Abstract
Nightjars represent a model taxon for investigating physiological limits of heat tolerance because of their habit of roosting and nesting in sunlit sites during the heat of the day. We investigated the physiological responses of Rufous-cheeked nightjars (Caprimulgus rufigena) and Freckled nightjars (Caprimulgus tristigma) to high air temperatures (T a) by measuring body temperature (T b), resting metabolic rate (RMR) and total evaporative water loss (TEWL) at T a ranging from 10 to 56 °C. Both species became hyperthermic at T a > T b. Lower critical limits of thermoneutrality occurred at T a between 35 and 37 °C, whereas we detected no clear upper critical limits of thermoneutrality. Between T a ≈ 37.0 and 39.9 °C, rates of TEWL increased rapidly with T a. At T a ≥ 40 °C, fractional increases in mass-specific TEWL rates were 78–106% of allometric predictions. Increasing evaporative heat dissipation incurred only small metabolic costs, with the RMR of neither species ever increasing by more than 20% above thermoneutral values. Consequently, both species displayed extremely efficient evaporative cooling; maximum evaporative heat dissipation was equivalent to 515% of metabolic heat production (MHP) at T a ≈ 56 °C in C. rufigena and 452% of MHP at T a ≈ 52 °C in C. tristigma. Our data reiterate that caprimulgids have evolved an efficient mechanism of evaporative cooling via gular fluttering, which minimizes metabolic heat production at high T a and reduces total heat loads. This likely aids in reducing TEWL rates and helps nightjars cope with some of the most thermally challenging conditions experienced by any bird.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Environmental temperature greatly influences the energy and water demands of an organism and, thereby, affects several components of fitness (McKechnie and Wolf 2010; du Plessis et al. 2012; Cunningham et al. 2013). When exposed to fluctuating air temperatures (T a), endotherms must physiologically and/or behaviorally adjust heat fluxes to maintain a body temperature (T b) setpoint (Porter and Gates 1969; Tattersall et al. 2012). Because they are primarily diurnal and non-fossorial, birds living in hot, arid environments routinely experience high T a and intense solar radiation, resulting in operative temperatures (T e) well above T b (Bartholomew and Dawson 1979; Walsberg and Voss-Roberts 1983). Under such conditions, evaporative cooling is the only mechanism whereby birds can dissipate heat and avoid lethal hyperthermia (Bartholomew and Cade 1963). As T a increases above T b, birds maintain sublethal T b through concomitant increases in total evaporative water loss (TEWL; Calder and Schmidt-Nielsen 1966; Dawson 1982). However, at very high T a, TEWL can become substantial (McKechnie and Wolf 2010) and lead to rapid depletion of body water. For example, Wolf and Walsberg (1996) showed that TEWL in Verdins (Auriparus flaviceps) increased nearly 14-fold as T a increased from 30 to 50 °C, and represented ~7% of body mass (M b) per hour at the latter T a. In hot, arid environments where drinking water is often scarce, birds thus face a fundamental tradeoff between balancing the need to evaporate large quantities of water to maintain sublethal T b while at the same time maintaining adequate hydration.
Whereas diurnal birds residing in hot environments can potentially replenish body water throughout the day by drinking and/or feeding on water-rich food, nocturnal species rest during the day and, consequently, can experience sustained periods of elevated TEWL that may lead to significant water deficits (Grant 1982). Many nocturnal taxa, such as owls (Strigidae), minimize their water requirements by occupying relatively cool, shaded sites in trees or cavities, thereby minimizing their need for evaporative cooling (Ligon 1968, 1969; Ganey 2004). Nightjars (Caprimulgidae), in contrast, often roost and nest on the ground in open, exposed sites where they are subjected to intense solar radiation and high T e that requires long periods with substantial evaporative cooling (e.g., Cowles and Dawson 1951; Dexter 1956; Weller 1958; Bartholomew et al. 1962; Steyn 1971; Grant 1982; Ingels et al. 1984). Grant (1982), for example, observed a female Lesser Nighthawk (Chordeiles acutipennis) spend almost 8 h incubating eggs in full sunlight where the black bulb temperature was ~58 °C. Moreover, T e is typically highest during summer months when day length is longest and periods without access to water may exceed 14 h.
Early studies found that some caprimulgids possess highly efficient evaporative cooling by dissipating large heat loads evaporatively while exhibiting only small increases in resting metabolic rate (RMR; Bartholomew et al. 1962; Dawson and Fisher 1969; Lasiewski and Seymour 1972). However, to the best of our knowledge, no studies evaluating phenotypic variation in RMR and TEWL between seasons (e.g., summer versus winter) or between climatically different sites (e.g., arid versus mesic) have been conducted among caprimulgids. Most comparative research on the thermal physiology of caprimulgids has focused on hypometabolism and the use of torpor as a mechanism for energy savings (Brigham et al. 2012). Given the proclivity of caprimulgids for roosting and nesting in exposed sites that can produce significant heat stress, they present an opportunity to examine the physiological mechanisms that animals use to cope with heat stress in the most energy and water-efficient manner. In addition, individuals of the same species can occur in a range of habitats and thus experience varying environmental temperatures (Cleere and Nurney 1998). Furthermore, some species are non-migratory and are subjected to seasonal fluctuations in T a. Consequently, they represent a good model taxon for investigating phenotypic flexibility and intraspecific variation in physiological variables related to heat tolerance, an area where avian data are limited (e.g., Noakes et al. 2016).
