Abstract
Plant roots associate with diverse communities of arbuscular mycorrhizal fungi (AMF), providing the plant with mineral nutrients in exchange for carbohydrates. We investigated how onion genotype and fungal species interact to determine the benefit of the symbiosis to the plant and the potential benefit of a mixed AMF community. Ten onion genotypes were inoculated with five different AMF species, or a mixture of all five, then plant growth/nutrient uptake was compared to non-inoculated controls. 18S ribosomal RNA (rRNA) terminal restriction fragment length polymorphism was used to compare the abundance of each AMF species between genotypes. Growth and nutrient uptake were significantly different between genotypes and AMF species, but no genotype × AMF interaction was observed, indicating a general response to AMF. Potentially useful pre-breeding material was identified for use in low-input systems. Inoculation of plants with AMF led to significant increases in the concentrations of N, P and Cu, whereas significant decreases in Ca, K, Na, Fe, Mn and Zn were observed. There were significant differences between AMF species in their effect on plant nutrition. Inoculation with Acaulospora spinosa led to a significant increase in shoot S concentration which may have implications for plant defence and pungency. No additive effect of a mixed community was observed. Contrasting genotypes showed subtly different preferences for associating with AMF from a mixed community, suggesting a selection process controlled by the plant and/or the fungi. The implications of this work for the development of sustainable, low-input systems are discussed.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Arbuscular mycorrhizal fungi (AMF; phylum Glomeromycota) form symbiotic relationships with plants that are widespread in the natural world and are observed with more than 80 % of land plants (Schüβler et al. 2001). Fungal hyphae penetrate the cortical cells of plant roots to form arbuscules, over which carbohydrates from the plant are traded for nutrients, particularly phosphorus (P), which the fungus assimilates from the soil (Morgan et al. 2005). Secondary benefits to the plant, such as increased disease resistance and drought tolerance, have also been reported (Augé et al. 1994; Newsham et al. 1995). Many studies have demonstrated plant growth effects associated with the arbuscular symbiosis (Gosling et al. 2006), particularly in soils with low P content. AMF colonisation is suppressed in soil with high P availability as the cost of the symbiosis to the plant outweighs the benefit of access to P via the fungal pathway, and plants reduce fungal access to carbohydrate (Gosling et al. 2013; Kahiluoto et al. 2001; Thingstrup et al. 1998). There is considerable interest in harnessing AMF in low-input and organic agricultural systems to promote plant P supply whilst reducing fertiliser usage (Gosling et al. 2006). This is particularly relevant at the current time as emphasis is being placed on sustainable intensification of agricultural systems.
Plant species have been shown to differ in their responsiveness to AMF (Klironomos 2003; Tawaraya 2003). Following colonisation by AMF, some species exhibit major growth benefits whereas others show major reductions in growth. This is often measured in terms of mycorrhizal dependency (MD) which is calculated using the following equation: (dry weight inoculated plant (AM) − dry weight control plant (NM)) / AM × 100 (Plenchette et al. 1983). Alternatively, it is expressed as mycorrhizal responsiveness (MR) which uses the same equation with the exception of replacing the denominator with dry weight of control plant (Baon et al. 1993). These dimensionless ratios indicate weight differences between AMF inoculated and non-inoculated plants as a percentage of either the inoculated (MD) or non-inoculated plant (MR). Differences in physiology between plant species have been shown to have a great influence on MD (Tawaraya 2003). Root dry weight (citrus), root length (hardwood) and root fibrousness (citrus) have all been shown to be negatively correlated with responsiveness to AMF (Graham and Syvertsen 1985; Menge et al. 1978; Pope et al. 1983). Length and density of root hairs are also negatively correlated with MD (Jasper and Davy 1993). Cultivated plant species generally have a lower MD than wild plant species (Klironomos 2003; Tawaraya 2003). Studies on barley have also shown that MR is negatively correlated with P use efficiency (Baon et al. 1993). In the case of graminaceous crops, this is probably a result of breeders selecting against cultivars which rely on AMF and rather selecting those which perform well in the absence of AMF (i.e. in highly fertilised soils). Alliums tend to be more dependent on AMF than other cultivated plants due to their thick, sparsely branched roots and lack of root hairs (Brewster 2008), and genetic evidence has shown that modern onion breeding has not selected against response to AMF (Galván et al. 2011).
Major differences in responsiveness to AMF have been observed between genotypes within a plant species. Such differences have been documented in wheat, maize, marigold and onion (Hetrick and Wilson 1992; Linderman and Davis 2004; Ortas and Akpinar 2011; Powell et al. 1982). However, such studies are limited often only assessing a small range of cultivars and/or AMF species and therefore do not encompass sufficient diversity to make robust conclusions about cultivar interactions with AMF. It is thought that there is a strong genetic influence on the difference in responsiveness to AMF and AMF colonisation (Linderman and Davis 2004). As with interspecific differences, morphological differences between genotypes have been shown to have a great influence on response to AMF (Tawaraya 2003). Recently, it has been suggested that MD and MR are not appropriate measures of the beneficial effects of inoculation with AMF as they result in a negative correlation between MD and plant weight in the non-mycorrhizal treatment (Galván et al. 2011; Sawers et al. 2010). Therefore, it is more appropriate, at least from a plant breeding perspective, to consider different indices for measuring mycorrhizal benefit. Two such indices, absolute responsiveness (R) and average performance (AP), have been proposed and used in studies on onion (Galván et al. 2011). R is calculated as dry weight inoculated plant − dry weight non-inoculated plant and AP is calculated as (dry weight inoculated plant + dry weight non-inoculated plant) / 2. AP may be the most appropriate index to use when assessing the value of genotypes to plant breeders as it would select for yield stability in conditions where AMF populations may vary (Galván et al. 2011).
