Abstract
Organismal temperature tolerance and metabolic responses are correlated to recent thermal history, but responses to thermal variability are less frequently assessed. There is great interest in whether organisms that experience greater thermal variability can gain metabolic or tolerance advantages through phenotypic plasticity. We compared thermal tolerance and routine aerobic metabolism of Convict cichlid acclimated for 2 weeks to constant 20 °C, constant 30 °C, or a daily cycle of 20 → 30 °C (1.7 °C/h). Acute routine mass-specific oxygen consumption (\(\dot{M}\)O2) and critical thermal maxima/minima (CTMax/CTMin) were compared between groups, with cycle-acclimated fish sampled from the daily minimum (20 °C, 0900 h) and maximum (30 °C, 1600 h). Cycle-acclimated fish demonstrated statistically similar CTMax at the daily minimum and maximum (39.0 °C, 38.6 °C) but distinct CTMin values, with CTMin 2.4 °C higher for fish sampled from the daily 30 °C maximum (14.8 °C) compared to the daily 20 °C minimum (12.4 °C). Measured acutely at 30 °C, \(\dot{M}\)O2 decreased with increasing acclimation temperature; 20 °C acclimated fish had an 85% higher average \(\dot{M}\)O2 than 30 °C acclimated fish. Similarly, acute \(\dot{M}\)O2 at 20 °C was 139% higher in 20 °C acclimated fish compared to 30 °C acclimated fish. Chronic \(\dot{M}\)O2 was measured in separate fish continually across the 20 → 30 °C daily cycle for all 3 acclimation groups. Chronic \(\dot{M}\)O2 responses were very similar between groups between average individual hourly values, as temperatures increased or decreased (1.7 °C/h). Acute \(\dot{M}\)O2 and thermal tolerance responses highlight “classic” trends, but dynamic, chronic trials suggest acclimation history has little effect on the relative change in oxygen consumption during a thermal cycle. Our results strongly suggest that the minimum and maximum temperatures experienced more strongly influence fish physiology, rather than the thermal cycle itself. This research highlights the importance of collecting data in both cycling and static (constant) thermal conditions, and further research should seek to understand whether ectotherm metabolism does respond uniquely to fluctuating temperatures.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Convict cichlid (Archocentrus nigrofasciatus, Günther, 1867) is a small freshwater teleost native to Central America, naturally occurring in El Salvador, Guatemala, and Honduras, and this species is a highly successful invasive species. Aquarium release and other methods of invasion have resulted in non-native populations in a large number of locations including Mexico, United States, Australia, the Middle East, Japan, and Italy (Esmaeili et al. 2013; Ishikawa and Tachihara 2010; Piazzini et al. 2010; Hovey and Swift 2012; Duffy et al. 2013; De La Torre Zavala et al. 2018). Successful invasive species provide opportunities to test hypotheses about physiological adaptation because invaders can be robust species that respond well to variable environmental conditions. Cichlids have been popular subjects for exploring phenotypic plasticity in response to changing temperature. A number of studies have detailed the thermal and metabolic physiology of related Cichlidae, and many species have demonstrated robust tolerance for high temperatures. For example, Mayan cichlid (Cichloasoma urophthalmus), an invasive species in Florida, that were acclimated to 25 °C chronically survived temperatures as high as 33 °C (Stauffer and Boltz 1994). African cichlid (Pseudocrenilabrus multicolor) acclimated to 26 °C demonstrated critical thermal maximum (CTMax) of ~ 38.5 °C and those acclimated to 32 °C had CTMax of ~ 41 °C; P. multicolor further showed similar oxygen (O2) consumption rates between 23 → 29 °C, with a significantly increased rate at 32 °C (McDonnell and Chapman 2015). Angelfish (Pterophyllum scalare) behaviorally selected temperatures between 28 → 30 °C and demonstrated upper critical thermal limits as high as 41.2 °C (Pérez et al. 2003). Many marine species found in warmer climates also have critical thermal limits approaching or exceeding 40 °C (Mora and Ospína 2001; Ospina and Mora 2004; Eme and Bennett 2009; Bennett 2010).
Most studies of fish thermal physiological responses emphasize plasticity caused by static temperature changes, rather than temperature cycling. Due to climate change, temperature regimes in aquatic environments are expected to show increases in average temperature and thermal variability. Although thermal acclimation commonly entails physiological reconstruction across multiple levels of biological organization (Hazel 1984; St-Pierre et al. 1998; Sollid and Nilsson 2006; Chadwick et al. 2015; Lu and Hsu 2015; Sandersfeld et al. 2015), some studies have shown that cycling thermal conditions compared to static (constant) temperatures do not affect whole-organismal aerobic energy requirements for certain fishes (Corey et al. 2017; Tunnah et al. 2017). Other fish species exposed to cycling thermoperiods have been shown to, for example, acclimate to the high cycle temperature (Otto and Gerking 1973; Feldmeth 1981; Threader and Houston 1983; Heath et al. 1993).
Thermal acclimation experiments that tightly control environmental conditions and minimize thermal flux do not mimic the thermal environments occupied by organisms, which are naturally variable and also changing due to climate change (Parmesan et al. 2000). What we know about “metabolic compensation” (e.g., reduced metabolic rate following acclimation to increased temperature or increased metabolic rate following acclimation to decreased temperature) based on static thermal experimental protocols may not predict responses to dynamic thermal environments (Dame and Vernberg 1978; Corey et al. 2017; Tunnah et al. 2017; Morash et al. 2018; Burggren 2019). If thermal cycling is important to metabolic compensation, then the recent acclimation history of a given organism could affect tolerance or metabolism; that is, it may be the temporal cycling of temperature, in addition to the minimum or maximum temperature, that determines physiological responses. However, relatively few studies have investigated physiological effects for fish of cycling temperature acclimation compared to constant temperature acclimation, and we do not have a clear picture of common physiological responses (e.g. Corey et al. 2017; Currie et al. 2004; Eme et al. 2011; Tunnah et al. 2017). There have been calls over the past 10 years to expand our understanding of responses for poikilotherms to stochastic and cycling temperatures (Somero 2012; Morash et al. 2018; Burggren 2019).
Much of the existing research quantifying organismal responses to cycling temperatures utilizes discrete, step-wise temperature levels for their thermal cycles (Burggren 2019). In these acclimation cycles, animals are held at a low temperature, then promptly or gradually stepped up to a high temperature, and then stepped back down after some time period. Acclimation protocols (similar to the present study) that use a cyclic pattern of temperature change or a temperature spike have been used less commonly (Burggren 2019). Studies that utilize truly stochastic thermal acclimation may more closely mimic natural conditions, but such experiments are logistically difficult to execute and statistically analyze. It has been suggested that stochastic variation and immediately recent thermal history may support a slightly increased thermal tolerance response as compared to cyclic temperatures (Drake et al. 2017). Whether or not animals are able to specially adapt to stochastic or predictable thermal change is crucial for understanding impacts of increased thermal variability.
Reduced thermal sensitivity through compensation could buffer increased energetic costs associated with thermal cycling (Gabriel et al. 2005; Seebacher et al. 2014). For example, while oxygen consumption increased with increasing temperature, Atlantic mud crab (Panopeus herbstii) demonstrated an increased level of metabolic control when acclimated to cycling temperatures (Dame and Vernberg 1978). In this case, constant-acclimated animals demonstrated a more classic thermal performance response, whereas cycle-acclimated animals demonstrated a broader thermal performance optimum peak. Broadly, others have suggested that following acclimation, species native to more variable climates may be better able to adjust physiologically to thermal variation than species native to more stable thermal environments (Seebacher et al. 2014). It is possible that species acclimated chronically to large temperature cycles could display compensatory metabolic phenotypic plasticity to the change in temperature itself, compared to species acclimated to static temperatures.
