas samples were determined using gas chromatography (Agilent 7890A, Agilent Technologies, Santa Clara, CA) equipped with an electron capture detector. Gas sample analysis was repeated or the column reconditioned and calibration was redone if quality control samples, placed every 20th position, showed a > 5% deviance from the expected concentration.
Column (0–65 cm) N2O accumulation, which refers to the total N2O accumulation over all soil depths and over the experimental period, was calculated as Gao et al. (2014), considering both aqueous and gaseous phases of N2O in the soil profile. It is used as an assessment of the magnitude and duration of N2O contents over the experimental period. For each sampling date, accumulation of the aqueous phase of N2O over the 0- to 65-cm column was calculated by multiplying soil VWC (20% or 35%) and solubility (Henry’s law) constant of 0.882 for N2O at 25 °C (Tiedje 1982), assuming the 0- to 65-cm soil profile had a constant VWC. Accumulation of the gaseous phase of N2O over 0–10, 10–20, 20–40, and 40–65 cm of soil layers were determined by multiplying N2O concentrations measured at 5, 15, 30 and 60 cm deep with atmosphere volume of each soil layer, which was calculated by subtracting VWC from soil porosity. Column N2O accumulation over the monitoring period was estimated as the sum of the daily accumulation of both aqueous and gaseous phases of N2O. Gap filling of column N2O accumulation for days with no determinations was done by linear interpolation of values from the day before and after the gap.
Surface N2O flux measurement and calculations
Surface N2O fluxes were measured at the time of sampling of silicon diffusion. For the surface flux determination, a PVC lid was placed on the column and sealed by adding water into the pre-installed PE groove. The headspace for sampling was 7.85 L. Four 30-mL gas samples from the headspace were collected through a serum stopper on the lid at 0, 15, 30, and 45 min after sealing, using a 35-mL airtight syringe. Gas samples were analyzed for N2O concentration by gas chromatography as previously described. Surface N2O emission rates (mg m−2 day−1) for each column were calculated by fitting a linear or non-linear regression model using the HMR package implemented with the R language (Pedersen 2011).
Similar to column N2O accumulation, cumulative emission for each column was estimated by summing daily estimates of N2O emissions obtained by linear interpolation between sampling dates for the monitoring period. The N2O emission factor (EF, %) on an applied N basis, expressed in percentage of fertilizer N emitted as N2O, was calculated as:
where N2OFert is the cumulative N2O emissions of the fertilizer treatments (mg N m−2), N2OControl is the cumulative N2O emissions of the unfertilized Control (mg N m−2), and applied N is the amount of N applied as fertilizer (mg N m−2).
Statistical analysis
Analysis of variance was performed using the PROC MIXED procedure in the Statistical Analysis Software (SAS Institute 2011) to determine the main and interactive effects of fertilizer treatment and soil WFPS on column N2O accumulation, cumulative emission, and emission factor. In the model, fertilizer treatment and soil WFPS were fixed effects, and least squares means of fixed effects were compared by Tukey’s honestly significant difference test. The column replicate was considered as a random factor. For a given sampling date, N2O concentrations at different depths and flux rates were subject to Fisher’s LSD to compare fertilizer treatments means. The Pearson correlation analysis was conducted to examine the relationships between daily N2O flux and soil N2O concentrations at different depths. Regression analysis was used to examine the relationship between column N2O accumulation and cumulative emission. The predetermined orthogonal contrasts were also used to test the differences in column N2O accumulation, cumulative emission, and emission factor between treatments. All data were tested for normality and homogeneity of variance. Before analysis, N2O flux data were log(10)-transformed to meet requirements for normality of residuals. Differences were declared to be significant at P < 0.05.
Results
Soil N2O concentrations
The soil concentrations of N2O in the control generally did not increase appreciably compared to the fertilizer treatments (Fig. 2). Concentrations of N2O in the control increased with depth after setup of the experiment but declined to background value (<1 μL L−1) by the end of the first week at 44% WFPS. In contrast, at the lower aeration status of 77% WFPS, concentrations in the control did not increase above background except at the shallowest depth (5 cm) at day 10.