The effect of different AMF species on plant growth is highly dependent on the specific AMF/plant species combination (Klironomos 2003). It has been suggested that the symbiosis between a plant and an AMF can range between mutualism and parasitism (Klironomos 2003). However, recent evidence has shown that this is an over simplistic view. Phosphorus uptake by plants occurs through two distinct pathways, the direct pathway (which occurs without AMF) and the mycorrhizal pathway (Smith and Smith 2011). Until recently, it was thought that negative growth responses associated with AMF were due to the plant taking up sufficient P through the direct pathway, and thus, the carbon costs associated with maintaining the fungus were greater than the P gain (Bethlenfalvay et al. 1983). However, it has been shown that the mycorrhizal pathway makes a significant contribution to P uptake regardless of growth response (Smith et al. 2004). In addition, negative growth responses are not associated with excessive carbon cost as was initially predicted (Smith and Smith 2011). This new evidence refutes claims that AMF are parasitic and raises the question of whether the plant or the fungus controls the mutualism. When a mixed inoculum of AM species is used to inoculate plants, there appears to be some level of competition for carbon supply and a single species often comes to dominate (Jansa et al. 2008). New evidence has shown that the control of mycorrhizal symbiosis is bidirectional (Kiers et al. 2011). Plants can provide fungal partners that deliver more P with more carbohydrates, and in turn, fungi can increase the flow of P to those plants which provide more carbohydrates.
Phosphorous fertilisers not only are costly but also represent a finite resource and a potential source of environmental contamination. In addition, crops only take up a small amount of the P which is applied (Galván et al. 2011). Therefore, gaining a greater understanding of onion genotype/AMF interactions is important for selecting (and breeding) genotypes which respond better to AMF and thus require less P fertiliser. In addition to this, different AMF species may provide different nutrient benefits to the host plant. This was illustrated in spring onion where colonisation with Glomus etunicatum (recently renamed Rhizophagus clarus) leads to a much greater increase in total S concentration than colonisation with Glomus versiforme (Shen et al. 2011). However, the actual identity of these fungi is unclear as neither had a strain number or source, and it has been shown that G. versiforme has not been cultured since its original description (Schüßler et al. 2011). A change in S uptake may have an effect on pungency as the major flavour precursors in onion are the alk(en)yl cysteine sulphoxides (Griffiths et al. 2002). Sulphur-containing compounds also have an important role in plant defence, so increasing S uptake may increase resistance to biotic and abiotic stresses (Rausch and Wachter 2005). Additionally, in maize, different AMF species have been shown to have a wide range of effects on Zn uptake (Ortas and Akpinar 2011).
The aim of this study was to elucidate the role of onion genotype in determining growth and nutritional responsiveness to AMF and the degree to which this is influenced by different AMF species. Furthermore, we investigated how selection of AMF species in the root zone is influenced by onion genotype and the potential benefit of a mixed AMF inoculum. This is the most in-depth study of onion/AMF interactions to date, using a wide range of both AMF species and onion cultivars.
Materials and methods
Plant and fungal material
Ten onion accessions (referred to as genotypes for this study, Table 1) were chosen from a diversity set of 96 accessions held in the Genetic Resources Unit at the University of Warwick. The ten genotypes were chosen to maximise diversity of geographical origin and morphological characteristics including bulb colour, shape and size. Seeds were sown in sand/terragreen (50/50, 20 % moisture), covered and placed in a glasshouse at 20/15 °C day/night for 2 weeks. Five AMF isolates were selected from a collection held at the University of Warwick. These isolates were selected to represent diversity from across the Glomeromycota. Funneliformis mosseae (BEG12), Rhizophagus manihotis (FL879) and Rhizophagus irregularis (BEG144) were selected from the Glomeraceae, whilst Diversispora epigaea (BEG47) and Acaulospora spinosa (WV861A) were selected from the Diversisporales (Redecker et al. 2013). AMF isolates were sub-cultured in 1-l pots using existing in-house stock cultures with sand/terragreen (S/T; 1:1, v/v) substrate moistened with 25 % Rorison’s nutrient solution (Hewitt 1966) with one-tenth strength phosphorus. Plantago lanceolata (ribwort plantain) was used as the host plant and cultures maintained in a growth chamber at 18 °C with a 10-h photoperiod (150 μmol m−1 s−1) for 6 months. The substrate of all AMF cultures was checked by microscopic examination for the presence of viable propagules including spores and mycelium.
Plant-fungus bioassays
A sandy-loam soil with low available P (5 mg l−1 analysed according to Olsen et al. 1954) and a P index of 0 was collected from Ryton Organic Gardens, Coventry, UK. The soil had a pH of 6.9, a K concentration of 146 mg l−1 and an Mg concentration of 145 mg l−1. Soil was passed through a 3–4-mm sieve and sterilised by γ-irradiation at 10–35 kGy using a cobalt-60 source (Isotron Ltd, Daventry, UK). The soil was inoculated with individual AMF species or a combination of all five species. Inoculum consisted of S/T substrate containing propagules and chopped P. lanceolata roots colonised by the fungus. The soil/inoculum mixture for each pot was prepared by mixing 900 g irradiated soil with 25 g AMF S/T culture. In the case of combined treatments, 5 g of each of the five AMF S/T cultures was mixed thoroughly then mixed with 900 g of irradiated soil. All pots received 10 ml of a soil filtrate obtained by filtering non-irradiated field soil through a 38-μm sieve to provide a resident microbial population free of other AMF propagules (Koide and Li 1989). Control pots also received 25 g twice-autoclaved mixed inoculum. Deionised water was added to adjust soil to 50 % water holding capacity (WHC).
Two-week-old onion seedlings of the ten genotypes were transplanted into the soil/inoculum mixture. The surface of the growing substrate was covered with a layer of sterilised perlite to reduce moisture loss and individual pots sealed in plastic Sunbags (Sigma Life Science, Poole, Dorset) to reduce the risk of cross-contamination of AMF (Walker and Vestberg 1994). Per genotype, four replicates (2 plants per pot) were used for each of the five individual AMF treatments and eight replicates for the combined treatments and controls. Pots were placed in a glasshouse and randomised using a split plot design with plot nested within blocks, replicates and bench. The glasshouse was maintained at 20 °C/15 °C day/night, and the soil was kept at approximately 50 % WHC by weighing pots and watering with deionised water on a bi-weekly basis. Sunbags were removed after 7 weeks once onion shoots started to outgrow the bags.
