Abstract
It is well known that nutrient availability and microbial biomass and activity in the soil following plant residue addition are influenced by residue composition. But, less is known about the effect of repeated addition of residue with different decomposability on these soil properties and how they are affected by the order in which the residues are added. In this study, low C/N residue (young kikuyu shoots) and high C/N residue (mature wheat shoots) were added four times (on days 0, 10, 20 and 30) at a rate of 7 g C kg−1 in a different order to a silt loam. Respiration was measured over 40 days and microbial biomass C (MBC), N (MBN) and P (MBP) and available N and P were measured on days 0, 10, 20, 30 and 40. Cumulative respiration in the 10 days following low C/N residue addition was higher than with high C/N residue addition. It decreased with increasing proportion of high C/N residue addition compared to four times addition of low C/N residue. Treatments with three or four additions of high C/N residue had lower MBC, MBN and MBP, and available N and P concentrations compared to treatments with three or four times addition of low C/N residue, irrespective of the order in which the residues were added. It can be concluded that with repeated residue addition, microbial biomass and N and P availability at the end of a given 10-day period are mainly influenced by the proportion of low or high C/N residue added previously, whereas the C/N ratio of residue added at the beginning of the period has little effect. Further nutrient availability was not affected by the order in which low or high C/N residue was added.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Net mineralisation-immobilisation patterns in soil amended with organic materials are influenced by environmental conditions (Saccone et al. 2013), the nature and abundance of the decomposers (Kristiansen et al. 2004) and the chemical composition of the organic amendments (Thomas and Asakawa 1993; Tian et al. 1992). Microorganisms have a low C/N ratio (typically <20); therefore, organic amendments with low C/N ratio can satisfy the nutrient demand of microbes and result in early net nutrient mineralisation (Hadas et al. 2004; Janssen 1996). In contrast, addition of plant residue with high C/N ratio can result in, at least temporary, net immobilisation of N in the microbial biomass (Moritsuka et al. 2004). Decomposition rate of organic materials is also influenced by the nature of the organic C. Water-soluble organic C can be rapidly decomposed because it is mostly in the form of simple compounds and readily accessible to soil microbes (Hadas et al. 2004). Breakdown of complex organic C compounds such as polyphenols or lignin requires the release of extracellular enzymes which can only be synthesised by a subset of the microbial population (Nannipieri et al. 2012; Sinsabaugh 2010). Due to this additional energy requirement, net energy gain of microbial decomposers is smaller with complex compared to simple C compounds. Therefore, the former are decomposed more slowly. Decomposition of the added organic matter is also affected by other factors such as clay type (Vogel et al. 2015) and soil moisture (Shi and Marschner 2015).
The effect of using organic amendments on soil nutrient availability has been studied extensively (e.g. Janssen 1996; Nicolardot et al. 2001; Partey et al. 2014; Sakala et al. 2000) but usually after a single addition of residues or residue mixtures (Barantal et al. 2011; Gartner and Cardon 2004; Wardle et al. 2006). In many ecosystems, plant residues from different species may be added repeatedly, for example, through leaf fall or root death. In a previous study, we determined the effect of frequent addition of a single residue type on mineralisation and nutrient availability (Duong et al. 2009). However, little is known about the effect of repeated addition of plant residues with different decomposability on soil respiration and nutrient release or immobilisation and how this is influenced by the order in which the residues are added. More recently, we showed that with two residue additions 20 days apart, nutrient availability after the second addition is influenced by the C/N ratio of the residue added first (Marschner et al. 2015).
The aim of this study was to investigate the effect of repeated addition of low and high C/N residues on soil respiration, microbial biomass and N and P availability. Specifically, we investigated if the order in which the residues are added influences the measured parameters. We tested the following hypothesis: With repeated residue addition cumulative respiration, microbial biomass and N and P availability at a given sampling date will increase with increasing proportion of low C/N residue added previously whereas the C/N ratio of the residue added at the start of the 10-day period has little effect.
