Abstract
Intracellular taurine is abundant in many animals and it influences an array of physiological processes, including osmoregulation, metabolism, and cardiac contractility. Taurine is an important osmolyte in teleost hearts, but its role in stress tolerance, cardiac metabolism, and contractility has not been assessed. The goal of this study was to determine if ventricular taurine concentration changes in response to environmental stress and to characterize its influence on contractility. Cardiac taurine concentrations varied in killifish (Fundulus heteroclitus) but were generally maintained following acute environmental challenges. In isometrically contracting ventricular strips, supplemental taurine (40 mmol L−1) protected peak tension development (F max) at high stimulation frequencies, an effect abolished by treatment with ryanodine, a blocker of sarcoplasmic reticulum Ca2+ release. In the presence of ryanodine, taurine-treated preparations were also better able to maintain F max at supraphysiological extracellular Ca2+ levels, but a prior anoxia exposure abolished this effect. Taurine had no impact on basal F max during or after anoxia, but it provided additive protection to high-frequency contractility post-anoxia. Tissue oxygen consumption and extracellular glucose utilization were unaffected by taurine in non-contracting preparations, indicating that it does not impact energy metabolism. Overall, the results suggest that cardiac taurine levels are well maintained on acute time scales in this highly stress-tolerant species. Supplemental taurine has no effect on aerobic metabolism in vitro, but it significantly improved cardiac contractility in a manner dependent upon sarcoplasmic reticulum Ca2+ cycling. The data indicate that taurine likely plays an important role in the regulation of cardiac performance in teleosts.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Taurine is a non-proteogenic amino acid implicated in a wide array of physiological processes in mammals, including osmoregulation, excitation–contraction (EC) coupling, bile salt conjugation, and the regulation of oxidative stress (reviewed by Schaffer et al. 2010). It is the most abundant amino acid in teleost ventricular muscle, accounting for 50% of all intracellular free amino acids. Concentrations range from 50 to 70 mmol kg−1 cell water (Fugelli and Vislie 1982), far exceeding extracellular levels, which are usually <1 µmol g−1 (Huxtable 1992). For example; in rainbow trout, ventricular taurine concentration is 48.7 µmol g−1, while plasma contains only 0.73 µmol g−1. In contrast, taurine appears to be distributed equally across intracellular and extracellular compartments in cephalopod hearts (Hochachka et al. 1983). Fish appear to lack the capacity for intracellular taurine synthesis and actively accumulate it from the extracellular fluid, despite the steep gradient across the sarcolemmal membrane (Goldstein et al. 1990). In euryhaline teleosts, taurine plays a crucial role as an osmolyte, allowing cell volume to remain constant as body fluid osmolality changes with environmental salinity (Evans 1979; Vislie 1983). Cellular taurine efflux increases under hypo-osmotic conditions, while intracellular accumulation occurs under hyper-osmotic conditions (Vislie 1983).
Taurine is considered cardioprotective in both mammalian (e.g., Bkaily et al. 1998) and cephalopod hearts (MacCormack et al. 2016). In the mammals, taurine differentially affects intracellular (Ca2+ i) and extracellular Ca2+ (Ca2+ e) handling to promote either Ca2+ efflux or influx, depending on the circumstances (Schaffer et al. 1994). In hypodynamic mammalian hearts, which are characterized by decreases in contractile strength, taurine promotes Ca2+ e influx to replenish depleted Ca2+ i pools (e.g., Sperelakis et al. 1992). In Ca2+ overloaded mammalian hearts, taurine exerts the opposite effect, promoting Ca2+ efflux to protect against arrhythmias (Satoh 1994) and prevent the initiation of apoptosis (Xu et al. 2006). A similar mechanism is observed in ischemia–reperfusion models, where supplemental taurine can prevent Ca2+ overload in ischemia–reperfusion models (Takahashi et al. 2002). The potential cardioprotective nature of taurine in fish has not been addressed. It is not clear whether physiological changes associated with environmental stressors such as salinity, hypoxia, or temperature extremes alter cardiac taurine levels in fish or how this may impact contractility.
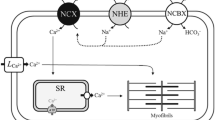
The mechanisms underlying cardiac excitation–contraction (EC) coupling in fish are generally similar to those in mammals, although the relative importance of Ca2+ i and Ca2+ e flux differs greatly across teleost species (Shiels and Sitsapesan 2015). In mammals, a relatively small Ca2+ influx across the sarcolemmal membrane triggers the release of a larger Ca2+ pool from the sarcoplasmic reticulum (SR), termed Ca2+-induced Ca2+ release (CICR). In most teleosts, CICR is small and SR Ca2+ flux plays only a minor role in routine contractile function, becoming increasingly important at higher contractile frequencies (Shiels and Galli 2014). The role of taurine in modulating Ca2+ flux and contractility in teleost hearts has not been addressed, but it does substantially impact cardiac performance in the cephalopod Sepia officinalis. In S. officinalis, extracellular taurine substantially reduces stroke volume and cardiac output and improves cardiac Ca2+ handling in vitro (MacCormack et al. 2016). Cephalopods have a well-developed SR that contributes to routine cardiac contractility (Gesser et al. 1997) and it is not clear if this is a requisite to facilitate taurine’s actions on EC coupling.
