Abstract
Circadian clocks are near universal among organisms and play a key role in coordinating physiological and metabolic functions to anticipate or coincide with predictable daily changes in the physical and social environment. However, whether circadian rhythms persist and are functionally important during hibernation in all mammals is currently unclear. We examined whether circadian rhythms of body temperature (T b) persist during multi-day, steady-state torpor and investigated the association between timing of animal emergence, exposure to light, and resumption of activity and T b rhythms in free-living and captive male arctic ground squirrels. High-resolution (0.02 °C) temperature loggers revealed that circadian rhythms of T b were not present during deep torpor in free-living arctic ground squirrels. Significant circadian rhythms of T b resumed, however, following the resumption of euthermia, but prior to emergence, though rhythms became much more robust coincident with aboveground emergence. Additionally, squirrels maintained in captivity under conditions of constant darkness spontaneously developed significant circadian rhythms of T b and activity soon after ending torpor. Exposing animals to a 5-s pulse of light within a week when they ended torpor increased the strength of rhythms. Our results are consistent with the hypothesis that circadian clock function is inhibited during hibernation in arctic ground squirrels, and we postulate that exposure to external stimuli, such as light in free-living animals, and meals or acute disturbance for captive squirrels, may enhance T b rhythmicity by synchronizing loosely coupled circadian oscillators within the suprachiasmatic nucleus.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Circadian systems provide animals with the ability to coordinate their physiological and metabolic functions in anticipation of predictable daily changes in their environment. In mammals, circadian rhythms are controlled by a master circadian clock formed by neurons within the suprachiasmatic nucleus (SCN) of the anterior hypothalamus; this master clock drives rhythms in body temperature (T b) and humoral cues that act to entrain individual “slave” oscillators located in peripheral tissues throughout the body (Reppert and Weaver 2002; Buhr et al. 2010). Even in the absence of zeitgeber, or external timing cues, endogenous circadian rhythms of physiology and behavior persist with a period of ~24 h, driven by all oscillators within the circadian system (Dibner et al. 2010). In nature, these oscillators remain in synchrony with the environment as the master clock within the SCN is entrained to the light–dark (LD) cycle.
The importance of circadian rhythms is evidenced by their ubiquity, occurring in bacteria, plants, fungi, and animals (Young and Kay 2001). In addition to playing a critical role in timing daily behaviors and physiology, it has long been proposed that circadian clocks are used to measure day length and initiate photoperiodic responses for seasonal timing (Bünning 1936), a concept that is now well supported in plants (Park et al. 1999) and animals (Ikeno et al. 2010; Wood and Loudon 2014). Despite the widespread importance of circadian rhythms for most organisms, chronobiologists have been interested in whether circadian function is compatible with all physiological states. For example, while circadian clocks play an important role in the timing of mechanisms that regulate seasonal hypometabolism or hibernation (Hut et al. 2014), either through their involvement in photoperiodic responses or by entraining circannual clocks, the persistence and potential function of circadian rhythms during prolonged intervals of hypometabolism and variable body temperature are less clear.
In insects, circadian genes are not only important in timing the initiation of diapause, but the major circadian clock genes continue to cycle throughout diapause (Meuti et al. 2015). In vertebrates, circadian clock function appears to be unimpaired by depressed metabolism associated with daily torpor (Herwig et al. 2006). However, the only study to examine clock gene expression during the multi-day torpor associated with seasonal hibernation in small mammals found evidence that clock genes in the SCN had ceased cycling (Revel et al. 2007). Given circadian clocks are mechanistically driven by transcription/translation feedback loops, the absence of cycling of circadian clock genes is consistent with findings that transcription and translation are globally suppressed during deep torpor in mammals (Storey and Storey 2004). Yet, selective transcription and translation can still occur during deep torpor (Hittel and Storey 2002), and circadian gene expression has been found to be resilient to large fluctuations in transcription rates (Dibner et al. 2009), though not at the very low temperatures that characterize hibernation in small mammals.
During hibernation, small mammals undergo periodic arousals to euthermic body temperatures each few days or several weeks, depending on ambient temperature and body size (Ruf and Geiser 2015, but see; Lovegrove et al. 2014). The function and trigger of such arousals are debated, and despite the lack of evidence for persistent cycles of circadian gene expression within the SCN during deep torpor, circadian clocks are hypothesized by some to function in timing arousals (Heller and Ruby 2004; Malan 2010). Recent studies indicate that many cellular components relevant to timekeeping are enzymatic or metabolic in nature, rather than transcriptional, indicating that the lack of continued cycling of clock genes observed by Revel et al. (2007) does not necessarily signify that circadian clocks are not functioning (reviewed in O’Neill et al. 2013).