We used cycling acclimation with CTM and metabolism measurements to address the importance and effects of cycling acclimation compared to constant thermal acclimation. Given the plasticity and thermal tolerance observed in other species of Cichlidae, Convict cichlid were hypothesized to be tolerant to increased temperatures and thermal variability and able to display “classic” metabolic compensation to static thermal acclimation. We hypothesized that measurements of CTM values would increase with increased acclimation temperature, where Convict cichlid acclimated to a statically high or cycling temperature regime would show higher CTM values than those acclimated to statically lower temperatures. In addition, we hypothesized that metabolic rates would acutely increase with increasing temperature but show reductions following acclimation (metabolic compensation). Finally, we hypothesized that this species would show less drastic metabolic change across the diel cycle when acclimated for 2-weeks to a 10 °C diel thermal cycle (20 → 30 °C), due to prior exposure and acclimation to the range of experienced temperatures. This study is unique because we compared methods likely to generate typical thermal tolerance and metabolic responses, constant thermal acclimation combined with thermal tolerance and static thermal metabolism measurements, to responses generated by cycling temperatures, cycling thermal acclimation combined with chronic metabolism measurements across that cycle, along with thermal tolerance measurements.
Materials and methods
Acclimation and maintenance of fish
Adult Convict cichlid, as denoted by size and coloration (Wisenden 1995), were sourced from a local aquarium distributor (Pet Kingdom, San Diego, CA). Fish for CTM and oxygen consumption trials were distributed evenly among replicate 25 L aquaria, with like-sized fish grouped together. Morgan et al. 2019 recently suggested that aquarium-reared zebrafish (Danio rerio), do not have a large change in CTMax trend compared to wild-caught fish, following laboratory acclimation to stable temperatures through four generations. This suggests thermal tolerance could be considered reflective of trends observed in wild populations. All individual fish were used in only a single trial, then sacrificed following the trial.
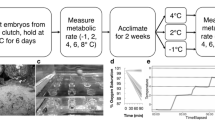
All fish (N = 143) were initially held at 25 °C for a 7 ± 2.1 days quarantine period (mean ± SD) while observed for proper health and absence of disease. Following quarantine, fish were divided into one of three treatment groups: constant 20 °C (20.1 ± 0.1, mean ± SD), constant 30 °C (30.0 ± 0.9, mean ± SD), and cycle-acclimated to a daily cycle of 20 → 30 °C (0900–1600 h; Fig. 1). For constant temperature groups, temperatures were increased or decreased at a rate of ~ 1 °C/day until desired acclimation temperature was reached, and then the temperature was held consistent for 15 ± 2.1 days (mean ± SD). For the cycle-acclimated group, cycle amplitude was gradually increased by changing the maximum and minimum temperatures by ~ 1 °C/day; that is, on day 1 following quarantine the daily cycle varied from 24 to 26 °C, day 2 following quarantine it varied from 23 to 27 °C, and so on until the cycle of 20 → 30 °C from ~ 0900 to 1600 h was reached. The final 20 → 30 °C cycle was maintained for 15 ± 2.1 days (mean ± SD; Fig. 2) at a rate of ~ 1.7 °C/h.
The experimental design including the experimental groups that were tested (in dashed boxes). Fish were acclimated to one of three experimental temperature groups, following which, \(\dot{M}\)O2 and critical thermal tolerance were measured for each acclimation group. Cycle-acclimated fish were two separate experimental groups (“cycle minimum and cycle maximum groups”) for acute \(\dot{M}\)O2 and CTM, with 7 (CTM) or 8 (acute \(\dot{M}\)O2) separate replicates sampled from the cycle minimum (20 °C) and the cycle maximum (30 °C) time point. Cycle-acclimated and constant acclimated fish were individual experimental groups for the chronic, cycling \(\dot{M}\)O2 measurements
For all groups, replicate 25 L aquaria were situated within a well-circulated 247 L water bath (aquarium pumps Lifegard Aquatics, Cerritos, CA). Fish were routinely collected and divided evenly among 8 replicate aquaria containing no more than 6 fish each. For the constant 30 °C group, the water bath was heated with a 300 W EHEIM Jager Aquarium Thermostat Heater (EHEIM GmbH & Co. Deizisau, Germany). For the constant 20 °C group, the water bath was cooled using a chiller (VWR, 13 L, Model 1156, ± 0.01 °C). For the cycle-acclimated group, the water bath was heated using a 200W and 300W EHEIM Jager Aquarium Thermostat Heater (EHEIM) and cooled using a chiller (JBJ 1/5 HP Arctica Aquarium Chiller, St. Charles, MO, Model DBA-150). For cycling acclimation temperatures, a diel cycle with one maximum and one minimum was created using timers that controlled heaters and chiller (Outdoor Mechanical Timer, Fosmon, Woodbury, MN).
All thermometers and loggers used in the study were calibrated against a VWR® Traceable® Digital Thermometer (± 0.05 °C; Avantor, VWR, Radnor, PA). This was done by measuring a range of temperatures (10 temperatures from 5 to 42 °C) with all thermometers and loggers to create a standard, and all reported temperatures have been corrected accordingly. Temperatures in all replicate aquaria were monitored manually, daily using a thermometer (VWR® Traceable® Lollipop™; ± 0.4 °C) for check for uniformity across aquaria and water changes. Aquaria temperatures were recorded every 30 min with waterproof loggers (HOBO® TidbiT®; UTBI-001, ± 0.21 °C; Onset Computer Corporation, Bourne, MA) and used to determine the average temperature for each acclimation group.
Aquaria were biologically filtered with 1 weekly water change of 15–30% with like-temperature water. No more than 50% of the water was changed in any tank at any one time. Water changes were performed using dechlorinated tap water that was carbon filtered for > 48 h. Excess food and waste were siphoned from the bottom of the tank using aquarium tubing during all routine water change processes. Ammonia, nitrite, and nitrate levels were monitored every ~ 10 days (API Freshwater Master Test Kit). Additionally, pH was monitored every week via electrode (Mettler Toledo Inc., InLab Expert Pro-ISM pH Electrode). Aquaria pH was maintained at 7.8 ± 0.03. Ammonia, nitrite, and nitrate values never exceeded 0.25 ppm, 0.05 ppm, and 80 ppm, respectively. Aquaria water was constantly bubbled with an air pump (JW Pet Company, Teterboro, NJ). Fish were fed ad libitum ground TetraMin® fish flakes (Spectrum Brands, Inc., Blacksburg, VA) once daily, and fasted for ~ 24 h prior to any tolerance or metabolism trials. Fish were left undisturbed whenever possible and maintained on 12:12 day/night cycle. Aquaria lights were turned on at 7:30 AM and off at 7:30 PM Pacific Standard Time. All procedures were approved by California State University San Marcos Institutional Animal Care and Use Committee approved protocol 17-004.
Thermal tolerance trials
Critical thermal maximum (CTMax) and critical thermal minimum (CTMin) were identified as the upper or lower temperature where fish were unable to maintain an upright position, termed ‘loss of equilibrium’ (LOE). Fish were evenly selected from replicate acclimation aquaria (N = 7, 1 fish from each of 7 replicates), with no more than one fish sampled from any replicate for any one trial. Fish were distributed individually into confined test chambers (250 mL Tri-Corner plastic beakers, Globe Scientific, Inc., Paramus, NJ) which were fixed in a 57 L water bath (‘CTM tank’) using a Styrofoam frame. For CTMax trials, the 57 L polystyrene-insulated CTM tank was heated by two 150W EHEIM Jager Aquarium Thermostat Heaters (EHEIM GmbH & Co.) set in excess of their maximum temperature. For CTMin trials, the CTM tank was cooled by chiller set to 0 °C (JBJ, Model DBA-150). Temperature stratification of the CTM tank was prevented by continuous circulation using aquarium pumps (Lifegard Aquatics). All individual test chambers were covered with netting during the trial, and each chamber was equipped with a small, individual tube for air bubbling to prevent thermal stratification.