Materials and methods
Soil and residues
A silt loam was collected from 0 to 15 cm at Waite Campus, The University of Adelaide (34° 58′ S, 138° 37′ E). The area is in a semi-arid region and has a Mediterranean climate with cool, wet winters and hot and dry summers. The soil is classified as red-brown earth according to Australian classification (Isbell 2002) and as Xeralf according to US Soil Taxonomy (Chittleborough and Oades 1979). The soil was managed for over 80 years in the Waite long-term rotation trial as permanent pasture. It has the following properties: sand 27 %, silt 51 % and clay 22 %, pH (1:5 soil/water) 7.3, electrical conductivity (EC 1:5) 742 μS cm−1, total N 134 mg kg−1 and total P 461 mg kg−1, total organic C (TOC) 15 g kg−1, available N 15 mg kg−1, available P 34 mg kg−1, microbial biomass C (MBC) 122 mg kg−1, microbial biomass N (MBN) 7 mg kg−1, microbial biomass P (MBP) 1 mg kg−1, maximum water holding capacity (WHC) 327 g kg−1 and bulk density 1.3 g cm−3. After collection, the soil was dried at 40 °C in a fan-forced oven. This temperature is not unnatural because daytime temperatures in summer often exceed 40 °C in the top soil and soils are air-dried for several weeks. Then, plant debris was removed, and the soil sieved to <2 mm.
Two residues with distinct properties were used (Table 1): low C/N young kikuyu shoots (Pennisetum clandestinum L.) and high C/N mature wheat shoots (Triticum aestivum L.). These two plant species were chosen because kikuyu is a common pasture species and used as green manure. Wheat is an important cereal, and its straw and stubble are often incorporated into the soil after harvest. The residues were dried at 40 °C in a fan-forced oven, ground and sieved to particle size 0.25 to 1 mm.
Experimental design
Before the start of the experiment, the air-dried soil was pre-incubated for 10 days at 25 °C at 50 % of WHC to reactivate the microbes and to stabilise their activity after rewetting. This water content was chosen based on Setia et al. (2011a) who found that microbial activity of a soil of this texture was maximal at 50 % WHC which was confirmed in other studies conducted with this soil in our group (Hatam, unpublished data).
Residues were added four times (on days 0, 10, 20 and 30) at a rate of 7 g C kg−1 soil. There were eight treatments (Fig. 1): soil amended with four times of low C/N residue (LLLL) or four times high C/N residue (HHHH). In three other treatments, low C/N residue was added on day 0, followed by high C/N residue added once then low C/N residue added twice (LHLL), followed by high C/N residue added twice then low C/N residue added once (LHHL) or high C/N residue added three times (LHHH). The three other treatments also included different proportions of high and low C/N residue, but high C/N residue was added on day 0 (HLHH, HLLH, HLLL). This design allowed studying both proportion of residue type in the repeated additions (from 25 to 100 %) and timing (start, middle or end).
After the initial residue addition, 30 g dry soil equivalent was filled into PVC cores with 1.85-cm radius, 5-cm height and a nylon net base (0.75 μm, Australian Filter Specialist) and packed to a bulk density of 1.3 g cm−3. Then, the cores were placed individually into 1-l jars with gas-tight lids equipped with septa to allow quantification of the headspace CO2 concentration as described below. The jars were incubated in the dark at 22–23 °C. Soil moisture was maintained at 50 % of WHC by checking the water content every few days by weight and adding reverse osmosis water if necessary. Cores were destructively sampled on days 0 (5 h after residue addition), 10 and 20, 30 and 40 (end of experiment) for analysis of available N and P, microbial biomass C, N and P, pH and EC. In a given 10-day period (0–10, 10–20, 20–30, 30–40 days), only the cores to be sampled at the end of the period were placed in jars. The remaining cores were incubated under the same conditions in large plastic tubs with loosely fitted lids. On days 10, 20 and 30, after removal of the cores from the jars for analysis, residues were added to the remaining cores. After thoroughly mixing the residues with the soil, bulk density was adjusted and the cores to be harvested at the next sampling time placed in the glass jars for respiration measurement.
Analyses and calculations
Soil texture was determined by the hydrometer method (Gee and Or 2002). The pH and electrical conductivity (EC) were determined in a 1:5 soil/water extract after 1 h end-over-end shaking at 25 °C (Rayment and Higginson 1992). Soil water holding capacity was determined using a sintered glass funnel connected to a 1 m water column (matric potential = −10 kPa) after (Wilke 2005). Total organic C content of soil and residues was determined by wet oxidation and titration (Walkley and Black 1934). To determine total N and P in soil and residues, the material was digested with H2SO4 and a mixture of HNO3 and HClO4, respectively. Total N was measured by a modified Kjeldahl method (Vanlauwe et al. 1996). Total P in the digest was measured by the phosphovanado-molybdate method according to Hanson (1950). Water-extractable organic C (WEOC) was determined by shaking 1 g residue with 30 ml reverse osmosis water for 1 h. The extract was centrifuged at 1509×g for 10 min and filtered through a Whatman# 42 filter paper. The WEOC concentration of the extract was determined after K2Cr2O7 and H2SO4 oxidation by titration with acidified (NH4)2Fe(SO4)2·6H2O (Anderson and Ingram 1993). Solutions with different sucrose concentrations (0.01–1 mg C ml−1) were used as standards.