At 44% WFPS, treatment of uniform urea showed the highest concentrations of N2O at all depths with concentrations returning to background value before week 3 (Fig. 2). Compared to uniform urea, uniform stabilized urea had comparable concentrations of N2O for the two shallowest depths but decreased N2O concentrations at the 30- and 60-cm depths. Compared to the control, polymer-coated urea increased concentrations of N2O at the 5-cm and 15-cm depths but not at deeper depths. An increase in N2O concentrations did not occur for other fertilizer treatments at 44% WFPS.
At 77% WFPS, treatment of uniform urea increased concentrations of N2O at all depths whereas polymer-coated urea increased N2O concentrations at only a 5-cm depth (Fig. 2). Concentrations were generally lower than peak concentrations observed at the higher aeration conditions of 44% WFPS but resided for a longer duration up to 53 days at all depths. For the polymer-coated urea treatment, most N2O accumulated at the shallowest depth with concentrations increasing up to 20 days and then decreasing to a near background value. Deep application of urea slightly increased N2O concentrations at all depths, whereas the magnitudes were much lower than the uniform placement. Deep application of stabilized urea did not increase N2O concentrations at all depths, showing a similar trend as the unfertilized control.
Column N2O accumulation
Column N2O accumulation, as the sum of gaseous and dissolved gas integrated over the five sample depths, indicated that it mainly occurred at both WFPS levels in the treatment of uniform urea and lesser in the treatment of uniform stabilized urea (Fig. 2). A significant interaction for treatment and aeration condition was observed for column N2O accumulation over the experimental period (Table 2). The interaction likely results because of lower N2O accumulation for stabilized urea than urea treatments at 77% but not 44% WFPS and greater accumulation for fertilizers placed uniformly throughout soil column depths and not placed solely closer to the soil surface at 44% than 77% WFPS. The magnitude of N2O accumulation was greater at the highest aeration status of 44% than 77% WFPS for uniformly applied urea and stabilized urea (Fig. 2). The duration of N2O accumulation was longer at 77% than 44% WFPS for the treatment of uniform urea. The N2O accumulation for treatment of deep urea occurred only at 77% WFPS with the appearance being after that for treatment of uniform urea.
Surface flux
Flux of N2O varied with aeration condition and fertilizer treatment (Fig. 3). Urea and stabilized urea with uniform placement showed the largest fluxes of N2O with those emissions occurring for the higher aeration condition of 44% WFPS and over the first 2 weeks of the study. Fluxes with polymer-coated urea occurred later, between weeks 2 and 3 and between weeks 3 and 6 at 44% and 77% WFPS, respectively. A significant interaction for treatment and aeration condition for N2O cumulative emissions was observed (Table 2). Cumulative emission with polymer-coated urea was higher than for urea treatments at 77% but not at 44% WFPS. Fertilizer deep placement had a slight increase in cumulative emission compared to the control without fertilizer but decrease compared to fertilizer uniformly placed throughout soil column.
Emission factor
Emission factors ranged between 0.11 and 1.10% at 44% WFPS and between 0.05 and 0.67% at 77% WFPS, respectively (Table 2). A significant interaction for treatment and aeration condition was observed, which likely resulted from that deep placement of urea and stabilized urea reduced emission factors compared to uniform placement at 44% but not at 77% WFPS. Based on the contrast analysis, emission factor with polymer-coated urea was higher than urea treatments at 77% but not at 44% WFPS. Across fertilizer sources of urea and stabilized urea, deep placement had a lower emission factor compared to fertilizer uniformly placed throughout soil column.
Relationship between soil profile N2O accumulation and surface emissions
At 44% WFPS, daily flux rates of N2O were positively related with soil N2O concentrations at all depths except treatments of deep urea and deep stabilized urea at 5 cm (Table 3). The positive relationships also existed between N2O flux and column N2O accumulation for all treatments. In contrast, at 77% WFPS, only a few significant relationships between surface flux and profile N2O concentration at different depths or at the 0- to 65-cm accumulation were observed for treatments of stabilized urea. Across fertilizer treatments, N2O cumulative emission increased exponentially (R2 = 0.61, P = 0.001) with column N2O accumulation at 44% WFPS while there was no significant relationship at 77% WFPS (Fig. 4).