Two lines of evidence support the persistence of circadian rhythms during deep torpor (multi-day torpor). First, captive golden-mantled ground squirrels (Callospermophilus lateralis) exhibit persistent low-amplitude (<0.1 °C) circadian T b rhythms of widely varying period lengths in deep torpor (Grahn et al. 1994). Florant et al. (2000) similarly found that captive marmots display circadian T b rhythms during torpor, although these rhythms did not occur in free-living animals, suggesting that rhythms could be an artifact of captivity. Similarly, no evidence for circadian T b rhythms during steady-state torpor was found in free-living European (Spermophilus citellus), Anatolian (S. xanthoprymnus), or arctic (Urocitellus parryii) ground squirrels (Hut et al. 2002a; Gür et al. 2009; Williams et al. 2012a, b). Because T b during deep torpor largely reflects ambient temperature (T a), T b rhythms can emerge in captive animals if there is a rhythm within the environmental chamber in which they are being housed (Florant et al. 2000). Ruby et al. (2002), however, found that while low-amplitude (<0.2 °C) T b rhythms were lost in SCN-ablated golden-mantled ground squirrels, they persisted in control animals, suggesting that such rhythms are endogenously generated. Additionally, Larkin et al. (2002) found that rhythms in brain temperature persist in golden-mantled ground squirrels maintained in an environmental chamber in which no rhythm in T a was detected. Although it is possible that low-amplitude T b rhythms in other species of ground squirrels were missed due to the limited resolution of the loggers used, it has also been noted that ground squirrels do not express a circadian T b rhythm when they first become euthermic in the spring. In captive European ground squirrels, rhythms emerge spontaneously within 5–15 days of the return to euthermia (Hut et al. 2002b), whereas previous studies indicate T b of free-living arctic ground squirrels is arrhythmic while animals remain in their constantly dark burrows for 4–5 weeks after torpor has ended until they first emerge to the surface (Williams et al. 2012a, b). The second major line of evidence for the persistence of circadian rhythms comes from studies examining the timing of periodic arousals where several studies have noted ~24-h periodicity in the duration of torpor bouts and/or arousals occurring at a particular time of day (Daan 1972; Grahn et al. 1994, Kӧrtner and Geiser 1998; Park et al. 2000). Several other studies have failed, however, to detect circadian timing in the occurrence of arousals from deep torpor (Thomas 1993; Waßmer and Woolnik 1997; Hut et al. 2002a), and such gating of arousals is very difficult to detect in mammals with multi-week-long torpor bouts (Grahn et al. 1994; Kӧrtner and; Geiser 2000).
In the present study, we examine patterns of T b rhythmicity during deep torpor and after spring arousal and emergence in free-living and captive arctic ground squirrels. First, we utilize very high-resolution (0.02 °C) data loggers to determine whether low-amplitude (<0.2 °C) circadian rhythms persist during deep torpor in free-living arctic ground squirrels. Such low-amplitude rhythms may have been missed due to the coarser (0.2 °C) resolution of loggers used in our prior studies (Williams et al. 2012a, b). We also examine whether free-living males develop circadian T b rhythms during their preemergent interval of euthermia in spring, or whether the recurrence of rhythmicity coincides with first exposure to light. We then examine whether circadian T b rhythms spontaneously develop under conditions of continuous darkness in captive arctic ground squirrels or whether light is needed as a cue to trigger the onset of rhythmicity in spring. We hypothesized that circadian function is inhibited by the very low brain and T b that accompanies deep torpor. We predicted that no T b rhythms would be present during torpor and post-hibernation T b rhythmicity would not return in spring until animals were exposed to light. In contrast to our previous studies in arctic ground squirrels, but consistent with studies of other ground squirrels, we found that circadian rhythms of T b can resume soon after torpor is complete prior to light exposure.
Materials and methods
Free-living animals
We recorded patterns of T b and post-hibernation exposure to light in free-living arctic ground squirrels residing near Toolik Field Station (68°38′N, 149°38′W) in northern Alaska. In August 2013, we captured and implanted 2 adult male ground squirrels with high-resolution abdominal T b loggers (TidbiT v2 Water Temperature Data Logger, Onset Computer Corporation, Bourne, MA, USA) that were removed from their casing and shrink wrapped prior to being coated in Elvax (DuPont, Wilmington, DE, USA). We restricted our sample size to two ground squirrels because these high-resolution loggers are heavier (20 g) and larger (4 cm diameter × 3 cm depth) than the loggers we typically use (Williams et al. 2011, 2012a). These loggers are accurate to within ±0.2 °C, have a resolution of 0.02 °C, and were programmed to record T b every 10 min. Both males were also equipped with light loggers affixed to collars (Intigeo-C56 geolocators, Migrate Technology Ltd, Cambridge, UK; see details in Williams et al. 2014). These loggers have a light sensor which reads once every minute with the highest recorded value per 5-min interval being saved to memory; thus, recording from these will indicate if animals are exposed to sunlight. The range of light readings is between 1 and 74,000 lx with a logarithmic resolution that contains 249 discrete levels (i.e., the devices have a much higher sensitivity at low light levels). We subsequently recaptured both males in May 2014, recovered the T b and light loggers, and downloaded the continuous 9-month record of T b and light exposure.
