Abstract
The fish gill is a multifunctional organ that is important in multiple physiological processes such as gas transfer, ionoregulation, and chemoreception. This characteristic organ of fishes has received much attention, yet an often-overlooked point is that larval fishes in most cases do not have a fully developed gill, and thus larval gills do not function identically as adult gills. In addition, large changes associated with gas exchange and ionoregulation happen in gills during the larval phase, leading to the oxygen and ionoregulatory hypotheses examining the environmental constraint that resulted in the evolution of gills. This review thus focuses exclusively on the larval fish gill of teleosts, summarizing the development of teleost larval fish gills and its function in gas transfer, ionoregulation, and chemoreception, and comparing and contrasting it to adult gills where applicable, while providing some insight into the oxygen vs ionoregulatory hypotheses debate.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The gill is a highly characteristic organ of fishes and crucial for their aquatic lifestyle. Agnathans have pouch-like gills that open through pores, but gills of all other fish species have arch-like structures situated on both sides of the pharynx with regularly spaced filaments (primary lamellae) projecting to the side and backwards from the arches. The filaments support the presence of secondary lamellae, or simply lamellae, which are semicircular or triangular-shaped plate-like structures running perpendicular to the filament’s long axis, greatly enhancing the surface area of the gill. Teleost fish in general possesses four pairs of gill arches spaced between five pairs of branchial slits, which allows for water to flow across the gills. Elasmobranch gills differ slightly, having an extra hemibranch on the anterior side of the first branchial slit. In adults of exclusively water-breathing fish, arguably the most crucial function of the gill is for gas transfer, particularly oxygen. Yet gas transfer is not the sole function of the gill; other functions include chemoreception, osmoregulation, acid–base balance, excretion of nitrogenous waste, hormone metabolism, and immune responses (Hughes 1984; Olson 1998; Dos Santos et al. 2001; Wilson and Laurent 2002; Evans et al. 2005; Perry et al. 2009, 2023; Zachar and Jonz 2012a).
Despite the wealth of information on adult fish gills, gills of larval fish are less well studied, reflected by the more than 16,000 “hits” when searching with the keywords fish AND gill on PubMed with only ~3000 of these “hits” containing the keywords larva, larvae, embryo, or ontogeny. This could in part be attributed to the fact that many species do not hatch with a fully functional gill. Larval gills in general are similar in morphology to adult gills, though all structures of the larval gill are underdeveloped. An exception to this would be elasmobranchs, which transition from external gill filaments to internal gills during embryonic development and hatch with fully developed gills (Pelster and Bemis Willian 1992; Gillis and Tidswell 2017). Additionally, larvae may rely predominantly on cutaneous respiration for oxygen uptake in early stages, questioning the main function of gills in larval fish. Thus in this review, I focus exclusively on larval fish gills, and more specifically larval gills of teleost fish, and attempt to synthesize previous works examining their structure and function. For the clarity of this review, larval stage will be defined here as the stage up to complete yolk sac absorption.
Structure of larval fish gills
Gill ontogeny
Differentiation of the gills begins early in development soon after gastrulation. The foregut endoderm iteratively evaginates in a rostral-to-caudal sequence, giving rise to a series of pharyngeal pouches that subsequently contact and fuse with overlying surface ectoderm, resulting in the perforation of pharyngeal slits and the delineation of pharyngeal arches (Shone and Graham 2014; Gillis and Tidswell 2017). The first (mandibular) pharyngeal arch develops into the jaw and pseudobranch (Thiruppathy et al. 2022; Hirschberger and Gillis 2022), whereas the second (hyoid) arch acts as a supporting structure for the jaws (Gillis et al. 2009a, b), giving rise to a filament bearing arch only in elasmobranchs (Wilson and Laurent 2002). Pharyngeal arches 3–7 give rise to the 5 branchial arches (branchial arches 1–5). The 4 anterior branchial arches will later support gill filaments whereas no filaments will develop on the 5th and last branchial arch (Schilling et al. 1996; Gillis et al. 2009a, b). Following the delineation of the branchial arches, primary branchial vessels arise from the ventral aorta and pass around the pharynx to connect with the dorsal aorta, before differentiating into afferent and efferent branchial arteries (Morgan 1974; Hughes 1984).
The molecular mechanism for branchial arch formation requires a complex interplay between transcription factors and signaling molecules (Fig. 1). The transcription factor paired box 1 (pax1) is expressed in the pharyngeal pouch-forming cells and has been implicated as a key factor of pharyngeal arch development (Liu et al. 2013, 2020; Okada et al. 2016). Pax1 acts as an upstream integrator to modulate expression of fibroblast growth factors (fgfs) and T-box transcription factors (tbx) that are themselves potent regulators of cell proliferation and differentiation. Fish lacking the expression of Fgf3 (David et al. 2002; Walshe and Mason 2003; Herzog et al. 2004; Crump et al. 2004), Fgf8 (Crump et al. 2004), Fgf receptor 1 (Leerberg et al. 2019), Fgf receptor-like 1 (Hall et al. 2006), Tbx1 (Piotrowski et al. 2003), and to a lesser extent Fgf4 (Jackman et al. 2004) all show defects in branchial arch formation. The underlying mechanism is that both Fgfs and Tbx1 can regulate hand2 and dlx genes that specify major patterning domains along the dorsoventral axis of pharyngeal arches (Talbot et al. 2010). This can be either through direct action on the dlx genes, such as dlx2a, or indirectly by acting on endothelin 1 (edn1) that can modulate hand2 and dlx3b/5a/6a (Liu et al. 2020). Regardless of the regulation pathway, loss of edn1, hand2 or dlx genes all result in the malformation of pharyngeal arches (Miller et al. 2003; Walker et al. 2006; Sperber et al. 2008; Talbot et al. 2010; Sasaki et al. 2013). In addition to Pax1 mediated signaling pathways, parathyroid hormone-related peptides (PTHrP) (Yan et al. 2012; Kwong and Perry 2015) and Wnt signaling, specifically Wnt11r and Wnt4a (Choe et al. 2013; Jin et al. 2018), are also important in pharyngeal pouch formation, and Wnt signaling seems to be in part mediated by Tbx1 (Choe and Crump 2014).
Following the formation of the brachial arches, filament folds begin to form midway along the branchial arch, which expand dorsally and ventrally over a period of growth (Morgan 1974). At the same time, blood vessels in the shape of a loop that connect the existing afferent and efferent branchial arteries form within the filament folds and a unidirectional flow of blood from afferent to efferent branchial arteries via the loop vessels is established (Morgan 1974; Hughes 1984; González et al. 1996). The budding of gill filaments requires the transcription factor glial cells missing 2 (Gcm2) (Hogan et al. 2004). Compared to branchial arch formation, filament budding is poorly examined at the molecular level though bone morphogenetic factors (BMPs), fibroblast growth factors (Fgfs), Notch signalling pathways, and Sonic Hedgehog (SHH) proteins likely are involved as they have been shown to be important in the regeneration of gill filaments following resection (Cadiz et al. 2023) (Fig. 1). Until recently, there has been much debate as to which germ layer gives rise to filaments in fish. The “ecto-endobranchiate hypothesis” suggests that gills in jawless and jawed fish evolved independently and thus arise from distinct embryonic epithelia (endoderm vs ectoderm, respectively) (Gillis and Tidswell 2017), through recent evidence is against this hypothesis. The endoderm origin of gills in jawless fish such as lamprey and hagfish has been well documented for decades through classical morphology studies of the gills (Stockard 1906; Damas 1944). However, more recent lineage tracing studies in both elasmobranchs and teleosts have shown that gill filaments also arise from the endodermal domains of the branchial arch, suggesting that gills evolved only once in fish (Warga and Nüsslein-Volhard 1999; Gillis and Tidswell 2017).