Trials were initiated at the appropriate temperature, and water temperature was changed at a rate of ~ 0.32 ± 0.04 °C/min (mean ± SD). Heating or cooling rate was monitored throughout the trial using waterproof loggers (UTBI-001, ± 0.21 °C; Onset) placed in blank beakers that recorded water temperature every 10 s. These data were used to determine average heating and cooling rates for trials. Fish were left undisturbed in chambers throughout the trial until LOE occurred, at which point the temperature in the respective test chamber was promptly recorded prior to removal. LOE temperature was recorded using a calibrated thermometer (VWR® Traceable® Lollipop™; ± 0.1 °C). Fish were quickly removed upon LOE and water immediately exchanged with acclimation temperature water for recovery. All fish were monitored ≥ 1 hr to assess recovery.
Oxygen consumption trials (\(\dot{M}\) O 2)
\(\dot{M}\)O2 was determined as the decrease in PO2 measured via intermittent respirometry using Loligo® Systems glass chambers and software (Loligo® Systems, DK-8800, Viborg, Denmark). Water-filled glass chambers with connective tubing provided a 200–500 mL closed system anchored within a reservoir tank (108 L). Prior to trials, the tank system and chambers were filled with clean, dechlorinated, carbon-filtered water equilibrated to the appropriate temperature for > 4 h, then held constant by a recirculating chiller (PolyScience 15L control, Model AP15R-30, Niles, IL; or VWR Model 1156; ± 0.01 °C). Fish were selected from replicate acclimation aquaria and placed into individual experimental chambers that were greater than tenfold the volume of individual fish, large enough to allow fish to turn around. Chambers were sealed under reservoir water and periodically flushed with fresh, oxygenated reservoir water between measurements. Reservoir water was continuously and vigorously bubbled for the length of all trials with air pumps (JW Pet Company). Length of measurement cycles was tailored to fish size with Po2 maintained between 13 and 21 kPa, and oxygen consumption rate was monitored throughout trials. Related and similarly distributed Mayan cichlid have a Pcrit of 4.7 kPa at 33 °C (Burggren et al. 2019), suggesting water oxygenation during trials were sufficient. O2 levels were measured for 240–300 s in repeating cycles every 630–660 s, between which the system was flushed until the restoration of initial levels of O2. Reservoir temperature was monitored with a Pt1000 temperature probe (Loligo® Systems, ± 0.15 °C, 1 Hz) and waterproof loggers (UTBI-001, ± 0.21 °C; Onset, every 30 min). Po2 was monitored using Witrox 4 and fiber optic mini sensors paired with an O2 sensor spot (Loligo® Systems) located within a flow-through cell directly connected to the chamber via a closed loop of tubing. O2 sensor spots were periodically zeroed with a solution of sodium sulfite, and 100% O2 saturation was taken as the Po2 following > 4 h of thermal equilibration immediately prior to the trial in bubbled reservoir water (JW Pet Company). Fish were left undisturbed during trials.
Acute oxygen consumption rate (\(\dot{M}\) O 2)
Acute oxygen consumption rate (\(\dot{M}\)O2) for both constant temperature acclimation groups was measured for ~ 4 h for fish at both static acclimation temperatures, 20 °C and 30 °C (N = 8 for each group, 1 fish from each of 8 replicates). For acute \(\dot{M}\)O2 of cycle-acclimated fish, separate replicate fish were sampled both at the trough (20 °C) or at the peak (30 °C) of the diel thermal cycle regimen (henceforth referred to as “cycle-acclimated 20 °C” and “cycle-acclimated 30 °C” groups; (N = 8 for each group, 1 fish from each of 8 replicates). Fish were placed into individual chambers (250 mL Tri-Corner beakers) that were allowed to come to equilibrium with the trial temperature for 10 min prior to carefully placing fish into experimental chambers. A single average \(\dot{M}\)O2 was taken as the average of 9–12 measurements across ~ 3 h at either a constant 20 °C or constant 30 °C, following 45–60 min of initial acclimation to the chamber. For 20 °C and 30 °C constant acclimation groups, separate replicate fish (N = 8) were measured at 20 °C and the 30 °C from both acclimation groups (32 total fish). For cycle-acclimated 20 °C and cycle-acclimated 30 °C groups, separate replicate fish (N = 8) were measured at 20 °C and 30 °C from both groups (32 total fish). Fish were taken from the respective acclimation temperature, or respective part of the diel thermal cycle, and placed in respirometry chambers at either 20 °C or 30 °C, as described above.
Chronic, cycling oxygen consumption rate (\(\dot{M}\) O 2)
Chronic \(\dot{M}\)O2 for constant and cycle-acclimated fish was measured continuously for 24–36 h across one complete 24 h diel acclimation cycle regimen (N = 7–8 for each group, 1 fish from each replicate). Average hourly \(\dot{M}\)O2 were taken as the average of 4–6 measurements for each hour of the trial, following 1 h of initial acclimation to the chamber. For all chronic trials, fish were initially introduced to the diel cycle at the point of the cycle closest to acclimation temperature, making time and initial temperature a factor that differed between acclimation groups. Fish were placed directly into experimental chambers and allowed 1 h to equilibrate before recording began. For the 20 °C constant and cycle-acclimated groups, fish were introduced to the cycle’s minimum, 20 °C at ~ 0700 h, prior to the increasing temperature portion of the diel cycle (20–30 °C 0900–1500 h) and were measured for the next 24–26 h. For the 30 °C constant group, fish were introduced to the cycle’s maximum, 30 °C at ~ 1500 h, prior to the decreasing temperature portion of the diel cycle (30–20 °C 1800–000 h), and were measured for the next 36–38 h (i.e., from a 30 to 20 °C, then 20–30 °C, then 30–20 °C again).
\(\dot{M}\) O 2 calculations
Each 240–300 s, \(\dot{M}\)O2 value (mg/O2 g of wet body/mass/h) was calculated as a function of decreased PO2 over time, relevant oxygen capacitance, and volume of the chamber minus the volume of the fish (estimated in mL from grams of wet body mass) using the following equation:
where PO2(t2) − PO2(t1) is the decrease in PO2 (kPa) in the chamber over the time period (t2 – t1, h), β is the oxygen capacitance of the water at the relevant temperature (mL O2/mL/kPa), and V is the chamber volume minus the organism volume (mL). Capacitance at the relevant temperature was used for acute \(\dot{M}\)O2 calculations. Capacitance for the chronic, cycling \(\dot{M}\)O2 was determined for the temperature during each individual 120 s measurement period and a regression of capacitance and water temperature to create individual capacitance values for each individual measurement. Blank trials were identically run and measured following all trials to blank-correct measurements. Average blank corrected values were generated for 20 °C and 30 °C and used for all measurements at those temperatures, and average blank corrected values were also generated for the upward and downward part of the temperature cycle, and used for all measurements during those changing temperatures. Blank correction revealed very small background \(\dot{M}\)O2, representing at maximum approximately 2% of a given fish’s \(\dot{M}\)O2. Dry masses were used to calculate dry mass-specific \(\dot{M}\)O2 measurements for each animal by dividing blank-corrected average \(\dot{M}\)O2 by the final dry mass of each individual. Similarly, wet mass of each animal were used to calculate wet mass-specific \(\dot{M}\)O2 for each individual. Wet mass-specific values were used for all statistical analysis, as there were no trends for differences in results between dry and wet mass-specific values, and wet mass-specific values allow for more ready comparison to previously published values.