Discussion
Soil profile N2O accumulation
The fertilizer N addition substantially increased N2O concentrations at all soil depths down to 65 cm, depending on placement and source of fertilizer and soil aeration conditions (Fig. 2, Table 2). The increase of N2O concentration in the soil in response to application of N fertilizer has been frequently reported (Gao et al. 2014; Reth et al. 2008; Wang et al. 2013), suggesting N application is the main trigger for N2O production. Concentration peaks were highest at the 30- and 60-cm depths, indicating that most of the N2O was produced at the 30- to 60-cm soil layer and diffused to the topsoil. In contrast, Wang et al. (2013) found the 0- to 30-cm topsoil dominates fertilizer-induced N2O production due to greater microbial activities. Our previous studies in Manitoba, Canada, also found that N2O production mainly occurred at the 0- to 5--cm surface soil following urea ammonium nitrate solution being side-dribbled on the soil surface (Gao et al. 2014). The difference in the distribution of N2O concentration in soil profile between this study and others could be associated with the different conditions between laboratory and field studies. It was likely the leaching process under field conditions that decreased the accumulation of NO3− and consequently the production and accumulation of N2O at low depths along the soil profile. The inconsistent result from the current study could also be related to differences in soil NO3− availability. Soils in the current study were collected from a cotton field which was under the drip-fertigated system for over 5 years and thus had an increasing NO3− concentration with depths due to the substantial downward movement of NO3− along the profile (Table 1). The greater NO3− availability at the 30- to 60-cm depth could have stimulated the microbial activity of nitrifier and denitrifier, resulting in a burst of N2O production at this soil layer. In addition, under conditions of this experiment, the added water likely moved down and accumulated at low layers of the soil profile. Such soil moisture gradient may have increased the microbial activities coinciding with the high NO3− concentrations and consequently increased N2O accumulation at deeper soil profiles.
Soil aeration is an important factor that controls the relative importance of nitrification and denitrification on N2O production. Our results clearly showed that the fertilizer-induced N2O concentration peaks at basically all depths were higher at 44% WFPS while the total accumulation of N2O over the experimental period was greater at 77% WFPS (Fig. 2, Table 2). The previous studies suggested nitrification dominates N2O production between 35 and 60% WFPS, whereas denitrification was the key N2O producing process when WFPS exceeds 70% (Bateman and Baggs 2005). In the current study, 44% WFPS represents field moisture level of topsoils (0–20 cm) under the drip-fertigated system, suggesting nitrification is the predominant process contributing to N2O production. In contrast, the prolonged and more accumulation of N2O at 77% WFPS was likely associated with the presence of active denitrifier under relatively anaerobic conditions (Davidson 1992). Further studies are needed to conclude the relative importance of nitrification and denitrification for the drip-fertigated system due to the temporal and spatial variability of soil aeration.
Compared to uniform placement, deep placement of urea and stabilized urea significantly reduced N2O accumulation at 44% WFPS which is the average soil moisture level under a drip-fertigated condition at field scale (Table 2). The N2O concentration peaks at all depths were also lower for the deep than for the uniform placement of urea and stabilized urea (Fig. 2). It is likely that nitrifier activity concentrated at topsoil layers due to more aerobic condition and higher soil organic C, resulting in higher nitrification rate and greater N2O production in topsoils at 44% WFPS (Liu et al. 2006). Initial soil properties confirmed that the lower soil layer (40–65 cm) had higher bulk density and lower organic C (Table 1). Thus, N2O production through nitrification was suppressed at the lower depths along the profile under aerobic conditions of 44% WFPS. These results suggested that fertilizer N accumulation at deep soil layers is less prone to N2O loss and confirmed previous field observations on generally low N2O emissions under drip-irrigated conditions (Guardia et al. 2017; Ma et al. 2018).
