Abstract
Euryhaline Mozambique tilapia (Oreochromis mossambicus) are native to estuaries where they encounter tidal fluctuations in environmental salinity. These fluctuations can be dramatic, subjecting individuals to salinities characteristic of fresh water (FW < 0.5‰) and seawater (SW 35‰) within a single tidal cycle. In the current study, we reared tilapia under a tidal regimen that simulated the dynamic conditions of their native habitat. Tilapia were sampled every 3 h over a 24 h period to temporally resolve how prolactin (PRL) signaling is modulated in parallel with genes encoding branchial effectors of osmoregulation. The following parameters were measured: plasma osmolality, plasma PRL177 and PRL188 concentrations, pituitary prl177 and prl188 gene expression, and branchial prl receptor (prlr1 and prlr2), Na+/Cl−-cotransporter (ncc2), Na+/K+/2Cl−-cotransporter (nkcc1a), Na+/K+-ATPase (nkaα1a and nkaα1b), cystic fibrosis transmembrane regulator (cftr), and aquaporin 3 (aqp3) gene expression. Throughout the 24 h sampling period, plasma osmolality reflected whether tilapia were sampled during the FW or SW phases of the tidal cycle, whereas pituitary prl gene expression and plasma PRL levels remained stable. Branchial patterns of ncc2, nkcc1a, nkaα1a, nkaα1b, cftr, and aqp3 gene expression indicated that fish exposed to tidally changing salinities regulate the expression of these gene transcripts in a similar fashion as fish held under static SW conditions. By contrast, branchial prlr1 and prlr2 levels were highly labile throughout the tidal cycle. We conclude that local (branchial) regulation of endocrine signaling underlies the capacity of euryhaline fishes, such as Mozambique tilapia, to thrive under dynamic salinity conditions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hydromineral balance requires the tight regulation of both solute and water transporting processes (Marshall and Grosell 2006). Teleost fishes typically maintain plasma osmolality at approximately one-third the osmolality of seawater (SW) through the coordinated activities of the gill, kidney, and gastrointestinal tract (Evans 2008; McCormick 2011). The extensive surface area of branchial epithelium is the primary site for Na+ and Cl− exchange with the external environment. In hyposmotic freshwater (FW) environments, teleosts counteract passive solute loss and excessive hydration by actively absorbing ions from the environment across branchial epithelium while simultaneously producing dilute urine. In hyperosmotic environments, teleosts mitigate water lost via osmosis and the diffusive gain of ions by drinking the surrounding SW and extruding Na+ and Cl− across the gill (Evans et al. 2005; McCormick 2011). As a euryhaline teleost indigenous to riverine and estuarine habitats of southeast Africa, some populations of Mozambique tilapia (Oreochromis mossambicus) are found within proximity to the tidal ebb and flow and thus subjected to marked variations in salinity (Trewavas 1983). Nonetheless, Mozambique tilapia are equipped with physiological systems that maintain plasma osmolality within narrow bounds, and thus allows them to reside in these dynamic locales (Trewavas 1983; Yamaguchi et al. 2018). Alternatively, some fishes that reside in intertidal zones, for instance the shanny (Lipophrys pholis), rely upon behavioral processes to avoid salinity stress associated with tidal cycles (Gibson 1984).
The pituitary hormone prolactin (PRL) is essential to the survival of euryhaline species in FW by stimulating ion uptake and diminishing osmotic permeability in key osmoregulatory tissues (Dharmamba et al. 1967; Manzon 2002; Pickford and Phillips 1959). In Mozambique tilapia, two isoforms of PRL are synthetized and released, PRL177 and PRL188 (Rentier-Delrue et al. 1989; Specker et al. 1985; Yamaguchi et al. 1988). Plasma levels of both PRLs rise in response to external hyposmotic (FW) conditions and pituitary mRNA levels of both prls are upregulated following the transfer of tilapia from SW to FW (Seale et al. 2002, 2012; Yada et al. 1994). The actions of PRL on target tissues are mediated through PRL receptors (denoted PRLR1 and -2), which are expressed in the gill, kidney, and distinct segments of the gastrointestinal tract (Fiol et al. 2009; Pierce et al. 2007; Seale et al. 2014). The branchial expression levels of prlr1 and prlr2 respond differently to osmotic stimuli and PRLs. While branchial prlr1 levels are stimulated by PRL177 and PRL188 (Inokuchi et al. 2015), prlr2 levels are not stimulated by either PRL, but are upregulated by an increase in environmental salinity (Inokuchi et al. 2015; Seale et al. 2012).
In Mozambique tilapia, at least four distinct ionocytes, denoted types I–IV, mediate ion transport by the gill (Hiroi et al. 2005; Kaneko et al. 2008; Furukawa et al. 2015). All four ionocyte sub-types are characterized by the presence of basolateral Na+/K+-ATPase that energizes both ion uptake and ion extrusion processes (Hiroi et al. 2008). Two isoforms of the NKA α1-subunit are expressed in the gill: nkaα1a expression is stimulated in response to decreased extracellular osmolality and increased by exposure to PRL (Inokuchi et al. 2015; Tipsmark et al. 2011), whereas nkaα1b expression increases when fish are transferred from FW to SW (Inokuchi et al. 2015; Tipsmark et al. 2011). Type II ionocytes are characterized by the presence of an apical Na+/Cl−-cotransporter (NCC2) that provides a conduit for the absorption of Na+ and Cl− from the external FW environment (Hiroi et al. 2008; Inokuchi et al. 2015). By contrast, type IV ionocytes express a basolateral Na+/K+/2Cl−-cotransporter (NKCC1a) and an apical Cl− channel (cystic fibrosis transmembrane conductance regulator; CFTR) (Hiroi et al. 2005) to support the excretion of Na+ and Cl− (via paracellular and transcellular routes, respectively) into the external SW environment. The aquaglyceroporin, AQP3, is expressed in the basolateral membrane of tilapia ionocytes, pavement cells, and mucous cells, and may confer osmosensitivity to these multiple cell types (Breves et al. 2016; Watanabe et al. 2005).