Animal sacrifice and size measurements
Following all CTM and \(\dot{M}\)O2 trials and recovery, fish were sacrificed using tricaine methanesulfonate. Immediately following sacrifice, standard length (± 0.1 mm; digital calipers) and wet body mass (± 0.01 mg, Mettler Toledo Inc., XS105) were taken for all fish (Table 1). Bodies were dried individually for 2–3 days at 65–70 °C (Quincy Lab Inc. Model 40 Lab Oven; Chicago, IL). Dry body masses (± 0.01 mg, Mettler Toledo Inc., XS105) were taken when final measurements changed by less than 1% over ~ 24 h.
Statistical analyses
Statistical analyses were performed using RStudio (RStudio, Inc., Version 1.2.5019) with R data analysis software (The R Foundation, Version 3.6.3). The parametric assumption of normality of residuals was tested using Shapiro–Wilk normality tests, and equality of variances was assessed using Bartlett’s test. Effects of temperature treatment on CTMax and CTMin were analyzed using two, separate one-way analysis of variance (ANOVA) analyses, one for CTMax and one for CTMin. The effects of temperature on acute \(\dot{M}\)O2 were analyzed using a single, two-way ANOVA, using trial temperature (20 °C and 30 °C) and acclimation treatment as predictors of \(\dot{M}\)O2, with \(\dot{M}\)O2 log-transformed to meet normality of residuals and HOV assumptions. Where significant differences were found, Tukey’s HSD post hoc for means comparison analysis was used to differentiate statistically distinct groups. A REPEATED measures ANOVA (RM-ANOVA) was similarly used to analyze the effects of temperature on chronic \(\dot{M}\)O2. Initial tests of sphericity revealed that univariate RM-ANOVA could not be used, so the multivariate form of RM-ANOVA was used. All statistical decisions were made based on α = 0.05, and values are provided as mean ± s.e.m. or mean ± SD.
Statistical analyses for thermal tolerance trials and acute \(\dot{M}\) O 2
For CTMax and CTMin trials, model residuals met the normality assumption (CTMax p = 0.59; CTMin p = 0.98). For acute \(\dot{M}\)O2 trials conducted at 20 °C and 30 °C, model residuals failed the normality assumption (p = 0.0012). Log-transforming \(\dot{M}\)O2 resolved the problem (p = 0.91). Data for CTMax (p = 0.56) and CTMin (p = 0.17) met the HOV assumption (Bartlett test). Data for acute, static temperature \(\dot{M}\)O2 rate trials also met the HOV assumption after log-transformation (p = 0.22; Bartlett test).
Effects of temperature on CTMax and CTMin were analyzed by comparing the means of experimental groups via separate one-way ANOVA analyses using acclimation temperature as a predictor of LOE. Effects of temperature on wet mass-specific log-transformed \(\dot{M}\)O2 were analyzed by comparing means of experimental groups via two-way ANOVA analysis using acclimation temperature and trial temperature as predictors of \(\dot{M}\)O2. Partitioning degrees of freedom for residuals was not necessary due to the balanced experimental design and orthogonal nature of the predictors; therefore, the non-significant interaction term was dropped for post hoc Tukey HSD comparisons of \(\dot{M}\)O2.
Statistical analyses for chronic, cycling \(\dot{M}\) O 2
Due to the dynamic nature of this experiment, with time and temperature simultaneously changing and both affecting the dependent variable (\(\dot{M}\)O2), results of HOV and normality assumption-tests showed multiple violations. Further, assumptions of sphericity were not met due to increased metabolic variability at higher temperatures. Therefore, we used a multivariate approach when performing a repeated measures ANOVA (RM-ANOVA), which does not assume sphericity. This multivariate approach tests for differences between treatment groups across all of the time points, with each time point treated as a separate variable, using multivariate analysis of variance (MANOVA); this tests for the main effect of acclimation treatment. Sequential differences between measured time points are tested against 0 with a one-sample MANOVA; this tests for the main effect of time. Sequential differences are compared between acclimation treatment groups with a MANOVA to determine if the amount of change over time differs between groups; this tests for interaction between acclimation treatment and time. The full set of 23 hourly measurements included several hours of measurements after the cycle had completed, which reduced the statistical power of the model by adding parameters at a time when differences were less likely to be detectable. We also separately analyzed the period in which the temperature cycle was occurring, measurements between 0800 and 2200. For the 20 °C constant and cycle-acclimated groups, the 24 h cycle began at 0900 h on one day and continued until the following day at 0900 h (N.B., fish were at the cycle’s minimum, 20 °C at 0800 h, prior to the increasing temperature portion of the diel cycle). For the 30 °C constant group, the 24 h cycle was taken as the decrease in temperature from 30 °C → 20 °C that began at 1700 h, and the increase in temperature from 0900–1600 (20 °C → 30 °C) the following day (N.B., fish were introduced to the cycle’s peak, 30 °C at ~ 1500 h, prior to the decreasing temperature portion of the diel cycle). No pattern of differences between the two decreasing temperature portions of the cycle experienced during the 36 h trial by the 30 °C constant group were detected.
Results
Critical thermal tolerance
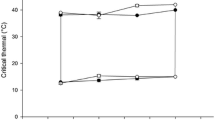
Acclimation temperature had a highly significant effect on both CTMax (p < 0.0001) and CTMin (one-way ANOVA, p < 0.0001; Supplementary Table S1). Acclimation to increased temperatures corresponded to increased CTMax (Fig. 3a) and CTMin (Fig. 3b). Fish acclimated to a daily cycle showed an intermediate CTMax response that did not statistically differ between fish sampled during exposure to the maximum or minimum of the cycle (Fig. 3a). Fish acclimated to a daily cycle showed distinct CTMin values dependent on sampling time (Fig. 3b). Cycle-acclimated fish sampled from the minimum of the cycle (20 °C) showed a statistically identical CTMin response to fish acclimated to a constant 20 °C. However, cycle-acclimated fish sampled from the maximum of the cycle (30 °C) showed an intermediate CTMin response distinct from fish acclimated to either constant 20 °C or 30 °C. When comparing fish acclimated to 20 °C or 30 °C, fish gained ~ 0.34 °C of upper thermal tolerance per degree of acclimation (Fig. 3a) and ~ 0.40 °C of lower thermal tolerance per degree of acclimation (Fig. 3b).