Here, we investigate the thermal physiology of caprimulgids exposed to high T a and ask: (1) is there a strong hyperthermic response within species and among populations when exposed to high T a? (2) Are there pronounced changes in RMR with increasing heat stress and do these values vary among populations within a species? (3) How do rates of TEWL vary with T a and under conditions of extreme heat within species and among populations? and (4) does the thermoregulatory performance of nightjars vary intraspecifically in a manner correlated with habitat characteristics, with birds from a hot, arid site exhibiting greater heat tolerance via reduced total heat loads (e.g., low RMR), and high ratios of evaporative heat lost (EHL) to metabolic heat production (MHP) at any given T a compared to conspecifics from a cooler, more mesic site? We also provide preliminary data on seasonal changes in thermoregulatory responses to heat within a species.
We studied two southern African nightjars that roost and nest in sites with high diurnal heat loads (R. S. O’Connor and A. E. McKechnie unpublished data). Rufous-cheeked nightjars (Caprimulgus rufigena) are common throughout the arid Nama Karoo and arid savanna regions of southern Africa, where they prefer open woodlands with clearings (Spottiswoode and Jackson 2005a). Rufous-cheeked nightjars nest in natural depressions in coarse soil where shade is minimal or absent (Cleere and Nurney 1998; Spottiswoode and Jackson 2005a). Freckled nightjars (Caprimulgus tristigma) are rupiculous breeders, laying their eggs in natural depressions on rocky outcroppings where incubating adults can experience daytime T e approaching 60 °C (Steyn 1971; Spottiswoode and Jackson 2005b). We investigated the thermoregulatory responses of two populations of Freckled nightjars, one from a hot, arid site that experiences predominantly winter rainfall and another from a more mesic site characterized by summer rainfall.
Materials and methods
Study species and sites
Rufous-cheeked nightjars were captured on the properties Dronfield (28°39′S, 24°48′E, ~1218 m ASL) and Benfontein (28°48′S, 24°48′E, ~1170 m ASL) near Kimberley, South Africa during three separate periods (October 2013, October 2014 and March 2015). These field sites are ~26 km apart, and Rufous-cheeked nightjars were found in similar habitat and vegetation on both reserves, consisting of arid savannah grassland interspersed with Vachellia erioloba woodlands. During October and March, average rainfall in Kimberley is approximately 40 mm and average daily maximum T a is 29.7 °C (South African Weather Service data from 2000 to present).
Freckled nightjars were captured at two sites over two summers and one additional winter period. The first site, which represents an arid climate and where individuals belong to the subspecies C. t. lentiginosus, was on a privately owned farm in the northern Kamiesberg Mountains in the Namaqualand region of the Northern Cape province, South Africa (30°02′S, 17°57′E, ~789 m ASL; same study site as described by Smit et al. 2011) during December 2013 and from December 2014 to March 2015. This site lies within the arid succulent Karoo ecoregion of western South Africa and is characterized by winter rainfall (Smit et al. 2011). Average rainfall in Namaqualand from December to March equals 8.5 mm and average daily maximum T a is 29.2 °C (South African Weather Service data from 2000 to present). The second summer site (subspecies C. t. granosus), which represents a mesic climate, was Ezemvelo Nature Reserve (25°42′S, 28°59′E, ~1415 m ASL), near Bronkhorstspruit, Gauteng province, South Africa, with birds captured during November 2014. We captured additional individuals at Ezemvelo during the austral winter between 10 May and 11 June 2014. Ezemvelo is situated between the grassland and savannah biomes and consists of open grassland interspersed with rocky sandstone outcroppings and isolated woodland (Boyles et al. 2012). Average rainfall in Bronkhorstspruit during November equals 94.9 mm and average daily maximum T a is 28.3 °C. During May and June, average rainfall in Bronkhorstspruit equals 12.3 mm and average daily maximum T a is 22.5 °C (South African Weather Service data from 2009 to present).
Rufous-cheeked nightjars and Freckled nightjars were captured at night by luring birds into mist nets using playback of conspecific calls or using a handheld net to catch birds resting on roads. Following capture, nightjars were held for 1–2 nights in an enclosure (60 × 71 cm) constructed of PVC piping and shade cloth. Individuals held for multiple nights were hand-fed a diet of wet dog food, mealworms and water, as nightjars do not readily feed in captivity (Dawson and Fisher 1969; Withers 1977). To minimize the time birds spent in captivity, all physiological measurements were conducted in the field using portable equipment.