Harvest protocol
The experiment was destructively harvested after 10 weeks and the number of green leaves recorded. The root mass was carefully removed from soil by washing under running tap water then rinsed thoroughly with deionised water. Roots were blotted dry and root lengths chopped into 1–2-cm pieces. Twenty to thirty random root sections were selected and stored in 20 % ethanol at 4 °C for assessing root colonisation. Approximately 200–300 mg of roots from mixed treatments and controls were stored at −20 °C for DNA extraction and analysis of root colonisation by AMF species using terminal restriction fragment length polymorphism (T-RFLP). Shoots and remaining roots were dried for 2 days at 80 °C and dry weights recorded. Mycorrhizal dependency (MD), mycorrhizal responsiveness (MR), absolute responsiveness (R) and average performance (AP) were calculated using the shoot dry weight data as described by (Baon et al. 1993; Galván et al. 2011; Plenchette et al. 1983).
Analyses of shoots and roots for nutrient content
Sub-samples (50 to 300 mg) of dried shoots were subjected to microwave-assisted nitric acid digestion and macro and trace elements (P, Ca, K, Mg, Na, S, Cu, Fe, Mn and Zn) quantified by an inductively coupled plasma optical emission spectrometer (ICP-OES; HORIBA Jobin-Yvon, France). Total N and C contents were determined via combustion of shoot samples (~50 to 300 mg) using a LECO CN 2000 analyser (LECO Corporation, USA).
A Kjeldahl digest was carried out with approximately 100 mg root material and macro elements (Ca, K, Mg, P and Na) quantified by ICP-OES. Root N content was determined by flow injection analysis (FOSS Analytical, Sweden). Percentage data was obtained, angular transformations carried out for N, Ca, K, P and S and log base e transformations were performed for Na, Cu, Fe, Mn and Zn.
Assessment of mycorrhizal colonisation
Root samples were cleared with 10 % KOH, acidified in 2 % HCl and stained with a solution of aniline blue (0.05 %) in 70 % glycerol (Grace and Stribley 1991). Ten stained root lengths were mounted in 70 % glycerol and assayed for mycorrhizal colonisation using a slide-intersect method adapted from that of McGonigle et al. 1990. The presence/absence of a mycorrhizal structure (arbuscule, coil, hypha, spore or vesicle) observed along ten intersects for each of the ten root lengths was recorded to give an estimation of percentage root length colonised.
Assessing genotype differences following inoculation with a mixture of AMF species
Terminal restriction fragment length polymorphism was used for assessing AMF colonisation in roots from mixed community treatments. This data was used to estimate how onion genotype affected the relative abundance of each AMF species. DNA was extracted from 200 to 300 mg root material using a Fast DNA Spin Kit for soil (MP Biomedicals). PCR was performed using 100 ng DNA and the AMF-specific 18S rRNA gene primer pair AML1 (labelled with the fluorescent dye 6-carboxyfluorescein) and unlabelled AML2 (Lee et al. 2008). PCR reactions were set up in 40-μl volumes containing 1 unit of KOD Hot Start polymerase (Novagen), 1 μM each primer, 2 mM MgSO4 and 0.2 μM each dNTP. Reactions were carried out using a 2-min initial denaturation at 95 °C followed by 35 cycles of denaturation at 95 °C for 20 s, annealing at 57 °C for 10 s and extension at 70 °C for 1 min followed by a final 4-min extension at 70 °C. PCR products were run on a 1 % agarose gel with 1× TBE at 100 V and visualised with Gel Red nucleic acid stain (Cambridge Bioscience). Products were purified using a QIAGEN (Crawley, UK) QIAquick PCR purification kit. T-RFLP digests were carried out in 10 μl containing 225 ng purified PCR product, 0.5 μl Hpy188I (New England Biolabs) and 1 μl 10× NEB Buffer 4. Reactions were incubated at 37 °C for 4 h and the enzyme was denatured by incubating at 95 °C for 15 min. T-RFLP analysis was performed in an Applied Biosciences 3130X1 automated capillary sequencer (Applied Biosystems, Foster City, CA, USA) using 1 μl of digested DNA and LIZ1200 as an internal size lane standard (Applied Biosystems). GeneMarker v1.5 (Soft Genetics, State College, PA, USA) was used to analyse the terminal restriction fragment (TRF) profiles and TRFs below 40 fluorescence units were excluded. The method differentiated each of the fungal species except D. epigaea/A. spinosa, which gave the same-sized TRF, although this was differentiated from TRF of the other species. The heights of peaks at 179, 385, 509.5 and 585 bp corresponding to R. irregularis, D. epigaea/A. spinosa, F. mosseae and R. manihotis, respectively, were recorded and used to estimate relative abundance of each AM fungus in mixed treatment root samples.
Statistical analysis
Statistical analyses were carried out using GenStat 12th edition (VSN International, Hemel Hempstead, UK). Analysis of variance (ANOVA) was carried out to assess the significance of the effect of onion genotype, AMF species and their interaction on plant growth and nutrient uptake. Missing values were present where plants did not survive until the end of the experiment or did not produce sufficient biomass to analyse. The significance of differences between individual means was assessed using LSD values (5 % level). Transformed data for each nutrient was analysed in Genstat using principal component analyses (PCAs). This allowed for the comparison of overall fingerprints for both data sets. Differences between genotypes in terms of T-RFLP profile (based on percentage of total peak height) following inoculation with a mixed community were assessed using ANOVA.