There were numerous studies investigating the effect of enhanced efficiency N fertilizers on N2O emissions from agricultural soils whereas few of them measured temporal change and depth distribution of N2O concentrations in the soil profile (Halvorson et al. 2014). To our knowledge, this is the first study providing concurrent measurements on N2O accumulation in the soil profile and surface flux, as affected by enhanced efficiency N fertilizers. Compared with conventional urea, both polymer-coated and stabilized urea products generally reduced N2O concentration peaks at all depths and accumulations over the experimental period, with the effectiveness being more prominent at 77% WFPS (Table 2). This is in agreement with our field studies in Manitoba in which polymer-coated urea and stabilized urea were more effective to reduce N2O emissions under wet and warm soil conditions (Gao et al. 2015). In a laboratory study, Di et al. (2014) found the nitrification inhibitor DCD effectively reduced N2O emissions at 100–130% field capacity (equivalent to 35–45% gravimetric water content) by inhibiting the growth of nitrifier communities. At events of irrigation or rainfall, nitrification inhibitors in soils are susceptible to vertical transport and can co-locate well with inorganic N to reduce rates of nitrification (Marsden et al. 2016). In this study, the downward movement of the urease and nitrification inhibitors would have suppressed N2O production at low depths along the profile, resulting in less N2O accumulation in the stabilized urea than urea when fertilizers were uniformly applied. These studies highlight the importance of soil moisture and fertilizer placement in controlling the benefits of stabilized urea or polymer-coated urea to reduce N2O production and emission from soils.
Surface N2O emissions as related to accumulation in soil profile
Cumulative N2O emissions and emission factors were lower at 77% WFPS, in spite of greater accumulation in the soil profile (Table 2). Low N2O emissions at WFPS over 70–75% were frequently reported in other studies and attributed to the reduction of N2O to N2 under the complete anaerobic conditions (Laville et al. 2011; Smith et al. 1998). Similar to the previous studies (Brummell et al. 2012; Gao et al. 2014), the current study revealed no relationship between N2O accumulation in the soil profile and surface emissions at high WFPS, suggesting the complexity of production, consumption, and transport of N2O along the soil profile. High accumulation in soil profile but low surface emission was likely related to reduced gas diffusion and increased reduction to N2 along soil profile at high WFPS. The previous studies also reported N2O in subsoil was likely consumed during upward diffusion due to longer residence time and diffusion path (Castle et al. 1998; Goldberg et al. 2008). The potential change of soil water content due to the downward water movement in each soil depth may also exert significant effects on the biological processes of nitrification/denitrification and gas diffusion coefficients. Such complexity of gas diffusivity results in the lack of relation between soil N2O accumulation and surface emissions at 77% WFPS (Table 3, Fig. 4). Instead, the exponential increase of cumulative emissions with soil N2O accumulation and the linear relationship between soil N2O concentrations and daily flux rate suggest soil N2O accumulation along the soil profile is the primary factor leading to substantial surface emissions under aerobic conditions of 44% WFPS. It needs to be noticed that the relationship between surface emissions and soil N2O accumulation was largely dependent on two measures with uniform urea treatment that had relatively high values. Still, excluding these two measures had resulted in similarly increasing the trend with R2 of 0.39 (P < 0.001).
Even though stabilized urea and polymer-coated urea generally reduced N2O accumulation in soil profiles compared with conventional urea, they were not effective to reduce surface emissions and emission factors at both levels of soil WFPS (Table 2). The inconsistency suggests N2O emissions to the atmosphere was predominantly determined by N2O produced near the surface of the soil. In the current study, even though the 30- to 65-cm layer produced most N2O, the diffusion to upward soil and then to the atmosphere was likely limited. Our results agree with other studies which reported the top surface (0–20 cm) soils play key roles in N2O emissions (Nan et al. 2016). This deduction is supported by the fact that deep placement of fertilizer caused little N2O emission though it had resulted in comparable N2O accumulation as uniform placement at 77% WFPS. For example, deep applications of conventional or stabilized urea significantly reduced N2O emissions than banding of polymer-coated urea in topsoil, due to the consumption of N2O to N2 through the diffusion process. These results suggest that deep application of fertilizers should be considered as an effective strategy to reduce N2O emissions from agricultural practices.