Available N (ammonium and nitrate) concentration was measured after 1 h end-over-end shaker with 2 M KCl at 1:10 soil extractant ratio. Ammonium-N was determined after Forster (1995). Nitrate-N was determined using a modification of Miranda et al. (2001) as described in Cavagnaro et al. (2006). Available P was extracted by the anion exchange resin method (Kouno et al. 1995), and the P concentration was determined colorimetrically (Murphy and Riley 1962).
Soil microbial biomass C (MBC), N and P were determined by the fumigation extraction method (Vance et al. 1987). Soil samples were exposed to 48-h chloroform fumigation followed by shaking at a 1:5 ratio with 0.5 M K2SO4. The C concentration in the extract of fumigated and non-fumigated soils was determined by adding 0.0667 M K2Cr2O7 and concentrated H2SO4. The remaining K2Cr2O7 was titrated with 0.033 M acidified (NH4)2Fe(SO4)2·6H2O (Anderson and Ingram 1993). The difference in C concentration between fumigated and non-fumigated soil was multiplied by 2.64 to calculate MBC (Vance et al. 1987). MBN was determined after Moore et al. (2000). Ammonium-N in the extracts was determined as described above for available N. MBN was calculated as the difference in ammonium-N concentration between fumigated and non-fumigated samples divided by 0.57 which is the proportionality factor to convert ammonium to microbial biomass N (Jenkinson 1988). For determination of soil MBP, the P concentration in the K2SO4 extracts was determined according to Murphy and Riley (1962). The difference in P concentration between fumigated and non-fumigated samples was multiplied by 2.5 (Brookes et al. 1982). A correction factor for P sorption was not used because an earlier study with this soil found that recovery of a P spike was 98 % (Butterly et al. 2010).
Soil respiration was determined by measuring the CO2 concentration in the headspace of the jars using a Servomex 1450 infrared gas analyser (Servomex, UK) as described in Setia et al. (2011b). After each measurement (T 1), the jars were vented to refresh the headspace using a fan and then resealed followed by determination of the CO2 concentration (T 0). The CO2 evolved during a given interval was calculated as the difference in CO2 concentration between T 1 and T 0. Due to the upper detection limit of the gas analyser (2 % CO2) and the decrease in respiration rate over time after residue addition, respiration was measured daily in the first 6 days after addition of residues and then every second day (days 8 and 10) until the following sampling times. Linear regression based on injection of known amounts of CO2 in the jars was used to define the relationship between CO2 concentration and detector reading. Cumulative respiration was calculated as the sum of respiration rates [in mg CO2-C (g soil and day)−1] for each 10-day period separately as well as over 40 days.
Statistical analysis
There were four replicates per treatment and sampling time. Data were analysed by one-way ANOVA for each sampling date separately using Genstat 15th edition (VSN Int. Ltd., UK). Tukey’s multiple comparison tests at 95 % confidence interval was used to determine significant differences among treatments.
Results
Residue properties
Low C/N residue (young kikuyu shoots) had higher total N and P, available N and P, water-extractable organic C concentration and electrical conductivity but lower C/N and C/P ratios than high C/N residue (mature wheat shoots). Total organic C and pH did not differ between the two residues (Table 1). Soil pH increased from days 0 to 40 (data not shown) and was about 6.7–7.5 on day 40 in all treatments.
Respiration
Respiration rates increased sharply on the first day after residue addition, particularly with addition of low C/N residue (Fig. S1). Respiration rates on the first day after residue addition were lower after the first than the subsequent additions. When low C/N residue was added after high C/N residue (day 10 in HL), the respiration rate on day 1 after residue addition was lower than when low C/N residue followed low C/N residue (LL). Previous low C/N residue addition increased respiration rate after high C/N residue addition (LH on day 11 or HLH on day 21) compared to high following high C/N residue (HH or HHH).
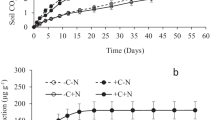
Cumulative respiration in the 10 days following addition of low C/N residue was about 30 % higher than after high C/N residue addition (Fig. 2). In the period between day 21 and 30, previous addition of high C/N residue decreased cumulative respiration after addition of low C/N residue (HL) compared to previous low C/N addition (LL). However, cumulative respiration after addition of high C/N residue in this period was not stimulated by prior low C/N residue addition (LH) compared to HH.