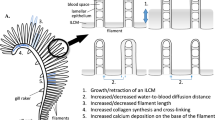
Formation of gill lamellae begins soon after the appearance of filaments, though variations in the timing do exist (Table 1). New lamellae are added towards the tips of the filaments during the entire period of growth and each lamella is comprised of a vascular core covered by a thin epithelium on either side, with pillar cells that are uniquely found in fish gills lining the lamellar blood space (Wilson and Laurent 2002). Lamellae appear first as ovoid surfaces that have a relatively thick water-blood barrier that gradually transition to a thinner triangular shaped structure, with the apex pointing toward the efferent filament artery (Hughes 1984). Blood flow direction in the lamellae is opposite to that of the water flowing through the gills, facilitating gas transfer through a counter current mechanism (van Dam 1938). In general, following the appearance and development of the lamellae, the larval gill, albeit smaller, is morphologically similar to the adult gill. Despite the importance of lamellae in facilitating gas transfer in fish, the underlying mechanisms that mediate lamellae formation are unknown.
Morphological plasticity of larval gills
The gills of adult fish show a remarkable degree of plasticity in response to osmorespiratory challenges. When oxygen availability decreases in hypoxic environments or when oxygen demand increases with exercise or high temperature, fish can increase the functional surface area of the gill to aid in oxygen uptake (Sollid and Nilsson 2006; Nilsson 2007; Nilsson et al. 2012; Gilmour and Perry 2018). Additionally, the gill functional surface area may be altered in the face of ionoregulatory challenges (Blair et al. 2017; Gilmour and Perry 2018; Giacomin et al. 2019). These examples of plasticity likely stem from the conflicting requirements for effective gas transfer and ion regulation. In the classic paper examining the osmorespiratory compromise (Randall et al. 1972), it was stated “conditions in the gills must be the result of continual compromise between the minimal rate of gas exchange required and the maximum rate of ion and water transfer that can be tolerated”. Gills can be modified either through reorganizing blood flow pathways such as flow redistribution within the lamellae, flow shunting between “respiratory” and “non-respiratory” pathways in the filament, and recruitment of distal lamellae, or through “reversible gill remodeling” (Sollid and Nilsson 2006) by utilizing the proliferation of the interlamellar gill cell mass, the proliferation of ionocytes up the sides of the lamellae, and by covering over the apical exposure of ionocytes by extension of pavement cells (See review by Wood and Eom 2021 for details).
Unlike in adults, current evidence suggests that the gills of larval fish are limited in terms of plasticity. When reared under hypoxic conditions, larvae of Arctic char and the blue gourami (Trichopodus trichopterus) did not show any changes in functional gill surface area compared to those reared under normoxic conditions (McDonald and McMahon 1977; Mendez-Sanchez and Burggren 2019). The surface area of gills in larval Siamese fighting fish (Betta splendens) did not change under mild hypoxia and only decreased under the most severe of hypoxic conditions, where the fish were also in poor physical conditions following this level of hypoxia treatment, complicating the interpretation of plasticity (Mendez-Sanchez and Burggren 2019). Similarly, no differences in the number and structural appearance of gill ionocytes were observed for larval rainbow trout (Oncorhynchus mykiss) reared from hatch to yolk sac absorption under varying salinities. (Shen and Leatherland 1978). Based on the current evidence available from the limited number of studies on a limited number of fish species, the overall conclusion is that gill plasticity in larval fish facing both oxygen and ionoregulatory challenges is limited. Whether this holds true requires more studies in a variety of larval fish species. The reasons for the seemingly absence of morphological plasticity in larval gills are unknown, although it may be that the larval gills are developing at near-maximum rates and thus do not have additional energetic scope for plasticity, especially to balance the reciprocal energetic costs on gas and ion fluxes when either is enhanced (Sackville and Brauner 2018). This in itself is another interesting topic worth examining.
Function of larval fish gills
Gas transfer
It is generally accepted that larval fish initially rely on cutaneous and not branchial respiration. There is also evidence suggesting that larval fish as a whole is a functional analog of a gill lamella serving as a countercurrent flow device for oxygen uptake, as flow of water across the surface of the larval fish is countercurrent to that of the blood flow through the cutaneous vasculature (Liem 1981; Zimmer et al. 2020). Measurements of oxygen levels in blood vessels leading towards and away from the gills in rainbow trout larvae just after hatch and mid-yolk absorption revealed that blood oxygen levels were lower in the vessels leading away from the gills, implying that at this stage the gill is more of a sink for oxygen rather than as a source for the rest of the body (Rombough 1992). However regardless of whether the gills are an oxygen sink or source, direct measurements of oxygen levels of inspired, buccal cavity, and expired water in zebrafish (Danio rerio) larvae show that at least the buccal cavity is capable of oxygen uptake (Pan et al. 2019). Functional gill ablation experiments that prevent ventilation in larval zebrafish also show that under normoxic conditions, cutaneous gas transfer alone is sufficient to meet oxygen demands up until 14 days post fertilization (dpf) (Rombough 2002), though branchial gas transfer is required to maintain oxygen demands under hypoxic conditions as early as 7 dpf (Pan et al. 2019). In studies with salmonid and Pacific lamprey (Entosphenus tridentatus) larvae, where ventilatory flow over the gills is physically separated from water flow over the rest of the body, i.e. placing the larva in a divided chamber where the head and the rest of the body is separated, at least 20% of oxygen uptake was accounted for by gills at hatch and gradually increased during develop reaching 50% of total uptake by mid-yolk absorption and 70–80% by the end of yolk absorption (Rombough and Ure 1991; Wells and Pinder 1996a; Rombough 1998; Fu et al. 2010; Zimmer et al. 2014; Sackville et al. 2022). However, the larvae that were used for these partitioning studies are relatively large compared to other fish larvae, with larval salmonids reaching the size of adult zebrafish. Whether data obtained from these partitioning studies can be extrapolated to most fish larvae would require further examination, which would be technically challenging due to the small larval sizes of most species.
To my knowledge, there is but a single study examining CO2 excretion in the gills of larval fish. In that study (Sackville et al. 2022) it was found that CO2 excretion by the gills of Pacific lamprey larvae accounted for ~30% of total CO2 excretion, a similar percentage as oxygen uptake at larval gills. One can perhaps find clues regarding CO2 excretion by examining larval amphibians, which also possess gills yet rely predominantly on cutaneous respiration. In the American bullfrog (Lithobates catesbeianus), gills contribute 40% of total oxygen uptake and CO2 excretion in the strictly aquatic stage tadpole, suggesting that as in larval Pacific lamprey, the relative contribution of the gill to CO2 excretion and O2 uptake are similar (Burggren and West 1982; Burggren 1984).
Ionoregulation
Ionoregulation at the gills is facilitated, in part, by specialized cells termed ionocytes that are often enriched with mitochondria. These ionocytes are further classified into different subtypes with distinct structures and functions that coordinate together for ionoregulation. This topic has been extensively reviewed in recent years (Bartels and Potter 2004; Hwang et al. 2011; Dymowska et al. 2012; Hiroi and McCormick 2012; Zimmer and Perry 2022; Shih et al. 2023; Tresguerres et al. 2023) and will again be reviewed in this special issue, and thus no further details will be provided here.
Despite the wealth of information on ionocyte subtypes on either the skin or adult gills, there is little information on the structure and function of ionocyte subtypes in larval fish gills. It is interesting, however, that most ionocytes are located on the filaments (Li et al. 1995; Schreiber and Specker 1999; Pisam et al. 2000; Katoh et al. 2000; Prescott et al. 2021) and thus first appear around the emergence of gill filaments, earlier than the appearance of lamellae that are important for gas transfer (Table 1). The distribution pattern of ionocytes between the gill and cutaneous surfaces is altered during development such that the relatively low percentage of ionocytes on the gills in larvae gradually increases with the gill becoming the predominant location of ionocytes in adults (Li et al. 1995; Rombough 1999).