Captive experiments
We conducted captive experiments using male arctic ground squirrels captured near Atigun River (68° 27′ N 149° 21′W) in northern Alaska. Animals were initially maintained in metal cages (48 × 31 × 30 cm, UnifabCages, Kalamazoo, MI, USA) on a 12L:12D photoperiod and at room temperature (20 ± 2 °C). Animals were given cotton batting for nesting (Perfect Fit, McDonald, Tukwila, WA, USA) with food (Mazuri Rodent Chow, Brentwood, MO, USA) and water provided ad libitum. At least one month prior to the onset of hibernation, squirrels were surgically implanted with T b and activity transmitters (~7 g, model TA10TA-F40-LF, Data Sciences International, St Paul, MN, USA). All transmitters were calibrated to within 0.1 °C at 0.0, 35, and 39 °C prior to implantation. In fall, after squirrels had undergone their pre-hibernation fattening, we transferred animals into 43 × 27 × 19 cm plastic tubs (Nalgene, Rochester, NY, USA) and placed them in environmental chambers maintained at 0 ± 1 °C. Chambers maintained constant darkness with animal husbandry performed using a headlamp with a red LED light; all food and water was removed once animals first became torpid. Animals were monitored throughout hibernation using the traditional sawdust method (Pengelley and Fisher 1961); wood shavings were placed on the dorsal surface of the animals and inspected daily to assess, by the presence or absence of sawdust, the duration of torpor bouts, and occurrence of arousal episodes.
To determine whether exposure to light is necessary for the resumption of circadian T b rhythms after torpor ends, we removed hibernating animals from the environmental chamber (n = 4), activated their implanted transmitters, placed them into larger (60 × 45 × 25 cm) plastic tubs, and transferred them into a room held at 20 ± 1 °C under conditions of continuous darkness. We provided animals with cotton bedding, pine shavings, and a 25-cm-long × 10-cm-diameter PVC tube for habitat enrichment. Water and gel packs (4 oz Napa Nectar, Systems Engineering Lab Group Inc., Napa, CA, USA) were provided ad libitum; 8–10 pellets of rodent chow were provided once daily between the hours of 7 am and midnight on an irregular schedule. We used night vision optics (Challenger monocular, Pulsar Vision, Brooklyn, NY) combined with a very low-intensity red light (M1 Infrared Illuminator, Surefire LLC, Fountain Valley, CA) for daily feedings and assessment of animal welfare during this phase of the experiment.
To determine whether light would induce or alter T b rhythmicity, squirrels were given a 5-s pulse of high-intensity white light for 5 s after 28 days of euthermy. Squirrels were individually moved in their tubs to a second dark chamber and pulsed at a distance of 20 cm using a surefire 320 lm flashlight for 5 s; all squirrels voluntarily stared into the light when being pulsed. Overhead full-spectrum lights in the room were turned on simultaneously with the flashlight pulse to ensure squirrels received a strong light signal. Following the pulse, animals were immediately returned to the DSI monitoring system for an additional 10 days of recording T b and activity under conditions of continuous darkness. Because squirrels became rhythmic prior to receiving the pulse 28 days into euthermia (see results), we repeated the experiment with 3 squirrels but pulsed them with light after only 3–6 days of euthermia. Individuals were pulsed on different days at different times of day to ensure they were not cueing into the activity of other squirrels in the experiment.
Data analysis
Circadian rhythmicity in T b during deep torpor for the two free-living individuals was assessed using both Chi-squared and Lomb–Scargle periodogram analyses on >10-day intervals of data during torpor bouts beginning in January, after the hibernacula had become frozen and ground squirrels were thermogenic during torpor, as evidenced by drops in core T b below −1 °C. The Lomb–Scargle algorithm has a better detection efficiency and accuracy in the presence of noise (Ruf 1999), but non-sinusoidal signals or outlying large-amplitude features can make the interpretation of Lomb–Scargle periodograms more difficult (Schimmel 2001). For example, the false alarm values (P values) in Lomb–Scargle are sensitive to non-Gaussian noise (Schimmel 2001; Refinetti et al. 2007). Lomb–Scargle analysis is also preferred when data are unevenly spaced or data points are missing (Ruf 1999), but this was not an issue for our dataset. For free-living and captive individuals, post-hibernation rhythmicity in T b and activity were assessed for each individual using Lomb–Scargle periodogram analysis (Chi-squared analysis provided equivalent results) on sliding 7-day intervals of data (e.g., interval 1: 1–7 days post-hibernation, interval 2: 2–8 days, …, interval 29: 29–35 days post-hibernation). We use the empirically calculated normalized power (PN) from the Lomb–Scargle periodogram analysis as a measure of the degree of rhythmicity for each 7-day interval. Actograms were constructed, and periodogram analysis was performed using Clocklab software (Actimetrics, Evanston, IL, USA).
Results
Free-living animals
During early bouts of torpor, prior to freezing of the active layer, T b of both males (#573 and #926) equipped with high-resolution (±0.02 °C) T b loggers gradually cooled throughout the torpor bout. During subsequent bouts of torpor when the active layer of soil surrounding the hibernacula was frozen and ground squirrels were thermogenic and thermoregulating (indicated by relatively stable T b well below 0 °C), T b exhibited some variability of <0.2 °C, but Chi-squared periodogram analysis indicated no significant circadian or rhythms (P > 0.25) in any of the 7 torpor bouts (3 bouts for male #926 and 4 bouts for #573; Fig. 1, Fig. S1). Lomb–Scargle periodogram analysis indicated no significant rhythms (P > 0.05) for any of the three torpor bouts for male #926 (Fig. S1); however, low-amplitude but significant rhythms (P < 0.001) were detected in all four of the torpor bouts for male #573, although the dominant rhythms identified were not circadian and instead had periods of ~19 and 31 h (Fig. 1).