Conclusion
Accumulation and emission of N2O were triggered by fertilizer treatments, with peaks of concentrations and flux rates which occurred within 3 weeks following fertilizer addition. Greater N2O accumulation in the subsoil layers (30–60 cm) was observed for all fertilizer treatments. High WFPS reduced surface N2O emissions, in spite of more N2O production and accumulation in soils, which is likely due to the consumption and low diffusivity along soil profile. Source and placement of N applied strongly affected N2O concentration and distribution in soil profiles. Compared with conventional urea, stabilized urea and polymer-coated urea generally reduced N2O accumulation in soil but did not affect or even increased surface emissions. Compared with uniformly applying fertilizers, deep placement reduced soil profile N2O accumulation at 44% WFPS but not at 77% WFPS. In contrast, deep placement reduced surface emissions by 56–71%, depending on soil WFPS and sources of fertilizers. These results confirmed our hypothesis that N fertilizers accumulated in deep soil layers are less prone to loss as N2O emission. Across fertilizer treatments, surface N2O emissions increased exponentially with soil N2O accumulation at 44% WFPS but not at 77% WFPS, demonstrating that soil moisture status dominates accumulation and emission of N2O in the soil profile. More studies are needed in understanding the contribution of nitrification and denitrification on fertilizer-induced N2O emissions and the linkage with activities of associated microbes.
References
Akiyama H, Yan X, Yagi K (2010) Evaluation of effectiveness of enhanced-efficiency fertilizers as mitigation options for N2O and NO emissions from agricultural soils: meta-analysis. Glob Chang Biol 16:1837–1846. https://doi.org/10.1111/j.1365-2486.2009.02031.x
Asgedom H, Tenuta M, Flaten DN, Gao X, Kebreab E (2014) Nitrous oxide emissions from a clay soil receiving granular urea formulations and dairy manure. Agron J 106:732–744. https://doi.org/10.2134/agronj2013.0096
Bateman EJ, Baggs EM (2005) Contributions of nitrification and denitrification to N2O emissions from soils at different water-filled pore space. Biol Fertil Soils 41:379–388. https://doi.org/10.1007/s00374-005-0858-3
Beauchamp EG (1997) Nitrous oxide emission from agricultural soils. Can J Soil Sci 77:113–123. https://doi.org/10.4141/S96-101
Behera SK, Panda RK (2009) Effect of fertilization and irrigation schedule on water and fertilizer solute transport for wheat crop in a sub-humid sub-tropical region. Agric Ecosyst Environ 130:141–155. https://doi.org/10.1016/j.agee.2008.12.009
Bouwman AF, Boumans LJM, Batjes NH (2002) Emissions of N2O and NO from fertilized fields: summary of available measurement data. Glob Biogeochem Cyles 16:6–1–6–13. https://doi.org/10.1029/2001GB001811
Brummell ME, Farrell RE, Siciliano SD (2012) Greenhouse gas soil production and surface fluxes at a high arctic polar oasis. Soil Biol Biochem 52:1–12. https://doi.org/10.1016/j.soilbio.2012.03.019
Carter MR (1993) Soil sampling and methods of analysis. Lewis Publ. CRC Press, Boca Raton
Castle K, Arah JRM, Vinten AJA (1998) Denitrification in intact subsoil cores. Biol Fertil Soils 28:12–18. https://doi.org/10.1007/s003740050457
Chilundo M, Joel A, Wesström I, Brito R, Messing I (2018) Influence of irrigation and fertilisation management on the seasonal distribution of water and nitrogen in a semi-arid loamy sandy soil. Agric Water Manag 199:120–137. https://doi.org/10.1016/j.agwat.2017.12.020
Cook WP, Sanders DC (1991) Nitrogen application frequency for drip-irrigated tomatoes. Hortic Sci 26:250–252