The importance of larval gills in ionoregulation appears to vary with species. Gill ablation experiments suggest that zebrafish larvae can rely solely on cutaneous ionocytes until 7 dpf, after which branchial ionocytes are required for ionoregulation (Rombough 2002), coinciding with the first appearance of ionocytes on the gill filaments at 5 dpf (Jonz and Nurse 2006). Partitioning studies have also shown that in rainbow trout, the underlying pattern for Na+ uptake was similar to that for oxygen, where branchial uptake accounts for only a low percentage of Na+ uptake at hatch and gradually increases through development until the gills become the predominant site for Na+ uptake (Fu et al. 2010; Zimmer et al. 2014; Zimmer and Wood 2015). A similar trend is also observed for ammonia excretion, where there is low branchial excretion of ammonia at hatch followed by a gradual increase with development (Zimmer et al. 2014; Zimmer and Wood 2015), which may reflect the fact that sodium uptake and ammonia excretion is a coupled process in freshwater fish (Zimmer et al. 2017). Similarly, larval fish gills likely play a role in acid–base regulation, as the branchial uptake of Na+ is linked to acid excretion either directly via Na+/H+ exchange or indirectly via the activity of H+-ATPase, and Cl− uptake at the gill is coupled to base excretion via Cl−/HCO3− exchange (Zimmer and Perry 2022). Gills seem to play an even greater role in ionoregulation in larval Pacific lamprey, as 100% of Na+ and Ca2+ uptake occurred at the gills, and there was no evidence for a shift from the skin (Sackville et al. 2022).
The oxygen vs ionoregulatory hypotheses
Gills are theorized to first appear in stem vertebrates, originating from the filter-feeding organs that are present in vertebrate ancestors which are small worm-like organisms that relied on the skin for breathing and ionoregulation (Gans and Northcutt 1983; Glenn Northcutt 2005). The oxygen hypothesis, popularized by August Krogh (1941), posits that at some point, the ability of the skin to supply the growing organism with oxygen becomes limiting and would lead to hypoxemia were it not for the development of the gills, implying oxygen uptake was the main driver for gill formation. The underlying reasoning is that body surface area (A) of a larva is roughly proportional to length squared, while its oxygen consumption rate (MO2) is proportional to the body volume, and thus length cubed. Therefore, as animals grow, the A:MO2 ratio tends to decline in proportion to body mass2/3 (Rubner 1883), resulting in restricted surface area for oxygen uptake to maintain increasing oxygen demands. Gills on the other hand do not face the same anatomical constraints as the skin and thus do not have to scale with length squared, providing an extra surface area for gas transfer to facilitate the growing oxygen demands in the larva. The ionoregulatory hypothesis proposed by Li et al. (1995) is different from the one we understand today. Based on the morphological observation that ionocytes on the filament develop prior to gill lamellae, Li et al. (1995) proposed that larval fish gills form a functional ionoregulatory organ before they start functioning as a gas-exchange organ. However, a more recent version of the ionoregulatory hypothesis suggests that evolution of the gills was primarily driven by constraints associated with ion regulation rather than oxygen uptake (Rombough 2007; Brauner and Rombough 2012; Sackville and Brauner 2018; Sackville et al. 2022). The two versions of the ionoregulatory hypothesis are not mutually exclusive and each is interesting in its own right. For clarity and to be consistent with the idea of the oxygen hypothesis, all mentions of the ionoregulatory hypothesis hence forth will refer to the idea that evolution of the gills was primarily associated with the constraints of ion regulation.
Although the ionoregulatory hypothesis has gained traction in recent years (Sackville et al. 2022), it remains an unproven “hypothesis” that requires further testing. There are currently three main arguments used to support the ionoregulatory hypothesis: (1) larval gills serve as a sink rather than a source for oxygen, (2) transition of ionoregulation from skin to gills occurs before that of oxygen uptake, and (3) gills of pre-vertebrate origin participate only in ion regulation and not oxygen uptake.
The first argument mainly stems from the fact that in larval rainbow trout, blood leaving the gills is lower in oxygen content compared to blood entering the gills (Rombough 1992). This result in itself only shows that gills are utilizing oxygen and cannot be used to discount that the gills are also taking up oxygen from inspired water, which is likely true at least in the case of larval zebrafish where expired water is lower in oxygen content compared to inspired water (Pan et al. 2019). More importantly, whether larval gills serve as a sink or source for oxygen cannot serve as an indicator of which function came first. Gills may have first appeared to uptake oxygen, and as ionoregulation appeared on the gills as well, the aerobic cost of ionoregulation resulted in the gills becoming a sink for oxygen. It could also be plausible that ionoregulation appeared first in the gills, and the aerobic cost of ionoregulation is what drove oxygen uptake in the gills.
The second argument that the transition of ionoregulation from skin to gills happens before that of oxygen uptake can be further broken down into three separate points:
-
a.
Ionocytes appear, with a few exceptions on larval gills before the functional oxygen uptake unit, the lamella (see Table 1). The caveat in this statement pertains to the assumption that the lamellar and filament epithelia are distinct and non-overlapping sites of oxygen uptake and ion exchanges, respectively (Girard and Payan 1980). Although in adult fish, the lamella is indeed the primary functional unit of oxygen uptake, this may not be the situation in the larval fish gill. In especially the early larval fish gill, lamellae are few and sparse in between, yet filaments are much better developed. The harmonic mean water-to-blood diffusion distance in larval Atlantic salmon gill filaments is 10.8–14 μm compared to 20 μm for the cutaneous epithelium and 14 μm for the yolk sac epithelium at hatch (Wells and Pinder 1996b). In addition, the filaments of larval zebrafish gills are vascularized (Mandic et al. 2020). Based on these, there is no reason why gill filaments alone could not serve as functional oxygen uptake units in the larval fish gill. With this assumption and because ionocytes and filaments usually appear around the same time during development (Table 1), the functional oxygen uptake units and the ionoregulatory cells appear around the same time.
-
b.
Functional gill ablation experiments indicate that gills are required for ionoregulation well before they become absolutely necessary for oxygen uptake. This conclusion arose from examining larval zebrafish that were prevented from ventilating (Rombough 2002). At 7 dpf, immersion in physiological saline but not hyperoxic water significantly improved survival, whereas both physiological saline and hyperoxia significantly improved survival in 14 dpf larvae, suggesting that cutaneous exchange alone was sufficient to meet the ionoregulatory requirements of larvae until about 7 dpf and the oxygen demand until 14 dpf. However, subsequent studies have shown that these findings apply only to normoxic conditions. Under hypoxic conditions, larval zebrafish require the participation of branchial respiration to meet oxygen demands (Pan et al. 2019). The same type of functional gill ablation study has also been carried out in rainbow trout, and findings suggest that cutaneous oxygen uptake and ion exchange become limiting at about the same time in rainbow trout (Rombough 2007). Thus, it remains unclear whether cutaneous ion exchange becomes limiting before cutaneous oxygen uptake.
-
c.
Cutaneous ion exchange transitions to gills before cutaneous oxygen uptake. There is unequivocal evidence from partitioning studies that larval gills take on the role of ion uptake before oxygen uptake (Fu et al. 2010; Zimmer et al. 2014; Zimmer and Wood 2015; Sackville et al. 2022). However, the only conclusion that can be drawn from this is that larval gills play a more important role in ion exchange compared to oxygen uptake but cannot discount the role of larval gills in oxygen uptake.
It should not go unnoticed that the first two arguments and the associated evidence support the original idea that Li et al. (1995) proposed in which larval fish gills form a functional ionoregulatory organ before they start functioning as a respiratory organ. However, whether these arguments can be used to support the current ionoregulatory hypothesis is questionable because an organ’s primary function does not have to be the function it was selected for during evolution. This is essentially the definition of an exaptation, where traits may have arisen not as the result of any benefit they provided at the time selection acted but as the result of their coexpression with traits that were acted on directly by the environment (Gould and Vrba 1982). Thus, looking at the oxygen vs ionoregulatory hypothesis debate from an evolutionary perspective, another way to frame the question is that between oxygen uptake and ionoregulation, which is an adaptation and which is an exaptation for gills. In other words, is it possible that ionic regulation arose as an exaptation following gill evolution that first occurred as an adaptation for oxygen uptake?