Taurine also influences energy metabolism in cephalopods, increasing glucose utilization without effecting aerobic metabolic rate (MacCormack et al. 2016). In mammals, taurine is linked to energy metabolism through the insulin-signaling pathway (Imae et al. 2014). In perfused rat hearts, taurine stimulates oxygen consumption and increases the rate of glucose utilization, an effect which is more pronounced in the presence of insulin (Lampson et al. 1983). Taurine’s involvement in cardiac energy metabolism has not been studied in teleosts, but the general conservation of glucoregulatory mechanisms with mammals (Polakof et al. 2012) suggests that taurine could play a similar role in fish.
Given the considerable gap in knowledge regarding taurine’s function(s) in fish hearts, we aimed to determine if cardiac taurine levels changed in fish exposed to common environmental stressors, including changes in salinity, temperature, and oxygen availability. We then used in vitro cardiac muscle preparations to assess the effects of supplemental extracellular taurine on cardiac contractility and energy metabolism. Experiments were carried out on killifish, Fundulus heteroclitus, as they represent a highly stress-tolerant model species. Cardiac taurine concentrations were generally maintained in killifish exposed to environmental stressors, but supplemental taurine did have significant effects on cardiac contractility in vitro. The latter effects were abolished by ryanodine, suggesting that taurine alters cardiac contractility by influencing SR Ca2+ cycling.
Materials and methods
Animals
Male and female killifish, Fundulus heteroclitus (body mass 8.5 ± 2.5 g), were caught by beach seine in brackish water (BW) in Little Shemogue, NB, Canada. They were transported to the Harold Crabtree Aqualab, Mount Allison University, and held in 750 L partially recirculated BW tanks (15 ppt, 15 °C) for a minimum of 2 weeks. A subset of fish were then transferred into a 100 L partially recirculated freshwater (FW) tank (15 °C) and acclimated for a minimum of 2 weeks prior to experimentation. All killifish were fed sinking trout chow (Corey Aquafeeds; Fredericton, NB, Canada) daily ad libitum. All studies followed Canadian Council on Animal Care guidelines and protocols were approved by Mount Allison University’s Animal Care Committee.
Cardiac taurine content
Measurements of cardiac taurine content closely followed the standard methods outlined in MacCormack et al. (2016), as originally modified from Shaffer and Kocsis (1981). It was necessary to pool ventricles from 5 fish to obtain the minimum sample mass required for the assay. Briefly, tissue was digested in 1:9 (w/v) 2% perchloric acid (PCA) overnight before an additional 1 in 10 dilution in 2% PCA. Taurine standards ranging from 0.198 to 7.02 mM were prepared in 2% PCA. 5 mL of each standard or sample dilution was passed through 4 mL of Dowex®50WX8 hydrogen form (100–200 mesh, Sigma–Aldrich, St. Louis, MO, USA) resin equilibrated in 2% PCA in a gravity-fed column (10GDG desalting column, Bio-Rad, Hercules, CA, USA). Contaminating amino acids were washed through with 2% PCA and taurine was then eluted with 2 mL ddH2O. 20 µL of eluate was added to 40 µL dinitrofluorobenzene (DNFB), 40 µL NaOH (1 M), 200 µL dimethylsulfoxide (DMSO), and vortexed for 3 × 10 s bursts. Next, 40 µL of HCl (3 M) and 1.660 mL of ddH2O were mixed into the reaction before the addition of 8 mL of ethyl acetate. The solutions were mixed for 10 min, after which the aqueous phase was removed and absorbance measured at 355 nm in quartz cuvettes in a spectrophotometer.
Isometrically contracting ventricular strip preparations
Isometrically contracting ventricular strip preparations were prepared as previously described (MacCormack et al. 2016) to assess the influence of extracellular taurine on cardiac contractility. Due to the limited quantity of heart tissue available, we were unable to quantify how supplemental taurine influenced intracellular taurine levels in vitro, but the data from these studies should still provide insight into taurine’s role in EC coupling. Killifish acclimated to FW were quickly transferred from their holding tank to a solution of buffered tricaine methanesulfonate (1.5 mmol L−1) until ventilation ceased and the spinal cord was severed. The heart was removed and placed into ice cold bathing media (modified from Marshall et al. 2002) containing (in mmol L−1): NaCl 140.0, KCl 2.6, CaCl2·2H2O 1.6, MgSO4 0.9, NaHCO3 11.9, NaH2PO4 3.0, glucose 5.6 (pH 7.8). Depending on the experimental condition, the media contained either 40.0 mmol L−1 taurine or sucrose, with osmolality closely matching that of killifish plasma at 340 mosmol kg−1. Sucrose was chosen as an osmotic control as it is a kosmotropic agent and, to our knowledge, there is no evidence that vertebrate cardiac muscle expresses the glycoside hydrolases required to metabolize it. Adrenaline (3.0 nmol L−1) was included in all preparations to maintain cardiac tonus (Shiels and Farrell 1997) and the media were equilibrated with 99.5% O2/0.5% CO2. The atrium and bulbus arteriosus were removed and the ventricle was sliced into two equal strips approximately 1 mm wide and 2 mm in length (3.8 ± 0.2 mg). Each strip was mounted in a Plexiglas chamber containing 20 mL of bathing media maintained at 15 °C and continuously gassed with 99.5% O2/0.5% CO2. Ventricular strips were tied to an isometric force transducer (Harvard Apparatus, South Natick, MA, USA) using suture thread and were stimulated to contract via two platinum electrodes using an SD9 Stimulator (Grass Instruments; Quincy, MA, USA) set at 50–70 V and 10 ms duration. Force transducers were interfaced to a PowerLab 8/26 data acquisition system (ADInstruments, Colorado Springs, CO, USA) and data were collected and analyzed using Chart V7.1 (ADInstruments). Resting tension was adjusted to achieve peak tension development (F max) and preparations were allowed to stabilize at a contraction frequency of 1.0 Hz for approximately 20 min. Preliminary electrocardiography studies on an anesthetized killifish indicated a heart rate of approximately 1.2 Hz (data not shown). The lower basal pacing frequency of 1.0 Hz was chosen to compensate for the additional demands associated with isometric contractions at F max.