Mean LOE (loss of equilibrium) for critical thermal maximum (a; CTMax) and critical thermal minimum (b; CTMin) as a function of acclimation temperature. Each point represents mean CTMax or CTMin from N = 7 fish, and error bars are ± SD. Uppercase letters denote statistically distinct means for groups within CTMax or CTMin (one-Way ANOVA; p < 0.001; Tukey’s post hoc; Supplementary Table S1)
Acute \(\dot{M}\) O 2
Qualitatively identical statistical results were obtained using un-transformed and log-transformed \(\dot{M}\)O2. The log of wet mass-corrected \(\dot{M}\)O2 was significantly affected by acclimation temperature (p = 0.006) and trial temperature (p < 0.0001), but not the interaction between acclimation temperature and trial temperature (p = 0.94) (two-way ANOVA, Supplementary Table S2). Post hoc Tukey’s test following removal of the nonsignificant interaction term (Supplementary Table S3) showed statistically identical wet-mass corrected \(\dot{M}\)O2 for the cycle minimum and cycle maximum groups when measured acutely at both 30 °C (Fig. 4a) and 20 °C (Fig. 4b). This response was intermediate to 20 °C and 30 °C constant acclimated fish which were statistically different from one another. Fish showed higher \(\dot{M}\)O2 when measured acutely at 30 °C compared to when measured acutely at 20 °C within the same acclimation treatment (Supplementary Table S4). Fish acclimated to a constant 20 °C showed a 194% increase in \(\dot{M}\)O2 when measured acutely at 30 °C. Similarly, fish sampled from the minimum of the daily cycle (20 °C) showed a 207% increase in \(\dot{M}\)O2 when measured acutely at 30 °C. Fish acclimated to a constant 30 °C showed a 60% decrease in \(\dot{M}\)O2 when measured acutely at 20 °C. Similarly, fish sampled from the minimum of the daily cycle (30 °C) showed a 61% decrease in \(\dot{M}\)O2 when measured acutely at 20 °C.
Acute wet mass-corrected \(\dot{M}\)O2 measurements taken at 30 °C (a) or 20 °C (b) for all acclimation treatments. Measurements for cycling acclimation fish were taken acutely by sampling fish both at the minimum and maximum of their cycle. Each bar represents mean \(\dot{M}\)O2 from N = 8 fish, and error bars are ± SD. Uppercase letters denote statistically distinct mean \(\dot{M}\)O2 (two-way ANOVA; p = 0.01; Tukey’s post hoc; Supplementary Tables S2, S3 and S4)
Chronic, cycling \(\dot{M}\) O 2
During the 20 → 30 °C temperature cycle, effects of time, treatment and the interaction between the two on hourly average \(\dot{M}\)O2 was measured over the course of 20 h, from 0800 through 0400 (RM-ANOVA; Fig. 5). Time coincides with temperature change, dynamically, during the 20 h trial. When all 20 h were included in the analyses (0800–0400), there was no significant effect of time, treatment, or the interaction between the two (Supplementary Table S5). If only the increase and decrease of \(\dot{M}\)O2 during the respective increase and decrease in temperature are compared (i.e., 0800–2200), there is a significant effect of time on \(\dot{M}\)O2 (i.e., temperature changes cause changes in \(\dot{M}\)O2), but there is no effect of treatment or the interaction between time (i.e., temperature) and treatment; that is to say, the rate of \(\dot{M}\)O2 change is very similar between all acclimation groups (Supplementary Table S6).
Hourly mean \(\dot{M}\)O2 of three acclimation treatments exposed to the 24 h temperature cycle (shown in insert). Measurements were taken as an hourly mean of > 4 individual, intermittently recorded measurements for each hour and averaged across 7–8 individuals. Open points designate fish acclimated to a constant 30 °C before naive exposure to the cycle. Closed points designate fish acclimated to a constant 20 °C before naive exposure to the cycle. Grey points designate fish acclimated to the same cycle for > 2 weeks prior to metabolic measurements. Error bars are ± SD. All three groups showed statistically similar \(\dot{M}\)O2 (repeated Measures ANOVA; Supplementary Tables S5 and S6)
Discussion
Research continues to highlight ongoing global temperature changes, and many species will continue to experience alterations to their thermal environments (Parmesan et al. 2000; Cutler et al. 2003; Hansen et al. 2006, 2010). Therefore, it is important to assess physiological impacts of fluctuating temperatures in ongoing research, and the present study uses chronic measurements of oxygen consumption during dynamic exposure to a large (10 °C) diel thermal cycle. The lack of a significant time-by-temperature interaction illustrates that the rate of \(\dot{M}\)O2 change was very similar between 20 → 30 °C cycle-acclimated fish and constant 20 °C or 30 °C acclimated fish. Overall, our study suggests that a robust, invasive freshwater fish does not appear to acclimate to the cycle itself following 2 weeks of 10 °C daily thermal changes (i.e., the interaction of time and acclimation temperature) but may instead be more heavily influenced by the minimum and maximum temperatures experienced.
Acute metabolic measurements revealed differences between acclimation groups and classic responses similar to what has been previously shown in static thermal acclimation protocols (Chipps et al. 2000; Das et al. 2004). Convict cichlid showed classic metabolic compensation: increased \(\dot{M}\)O2 at lower temperatures (20 °C) following chronic low-temperature acclimation (20 °C), and decreased \(\dot{M}\)O2 at higher temperatures (30 °C) following chronic high-temperature acclimation (30 °C). Related species such as the dwarf cichlid (Apistogramma agassizii) have similarly demonstrated metabolic compensation across their thermal tolerance range (Kochhann et al. 2015). For example, A. agassizii maintained metabolic rates around 0.4–0.5 mg O2/g/h even after rapid temperature increases of 3 °C from 26 °C to 29 °C. In addition, P. multicolor showed \(\dot{M}\)O2 of ~ 0.3 mg O2/g/h across acclimation temperatures between 23 and 29 °C, until a significant spike to ~ 0.5 mg O2/g/h was observed in fish acclimated to 32 °C (McDonnell and Chapman 2015). Our study suggests substantial physiological adaptation between Convict cichlid acclimated to 20 °C or 30 °C that may facilitate its success as an invasive species.
However, when chronic \(\dot{M}\)O2 was examined on a longer timescale, during the daily 20 °C → 30 °C cycle, fish demonstrated similar routine aerobic metabolism (Fig. 5). Chronic \(\dot{M}\)O2 measured during this daily cycle showed that thermal acclimation to the temperature cycle itself may not be a significant influence on aerobic metabolism of Convict cichlid. The lack of a significant interaction between time and temperature in our chronic, cycling temperature \(\dot{M}\)O2 experiment (Fig. 5) strongly suggests that cycle-acclimated fish did not physiologically acclimatize to the cycle in particular, i.e., the repeated rate of change in temperature. Our analysis shows no differences between our thermal acclimation groups across these hourly \(\dot{M}\)O2 values. Given that the rate of change in \(\dot{M}\)O2 was very similar between acclimation groups, the minimum and maximum temperatures experienced may play a more significant role than the rate of thermal change or the thermal cycle itself. With the logistical and analytical difficulties in potential stochastic thermal acclimation experiments (Burggren 2019), our multivariate RM-ANOVA analysis and experimental design provide a ready avenue to examine changes across successive average hourly \(\dot{M}\)O2 generated while experimental temperature changes (Fig. 5). Future studies implementing cycling thermal acclimation could further our analyses to pinpoint sections of the thermal change where differences in energy usage between different thermal acclimation groups may occur.
Heat hardening, or increased tolerance following sub lethal exposure, similarly indicates that maximum temperatures experienced may play a more critical role in the acclimation response than thermal variability or specific cycle (e.g., Maness and Hutchison 1980). Heat hardening through short-term exposure to sub-lethal temperatures can induce an adaptive, protective response against future thermal stress (Maness and Hutchison 1980; Loeschcke et al. 2007). This acutely induced response only persists on the scale of hours to days but can provide an additional buffer of tolerance against extreme temperatures (Maness and Hutchison 1980; Bilyk et al. 2012). Heat hardening can result in a temporarily elevated CTM response. For example, repeated CTM measurements were found to have a heat-hardening response for temperate P. promelas, and C. lutrensis, where secondary CTM responses were higher than the first within hours (Maness and Hutchison 1980). Similarly, Antarctic notothenioids demonstrated a heat-hardening response with an average 1 °C increase in CTM (Bilyk et al. 2012). Thermal cycling can induce elevated levels of HSP70 in Atlantic salmon (Salmo salar) white muscle, heart, and liver tissues that were not detectable prior to cycling exposure (Tunnah et al. 2017). However, the linkage, if any, between heat-shock protein expression and heat-hardening remains unclear (Bilyk et al. 2012; Tunnah et al. 2017). It is possible that, while energetic demands are not reflected in whole animal metabolism, other biological mechanisms undergo reconstruction to reflect exposure to cycling and extreme temperatures (Bullock 1955; Tunnah et al. 2017).