Extensive study of Mozambique tilapia has focused on steady-state FW- and SW-acclimated fish, as well as those transferred in a unidirectional fashion from FW to SW or vice versa; little is known about how these fish respond to the tidally driven fluctuations in salinity they may encounter in their native estuaries. Previously, we reported osmoregulatory parameters in 4-month old tilapia reared under a tidal regimen (TR). Fish were exposed to salinities that ranged between FW and SW every 6 h (h) (Moorman et al. 2014, 2015). Our findings from these studies were: (1) circulating PRL188 levels were decoupled from fluctuations in plasma osmolality during the tidal cycle in TR-acclimated fish; (2) the gene expression of ncc, nkaα1a, nkaα1b, and aqp3 in fish acclimated to a TR was higher than that of fish acclimated to SW but lower than that of FW fish; and (3) the morphologies of ionocytes in TR-acclimated fish largely resembled those of SW-acclimated fish (Moorman et al. 2014). Recent examination of the ability for adult, 2-year old, tilapia to acclimate to a TR over the course of 1 week revealed an osmoregulatory profile that resembled fish raised in a TR from the fry stage (Pavlosky et al. 2019). By sampling fish near the end of the FW and SW phases of the tidal cycle (TF and TS, respectively), the results of these two previous studies provided a baseline osmoregulatory profile for TR-acclimated fish, and an approximation of how fish responded to cyclical changes in environmental salinity.
The primary objective of the current study was to provide a detailed profile of osmoregulatory endpoints in fish acclimated to a TR at a greater resolution than previous approaches. To accomplish this, tilapia were subjected to salinity changes that closely approximate natural tidal conditions and sampled every 3 h (midway and at the terminus of a tidal cycle) over a continuous 24 h period. Fish reared under a TR were compared with fish that remained in continuous FW or SW conditions. Here, we report the following endpoints: plasma osmolality, plasma levels of PRL177 and PRL188; pituitary expression of prl177 and prl188 and branchial mRNA expression of prlr1, prlr2, ncc2, nkcc1a, nkaα1a, nkaα1b, cftr, and aqp3. By characterizing this suite of endpoints in tilapia reared under a TR, we revealed how the environment directs PRL signaling through the local regulation of its receptors.
Materials and methods
Fish rearing
Mozambique tilapia (O. mossambicus) yolk-sac larvae were collected from broodstock maintained in FW (0.1 ± 0.1‰) tanks at the Hawai‘i Institute of Marine Biology. Fourteen days post-collection, the yolk sacs were fully absorbed, and the fry were seeded to 700-l, outdoor, tanks filled with 140 l of FW, at a density of 120 fish per tank. Water temperature was maintained at 27 ± 2 °C and fish were held under natural photoperiod. Two days after seeding, tanks were transitioned to brackish water (BW) of 10‰ by the addition of SW (34 ± 1‰; Kaneohe Bay, Oahu, HI, USA). Five days after seeding, the salinity was further increased to 18 ± 2‰, and then 8 days after seeding, two BW tanks were transitioned back to FW, two were transitioned to SW, and the remaining four tanks put under TR, where salinities alternated between FW and SW every 6 h (Moorman et al. 2014, 2015). Prior to their transition from BW to FW, SW, or the tidal paradigm, fish were fed ground trout chow pellets (Skretting, Tooele, UT, USA) ad libitum daily. After transitions, fish were provided fixed rations of 18% mean body weight divided over two daily feedings (mean body weight 24 ± 1 mg). Rations were decreased by 4% every 21–25 days until they were equivalent to 4% mean body weight. The fish were reared under these conditions until the time of sampling. Fish were fasted during the 24 h sampling period; the final feeding of all treatment groups occurred immediately prior to the first sampling time point.
Four males and four females reared under the TR were sampled at each time point (every 3 h). For each time point at which TR fish were sampled, corresponding FW- and SW-control groups were also sampled (four males and four females per treatment). Fish were collected at each time point from across all of the replicate tanks for the FW, SW, and TR treatments. Salinity was measured hourly in all tanks over the course of the 24 h sampling period (Figs. 1, 2, 3, 4, 5, 6, 7). Salinity ranged between 0.1 and 0.2‰ in FW-control tanks, 34.5–35.2‰ in SW-control tanks, and 0.2–35.2‰ in TR tanks. In TR tanks, salinity changed completely from FW to SW and vice versa within 2 h of switching the source of water. All experiments were conducted in accordance with the principles and procedures approved by the Institutional Animal Care and Use Committee, University of Hawai‘i.
Plasma osmolality of Mozambique tilapia (Oreochromis mossambicus) reared in fresh water (FW black dashed), seawater (SW black dotted) and a tidal regimen (TR solid black) and sampled over 24 h. Values represent mean ± SEM (n = 6–8). Shading denotes dark hours. Black lines and symbols denote plasma osmolality (left y-axis). Grey lines and symbols denote mean water salinity measured hourly in FW, SW, and TR tanks (right y-axis). Salinity and time effects were analyzed by two-way ANOVA, followed by Bonferroni’s test when main or interaction effects were detected (*, **, ***P < 0.05, 0.01 and 0.001, respectively). Mean values not sharing the same letter are different (P < 0.05); uppercase letters indicate differences across treatments at a given time point; differences over time within each treatment are reported in Supplementary Table 1
Plasma PRL177 (a) and plasma PRL188 (b) of Mozambique tilapia reared in FW (black dashed), SW (black dotted) and a TR (solid black) and sampled over 24 h. Values represent mean ± SEM (n = 6–8). Shading denotes dark hours. Black lines and symbols denote plasma PRL177 or PRL188 (left y-axis). Grey lines and symbols denote mean water salinity measured hourly in FW, SW, and TR tanks (right y-axis). Salinity and time effects were analyzed by two-way ANOVA, followed by Bonferroni’s test when main or interaction effects were detected (*, **, ***P < 0.05, 0.01 and 0.001, respectively). Mean values not sharing the same letter are different (P < 0.05); uppercase letters indicate differences across treatments at a given time point; differences over time within each treatment are reported in Supplementary Table 1