Experiment 1: environmental effects on cardiac taurine content
To examine if taurine levels change in response to environmental change or physiological stress, cardiac taurine concentrations were measured in BW and FW acclimated killifish, and FW acclimated killifish exposed to acute hypoxia, or to an acute heat stress (15 fish per treatment group). For both hypoxia and heat stress trials, fish were transferred from their holding tank to cooler containing 30 L of aerated FW held at 15 °C and allowed to acclimate overnight. For the acute hypoxia exposure, dissolved oxygen saturation (DO2) was reduced from 100 to 10% over 5 h by bubbling with N2 and then held at 10% DO2 for 3 h. For the acute heat stress, temperature was increased linearly from 15 to 33 °C over 8 h, before fish were sampled. Following treatment, fish were sacrificed in a solution of buffered tricaine methanesulfonate (1.5 mmol L−1) and the spinal cord severed. Heart ventricular tissue was then quickly sampled and immediately flash frozen in liquid N2 and stored at −80 °C until use.
Experiment 2: effects of extracellular taurine and ryanodine on cardiac contractility
Each preparation was subject to a single treatment and run in parallel with the appropriate control from the same fish. Following the 20 min stabilization period, a force–frequency trial was performed where frequency was increased in 0.2 Hz increments at 1 min intervals until frequency reached 3.5 Hz or the strip stopped responding to stimulation. After this, frequency was reset to 1.0 Hz and the preparations were left to recover for 10 min. Preparations were then challenged by incrementally raising extracellular Ca2+ (Ca2+ e) levels by 1.0 mmol L−1 every 4 min, up to a maximum of 5.6 mmol L−1. In a second set of experiments, ventricular strip preparations were allowed to stabilize for 20 min before being treated with 10 µmol L−1 ryanodine (in DMSO). Preparations were left to stabilize for an additional 20 min before being challenged with the same force-frequency and Ca2 + e titrations described above. At this concentration, ryanodine inhibits the release of Ca2+ from the sarcoplasmic reticulum (SR) in fish cardiac muscle (Vornanen et al. 2002). All other tissue preparations in this experimental group were exposed to 10 µL DMSO as a vehicle control.
Experiment 3: cardioprotective effects of taurine during anoxia and reoxygenation
Contractility in killifish ventricular strip preparations exposed to hypoxia and reoxygenation was characterized to investigate a potential cardioprotective role of taurine under these conditions. Ventricular strips were prepared as described above and allowed to stabilize at 1.0 Hz for 20 min under oxygenated conditions. Preparations were first challenged with a force–frequency trial, as above, and subsequently allowed to recover at 1.0 Hz stimulation frequency for 5 min. The tissue chambers were then gassed with 99.5% N2/0.5% CO2 and held for 15 min under anoxia before reoxygenation with 99.5% O2/0.5% CO2. Preparations were allowed to recover from anoxia for 10 min, subjected to a second force-frequency trial, then allowed 5 min to recover before being challenged with increasing concentrations of Ca2+ e challenge, as described above.
Experiment 4: effects of extracellular taurine on cardiac metabolism
To assess the influence of taurine on aerobic metabolism in killifish heart, oxygen consumption (ṀO2) and glucose utilization rates were measured in non-contracting tissue preparations. Ventricular muscle strips were prepared from 2 to 3 animals as described above, and pooled into one of two identical respirometry cuvettes (OX1LP, Qubit Systems Inc., Kingston, ON, Canada), each with a total tissue mass of ~25 mg. The cuvettes contained 1.0 mL of bathing media (including 3.0 nmol L−1 adrenaline) with either 40 mmol L−1 taurine or an equivalent concentration of sucrose and were maintained at 15 °C with a recirculating refrigerated water bath. The preparations received no stimulation and spontaneous contractions were not observed. DO2 data were collected with a LabQuest Mini data acquisition system and LoggerPro software (Vernier Software & Technology, Beaverton, OR, USA) and mass-specific ṀO2 was calculated from the initial linear decline in DO2.
The rate of tissue glucose utilization was also assessed according to MacCormack et al. (2016). An initial sample of bathing media was taken just after ventricular strip preparations were added to the respirometer cuvettes and a subsequent sample was collected after 25 min. Glucose concentration was quantified in media samples according to Clow et al. (2004).