Convict cichlid gain upper and lower temperature tolerance in a fashion similar to other fish species. Cycling temperature acclimation can drive up thermal tolerance (Otto and Gerking 1973; Feldmeth 1981; Threader and Houston 1983), and it has been shown to improve thermal tolerance, with the gain of tolerance driven by the mean and amplitude of the thermal cycle (Colinet et al. 2015). For example, eurythermal mosquitofish (Gambusia affinis) acclimated to cycling temperatures showed increased CTMax similar to fish that had been statically acclimated to peak temperatures of the respective cycles (Otto 1973). Other research indicates that cycle-acclimated fish may acclimate to the mean of their thermal cycle and not show distinct CTM compared to fish acclimated to static temperatures, or species might acquire enhanced upper thermal tolerance only following long-term (at least one month) acclimation to a high-temperature thermal cycle (Currie et al. 2004; Eme et al. 2011). Our results show that Convict cichlid acclimated to a daily 20 → 30 °C cycle gain lower thermal tolerance during the 20 °C temperature portion of the cycle while retaining upper thermal tolerance, and that no upper tolerance is gained during the 30 °C temperature portion of the cycle relative to 30 °C-acclimated fish. It has been shown that acclimation to higher temperatures is a more rapidly occurring process than acclimation to lower temperatures, particularly for tropical fishes (Brett 1956; Brattstrom and Lawrence 1962; Allen and Strawn 1971; Chung 2000, 2001). Convict cichlid and other warm-water species are commonly geographically limited by low temperatures, and our data suggest that recent cool-water exposure enhances CTMin of this successful invasive species (Eme and Bennett 2008; Schofield et al. 2010).
Higher temperatures will benefit cold-limited species by increasing performance if current levels of performance are below their optimum temperature (Seebacher et al. 2014). Cycling temperatures have been shown to decrease lower thermal tolerance (gain of cold tolerance) in comparison to static thermal acclimation protocols (Colinet et al. 2015), and this finding by others is similar to the present study’s observation that the cycle-acclimated group had increased cold tolerance during the 20 °C portion of their cycle (Fig. 3B). Further, exposure to these cooler temperatures could potentially offset the biological cost of thermal stress imposed by prolonged exposure to increased temperatures and extreme thermal events (Colinet et al. 2015; Corey et al. 2017; Davis et al. 2019). Nonetheless, the high cost of immediate performance could cause the cost of thermal variability to outweigh the benefits (Colinet et al. 2015; Dowd et al. 2015). Exposure to cycling temperatures imposes a greater energetic cost than lower, stable thermal environments (Schaefer and Ryan 2006). For example, Zebrafish (Danio rerio) reared in variable thermal environments show decreased body size (indicative of increased energetic cost) and increased CTMax, compared to fish reared in constant thermal environments (Schaefer and Ryan 2006). Similarly, tadpoles were not able to adjust metabolic responses to offset energetic demands associated with thermal variability and instead allocated less energy toward growth (Kern et al. 2015). Metabolic rates may increase overall for freshwater species, which experience a greater magnitude of change relative to marine species, regardless of the potential to acclimatize to changing conditions (Seebacher et al. 2014). Therefore, Convict cichlid may not be able to decrease thermal sensitivity or reduce metabolic change, enough to offset the energetic demands of experiencing regular and increasingly rapid thermal change.
Our research highlights the need to examine whole-animal fish thermal and metabolic physiology on multiple time scales (Enders and Boisclair 2016; Holsman and Danner 2016; Clark et al. 2020). Changes in gene expression, particularly of molecular chaperones and heat shock proteins, and posttranslational modification may be mechanisms that fish utilize to combat thermal variability (Somero 2012). It has been suggested that more thermally tolerant corals frontload stress response mechanisms, including upregulated heat shock protein (Hsp70) and antioxidant enzyme gene expression, to more efficiently respond to increased stress (Barshis et al. 2013). Similarly, marine oysters (Isognomon nucleus) exposed to prior bouts of thermal stress showed an enhanced heat shock response through elevated levels of Hsp70 (Giomi et al. 2016). While it may be that cellular and subcellular components seem predisposed to cycling temperatures for certain species (Barshis et al. 2013; Corey et al. 2017; Tunnah et al. 2017), without those data paired with whole-animal measurements, it is unclear what fitness-level affects cycling temperatures may have on animals.
Cycling temperatures impose increased energetic costs, and species that could decrease metabolic thermal sensitivity could possess physiological buffers (i.e., plasticity) against unpredictable environmental variability (Williams et al. 2012). Organisms could further benefit from plasticity in the breadth of physiological performance, so as to maintain performance across an increased range of environmental variability (Gabriel et al. 2005). However, our study suggests that while thermal tolerance and metabolism may be plastic responses, Convict cichlid does not necessarily gain any additional benefit from acclimation to a cycling regime, with exposure to thermal variability generally causing an intermediate response. It is possible that fish reared under more variable conditions may be more robust to environmental change than those reared under constant thermal conditions (Schaefer and Ryan 2006). Therefore, it is also possible that the sourcing of the fish in our study (aquarium hatched) may partially drive down the phenotypic plasticity observed when exposed to cycling temperatures in later life stages compared to wild populations. Wild populations may be highly adapted to local, specific thermal experience; captive breeding or domestication can significantly shift genetic mechanisms both through intentional (e.g. selective) and unintentional biological manipulation (Lorenzen et al. 2012; Whiteley et al. 2011). Genetic strain has the potential to alter response mechanisms for domestic strains, both compared to one another and to wild strains (Carline and Machung 2001; Kristensen et al. 2002); such shifts may then alter key behaviors such as flightiness that mediate interactions between fish and their environment (Robison and Rowland 2005; Vincent 1960). For example, wild strains of brown trout (Slamo trutta), brook trout (Salvelinus fontinalis), and rainbow trout (Oncorhynchus mykiss) all had significantly higher CTMax compared to domesticated strains, with the largest difference at 1.6 °C greater for wild-strains (Carline and Machung 2001; Vincent 1960). In contrast, wild-caught zebrafish (Danio rerio) demonstrated ~ 1 °C lower thermal tolerance, suggesting the propensity for captive-bred animals to either under or overestimate discreet responses may be species, strain, and context dependent (Morgan et al. 2019). Generally, captive bred and domesticated individuals often perform less efficiently when released compared to wild populations, with notably lower fitness and reproductive success (Lorenzen et al. 2012). However, while discreet values of thermal tolerance are comparatively different between wild-caught and laboratory populations, overall trends persist, and those alterations to discreet values are conserved through several generations of domestication (Morgan et al. 2019). Future research using similar analysis applied toward comparing fish reared in variable environments to static environments may provide insight on the influence of variability in the context of life history. Further, such research could highlight potential effects in relation to timing or duration of exposure.