The third argument for the ionoregulatory hypothesis relates to the gills of pre-vertebrate origin participating only in ion movements and not oxygen uptake (Sackville et al. 2022). Although supportive of the ionoregulatory hypothesis, the study of Sackville et al. (2022) was limited in that while showing that gills of pre-vertebrate origin did not participate in oxygen uptake, no evidence was presented that pre-vertebrate gills participate in ion regulation; such experiments should be a focus of future studies.
Chemoreception
Chemoreception is the process by which organisms respond to environmental chemical stimuli. Of the various sensory stimuli, oxygen has received the greatest attention and thus oxygen chemoreception has been reviewed extensively in recent years (Pan and Perry 2020, 2023; Kameda 2021; Reed and Jonz 2022; Perry et al. 2023) including an up-to-date synthesis in this special issue on the fish gill (ref to follow). Thus only a brief overview is provided here with a focus on the role of larval gills. In adult fish, neuroepithelial cells (NECs) present at the tips of gill filaments are generally accepted to be the oxygen chemoreceptor cells because NECs isolated from the gill and tested in vitro were found to be oxygen sensitive (Jonz et al. 2004; Burleson et al. 2006; Zachar and Jonz 2012b). However, while compelling, there is a lack of direct evidence for this claim. A chemoreceptor cell is defined by its intrinsic sensitivity to physiologically relevant changes in the chemical it is sensing, enabling cellular signalling responses in vivo to facilitate appropriate downstream reflexes. With respect to NECs, their intrinsic capability to respond to changes in oxygen was elegantly demonstrated in isolated NECs in vitro (Jonz et al. 2004; Burleson et al. 2006; Zachar and Jonz 2012b). What is lacking, however, is evidence for their oxygen sensitivity in vivo and a direct link between NEC activity and the modulation of ventilation. For a detailed discussion on this topic, see the commentary by Pan and Perry (2023). Thus, until further evidence is acquired, NECs should be considered as putative oxygen chemoreceptor cells.
In the gills of larval zebrafish, NECs are not innervated until 7 dpf (Jonz and Nurse 2005) and thus may be unable to serve as oxygen chemoreceptor cells prior to this developmental age. Yet, robust ventilatory responses already occur in 4 dpf zebrafish larvae exposed to hypoxia (Jonz and Nurse 2005; Pan et al. 2019, 2021, 2023). Because peripheral sensory neurons of 4 dpf zebrafish innervating the branchial region also respond to hypoxia in vivo (Pan et al. 2023), it is likely that the ventilatory responses in these early larvae arise from the activation of branchial oxygen chemoreceptor cells linked to the peripheral sensory neurons. A possible candidate is the Merkel-like cell (MLC), which is a basal serotonergic cell sitting below taste receptor cells forming the taste bud complex. These MLCs in larval zebrafish are activated by the chemical hypoxia mimic, sodium cyanide, in vivo (Pan et al. 2023). In addition, MLCs are also activated by hypoxia in vivo and can transmit subsequent signals to the central nervous system via peripheral sensory neurons to drive the hypoxic ventilatory response (Pan 2021). Thus, MLCs fulfill the criteria of oxygen chemoreceptor cells in being sensitive to hypoxia in vivo and able to drive downstream reflexes induced by hypoxia.
Chemoreception of other respiratory gases such as CO2 and NH3 has received much less attention compared to oxygen. Although there is evidence that isolated NECs from adult gill filaments are responsive to CO2 and NH3 (Qin et al. 2010; Zhang et al. 2011; Porteus et al. 2021), there is no direct in vivo evidence that NECs function as CO2 or NH3 chemoreceptors. Even less is known about CO2 and NH3 sensing in the gills of larval fish, though both CO2 (Kunert et al. 2022) and NH3 (Porteus et al. 2021) are able to elicit ventilatory responses in larval fish, suggesting the presence of CO2 and NH3 chemoreceptor cells in larval fish.
In addition to respiratory gas chemoreception, chemoreception of taste molecules also occurs in larval fish gills. Taste buds in fish are onion-shaped structures composed of taste receptor cells (TRCs) sitting on top of a basal MLC and are found on the lips, orobranchial cavity, and gill arch of larvae (Kapsimali et al. 2011; Zachar and Jonz 2012c). Formation of TRCs require the expression of Fgf8a that drives Delta-Notch signaling and subsequently miR-200 activity in order to promote taste bud cell type differentiation (Kapsimali et al. 2011; Soulika et al. 2016). Zebrafish TRCs express dedicated G protein-coupled receptors as taste receptors for detecting tastants and express components of the PLCβ2-signaling stream for the transduction of taste information (Ishimaru et al. 2005; Aihara et al. 2007; Yoshida et al. 2007). Histological studies have confirmed the presence of distinct cell types expressing specific combinations of taste receptors, though the identities of all TRCs within a single taste bud are not fully known (Ishimaru et al. 2005; Beppu et al. 2022). The specific tastants of TRCs have also not been characterized in larval fish, but adult fish are known to be responsive to various amino acids and bitter compounds, and this likely will translate to TRCs on larval gills as well (Oike et al. 2007). The field of taste reception in fish as a whole is understudied and much-needed future studies are likely to yield exciting new discoveries.
Conclusions and perspectives
Larval fish gills provide adequate reasons to examine the structure and function of them on their own. What are the underlying mechanisms driving the development of lamellae? To what extent are larval fish gills plastic to environmental changes? What cell types patriciate in O2, CO2, and NH3 chemoreception? How do larval gills taste different compounds? And ultimately, what is the environmental constraint that drove the evolution of fish gills? All these are interesting questions that await an answer, and in this era of genetic modification (Zimmer et al. 2019; Pan and Perry 2023) and omics-based research (Melzner et al. 2022), techniques such as CRISPR/Cas9 knock out can be used to identify novel transcription factors that drive gill development, transcriptomic approaches can be used to probe plasticity, and in vivo calcium imaging approaches can be used identify novel chemoreceptors. Even more tools required to answer these questions are being developed and ready to be utilized.
Data availability
No datasets were generated or analysed during the current study.