Data analysis and statistics
All the values are expressed as mean ± SEM. In the ventricular strip procedure, resting tension (RT) was measured between beats and quantified as percent initial from the RT at 1.0 Hz and 1.6 mM Ca2+ e. F max represented the difference between resting and peak tension developed during contraction and was normalized to initial F max for each strip. Due to their small size, it was not possible to accurately measure the dimensions of ventricular strips while mounted in the tissue baths, so absolute force development was not calculated. Statistical analyses were carried out using either Prism 6 (GraphPad Software Inc., La Jolla, CA, USA) or R 3.2.1 (RStudio, Boston, MA, USA). Differences in plasma osmolality, tissue ṀO2 and tissue glucose utilization rates were assessed with paired or unpaired t tests, as appropriate. Differences in the frequency at which ventricular strips failed to respond to stimulation were assessed using a one-way analysis of variance (ANOVA) followed by a Gehan–Breslow–Wilcoxon test with a Bonferroni correction. The effect of Ca2+ e or changes in stimulation frequency on RT and F max were assessed using a two-way repeated-measures ANOVA. The effects of anoxia exposure and taurine supplementation on the force-frequency relationship were assessed using a three-way split plot ANOVA, with repeated measures for oxygenation condition. Not all taurine/sucrose supplemented ventricular strips represented paired preparations, so repeated measures was not appropriate in this instance. When significant interactions were observed, data were split and analyzed by one-way ANOVA and Tukey post hoc tests with a Bonferroni correction. Shapiro’s test was performed to check for normality, and Levene’s test was used to check for homogeneity. P values <0.05 were considered significant unless otherwise noted.
Results
Experiment 1: environmental effects on cardiac taurine content
Plasma osmolality in killifish acclimated to BW was marginally, but significantly higher than in fish acclimated to FW (354.6 ± 3.0 vs. 333.7 ± 4 mOsm/kg respectively, P < 0.001, n = 10–11). This agrees well with the previous studies showing killifish effectively maintain plasma ion levels over a range of salinities (Scott et al. 2008). No statistically significant differences in cardiac taurine concentrations were noted between BW and FW killifish or between FW killifish acutely exposed to high temperatures or hypoxia (Fig. 1; P = 0.406).
Experiment 2: effects of taurine and ryanodine on cardiac contractility
The influence of supplemental taurine on cardiac contractility was assessed using isometrically contracting ventricular strip preparations (Fig. 2). For F max, no significant interaction was detected between treatment and stimulation frequency (P = 0.08) and significant effects of both treatment (P < 0.001) and frequency (P < 0.001) were noted. A significant negative force–frequency relationship was observed between 1.0 and 2.0 Hz, after which F max stabilized somewhat in strips that continued to respond to stimulation. Taurine (40 mmol L−1) had a protective effect on contractility, significantly attenuating the decline in F max relative to preparations treated with 40 mmol L−1 sucrose. For RT, no interaction was noted between treatment and stimulation frequency (P = 1.00), and no significant effects of either were parameter were observed (Fig. 3).
a Relative peak tension development (F max) and b resting tension (RT) in killifish ventricular strip preparations challenged with increasing stimulation frequency. Preparations were treated with either 40 mM taurine or 40 mM sucrose. c F max and d RT in similar preparations treated with 10 µmol L−1 ryanodine (Ry). Initial n = 5–7, with sample size decreasing with increased frequency as preparations failed to respond to stimulation. Statistically significant differences between taurine and sucrose-treated hearts are denoted with an asterisk
Impact of 10 µmol L−1 ryanodine (Ry) for the proportion of killifish ventricular strip preparations responding to stimulation with increased pacing frequency (a) and the maximum pacing frequency (b) for each treatment group. Maximum pacing frequency was significantly depressed in ryanodine treated preparations, as denoted by an asterisk
The force–frequency trial was repeated with the addition of ryanodine (10 µmol L−1) on a separate set of preparations to assess the affects of supplemental taurine on SR Ca2+ cycling (Fig. 2). Exposure to ryanodine for 20 min pacing at 1.0 Hz had no effect on F max in either taurine or sucrose-treated preparations (P = 0.870, data not shown). F max at higher frequencies was generally depressed for all preparations in the presence of ryanodine and strips failed to contract at lower imposed stimulation frequencies (see below). No interaction was noted between treatment and stimulation frequency (P = 0.240). Increasing pacing frequently significantly decreased F max (P < 0.001) overall, and ryanodine abolished the protective effect of taurine on F max, with no significant difference noted between treatments (P = 0.103). No changes in RT were observed.
The frequency at which the strips failed to contract upon stimulation was examined to infer the contribution of SR Ca2+ handling to cardiac contractility (Fig. 3). No significant interaction was detected between sucrose/taurine treatment and ryanodine exposure (P = 0.280), and no differences in maximum pacing frequency were noted between sucrose- and taurine-treated preparations in the absence of ryanodine (P = 0.425). Ryanodine addition significantly reduced maximum pacing frequency (P < 0.001) by ~40%, indicating that the importance of SR Ca2+ cycling increases at higher pacing frequencies, regardless of whether taurine is present.