Body temperature (T b) during a 14-day-long torpor bout of free-living arctic ground squirrel male #573 (a). T b between the vertical dashed bars was used to create an actogram (b) and for Chi-squared (c) and Lomb–Scargle (d) periodogram analyses. No significant rhythms were found using Chi-squared periodogram analysis; for Lomb–Scargle periodogram analysis, rhythms above the horizontal line are significant at α = 0.05
Male #573 terminated torpor on March 21 and spent 25 days below ground prior to emerging to the surface during daylight hours on April 14 (based on exposure to high-intensity light); however, light logger data indicated this male was regularly exposed to low-intensity light (8–140 L beginning on March 28, 7 days after completing torpor (Fig. 2a). Lomb–Scargle periodogram analysis revealed a strong ultradian rhythm (tau = 7.2–7.5 h), but no circadian rhythm, during the first 7-day interval; a significant circadian rhythm first emerged in the March 29 to April 4 interval, after exposure to low-intensity light but well before emergence to the surface and exposure to high-intensity light. By the March 31–April 6 interval, the ultradian T b rhythm remained significant but weak, whereas the circadian T b rhythm became progressively stronger (Fig. 2). The second male (#926) terminated torpor on March 14 and did not emerge to the surface until 21 days later on April 4; no light exposure was measured until the male first emerged on April 4 (Fig. 3). This male, however, exhibited significant (P < 0.001) ultradian (8.5 h) and free-running circadian (23.55 h) rhythms during the first 7 days after terminating torpor (Fig. 3). Additionally, the ultradian rhythms became progressively weaker and the circadian rhythm became progressively stronger during the interval of belowground euthermia (Fig. 3). Rhythms of T b for both males, however, became entrained and dramatically increased in amplitude immediately following emergence from the hibernacula and resumption of daily surface activity (Figs. 2e, 3e).
Body temperature (T b; black line) and exposure to light (vertical lines) immediately following the termination of torpor in spring in male #573 (a). Dim light exposure (dashed vertical lines) is shown on a scale of 0 to 1000 L, whereas the bright light (solid vertical lines) is on a scale of 0 to 100,000 L PN (circles) indicates the strength of rhythms for 7-day intervals, based on Lomb–Scargle periodogram analysis. An actogram based on body temperature during the 35 days following the completion of torpor (b). Lomb–Scargle periodograms of the first 7 days following the completion of torpor (c), the interval from 8 to 14 days after torpor was complete (d), and the first 7 days following emergence (e). Note the different scales of the y-axes for PN. The horizontal line in the periodograms indicates the threshold for significance at α = 0.05
Body temperature (T b; black line) and exposure to light (vertical lines) immediately following the termination of torpor in spring in male #926 (a). PN (circles) indicates the strength of rhythms for 7-day intervals based on Lomb–Scargle periodogram analysis. An actogram based on body temperature during the 35 days following the completion of torpor (b). Lomb–Scargle periodograms of the first 7 days following the completion of torpor (c), the interval from 8 to 14 days after torpor was complete (d), and the first 7 days following emergence (e). Note the different scales of the y-axes for PN. The horizontal line in the periodograms indicates the threshold for significance at α = 0.05
Captive animals
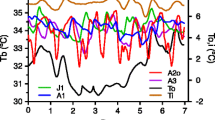
Following the termination of torpor in captivity, all 4 animals held in constant darkness developed significant circadian rhythms prior to being given a pulse of light on day 28 (Fig. 4). Three of the animals exhibited significant (P < 0.05) T b rhythms in the first 7-day interval of euthermia following their final bout of torpor and significant rhythms persisted in all subsequent 7-day intervals; the fourth animal (12–14) did not exhibit a significant T b rhythm until the second 7-day interval (days 2–8 post-hibernation) and then significant rhythms persisted in all subsequent intervals (Fig. 4).The strength of rhythmicity, based on the PN value from the Lomb–Scargle periodogram analysis and visual observations of the data (Fig. 4), increased substantially 2–3 weeks after torpor was terminated, but prior to the light pulse. Three of the animals had free-running T b rhythms with a period <24 h (tau = 23.35, 23.50, and 23.80 h), whereas the period was >24 h for the fourth animal (tau = 24.05 h). In one animal (12–18), activity and T b rhythmicity became more robust coincident with exposure to the pulse of light on day 28 (Fig. 4). However, the phase of the rhythm was unaffected by the light pulse on day 28 in all animals (data not shown). We repeated this experiment with three additional squirrels; once again, all squirrels became spontaneously rhythmic in the constant dark without exposure to light (data not shown). When the experiment was repeated a third time, but with squirrels receiving a pulse of light after only 4–6 days of euthermia, the strength of circadian T b rhythms appeared to increase more rapidly, immediately following the pulse (Fig. 5; Fig. S2). A comparative quantitative assessment of rhythmicity in these squirrels was not possible however, because of a loss of ~24 h of data due to a system failure shortly preceding or following the pulse in all three squirrels. Although one ground squirrel developed a very strong rhythm coincident with the day he was pulsed with light for 5 s, his activity levels and body temperature had both already risen substantially in the hours preceding the pulse (Fig. 5). Further, because circadian rhythms were already present within the first 7 days of terminating torpor in animals maintained in constant darkness, and these rhythms became more robust prior to exposure to light, we cannot be certain that the light pulse actually caused the increase in the amplitude or strength of the rhythm.