References
Aihara Y, Yasuoka A, Yoshida Y et al (2007) Transgenic labeling of taste receptor cells in model fish under the control of the 5′-upstream region of medaka phospholipase C-beta 2 gene. Gene Expr Patterns 7:149–157. https://doi.org/10.1016/j.modgep.2006.06.004
Bartels H, Potter IC (2004) Cellular composition and ultrastructure of the gill epithelium of larval and adult lampreys: Implications for osmoregulation in fresh and seawater. J Exp Biol 207:3447–3462. https://doi.org/10.1242/jeb.01157
Beppu K, Tsutsumi R, Ansai S et al (2022) Development of a screening system for agents that modulate taste receptor expression with the CRISPR-Cas9 system in medaka. Biochem Biophys Res Commun 601:65–72. https://doi.org/10.1016/j.bbrc.2022.02.082
Blair SD, Matheson D, Goss GG (2017) Physiological and morphological investigation of Arctic grayling (Thymallus arcticus) gill filaments with high salinity exposure and recovery. Conserv Physiol 5:cox040. https://doi.org/10.1093/conphys/cox040
Bodinier C, Boulo V, Lorin-Nebel C, Charmantier G (2009) Influence of salinity on the localization and expression of the CFTR chloride channel in the ionocytes of Dicentrarchus labrax during ontogeny. J Anat 214:318–329. https://doi.org/10.1111/j.1469-7580.2009.01050.x
Brauner CJ, Rombough PJ (2012) Ontogeny and paleophysiology of the gill: new insights from larval and air-breathing fish. Respir Physiol Neurobiol 184:293–300. https://doi.org/10.1016/j.resp.2012.07.011
Burggren W (1984) Transition of respiratory processes during amphibian metamorphosis: from egg to adult. In: Seymour RS (ed) Respiration and metabolism of embryonic vertebrates. Springer, Dordrecht, pp 31–53
Burggren WW, West NH (1982) Changing respiratory importance of gills, lungs and skin during metamorphosis in the bullfrog rana catesbeiana. Respir Physiol 47:151–164. https://doi.org/10.1016/0034-5687(82)90108-6
Burleson ML, Mercer SE, Wilk-Blaszczak MA (2006) Isolation and characterization of putative O2 chemoreceptor cells from the gills of channel catfish (Ictalurus punctatus). Brain Res 1092:100–107. https://doi.org/10.1016/j.brainres.2006.03.085
Cadiz L, Reed M, Monis S et al (2023) Identification of signalling pathways involved in gill regeneration in zebrafish. https://doi.org/10.1242/jeb.246290
Choe CP, Crump JG (2014) Tbx1 controls the morphogenesis of pharyngeal pouch epithelia through mesodermal Wnt11r and Fgf8a. Development 141:3583–3593. https://doi.org/10.1242/dev.111740
Choe CP, Collazo A, Trinh LA et al (2013) Wnt-dependent epithelial transitions drive pharyngeal pouch formation. Dev Cell 24:296–309. https://doi.org/10.1016/j.devcel.2012.12.003
Crump JG, Maves L, Lawson ND et al (2004) An essential role for Fgfs in endodermal pouch formation influences later craniofacial skeletal patterning. Development 131:5703–5716. https://doi.org/10.1242/dev.01444
Damas H (1944) Recherches sur le developpement de Lampetra fluviatilis L.—contribution a l’etude de la cephalogenese des vertebres. Arch Biol (paris) 55:1–289
David NB, Saint-Etienne L, Tsang M et al (2002) Requirement for endoderm and FGF3 in ventral head skeleton formation. Development 129:4457–4468. https://doi.org/10.1242/dev.129.19.4457
Dos Santos NMS, Taverne-Thiele JJ, Barnes AC et al (2001) The gill is a major organ for antibody secreting cell production following direct immersion of sea bass (Dicentrarchus labrax, L.) in a Photobacterium damselae ssp. piscicida bacterin: an ontogenetic study. Fish Shellfish Immunol 11:65–74. https://doi.org/10.1006/fsim.2000.0295
Dunaevskaya E (2010) Histological investigations of organs and tissues development of ballan wrasse larvae during ontogenesis. Master, Bodø University College
Dymowska AK, Hwang P-P, Goss GG (2012) Structure and function of ionocytes in the freshwater fish gill. Respir Physiol Neurobiol 184:282–292. https://doi.org/10.1016/j.resp.2012.08.025
El-Fiky N, Hinterleitner S, Wieser W (1987) Differentiation of swimming muscles and gills, and development of anaerobic power in the larvae of cyprinid fish (Pisces, Teleostei). Zoomorphology 107:126–132. https://doi.org/10.1007/BF00312122
Evans DH, Piermarini PM, Choe KP (2005) The multifunctional fish gill: dominant site of gas exchange, osmoregulation, acid-base regulation, and excretion of nitrogenous waste. Physiol Rev 85:97–177
Finnerty SH (2019) Tracking ontogenetic changes of ionocyte distribution and morphology in larval white seabass (Atractoscion nobilis). M.S., University of California, San Diego
Foscarini R (1989) A comparative study of the skin and gill structure in oviparous and viviparous freshwater fish larvae. J Fish Biol 34:31–40. https://doi.org/10.1111/j.1095-8649.1989.tb02955.x
Fu C, Wilson JM, Rombough PJ, Brauner CJ (2010) Ions first: Na+ uptake shifts from the skin to the gills before O2 uptake in developing rainbow trout, Oncorhynchus mykiss. Proc R Soc B Biol Sci 277:1553–1560. https://doi.org/10.1098/rspb.2009.1545
Gans C, Northcutt RG (1983) Neural crest and the origin of vertebrates: a new head. Science 220:268–273. https://doi.org/10.1126/science.220.4594.268
Gao X, Hong L, Liu Z et al (2016) An integrative study of larval organogenesis of American shad Alosa sapidissima in histological aspects. Chin J Ocean Limnol 34:136–152. https://doi.org/10.1007/s00343-016-5008-2
Giacomin M, Bryant HJ, Val AL et al (2019) The osmorespiratory compromise: physiological responses and tolerance to hypoxia are affected by salinity acclimation in the euryhaline Atlantic killifish (Fundulus heteroclitus). J Exp Biol 222:jeb206599. https://doi.org/10.1242/jeb.206599
Gillis JA, Tidswell ORA (2017) The origin of vertebrate gills. Curr Biol 27:729–732. https://doi.org/10.1016/j.cub.2017.01.022
Gillis JA, Dahn RD, Shubin NH (2009a) Chondrogenesis and homology of the visceral skeleton in the little skate, Leucoraja erinacea (Chondrichthyes: Batoidea). J Morphol 270:628–643. https://doi.org/10.1002/jmor.10710
Gillis JA, Dahn RD, Shubin NH (2009b) Shared developmental mechanisms pattern the vertebrate gill arch and paired fin skeletons. Proc Natl Acad Sci 106:5720–5724. https://doi.org/10.1073/pnas.0810959106
Gilmour KM, Perry SF (2018) Conflict and compromise: using reversible remodeling to manage competing physiological demands at the fish gill. Physiology 33:412–422. https://doi.org/10.1152/physiol.00031.2018
Girard JP, Payan P (1980) Ion exchanges through respiratory and chloride cells in freshwater- and seawater-adapted teleosteans. Am J Physiol Regul Integr Comp Physiol 238:R260–R268. https://doi.org/10.1152/ajpregu.1980.238.3.R260
Glenn Northcutt R (2005) The new head hypothesis revisited. J Exp Zool B Mol Dev Evol 304B:274–297. https://doi.org/10.1002/jez.b.21063
González ME, Blánquez MJ, Rojo C (1996) Early gill development in the rainbow trout, Oncorhynchus mykiss. J Morphol 229:201–217. https://doi.org/10.1002/(SICI)1097-4687(199608)229:2<201::AID-JMOR5>3.0.CO;2-3
Gould SJ, Vrba ES (1982) Exaptation—a missing term in the science of form. Paleobiology 8:4–15. https://doi.org/10.1017/S0094837300004310
Hachero-Cruzado I, Ortiz-Delgado JB, Borrega B et al (2009) Larval organogenesis of flatfish brill Scophthalmus rhombus L: histological and histochemical aspects. Aquaculture 286:138–149. https://doi.org/10.1016/j.aquaculture.2008.09.039
Hall C, Flores MV, Murison G et al (2006) An essential role for zebrafish Fgfrl1 during gill cartilage development. Mech Dev 123:925–940. https://doi.org/10.1016/j.mod.2006.08.006