Ventricular strip preparations were also challenged with increasing concentrations of Ca2+ e to assess Ca2+ sensitivity. In the absence of ryanodine, F max generally remained stable in both taurine and sucrose-treated preparations, while it tended to decrease in sucrose-treated strips in the presence of ryanodine. No interaction was detected between treatment and Ca2+ e concentration (P = 0.161) and no significant effect of increasing Ca2+ e was observed overall (P = 0.914). A significant effect of treatment was apparent (P < 0.001), and post hoc tests showed that in the presence of ryanodine, F max at 4.6 mmol L−1 Ca2+ e was significantly higher in preparations supplemented with taurine than those supplemented with sucrose (P = 0.036). F max remained elevated at 5.6 mmol L−1 Ca2+ e, although not significantly, so (P = 0.076). RT was unaffected by increases in Ca2+ e under any treatment condition (data not shown).
Experiment 3: cardioprotective effects of taurine during anoxia and reoxygenation
Ventricular strip preparations were supplemented with either taurine or sucrose and basal contractility (at 1.0 Hz pacing frequency) was assessed before and after 15 min anoxia, and after 20 min recovery under oxygenated conditions (Fig. 5). No significant interaction was noted between treatment and oxygenation (P = 0.166) and no significant effect of taurine supplementation was observed (P = 0.337). Oxygenation significantly influenced basal F max overall (P < 0.001), so taurine and sucrose data were pooled and the specific effects of oxygenation examined. A 15 min anoxia exposure significantly decreased F max by 38% (P < 0.001). Following reoxygenation, F max tended to be higher than that observed pre-anoxia, potentially indicating Ca2+ i accumulation. This trend was more evident in taurine-treated preparations that those supplemented with sucrose, but the effect was not statistically significant (P = 0.082).
In force–frequency challenges, a significant interaction was noted between exposure to anoxia and taurine supplementation (P < 0.001). A prior 15 min exposure to anoxia significantly impaired performance in a subsequent force-frequency challenge (P < 0.001) and supplemental taurine had a significant protective effect on F max during this period (P = 0.020, Fig. 5b). A prior anoxia exposure had no effect on maximum pacing frequency (P = 0.470; data not shown) and RT was unaffected under any test condition (data not shown).
Following the post-anoxia force–frequency challenge, preparations were exposed to increasing levels of Ca2+ e. No interaction was noted between taurine treatment and Ca2+ e (P = 0.956) and no effect of either supplemental taurine (P = 0.338) or increasing Ca2+ e (P = 0.178) was noted (Fig. 5c).
Experiment 4: effects of extracellular taurine on cardiac metabolism
In invertebrates, taurine can increase extracellular glucose utilization without impacting tissue ṀO2 (MacCormack et al. 2016). In the current study, ṀO2 (Fig. 6) in non-contracting ventricular strip preparations were ~fourfold higher than those observed in similar preparations from cuttlefish systemic heart (MacCormack et al. 2016) and were close to rates measured in rainbow trout hearts contracting at 0.5 Hz (Kalinin and Gesser 2002). ṀO2 was similar in both sucrose- and taurine-treated ventricles (P = 0.901). Rates of extracellular glucose utilization were also higher than those seen in cuttlefish and no statistically significant differences were noted between treatments (P = 0.408). These data also support the validity of sucrose as an appropriate osmotic control for these studies. If sucrose was cytotoxic or metabolized by the tissue, we would expect ṀO2 and glucose utilization rates to differ greatly between treatment groups.
Discussion
The goal of the current study was to determine if ventricular taurine concentration changes in response to common environmental stressors and to characterize the influence of taurine on cardiac contractility in a model teleost, the killifish.
Heart taurine levels are maintained in killifish
The sensitivity of the taurine analysis in the current study was limited by the need to pool samples from 5 fish to obtain sufficient ventricular tissue for the taurine assay. This limited our capacity for replication and statistical power, and thus, the associated data should be considered accordingly. Killifish exhibited no significant alterations in ventricular taurine levels in response to changes in salinity (Fig. 1). Cardiac taurine levels vary with plasma osmolality in flounder (Vislie and Fugelli 1975) and brown trout (Fugelli and Vislie 1982), but no such relationship was noted in killifish. The <6% change in plasma osmolality that we observed between FW and BW acclimated killifish was likely insufficient to induce a detectable change in tissue taurine content. A similar maintenance of taurine content was observed in vivo and in vitro in ventricular tissue from little skate, which exhibits an equivalent concentration of taurine (Boyd et al. 1977; Forster et al. 1978). Increases in gill perfusion and ventilation during high temperatures and hypoxia can reduce plasma osmolality in some FW fish (Sardella and Brauner 2007), so taurine levels may be expected to change under such conditions. This was not the case in the current study, as ventricular taurine levels were also maintained in FW killifish following acute high temperature and hypoxia exposures. The severity and/or duration of the challenges was likely insufficient to disrupt plasma osmolality in this euryhaline, eurythermal, and euryoxic species (Schulte 2014). Addressing this question in more stress-sensitive species or those exhibiting unique physiological coping strategies may lend greater insight into the influence of environmental factors on cardiac taurine dynamics. In some fish species, stress responses can profoundly alter extracellular fluid composition, which may trigger associated changes in intracellular taurine levels. More information is needed to understand how taurine varies under such conditions and how this subsequently impacts cardiac physiology.