Results
Plant growth parameters
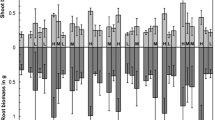
Inoculation with AMF led to a significant increase in shoot dry weight (Table 2/Fig. A1, P < 0.05). For example, Bedfordshire Champion (BC) plants inoculated with F. mosseae produced an average shoot dry weight of 2.14 g compared to 0.26 g in the non-inoculated controls. All the genotypes tested showed significant growth effects (Table 2, P < 0.001), and ANOVA showed that there were significant differences between the onion genotypes (P < 0.001). MD, MR, R and AP in terms of shoot dry weight were calculated for each onion genotype × AMF species combination. MD and MR were found to be significantly correlated (r = −0.56 to −0.91, P < 0.001) with shoot dry weight in the absence of AMF but not normally correlated with shoot dry weight in the presence of AMF, so they were considered not useful (Table 3). Both R (r = 0.90–1.0, P < 0.001) and AP (r = 0.73–0.92, P < 0.001) were significantly correlated with dry weight in the inoculated treatment (Table 3). In addition, both were correlated with shoot dry weight in the non-inoculated treatments with AP showing the strongest correlations. In terms of both R and AP, the best performing genotypes were White Ebenezer (WE) and Owa (OW) (Fig. 1). This was consistent across the different AMF species. The worst performing genotypes were Morada de Amposta (MA) and Candy F1 (CF1). This is in stark contrast to the data for MD which would rank MA and CF1 as two of the most responsive genotypes.
Average performance (AP, white bars) and absolute responsiveness (R, black bars) and of ten onion genotypes following inoculation with five individual AMF species or a mixture of all five. a F. mosseae; b R. manihotis; c D. epigaea; d R. irregularis; e A. spinosa; f Mixture. Error bars represent the SEM of four replicates
All the AMF species produced significant growth effects (Table 2, P < 0.05). Inoculation with F. mosseae, R. manihotis, D. epigaea and the mixed community significantly increased shoot dry weight in all the onion genotypes. Inoculation with A. spinosa only significantly increased shoot dry weight in the genotypes White Lisbon (WL), CF1, OW, Creamgold (CG), MA and Pukekohe Longkeeper (PL) with non-significant weight increases seen in the other four genotypes. There were significant differences between the effects of AMF species (P < 0.001) with F. mosseae producing the strongest growth effect and A. spinosa the weakest. However, no onion genotype × AMF interaction was observed (P = 0.319). Inoculation with a mixed community (consisting of all five AMF species) did not increase the shoot weight by a greater amount than the strongest individual species.
Inoculation with AMF also had a significant effect on root dry weight (P < 0.05, Table A1). Significant differences between onion genotypes (P = 0.001) and AMF species (P < 0.001) were observed. An onion genotype × AMF interaction was also observed (P = 0.012) suggesting that genotypes respond differently to AMF species in terms of root weight. Inoculation with F. mosseae had the strongest stimulation of root dry weight. In contrast, inoculation with A. spinosa only led to a significant increase in root dry weight in the genotypes OW and MA. The data followed a broadly similar pattern to the shoot dry weight data. Inoculation with a mixed community did not increase root growth by a greater degree than the strongest individual species.
Inoculation with AMF also had a significant effect on the number of green leaves (P < 0.05, see Table A2). Significant differences between genotypes (P = 0.001) and AMF species (P = 0.001) were seen, but no genotype × AMF interaction was observed (P = 0.565). Significant increases in the number of green leaves occurred with almost all of the onion genotype/AMF species combinations. In comparison with the shoot and root dry weight data, the differences between species were relatively small, varying from a mean of 7.60 with R. irregularis to 8.85 with D. epigaea. Although this is only a difference of one leaf, it is likely to have a significant effect on yield and/or harvest date. Overall, control plants produced four to five leaves per pot, whereas inoculated plants produced eight to nine leaves per pot.
Plant nutrient composition
Owing to the large volume of data, PCAs were carried out on shoot and root nutrient data to elucidate genotype and fungal effects on overall nutrient composition. In the shoots, two principal components accounted for 54 % of the total variation (35 % for PC1 and 19 % for PC2). It was clear that inoculation with any AMF species altered the nutrient profile of the plant, regardless of onion genotype (Fig. 2a). The lowest effect was seen when plants were inoculated with A. spinosa, which had a nutrient profile closer to that of the controls. Inoculation with all other species, including the mixture, produced very similar nutrient profiles. However, the differences between AMF species were shown to be significant for both PC1 (P < 0.001) and PC2 (P < 0.001). This was mainly due to the different profiles produced by A. spinosa. There were subtle, but significant, differences between the genotypes with White Lisbon producing a noticeably different profile. The differences between genotypes were shown to be significant in terms of both PC1 (P < 0.001) and PC2 (P < 0.001). A significant genotype × AMF interaction was observed for PC2 (P < 0.001) but not PC1 (P = 0.288). However, PC2 only accounted for 19 % of the overall variation so onion genotype × AMF interactions were not particularly influential.
Principal component analysis of onion nutrient composition following inoculation with five individual AMF species or a mixture of all five. a Shoot nutrient PCA; b root nutrient PCA. Orange—control, green—D. epigaea, black—G. mosseae, blue—R. irregularis, turquoise—A. spinosa, pink—mixture, red—R. manihotis. 1 BC, 2 RF, 3 WE, 4 GR, 5 OW, 6 CG, 7 CF1, 8 MA, 9 PL, 10 WL
In terms of root nutrient profiles, the two principal components accounted for 65 % of the total variation (45 % for PC1 and 20 % for PC2, Fig. 2b). The root data followed a similar pattern to the shoot data with significant differences between AMF species for both PC1 (P < 0.001) and PC2 (P = 0.002). Inoculation with A. spinosa or R. irregularis produced a nutrient profile closer to that of the controls. Inoculation with any of the other species had a similar effect and resulted in a significantly different profile. Differences between onion genotypes were observed for PC1 (P = 0.002) but not PC2 (P = 0.294). A significant interaction was observed for PC1 (P < 0.001) but not PC2 (P = 0.081). As PC1 accounted for 45 % of the overall variation, the presence of an interaction here is important.