Hamada K (1968) Development of a goby, [Chaenogobius urotaenia], with special reference to the gill and the chloride cell. Bull Fac Fish Hokkaido Univ 19:185–197
Herzog W, Sonntag C, von der Hardt S et al (2004) Fgf3 signaling from the ventral diencephalon is required for early specification and subsequent survival of the zebrafish adenohypophysis. Development 131:3681–3692. https://doi.org/10.1242/dev.01235
Hiroi J, McCormick SD (2012) New insights into gill ionocyte and ion transporter function in euryhaline and diadromous fish. Respir Physiol Neurobiol 184:257–268. https://doi.org/10.1016/j.resp.2012.07.019
Hiroi J, Kaneko T, Seikai T, Tanaka M (1998) Developmental sequence of chloride cells in the body skin and gills of Japanese flounder (Paralichthys olivaceus) larvae. jzoo 15:455–460. https://doi.org/10.2108/0289-0003(1998)15[455:DSOCCI]2.0.CO;2
Hirschberger C, Gillis JA (2022) The pseudobranch of jawed vertebrates is a mandibular arch-derived gill. Development 149:dev200184. https://doi.org/10.1242/dev.200184
Hogan BM, Hunter MP, Oates AC et al (2004) Zebrafish gcm2 is required for gill filament budding from pharyngeal ectoderm. Dev Biol 276:508–522. https://doi.org/10.1016/j.ydbio.2004.09.018
Huang C-Y, Lin C-H, Lin H-C (2015) Development of gas exchange and ion regulation in two species of air-breathing fish, Betta splendens and Macropodus opercularis. Comp Biochem Physiol a: Mol Integr Physiol 185:24–32. https://doi.org/10.1016/j.cbpa.2015.03.008
Hughes GM (1984) 1 general anatomy of the gills. In: Hoar WS, Randall DJ (eds) Fish physiology, vol 10, part A. Academic Press, pp 1–72
Hwang PP (1990) Salinity effects on development of chloride cells in the larvae of ayu (Plecoglossus altivelis). Mar Biol 107:1–7. https://doi.org/10.1007/BF01313236
Hwang P-P, Lee T-H, Lin L-Y (2011) Ion regulation in fish gills: recent progress in the cellular and molecular mechanisms. Am J Physiol Regul Integr Comp Physiol 301:R28–R47. https://doi.org/10.1152/ajpregu.00047.2011
Ishimaru Y, Okada S, Naito H et al (2005) Two families of candidate taste receptors in fishes. Mech Dev 122:1310–1321. https://doi.org/10.1016/j.mod.2005.07.005
Iwai T, Hughes GM (1977) Preliminary morphometric study on gill development in black sea bream (Acanthopagrus schlegeli). Nippon Suisan Gakkaishi 43:929–934. https://doi.org/10.2331/suisan.43.929
Jackman WR, Draper BW, Stock DW (2004) Fgf signaling is required for zebrafish tooth development. Dev Biol 274:139–157. https://doi.org/10.1016/j.ydbio.2004.07.003
Jin S, Jiyun O, Stellabotte F, Choe CP (2018) Foxi1 promotes late-stage pharyngeal pouch morphogenesis through ectodermal Wnt4a activation. Dev Biol 441:12–18. https://doi.org/10.1016/j.ydbio.2018.06.011
Jonz MG, Nurse CA (2005) Development of oxygen sensing in the gills of zebrafish. J Exp Biol 208:1537–1549. https://doi.org/10.1242/jeb.01564
Jonz MG, Nurse CA (2006) Epithelial mitochondria-rich cells and associated innervation in adult and developing zebrafish. J Comp Neurol 497:817–832. https://doi.org/10.1002/cne.21020
Jonz MG, Fearon IM, Nurse CA (2004) Neuroepithelial oxygen chemoreceptors of the zebrafish gill. J Physiol 560:737–752. https://doi.org/10.1113/jphysiol.2004.069294
Kameda Y (2021) Comparative morphological and molecular studies on the oxygen-chemoreceptive cells in the carotid body and fish gills. Cell Tissue Res 384:255–273. https://doi.org/10.1007/s00441-021-03421-y
Kapsimali M, Kaushik A-L, Gibon G et al (2011) Fgf signaling controls pharyngeal taste bud formation through miR-200 and Delta-Notch activity. Development 138:3473–3484. https://doi.org/10.1242/dev.058669
Katoh F, Shimizu A, Uchida K, Kaneko T (2000) Shift of chloride cell distribution during early life stages in seawater-adapted killifish. Fundulus Heteroclitus Jzoo 17:11–18. https://doi.org/10.2108/zsj.17.11
Krogh A (1941) The comparative physiology of respiratory mechanisms. University of Pennsylvania Press, Philadelphia
Kunert E, Joyce W, Pan YK et al (2022) Role of cytosolic carbonic anhydrase Ca17a in cardiorespiratory responses to CO2 in developing zebrafish (Danio rerio). Am J Physiol Regul Integr Comp Physiol 323:R532–R546. https://doi.org/10.1152/ajpregu.00050.2022
Kwan GT, Wexler JB, Wegner NC, Tresguerres M (2019) Ontogenetic changes in cutaneous and branchial ionocytes and morphology in yellowfin tuna (Thunnus albacares) larvae. J Comp Physiol B 189:81–95. https://doi.org/10.1007/s00360-018-1187-9
Kwong RWM, Perry SF (2015) An essential role for parathyroid hormone in gill formation and differentiation of ion-transporting cells in developing zebrafish. Endocrinology 156:2384–2394. https://doi.org/10.1210/en.2014-1968
Leerberg DM, Hopton RE, Draper BW (2019) Fibroblast growth factor receptors function redundantly during zebrafish embryonic development. Genetics 212:1301–1319. https://doi.org/10.1534/genetics.119.302345
Li J, Eygensteyn J, Lock RAC et al (1995) Branchial chloride cells in larvae and juveniles of freshwater tilapia Oreochromis mossambicus. J Exp Biol 198:2177–2184. https://doi.org/10.1242/jeb.198.10.2177
Liem KF (1981) Larvae of air-breathing fishes as countercurrent flow devices in hypoxic environments. Science 211:1177–1179. https://doi.org/10.1126/science.7466391
Liu X, Wang H, Li G et al (2013) The function of DrPax1b gene in the embryonic development of zebrafish. Genes Genet Syst 88:261–269. https://doi.org/10.1266/ggs.88.261
Liu Y-H, Lin T-C, Hwang S-PL (2020) Zebrafish Pax1a and Pax1b are required for pharyngeal pouch morphogenesis and ceratobranchial cartilage development. Mech Dev 161:103598. https://doi.org/10.1016/j.mod.2020.103598
Lorin-Nebel C, Boulo V, Bodinier C, Charmantier G (2006) The Na+/K+/2Cl- cotransporter in the sea bass Dicentrarchus labrax during ontogeny: involvement in osmoregulation. J Exp Biol 209:4908–4922. https://doi.org/10.1242/jeb.02591
Mandic M, Pan YK, Gilmour KM, Perry SF (2020) Relationships between the peak hypoxic ventilatory response and critical O2 tension in larval and adult zebrafish (Danio rerio). J Exp Biol 223:jeb213942. https://doi.org/10.1242/jeb.213942
McDonald DG, McMahon BR (1977) Respiratory development in Arctic char Salvelinus alpinus under conditions of normoxia and chronic hypoxia. Can J Zool 55:1461–1467. https://doi.org/10.1139/z77-189
Melzner F, Podbielski I, Mark FC, Tresguerres M (2022) The silent loss of cell physiology hampers marine biosciences. PLoS Biol 20:e3001641. https://doi.org/10.1371/journal.pbio.3001641
Mendez-Sanchez JF, Burggren WW (2019) Hypoxia-induced developmental plasticity of larval growth, gill and labyrinth organ morphometrics in two anabantoid fish: The facultative air-breather Siamese fighting fish (Betta splendens) and the obligate air-breather the blue gourami (Trichopodus trichopterus). J Morphol 280:193–204. https://doi.org/10.1002/jmor.20931
Miller CT, Yelon D, Stainier DYR, Kimmel CB (2003) Two endothelin 1 effectors, hand2 and bapx1, pattern ventral pharyngeal cartilage and the jaw joint. Development 130:1353–1365. https://doi.org/10.1242/dev.00339
Morgan M (1974) The development of gill arches and gill blood vessels of the rainbow trout, Salmo gairdneri. J Morphol 142:351–363. https://doi.org/10.1002/jmor.1051420309