Taurine protects normoxic contractility by enhancing SR function
In isometrically contracting ventricular strips, taurine significantly protected F max at high pacing frequencies, an effect that was abolished by ryanodine (Fig. 2). SR Ca2+ cycling contributes little to contractility at low frequencies in most fish hearts (<2 Hz; Vornanen et al. 2002), but its importance increases with increasing pacing rate (reviewed by Shiels and Galli 2014). This is clearly the case in killifish as well, as ryanodine reduced maximum pacing frequency by 40% (Fig. 3). In mammalian studies, extracellular taurine supplementation has been shown to trigger sustained increases in Ca2+ i, likely via increased Na+ entry through a Na+-taurine symporter, which then promotes Ca2+ influx through the NCX (Bkaily et al. 1998). Taurine’s direct influences on mammalian cardiomyocyte Ca2+ flux are largely limited to the sarcolemmal membrane, as it inhibits Ca2+ i loading associated with ouabain treatment but not with ryanodine (Rao and Tau 1998). Fish cardiomyocytes generally exhibit little Ca2+ induced Ca2+ release (CICR), but enhanced sarcolemmal Ca2+ influx may recruit additional ryanodine receptors and promote CICR under certain conditions (Shiels and Galli 2014). It is possible that taurine-mediated increases in Ca2+ i sensitized SR Ca2+ cycling to protect F max at higher stimulation frequencies. In mammalian skeletal muscle, in vivo taurine supplementation increased calsequestrin expression (Goodman et al. 2009), an effect that should sensitize the ryanodine receptor to changes in Ca2+ i (Györke and Terentyev 2008). It is unclear that if a similar mechanism could be induced under the acute conditions tested here, but the lack of an effect on maximum pacing frequency supports the conclusion that taurine strictly influences the magnitude of the Ca2+ flux and not the kinetics, which is in keeping with sensitizing the SR to changes in Ca2+ i.
Contractility in killifish ventricular strip preparations was remarkably insensitive to increases in Ca2+ e and no effect of taurine on F max or RT was noted in the absence of ryanodine. In the presence of ryanodine, F max tended to decrease with increasing Ca2+ e levels in sucrose-treated preparations, while it was significantly protected in taurine-treated hearts (Fig. 4). The relative decrease in F max in sucrose-treated preparations is difficult to explain based on the available data. If taurine supplementation improves cardiac Ca2+ handling, as it does in cuttlefish (MacCormack et al. 2016), sucrose-treated hearts may experience Ca2+ i overload and the accompanying cytotoxicity would impair contractility. Taurine can also be cytoprotective in this instance by preventing the initiation of apoptosis at high Ca2+ i (Schaffer et al. 2014). These possibilities seem unlikely given that RT varied <2% as Ca2+ e increased in both ryanodine treatment groups (data not shown), indicating that Ca2+ i homeostasis was well regulated. Additional studies are necessary to characterize the mechanisms underlying this response.
Taurine provides additional cardioprotection from anoxia
Killifish cardiac muscle exhibited a 38% decrease in F max after a 15 min exposure to anoxia but fully recovered upon reoxygenation (Fig. 5). This species shows considerable whole animal hypoxia tolerance (e.g., Borowiec et al. 2015), so it is not surprising that it translates to the tissue level, as well. Taurine had no effect on basal F max (at 1.0 Hz) post-anoxia, but it significantly improved performance of hearts in the force–frequency trial following anoxia compared to sucrose-treated controls. The SR’s role in cardiac contractility increases under stressful conditions, supplementing the total Ca2+ transient (Cros et al. 2014). In this experiment, the combined energetic stresses of anoxia and high pacing frequencies may have reduced ATP availability and inhibited active membrane processes associated with Ca2+ cycling (Vornanen and Tuomennoro 1999). It is possible that SR function is preferentially protected under these conditions, increasing its relative contribution to the total Ca2+ transient; in P. pardalis hearts, some glycolytic enzymes reversibly bind to particulate components under anoxia, likely to preferentially fuel specific processes (Treberg et al. 2007). Ryanodine reduces F max under anoxia this species (MacCormack et al. 2003), suggesting that SR function may be one such process. Overall, our data suggest that taurine may further enhance the SR’s contribution to Ca2+ flux under anoxia, but additional work is needed to determine the exact mechanisms underlying this response.