Cumulative respiration for 10-day periods (1–10, 11–20, 21–30 and 30–40 days after start of the experiment) in soil amended with low and high C/N residue on days 0, 10, 20, 30 and 40 (n = 4). For a given 10-day interval, column sections with different letters are significantly different (P ≤ 0.05). For treatment abbreviations, see Fig. 1
Cumulative respiration on day 40 was highest in LLLL and lowest in HHHH (Table 2). Compared to LLLL, cumulative respiration decreased with increasing proportion of high C/N residue and was 33 % lower in LHHH than in LLLL. Similarly, cumulative respiration compared to HHHH increased with increasing proportion of low C/N residue; it was 72 % higher in HLLL compared to HHHH.
Microbial biomass
MBC was lower on days 0 and 10 than at the later harvest dates (Fig. 3). From day 10 to day 40, MBC increased about 2-fold. There were no significant differences among treatments on days 0, 10 and 20 (Fig. 3a, b, c). On day 30, MBC also differed little among treatments but was significantly lower in LHH than in LHL (Fig. 3d). On day 40, treatments with three or four additions of high C/N residue (LHHH, HLHH, HHHH) had lower MBC than those with three or four additions of low C/N residue (LHLL, HLLLL, LLLLL) (Fig. 3e). At a given proportion of low or high C/N residue in the treatment (e.g. LHHL or HLLH), MBC on day 40 was not influenced by the order in which the residues were added.
Microbial biomass C on days 0 (a), 10 (b), 20 (c), 30 (d) and 40 (e) in soil amended with low C/N and high C/N residue on days 0, 10, 20 and 30 (n = 4). At a given sampling day, bars with different letters are significantly different (P ≤ 0.05). ns not significant. For treatment abbreviations, see Fig. 1
MBN was lowest on day 0 and highest on day 30 (Fig. 4). On day 0 and particularly day 10, MBN was about 2-fold higher with low C/N residues than with high C/N residue the latter (Fig. 4a, b). On day 20, MBN was lower in HH than in all other treatments which had received low C/N residue at least once (LL, LH, HL). MBN was not influenced by the order in which the high and low C/N residues were added (HL or LH). On day 30, the MBN was lowest in HHH. MBN was highest in treatments with two or three low C/N residue additions (LHL, HLL, LLL). MBN was not influenced by the order in which the residues were added. On day 40, MBN was influenced by the proportion of low C/N residues (25–100 %) but not by the order in which the residues were added. MBN increased with the proportion of low C/N residue.
Microbial biomass N on days 0 (a), 10 (b), 20 (c), 30 (d) and 40 (e) in soil amended with low C/N and high C/N residue on days 0, 10, 20 and 30 (n = 4). At a given sampling day, bars with different letters are significantly different (P ≤ 0.05). For treatment abbreviations, see Fig. 1
MBP increased from day 0 to day 40 (Fig. 5). On days 0 and 10, MBP was generally lower in soil amended with high C/N residue than with low C/N residue (Fig. 5a, b). On day 20, MBP was lowest in HH, followed by treatments where high and low C/N residues were added (HL or LH) and was highest in LL. The order in which high and low C/N residues were added did not influence the MBP. MBP on day 30 was 3-fold higher in LLL than in HHH (Fig. 5d). On day 40, the differences in MBP among treatments were more pronounced than on day 30 (Fig. 5e). MBP was about 4-fold higher in LLLL than in HHHH and increased with proportion of low C/N residue. In general, the order in which the residues were added did not influence MBP (e.g. LHLL compared to HLLL).
Microbial biomass P on days 0 (a), 10 (b), 20 (c), 30 (d) and 40 (e) in soil amended with low C/N and high C/N residue on days 0, 10, 20 and 30 (n = 4). At a given sampling day, bars with different letters are significantly different (P ≤ 0.05). For treatment abbreviations, see Fig. 1
The MBC/MBN ratio ranged between 10 and 30 (Fig. S2). It was highest on day 0 and low on days 20 and 30. On day 0, the MBC/MBN ratio did not differ consistently between soils with high and low C/N residue addition (Fig. S2A). At the following sampling days, it was highest in the treatments that received only high C/N residue and lowest in the treatments with only low C/N residues. On days 20 and 30, the MBC/MBN ratio was higher in treatments that received high C/N residue last compared to those receiving low C/N last (e.g. on day 30 LHH compared to HLL). On day 40, the MBC/MBN ratio of all treatments with low C/N residue at least once was lower than in HHHH but higher than in LLLL (Fig. S2E).