Morrison CM, Miyake T, Wright JR Jr (2001) Histological study of the development of the embryo and early larva of Oreochromis niloticus (Pisces: Cichlidae). J Morphol 247:172–195. https://doi.org/10.1002/1097-4687(200102)247:2%3c172::AID-JMOR1011%3e3.0.CO;2-H
Nilsson GE (2007) Gill remodeling in fish—a new fashion or an ancient secret? J Exp Biol 210:2403–2409. https://doi.org/10.1242/jeb.000281
Nilsson GE, Dymowska A, Stecyk JAW (2012) New insights into the plasticity of gill structure. Respir Physiol Neurobiol 184:214–222. https://doi.org/10.1016/j.resp.2012.07.012
Oğuz AR (2018) Development of osmoregulatory tissues in the Lake van fish (Alburnus tarichi) during larval development. Fish Physiol Biochem 44:227–233. https://doi.org/10.1007/s10695-017-0427-3
Oikawa S, Hirata M, Kita J, Itazawa Y (1999) Ontogeny of respiratory area of a marine teleost, porgy, Pagrus major. Ichthyol Res 46:233–244. https://doi.org/10.1007/BF02678509
Oike H, Nagai T, Furuyama A et al (2007) Characterization of ligands for fish taste receptors. J Neurosci 27:5584–5592. https://doi.org/10.1523/JNEUROSCI.0651-07.2007
Okada K, Inohaya K, Mise T et al (2016) Reiterative expression of pax1 directs pharyngeal pouch segmentation in medaka. Development 143:1800–1810. https://doi.org/10.1242/dev.130039
Olson KR (1998) Hormone metabolism by the fish gill. Comp Biochem Physiol A Mol Integr Physiol 119:55–65. https://doi.org/10.1016/S1095-6433(97)00406-6
Padrós F, Villalta M, Gisbert E, Estévez A (2011) Morphological and histological study of larval development of the Senegal sole Solea senegalensis: an integrative study. J Fish Biol 79:3–32. https://doi.org/10.1111/j.1095-8649.2011.02942.x
Pan Y (2021) Oxygen chemoreception in larval zebrafish: From signal initiation to the hypoxic ventilatory response. PhD Thesis, Université d’Ottawa/University of Ottawa
Pan YK, Perry SF (2020) Neuroendocrine control of breathing in fish. Mol Cell Endocrinol 509:110800. https://doi.org/10.1016/j.mce.2020.110800
Pan YK, Perry SF (2023) The control of breathing in fishes—historical perspectives and the path ahead. J Exp Biol 226:jeb245529. https://doi.org/10.1242/jeb.245529
Pan YK, Mandic M, Zimmer AM, Perry SF (2019) Evaluating the physiological significance of hypoxic hyperventilation in larval zebrafish (Danio rerio). J Exp Biol 222:jeb204800. https://doi.org/10.1242/jeb.204800
Pan YK, Jensen G, Perry SF (2021) Disruption of tph1 genes demonstrates the importance of serotonin in regulating ventilation in larval zebrafish (Danio rerio). Respir Physiol Neurobiol 285:103594. https://doi.org/10.1016/j.resp.2020.103594
Pan YK, Julian T, Garvey K, Perry SF (2023) Catecholamines modulate the hypoxic ventilatory response of larval zebrafish (Danio rerio). J Exp Biol 226:jeb245051. https://doi.org/10.1242/jeb.245051
Pelster B, Bemis William E (1992) Structure and function of the external gill filaments of embryonic skates (Raja erinacea). Respir Physiol 89:1–13. https://doi.org/10.1016/0034-5687(92)90066-6
Perry SF, Esbaugh A, Braun M, Gilmour KM (2009) Gas transport and gill function in water-breathing fish. In: Glass ML, Wood SC (eds) Cardio-respiratory control in vertebrates: comparative and evolutionary aspects. Springer, Berlin, Heidelberg, pp 5–42
Perry SF, Pan YK, Gilmour KM (2023) Insights into the control and consequences of breathing adjustments in fishes-from larvae to adults. Front Physiol 14:1065573
Piotrowski T, Ahn D, Schilling TF et al (2003) The zebrafish van gogh mutation disrupts tbx1, which is involved in the DiGeorge deletion syndrome in humans. Development 130:5043–5052. https://doi.org/10.1242/dev.00704
Pisam M, Massa F, Jammet C, Prunet P (2000) Chronology of the appearance of β, A, and α mitochondria-rich cells in the gill epithelium during ontogenesis of the brown trout (Salmo trutta). Anat Rec 259:301–311. https://doi.org/10.1002/1097-0185(20000701)259:3%3c301::AID-AR70%3e3.0.CO;2-1
Pittman K, Skiftesvik AB, Berg L (1990) Morphological and behavioural development of halibut, Hippoglossus hippoglossus (L.) larvae. J Fish Biol 37:455–472. https://doi.org/10.1111/j.1095-8649.1990.tb05876.x
Porteus C, Kumai Y, Abdallah SJ et al (2021) Respiratory responses to external ammonia in zebrafish (Danio rerio). Comp Biochem Physiol A Mol Integr Physiol 251:110822. https://doi.org/10.1016/j.cbpa.2020.110822
Prescott LA, Regish AM, McMahon SJ et al (2021) Rapid embryonic development supports the early onset of gill functions in two coral reef damselfishes. J Exp Biol 224:jeb242364. https://doi.org/10.1242/jeb.242364
Qin Z, Lewis JE, Perry SF (2010) Zebrafish (Danio rerio) gill neuroepithelial cells are sensitive chemoreceptors for environmental CO2. J Physiol 588:861–872. https://doi.org/10.1113/jphysiol.2009.184739
Randall DJ, Baumgarten D, Malyusz M (1972) The relationship between gas and ion transfer across the gills of fishes. Comp Biochem Physiol A Physiol 41:629–637. https://doi.org/10.1016/0300-9629(72)90017-5
Reed M, Jonz MG (2022) Neurochemical signalling associated with gill oxygen sensing and ventilation: a receptor focused mini-review. Front Physiol 13:940020
Rombough PJ (1992) Intravascular oxygen tensions in cutaneously respiring rainbow trout (Oncorhynchus mykiss) larvae. Comp Biochem Physiol A Physiol 101:23–27. https://doi.org/10.1016/0300-9629(92)90622-W
Rombough PJ (1998) Partitioning of oxygen uptake between the gills and skin in fish larvae: a novel method for estimating cutaneous oxygen uptake. J Exp Biol 201:1763–1769. https://doi.org/10.1242/jeb.201.11.1763
Rombough PJ (1999) The gill of fish larvae. Is it primarily a respiratory or an ionoregulatory structure? J Fish Biol 55:186–204. https://doi.org/10.1111/j.1095-8649.1999.tb01055.x
Rombough P (2002) Gills are needed for ionoregulation before they are needed for O2 uptake in developing zebrafish, Danio rerio. J Exp Biol 205:1787–1794. https://doi.org/10.1242/jeb.205.12.1787
Rombough P (2007) The functional ontogeny of the teleost gill: which comes first, gas or ion exchange? Comp Biochem Physiol a: Mol Integr Physiol 148:732–742. https://doi.org/10.1016/j.cbpa.2007.03.007
Rombough PJ, Ure D (1991) Partitioning of oxygen uptake between cutaneous and branchial surfaces in larval and young juvenile chinook salmon Oncorhynchus tshawytscha. Physiol Zool 64:717–727. https://doi.org/10.1086/physzool.64.3.30158203
Rubner M (1883) Über den Einfluss der Körpergrösse auf Stoff- und Kraftwechsel. Z Biol 19:536
Sackville MA, Brauner CJ (2018) case study: gill plasticity in larval fishes. In: Burggren W, Dubansky B (eds) Development and environment. Springer, Cham, pp 377–400
Sackville MA, Cameron CB, Gillis JA, Brauner CJ (2022) Ion regulation at gills precedes gas exchange and the origin of vertebrates. Nature 610:699–703. https://doi.org/10.1038/s41586-022-05331-7
Sánchez-Amaya MI, Ortiz-Delgado JB, García-López Á et al (2007) Larval ontogeny of redbanded seabream Pagrus auriga Valenciennes, 1843 with special reference to the digestive system. A histological and histochemical approach. Aquaculture 263:259–279. https://doi.org/10.1016/j.aquaculture.2006.10.036
Santamarı́a CA, Marı́n de Mateo M, Traveset R et al (2004) Larval organogenesis in common dentex Dentex dentex L. (Sparidae): histological and histochemical aspects. Aquaculture 237:207–228. https://doi.org/10.1016/j.aquaculture.2004.03.020
Sasaki MM, Nichols JT, Kimmel CB (2013) edn1 and hand2 interact in early regulation of pharyngeal arch outgrowth during zebrafish development. PLoS ONE 8:e67522. https://doi.org/10.1371/journal.pone.0067522