Body temperature (T b) and activity patterns of four captive arctic ground squirrels kept in constant dark during the 36 days immediately following the termination of torpor (a–d). PN values (open circles), which indicate the strength of circadian rhythms, are shown at the midpoint of the 7-day intervals analyzed using Lomb–Scargle periodograms; note the different scales of PN axes among individuals. All animals exhibited significant rhythms prior to exposure to a 5-s pulse of bright light (vertical dashed red line), although the T b rhythm of individual 12–18 became more robust coincident with exposure to light. e–h show the actogram-based T b
Body temperature (T b) and activity for an arctic ground squirrel given a 5-s pulse of bright light (vertical line) after less than 4 days of post-hibernation euthermia. Although the initiation of the rhythm coincides with the day the pulse was given, activity levels and T b were both high in the hours preceding the pulse of light
Discussion
Using high-resolution (0.02 °C) T b loggers, we show that the apparent loss of circadian T b rhythms during multi-day, deep torpor that we previously reported (Williams et al. 2012a, b) was not due to a lack of sensitivity of our data loggers. The low-amplitude (<0.1 °C) circadian T b rhythms observed in captive golden-mantled ground squirrels (Grahn et al. 1994; Ruby et al. 2002) are not present in free-living arctic ground squirrels though one animal consistently exhibited rhythms <19 and >30 h during deep torpor. Consistent with our previous studies, we also found that timing of emergence in free-living arctic ground squirrels can be readily identified by the rapid development of more robust circadian T b rhythm (Williams et al. 2011). However, we also found that weak, but significant, circadian T b rhythms can be re-initiated prior to emergence. In one free-living individual, this occurred coincident with exposure to low-intensity light, presumably through the burrow entrance and possibly through the snowpack. However, the second free-living individual developed T b rhythms immediately after torpor ended without any measured exposure to light at a sampling rate of once per minute. Although it is possible that circadian rhythms in this individual were initiated by exposure to unmeasured pulse(s) of light <1 min in duration, we also found that animals housed in captivity will spontaneously become rhythmic without exposure to light. This is similar to what was previously observed in captive European ground squirrels (Hut et al. 2002b) and suggests that many of the differences in results among studies could reflect experimental conditions rather than physiological differences among species.
Given the lack of circadian clock gene cycling previously found in hibernating European hamsters (Revel et al. 2007), we postulate the circadian clock is arrested during deep torpor and thus is not functional in triggering spontaneous arousals. Assuming clock gene/protein cycling is arrested only at the low T b of deep torpor, neurons within the SCN would undergo ~9–13 stop–start cycles in the absence of external entraining cues as animals undergo periodic arousal episodes throughout hibernation. We speculate that in the absence of external synchronizing light cues, these repeated stop–start cycles could lead to desynchrony of neurons within the SCN. In free-living animals, desynchrony could persist until exposure to an external light cue acts as a strong synchronizing agent, as we previously observed (Williams et al. 2012b). However, our current results suggest that at least some animals immediately exhibit circadian T b rhythms following the resumption of euthermia, though rhythms become much stronger following emergence (Fig. 2). We suggest that the ultradian rhythms in T b of 6–7 h that occurred in the free-living males remaining in their burrows after their last arousal may represent feeding cycles of gut fill and diet-induced thermogenesis. Males but not female arctic ground squirrels cache food in their hibernacula that fuel the ~3-week-long preemergence interval of euthermia that is necessary for gonadal growth and spermatogenesis (Barnes 1996).
The results for captive ground squirrels, including arctic (present study) and European (Hut et al. 2002a, b) species, seem to contradict the hypothesis that light is needed to trigger the resumption of strong circadian rhythmicity, as robust T b and activity rhythms developed in the absence of a light cue. However, captive animals are exposed to other external stimuli that might act as synchronizing agents, including meals presented each day (albeit not in a rhythmic matter) and episodes of acute disturbance that results in stress as they are moved into new clean tubs every few days. The variability among individuals that we observed may be a consequence of the timing of these external stimuli relative to the phase of the circadian rhythm, determining whether this is the case would require further study with much larger sample sizes. Free-living males have a food cache, but the amount of food available is only affected by the rate at which they consume the cache. Presentation of food and feeding can be entraining agents for peripheral circadian clocks (Stokkan et al. 2001; Schlibler et al. 2003) and scheduled meals can even act to entrain the master circadian clock (Castillo et al. 2004). Thus, it seems plausible that meals might act to synchronize oscillators within the SCN, even if there is an absence of scheduled feeding to maintain entrainment once the clock is re-synchronized. Stress hormones (glucocorticoids) can also act as zeitgeber (timing cues) for peripheral clocks (Pezük et al. 2012) and, perhaps more importantly, acute stress is known to trigger the release of vasopressin within the SCN (Engelmann et al. 1998). Vasopressin plays an important role in synchronizing neurons within the SCN (Yamaguchi et al. 2013), and the re-establishment of rhythmicity in captive European ground squirrels is correlated with the number of vasopressin-containing neurons within the SCN (Hut et al. 2002b). Thus, while captive experiments are generally regarded as “more controlled,” it is possible that husbandry associated with maintaining animals under captive conditions triggers the earlier post-hibernation resumption of strong circadian T b rhythms.