The MBC/MBP ratio ranged between 100 and 600 and was highest on day 0 (Fig. S3). On day 0, the MBC/MBP ratio was higher in soil with high C/N residues than with low C/N residues (Fig. S3A). Later, the ratio was highest in treatments receiving only high C/N residues and lowest in treatments with only low C/N residues.
Available N and P
Available N in soils with residues was mainly in the form of NH4 +-N; the proportion as NO3 −-N was <37 % (data not shown). On days 0 and 10, the available N concentration was higher in soil with low C/N residue addition compared to high C/N residue-amended soil (Fig. 6a, b). The available N concentration doubled from day 0 to day 10 in soil amended with low C/N residue, whereas it decreased by about 70 % in soil with high C/N residue. Consequently, the available N concentration was about 2-fold higher with low C/N compared to high C/N residue on day 0 but more than 10-fold higher on day 10. From day 20 to day 40, the available N concentration was highest in the treatment with only low C/N residue added and lowest in soil with only high C/N residue amendment. On days 30 and 40, the available N concentration increased with proportion of low C/N residue but was not influenced by the order in which the residues were added (Fig. 6d, e).
Available N (NH4 + + NO3 −) concentration (mg N kg−1) on days 0 (a), 10 (b), 20 (c), 30 (d) and 40 (e) in soil amended with low C/N and high C/N residue on days 0, 10, 20 and 30 (n = 4). At a given sampling day, bars with different letters are significantly different (P ≤ 0.05). For treatment abbreviations, see Fig. 1
The available P concentration remained within the same range throughout the experiment (Fig. 7). On days 0 and 10, the available P concentration was about 30 % higher in soil with low C/N residue than when amended with high C/N residue (Fig. 7a, b). At the following harvest dates, the available P concentration was highest in treatments with only low C/N residues and lowest in those with only high C/N residues. On days 30 and 40 (Fig. 7d, e) the available P concentration increased with proportion of low C/N residue in the treatments (e.g. LHL compared to LHH) and was not influenced by the order in which the residues were added.
Available P concentration (mg P kg−1) on days 0 (a), 10 (b), 20 (c), 30 (d) and 40 (e) in soil amended with low C/N and high C/N residue on days 0, 10, 20 and 30 (n = 4). At a given sampling day, bars with different letters are significantly different (P ≤ 0.05). For treatment abbreviations, see Fig. 1
Discussion
Based on this experiment we confirm the hypothesis because cumulative respiration, microbial biomass and N and P availability with repeated residue addition at a given sampling date increased with increasing proportion of low C/N residue added previously whereas the C/N ratio of the residue added at the start of the period has little effect. The following discussion is separated into periods after residue addition.
Period after first residue addition (days 0–10)
As expected from previous studies examining the effect of C/N ratio of organic amendments (Moritsuka et al. 2004; Partey et al. 2014), respiration rate during this period and cumulative respiration and MBN, MBP, available N and P concentrations on day 10 (Fig. S1, Figs. 2, 3, 4, 5, 6 and 7) were higher in soil amended with low C/N residue than after addition of high C/N residue. This can be explained by the higher N and P concentrations of the low C/N residue. The strong increase of the available N concentration from day 0 to day 10 indicates that N supply from residues exceeded microbial demand. The high concentration of water-extractable organic C in the low C/N residue may also have contributed to its high decomposability (Abiven et al. 2005; Hasbullah and Marschner 2014). It should be noted that the MBC, MBN and MBP values on day 0 could also include chloroform labile C, N and P from the residues added just 5 h before sampling which may explain the high MBC/MBN ratio on day 0 (Fig. S2). The higher available N and P concentrations on day 0 with low compared to high C/N residues are likely to be due to release of inorganic N and P from the low C/N residues, not due to mineralisation of residue N and P because there were only a few hours between residue addition and sampling on day 0. The following samples were taken at the end of a given period, before adding the next residue. Therefore, the direct effect of residue C, N and P should be small.
Period after second residue addition (days 10–20)
The previously added low C/N residue increased initial respiration after addition of high C/N residue whereas the previous high C/N residue had a negative effect on initial respiration after addition of low C/N residue (Fig. S1). This is in agreement with our previous study where residues were added only twice (Marschner et al. 2015). Available N and P concentrations and MBP in the treatments in which the C/N ratio of the residue differed between the first and second addition (HL and LH) were influenced by the C/N ratio of both additions because they increased in the following order: HH < LH, HL < LL (Figs. 5, 6 and 7). In all treatments receiving low C/N residue at least once (LL, HL, LH), MBN was higher than in HH (Fig. 4). This indicates that N mineralised from the low C/N residue added on day 10 exceeded microbial demand in this period. The decrease in available N concentration from day 10 to day 20 in treatments in which low C/N residue was added first (LL and LH) cannot be explained by immobilisation in the microbial biomass because MBN did not increase. The decrease in available N may be due to denitrification in anaerobic microsites (Burger et al. 2005). The lower available N and P concentrations in LH compared to LL on day 20 could be due to N and P uptake by microbes decomposing the high C/N residue added on day 10. In case of P, this argument is supported by the increase in MBP from day 10 to day 20 in the LH treatment.
