Abstract
Incubation conditions are critical in determining numerous traits in reptilian neonates. This is particularly significant in species with low offspring survival such as sea turtle species, because of the extremely high predation rates that hatchlings face during their initial dispersal from nesting beaches. Hatchlings that develop in suboptimal nest environments are likely to be smaller, slower and more susceptible to predation than hatchlings from optimal nest environments. Previous studies have focused on the effects of temperature on hatchling traits, but few have investigated the effects of moisture concentrations, despite moisture levels in nests influencing hatchling size, sex, incubation duration, and hatching success. Here, we incubated eggs of three sea turtle species at various moisture levels and tested the terrestrial and aquatic locomotor performance of the resultant hatchlings during the frenzy and post-frenzy period. We also compared and evaluated the ontogeny of early locomotor performance for each species over the first months of life. Drier incubation conditions produced hatchlings that crawled more slowly and took longer to self-right than hatchlings from wetter incubation conditions. There was no difference in swimming performance associated with moisture treatments. We suggest that moisture in the nest environment during incubation may influence hatchling performance via their initial hydration levels. Thus, nest moisture influences terrestrial performance (i.e., escaping from the nest and dispersing across the beach), although upon entering the ocean hatchlings have the opportunity to rehydrate by drinking and thus, differences in locomotor performance associated with moisture treatments cease.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Many oviparous species lay their eggs in nests to reduce environmental fluctuations and optimise nest conditions (Blackburn 1999). However, embryos can still experience considerable environmental variation in nest conditions as a result of local weather and climatic variation (Ackerman et al. 1997; Cagle et al. 1993). Additionally, nest location can result in considerable differences in incubation environments based on shade availability or proximity to water sources (Hill et al. 2015; van de Merwe et al. 2006; Wood et al. 2014).
Within the vertebrates whose parental care ends with nest site selection, and hence whose eggs are exposed to the external environment, sea turtles have been the focus of numerous studies on the effects of incubation conditions on embryonic development and hatchling traits (Booth 1998, 2006, 2017; Caut et al. 2010; Lolavar and Wyneken 2015, 2017). Sea turtle nesting seasons can last for many months, often starting in cool, wet conditions and lasting until conditions become warm and dry (Dornfeld et al. 2015). Additionally, their nesting takes place on coastal beaches, that under climate change scenarios, are predicted to be affected by increased air and sea temperatures, sea level rise, altered rainfall patterns and increased storm frequency and intensity (Fuentes et al. 2010a, b; IPCC 2014). The majority of studies on the effects of incubation conditions on sea turtles have focused on temperature. These studies showed that warmer incubation temperatures increase female hatchling production (Godfrey and Mrosovsky 2006; Godley et al. 2002) and produce smaller, weaker hatchlings (Booth 2006, 2017) than cooler incubation temperatures.
Despite the strong effect of incubation temperature on hatchling traits, few studies have investigated the effects of other environmental factors, such as moisture. Moisture of the incubation environment has been shown to influence hatchling morphology and hatching success (Kraemer and Bell 1980; McGehee 1990; Ragotzkie 1959), while more recent studies have begun to investigate how moisture influences hatchling sex ratios (Lolavar and Wyneken 2017; Wyneken and Lolavar 2015). In addition to potential direct effects, moisture can exert an indirect effect via alteration of other environmental factors, such as temperature (Lolavar and Wyneken 2015). However, compared to other hatchling traits, the effect of moisture during incubation on locomotor performance of sea turtle neonates has been relatively unstudied.
Understanding the factors that determine locomotor performance in sea turtle hatchlings is important because of the importance of a brief period of extreme activity termed the ‘frenzy period’ (Carr and Ogren 1960). The frenzy is characterised by heightened activity, lasting approximately 24 h, that sea turtle hatchlings undergo as they emerge from their nest, crawl from the nest to the water and then swim rapidly and continuously to reach offshore waters as quickly as possible (Wyneken and Salmon 1992). Hatchlings that are slower crawlers spend more time exposed to terrestrial predators and hatchlings that spend less time swimming or are slower swimmers spend more time in nearshore, predator dense zones (Whelan and Wyneken 2007). Therefore, slower crawlers and swimmers are more likely to be preyed upon (Gyuris 1994). Variation in hatchling performance, as a result of incubation conditions, can alter hatchling survival rates (Cavallo et al. 2015). Altered hatchling recruitment may result in changed population dynamics and impacts to population viability.
The majority of hatchling dispersal occurs in the ocean and thus, hatchling swimming performance has the greatest influence on hatchling survival. Sea turtle hatchlings generally exhibit four swimming behaviours: power stroking, dog paddling, ‘rearflipper kicking’ and resting (Wyneken 1997). Power stroking is described by swimming with both flippers flapping in unison and generates thrust on the down stroke and occasionally on the up stroke (Booth 2014). The dog paddling is a ‘front crawl’ type stroke where the hatchlings alternate protraction and retraction of diagonally opposite flippers and hind feet as they swim. This behaviour is generally used by hatchlings as they orient or breathe. Rear flipper kicking produces thrust by the hind limbs alone and is used after the frenzy. The last behaviour is resting, characterized by hatchlings flexing the flippers over the carapace and tucking the hind limbs as they passively float at the surface. This behaviour is seldom seen during the initial stages of dispersal, but hatchlings spend more time resting as they tire.
One overall measure of swimming performance is mean swim thrust, or the mean amount of thrust produced over the entire swimming trial, because this measure incorporates other attributes of swimming performance into a single value (Booth 2009; Booth and Evans 2011). Other attributes indicate the amount of time that hatchlings spend exhibiting certain swimming forms, such as the proportion of time spent powerstroking over an entire swimming trial, and the duration of individual powerstroking bouts. Hatchlings that spend a higher proportion of their swimming trial powerstroking or have longer powerstroking bouts are able to complete more powerstrokes and thus, are likely to produce higher mean thrust. Another attribute of swimming performance is stroke frequency during powerstroking bouts or stroke-rate during powerstroking bouts. Hatchlings that powerstroke at higher frequencies complete more powerstrokes and are likely to produce higher mean thrust. Lastly, mean maximum thrust is a measure of the maximum thrust production per powerstroke. Producing more thrust per powerstroke allows hatchlings to produce higher mean thrust. Thus, mean swim thrust provides an overall measure of swimming performance while the other attributes reflect the amount of time that hatchlings spend performing specific behaviours, the rate at which they stroke and the amount of thrust that they can produce per stoke (Booth 2009; Booth and Evans 2011). This allows us to directly compare hatchlings and to analyse the differences between hatchlings that result in altered swimming performance.