Davidson EA (1992) Sources of nitric oxide and nitrous oxide following wetting of dry soil. Soil Sci Soc Am J 56:95–102. https://doi.org/10.2136/sssaj1992.03615995005600010015x
Deare FM, Ahmad N, Ferguson TU (1995) Downward movement of nitrate and ammonium nitrogen in a flatland ultisol. Fert Res 42:175–184. https://doi.org/10.1007/BF00750512
Di H, Cameron KC, Podolyan A, Robinson A (2014) Effect of soil moisture status and a nitrification inhibitor, dicyandiamide, on ammonia oxidizer and denitrifier growth and nitrous oxide emissions in a grassland soil. Soil Biol Biochem 73:59–68. https://doi.org/10.1016/j.soilbio.2014.02.011
Fanish SA, Muthukrishnan P (2013) Nutrient distribution under drip fertigation systems. World J Agri Sci 9:277–283. https://doi.org/10.5829/idosi.wjas.2013.9.3.2941
Gao X, Asgedom H, Tenuta M, Flaten DN (2015) Enhanced efficiency urea sources and placement effects on nitrous oxide emissions. Agron J 107:265–277. https://doi.org/10.2134/agronj14.0213
Gao X, Rajendran N, Tenuta M, Dunmola A, Burton DL (2014) Greenhouse gas accumulation in the soil profile is not always related to surface emissions in a prairie pothole agricultural landscape. Soil Sci Soc Am J 78:805–817. https://doi.org/10.2136/sssaj2013.05.0157
Goldberg SD, Knorr KH, Gebauer G (2008) N2O concentration and isotope signature along profiles provide deeper insight into the fate of N2O in soils. Isot Environ Health Stud 44:377–391. https://doi.org/10.1080/10256010802507433
Granli T, Bockman OC (1994) Nitrous oxide from agriculture. Nor J Agric Sci 12(Suppl):1–128
Guardia G, Cangani MT, Andreu G, Sanz-Cobena A, García-Marco S, Álvarez JM, Recio-Huetos J, Vallejo A (2017) Effect of inhibitors and fertigation strategies on GHG emissions, NO fluxes and yield in irrigated maize. Field Crop Res 204:135–145. https://doi.org/10.1016/j.fcr.2017.01.009
Halvorson AD, Snyder CS, Blaylock AD, Del Grosso SJ (2014) Enhanced-efficiency nitrogen fertilizers: potential role in nitrous oxide emission mitigation. Agron J 106:715–722. https://doi.org/10.2134/agronj2013.0081
Huang T, Gao B, Hu XK, Lu X, Well R, Christie P, Bakken LR, Ju XT (2014) Ammonia-oxidation as an engine to generate nitrous oxide in an intensively managed calcareous Fluvo-aquic soil. Sci Rep 4:3950. https://doi.org/10.1038/srep03950
IPCC (2013) Climate change 2013: the physical science basis. Cambridge University Press, New York
Laville P, Lehuger S, Loubet B, Chaumartin F, Cellier P (2011) Effect of management, climate and soil conditions on N2O and NO emissions from an arable crop rotation using high temporal resolution measurements. Agric For Meteorol 151:228–240. https://doi.org/10.1016/j.agrformet.2010.10.008
Liu R, Hayden HL, Suter H, Hu H, Lam SK, He J, Mele PM, Chen D (2017) The effect of temperature and moisture on the source of N2O and contributions from ammonia oxidizers in an agricultural soil. Biol Fertil Soils 53:141–152. https://doi.org/10.1007/s00374-016-1167-8
Liu X, Mosier AR, Halvorson AD, Zhang F (2006) The impact of nitrogen placement and tillage on NO, N2O, CH4 and CO2 fluxes from a clay loam soil. Plant Soil 280:177–188. https://doi.org/10.1007/s11104-005-2950-8
Loveland PJ, Whalley WR (1991) Particle size analysis. In: Smith KA, Mullins CE (eds) Soil analysis: physical methods. Marcel Dekker, Inc., New York, pp 271–328
Ma Z, Gao X, Tenuta M, Kuang W, Gui D, Zeng F (2018) Urea fertigation sources affect nitrous oxide emission from a drip-fertigated cotton field in northwestern China. Agric Ecosyst Environ 265:22–30. https://doi.org/10.1016/j.agee.2018.05.021
Marsden KA, Marín-Martínez AJ, Vallejo A, Hill PW, Jones DL, Chadwick DR (2016) The mobility of nitrification inhibitors under simulated ruminant urine deposition and rainfall: a comparison between DCD and DMPP. Biol Fertil Soils 52(4):491–503. https://doi.org/10.1007/s00374-016-1092-x