The effect of the previously added residue on nutrient dynamics after the second amendment can be explained by the large proportion of previously added residue still in the soil at the time of the second amendment. Based on cumulative respiration in the first period and without considering a priming effect (Kuzyakov et al. 2000), it can be calculated that about 80 % of the low C/N residue added at the start of the experiment and 90 % of the high C/N residue was still in the soil on day 10. This previously added residue would be decomposed from day 10 to 20 along with the residue added on day 10.
Period after third (days 20–30) and fourth residue addition (days 30–40)
In the period after the third and fourth residue addition, the C/N ratio of the residue added on day 10 no longer influenced respiration rates (Fig. S1). In most treatments, cumulative respiration was influenced by the C/N ratio of the residue added at the start of the period, being higher when low C/N residue was added than with high C/N residue (Fig. 2). Cumulative respiration, available N and P concentrations, and MBN and MBP increased in the following order: only H added < L once < L twice < only L added confirming that these parameters are influenced by the proportion of low or high C/N residue added.
Of the parameters assessed, MBC was the least affected by the treatments (Fig. 3). Clear treatment effects were evident only on day 40 and differences less pronounced than in cumulative respiration, MBN and MBP on day 40 (Figs. 4 and 5). This suggests that microbial activity and nutrient uptake as well as MBC/MBN or MBC/MBP ratios are more strongly influenced by residue C/N ratio than MBC. The small effect on MBC may be because the difference between the two residues in organic C concentration was smaller than in N and P concentration (Table 1). Apparently, both high and low C/N residues supply sufficient C for microbial growth.
Assuming that only C from low C/N residue was respired (no priming effect), it can be calculated that about 50 % of the previously added residue was still present at the time of the fourth addition. The nutrients released from microbes decomposing the low C/N residues could be taken up by microbes decomposing the high C/N residue and accelerate their decomposition rate. A greater proportion of previously added low C/N residue would also mean a greater amount of that residue in the soil at the time when the high C/N residue is added. This would increase the likelihood of close proximity of microbes decomposing high and low C/N residues and thus nutrient transfer to the former.
The strong effect of the previously added residue on respiration, nutrient uptake of soil microbes and nutrient availability is likely to be due to the short interval between residue additions in this study. After 10 days, only a small proportion of the previously added residue will have been decomposed (in this study 10–20 %). With longer intervals, the effect of the previously added residue is likely to become smaller because less will be left in the soils when the new residue is added. A short interval between residue additions may occur, for example, during litter fall in autumn or with short intervals between strong rainfall events which mix litter by surface erosion.
Conclusion
This study showed that with repeated residue addition, nutrient availability may be very different from that predicted based on single residue additions. When residues are added repeatedly, the C/N ratio and amount of the residue present in the soil at the time of the next residue addition determine nutrient availability whereas the C/N ratio of the residue at the beginning of the 10-day period has little effect. In the present study, the source of C, N and P in respired CO2, microbial biomass and available nutrients could not be determined. In future studies with repeated residue additions, one of the addition could be in the form of 13C, 15N or 32P-labelled residues. The observed differences in soil respiration and microbial biomass C, N and P are likely to be accompanied by changes in microbial community structure which could also be investigated in the future.