a Relative peak tension development (F max) prior to, during, and after 15 min anoxia in killifish ventricular strip preparations supplemented with either 40 mmol L−1 taurine or sucrose (n = 6, both groups). b Force–frequency relationships before and after anoxia from ventricular strip preparations receiving supplemental taurine or sucrose. c Extracellular Ca2+ sensitivity following 15 anoxia and a subsequent force–frequency challenge. In a, no effect of taurine treatment was noted, so data from taurine or sucrose-treated preparations were pooled to analyze the effects of anoxia and reoxygenation. Statistically significant differences from pre-anoxia F max are indicated by dissimilar letters. In b, anoxia exposure significantly impaired F max in both treatment groups in the subsequent force–frequency challenge and taurine significantly increased F max under those conditions (as indicated by an asterisk)
Energy metabolism is not influenced by supplemental taurine
We observed no effect of taurine supplementation on either resting ventricular ṀO2 or extracellular glucose utilization rates (Fig. 6). In similar systemic heart preparations from the cephalopod Sepia officinalis, taurine doubled the rate of extracellular glucose utilization without impacting tissue ṀO2, suggesting that it shifted the metabolic fuel preference of the tissue (MacCormack et al. 2016). In perfused rat hearts, taurine stimulates both glucose utilization and ṀO2 in an insulin dependent manner (Lampson et al. 1983; Imae et al. 2014). The lack of insulin or alternative substrates in the simplified experimental system used here may have masked subtle regulatory effects of taurine on metabolism. Increasing tissue energy demand by stimulating contractions may also impact this process and such issues should be addressed in future studies.
Conclusions
Although tissue taurine levels did not change in killifish exposed to environmental challenges, supplemental taurine significantly influenced cardiac contractility in vitro, likely by modulating SR function. Taurine’s effects were additive to those elicited by anoxia and reoxygenation, suggesting that it alters Ca2+ cycling via a different mechanism than the latter stressor. Characterizing how taurine influences SR function may also improve our incomplete understanding of the role of the SR in routine cardiac Ca2+ cycling in ectotherms and its importance in maintaining heart function over a broad range of temperatures (Shiels and Galli 2014; Shiels and Sitsapesan 2015).
References
Bkaily G, Jaalouk D, Sader S, Shbaklo H, Pothier P, Jacques D, D’Orleans-Juste P, Cragoe EJ, Bose R (1998) Taurine indirectly increases [Ca]i by inducing Ca2+ influx through the Na(+)-Ca2+ exchanger. Mol Cell Biochem 188:187–197
Borowiec BG, Darcy KL, Gillette DM, Scott GR (2015) Distinct physiological strategies are used to cope with constant hypoxia and intermittent hypoxia in killifish (Fundulus heteroclitus). J Exp Biol 218:1198–1211
Boyd TA, Cha CJ, Forster RP, Goldstein L (1977) Free amino acids in tissues of the skate Raja erinacea and the stingray Dasyatis sabina: effects of environmental dilution. J exp Zool 199:435–442
Clow KA, Rodnick KJ, MacCormack TJ, Driedzic WR (2004) The regulation and importance of glucose uptake in the isolated Atlantic cod heart: rate-limiting steps and effects of hypoxia. J Exp Biol 207:1865–1874
Cros C, Sallé L, Warren DE, Shiels HA, Brette F (2014) The calcium stored in the sarcoplasmic reticulum acts as a safety mechanism in rainbow trout heart. Am J Physiol 307:R1493–R1501
Evans DH (1979) Fish. In: Maloiy GMO (ed) Osmotic and ionic regulation in animals. Academic Press, London, pp 305–390
Forster RP, Hannafin JA, Shiffrin JS (1978) Characterization of taurine uptake in vitro by heart of the little skate, Raja erinacea. Bull Mt Desert Isl Biol Lab Salisb Cove Maine 18:1–4
Fugelli K, Vislie T (1982) Physiological response to acid water in brown trout (Salmo trutta L.): cell volume regulation in heart ventricle tissue. J Exp Biol 101:71–82
Gesser H, Driedzic WR, Rantin FT, de Freitas JC (1997) Ca2+ regulation of heart contractility in Octopus. J Comp Physiol B 167:474–480
Goldstein L, Luer CA, Blum PC (1990) Taurine accumulation by the heart of embryonic skates, Raja eglanteria. J Exp Biol 150:449–452
Goodman CA, Horvath D, Stathis C, Mori T, Croft K, Murphy RM, Hayes A (2009) Taurine supplementation increases skeletal muscle force production and protects muscle function during and after high-frequency in vitro stimulation. J Appl Physiol 107:144–154
Györke S, Terentyev D (2008) Modulation of ryanodine receptor by luminal calcium and accessory proteins in health and cardiac disease. Cardiovasc Res 77:245–255
Hochachka PW, Mommsen TP, Storey J, Storey KB, Johansen K, French CJ (1983) The relationship between arginine and proline metabolism in cephalopods. Mar Biol Lett 4:1–21
Huxtable RJ (1992) Physiological actions of taurine. Physiol Rev 72:101–163
Imae M, Asano T, Murakami S (2014) Potential role of taurine in the prevention of diabetes and metabolic syndrome. Amino Acids 46:81–88
Kalinin A, Gesser H (2002) Oxygen consumption and force development in turtle and trout cardiac muscle during acidosis and high extracellular potassium. J Comp Physiol B 172:145–151