Nan W, Yue S, Li S, Huang H, Shen Y (2016) Characteristics of N2O production and transport within soil profiles subjected to different nitrogen application rates in China. Sci Total Environ 542:864–875. https://doi.org/10.1016/j.scitotenv.2015.10.147
Pedersen AR (2011) HMR: flux estimation with static chamber data. R package version 0.3.1. [Online] Available: https://cran.r-project.org/web/packages/HMR/index.html. Accessed 10 Feb 2018
Qin S, Ding K, Clough TJ, Hu C, Luo J (2017) Temporal in situ dynamics of N2O reductase activity as affected by nitrogen fertilization and implications for the N2O/(N2O + N2) product ratio and N2O mitigation. Biol Fertil Soils 53:723–727. https://doi.org/10.1007/s00374-017-1232-y
Ravishankara AR, Daniel JS, Portmann RW (2009) Nitrous oxide (N2O): the dominant ozone-depleting substance emitted in the 21st century. Science 326:123–125. https://doi.org/10.1126/science.1176985
Reth S, Graf W, Gefke O, Schilling R, Seidlitz HK, Munch JC (2008) Whole year-round observation of N2O profiles in soil: a lysimeter study. Water Air Soil Pollut Focus 8:129–137. https://doi.org/10.1007/s11267-007-9165-3
Sanz-Cobena A, Abalos D, Meijide A, Sanchez-Martin L, Vallejo A (2016) Soil moisture determines the effectiveness of two urease inhibitors to decrease N2O emission. Mitig Adapt Strateg Gl 21:1131–1144. https://doi.org/10.1007/s11027-014-9548-5
SAS Institute (2011) SAS for Windows. Release 9.3. SAS Inst. Inc., Cary
Smith KA, Thomson PE, Clayton H, McTaggart IP, Conen F (1998) Effects of temperature, water content and nitrogen fertilisation on emissions of nitrous oxide by soils. Atmos Environ 32:3301–3309. https://doi.org/10.1016/S1352-2310(97)00492-5
Stark JM, Firestone MK (1995) Mechanisms for soil moisture effects on activity of nitrifying bacteria. Appl Environ Microbiol 61:218–221
Tiedje J (1982) Denitrification. In: A.L. Page (Ed) Methods of soil analysis. Part 2. 2nd ed. Agron. Monogr. 9. ASA and SSSA, Madison. p.1011–1026
Wang Q, Zhang L, Shen J, Du S, Han L, He J (2016) Nitrogen fertiliser-induced changes in N2O emissions are attributed more to ammonia-oxidising bacteria rather than archaea as revealed using 1-octyne and acetylene inhibitors in two arable soils. Biol Fertil Soils 52:1163–1171. https://doi.org/10.1007/s00374-016-1151-3
Wang Y, Hu C, Ming H, Zhang Y, Li X, Dong W, Oenema O (2013) Concentration profiles of CH4, CO2 and N2O in soils of a wheat–maize rotation ecosystem in North China Plain, measured weekly over a whole year. Agric Ecosyst Environ 164:260–272. https://doi.org/10.1016/j.agee.2012.10.004
Yao P, Li X, Liu J, Shen Y, Yue S, Li S (2018) The role of maize plants in regulating soil profile dynamics and surface emissions of nitrous oxide in a semiarid environment. Biol Fertil Soils 54:119–135. https://doi.org/10.1007/s00374-017-1243-8
Acknowledgements
We thank Nutrien Ltd. and Koch Fertilizer Ltd. for providing polymer-coated urea and stabilized urea used in this study. The assistance of Zhiwen Ma on gas sampling is greatly appreciated.
Funding
This study was funded by National Natural Science Foundation of China (No. 31570002, 31870499), the China 1000 Talent Program (Y472171), and China Scholarship Council (201704910732).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kuang, W., Gao, X., Tenuta, M. et al. Relationship between soil profile accumulation and surface emission of N2O: effects of soil moisture and fertilizer nitrogen. Biol Fertil Soils 55, 97–107 (2019). https://doi.org/10.1007/s00374-018-01337-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00374-018-01337-4