Here, we investigated how moisture levels during incubation influence locomotor performance by incubating eggs from three species of sea turtle in different moisture conditions. We also evaluated the ontogeny of each species’ locomotor performance and its potential consequences for population dynamics and viability. Finally, we compared the locomotor performance of the three species to identify potential differences in life history and how this may influence the impact of moisture levels during incubation. Our goal was to develop a greater understanding of how changes in moisture levels during incubation may alter hatchling recruitment and population dynamics.
Methods
Egg collection
We collected eggs from Australian populations of green sea turtles (Chelonia mydas) from Heron Island, Queensland, flatback sea turtles (Natator depressus) from Curtis Island, Queensland and olive ridley sea turtles (Lepidochelys olivacea) from Tiwi Islands, Northern Territory. We patrolled nesting beaches at night and collected the eggs as they were laid or just after the female finished laying if we found the female covering her nest.
Egg transportation
Eggs were placed in plastic bags that were vacuum-sealed within 1 h of being laid following the protocol of Williamson et al. (2017b). Vacuum sealing soon after oviposition delays the breaking of embryonic diapause by preventing eggs from being exposed to atmospheric oxygen, and ensures that embryos do not experience movement-induced mortality during transport (Rafferty et al. 2013; Williamson et al. 2017a). Bags of eggs were then placed in an insulated container lined with vermiculite or bubble wrap. Each container contained ice packs to maintain the temperature at 10–12 °C during transport to Monash University, Melbourne, where eggs were placed into incubators filled with sand. While in transport, green sea turtle eggs were sealed for approximately 30 h; three olive ridley clutches were sealed for 72 h and the other three were sealed for approximately 24 h; one flatback clutch was sealed for 48 h and the other five were sealed for 24 h.
Experimental design
Each clutch of eggs was divided into three equal groups and allocated to a moisture treatment (detailed below). We collected 75 eggs from 3 green turtle females and 68 from a fourth female (293 eggs total). Twenty-five eggs were allocated to each moisture treatment for the first 3 females but for the fourth, 23 eggs were allocated to the 4% moisture treatment, 23 to the 6% moisture treatment and 22 to the 8% moisture treatment. For olive ridleys and flatbacks (180 eggs per species), we collected 30 eggs from 6 females of each species and allocated 10 eggs per clutch to each moisture treatment.
Incubation conditions
We allocated eggs from each clutch to three moisture treatments (4%, 6% and 8% w/w). These three moisture contents represent low, intermediate and high values in natural nests, while still ensuring successful embryonic development (Patino-Martinez et al. 2014). All eggs were incubated at each species’ pivotal temperature: 27.6 °C for green turtles and 29.3 °C for flatback turtles (Limpus 2008). Olive ridley pivotal temperatures vary significantly globally (Plotkin 2007) and are unknown for the Tiwi Island population. Thus, we maintained olive ridley eggs at the pivotal temperature of the (geographically) closest sea turtle population with measured pivotal temperatures, which in this case was 29.4 °C measured for the Cape Domett flatback population (Stubbs et al. 2014). Each group of eggs from every moisture treatment and clutch combination was placed in their own incubator (Hovabator 1602 N, GQF Manufacturing, Georgia, USA). All incubators were housed in a temperature-controlled room set to 25 °C. Eggs were buried in sand (Richgro Play Sand, 98% crystalline silica) with the top of the egg exposed so that we could monitor white spot formation as an indicator of embryonic development. Eggs that began to turn yellow, indicating embryonic death, were removed from the incubator. Once all remaining eggs had formed white spots, we covered the eggs fully with sand. As we were unable to determine whether eggs died from natural causes or from transport-induced causes, hatching success was calculated from the number of eggs that were collected. Incubator temperature was monitored daily using temperature probes (Pasco PS-2135, Roseville, California USA) buried next to the eggs.
Each incubator was filled with a known mass of dry sand and we added water to the sand to create the appropriate moisture concentration. We took multiple samples of sand (2–3 g total) from around the eggs each day and calculated the moisture concentration of the sand by weighing, drying and then reweighing the sand. Moisture concentration was calculated using the following formula, where weight is measured in grams:
Using a spray bottle, we then added the amount of distilled water required to maintain the appropriate sand moisture concentration. All sand was replaced after drying to ensure total sand volume and mass did not decrease.
Hatchling testing
We gave hatchlings, depending on their activity levels, 24–48 h post-emergence to internalize their yolk sac before removing them from incubators. Hatchlings were marked on the carapace with non-toxic nail polish with unique patterns for identification, then we measured head width, straight carapace length (SCL), straight carapace width (SCW), flipper length (± 0.01 mm) using digital callipers and measured mass using electronic scales (± 0.01 g). We then selected five hatchlings at random from each incubator to be tested. Thus, five hatchlings were tested from each combination of moisture treatment and clutch.
Locomotor and self-righting performance testing occurred during daylight hours in a darkened room without windows and with the air temperature set at 25 °C (range: 23.8–25.5°). First, we tested hatchling self-righting ability by placing hatchlings upside-down onto their carapace. Hatchlings were tested on moist, level sand and we recorded the time it took hatchlings to right themselves. Each hatchling was tested five times and was considered to have failed the trial if it did not right itself within 30 s (Rings et al. 2014). We then determined the mean time it took hatchlings to self-right (failed trials were counted as 30 s) and the number of times a hatchling was able to successfully self-right within 30 s.
Next, we tested hatchling crawling ability along a level 2.4 m ‘racetrack’ using PVC guttering lined with moist sand and a white light at one end. Hatchlings were placed at the opposite end of the racetrack to the light and were timed as they crawled towards the light. Each hatchling was tested twice to simulate a minimal crawl to the water, and we report the mean of the two trials here.
Lastly, we tested hatchling swimming ability at hatching and when the hatchlings were 4 weeks old, following the protocol of Gatto and Reina (2020). We placed hatchlings into Lycra® ‘swimsuit’ harnesses that did not impede their flipper movements. Each vest was attached to a load cell (PS-2201, Pasco, USA) with fishing line so that the load cell recorded the amount of thrust (Newtons) produced with each stroke taken by the hatchling. Hatchlings were encouraged to swim unidirectionally using a white light and the load cells measured thrust production 20 times per second. Swimming performance recordings were started as soon as the hatchlings began to powerstroke. Load cells were calibrated by hanging a weight of known mass from each load cell, while water temperature was recorded using a digital aquarium thermometer. Water temperature ranged from 25.0 to 28.6 °C.