Using Lomb–Scargle periodogram analysis, we identified several rhythms during bouts of deep torpor in one of the free-living males. However, the dominant rhythms identified were not circadian (tau = 19.1 and 31 h) and, because they were not identified using Chi-squared periodogram analysis and the Lomb–Scargle method is sensitive to non-normality in the residuals and large-amplitude features (Schimmel 2001), we speculate these rhythms may be false positives. Lomb–Scargle algorithms, however, have better detection efficiency and accuracy in the presence of noise (Ruf 1999), and thus it is also possible that the low signal-to-noise ratio at these low T b explains the lack of detection based on the Chi-squared analysis. If the stronger <19 and >31 h rhythms are real, whether the weaker peaks are true rhythms is unclear as the detection of harmonics that do not actually exist in the dataset is a well-described issue for Lomb–Scargle analysis (Refinetti et al. 2007). Alternatively, in cases where noise is not normally distributed, other power spectra can hide the 24-h periodicity (Schimmel 2001), which could be happening here in the male with <19 and >31 h rhythms. In the second male, we failed to detect rhythms of any period, regardless of the approach used. The only study to measure patterns of clock gene expression during the deep torpor associated with hibernation failed to find evidence for continued cycling of clock genes (Revel et al. 2007). Nevertheless, circadian clocks are still proposed by many to play a role in triggering spontaneous arousals (Ruby 2003; Heller and Ruby 2004; Malan 2010). The attractiveness of this hypothesis is that (1) it would explain why the rhythmicity persists in the T b of SCN-intact, but not SCN-ablated, golden-mantled ground squirrels during deep torpor (Ruby et al. 2002), and (2) it could explain how some species appear to be able to time their arousals from deep torpor to occur at a particular time of day (Kӧrtner and Geiser 1998; Park et al. 2000). However, for at least some of these species, it is possible that the timing of arousals reflects a direct behavioral response to 24-h fluctuations in ambient thermal conditions experienced in the hibernacula, independent of circadian systems (i.e., masking). Thermoreceptors in the skin can relay information via dorsal root ganglion and lamina I neurons in the spinal cord to the hypothalamus (Morrison and Nakamura 2011) providing the pathway for such a direct effect. In bats, it has been noted that while arousal in southern populations is timed to coincide with sunset, this does not occur in the more northern ranges where flying insects are almost never available during winter (Czenze et al. 2013). More southern populations have an ecological need to time their arousals with sunset, but whether this is timed based on a circadian clock or in response to daily fluctuations in ambient conditions requires more research. Alternatively, the increase in arousal frequency at a particular time of day in these populations may be due to disturbance from euthermic and active conspecifics (Czenze and Willis 2015).
We suggest the lack of circadian T b rhythmicity during deep torpor is indicative of an arrested circadian clock, though we concede it is possible that circadian clocks remain functional during deep torpor and it is instead the output from these clocks (i.e., T b rhythms) that are suppressed or eliminated during hibernation. Arctic ground squirrels have very low T b and brain temperatures during hibernation as they defend a thermal gradient between T b and T a (Richter et al. 2015), and it is possible that circadian T b rhythms are not present at these lower T b. Even for golden-mantled ground squirrels, where there is evidence for the persistence of circadian rhythms during torpor at T b of 8–10 °C (Larkin et al. 2002; Ruby et al. 2002), there have been no studies to determine whether these T b rhythms persist at the lower minimum torpid T b (0 ± 1 °C) measured in free-living individuals (Healy et al. 2012). Our study provides insight into some of the discrepancies between earlier studies of captive and free-living ground squirrels. However, additional studies focused on measuring cycling within the molecular clockwork itself are needed to further unravel the persistence and functionality of circadian rhythms during deep torpor in obligate seasonal hibernators. If central and peripheral circadian rhythms are truly arrested, alternative hypotheses regarding regulation of the periodicity of arousal episodes and any role of the circadian clock in generating circannual rhythms will need to be investigated further.
References
Barnes BM (1996) Relationships between hibernation and reproduction in male ground squirrels. In: Adaptations to the Cold: Tenth International Hibernation Symposium. Geiser F et al, eds. University of New England Press, Armidale, pp 71–80.