Inoculation with AMF led to changes in the concentrations of many of the nutrients tested. The major changes in shoot nutrient content are summarised in Table 4. Significant increases in the concentrations of N, P and Cu were observed (P < 0.05). In contrast, significant decreases in the Ca, K, Na, Fe, Mn and Zn concentrations were seen (P < 0.05). The concentrations of P were significantly higher in plants from the mixed inoculum treatment than any other treatment. However, there was no significant difference in total shoot P between the mixture and the best individual species, F. mosseae (Table 4). Significant decreases in S concentrations (relative to non-inoculated controls) were observed when plants were inoculated with all AMF species with the exception of A. spinosa, which caused a significant increase in S relative to non-inoculated control plants (P < 0.05). In addition, no significant decrease in K was observed with A. spinosa inoculation. These differences contribute to the different PCA profiles observed in Fig. 2. Differences in nutrient profile between onion genotypes are summarised in Table A3.
AMF colonisation
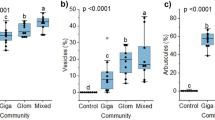
Mycorrhizal structures were observed in all the different onion/AMF combinations. Significant differences in percentage colonisation (angular transformed data) were observed between genotypes (P = 0.020, Fig. 3a). The highest amount of colonisation was observed in OW (43.1 %), whereas the lowest colonisation was observed in MA (33.6 %). Significant differences between AMF species were also observed (P < 0.001, Fig. 3b). The highest percentage colonisation was observed with R. manihotis (57.7 %), whereas the lowest was observed with A. spinosa (38.2 %). No onion/AMF interaction was observed (P = 0.359). For inoculated plants, a weak (but significant) correlation between shoot dry weight and percentage colonisation was observed (Fig. 3c, r = 0.53, P < 0.001). However, this is mainly due to the influence of the A. spinosa data, and when this is removed, the correlation is not significant (r = 0.25). No significant correlations were observed when the AM species were correlated individually (data not shown). No correlations were observed between percentage colonisation and any plant growth or nutrient uptake parameter (data not shown).
Composition of root AMF communities in plants inoculated with a five-species fungus mixture
The proportion of each AMF species in the roots of the onion plants was estimated using T-RFLP. This allowed direct comparison of genotypes. The primers led to efficient amplification from all the AMF species (Fig. A2). All AMF species were detected in the roots of all the onion genotypes. In terms of percentage of total peak height for each AMF species (Fig. 4), significant differences between the genotypes were observed for R. irregularis (P = 0.017), R. manihotis (P = 0.006), F. mosseae (P = 0.005) and A. spinosa/D. epigaea (same peak, P = 0.007). The largest difference was seen in the genotype BC which had a larger proportion of R. manihotis and R. irregularis and a lower proportion of F. mosseae in its roots when compared to the other genotypes. A summary of all ANOVAs can be found in Table 5.
Discussion
In terms of growth and nutritional responses to AMF, significant differences were observed between the genotypes tested. Such differences in growth response have been previously reported to exist between plant species and genotypes (Klironomos 2003; Tawaraya 2003). However, many studies use MD or MR to compare genotypes, indices which suffer from the unavoidable negative correlation between responsiveness and control plant weight (Galván et al. 2011; Sawers et al. 2010). From our data, it appears that AP is the most appropriate index for measuring the performance of a genotype in terms of biomass production. Our data indicate that there is potential to breed onion cultivars with increased responsiveness to AMF and that the genotypes WE and OW have potential as sources of beneficial alleles for breeding cultivars for sustainable onion production. Both perform well in the presence or absence of AM in a low-nutrient soil. The precise reasons for the differences between genotypes are unclear. Weak but significant positive correlations between control plant root dry weight for both R (r = 0.33, P < 0.05) and AP (r = 0.51, P < 0.001) could partly explain the differences, and this has been suggested previously (Menge et al. 1978). However, this cannot fully explain the differences. Differences may also be related to different nutrient use efficiencies, as was found to be the case for barley where a negative correlation between P use efficiency and MD was observed (Baon et al. 1993). Alternatively, there may be differences in other traits which have a pleiotropic effect on AMF colonisation, e.g. root morphology. Our future work will aim to identify genetic loci specifically related to differences in R and AP.
Significant differences between the AMF species were observed with F. mosseae producing the strongest growth effects. The growth effects of F. mosseae on onion have been previously documented (Khan 1988; Vosátka 1995). However, this is the first report that identifies F. mosseae as the most beneficial symbiont for onion. A previous study on onion tested a range of AMF species in non-sterilised soil found that R. irregularis (formerly called Glomus intraradices) had a larger effect on bulb weight and diameter than F. mosseae (Vosátka 1995). However, this study used an uncharacterised isolate of R. irregularis so it is difficult to compare with our work. Other work on onion failed to use individual AMF species, so cannot be directly compared to our study (Powell et al. 1982). It should be noted that there may be genetic variation within F. mosseae, and it would be interesting to assess the impact this may have. Inoculation with A. spinosa produced the smallest growth enhancement. The growth-promoting effects of A. spinosa have been less widely reported than other AMF, although it has been shown to promote growth in rice and white yam (Ammani and Rao 1996; Tchabi et al. 2010). Inoculation with A. spinosa did promote growth in this trial, but to a lesser extent than inoculation with the other AMF species. The large growth responses observed in this trial were expected as the trial was carried out in low P soil, conditions which favour plant-AMF interactions (Gosling et al. 2006).