Using this technique, we measured five attributes of swimming performance. First, mean swim thrust (N) produced over an entire swimming trial. Second, the proportion of time that hatchlings spent power-stroking over an entire swimming trial (%). Third, the powerstroke frequency of hatchlings during power-stroking bouts (strokes per minute). Fourth, the duration of power-stroking bouts (s) and fifth, the mean maximum thrust (N). After hatchlings were tested during the frenzy, they were housed (conditions described below) until they were 4 weeks old. Swimming trials during the frenzy lasted for 2 h, because predation rates are generally highest within the first few hours of dispersal because of higher predator densities in near-shore waters (Whelan and Wyneken 2007). This means that swimming performance within the first few hours is likely to have a considerable effect on survival rates. Once hatchlings enter pelagic waters, predator densities decrease (Whelan and Wyneken 2007), and so do hatchling activity levels (Booth 2009; Wyneken and Salmon 1992). Therefore, during post-frenzy testing when hatchlings were four weeks of age, swimming trials lasted for 30 min to reflect ecologically relevant periods of swimming activity. We used the same hatchlings at both 0 and 4 weeks of age but we replaced hatchlings that died at random from within the same combination of clutch and moisture treatment.
Hatchling housing
Hatchlings were housed in 3 L and 10 L plastic tanks or in glass tanks divided with plastic mesh (12.5 mm grid, Aquasonic, Australia). Tanks were kept clean by a continuous flow-through system consisting of a drum filter (Faivre 60 series, Faivre, France), fluid sand bed filters (RK2 systems, USA), a protein skimmer (RK10AC, RK2 systems, USA), a UV filter (240 W UV steriliser, Emperor Aquatics, USA) and an ozone steriliser (RK300MG, RK2 systems, USA). Water quality was monitored daily using OxyGuard hand-held monitors (Technolab, Australia). Water temperature was maintained between 26 and 27 °C using a heater (3 kW heater, Shego, Germany) and a chiller (FBT175SSD, Toyesi, Australia). Animals were maintained under a 12:12 day/night cycle and provided with UV lighting (Exo Terra Repti Glo 5.0 25 W). Turtles were fed ~ 2% of their body mass daily (Higgins 2003) with commercial turtle pellets (4 mm Marine float range, Ridley Aquafeed).
Hatchling release
After the second round of testing at 4 weeks of age, hatchlings were placed into plastic containers with holes drilled in the sides and lid and with foam lining the bottom of the containers. The hatchlings then were transported back to their natal beach and released offshore.
Statistical analysis
All statistical tests were performed in R (R Core Team 2014).
Differences in incubation conditions between treatment groups were tested for normality and were analysed using ANOVA and Tukey’s HSD.
We used linear mixed-effects models in the lme4 package (Bates 2007) to compare hatching success and incubation duration between moisture treatments. We used treatment as the fixed effect and clutch was the random effect.
We analysed the effect of moisture treatment on hatchling morphology using linear mixed effects models with moisture treatment as the fixed effect and clutch as the random effect.
When evaluating the effect of moisture treatment on hatchling locomotor performance, we used linear mixed-effects models with moisture treatment as the fixed effect. Our random effects were clutch and test temperature. Test temperature was the air temperature for self-righting and crawling tests and was the water temperature for swimming tests. When testing the effect of moisture treatment on the ability of hatchlings to self-right, we analysed the number of times a hatchling was able to successfully self-right as a binomial where 1 was 5 successful attempts, 0.6 was 3 successful attempts and 0 was no successful attempts.
We analysed the change in swimming performance over time using linear mixed-effects models with behavioural stage (frenzy or post-frenzy) as the fixed effect and hatchling ID, clutch, moisture treatment and water temperature as the random effects. Our hatchling ID random effect accounted for repeated measures by allowing each individual’s y-intercept to vary, which accounts for differences between those individuals.
Lastly, we compared the locomotor performance among species during the frenzy and post-frenzy periods, respectively, using linear mixed-effects models. Species was the fixed effect and clutch, moisture treatment and test temperature were the random effects.
The response of each species and each measure of terrestrial locomotor performance to moisture levels during incubation was inconsistent. To determine the overall response of sea turtle terrestrial locomotor performance to moisture levels during incubation, we performed a within-study multivariate meta-analysis following the protocol of McQueen et al. (2017). We excluded swimming performance from the analysis, because we did not observe a response to moisture treatment in any of our swimming performance indicators. We used the R package ‘metafor’ (Viechtbauer 2010) and equations described in Nakagawa and Cuthill (2007) to run our weighted model with restricted maximum-likelihood to account for variation in sample sizes between tests. To account for the non-independence caused by measuring multiple locomotor performance indicators in the same hatchlings, we incorporated a variance–covariance matrix. The matrix included the within-species variance associated with each measure of terrestrial locomotor performance, and the covariances between dependent variables. The covariances were calculated using the correlation coefficients for each combination of response variables that measured the same hatchlings (i.e., between crawling speed and average time to self-right within species). To make interpretation of the results clearer, our response variables were the average time to self-right, the number of failed self-righting attempts and the average time it took hatchlings to complete crawling trials. Positive values are, therefore, associated with poorer locomotor performance (i.e., longer crawling times, longer self-righting times and more failed self-righting attempts). Thus, negative Zr values support the hypothesis that higher moisture levels produce faster crawlers and self-righters, while positive Zr values support the hypothesis that lower moisture levels produce faster crawlers and self-righters.
Animal ethics and permits
Eggs were collected under Queensland scientific purposes permit WITK17747816 (Chelonia mydas) and WITK18685417 (Natator depressus) and Northern Territory permit to take wildlife 62703 (Lepidochelys olivacea). Hatchlings were housed and tested under research permit 10008208 and all procedures were approved by the Monash University Biological Sciences Animal Ethics Committee (BSCI/2016/23).
Results
Incubation conditions
The actual incubation moisture percentages in our experimental treatments (nominally 4%, 6% and 8% moisture) were statistically different within each species (Green (GR) t10 = 16.569, p < 0.001; Olive ridley (OR) t16 = 34.629, p < 0.002; Flatback (FL) t15 = 22.872, p < 0.001, Table 1). There was no difference in incubation temperatures between moisture treatments within any of the three species (GR t10 = 1.43, p = 0.183; OR t16 = 0.919, p = 0.372; FL t15 = − 0.385, p = 0.706, Table 1).
Hatching success and incubation duration
Moisture treatment did not influence incubation duration for green hatchlings (F1,7 = 0.473, p = 0.514), olive ridley hatchlings (F1,9.791 = 0.782, p = 0.398) or flatbacks (F1,11.061 = 2.115, p = 0.174). Clutch effects explained 0.88% of the variance in incubation duration in green hatchlings, 23.97% in olive ridleys and 2.43% in flatback hatchlings.