Lampson WG, Kramer JH, Schaffer SW (1983) Potentiation of the actions of insulin by taurine. Can J Physiol Pharmacol 61:457–463
MacCormack TJ, Treberg JR, Almeida-Val VMF, Val AL, Driedzic WR (2003) Mitochondrial KATP channels and sarcoplasmic reticulum influence cardiac force development under anoxia in the Amazonian armored catfish Liposarcus pardalis. Comp Biochem Physiol A 134:441–448
MacCormack TJ, Callaghan NI, Sykes AV, Driedzic WR (2016) Taurine depresses contractility and enhances systemic heart glucose utilization in the cuttlefish, Sepia officinalis. J Comp Physiol B 186:215–227
Marshall WS, Howard JA, Cozzi RRF, Lynch EM (2002) NaCl and fluid secretion by the intestine of the teleost Fundulus heteroclitus: involvement of CFTR. J Exp Biol 205:745–758
Polakof S, Panserat S, Soengas JL, Moon TW (2012) Glucose metabolism in fish: a review. J Comp Physiol B 182:1015–1045
Rao MR, Tao L (1998) Effects of taurine on signal transduction steps induced during hypertrophy of rat heart myocytes. In: Schaffer SW, Lombardini JB, Huxtable RJ (eds) Taurine 3: cellular and regulatory mechanisms. Springer, New York, pp 137–140
Sardella BA, Brauner CJ (2007) The osmo-respiratory compromise in fish. In: Fernandes MN (ed) Fish respiration and environment. Science Publishers, Enfield, pp 147–165
Satoh H (1994) Regulation of the action potential configuration by taurine in guinea-pig ventricular muscles. Gen Pharmacol 25:47–52
Schaffer SW, Ballard C, Azuma J (1994) Mechanisms underlying physiological and pharmacological actions of taurine on myocardial calcium transport. In: Huxtable R, Michalk DV (eds) Taurine in health and disease. Plenum Press, NY, pp 171–196
Schaffer SW, Jong CJ, Ramila KC, Junichi A (2010) Physiological roles of taurine in heart and muscle. J Biochem Sci 17:1–8
Schaffer SW, Jong CJ, Ito T, Azuma J (2014) Effect of taurine on ischemia–reperfusion injury. Amino Acids 46:21–30
Schulte PM (2014) What is environmental stress? Insights from fish living in a variable environment. J Exp Biol 217:23–34
Scott GR, Baker DW, Schulte PM, Wood CM (2008) Physiological and molecular mechanisms of osmoregulatory plasticity in killifish after seawater transfer. J Exp Biol 211:2450–2459
Shaffer J, Kocsis J (1981) Taurine mobilizing effects of beta alanine and other inhibitors of taurine transport. Life Sci 28:2727–2736
Shiels HA, Farrell AP (1997) The effect of temperature and adrenaline on the relative importance of the sarcoplasmic reticulum in contributing Ca2+ to force development in isolated ventricular trabeculae from rainbow trout. J Exp Biol 200:1607–1621
Shiels HA, Galli GL (2014) The sarcoplasmic reticulum and the evolution of the vertebrate heart. Physiology (Bethesda) 29:456–469
Shiels HA, Sitsapesan R (2015) Is there something fishy about the regulation of the ryanodine receptor in the fish heart? Exp Physiol 100(12):1412–1420
Sperelakis N, Satoh H, Bkaily G (1992) Taurine effects on ionic currents in myocardial cells. Adv Exp Med Biol 315:129–143
Takahashi K, Ohyabu Y, Schaffer SW, Azuma J (2002) Taurine prevents ischemia damage in cultured neonatal rat cardiomyocytes. In: Della Corte L, Huxtable RJ, Sgaragli G, Tipton KF (eds) Taurine 4. Taurine and excitable tissues. Kluwer Academic, New York, pp 109–116
Treberg JR, MacCormack TJ, Lewis JM, Almeida-Val VMF, Val AL, Driedzic WR (2007) Intracellular glucose and binding of hexokinase and phosphofructokinase to particulate fractions increase under hypoxia in heart of the Amazonian armoured catfish (Liposarcus pardalis). Physiol Biochem Zool 80:542–550
Vislie T (1983) Cell volume regulation in fish heart ventricles with special reference to taurine. Comp Biochem Physiol 76A:507–514
Vislie T, Fugelli K (1975) Cell volume regulation in flounder (Platichthys flesus) heart muscle accompanying an alteration in plasma osmolality. Comp Biochem Physiol A 52:415–418
Vornanen M, Tuomennoro J (1999) Effects of acute anoxia on heart function in crucian carp: importance of cholinergic and purinergic control. Am J Physiol 277:R465–R475
Vornanen M, Shiels HA, Farrell AP (2002) Plasticity of excitation-contraction coupling in fish cardiac myocytes. Comp Biochem Physiol A 132:827–846
Xu YJ, Saini HK, Zhang M, Elimban V, Dhalla NS (2006) MAPK activation and apoptotic alterations in hearts subjected to calcium paradox are attenuated by taurine. Cardiovasc Res 72:163–174
Acknowledgements
EFH was supported by a Natural Sciences and Engineering Research Council of Canada (NSERC) Undergraduate Student Research Award. TJM was supported by an NSERC Discovery grant. The authors wish to thank Mr. Wayne Anderson for animal care and Mr. Neal Callaghan and Ms. Louise Tunnah for the assistance with statistical analyses.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by H. V. Carey.
Rights and permissions
About this article
Cite this article
Henry, E.F., MacCormack, T.J. Taurine protects cardiac contractility in killifish, Fundulus heteroclitus, by enhancing sarcoplasmic reticular Ca2+ cycling. J Comp Physiol B 188, 89–99 (2018). https://doi.org/10.1007/s00360-017-1107-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00360-017-1107-4