Although some combinations of genotypes/AMF species have been previously investigated in other crops such as maize (Ortas and Akpinar 2011), the question of a genotype × AMF interaction has remained unanswered. Our study included a large number of genotype × AMF combinations, allowing for the possibility of an interaction between onion genotype and AMF species to be investigated using a robust statistical analysis. Previously, it had been suggested that an interaction may exist leading to the suggestion of specific fungus selection by onion host genotypes (Powell et al. 1982). However, this work utilised a limited number of genotypes and no clearly defined AMF species making it difficult to robustly assess the presence of a genotype × AMF interaction. Our data suggests that no interaction exists (P = 0.319) in terms of the shoot dry weight and number of green leaves (P = 0.565). There was also no significant interaction for shoot nutrient PC1 (P = 0.288). Although a significant interaction was observed for shoot nutrient PC2, this PC only accounted for 19 % of the overall variation. This suggests a general growth response to inoculation and that different plant genotypes respond to AMF species in the same way refuting the suggestion of a high level of specificity in host-fungus selection (Powell et al. 1982). This is an important discovery in terms of onion breeding for sustainable production systems as it means breeders can select for general responsiveness to AMF. An interaction was observed for root dry weight (P = 0.012) and the major root nutrient PC (P < 0.001). Although these interactions clearly exist in the roots, they do not appear to affect the growth of above ground parts of the plant.
Inoculation with AMF changed the composition of most plant nutrients. Such changes have been documented in several plant species including onion (Charron et al. 2001). However, no previous study has investigated such a comprehensive range of macro- and micro-nutrients. Our results suggest that different AMF species have similar effects on the overall nutrient profile, with the exception of A. spinosa. This data supports the theory that different AMF species may have effects on different nutrients. It may then be possible to promote crop growth in a field soil with a low concentration of a particular nutrient via inoculation of a specific AMF species. We observed significant increases in N content following inoculation, consistent with the finding that soils that are rich in AMF have a higher level of available N (Negrete-Yankelevich et al. 2013). A significant decrease in onion S content was observed when plants were inoculated with all species except A. spinosa (which led to a significant increase). This is the first report of a decrease in onion sulphur content following inoculation. Increases in shoot S concentration following mycorrhizal inoculation have been previously reported in bulb onion (Guo et al. 2006) and also spring onion (Shen et al. 2011). A change in S uptake may have an effect on pungency as the major flavour precursors in onion are the alk(en)yl cysteine sulphoxides (Griffiths et al. 2002). It may be possible to select AMF to produce a desired pungency of onion. In addition to this, the downstream products of ASCO biosynthesis have been shown to have health-enhancing properties such as anticarcinogenic and antiplatelet activity (Griffiths et al. 2002). It may be possible to select AMF that increase the concentrations of such compounds thus producing an onion with added value. Sulphur compounds also have an important role in plant defence (Rausch and Wachter 2005), so it may be possible to select AMF species that help protect plants against biotic and abiotic stresses. It should be noted however that there may be a trade-off between S uptake for a desired pungency and for plant defence. When plants were inoculated with a mixture of AMF species, the observed decrease in S induced by A. spinosa was negated and an increase in S (compared to controls) was observed. This illustrates the importance of selecting a suitable AMF species for the conditions.
There is a great degree of disparity in the literature regarding the possible beneficial effect of a mixed AMF inoculum for plant growth. Some research has suggested that inoculation with a mixed community of AMF species could potentially be more beneficial than use of a single isolate (Jansa et al. 2008; Maherali and Klironomos 2007), whereas other research suggest no additive effect (Daft 1983; Gavito and Varela 1995; Janoušková et al. 2009). No previous work has tested the effect of a mixed inoculum in a range of plant genotypes. We tested ten diverse genotypes and found that there was no increased benefit of a mixed inoculum. For all ten genotypes tested, inoculation with a mixed community never produced a stronger growth effect than the best individual inoculant (Table 2). There was also no extra increase in total shoot P content (Table 4). Other work has shown that increasing species richness leads to higher levels of available P, but this was on a whole field scale using maize as a model (Negrete-Yankelevich et al. 2013). It has been suggested that the lack of an additive effect of mixed inoculations is due to one species becoming dominant in a mixed pot culture (Jansa et al. 2008). However, this was not the case in our tests as root AMF community profiling showed that a mixture of species was frequently detected. There was however no disadvantage of a mixed inoculum. Again, this is an important finding for breeding, reinforcing the suggestion of a general compatibility between onion and AMF rather than a specific interaction that would be influenced by competition between AMF species.
T-RFLP was used to compare the AMF composition of onion roots following inoculation with a mixed community. This allowed the direct comparison of fungal-genotype specificity, and significant differences were observed. Most notably, BC roots contained a higher proportion of R. irregularis/R. manihotis and a lower proportion of F. mosseae compared to the other genotypes. These differences were not due to an incompatibility between F. mosseae and BC as this species colonised the roots of this genotype well in the single inocula pots (49.4 % mean colonisation, higher than WL, CF1 and MA). It therefore appears that there was a process of selection occurring. It may be the plant or the fungus (or both) which drives the selection process. Recent work has suggested that both the fungus and the plant have a role in controlling the mutualism, and symbionts offering the greatest nutrient transfer are preferentially rewarded with C (Kiers et al. 2011). The only limitation of the T-RFLP was that differences between AMF species could not be quantitatively compared (due to possible differences in PCR efficiency, these appear to be small differences (Fig. A2) but may still be significant). However, this does not affect the direct comparison of genotypes.
Our study is the most in-depth study of onion/AMF interactions to date, using a wide range of both AMF species and onion cultivars. Understanding the dynamics of the symbiosis between plants and AMF is important if AMF are to be exploited in the development and implementation of more sustainable, low-input systems which maintain yield. Growing a more responsive genotype could lead to reduced fertiliser inputs, this could be enhanced if the fungal community in a field can be manipulated. In addition to a general increase in yield, it may be possible to select an AMF species which is efficient in delivering a certain nutrient (in addition to P) thus decreasing the need for extra fertilisers and/or enhancing crop quality through changes in traits such as pungency or nutrient content.