Convict cichlid is a eurythermal species capable of living in a wide range of thermal environments (Esmaeili et al. 2013; Ishikawa and Tachihara 2010; Piazzini et al. 2010; Duffy et al. 2013; De La Torre Zavala et al. 2018). The similarity of metabolic rate during cycling temperatures, across the acclimation treatments, could be reflective of this nature. Future studies should examine if this trend persists in other invasive, “robust” species, or in fishes more broadly. This study builds on our understanding of impact for “more natural” thermal cycling acclimation compared to commonly used constant-temperature acclimation techniques. Few studies assess immediate and continuous metabolic measurements, particularly in the context of dynamic temperatures. Recent thermal history (within a range of 1–14 days) in particular may have a significant impact on thermal tolerance and cardiac performance (Drake et al. 2017). Further investigation into this immediate time period could highlight how readily different species may respond to rapid thermal change.
Code availability
R Studio.
References
Allen KO, Strawn K (1971) Rate of acclimation of juvenile channel catfish, Ictalurus punctatus, to high temperatures. Trans Am Fish Soc 100:665–671
Barshis DJ, Ladner JT, Oliver TA et al (2013) Genomic basis for coral resilience to climate change. Proc Natl Acad Sci USA 110:1387–1392
Bennett WA (2010) Extreme physiology of intertidal fishes of the Wakatobi. Marine research and conservation in the Coral Triangle. Nova Science Publishers, New York
Bilyk KT, Evans CW, DeVries AL (2012) Heat hardening in Antarctic notothenioid fishes. Polar Biol 35:1447–1451
Brattstrom BH, Lawrence P (1962) The rate of thermal acclimation in anuran amphibians. Physiol Zool 35:148–156
Brett JR (1956) Some principles in the thermal requirements of fishes. Q Rev Biol 31:75–87
Bullock TH (1955) Compensation for temperature in the metabolism and activity of poikilotherms. Biol Rev 30:311–342
Burggren WW (2019) Inadequacy of typical physiological experimental protocols for investigating consequences of stochastic weather events emerging from global warming. Am J Physiol Regul Integr Comp Physiol 316:R318–R322
Burggren WW, Arriaga-Bernal JC, Méndez-Arzate PM, Méndez-Sánchez JF (2019) Metabolic physiology of the Mayan cichlid fish (Mayaheros uropthalmus): re-examination of classification as an oxyconformer. Comp Biochem Physiol Part A Mol Integr Physiol 237:110538
Carline RF, Machung JF (2001) Critical thermal maxima of wild and domestic strains of trout. Trans Am Fish Soc 130:1211–1216
Chadwick JG, Nislow KH, McCormick SD (2015) Thermal onset of cellular and endocrine stress responses correspond to ecological limits in brook trout, an iconic cold-water fish. Conserv Physiol 3:cov017
Chipps SR, Clapp DF, Wahl DH (2000) Variation in routine metabolism of juvenile muskellunge: evidence for seasonal metabolic compensation in fishes. J Fish Biol 56:311–318
Chung KS (2000) Heat resistance and thermal acclimation rate in tropical tetra Astyanax bimaculatus of Venezuela. Environ Biol Fish 57:459–463
Chung KS (2001) Critical thermal maxima and acclimation rate of the tropical guppy Poecilla reticulata. Hydrobiologia 462:253–257
Clark TD, Raby GD, Roche DG et al (2020) Ocean acidification does not impair the behaviour of coral reef fishes. Nature 577:370–375
Colinet H, Sinclair B, Vernon P, Renault D (2015) Insects in fluctuating thermal environments. Annu Rev Entomol 60:123–140
Corey E, Linnansaari T, Cunjak RA, Currie S (2017) Physiological effects of environmentally relevant, multi-day thermal stress on wild juvenile Atlantic salmon (Salmo salar). Conserv Physiol 5:cox14
Currie RJ, Bennett WA, Beitinger TL, Cherry DS (2004) Upper and lower temperature tolerances of juvenile freshwater game-fish species exposed to 32 days of cycling temperatures. Hydrobiologia 523:127–136
Cutler KB, Edwards RL, Taylor FW et al (2003) Rapid sea-level fall and deep-ocean temperature change since the last interglacial period. Earth Planet Sc Lett 206:253–271
Dame RF, Vernberg FJ (1978) The influence of constant and cyclic acclimation temperatures on the metabolic rates of Panopeus herbstii and Uca pugilator. Biol Bull 154:188–197
Das T, Pal AK, Chakraborty SK et al (2004) Thermal tolerance and oxygen consumption of Indian major carps acclimated to four temperatures. J Therm Biol 29:157–163
Davis BE, Hansen MJ, Cocherell DE et al (2019) Consequences of temperature and temperature variability on swimming activity, group structure, and predation of endangered delta smelt. Freshw Biol 64:2156–2175
De La Torre Zavala AM, Arce E, Luna-Figueroa J, Córdoba-Aguilar A (2018) Native fish, Cichlasoma istlanum, hide for longer, move and eat less in the presence of a non-native fish, Amatitlania nigrofasciata. Environ Biol Fish 101:1077–1082
Dowd WW, King FA, Denny MW (2015) Thermal variation, thermal extremes and the physiological performance of individuals. J Exp Biol 218:1956–1967
Drake MJ, Miller NA, Todgham AE (2017) The role of stochastic thermal environments in modulating the thermal physiology of an intertidal limpet, Lottia digitalis. J Exp Biol 220:3072–3083
Duffy R, Snow M, Bird C (2013) The convict cichlid Amatitlania nigrofasciata (Cichlidae): first record of this non-native species in Western Australian waterbodies. Rec West Aust Mus 28:7–12
Eme J, Bennett WA (2008) Low temperature as a limiting factor for introduction and distribution of Indo-Pacific damselfishes in the eastern United States. J Therm Biol 33:62–66
Eme J, Bennett WA (2009) Critical thermal tolerance polygons of tropical marine fishes from Sulawesi, Indonesia. J Therm Biol 34:220–225
Eme J, Dabruzzi TF, Bennett WA (2011) Thermal responses of juvenile squaretail mullet (Liza vaigiensis) and juvenile crescent terapon (Terapon jarbua) acclimated at near-lethal temperatures, and the implications for climate change. J Exp Mar Biol Ecol 399:35–38
Enders EC, Boisclair D (2016) Effects of environmental fluctuations on fish metabolism: Atlantic salmon Salmo salar as a case study. J Fish Biol 88:344–358
Esmaeili HR, Gholamifard A, Sayyadzadeh G et al (2013) New record of the convict cichlid, Amatitlania nigrofasciata (Günther, 1867), from the Middle East (Actinopterygii: Cichlidae). Aqua, Int J Ichthyol 19:225–229
Feldmeth CR (1981) The evolution of thermal tolerance in desert pupfish (genus Cyprinodon)
Gabriel W, Luttbeg B, Sih A, Tollrian R (2005) Environmental tolerance, heterogeneity, and the evolution of reversible plastic responses. Am Nat 166:339–353
Giomi F, Mandaglio C, Ganmanee M et al (2016) The importance of thermal history: costs and benefits of heat exposure in a tropical, rocky shore oyster. J Exp Biol 219:686–694
Hansen J, Sato M, Ruedy R et al (2006) Global temperature change. Proc Natl Acad Sci USA 103:14288–14293
Hansen J, Ruedy R, Sato M, Lo K (2010) Global surface temperature change. Rev Geophys 48:RG4004
Hazel JR (1984) Effects of temperature on the structure and metabolism of cell membranes in fish. Am J Physiol Regul Integr Comp Physiol 246:R460–R470
Heath AG, Turner BJ, Davis WP (1993) Temperature preferences and tolerances of three fish species inhabiting hyperthermal ponds on mangrove islands. Hydrobiologia 259:47–55