Pituitary gene expression of prl177 (a) and prl188 (Bb) of Mozambique tilapia reared in FW (black dashed), SW (black dotted) and a TR (solid black) and sampled over 24 h. Values represent mean ± SEM (n = 6–8). Shading denotes dark hours. Black lines and symbols denote pituitary prl177 or prl188 expression (left y-axis). Grey lines and symbols denote mean water salinity measured hourly in FW, SW, and TR tanks (right y-axis). Salinity and time effects were analyzed by two-way ANOVA, followed by Bonferroni’s test when main or interaction effects were detected (*, **, ***P < 0.05, 0.01 and 0.001, respectively). Mean values not sharing the same letter are different (P < 0.05); uppercase letters indicate differences across treatments at a given time point; differences over time within each treatment are reported in Supplementary Table 1
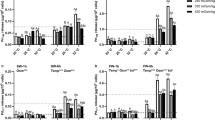
Branchial gene expression of prlr1 (a) and prlr2 (b) in Mozambique tilapia reared in FW (black dashed), SW (black dotted) and a TR (solid black) and sampled over 24 h. Values represent mean ± SEM (n = 6–8). Shading denotes dark hours. Black lines and symbols denote branchial prlr1 or prlr2 expression (left y-axis). Grey lines and symbols denote mean water salinity measured hourly in FW, SW, and TR tanks (right y-axis). Salinity and time effects were analyzed by two-way ANOVA, followed by Bonferroni’s test when main or interaction effects were detected (*, **, ***P < 0.05, 0.01 and 0.001, respectively). Mean values not sharing the same letter are different (P < 0.05); uppercase letters indicate differences across treatments at a given time point; differences over time within each treatment are reported in Supplementary Table 1
Branchial gene expression of ncc2 (a) and nkcc1a (b) in Mozambique tilapia reared in FW (black dashed), SW (black dotted) and a TR (solid black) and sampled over 24 h. Values represent mean ± SEM (n = 6–8). Shading denotes dark hours. Black lines and symbols denote branchial ncc2 or nkcc1a expression (left y-axis). Grey lines and symbols denote mean water salinity measured hourly in FW, SW, and TR tanks (right y-axis) Salinity and time effects were analyzed by two-way ANOVA, followed by Bonferroni’s test when main or interaction effects were detected (*, **, ***P < 0.05, 0.01 and 0.001, respectively). Mean values not sharing the same letter are different (P < 0.05); uppercase letters indicate differences across treatments at a given time point; differences over time within each treatment are reported in Supplementary Table 1
Branchial gene expression of nkaα1a (a) and nkaα1b (b) in Mozambique tilapia reared in FW (black dashed), SW (black dotted) and a TR (solid black) and sampled over 24 h. Values represent mean ± SEM (n = 6–8). Shading denotes dark hours. Black lines and symbols denote branchial nkaα1a and nkaα1b expression (left y-axis). Grey lines and symbols denote mean water salinity measured hourly in FW, SW, and TR tanks (right y-axis). Salinity and time effects were analyzed by two-way ANOVA, followed by Bonferroni’s test when main or interaction effects were detected (*, **, ***P < 0.05, 0.01 and 0.001, respectively). Mean values not sharing the same letter are different (P < 0.05); uppercase letters indicate differences across treatments at a given time point; differences over time within each treatment are reported in Supplementary Table 1
Branchial gene expression of cftr (a) and aqp3 (b) in Mozambique tilapia reared in FW (black dashed), SW (black dotted) and a TR (solid black) and sampled over 24 h. Values represent mean ± SEM (n = 6–8). Shading denotes dark hours. Black lines and symbols denote branchial cftr and aqp3 expression (left y-axis). Grey lines and symbols denote mean water salinity measured hourly in FW, SW, and TR tanks (right y-axis). Salinity and time effects were analyzed by two-way ANOVA, followed by Bonferroni’s when main or interaction effects were detected (*, **, ***P < 0.05, 0.01 and 0.001, respectively). Mean values not sharing the same letter are different (P < 0.05); uppercase letters indicate differences across treatments at a given time point; differences over time within each treatment are reported in Supplementary Table 1
Treatments and sampling
At the time of sampling, fish were netted and lethally anesthetized with 2-phenoxyethanol (0.3 ml/l). After fish were weighed, blood was collected with a needle and syringe coated with sodium heparin (200 U/ml, Sigma-Aldrich, St. Louis, MO, USA). Plasma was separated by centrifugation and stored at − 20 °C until further analyses. Pituitaries and gill filaments (from the second gill arch on the left side of the fish) were collected into empty tubes, frozen in liquid nitrogen, and stored at − 80 °C.
Quantitative real-time PCR (qRT-PCR)
Total RNA was extracted from pituitary and gill samples using TRI Reagent according to the manufacturer’s instructions (Molecular Research Center, Cincinnati, OH, USA). Using a High Capacity cDNA Reverse Transcription kit (Life Technologies, Carlsbad, CA, USA), 30 ng of total RNA from pituitary and 400 ng from gill were reverse transcribed into cDNA. Quantitative real-time PCR (qRT-PCR) assays were set up as previously described (Pierce et al. 2007), using the StepOnePlus real-time PCR system (Applied Biosystems, Carlsbad, CA, USA). The PCR mixture (15 ul) contained Power SYBR Green PCR Master Mix (Applied Biosystems), 200 nM of each primer, and 1 µl and 2 µl of cDNA (equivalent to 1.5 ng and 40 ng total RNA from pituitary and gill, respectively). PCR cycling parameters were 50 °C for 2 min and 95 °C for 10 min, followed by 40 cycles of 95 °C for 15 s and 60 °C for 1 min. The mRNA levels of reference and target genes were determined by an absolute quantification standard curve. Elongation factor 1α (ef1α) levels used to normalize the mRNA levels of target genes. All primer pairs are listed in Table 1.
Plasma parameters
Ten microliters of plasma were used to measure plasma osmolality; 10 µl and 25 µl of plasma were used to measure PRL177 and PRL188, respectively. Samples were measured in duplicates for all assays. Plasma osmolality was measured using a vapor pressure osmometer (Wescor 5100C; Wescor, Logan, UT, USA). Plasma PRL177 and PRL188 were measured by homologous radioimmunoassay as previously described (Ayson et al. 1993; Yamaguchi et al. 2016).
Statistical analyses
Statistical analyses were conducted by two-way analysis of variance (ANOVA) with time and salinity treatment (FW-controls, SW-controls, and TR fish) as main effects. Significant interaction effects of time and salinity (P < 0.05) were followed up by Bonferroni’s test. Differences across salinity treatments are shown in Figs. 1, 2, 3, 4, 5, 6, 7; differences across time points within a given treatment are reported in Supplementary Table 1. Data are expressed as mean ± SEM. When appropriate, individual values were log-transformed to meet assumptions of normality and homogeneity of variance. Statistical analyses were performed using Prism 8.0 software (GraphPad, La Jolla, CA, USA).