References
Ammani K, Rao AS (1996) Effect of two arbuscular mycorrhizal fungi Acaulospora spinosa and A. scrobiculata on upland rice varieties. Microbiol Res 151:235–237
Augé RM, Duan X, Ebel RC, Stodola AJW (1994) Nonhydraulic signalling of soil drying in mycorrhizal maize. Planta 193:74–82. doi:10.1007/bf00191609
Baon JB, Smith SE, Alston AM (1993) Mycorrhizal responses of barley cultivars differing in P efficiency. Plant Soil 157:97–105. doi:10.1007/bf00038752
Bethlenfalvay GJ, Bayne HG, Pacovsky RS (1983) Parasitic and mutualistic associations between a mycorrhizal fungus and soybean: the effect of phosphorus on host plant-endophyte interactions. Physiol Plant 57:543–548. doi:10.1111/j.1399-3054.1983.tb02783.x
Brewster JL (2008) Onions and other vegetable alliums. Crop production in horticulture, 2 edn. CAB International, Wallingford
Charron, Furlan, Bernier C, Doyon (2001) Response of onion plants to arbuscular mycorrhizae. Mycorrhiza 11:145–150. doi:10.1007/s005720100122
Daft M (1983) The influence of mixed inocula on endomycorrhizal development. Plant Soil 71:331–337
Galván G, Kuyper T, Burger K, Keizer L, Hoekstra R, Kik C, Scholten O (2011) Genetic analysis of the interaction between Allium species and arbuscular mycorrhizal fungi. Theor Appl Genet 122:947–960. doi:10.1007/s00122-010-1501-8
Gavito ME, Varela L (1995) Response of “criollo” maize to single and mixed species inocula of arbuscular mycorrhizal fungi. Plant Soil 176:101–105. doi:10.1007/bf00017680
Gosling P, Hodge A, Goodlass G, Bending GD (2006) Arbuscular mycorrhizal fungi and organic farming. Agric Ecosyst Environ 113:17–35. doi:10.1016/j.agee.2005.09.009
Gosling P, Mead A, Proctor M, Hammond JP, Bending GD (2013) Contrasting arbuscular mycorrhizal communities colonizing different host plants show a similar response to a soil phosphorus concentration gradient. New Phytol 198:546–556. doi:10.1111/nph.12169
Grace C, Stribley DP (1991) A safer procedure for routine staining of vesicular-arbuscular mycorrhizal fungi. Mycol Res 95:1160–1162. doi:10.1016/s0953-7562(09)80005-1
Graham JH, Syvertsen JP (1985) Host determinants of mycorrhizal dependency of citrus rootstock seedlings. New Phytol 101:667–676. doi:10.1111/j.1469-8137.1985.tb02872.x
Griffiths G, Trueman L, Crowther T, Thomas B, Smith B (2002) Onions—a global benefit to health. Phytother Res 16:603–615
Guo T, Zhang J, Christie P, Li X (2006) Effects of arbuscular mycorrhizal fungi and ammonium: nitrate ratios on growth and pungency of onion seedlings. J Plant Nutr 29:1047–1059
Hetrick BAD, Wilson GWT (1992) Mycorrhizal dependence of modern wheat varieties, landraces, and ancestors. Can J Bot 70:2032–2040
Hewitt EJ (1966) Sand and water culture methods used in the study of plant nutrition, 2nd edition. Commonwealth Agricultural Bureaux, technical communication no. 22, East Malling, Kent
Janoušková M, Seddas P, Mrnka L, van Tuinen D, Dvořáčková A, Tollot M, Gianinazzi-Pearson V, Vosátka M, Gollotte A (2009) Development and activity of Glomus intraradices as affected by co-existence with Glomus claroideum in one root system. Mycorrhiza 19:393–402. doi:10.1007/s00572-009-0243-4
Jansa J, Smith FA, Smith SE (2008) Are there benefits of simultaneous root colonization by different arbuscular mycorrhizal fungi? New Phytol 177:779–789. doi:10.1111/j.1469-8137.2007.02294.x
Jasper DA, Davy JA (1993) Root characteristics of native plant species in relation to the benefit of mycorrhizal colonization for phosphorus uptake. Plant Soil 155–156:281–284. doi:10.1007/BF00025037
Kahiluoto H, Ketoja E, Vestberg M, Saarela I (2001) Promotion of AM utilization through reduced P fertilization 2 field studies. Plant Soil 231:65–79. doi:10.1023/a:1010366400009
Khan AG (1988) Inoculum density of Glomus mosseae and growth of onion plants in unsterilized bituminous coal spoil. Soil Biol Biochem 20:749–753. doi:10.1016/0038-0717(88)90162-9
Kiers ET, Duhamel M, Beesetty Y, Mensah JA, Franken O, Verbruggen E, Fellbaum CR, Kowalchuk GA, Hart MM, Bago A, Palmer TM, West SA, Vandenkoornhuyse P, Jansa J, Bücking H (2011) Reciprocal rewards stabilize cooperation in the mycorrhizal symbiosis. Science 333:880–882. doi:10.1126/science.1208473
Klironomos JN (2003) Variation in plant response to native and exotic arbuscular mycorrhizal fungi. Ecology 84:2292–2301. doi:10.1890/02-0413
Koide RT, Li M (1989) Appropriate controls for vesicular–arbuscular mycorrhiza research. New Phytol 111:35–44. doi:10.1111/j.1469-8137.1989.tb04215.x
Lee J, Lee S, Young JPW (2008) Improved PCR primers for the detection and identification of arbuscular mycorrhizal fungi. FEMS Microbiol Ecol 65:339–349. doi:10.1111/j.1574-6941.2008.00531.x
Linderman RG, Davis EA (2004) Varied response of marigold (Tagetes spp.) genotypes to inoculation with different arbuscular mycorrhizal fungi. Sci Hortic 99:67–78. doi:10.1016/s0304-4238(03)00081-5
Maherali H, Klironomos JN (2007) Influence of phylogeny on fungal community assembly and ecosystem functioning. Science 316:1746–1748. doi:10.1126/science.1143082
McGonigle TP, Miller MH, Evans DG, Fairchild GL, Swan JA (1990) A new method which gives an objective measure of colonization of roots by vesicular–arbuscular mycorrhizal fungi. New Phytol 115:495–501. doi:10.1111/j.1469-8137.1990.tb00476.x