Schilling TF, Piotrowski T, Grandel H et al (1996) Jaw and branchial arch mutants in zebrafish I: branchial arches. Development 123:329–344. https://doi.org/10.1242/dev.123.1.329
Schreiber AM, Specker JL (1999) Metamorphosis in the summer flounder Paralichthys dentatus: changes in gill mitochondria-rich cells. J Exp Biol 202:2475–2484. https://doi.org/10.1242/jeb.202.18.2475
Segner H, Storch V, Reinecke M et al (1994) The development of functional digestive and metabolic organs in turbot, Scophthalmus maximus. Marine Bioliogy 119:471–486. https://doi.org/10.1007/BF00347544
Shadrin AM, Ozernyuk ND (2002) Development of the gill system in early ontogenesis of the zebrafish and ninespine stickleback. Russ J Dev Biol 33:91–96. https://doi.org/10.1023/A:1014916229219
Shen ACY, Leatherland JF (1978) Effect of ambient salinity on ionic and osmotic regulation of eggs, larvae, and alevins of rainbow trout (Salmo gairdneri). Can J Zool 56:571–577. https://doi.org/10.1139/z78-081
Shih S-W, Yan J-J, Chou M-Y, Hwang P-P (2023) Recent progress and debates in molecular physiology of Na+ uptake in teleosts. Front Mar Sci 10:1066929
Shone V, Graham A (2014) Endodermal/ectodermal interfaces during pharyngeal segmentation in vertebrates. J Anat 225:479–491. https://doi.org/10.1111/joa.12234
Sollid J, Nilsson GE (2006) Plasticity of respiratory structures—adaptive remodeling of fish gills induced by ambient oxygen and temperature. Respir Physiol Neurobiol 154:241–251. https://doi.org/10.1016/j.resp.2006.02.006
Soulika M, Kaushik A-L, Mathieu B et al (2016) Diversity in cell motility reveals the dynamic nature of the formation of zebrafish taste sensory organs. Development 143:2012–2024. https://doi.org/10.1242/dev.134817
Sperber SM, Saxena V, Hatch G, Ekker M (2008) Zebrafish dlx2a contributes to hindbrain neural crest survival, is necessary for differentiation of sensory ganglia and functions with dlx1a in maturation of the arch cartilage elements. Dev Biol 314:59–70. https://doi.org/10.1016/j.ydbio.2007.11.005
Stockard CR (1906) The development of the mouth and gills in Bdellostoma stouti. Am J Anat 5:481–517. https://doi.org/10.1002/aja.1000050405
Talbot JC, Johnson SL, Kimmel CB (2010) hand2 and Dlx genes specify dorsal, intermediate and ventral domains within zebrafish pharyngeal arches. Development 137:2507–2517. https://doi.org/10.1242/dev.049700
Tang S-L, Liang X-F, Wang Y et al (2022) Development of gill rakers may influence the prey choice in Chinese perch (Siniperca chuatsi) larvae. Aquac Res 53:1973–1980. https://doi.org/10.1111/are.15726
Thiruppathy M, Fabian P, Gillis JA, Crump JG (2022) Gill developmental program in the teleost mandibular arch. eLife 11:e78170. https://doi.org/10.7554/eLife.78170
Tresguerres M, Kwan GT, Weinrauch A (2023) Evolving views of ionic, osmotic and acid–base regulation in aquatic animals. J Exp Biol 226:jeb245747. https://doi.org/10.1242/jeb.245747
Valerio PF (1995) The development of embryos and larvae of the Atlantic cod, Gadus morhua, with particular emphasis on the ontogeny of chloride cells. Doctoral, Memorial University of Newfoundland
van Dam L (1938) On the utilization of oxygen and regulation of breathing in some aquatic animals. PhD, University of Groningen
Walker MB, Miller CT, Coffin Talbot J et al (2006) Zebrafish furin mutants reveal intricacies in regulating Endothelin1 signaling in craniofacial patterning. Dev Biol 295:194–205. https://doi.org/10.1016/j.ydbio.2006.03.028
Walshe J, Mason I (2003) Fgf signalling is required for formation of cartilage in the head. Dev Biol 264:522–536. https://doi.org/10.1016/j.ydbio.2003.08.010
Warga RM, Nüsslein-Volhard C (1999) Origin and development of the zebrafish endoderm. Development 126:827–838. https://doi.org/10.1242/dev.126.4.827
Wells PR, Pinder AW (1996a) The respiratory development of Atlantic salmon: II. Partitioning of oxygen uptake among gills, yolk sac and body surfaces. J Exp Biol 199:2737–2744. https://doi.org/10.1242/jeb.199.12.2737
Wells PR, Pinder AW (1996b) The respiratory development of Atlantic salmon: I. Morphometry of gills, yolk sac and body surface. J Exp Biol 199:2725–2736. https://doi.org/10.1242/jeb.199.12.2725
Wilson JM, Laurent P (2002) Fish gill morphology: inside out. J Exp Zool 293:192–213. https://doi.org/10.1002/jez.10124
Wood CM, Eom J (2021) The osmorespiratory compromise in the fish gill. Comp Biochem Physiol A Mol Integr Physiol 254:110895. https://doi.org/10.1016/j.cbpa.2021.110895
Yan Y-L, Bhattacharya P, He XJ et al (2012) Duplicated zebrafish co-orthologs of parathyroid hormone-related peptide (PTHrP, Pthlh) play different roles in craniofacial skeletogenesis. J Endocrinol 214:421–435. https://doi.org/10.1530/JOE-12-0110
Yoshida Y, Saitoh K, Aihara Y et al (2007) Transient receptor potential channel M5 and phospholipaseC-β2 colocalizing in zebrafish taste receptor cells. NeuroReport 18:1517. https://doi.org/10.1097/WNR.0b013e3282ec6874
Zachar PC, Jonz MG (2012a) Neuroepithelial cells of the gill and their role in oxygen sensing. Respir Physiol Neurobiol 184:301–308. https://doi.org/10.1016/j.resp.2012.06.024
Zachar PC, Jonz MG (2012b) Oxygen sensitivity of gill neuroepithelial cells in the anoxia-tolerant goldfish. In: Nurse CA, Gonzalez C, Peers C, Prabhakar N (eds) Arterial chemoreception. Springer, Dordrecht, pp 167–172
Zachar PC, Jonz MG (2012c) Confocal imaging of Merkel-like basal cells in the taste buds of zebrafish. Acta Histochem 114:101–115. https://doi.org/10.1016/j.acthis.2011.03.006
Zhang L, Nurse CA, Jonz MG, Wood CM (2011) Ammonia sensing by neuroepithelial cells and ventilatory responses to ammonia in rainbow trout. J Exp Biol 214:2678–2689. https://doi.org/10.1242/jeb.055541
Zimmer AM, Wood CM (2015) Ammonia first? The transition from cutaneous to branchial ammonia excretion in developing rainbow trout is not altered by exposure to chronically high NaCl. J Exp Biol 218:1467–1470. https://doi.org/10.1242/jeb.119362
Zimmer AM, Perry SF (2022) Physiology and aquaculture: a review of ion and acid-base regulation by the gills of fishes. Fish Fish 23:874–898. https://doi.org/10.1111/faf.12659
Zimmer AM, Wright PA, Wood CM (2014) What is the primary function of the early teleost gill? Evidence for Na+/NH+4 exchange in developing rainbow trout (Oncorhynchus mykiss). Proc R Soc B Biol Sci 281:20141422. https://doi.org/10.1098/rspb.2014.1422
Zimmer AM, Wright PA, Wood CM (2017) Ammonia and urea handling by early life stages of fishes. J Exp Biol 220:3843–3855. https://doi.org/10.1242/jeb.140210
Zimmer AM, Pan YK, Chandrapalan T et al (2019) Loss-of-function approaches in comparative physiology: is there a future for knockdown experiments in the era of genome editing? J Exp Biol 222:jeb175737. https://doi.org/10.1242/jeb.175737
Zimmer AM, Mandic M, Rourke KM, Perry SF (2020) Breathing with fins: do the pectoral fins of larval fishes play a respiratory role? Am J Physiol Regul Integr Comp Physiol 318:R89–R97. https://doi.org/10.1152/ajpregu.00265.2019
Funding
Michael Smith Health Research BC.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Steve Perry.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Pan, Y.K. Structure and function of the larval teleost fish gill. J Comp Physiol B (2024). https://doi.org/10.1007/s00360-024-01550-8
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00360-024-01550-8