Holsman K, Danner E (2016) Numerical integration of temperature-dependent functions in bioenergetics models to avoid overestimation of fish growth. Trans Am Fish Soc 145:334–347
Hovey TE, Swift CC (2012) First record of an established population of the convict cichlid (Archocentrus nigrofasciatus) in California. Calif Fish Game 98:125–128
Ishikawa T, Tachihara K (2010) Life history of the nonnative convict cichlid Amatitlania nigrofasciata in the Haebaru Reservoir on Okinawa-jima Island, Japan. Environ Biol Fish 88:283–292
Kern P, Cramp RL, Franklin CE (2015) Physiological responses of ectotherms to daily temperature variation. J Exp Biol 218:3068–3076
Kochhann D, Campos DF, Val AL (2015) Experimentally increased temperature and hypoxia affect stability of social hierarchy and metabolism of the Amazonian cichlid Apistogramma agassizii. Comp Biochem Physiol C 190:54–60
Kristensen T, Dahlgaard J, Loeschcke V (2002) Inbreeding affects Hsp70 expression in two species of Drosophila even at benign temperatures. Evol Ecol Res 4:1209–1216
Loeschcke V, Hoffmann AA, Huey AERB, Whitlock EMC (2007) Consequences of heat hardening on a field fitness component in Drosophila depend on environmental temperature. Am Nat 169:175–183
Lorenzen K, Beveridge MCM, Mangel M (2012) Cultured fish: integrative biology and management of domestication and interactions with wild fish. Biol Rev 87:639–660
Lu C-Y, Hsu C-Y (2015) Ambient temperature reduction extends lifespan via activating cellular degradation activity in an annual fish (Nothobranchius rachovii). Age 37:33
Maness JD, Hutchison VH (1980) Acute adjustment of thermal tolerance in vertebrate ectotherms following exposure to critical thermal maxima. J Therm Biol 5:225–233
McDonnell LH, Chapman LJ (2015) At the edge of the thermal window: effects of elevated temperature on the resting metabolism, hypoxia tolerance and upper critical thermal limit of a widespread African cichlid. Conserv Physiol 3:cov050
Mora C, Ospína A (2001) Tolerance to high temperatures and potential impact of sea warming on reef fishes of Gorgona Island (tropical eastern Pacific). Mar Biol 139:765–769
Morash AJ, Neufeld C, MacCormack TJ, Currie S (2018) The importance of incorporating natural thermal variation when evaluating physiological performance in wild species. J Exp Biol 221:164673
Morgan R, Sundin J, Finnøen MH et al (2019) Are model organisms representative for climate change research? Testing thermal tolerance in wild and laboratory zebrafish populations. Conserv Physiol 7:coz036
Ospina AF, Mora C (2004) Effect of body size on reef fish tolerance to extreme low and high temperatures. Environ Biol Fish 70:339–343
Otto RG (1973) Temperature tolerance of the mosquitofish, Gambmia affinis (Baird and Girard). J Fish Biol 5:575–585
Otto RG, Gerking SD (1973) Heat tolerance of a Death Valley pupfish (Genus Cyprinodon). Physiol Zool 46:43–49
Parmesan C, Root TL, Willig MR (2000) Impacts of extreme weather and climate on terrestrial biota. B Am Meteorol Soc 8:443–450
Pérez E, Dı́az F, Espina S, (2003) Thermoregulatory behavior and critical thermal limits of the angelfish Pterophyllum scalare (Lichtenstein) (Pisces: Cichlidae). J Therm Biol 28:531–537
Piazzini S, Lori E, Favilli L et al (2010) A tropical fish community in thermal waters of southern Tuscany. Biol Invasions 12:2959–2965
Robison BD, Rowland W (2005) A potential model system for studying the genetics of domestication: behavioral variation among wild and domesticated strains of zebra danio (Danio rerio). Can J Fish Aquat Sci 62:2046–2054
Sandersfeld T, Davison W, Lamare MD et al (2015) Elevated temperature causes metabolic trade-offs at the whole-organism level in the Antarctic fish Trematomus bernacchii. J Exp Biol 218:2373–2381
Schaefer J, Ryan A (2006) Developmental plasticity in the thermal tolerance of zebrafish Danio rerio. J Fish Biol 69:722–734
Schofield PJ, Loftus WF, Kobza RM et al (2010) Tolerance of nonindigenous cichlid fishes (Cichlasoma urophthalmus, Hemichromis letourneuxi) to low temperature: laboratory and field experiments in south Florida. Biol Invasions 12:2441–2457
Seebacher F, White C, Franklin C (2014) Physiological plasticity increases resilience of ectothermic animals to climate change. Nat Clim Change 5:61–66
Sollid J, Nilsson GE (2006) Plasticity of respiratory structures—adaptive remodeling of fish gills induced by ambient oxygen and temperature. Resp Physiol Neurobi 154:241–251
Somero G (2012) The physiology of global change: linking patterns to mechanisms. Ann Rev Mar Sci 4:39–61
Stauffer JR, Boltz SE (1994) Effect of salinity on the temperature preference and tolerance of age-0 Mayan cichlids. Trans Am Fish Soc 123:101–107
St-Pierre J, Charest P-M, Guderley H (1998) Relative contribution of quantitative and qualitative changes in mitochondria to metabolic compensation during seasonal acclimatisation of rainbow trout Oncorhynchus mykiss. J Exp Biol 201:2961–2970
Threader RW, Houston AH (1983) Heat tolerance and resistance in juvenile rainbow trout acclimated to diurnally cycling temperatures. Comp Biochem Phys A 75:153–155
Tunnah L, Currie S, MacCormack TJ (2017) Do prior diel thermal cycles influence the physiological response of Atlantic salmon (Salmo salar) to subsequent heat stress? Can J Fish Aquat Sci 74:127–139
Vincent RE (1960) Some influences of domestication upon three stocks of brook trout (Salvelinus fontinalis Mitchill). Trans Am Fish Soc 89:35–52
Whiteley AR, Bhat A, Martins EP, Mayden RL, Arunachalam M, Uusi-Heikkilä S, Ahmed ATA, Shrestha J, Clark M, Stemple D, Bernatchez L (2011) Population genomics of wild and laboratory zebrafish (Danio rerio). Mol Ecol 20:4259–4276
Williams CM, Marshall KE, MacMillan HA et al (2012) Thermal variability increases the impact of autumnal warming and drives metabolic depression in an overwintering butterfly. PLoS ONE 7:e34470
Wisenden BD (1995) Reproductive behaviour of free-ranging convict cichlids, Cichlasoma nigrofasciatum. Environ Biol Fish 43:121–134
Acknowledgements
The authors wish to express their gratitude to the Eme Physiology lab members for their help in CTM trials. Thanks to Dr. Casey Mueller for help with oxygen consumption analyses. This work was partly supported by startup funds from California State University San Marcos to J. E.
Funding
CSUSM startup funds to JE.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author(s) declare no competing interests.
Ethics approval
IACUC Protocol 17-004, CSUSM.
Availability of data and material
Data is archived in the data repository Dryad (https://datadryad.org/stash/share/sMIFBjzyNmhCGD6STtHbgpt5gOxgqCwjAx4RWlFBkPo).
Additional information
Communicated by K.H. Dausmann.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Cooper, C.J., Kristan, W.B. & Eme, J. Thermal tolerance and routine oxygen consumption of convict cichlid, Archocentrus nigrofasciatus, acclimated to constant temperatures (20 °C and 30 °C) and a daily temperature cycle (20 °C → 30 °C). J Comp Physiol B 191, 479–491 (2021). https://doi.org/10.1007/s00360-021-01341-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00360-021-01341-5