Results
Plasma osmolality, PRL177, and PRL188
Throughout the 24 h sampling period, plasma osmolalities in TR fish were elevated in TS (1545 and 0345) compared with TF (1900 and 0945), with mid-phase (1300, 0100 and 0700) values intermediate to TS and TF (Fig. 1). A sharp drop in plasma osmolality in TR fish began after 0400 and continued through the final 6 h of the 24 h period. Plasma osmolality in FW- and SW-controls did not differ for the first 6 h of the experiment but began to diverge at 1900 toward maximum and minimum values, respectively. Plasma PRL177 levels in TR fish remained below 4 ng/ml for the initial 18 h of the experiment, after which time a rise to ~ 9 ng/ml in TR fish occurred during the second half of the dark phase which coincided with a drop in ambient salinity (Fig. 2a). The onset of this rise was delayed by 3 h, but resembled the rise observed in FW-controls that also began in the second half of the dark hours, spanned 6 h, and peaked just after the onset of daylight (0700). After the peak in PRL177 in FW-controls at 0700, a tendency to drop was observed at 0945. PRL177 levels in SW-controls remained steady throughout the 24 h period. Plasma PRL188 in FW-controls was elevated above SW-controls for the majority of the time-course. PRL188 levels in TR fish remained steady and similar to those in SW-controls for the initial 18 h, after which time PRL188 rose to ~ 14 ng/ml. The rise in PRL188 coincided with a drop in ambient salinity associated with the tidal cycle (Fig. 2b). This rise in PRL188 (similar to PRL177) in TR fish was delayed by 3 h relative to the onset of the rise observed in FW-controls. Like PRL177, the peak in PRL188 in FW-controls at 0700 was also followed by a fall. PRL188 levels in SW-controls remained steady throughout the 24 h period.
Pituitary prl 177 and prl 188 gene expression
Pituitary mRNA expression of prl177 was approximately threefold higher in FW- versus SW-controls (Fig. 3a). prl177 levels in TR fish were intermediate to the FW- and SW-controls, although more closely resembling the values observed in SW.
The expression of prl188 in FW-controls was elevated compared with SW-controls for the entire experiment (Fig. 3b). The difference in prl188 levels between FW- and SW-controls was ~ tenfold greater than that observed for prl177; prl188 levels in TR fish were generally higher than levels in SW-controls. While the expression of prl177 gradually rose in both TR fish and FW-controls throughout the 24 h period, there was no time effect on prl188.
Branchial prlr1 and prlr2 gene expression
Branchial gene expression of prlr1 in FW-controls was consistently elevated over SW-controls throughout the 24 h period (Fig. 4a). prlr1 levels in TR fish were similar to levels in SW-controls in the TS phase (1545 and 0345), and to levels in FW-controls during the TF phase in both light (0945) and dark (2145) h. Contrasting with prlr1 patterns, prlr2 expression in SW-controls was generally elevated over FW-controls throughout the sampling period (Fig. 4b). Similar to patterns observed for prlr1 expression, prlr2 levels in TR fish resembled expression patterns in SW-controls during the TS phase (0345) and intermediate phases of the tidal cycle where salinity was similar to that of SW (1300 and 0100), and with the expression in FW-controls during TF phases (0945 and 2145) of the tidal cycle (Fig. 4b).
Branchial ncc2, nkcc1a, nkaα1a, nkaα1b, cftr, and aqp3 gene expression
Throughout the 24 h sampling period, branchial gene expression of ncc2 was elevated in FW- versus SW-controls. ncc2 expression in TR fish varied minimally from the SW-controls over the entire experiment (Fig. 5a). At 0345, ncc2 expression in FW-controls showed a tendency to increase, remaining elevated through the final sampling time point (0945). This rise in ncc2 coincided with increases in plasma PRL177 and PRL188 levels in time-matched FW-controls (Fig. 2a and b). On the other hand, nkcc1a mRNA levels in SW-controls ranged from two to nearly fivefold higher than levels in FW-controls throughout the experiment (Fig. 5b). nkcc1a expression in TR fish resembled nkcc1a patterns in SW-controls at all time points except for 1900. However, there was no significant time effect on nkcc1a expression (Fig. 5b).
Branchial nkaα1a expression was 10–30-fold higher in FW- versus SW-controls; nkaα1a levels in TR fish were similar to those in FW-controls throughout most of the sampling period (Fig. 6a). nkaα1a expression in FW-controls reached peak levels compared with SW-controls at the first 0945 time point, and again at the second 0945 time point. The onset of the rise in nkaα1a expression leading up to the second 0945 time point occurred during the dark hours at 0345, similarly timed to the observed onset of increases in plasma PRL177 and PRL188 levels in FW-controls (Fig. 2a and b). There was no significant main effect of salinity treatment on branchial nkaα1b expression (Fig. 6b). A significant effect of time was detected (Supplementary Table 1), with slight reductions in nkaα1b expression in SW-controls and TR fish during the dark hours (Fig. 6b).
Branchial cftr expression was consistently higher in SW- versus FW-controls; cftr levels in TR fish were similar to levels in SW-controls at most time points (Fig. 7a). Branchial aqp3 expression, on the other hand, was higher in FW-controls compared with SW-controls at all time points (Fig. 7b). In TR fish, aqp3 levels were intermediate to levels in FW- and SW-controls. However, there was a peak in aqp3 expression at 0945 in TR fish, which was timed with the aforementioned rises in plasma PRL177 and PRL188. This rise in aqp3 expression in TR fish mirrored a similar rise that occurred in FW-controls.
Discussion
The main objective of this study was to compare the nature of PRL signaling in a euryhaline teleost at the systemic and tissue levels in relation to the phases of a tidal cycle. We approached this objective by rearing tilapia under a TR and then assessing with high temporal resolution plasma osmolality and circulating PRLs, as well as branchial prlr1 and prlr2 gene expression. We considered these aspects of PRL signaling in parallel with branchial patterns of ncc2, nkcc1a, nkaαla, nkaαlb, cftr, and aqp3 gene expression. To our knowledge, this is the first study that has examined these parameters in a euryhaline teleost with sufficient temporal resolution (every 3 h for 24 h) to contrast systemic and local modes of endocrine signaling under tidal conditions.
It has been repeatedly demonstrated that pituitary prl and/or plasma PRL levels are elevated in teleosts held under steady-state FW versus SW conditions (Ayson et al. 1994; Ball and Ingleton 1973; Batten and Ball 1976; Chang et al. 2007; Laiz-Carrion et al. 2009; Ogasawara et al. 1989; Seale et al. 2012; Varsamos et al. 2006; Wigham and Ball 1977). In Mozambique tilapia, PRL release from the pituitary is stimulated by a fall in extracellular osmolality both in vivo and in vitro (Borski et al. 1992; Grau et al. 1981; Seale et al. 2002, 2006, 2012; Yada et al. 1994). In turn, we were surprised to recently observe that plasma PRL188 in TR tilapia was unchanged throughout the FW and SW phases of the tidal cycle despite fluctuations in plasma osmolality (Moorman et al. 2014). In this earlier study, we showed that PRL177, like PRL188, was decoupled from plasma osmolality in TR tilapia during the first 18 h of the cycle. Accordingly, both pituitary prl177 and prl188 mRNA levels were stable in TR tilapia and expressed at levels intermediate to FW- and SW-controls.