References
Abiven S, Recous S, Reyes V, Oliver R (2005) Mineralisation of C and N from root, stem and leaf residues in soil and role of their biochemical quality. Biol Fertil Soils 42:119–128. doi:10.1007/s00374-005-0006-0
Anderson JM, Ingram JSI (1993) Tropical soil biology and fertility: a handbook of methods, 2nd edn. CAB International, Wallingford
Barantal S, Roy J, Fromin N, Schimann H, Hattenschwiler S (2011) Long-term presence of tree species but not chemical diversity affect litter mixture effects on decomposition in a neotropical rainforest. Oecologia 167:241–252
Brookes PC, Powlson DS, Jenkinson DS (1982) Measurement of microbial biomass phosphorus in soil. Soil Biol Biochem 14:319–329. doi:10.1016/0038-0717(82)90001-3
Burger M, Jackson L, Lundquist E, Louie D, Miller R, Rolston D, Scow K (2005) Microbial responses and nitrous oxide emissions during wetting and drying of organically and conventionally managed soil under tomatoes. Biol Fertil Soils 42:109–118. doi:10.1007/s00374-005-0007-z
Butterly C, Marschner P, McNeill A, Baldock J (2010) Rewetting CO2 pulses in Australian agricultural soils and the influence of soil properties. Biol Fertil Soils 46:739–753. doi:10.1007/s00374-010-0481-9
Cavagnaro T, Jackson L, Six J, Ferris H, Goyal S, Asami D, Scow K (2006) Arbuscular mycorrhizas, microbial communities, nutrient availability, and soil aggregates in organic tomato production. Plant Soil 282:209–225
Chittleborough DJ, Oades JM (1979) Development of a red-brown earth. 1 A reinterpretation of published data. Aust J Soil Res 17:371–381. doi:10.1071/sr9790371
Duong TTT, Baumann K, Marschner P (2009) Frequent addition of wheat straw residues to soil enhances carbon mineralization rate. Soil Biol Biochem 41:1475–1482. doi:10.1016/j.soilbio.2009.04.003
Forster J (1995) Soil nitrogen. In: Alef K, Nannipieri P (eds) Methods in applied soil microbiology and biochemistry. Acadamic Press, London, pp 79–87
Gartner TB, Cardon ZG (2004) Decomposition dynamics in mixed-species leaf litter. Oikos 104:230–246. doi:10.1111/j.0030-1299.2004.12738.x
Gee GW, Or D (2002) Particle size analysis. In: Dane JH, Topp GC (eds) Methods of soil analysis part 4 physical methods. Soil Science Society of America, Madison, pp 255–294
Hadas A, Kautsky L, Goek M, Erman Kara E (2004) Rates of decomposition of plant residues and available nitrogen in soil, related to residue composition through simulation of carbon and nitrogen turnover. Soil Biol Biochem 36:255–266. doi:10.1016/j.soilbio.2003.09.012
Hanson WC (1950) The photometric metermination of phosphorus in fertilizers using the phosphovanado-molybdate complex. J Sci Food Agric 1:172–173
Hasbullah H, Marschner P (2014) Residue properties influence the impact of salinity on soil respiration. Biol Fertil Soils :1–13. doi:10.1007/s00374-014-0955-2
Isbell RF (2002) The Australian soil classification. Revised edn. CSIRO, Collingwood
Janssen BH (1996) Nitrogen mineralization in relation to C:N ratio and decomposability of organic materials. Plant Soil 181:39–45. doi:10.1007/BF00011290
Jenkinson DS (1988) Determination of microbial biomass in soil: measurement and turnove. In: Wilson JR (ed) Advances in nitrogen cycling in agricultural ecosystems. CAB International, Wallingford, pp 368–386
Kouno K, Tuchiya Y, Ando T (1995) Measurement of soil microbial biomass phosphorus by an anion exchange membrane method. Soil Biol Biochem 27:1353–1357
Kristiansen SM, Brandt M, Hansen EM, Magid J, Christensen BT (2004) 13C signature of CO2 evolved from incubated maize residues and maize-derived sheep faeces. Soil Biol Biochem 36:99–105. doi:10.1016/j.soilbio.2003.07.002
Kuzyakov Y, Friedel JK, Stahr K (2000) Review of mechanisms and quantification of priming effects. Soil Biol Biochem 32:1485–1498. doi:10.1016/S0038-0717(00)00084-5
Marschner P, Hatam Z, Cavagnaro T (2015) Soil respiration, microbial biomass and nutrient availability after the second amendment are influenced by legacy effects of prior residue addition. Soil Biol Biochem 88:169–177
Miranda KM, Espey MG, Wink DA (2001) A rapid, simple spectrophotometric method for simultaneous detection of nitrate and nitrite. Nitric Oxide 5:62–71
Moore JM, Klose S, Tabatabai MA (2000) Soil microbial biomass carbon and nitrogen as affected by cropping systems. Biol Fertil Soils 31:200–210. doi:10.1007/s003740050646