Buhr ED, Yoo SH, Takahashi JS (2010) Temperature as a universal resetting cue for mammalian circadian oscillators. Science 330:379–385
Bünning E (1936) Die endogene tagersrhythmik als grundlage der photoperiodischen reaktion. Ber Dtsch Bot Ges 54:590–607
Castillo MR, Hochstetler KJ, Tavernier RJ, Greene DM, Bult-Ito A (2004) Entrainment of the master circadian clock by scheduled feeding. Am J Physiol-Reg Integr Comp Physiol 287:R551–R555
Czenze ZJ, Willis CK (2015) Warming up and shipping out: arousal and emergence timing in hibernating little brown bats (Myotis lucifugus). J Comp Physiol B 185:575–586
Czenze ZJ, Park AD, Willis CK (2013) Staying cold through dinner: cold-climate bats rewarm with conspecifics but not sunset during hibernation. J Comp Physiol B 183:859–866
Daan S (1972) Periodicity of heterothermy in the garden dormouse, Eliomys quercinus (L.). Netherlands. J Zool 23:237–265
Dibner C, Sage D, Unser M, Bauer C, d’Eysmond T, Naef F, Schibler U (2009) Circadian gene expression is resilient to large fluctuations in overall transcription rates. EMBO J 28:123–134
Dibner C, Schibler U, Albrecht U (2010) The mammalian circadian timing system: organization and coordination of central and peripheral clocks. Ann Rev Physiol 72:517–549
Engelmann M, Ebner K, Landgraf R, Wotjak CT (1998) Swim stress triggers the release of vasopressin within the suprachiasmatic nucleus of male rats. Brain Res 792:343–347
Florant GL, Hill V, Ogilvie MD (2000) Circadian rhythms of body temperature in laboratory and field marmots (Marmota flaviventris). In: Heldmaier G, Klingenspor (eds) Life in the cold. Springer Berlin, pp 223–231
Grahn DA, Miller JD, Houng VS, Heller HC (1994) Persistence of circadian rhythmicity in hibernating ground squirrels. Am J Physiol-Reg Integr Comp Physiol 266:R1251–R1258
Gür MK, Refinetti R, Gür H (2009) Daily rhythmicity and hibernation in the Anatolian ground squirrel under natural and laboratory conditions. J Comp Physiol B 179:155–164
Healy JE, Burdett KA, Buck CL, Florant GL (2012) Sex differences in torpor patterns during natural hibernation in golden-mantled ground squirrels (Callospermophilus lateralis). J Mammal 93:751–758
Heller HC, Ruby NF (2004) Sleep and circadian rhythms in mammalian torpor. Annu Rev Physiol 66:275–289
Herwig A, Revel F, Saboureau M, Pévet P, Steinlechner S (2006) Daily torpor alters multiple gene expression in the suprachiasmatic nucleus and pineal gland of the Djungarian hamster (Phodopus sungorus). Chronobiol Int 23:269–276
Hittel D, Storey KB (2002) The translation state of differentially expressed mRNAs in the hibernating 13-lined ground squirrel (Spermophilus tridecemlineatus). Arch Biochem Biophys 401:244–254
Hut R, Barnes B, Daan S (2002a) Body temperature patterns before, during, and after semi-natural hibernation in the European ground squirrel. J Comp Physiol B 172:47–58
Hut R, Van der Zee E, Jansen K, Gerkema M, Daan S (2002b) Gradual reappearance of post-hibernation circadian rhythmicity correlates with numbers of vasopressin-containing neurons in the suprachiasmatic nuclei of European ground squirrels. J Comp Physiol B 172:59–70
Hut R, Dardente H, Riede SJ (2014) Seasonal timing: how does a hibernator know when to stop hibernating? Curr Biol 24:R602–R605
Ikeno T, Tanaka SI, Numata H, Goto SG (2010) Photoperiodic diapause under the control of circadian clock genes in an insect. BMC Biol 8:116
Körtner G, Geiser F (1998) Ecology of natural hibernation in the marsupial mountain pygmy-possum (Burramys parvus). Oecologia 113:170–178
Körtner G, Geiser F (2000) The temporal organization of daily torpor and hibernation: circadian and circannual rhythms. Chronobiol Int 17:103–128
Larkin JE, Franken P, Heller HC (2002) Loss of circadian organization of sleep and wakefulness during hibernation. Am J Physiol - Reg Int Comp Physiol 282:R1086–R1095
Lovegrove BG, Lobban KD, Levesque DL (2014) Mammal survival at the Cretaceous–Palaeogene boundary: metabolic homeostasis in prolonged tropical hibernation in tenrecs. Proc R Soc B 281:20141304
Malan A (2010) Is the torpor-arousal cycle of hibernation controlled by a non-temperature-compensated circadian clock? J Biol Rhythms 25:166–175
Meuti ME, Stone M, Ikeno T, Denlinger DL (2015) Functional circadian clock genes are essential for the overwintering diapause of the Northern house mosquito, Culex pipiens. J Exp Biol 218:412–422
Morrison SF, Nakamura K (2011) Central neural pathways for thermoregulation. Front Biosci 16:74–104
O’Neill JS, Maywood ES, Hastings MH (2013) Cellular mechanisms of circadian pacemaking: beyond transcriptional loops. In: Circadian clocks. Springer, Berlin Heidelberg, pp 67–103
Park DH, Somers DE, Kim YS, Choy YH, Lim HK, Soh MS, Kim HJ, Kay SA, Nam HG (1999) Control of circadian rhythms and photoperiodic flowering by the Arabidopsis GIGANTEA gene. Science 285:1579–1582
Park KJ, Jones G, Ransome RD (2000) Torpor, arousal and activity of hibernating greater horseshoe bats (Rhinolophus ferrumequinum). Funct Ecol 14:580–588
Pengelley ET, Fisher KC (1961) Rhythmical arousal from hibernation in the golden-mantled ground squirrel, Citellus lateralis tescorum. Can J Zool 39:105–120
Pezük P, Mohawk JA, Wang LA, Menaker M (2012) Glucocorticoids as entraining signals for peripheral circadian oscillators. Endocrinol 153:4775–4783
Refinetti R, Cornélissen G, Halberg F (2007) Procedures for numerical analysis of circadian rhythms. Biol Rhythm Res 38:275–325.