For green (F1 = 0.628, p = 0.428) and olive ridley sea turtles (F1 = 0.227, p = 0.633), moisture treatment did not influence hatching success, but flatback eggs incubated at 4% moisture had significantly lower hatching success than eggs incubated at 6% or 8% moisture (F1 = 14.713, p < 0.001, Table 1).
Hatchling morphometrics
The effect of moisture during incubation on hatchling morphometrics varied with species. Moisture concentrations did not correlate with green hatchling morphometrics at any age.
In 4-week-old olive ridleys, turtles incubated at 4% moisture had narrower heads than turtles incubated at 6% or 8% moisture (F1,101.88 = 12.584, p < 0.001). Four-week-old olive ridleys incubated at 8% moisture were longer (F1,102.14 = 10.727, p = 0.001) and heavier (F1,102.98 = 4.431, p = 0.038) than hatchlings incubated at 4%, but neither moisture treatment differed from turtles incubated at 6% moisture. Four-week-old olive ridleys incubated at 6% moisture were wider than turtles incubated at 4% moisture (F1,103.27 = 4.435, p = 0.038), but neither the 6% nor 4% moisture treatments differed from the 8% moisture treatment. Lastly, 0-week-old olive ridley hatchlings incubated at 6% moisture had longer flippers than those incubated at 4% moisture (F1,114.52 = 6.262, p = 0.014), but hatchlings incubated at 8% moisture did not differ from the other treatment groups.
In flatbacks, 0-week-old hatchlings incubated at 8% moisture had narrower heads than those incubated at 6% or 4% moisture (F1,121 = 7.866, p < 0.001). At 4 weeks old, turtles incubated at 4% moisture were heavier than those incubated at 6% or 8% moisture (F1,112.85 = 4.918, p = 0.029).
The statistical differences between moisture concentrations and variance explained by our random effect (clutch) can be found in Table 2.
Effect of moisture on locomotor performance
Statistical results of linear mixed effects models evaluating differences in locomotor performance between moisture treatments are shown in Supplementary Table 1, Fig. 1 and Table 3.
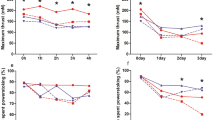
The effect of moisture treatment on our measures of hatchling terrestrial locomotor performance (mean ± standard error). Each hatchling was tested five times for self-righting ability and were tested twice on a 2.4 m racetrack. Letters represent differences between moisture treatments within each species
Moisture treatment did not influence the time it took green turtle hatchlings to self-right, how often a green hatchling was able to successfully self-right in less than 30 s or crawling speed (Fig. 1, Table 3).
Olive ridley hatchlings incubated at 4% moisture were slower to self-right, failed to self-right more often and were slower crawlers than those incubated at 6% or 8% moisture. Hatchlings incubated at 6% were slower to self-right, failed to self-right more often and were slower crawlers than those incubated at 8% moisture (Fig. 1, Table 3).
Flatback hatchlings incubated at 4% moisture were slower to self-right and failed to self-right more often than hatchlings incubated at 6% or 8% moisture. There was no difference between hatchlings incubated at 6% and 8% moisture. Moisture treatment did not influence flatback hatchling crawling speed (Fig. 1, Table 3).
Moisture treatment did not affect swimming performance at hatching or at 4 weeks of age in any of the three species, with no difference in mean swim thrust, mean maximum thrust, powerstroke frequency, the duration of powerstroking bouts or the proportion of time spent powerstroking in hatchlings of the same species (Table 3).
Change in swimming attributes over time
Our swimming performance attributes in green and flatback hatchlings changed considerably from the frenzy to post-frenzy period, with mean swim thrust increasing in green hatchlings but decreasing in flatback hatchlings over time. This change in mean swim thrust was the same as the change in the proportion of time spent power-stroking in both species with flatback hatchlings spending less time power stroking post-frenzy, and green hatchlings, spending more time power stroking post-frenzy, compared to the frenzy. However, post-frenzy flatback hatchlings exhibited faster powerstroke frequencies and post-frenzy, green hatchlings exhibited slower powerstroke frequencies compared to frenzy hatchlings. While post-frenzy flatback hatchlings exhibited shorter powerstroke bout durations compared to the frenzy, we did not observe a change in the duration of green hatchling power stroking bouts over time. Both flatback and green hatchlings were able to produce greater mean maximum thrust post-frenzy compared to the frenzy. Unlike green or flatback hatchlings, olive ridley hatchling swimming performance attributes did not change over time (Fig. 2, Table 4).
The ontogenetic change and species’ comparisons of swimming performance attributes in flatback, green and olive ridley hatchlings (mean ± standard error). Asterisks represent statistical differences between frenzy and post-frenzy mean swim thrust within each species. Letters and numbers represent differences between species during the frenzy and post-frenzy, respectively. Frenzy values are presented as circles with solid lines and post-frenzy values are triangles with dashed lines
Statistical results of linear mixed effects models evaluating change in swimming performance attributes over time are shown in Supplementary Table 2.
Difference in locomotor performance among species
There was no difference in the time it took hatchlings of different species to self-right or in the number of successful self-righting attempts.
Olive ridley hatchlings were the slowest crawlers but there was no difference between green and flatback hatchlings (Table 4).
During the frenzy period, there was no difference between green and flatback hatchlings, although post-frenzy, green hatchlings produced higher mean swim thrust than flatback hatchlings. During both the frenzy and post-frenzy, olive ridley hatchlings produced the lowest mean swim thrust (Fig. 2, Table 4).
During the frenzy, there was no difference among any of the three species in the proportion of time spent powerstroking, although post-frenzy, green hatchlings spent a greater proportion of time powerstroking than olive ridleys, which spent more time powerstroking than flatback hatchlings (Fig. 2).
During the frenzy, olive ridley hatchlings had the highest powerstroke frequencies, followed by green hatchlings and lastly by flatback hatchlings. Post-frenzy, flatbacks had the highest powerstroke frequencies, followed by olive ridleys and lastly by green hatchlings (Fig. 2).
There was no difference in powerstroking bout duration among species during the frenzy, but post-frenzy, flatbacks had the shortest powerstroke bout durations, and there was no difference between green and olive ridley hatchlings (Fig. 2).
Flatback hatchlings produced the greatest mean maximum thrust during the frenzy, followed by green hatchlings, followed by olive ridley hatchlings. Post-frenzy, olive ridley hatchlings still produced the least mean maximum thrust, but there was no difference between green and flatback hatchlings (Fig. 2, Table 4).
Statistical results of linear mixed effects models evaluating differences in locomotor performance among species can be found in Supplementary Table 3.