After the first 18 h of sampling, and during the second FW phase (0945), plasma osmolality dropped markedly in TR fish (Fig. 1). Attendant increases in plasma PRL177 and PRL188 occurred over the same period (Fig. 2). While these PRL responses to decreases in extracellular osmolality are consistent with previous studies (Grau et al. 1981; Helms et al. 1991; Seale et al. 2002, 2006; Wigham and Ball 1977), it is unclear what may have precipitated the robust drop in plasma osmolality during the second FW phase of the sampling period. Interestingly, we observed peaks in plasma PRLs in the FW-controls at 0700. This pattern resembled a similarly timed peak in PRL levels in Gulf killifish (Fundulus grandis) maintained in FW under comparable photoperiod and temperatures (Spieler et al. 1978). This suggests that the peak in plasma PRL levels observed in TR fish in the current study could be associated with a diurnal rhythm of PRL secretion. Circulating PRL levels in the SW-controls, however, did not change between 0345 and 0700, perhaps due to an overriding effect of the high environmental salinity (Fig. 2). Whether or not associated with a diurnal rhythm, it is worth noting that the peaks in circulating PRLs in TR fish were delayed relative to those in FW-controls, a likely reflection of the exposure of TR fish to elevated salinity (during the SW phase) immediately prior to 0700.
Previously, branchial expression of prlr1 mRNA was stimulated in a dose-dependent manner by PRL177 and PRL188 (Inokuchi et al. 2015) and by transfer from SW to FW (Breves et al. 2011; Fiol et al. 2009). Consistent with these patterns, we observed that branchial prlr1 expression was elevated in FW- versus SW-controls (Fig. 4a). prlr1 levels in TR fish fluctuated between levels observed in FW- and SW-controls during the 24 h period; prlr1 levels were elevated during the FW phase of the tidal cycle (Fig. 4a). Recall that neither plasma PRL177 nor PRL188 fluctuated in TR fish (Fig. 2). Thus, the enhancement of PRL signaling in TR fish to promote phenotypes supportive of FW acclimation seemingly occurs at the tissue level through the modulated expression of prlr1. On the other hand, branchial prlr2 expression was shown to increase following rises in extracellular osmolality both in vivo (Fiol et al. 2009) and in vitro (Inokuchi et al. 2015). In the current study, prlr2 levels were higher in the gill of SW- versus FW-controls (Fig. 4b). Additionally, the pattern of prlr2 expression in TR fish was opposite to that of prlr1. This pattern was consistent with previous findings in TR-acclimated tilapia (Moorman et al. 2014). Fiol et al. (2009) proposed that cells expressing prlr2 have an improved tolerance to hyperosmotic extracellular conditions and/or capacities to sequester circulating PRLs to attenuate PRL signaling. The tilapia prlr2 gene can be spliced into long and short variants; the short variant may prevent the binding of PRL to PRLR1 (Fiol et al. 2009). The tight control of PRL receptors revealed in the current study indicates that target tissues directly modulate PRL’s effects under tidal conditions.
Elevated ncc2 expression in FW- versus SW-controls throughout the 24 h period was consistent with the role of ncc2-expressing ionocytes in ion uptake (Fig. 5a) (Breves et al. 2010; Hiroi et al. 2008; Inokuchi et al. 2008; Kaneko et al. 2008). As TR fish have previously been shown to maintain branchial ionocytes that morphologically resemble SW-type ionocytes (Moorman et al. 2014), it was not surprising that ncc2 levels in TR fish were similar to levels in SW-controls. Moreover, the robust expression of nkcc1a and cftr in TR fish (Figs. 5b and 7a) indicated the presence of SW-type ionocytes (Hiroi et al. 2005; Madsen et al. 2007; Marshall and Singer 2002; Singer et al. 1998). Thus, the gene expression levels of ncc2, nkcc1a, and cftr reported here and in previous studies (Moorman et al. 2014, 2015) are consistent with the abundance of their translated proteins based on immunohistochemical analysis (Moorman et al. 2014). Consistent with our previous reports of elevated nkaα1a expression in response to FW transfer, decreases in extracellular osmolality, and PRL administration (Inokuchi et al. 2015; Tipsmark et al. 2011), nkaα1a expression was higher in FW- versus SW-controls over the entire experiment (Fig. 6a). In contrast to the low expression of ncc2, TR fish maintained nkaα1a at levels resembling those observed in FW-controls. Because nkaα1a expression in TR fish remained stable despite elevations in plasma PRLs during the second FW phase, the capacities for PRL and external salinity to stimulate nkaα1a expression were seemingly muted under a tidal regimen. This clearly contrasts with nkaα1a patterns observed under unidirectional salinity transfer paradigms (Tipsmark et al. 2011).
Branchial aqp3 expression in FW-controls was elevated over SW-controls at all time points (Fig. 7b) consistent with patterns described in European eel (Anguilla anguilla), Japanese eel (Anguilla japonica), Japanese medaka (Oryzias latipes), Atlantic killifish (Fundulus heteroclitus), Atlantic salmon (Salmo salar), and Mozambique tilapia (Cutler and Cramb 2002; Jung et al. 2012; Lignot et al. 2002; Madsen et al. 2014; Moorman et al. 2014, 2015; Tipsmark et al. 2010; Tse et al. 2006). In contrast to ncc2, aqp3 expression rose in FW-controls and TR fish with the rises in plasma PRLs beginning at 0100 and 0345, respectively. This is consistent with PRL acting as a stimulator of aqp3 expression in tilapia (Breves et al. 2016). At least under a tidal paradigm, ncc2 and aqp3 exhibit different sensitivities to circulating PRLs.
The regulation of genes encoding branchial effectors of ion and water movements is tied to osmosensory transduction networks (Fiol and Kultz 2007). While ncc2, nkcc1a, nkaαla, nkaαlb, cftr, and aqp3 are highly responsive to unidirectional changes in extracellular osmolality and PRL in tilapia (Breves et al. 2010, 2016; Inokuchi et al. 2015; Seale et al. 2012; Tipsmark et al. 2011), to our knowledge, rearing fish under a TR has been the only approach that allows for the examination of these parameters in a paradigm that decouples PRL from plasma osmolality in vivo (Moorman et al. 2014, 2015). The observed fluctuations in the expression of prlrs in TR fish, however, indicated that PRL sensitivity is locally mediated by osmotic conditions. Recently, we found that the sensitivity of PRL cells to both PRL177 and PRL188 is modulated by extracellular osmolality (Yamaguchi et al. 2016). This modulation may be mediated, at least in part, by osmotically induced changes in the expression of prlrs. Similar to the patterns observed in the gill, prlr2 expression in the pituitary is upregulated by an increase in extracellular osmolality in vitro and in vivo (Seale et al. 2012). Thus, while most studies have focused on the function of ion transporters, pumps, and channels in the context of ion uptake and secretion (Marshall and Grosell 2006), our current approach provides insight into the integrated local osmotic and endocrine control of these targets, where the environmental regulation of PRL signaling is shifted from ligands to receptors.