Menge JA, Johnson ELV, Platt RG (1978) Mycorrhizal dependency of several citrus cultivars under three nutrient regimes. New Phytol 81:553–559. doi:10.1111/j.1469-8137.1978.tb01628.x
Morgan JAW, Bending GD, White PJ (2005) Biological costs and benefits to plant–microbe interactions in the rhizosphere. J Exp Bot 56:1729–1739. doi:10.1093/jxb/eri205
Negrete-Yankelevich S, Maldonado-Mendoza I, Lázaro-Castellanos J, Sangabriel-Conde W, Martínez-Álvarez J (2013) Arbuscular mycorrhizal root colonization and soil P availability are positively related to agrodiversity in Mexican maize polycultures. Biol Fertil Soils 49:201–212. doi:10.1007/s00374-012-0710-5
Newsham KK, Fitter AH, Watkinson AR (1995) Multi-functionality and biodiversity in arbuscular mycorrhizas. Trends Ecol Evol 10:407–411. doi:10.1016/s0169-5347(00)89157-0
Olsen S, Cole C, Watanabe F, Dean L (1954) Estimation of available phosphorous in soils by extraction with sodium carbonate US Department of Agriculture Circular no. 939
Ortas I, Akpinar Ç (2011) Response of maize genotypes to several mycorrhizal inoculums in terms of plant growth, nutrient uptake and spore production. J Plant Nutr 34:970–987. doi:10.1080/01904167.2011.555580
Plenchette C, Fortin JA, Furlan V (1983) Growth-responses of several plant-species to mycorrhizae in a soil of moderate P-fertility. 1 mycorrhizal dependency under field conditions. Plant Soil 70:199–209. doi:10.1007/bf02374780
Pope PE, Chaney WR, Rhodes JD, Woodhead SH (1983) The mycorrhizal dependency of four hardwood tree species. Can J Bot 61:412–417. doi:10.1139/b83-048
Powell CL, Clark GE, Verberne NL (1982) Growth response of four onion cultivars to several isolates of VA mycorrhizal fungi. N Z J Agr Res 25:465–470. doi:10.1080/00288233.1982.10417914
Rausch T, Wachter A (2005) Sulfur metabolism: a versatile platform for launching defence operations. Trends Plant Sci 10:503–509. doi:10.1016/j.tplants.2005.08.006
Redecker D, Schussler A, Stockinger H, Sturmer SL, Morton JB, Walker C (2013) An evidence-based consensus for the classification of arbuscular mycorrhizal fungi (Glomeromycota). Mycorrhiza 23:515–531. doi:10.1007/s00572-013-0486-y
Sawers RH, Gebreselassie M, Janos D, Paszkowski U (2010) Characterizing variation in mycorrhiza effect among diverse plant varieties. Theor Appl Genet 120:1029–1039. doi:10.1007/s00122-009-1231-y
Schüßler A, Krüger M, Walker C (2011) Revealing natural relationships among arbuscular mycorrhizal fungi: culture line BEG47 represents Diversispora epigaea, not Glomus versiforme. PLoS ONE 6:e23333. doi:10.1371/journal.pone.0023333
Schüβler A, Schwarzott D, Walker C (2001) A new fungal phylum, the Glomeromycota: phylogeny and evolution. Mycol Res 105:1413–1421. doi:10.1017/S0953756201005196
Shen H, Yang H, Guo T (2011) Influence of arbuscular mycorrhizal fungi and ammonium:nitrate ratios on growth and pungency of spring onion plants. J Plant Nutr 34:743–752
Smith SE, Smith FA (2011) Roles of arbuscular mycorrhizas in plant nutrition and growth: new paradigms from cellular to ecosystem scales. Annu Rev Plant Biol 62:227–250. doi:10.1146/annurev-arplant-042110-103846
Smith SE, Smith FA, Jakobsen I (2004) Functional diversity in arbuscular mycorrhizal (AM) symbioses: the contribution of the mycorrhizal P uptake pathway is not correlated with mycorrhizal responses in growth or total P uptake. New Phytol 162:511–524. doi:10.1111/j.1469-8137.2004.01039.x
Tawaraya K (2003) Arbuscular mycorrhizal dependency of different plant species and cultivars. Soil Sci Plant Nutr 49:655–668. doi:10.1080/00380768.2003.10410323
Tchabi A, Coyne D, Hountondji F, Lawouin L, Wiemken A, Oehl F (2010) Efficacy of indigenous arbuscular mycorrhizal fungi for promoting white yam (Dioscorea rotundata) growth in West Africa. Appl Soil Ecol 45:92–100. doi:10.1016/j.apsoil.2010.03.001
Thingstrup I, Rubaek G, Sibbesen E, Jakobsen I (1998) Flax (Linum usitatissimum L.) depends on arbuscular mycorrhizal fungi for growth and P uptake at intermediate but not high soil P levels in the field. Plant Soil 203:37–46. doi:10.1023/a:1004362310788
Vosátka M (1995) Influence of inoculation with arbuscular mycorrhizal fungi on the growth and mycorrhizal infection of transplanted onion. Agric Ecosyst Environ 53:151–159. doi:10.1016/0167-8809(94)00563-T
Walker C, Vestberg M (1994) A simple and inexpensive method for producing and maintaining closed pot cultures of arbuscular mycorrhizal fungi. Agric Sci Finl 3:233–240
Acknowledgments
We thank Chris Walker (Royal Botanic Garden, Edinburgh) for checking the purity of our AMF cultures as well as Andrew Jukes, Matthew Mitchell and Joan Yurkwich (Warwick Crop Centre) for carrying out the nutrient analyses and the Warwick Crop Centre Horticultural Services team for their help with plant-fungus bioassay.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Taylor, A., Pereira, N., Thomas, B. et al. Growth and nutritional responses to arbuscular mycorrhizal fungi are dependent on onion genotype and fungal species. Biol Fertil Soils 51, 801–813 (2015). https://doi.org/10.1007/s00374-015-1027-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00374-015-1027-y