Within study meta-analysis
Zr values that incorporate 0 indicate that moisture has no effect on that measure of terrestrial locomotor performance in that species. Thus, flatback crawling speed and all measures of green sea turtle hatchling terrestrial locomotor performance did not respond to moisture treatment during incubation. Negative Zr values indicate that wet incubation conditions produce hatchlings that are faster crawlers and are faster, more successful self-righters. Thus, higher moisture concentrations produced flatback hatchlings that were faster self-righters and also produced olive ridley hatchlings that were faster crawlers and self-righters. Overall, our within study meta-analysis confirmed that among species, hatchlings incubated at higher moisture levels were generally faster crawlers and self-righters (β = − 0.224, SE = 0.092, p < 0.05) (Fig. 3).
Results from our within study meta-analysis on the response of hatchling terrestrial locomotor performance to moisture levels during incubation. We report standardised effect sizes (Zr) with positive values supporting the hypothesis that lower moisture levels during incubation produce hatchlings that are faster crawlers and self-righters and negative values supporting the hypothesis that higher moisture values produce hatchlings that are faster crawlers and self-righters. Values that overlap with 0 indicate that moisture does not influence that measure of terrestrial locomotion. We present the effect sizes of each individual locomotor test and species as well as the overall effect size among species and tests
Discussion
Moisture influences terrestrial locomotion but not aquatic locomotion
Wetter incubation conditions of 6% and 8% moisture (w/w) produced flatback and olive ridley hatchlings that were able to self-right successfully more often and took less time to self-right than hatchlings incubated at 4% moisture. Olive ridley hatchlings incubated under more moist conditions (≥ 6% moisture) were faster crawlers than hatchlings incubated in drier conditions (4% moisture). Despite the relatively consistent influence of moisture on terrestrial locomotion as shown by our meta-analysis, moisture concentration during incubation had no effect on any of the swimming performance attributes. A potential explanation is that differences between moisture treatments can only be observed on land, because sea turtle hatchlings are largely suited for aquatic locomotion where they are supported by water (Wyneken 1997). Their different locomotion on land may reveal differences in physiology among hatchlings that aquatic locomotion does not. Alternatively, the effect of moisture on locomotion may reflect physiological effects that disappear once hatchlings enter the ocean. Sea turtle hatchlings are dehydrated when they emerge from the nest but they can recover lost water by drinking up to 12% of their body mass within the first 48 h of entering the ocean (Reina et al. 2002) and excrete excess salt through an efficient salt-secreting gland (Reina 2000). Thus, low moisture concentrations during incubation may have led to less hydrated hatchlings (Finkler 1999; Hewavisenthi et al. 2001) that were slower crawlers and self-righters than hatchlings from wet nests. However, once hatchlings entered the water during swimming performance testing, they could quickly rehydrate and the differences between moisture treatments disappeared (Bennett et al. 1986; Reina et al. 2002). Mass-specific salt gland secretion rates and concentrations are similar among sea turtle species (Reina et al. 2002), suggesting that the ability of hatchlings to rehydrate is high regardless of species. Potentially, differences in hydration may also alter locomotor performance by influencing lactate accumulation as observed in snapping turtles (Chelydra serpentina) (Miller et al. 1987). Additionally, previous studies in freshwater turtles have shown that differences in locomotor performance between moisture treatments remained even after hatchlings became fully hydrated, suggesting, at least in freshwater turtles, that incubation moisture concentrations may have a long-term effect on development (Miller et al. 1987). It is important to consider that sea turtle hatchlings can take up to a week to emerge from the nest after ‘pipping’ from the egg (Rusli et al. 2016) and that they slowly dehydrate within the nest during this time (Reina et al. 2002). Thus, in natural nests, hatchling hydration levels change considerably from pipping to emergence and this may alter the effect of moisture during incubation on locomotor performance. Smaller olive ridley hatchlings may be more susceptible to water loss and dehydration post-emergence, compared to larger hatchlings, because of their greater surface area to volume ratio (Foley and Spotila 1978; Hertz 1980). While there was no statistically significant effect of moisture on green sea turtle hatchlings, our meta-analysis showed that among species, there was a significant positive correlation of moisture concentration during incubation with crawling speed and self-righting ability, at least within the range of moisture we examined.
Differences in ontogeny reflect life history variation
During the frenzy, all sea turtle hatchlings are benefitted by entering the ocean and escaping predator-dense nearshore waters as quickly as possible (Wyneken and Salmon 1992), though the duration and intensity of the frenzy differs among species (Chung et al. 2009a, b; Salmon et al. 2009). Thus, species did not differ in the amount of time they spent power-stroking during the frenzy. However, post-frenzy flatback hatchlings showed reductions in the proportion of time spent powerstroking and the duration of powerstroking bouts. They also exhibited increased powerstroke frequencies compared to frenzied flatbacks. These behaviours may facilitate short, high intensity bursts of swimming to escape predators (Pereira et al. 2012; Salmon et al. 2009) in a species that has a completely neritic life history (Bolten 2003). In comparison, post-frenzy green hatchlings spent more time powerstroking, yet had slower strokes rates during power-stroking bouts than frenzied green hatchlings. Thus, green hatchlings may maximise the proportion of time spent powerstroking post-frenzy to facilitate extended dispersals into pelagic waters (Bolten 2003) compared to flatbacks that maximise stroke rates post-frenzy. Compared to the frenzy, flatback hatchlings experience smaller reductions in maximal metabolic rate post-frenzy than green hatchlings (Gatto et al., unpublished data). This may reflect flatback hatchlings transitioning to short, high intensity bursts of swimming activity to escape predation in neritic waters (Pereira et al. 2012; Salmon et al. 2009), compared to post-frenzy green hatchlings that experience reductions in swimming intensity but remain highly active post-frenzy as they undertake extended dispersal migrations (Bolten 2003). Though olive ridley swimming attributes did not change statistically from the frenzy to post-frenzy, changes in these attributes matched those of green turtles, potentially reflecting that their life history more closely resembles that of green turtles than flatback turtles (Bolten 2003). Ontogenetic differences among species in their swimming performance largely appear to reflect life history variation. These life history differences lead to divergent foraging behaviours and predation pressures (Bolten 2003; Salmon et al. 2009), partially driving the variation in locomotor performance that we observed here.
Olive ridleys are the slowest locomotors
Among species, there was no difference in self-righting ability, although olive ridleys were slower crawlers and the slowest swimmers, as indicated by mean swim thrust compared to flatback or green hatchlings. The lower mean swim thrust of olive ridleys appears to be largely driven by their lower mean maximum thrust production, both during and post-frenzy. The considerably smaller body size of olive ridley hatchlings likely makes them less capable than larger species of producing thrust during terrestrial and aquatic locomotion, resulting in slower crawling and swimming speeds (Burgess et al. 2006; Pereira et al. 2012). Interestingly, olive ridley hatchlings exhibited the highest stroke rates during power-stroking bouts at emergence compared to the other species, potentially a strategy that olive ridleys use to offset their lower thrust production per stroke (Booth 2009; Burgess et al. 2006). Increases in crawling speed resulting from wetter incubation conditions may be more beneficial to olive ridley hatchlings because of their small body size and slower crawling speeds compared to other species.