Reppert SM, Weaver DR (2002) Coordination of circadian timing in mammals. Nature 418:935–941
Revel FG, Herwig A, Garidou ML, Dardente H, Menet JS, Masson-Pévet M, Simonneaux V, Saboureau M, Pévet P (2007) The circadian clock stops ticking during deep hibernation in the European hamster. Proc Natl Acad Sci USA 104:13816–13820
Richter MM, Williams CT, Lee TN, Tøien Ø, Florant GL, Barnes BM, Buck CL (2015) Thermogenic capacity at subzero temperatures: How low can a hibernator go? Physiol Biochem Zool 88:81–89
Ruby NF (2003) Hibernation: when good clocks go cold. J Biol Rhythms 18:275–286
Ruby NF, Dark J, Burns DE, Heller HC, Zucker I (2002) The suprachiasmatic nucleus is essential for circadian body temperature rhythms in hibernating ground squirrels. J Neurosci 22:357–364
Ruf T (1999) The Lomb-Scargle periodogram in biological rhythm research: analysis of incomplete and unequally spaced time-series. Biol Rhythm Res 30:178–201.
Ruf T, Geiser F (2015) Daily torpor and hibernation in birds and mammals. Biol Rev 90:891–926
Schibler U, Ripperger J, Brown SA (2003) Peripheral circadian oscillators in mammals: time and food. J Biol Rhythms 18:250–260
Schimmel M (2001) Emphasizing difficulties in the detection of rhythms with Lomb-Scargle periodograms. Biol Rhythm Res 32:341–346
Stokkan KA, Yamazaki S, Tei H, Sakaki Y, Menaker M (2001) Entrainment of the circadian clock in the liver by feeding. Science 291:490–493
Storey KB, Storey JM (2004) Metabolic rate depression in animals: transcriptional and translational controls. Biol Rev 79:207–233
Thomas DW (1993) Lack of evidence for a biological alarm clock in bats (Myotis spp.) hibernating under natural conditions. Can J Zool 71:1–3
Waßmer T, Wollnik F (1997) Timing of torpor bouts during hibernation in European hamsters (Cricetus cricetus L.). J Comp Physiol B 167:270–279
Williams CT, Sheriff MJ, Schmutz JA, Kohl F, Tøien Ø, Buck CL, Barnes BM (2011) Data logging of body temperatures provides precise information on phenology of reproductive events in a free-living arctic hibernator. J Comp Physiol B 181:1101–1109
Williams CT, Barnes BM, Buck CL (2012a) Daily body temperature rhythms persist under the midnight sun but are absent during hibernation in free-living arctic ground squirrels. Biol Lett 8:31–34
Williams CT, Barnes BM, Richter M, Buck CL (2012b) Hibernation and circadian rhythms of body temperature in free-living Arctic ground squirrels. Physiol Biochem Zool 85:397–404
Williams CT, Wilsterman K, Kelley AD, Breton AR, Stark H, Humphries MM, McAdam AG, Barnes BM, Boutin SB, Buck CL (2014) Light loggers reveal weather-driven changes in the daily activity patterns of arboreal and semifossorial rodents. J Mamm 95:1230–1239
Wood S, Loudon A (2014) Clocks for all seasons: unwinding the roles and mechanisms of circadian and interval timers in the hypothalamus and pituitary. J Endocrin 222:R39–R59
Yamaguchi Y, Suzuki T, Mizoro Y, Kori H, Okada K, Chen Y, Dong X (2013) Mice genetically deficient in vasopressin V1a and V1b receptors are resistant to jet lag. Science 342:85–90
Young MW, Kay SA (2001) Time zones: a comparative genetics of circadian clocks. Nat Rev Gen 2:702–715
Acknowledgements
This research was supported by grants from the National Science Foundation to CTW (IOS-1147232), CLB (IOS-1147232), and BMB (IOS-1147187). We thank Jeanette Moore and staff of Toolik Field Station for assistance with this research. Any opinions, findings, and conclusions or recommendations expressed in this material are those of the authors and do not necessarily reflect the views of the National Science Foundation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
Protocols involving animals were approved by the University of Alaska Fairbanks Institutional Animal Care and Use Committee (IACUC # 340270-41). The bureau of land management provided permission to work at our study sites (Permit F-94817).
Additional information
Communicated by F. Breukelen.
This manuscript is part of the special issue Hibernation—Guest Editors: Frank van Breukelen and Jenifer C. Utz.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Williams, C.T., Radonich, M., Barnes, B.M. et al. Seasonal loss and resumption of circadian rhythms in hibernating arctic ground squirrels. J Comp Physiol B 187, 693–703 (2017). https://doi.org/10.1007/s00360-017-1069-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00360-017-1069-6