Moritsuka N, Yanai J, Mori K, Kosaki T (2004) Biotic and abiotic processes of nitrogen immobilization in the soil-residue interface. Soil Biol Biochem 36:1141–1148. doi:10.1016/j.soilbio.2004.02.024
Murphy J, Riley JP (1962) A modified single solution for determination of phosphate in natural waters. Anal Chim Acta 27:31–36
Nannipieri P, Giagnoni L, Renella G, Puglisi E, Ceccanti B, Masciandaro G, Fornasier F, Moscatelli MC, Marinari S (2012) Soil enzymology: classical and molecular approaches. Biol Fertil Soils 48:743–762. doi:10.1007/s00374-012-0723-0
Nicolardot B, Recous S, Mary B (2001) Simulation of C and N mineralisation during crop residue decomposition: a simple dynamic model based on the C:N ratio of the residues. Plant Soil 228:83–103. doi:10.1023/A:1004813801728
Partey ST, Preziosi RF, Robson GD (2014) Improving maize residue use in soil fertility restoration by mixing with residues of low C-to-N ratio: effects on C and N mineralization and soil microbial biomass. J Soil Sci Plant Nutr 14:518–531
Rayment GE, Higginson FR (1992) Australian laboratory handbook of soil and water chemical methods. Inkata Press, Melbourne
Saccone P, Morin S, Baptist F, Bonneville J-M, Colace M-P, Domine F, Faure M, Geremia R, Lochet J, Poly F, Lavorel S, Clément J-C (2013) The effects of snowpack properties and plant strategies on litter decomposition during winter in subalpine meadows. Plant Soil 363:215–229. doi:10.1007/s11104-012-1307-3
Sakala WD, Cadisch G, Giller KE (2000) Interactions between residues of maize and pigeonpea and mineral N fertilizers during decomposition and N mineralization. Soil Biol Biochem 32:679–688. doi:10.1016/S0038-0717(99)00204-7
Setia R, Marschner P, Baldock J, Chittleborough D, Verma V (2011a) Relationships between carbon dioxide emission and soil properties in salt-affected landscapes. Soil Biol Biochem 43:667–674. doi:10.1016/j.soilbio.2010.12.004
Setia R, Smith P, Marschner P, Baldock J, Chittleborough D, Smith J (2011b) Introducing a decomposition rate modifier in the rothamsted carbon model to predict soil organic carbon stocks in saline soils. Environ Sci Technol 45:6396–6403
Shi A, Marschner P (2015) The number of moist days determines respiration in drying and rewetting cycles. Biol Fertil Soils 51:33–41. doi:10.1007/s00374-014-0947-2
Sinsabaugh RL (2010) Phenol oxidase, peroxidase and organic matter dynamics of soil. Soil Biol Biochem 42:391–404. doi:10.1016/j.soilbio.2009.10.014
Thomas RJ, Asakawa NM (1993) Decomposition of leaf litter from tropical forage grasses and legumes. Soil Biol Biochem 25:1351–1361. doi:10.1016/0038-0717(93)90050-L
Tian G, Kang BT, Brussaard L (1992) Biological effects of plant residues with contrasting chemical compositions under humid tropical conditions—decomposition and nutrient release. Soil Biol Biochem 24:1051–1060. doi:10.1016/0038-0717(92)90035-V
Vance ED, Brookes PC, Jenkinson DS (1987) An extraction method for measuring soil microbial biomass C. Soil Biol Biochem 19:703–707. doi:10.1016/0038-0717(87)90052-6
Vanlauwe B, Nwoke OC, Sanginga N, Merckx R (1996) Impact of residue quality on the C and N mineralization of leaf and root residues of three agroforestry species. Plant Soil 183:221–231. doi:10.1007/BF00011437
Vogel C, Heister K, Buegger F, Tanuwidjaja I, Haug S, Schloter M, Kögel-Knabner I (2015) Clay mineral composition modifies decomposition and sequestration of organic carbon and nitrogen in fine soil fractions. Biol Fertil Soils 51:427–442. doi:10.1007/s00374-014-0987-7
Walkley A, Black IA (1934) An examination of the Degtjareff method for determining soil organic matter and a proposed modification of the chromic acid titration method. Soil Sci 37:29–38
Wardle DA, Yeates GW, Barker GM, Bonner KI (2006) The influence of plant litter diversity on decomposer abundance and diversity. Soil Biol Biochem 38:1052–1062. doi:10.1016/j.soilbio.2005.09.003
Wilke BM (2005) Determination of chemical and physical soil properties. In: Margesin R, Schinner F (eds) Manual for soil analysis—monitoring and assessing soil bioremediation. Springer, Berlin, pp 47–93
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nguyen, T.T., Marschner, P. Soil respiration, microbial biomass and nutrient availability in soil after repeated addition of low and high C/N plant residues. Biol Fertil Soils 52, 165–176 (2016). https://doi.org/10.1007/s00374-015-1063-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00374-015-1063-7