Ecological ramifications of moisture
Although the influence of moisture during incubation on hatchling locomotor performance is limited to terrestrial locomotion, variation in moisture level on nesting beaches is likely to influence sea turtle populations. Not only are higher moisture levels, as a result of higher rainfall and sea level rise, likely to reduce nest temperatures (Lolavar and Wyneken 2015), our data show that they will also produce hatchlings that are faster crawlers and are possibly more likely to survive initial, terrestrial phases of dispersal. Conversely, drier nests are likely to be hotter and may produce hatchlings with reduced terrestrial locomotor ability. However, the impact of moisture variation will not influence species equally. Green sea turtles appear to be considerably less sensitive to moisture levels during incubation than either flatback or olive ridley hatchlings, potentially reflecting their generally greater tolerance of extreme temperatures compared to other species (Howard et al. 2014). The greater sensitivity to moisture of olive ridley hatchlings compared to other species may result from their smaller egg size and thus, greater egg surface area to volume ratio (Ackerman et al. 1985). However, the role of egg size on the sensitivity of developing sea turtle embryos to moisture requires further investigation, particularly considering that the intermediate sized eggs of green turtles were less response to moisture during incubation than the large eggs of flatback turtles. Eggshell structure is similar among sea turtle species and is unlikely to contribute to species’ sensitivity to moisture (Phillott and Parmenter 2006). Within species, populations are likely to experience significantly different changes in moisture levels because changes in precipitation will vary regionally (IPCC 2014). Thus, populations that experience an increase in moisture may experience greater hatchling survival during the crawl from nest to ocean and those in drier areas may experience decreases in hatchling survival. Within populations, moisture concentrations and thus, hatchling terrestrial locomotor performance, will vary both temporally throughout the nesting season and spatially depending on proximity to the ocean and to vegetation (Dornfeld et al. 2015; Wood et al. 2000). Overall, sea turtle population responses to moisture will vary between species, populations, beach characteristics and even between nest locations. Differences in beach characteristics and nest location can result in variation in substrate grain size (Chen et al. 2007; Karavas et al. 2005), vegetation type and density (Hays et al. 1995) and can alter the elevation of the nest relative to the ocean (Wood et al. 2000), all of which influence the amount of moisture in the nest and can influence the availability of moisture to developing embryos (Bouchard and Bjorndal 2000; Foley et al. 2006; Kraemer and Bell 1980). Sea turtles have been shown to shift their nesting phenology and nest-site selection in response to altered air and sea temperatures (Lamont and Fujisaki 2014; Mazaris et al. 2013). Whether nesting females will do the same in response to moisture or indeed whether they are capable of detecting these differences remains to be seen. However, sand moisture concentrations can rapidly vary, both spatially with depth and temporally in response to rainfall, making moisture an unreliable cue for nesting females (Wood et al. 2000). Females that do shift their nest sites are likely to experience fitness advantages as a result of increased hatchling survival during dispersal (Lamont and Fujisaki 2014).
Conclusion
In conclusion, wetter incubation conditions produce sea turtle hatchlings that crawl faster, take less time to right themselves when over-turned, and are able to successfully right themselves more often than hatchlings from dry incubation conditions. Green hatchlings were the least sensitive to moisture and did not respond to incubation moisture concentrations in any of our performance tests. None of the three species we tested varied in their swimming performance in response to moisture concentrations. Flatbacks were the largest hatchlings and thus, required more water to be normally hydrated. In comparison, olive ridleys were the smallest hatchlings and could dehydrate more quickly in air compared to other, larger species. Differences in hydration potentially influence terrestrial locomotion, but these differences disappear once hatchlings enter the ocean and likely rehydrate. Future studies on the effects of moisture during incubation should focus on pinpointing the mechanisms behind the effect of moisture on crawling speeds, and consider incubating eggs at higher moisture levels that may highlight differences between hatchlings and reflect potential incubation conditions under climate change scenarios. Research should also investigate multiple, interacting environmental variables, such as temperature and moisture, that more realistically reflect natural nests. When comparing species, the divergent behaviours of all three species we examined largely reflected differences in life history.
Data availability
Data will be made available from the authors upon request.
References
Ackerman RA, Lutz P, Musick J (1997) The nest environment and the embryonic development of sea turtles. In: Lutz PL, Musick J (eds) The biology of sea turtles, vol 1. CRC Press, Boca Raton, Florida, pp 83–106
Ackerman RA, Seagrave RC, Dmi'el R, Ar A (1985) Water and heat exchange between parchment-shelled reptile eggs and their surroundings. Copeia 3:703–711
Bates D (2007) Linear mixed model implementation in lme4. University of Wisconsin, vol 15 (Unpublished manuscript)
Bennett JM, Taplin LE, Grigg GC (1986) Sea water drinking as a homeostatic response to dehydration in hatchling loggerhead turtles Caretta caretta. Comp Biochem Physiol A Physiol 83(3):507–513
Blackburn DG (1999) Viviparity and oviparity: evolution and reproductive strategies. Encyclopedia of reproduction, vol 3. Academic Press, San Diego, California, pp 994–1003
Bolten AB (2003) Variation in sea turtle life history patterns: neritic vs. oceanic developmental stages. Biol Sea Turtles 2:243–257
Booth D (1998) Incubation of turtle eggs at different temperatures: do embryos compensate for temperature during development? Physiol Biochem Zool 71(1):23–26
Booth D (2006) Influence of incubation temperature on hatchling phenotype in reptiles. Physiol Biochem Zool 79(2):274–281
Booth D (2009) Swimming for your life: locomotor effort and oxygen consumption during the green turtle (Chelonia mydas) hatchling frenzy. J Exp Biol 212(1):50–55
Booth D (2014) Kinematics of swimming and thrust production during powerstroking bouts of the swim frenzy in green turtle hatchlings. Biol Open 3(10):887–894
Booth D (2017) The influence of incubation temperature on sea turtle hatchling quality. Integr Zool 12(5):352–360
Booth D, Evans A (2011) Warm water and cool nests are best. How global warming might influence hatchling green turtle swimming performance. PLoS ONE 6(8):e23162