Two general patterns of gene expression in TR-acclimated fish were identified in this study. While the expression levels of most genes were stable throughout the tidal cycle, others, especially prlr1 and -2, responded acutely to changes in salinity. Moreover, tilapia reared under a TR were largely able to withstand wide fluctuations in external salinity while maintaining plasma osmolality and circulating PRL177 and PRL188 levels within narrow ranges. These findings support the notion that, rather than adjusting circulating PRLs with each tidal cycle, TR fish regulate PRL signaling via the transcriptional control of both prlrs. We propose that this mode of regulation aligns the magnitude and nature of PRL’s effects with a given phase of the tidal cycle. These effects may include the regulation of branchial permeability (perhaps via tight-junctions) given that mRNAs encoding ion channels, pumps, and co-transporters were mostly unchanged in TR fish. A suite of branchial genes in teleosts are known to be directly osmosensitive (Inokuchi et al. 2015), and their expression may be entirely regulated locally, rather than through systemic hormones, under tidal conditions. Future studies employing tidal paradigms in a broader suite of euryhaline species will unveil how the coupling of ambient salinity with endocrine signaling is dependent upon the experimental paradigm.
References
Ayson FG, Tagawa M, Kaneko T, Hasegawa S, Grau EG, Nishioka RS, Bern HA, Hirano T (1993) Homologous radioimmunoassays for tilapia prolactins and growth hormone. Gen Comp Endocrinol 89:138–148
Ayson FG, Kaneko T, Hasegawa S, Hirano T (1994) Differential expression of two prolactin and growth hormone genes during early development of tilapia (Oreochromis mossambicus) in fresh water and seawater: implications for possible involvement in osmoregulation during early life stages. Gen Comp Endocrinol 95(1):143–152. https://doi.org/10.1006/gcen.1994.1111
Ball JN, Ingleton PM (1973) Adaptive variations in prolactin secretion in relation to external salinity in the teleost Poecilia latipinna. Gen Comp Endocrinol 20(2):312–325
Batten TF, Ball JN (1976) Circadian changes in prolactin cell activity in the pituitary of the teleost Poecilia latipinna in freshwater. Cell Tissue Res 165(2):267–280
Borski RJ, Hansen MU, Nishioka RS, Grau EG (1992) Differential processing of the two prolactins of the tilapia (Oreochromis mossambicus), in relation to environmental salinity. J Exp Zool 264(1):46–54
Breves JP, Watanabe S, Kaneko T, Hirano T, Grau EG (2010) Prolactin restores branchial mitochondrion-rich cells expressing Na +/Cl− cotransporter in hypophysectomized mozambique tilapia. Am J Physiol 299(2):R702–R710. https://doi.org/10.1152/ajpregu.00213.2010 (ajpregu.00213.2010 [pii])
Breves JP, Seale AP, Helms RE, Tipsmark CK, Hirano T, Grau EG (2011) Dynamic gene expression of GH/PRL-family hormone receptors in gill and kidney during freshwater-acclimation of Mozambique tilapia. Comp Biochem Physiol 158(2):194–200. https://doi.org/10.1016/j.cbpa.2010.10.030 (S1095-6433(10)00518-0 [pii])
Breves JP, Inokuchi M, Yamaguchi Y, Seale AP, Hunt BL, Watanabe S, Lerner DT, Kaneko T, Grau EG (2016) Hormonal regulation of aquaporin 3: opposing actions of prolactin and cortisol in tilapia gill. J Endocrinol 230(3):325–337. https://doi.org/10.1530/JOE-16-0162
Chang YJ, Min BH, Choi CY (2007) Black porgy (Acanthopagrus schlegeli) prolactin cDNA sequence: mRNA expression and blood physiological responses during freshwater acclimation. Comp Biochem Physiol 147(1):122–128. https://doi.org/10.1016/j.cbpb.2007.01.006
Cutler CP, Cramb G (2002) Branchial expression of an aquaporin 3 (AQP-3) homologue is downregulated in the European eel Anguilla anguilla following seawater acclimation. J Exp Biol 205(Pt 17):2643–2651
Dharmamba M, Handin RI, Nandi J, Bern HA (1967) Effect of prolactin on freshwater survival and on plasma osmotic pressure of hypophysectomized Tilapia mossambica. Gen Comp Endocrinol 9(2):295–302
Evans DH (2008) Teleost fish osmoregulation: what have we learned since August Krogh, Homer Smith, and Ancel Keys. Am J Physiol 295(2):R704–R713. https://doi.org/10.1152/ajpregu.90337.2008
Evans DH, Piermarini PM, Choe KP (2005) The multifunctional fish gill: dominant site of gas exchange, osmoregulation, acid-base regulation, and excretion of nitrogenous waste. Physiol Rev 85(1):97–177. https://doi.org/10.1152/physrev.00050.2003
Fiol DF, Kultz D (2007) Osmotic stress sensing and signaling in fishes. FEBS J 274(22):5790–5798. https://doi.org/10.1111/j.1742-4658.2007.06099.x
Fiol DF, Sanmarti E, Sacchi R, Kultz D (2009) A novel tilapia prolactin receptor is functionally distinct from its paralog. J Exp Biol 212(Pt 13):2007–2015. https://doi.org/10.1242/jeb.025601
Furukawa F, Watanabe S, Seale AP, Breves JP, Lerner DT, Grau EG, Kaneko T (2015) In vivo and in vitro effects of high-K(+) stress on branchial expression of ROMKa in seawater-acclimated Mozambique tilapia. Comp Biochem Physiol 187:111–118. https://doi.org/10.1016/j.cbpa.2015.05.017
Gibson RN (1984) Hydrostatic pressure and the rhythmic behaviour of intertidal marine fishes. Trans Am Fish Soc 113(4):479–483
Grau EG, Nishioka RS, Bern HA (1981) Effects of osmotic pressure and calcium ion on prolactin release in vitro from the rostral pars distalis of the tilapia Sarotherodon mossambicus. Gen Comp Endocrinol 45(3):406–408
Helms LM, Grau EG, Borski RJ (1991) Effects of osmotic pressure and somatostatin on the cAMP messenger system of the osmosensitive prolactin cell of a teleost fish, the tilapia (Oreochromis mossambicus). Gen Comp Endocrinol 83(1):111–117