Bouchard SS, Bjorndal KA (2000) Sea turtles as biological transporters of nutrients and energy from marine to terrestrial ecosystems. Ecology 81(8):2305–2313
Burgess EA, Booth DT, Lanyon JM (2006) Swimming performance of hatchling green turtles is affected by incubation temperature. Coral Reefs 25(3):341–349
Cagle K, Packard G, Miller K, Packard M (1993) Effects of the microclimate in natural nests on development of embryonic painted turtles, Chrysemys picta. Funct Ecol 7:653–660
Carr AF, Ogren LH (1960) The ecology and migrations of sea turtles, IV: the green turtle in the Caribbean Sea. Bulletin of the AMNH 121:1–48
Caut S, Guirlet E, Girondot M (2010) Effect of tidal overwash on the embryonic development of leatherback turtles in French Guiana. Mar Environ Res 69(4):254–261
Cavallo C, Dempster T, Kearney MR, Kelly E, Booth D, Hadden KM, Jessop TS (2015) Predicting climate warming effects on green turtle hatchling viability and dispersal performance. FunctEcol 29(6):768–778
Chen H-C, Cheng I, Hong E (2007) The influence of the beach environment on the digging success and nest site distribution of the green turtle, Chelonia mydas, on Wan-an Island, Penghu Archipelago, Taiwan. J Coast Res 23(5(235)):1277–1286
Chung F, Pilcher N, Salmon M, Wyneken J (2009a) Offshore migratory activity of hawksbill turtle (Eretmochelys imbricata) hatchlings, I. Quantitative analysis of activity, with comparisons to green turtles (Chelonia mydas). Chelonian Conserv Biol 8(1):28–34
Chung F, Pilcher N, Salmon M, Wyneken J (2009b) Offshore migratory activity of hawksbill turtle (Eretmochelys imbricata) hatchlings, II. Swimming gaits, swimming speed, and morphological comparisons. Chelonian Conserv Biol 8(1):35–42
Dornfeld TC, Robinson NJ, Tomillo PS, Paladino FV (2015) Ecology of solitary nesting olive ridley sea turtles at Playa Grande, Costa Rica. Mar Biol 162(1):123–139
Finkler MS (1999) Influence of water availability during incubation on hatchling size, body composition, desiccation tolerance, and terrestrial locomotor performance in the snapping turtle Chelydra serpentina. Physiol Biochem Zool 72(6):714–722
Foley RE, Spotila JR (1978) Effect of wind speed, air temperature, body size and vapor density difference on evaporative water loss from the turtle Chrysemys scripta. Copeia 4:627–634
Foley AM, Peck SA, Harman GR (2006) Effects of sand characteristics and inundation on the hatching success of loggerhead sea turtle (Caretta caretta) clutches on low-relief mangrove islands in southwest Florida. Chelonian Conserv Biol 5(1):32–41
Fuentes M, Hamann M, Limpus C (2010a) Past, current and future thermal profiles of green turtle nesting grounds: implications from climate change. J Exp Mar Biol Ecol 383(1):56–64
Fuentes MMB, Limpus CJ, Hamann M, Dawson J (2010b) Potential impacts of projected sea-level rise on sea turtle rookeries. AquatConserv Mar FreshwEcosyst 20(2):132–139
Gatto CR, Reina RD (2020) The ontogeny of sea turtle hatchling swimming performance. Biol J Linn Soc 131(1):172–182
Godfrey MH, Mrosovsky N (2006) Pivotal temperature for green sea turtles, Chelonia mydas, nesting in Suriname. Herpetol J 16(1):55–61
Godley B, Broderick A, Glen F, Hays G (2002) Temperature-dependent sex determination of Ascension Island green turtles. Mar Ecol Prog Ser 226:115–124
Gyuris E (1994) The rate of predation by fishes on hatchlings of the green turtle (Chelonia mydas). Coral Reefs 13(3):137–144
Hays GC, Mackay A, Adams C, Mortimer J, Speakman J, Boerema M (1995) Nest site selection by sea turtles. J Mar Biol Assoc UK 75(3):667–674
Hertz PE (1980) Responses to dehydration in Anolis lizards sampled along altitudinal transects. Copeia 1980:440–446
Hewavisenthi S, Parmenter CJ, Gatten, Jr R (2001) Influence of incubation environment on the development of the flatback turtle (Natator depressus). Copeia 3:668–682
Higgins BM (2003) Sea turtle husbandry, vol 2. The biology of sea turtles. CRC Press, Boca Raton, pp 411–440
Hill JE, Paladino FV, Spotila JR, Tomillo PS (2015) Shading and watering as a tool to mitigate the impacts of climate change in sea turtle nests. PLoS ONE 10(6):e0129528
Howard R, Bell I, Pike DA (2014) Thermal tolerances of sea turtle embryos: current understanding and future directions. Endang Species Res 26(1):75–86
IPCC (2014) Climate change 2014: synthesis report. In: Core Writing Team, Pachauri RK, Meyer LA (eds) Contribution of working groups I, II and III to the fifth assessment report of the intergovernmental panel on climate change. IPCC, Geneva, Switzerland, p 151
Karavas N, Georghiou K, Arianoutsou M, Dimopoulos D (2005) Vegetation and sand characteristics influencing nesting activity of Caretta caretta on Sekania beach. Biol Conserv 121(2):177–188
Kraemer JE, Bell R (1980) Rain-induced mortality of eggs and hatchlings of loggerhead sea turtles (Caretta caretta) on the Georgia coast. Herpetologica 36(1):72–77
Lamont MM, Fujisaki I (2014) Effects of ocean temperature on nesting phenology and fecundity of the loggerhead sea turtle (Caretta caretta). J Herpetol 48(1):98–102
Limpus CJ (2008) A biological review of Australian marine turtles. In: The state of queensland, environmental protection agency, vol 1. Queensland Government, Brisbane, Queensland
Lolavar A, Wyneken J (2015) Effect of rainfall on loggerhead turtle nest temperatures, sand temperatures and hatchling sex. Endang Species Res 28(3):235–247
Lolavar A, Wyneken J (2017) Experimental assessment of the effects of moisture on loggerhead sea turtle hatchling sex ratios. Zoology 123:64–70
Mazaris AD, Kallimanis AS, Pantis JD, Hays GC (2013) Phenological response of sea turtles to environmental variation across a species' northern range. Proc R SocLond B Biol Sci 280(1751):20122397
McGehee MA (1990) Effects of moisture on eggs and hatchlings of loggerhead sea turtles (Caretta caretta). Herpetologica 46(3):251–258
McQueen A, Naimo AC, Teunissen N, Magrath RD, Delhey K, Peters A (2017) Bright birds are cautious: seasonally conspicuous plumage prompts risk avoidance by male superb fairy-wrens. Proc R SocLond B Biol Sci 284(1857):20170446
Miller K, Packard GC, Packard MJ (1987) Hydric conditions during incubation influence locomotor performance of hatchling snapping turtles. J Exp Biol 127(1):401–412
Nakagawa S, Cuthill IC (2007) Effect size, confidence interval and statistical significance: a practical guide for biologists. Biol Rev 82(4):591–605
Patino-Martinez J, Marco A, Quiñones L, Hawkes LA (2014) The potential future influence of sea level rise on leatherback turtle nests. J Exp Mar Biol Ecol 461:116–123
Pereira CM, Booth DT, Limpus CJ (2012) Swimming performance and metabolic rate of flatbackNatator depressus and loggerhead Caretta caretta sea turtle hatchlings during the swimming frenzy. Endang Species Res 17(1):43–51
Phillott AD, Parmenter CJ (2006) The ultrastructure of sea turtle eggshell does not contribute to interspecies variation in fungal invasion of the egg. Can J Zool 84(9):1339–1344
Plotkin PT (2007) Biology and conservation of ridley sea turtles. JHU Press, Baltimore
R Core Team (2014) R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2014.
Rafferty AR, Evans RG, Scheelings TF, Reina RD (2013) Limited oxygen availability in utero may constrain the evolution of live-birth in reptiles. Am Nat 181(2):245–253
Ragotzkie RA (1959) Mortality of loggerhead turtle eggs from excessive rainfall. Ecology 40(2):303–305
Reina R (2000) Salt gland blood flow in the hatchling green turtle, Chelonia mydas. J Comp Physiol B 170(8):573–580
Reina RD, Jones TT, Spotila JR (2002) Salt and water regulation by the leatherback sea turtle Dermochelys coriacea. J Exp Biol 205(13):1853–1860
Rings CC, Rafferty AR, Guinea ML, Reina RD (2014) The impact of extended preovipositional arrest on embryonic development and hatchling fitness in the flatback sea turtle. Physiol Biochem Zool 88(2):116–127
Rusli MU, Booth DT, Joseph J (2016) Synchronous activity lowers the energetic cost of nest escape for sea turtle hatchlings. J Exp Biol 219(10):1505–1513
Salmon M, Hamann M, Wyneken J, Schauble C (2009) Early swimming activity of hatchling flatback sea turtles Natator depressus: a test of the ‘predation risk’hypothesis. Endang Species Res 9(1):41–47
Stubbs JL, Kearney MR, Whiting SD, Mitchell NJ (2014) Models of primary sex ratios at a major flatback turtle rookery show an anomalous masculinising trend. Clim Change Responses 1(1):1
van de Merwe J, Ibrahim K, Whittier J (2006) Effects of nest depth, shading, and metabolic heating on nest temperatures in sea turtle hatcheries. Chelonian Conserv Biol 5(2):210–215. https://doi.org/10.2744/1071-8443(2006)5[210:EONDSA]2.0.CO;2
Viechtbauer W (2010) Conducting meta-analyses in R with the metafor package. J Stat Softw 36(3):1–48
Whelan CL, Wyneken J (2007) Estimating predation levels and site-specific survival of hatchling loggerhead seaturtles (Caretta caretta) from south Florida beaches. Copeia 3:745–754
Whelan CL (2007) Estimating predation levels and site-specific survival of hatchling loggerhead seaturtles (Caretta caretta) from South Florida beaches. Copeia 3:745–754
Williamson SA, Evans RG, Reina RD (2017a) Use of hypoxia to extend embryonic arrest in turtle eggs and prevent movement-induced mortality. Biol Cons 216:86–92. https://doi.org/10.1016/j.biocon.2017.10.009
Williamson SA, Evans RG, Robinson NJ, Reina RD (2017b) Hypoxia as a novel method for preventing movement-induced mortality during translocation of turtle eggs. Biol Conserv 216:86–92
Wood DW, Bjorndal KA (2000) Ross S (2000) Relation of temperature, moisture, salinity, and slope to nest site selection in loggerhead sea turtles. Copeia 1:119–119
Wood A, Booth DT, Limpus CJ (2014) Sun exposure, nest temperature and loggerhead turtle hatchlings: Implications for beach shading management strategies at sea turtle rookeries. J Exp Mar Biol Ecol 451:105–114
Wyneken J (1997) Sea turtle locomotion: mechanisms, behavior, and energetics. In: Lutz PL, Musick J (eds) The Biology of Sea Turtles, vol 1. CRC Press, Boca Raton, Florida, pp 165–198
Wyneken J, Lolavar A (2015) Loggerhead sea turtle environmental sex determination: implications of moisture and temperature for climate change based predictions for species survival. J Exp Zool Part B Mol Dev Evol 324(3):295–314
Wyneken J, Salmon M (1992) Frenzy and postfrenzy swimming activity in loggerhead, green, and leatherback hatchling sea turtles. Copeia 2:478–484
Acknowledgements
We thank everyone at the Heron Island research station, the Curtis Island flatback research team and James and the Tiwi Land Council for their help collecting eggs and releasing turtles. Thanks to Jan, Rod and Neal from Aquacore for all of their help housing the turtles and to Fran, Vanessa and Jade for their help measuring and caring for the turtles. Thanks to Jeanette Wyneken, Amanda Southwood Williard, Stephen Dunbar and two anonymous reviewers for their insightful and useful comments on a draft of this manuscript. Thanks to the Herpetological Conservation International Michael Dee grant, the Australian Wildlife Society University Student grant, the Great Barrier Reef Marine Park Authority Reef Guardians grant and the Monash University School of Biological Sciences for their financial support. CG was also supported by an Australian Government Research Training Program (RTP) scholarship.
Funding
CG was supported by the Herpetological Conservation International Michael Dee grant, the Australian Wildlife Society University Student grant, the Great Barrier Reef Marine Park Authority Reef Guardians grant and the Monash University School of Biological Sciences. CG was also supported by an Australian Government Research Training Program (RTP) scholarship.
Author information
Authors and Affiliations
Contributions
CG and RR developed the experimental design. CG conducted the experiment and performed the statistical analysis. CG and RR discussed the results and contributed to final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Ethical approval
Eggs were collected under Queensland scientific purposes permit WITK17747816 (Chelonia mydas) and WITK18685417 (Natator depressus) and Northern Territory permit to take wildlife 62703 (Lepidochelys olivacea). Hatchlings were housed and tested under research permit 10008208 and all procedures were approved by the Monash University Biological Sciences Animal Ethics Committee (BSCI/2016/23).
Additional information
Communicated by Philip Withers.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Gatto, C.R., Reina, R.D. Sea turtle hatchling locomotor performance: incubation moisture effects, ontogeny and species-specific patterns. J Comp Physiol B 190, 779–793 (2020). https://doi.org/10.1007/s00360-020-01307-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00360-020-01307-z