Hiroi J, McCormick SD, Ohtani-Kaneko R, Kaneko T (2005) Functional classification of mitochondrion-rich cells in euryhaline Mozambique tilapia (Oreochromis mossambicus) embryos, by means of triple immunofluorescence staining for Na+/K+ -ATPase, Na+/K+/2Cl− cotransporter and CFTR anion channel. J Exp Biol 208(Pt 11):2023–2036. https://doi.org/10.1242/jeb.01611
Hiroi J, Yasumasu S, McCormick SD, Hwang PP, Kaneko T (2008) Evidence for an apical Na–Cl cotransporter involved in ion uptake in a teleost fish. J Exp Biol 211(Pt 16):2584–2599. https://doi.org/10.1242/jeb.018663
Inokuchi M, Hiroi J, Watanabe S, Lee KM, Kaneko T (2008) Gene expression and morphological localization of NHE3, NCC and NKCC1a in branchial mitochondria-rich cells of Mozambique tilapia (Oreochromis mossambicus) acclimated to a wide range of salinities. Comp Biochem Physiol 151(2):151–158. https://doi.org/10.1016/j.cbpa.2008.06.012
Inokuchi M, Breves JP, Moriyama S, Watanabe S, Kaneko T, Lerner DT, Grau EG, Seale AP (2015) Prolactin 177, prolactin 188, and extracellular osmolality independently regulate the gene expression of ion transport effectors in gill of Mozambique tilapia. Am J Physiol 309(10):R1251–R1263. https://doi.org/10.1152/ajpregu.00168.2015
Jung D, Sato JD, Shaw JR, Stanton BA (2012) Expression of aquaporin 3 in gills of the Atlantic killifish (Fundulus heteroclitus): effects of seawater acclimation. Comp Biochem Physiol 161(3):320–326. https://doi.org/10.1016/j.cbpa.2011.11.014
Kaneko T, Watanabe S, Lee KM (2008) Functional morphology of mitochondrion-rich cells in euryhaline and stenohaline teleosts. Aqua-BioSci Monogr 1:1–68
Laiz-Carrion R, Fuentes J, Redruello B, Guzman JM, Martin del Rio MP, Power D, Mancera JM (2009) Expression of pituitary prolactin, growth hormone and somatolactin is modified in response to different stressors (salinity, crowding and food-deprivation) in gilthead sea bream Sparus auratus. Gen Comp Endocrinol 162(3):293–300. https://doi.org/10.1016/j.ygcen.2009.03.026
Lignot JH, Cutler CP, Hazon N, Cramb G (2002) Immunolocalisation of aquaporin 3 in the gill and the gastrointestinal tract of the European eel Anguilla anguilla (L.). J Exp Biol 205(Pt 17):2653–2663
Madsen SS, Jensen LN, Tipsmark CK, Kiilerich P, Borski RJ (2007) Differential regulation of cystic fibrosis transmembrane conductance regulator and Na+, K+-ATPase in gills of striped bass, Morone saxatilis: effect of salinity and hormones. J Endocrinol 192(1):249–260. https://doi.org/10.1677/JOE-06-0016
Madsen SS, Bujak J, Tipsmark CK (2014) Aquaporin expression in the Japanese medaka (Oryzias latipes) in freshwater and seawater: challenging the paradigm of intestinal water transport? J Exp Biol 217(Pt 17):3108–3121. https://doi.org/10.1242/jeb.105098
Magdeldin S, Uchida K, Hirano T, Grau EG, Abdelfattah A, Nozaki M (2007) Effects of environmental salinity on somatic growth and growth hormone/insulin-like growth factor-I axis in juvenile tilapia, Oreochromis mossambicus. Fish Sci 73:1023–1032
Manzon LA (2002) The role of prolactin in fish osmoregulation: a review. Gen Comp Endocrinol 125:291–310
Marshall WS, Grosell M (2006) Ion transport, osmoregulation, and acid-base balance. In: Evans DH, Clairborne JB (eds) The physiology of fishes, 3rd edn. CRC Press, Boca Raton, pp 177–230
Marshall WS, Singer TD (2002) Cystic fibrosis transmembrane conductance regulator in teleost fish. Biochim Biophys Acta 1566(1–2):16–27
McCormick SD (2011) The Hormonal Control of Osmoregulation in Teleost Fish. In: Farrell AP (ed) Encyclopedia of fish physiology: from genome to environment, vol 2. Academic Press, San Diego, pp 1466–1473
Moorman BP, Inokuchi M, Yamaguchi Y, Lerner DT, Grau EG, Seale AP (2014) The osmoregulatory effects of rearing Mozambique tilapia in a tidally changing salinity. Gen Comp Endocrinol 207:94–102. https://doi.org/10.1016/j.ygcen.2014.03.013
Moorman BP, Lerner DT, Grau EG, Seale AP (2015) The effects of acute salinity challenges on osmoregulation in Mozambique tilapia reared in a tidally changing salinity. J Exp Biol 218(Pt 5):731–739. https://doi.org/10.1242/jeb.112664
Ogasawara T, Hirano T, Akiyama T, Arai S, Tagawa M (1989) Changes in plasma prolactin and growth hormone concentrations during freshwater adaptation of juvenile chum salmon (Oncorhynchus keta) reared in seawater for a prolonged period. Fish Physiol Biochem 7(1–6):309–313. https://doi.org/10.1007/BF00004722
Pavlosky KK, Yamaguchi Y, Lerner DT, Seale AP (2019) The effects of transfer from steady-state to tidally-changing salinities on plasma and branchial osmoregulatory variables in adult Mozambique tilapia. Comp Biochem Physiol A 227:134–145. https://doi.org/10.1016/j.cbpa.2018.10.005
Pickford GE, Phillips JG (1959) Prolactin, a factor in promoting survival of hypophysectomized killifish in fresh water. Science 130(3373):454–455
Pierce AL, Fox BK, Davis LK, Visitacion N, Kitahashi T, Hirano T, Grau EG (2007) Prolactin receptor, growth hormone receptor, and putative somatolactin receptor in Mozambique tilapia: tissue specific expression and differential regulation by salinity and fasting. Gen Comp Endocrinol 154(1–3):31–40. https://doi.org/10.1016/j.ygcen.2007.06.023
Rentier-Delrue F, Swennen D, Prunet P, Lion M, Martial JA (1989) Tilapia prolactin: molecular cloning of two cDNAs and expression in Escherichia coli. DNA 3:261–270
Seale AP, Riley LG, Leedom TA, Kajimura S, Dores RM, Hirano T, Grau EG (2002) Effects of environmental osmolality on release of prolactin, growth hormone and ACTH from the tilapia pituitary. Gen Comp Endocrinol 128:91–101
Seale AP, Fiess JC, Hirano T, Cooke IM, Grau EG (2006) Disparate release of prolactin and growth hormone from the tilapia pituitary in response to osmotic stimulation. Gen Comp Endocrinol 145(3):222–231. https://doi.org/10.1016/j.ygcen.2005.09.006
Seale AP, Moorman BP, Stagg JJ, Breves JP, Lerner D, Grau G (2012) Prolactin 177, prolactin 188 and prolactin receptor 2 in the pituitary of the euryhaline tilapia, Oreochromis mossambicus, are differentially osmosensitive. J Endocrinol 213:89–98. https://doi.org/10.1530/JOE-11-0384
Seale AP, Stagg JJ, Yamaguchi Y, Breves JP, Soma S, Watanabe S, Kaneko T, Cnaani A, Harpaz S, Lerner DT, Grau EG (2014) Effects of salinity and prolactin on gene transcript levels of ion transporters, ion pumps and prolactin receptors in Mozambique tilapia intestine. Gen Comp Endocrinol 206:146–154. https://doi.org/10.1016/j.ygcen.2014.07.020
Singer TD, Tucker SJ, Marshall WS, Higgins CF (1998) A divergent CFTR homologue: highly regulated salt transport in the euryhaline teleost F. heteroclitus. Am J Physiol 274(3 Pt 1):C715–C723
Specker JL, King DS, Nishioka RS, Shirahata K, Yamaguchi K, Bern HA (1985) Isolation and partial characterization of a pair of prolactins released in vitro by the pituitary of cichlid fish, Oreochromis mossambicus. Proc Nat Acad Sci USA 82:7490–7494
Spieler RE, Meier AH, Noeske TA (1978) Temperature-induced phase shift of daily rhythm of serum prolactin in gulf killifish. Nature 271(5644):469–470
Tipsmark CK, Sorensen KJ, Madsen SS (2010) Aquaporin expression dynamics in osmoregulatory tissues of Atlantic salmon during smoltification and seawater acclimation. J Exp Biol 213(3):368–379. https://doi.org/10.1242/jeb.034785
Tipsmark CK, Breves JP, Seale AP, Lerner DT, Hirano T, Grau EG (2011) Switching of Na+, K+-ATPase isoforms by salinity and prolactin in the gill of a cichlid fish. J Endocrinol 209(2):237–244. https://doi.org/10.1530/JOE-10-0495
Trewavas E (1983) Tilapiine fishes of the genera Sarotherodon, Oreochromis and Danakilia. British Museum (Natural History) Publication Number 878. Cornell University Press, Ithaca
Tse WK, Au DW, Wong CK (2006) Characterization of ion channel and transporter mRNA expressions in isolated gill chloride and pavement cells of seawater acclimating eels. Biochem Biophys Res Commun 346(4):1181–1190. https://doi.org/10.1016/j.bbrc.2006.06.028
Varsamos S, Xuereb B, Commes T, Flik G, Spanings-Pierrot C (2006) Pituitary hormone mRNA expression in European sea bass Dicentrarchus labrax in seawater and following acclimation to fresh water. J Endocrinol 191(2):473–480. https://doi.org/10.1677/joe.1.06847
Watanabe S, Kaneko T, Aida K (2005) Aquaporin-3 expressed in the basolateral membrane of gill chloride cells in Mozambique tilapia Oreochromis mossambicus adapted to freshwater and seawater. J Exp Biol 208(Pt 14):2673–2682. https://doi.org/10.1242/jeb.01684
Wigham T, Ball JN (1977) Effect of environmental salinity changes on the secretory activity of prolactin cells in ocular pituitary transplants in Poecilia latipinna (Teleostei). Gen Comp Endocrinol 31(1):148–153
Yada T, Hirano T, Grau EG (1994) Changes in plasma levels of the two prolactins and growth hormone during adaptation to different salinities in the euryhaline tilapia (Oreochromis mossambicus). Gen Comp Endocrinol 93:214–223
Yamaguchi K, Specker JL, King DS, Yokoo Y, Nishioka RS, Hirano T, Bern HA (1988) Complete amino acid sequences of a pair of fish (tilapia) prolactins, tPRL177 and tPRL188. J Biol Chem 263:9113–9121
Yamaguchi Y, Moriyama S, Lerner DT, Grau EG, Seale AP (2016) Autocrine positive feedback regulation of prolactin release from tilapia prolactin cells and its modulation by extracellular osmolality. Endocrinology 157(9):3505–3516. https://doi.org/10.1210/en.2015-1969
Yamaguchi Y, Breves JP, Haws MC, Lerner DT, Grau EG, Seale AP (2018) Acute salinity tolerance and the control of two prolactins and their receptors in the Nile tilapia (Oreochromis niloticus) and Mozambique tilapia (O. mossambicus): a comparative study. Gen Comp Endocrinol 257:168–176. https://doi.org/10.1016/j.ygcen.2017.06.018
Acknowledgements
We are grateful to Mr. Julian Leon for laboratory assistance and Dr. Lucia A. Seale and Ms. Amanda Reyes for sampling assistance.
Funding
This work was funded in part by grants from the National Science Foundation (IOS-1755016 and IOS-1755131 to APS and JPB), the National Oceanic and Atmospheric Administration (NA18OAR4170347 to DTL and APS and NA14OAR4170071, which is sponsored by the University of Hawai‘i Sea Grant College Program projects R/SS-12 and R/SB-18 to APS), the National Institutes of Diabetes and Digestive and Kidney Diseases 1R21DK111775-01 from the National Institutes of Health to APS, and the National Institute of Food and Agriculture Hatch No. HAW02051-H to APS. The views expressed herein are those of the authors and do not necessarily reflect the views of the aforementioned granting agencies. University of Hawai‘i Sea Grant publication number UNIHI-SEAGRANT-JC-14-62.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
There is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Additional information
Communicated by B. Pelster.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Seale, A.P., Pavlosky, K.K., Celino-Brady, F.T. et al. Systemic versus tissue-level prolactin signaling in a teleost during a tidal cycle. J Comp Physiol B 189, 581–594 (2019). https://doi.org/10.1007/s00360-019-01233-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00360-019-01233-9