Measurements of body temperature, resting metabolic rate, and evaporative water loss
Body temperature was measured using temperature-sensitive passive integrated transponder (PIT) tags (Biomark, Boise ID, USA) injected into the peritoneal cavity. Prior to the study, a subset of 70 PIT tags was compared to thermocouple values (type T, Physitemp, Clifton, NJ, USA) in a circulating water bath at temperatures ranging from 39 to 46 °C (Whitfield et al. 2015). PIT tags deviated from thermocouple readings by 0.02 ± 0.09 °C (mean ± SD; Whitfield et al. 2015). We recorded T b using a portable transceiver system (model 2001F-ISO, Biomark, Boise, ID, USA) connected to an external racket antenna placed within 10 cm of the metabolic chamber housing the study animal. PIT tag readings were logged every 15 s on a personal computer during respirometry trials.
We used open flow-through respirometry to measure the rates of carbon dioxide production (\( \dot{V}_{{{\text{CO}}_{2} }} \)) and TEWL of nightjars over a range of T a spanning 10–56 °C. Individuals were placed in metabolic chambers with volumes of 9 l (C. tristigma and C. rufigena) or 4.6 l (C. rufigena) constructed from plastic containers. A 1-cm layer of mineral oil at the bottom of each metabolic chamber prevented evaporation from urine and feces affecting evaporative water loss estimates. A mesh platform with spaces large enough for feces to fall through was positioned above the mineral oil. Metabolic chambers were placed inside a modified ice chest (~75 l) fitted with a Peltier device (model AC-162, TE Technology Inc., Traverse City, MI, USA) and a custom-built controller (TC-36-25-RS486 and MP-2986) to regulate T a (typically ±0.1 °C) inside the animal chamber. Air temperature inside each metabolic chamber was measured using a thermistor probe (model TC-100, Sable Systems, Las Vegas, NV, USA) inserted through a small hole in the lid and secured in place with a rubber grommet.
Atmospheric air was supplied to metabolic chambers by an air pump (model DOA-P13- BN, Gast Air Pumps, Benton Harbor, MI, USA) with a maximum output of ~30 L min−1. Both before entering and after exiting the pump, atmospheric air was scrubbed of water vapor by passing the air stream through columns of silica gel and drierite connected in series. Once scrubbed of water vapor, the airstream was split into two channels with one used as a baseline gas and the other directed to the animal chamber. Baseline flow rates were controlled using a needle valve (model RFU 482-1/8, Camozzi, Brescia, Italy), whereas chamber flow rates were controlled with an Alicat mass-flow controller (model MCP-50SLpm-D/5M, Alicat Scientific Inc., Tucson, AZ, USA). To enhance the mixing of air within each chamber, a 90° fitting was attached at the air inlet and directed air towards the corner of the chamber. This directed the incurrent air stream away from the air outlet port, as well as preventing incurrent air from blowing directly onto the bird. Flow rates varied between approximately 1 and 30 l min−1, depending on the experimental T a. At lower T a values when birds were not heat stressed, we used Eq. 4 of McNab (2006) to determine the “critical” flow rate, the minimum flow rate required for the complete mixing of gases in a given chamber volume. Flow accuracy ranged from ±11% at 1 l min−1 to ≤±3% at flow rates above 5 l min−1. At higher T a approaching and exceeding T b, flow rates were regulated to keep chamber humidity below a dew point of −5 °C (relative humidity of ≈10% at 30 °C). Higher flow rates (and hence lower chamber humidity) aided in keeping birds calm when exposed to high T a (Whitfield et al. 2015). Using Lasiewski’s et al. (1966) equation for estimating chamber equilibrium times indicated that at the flow rates used at T a ≥ 30 °C mean chamber equilibrium times averaged 4.0 ± 2.0 min for the 4.6 l chamber and 7.0 ± 3.9 min for the 9 l chamber.
Excurrent air from the metabolic chamber and a baseline channel of incurrent air were sequentially subsampled using a MUX Flow-Multiplexer (Sable Systems, Las Vegas, NV, USA) in manual mode. At the start of each set of gas exchange measurements, baseline air was first subsampled for 5 min, followed by excurrent air from the metabolic chamber for 10–15 min. Birds experienced a ramped profile of increasing T a (see below), and we subsampled baseline air for 3 min during each period when T a was increasing, resulting in approximately four baselines per run. The subsampled air was pulled through a CO2/H2O gas analyzer (model LI-840A, LI-COR, Lincoln, NE, USA) and an O2 analyzer that also measured barometric pressure (FC—10 Oxygen Analyzer, Sable Systems, Las Vegas, NV, USA). Gas analysers were calibrated using the same techniques as Whitfield et al. (2015). All tubing used in the system was Bev-A-Line tubing (Thermoplastic Processes Inc., Warren, NJ, USA). Voltage outputs from analyzers were digitized using a Universal Interface (model UI-2, Sable Systems, Las Vegas, NV, USA) and recorded at a sampling interval of 5 s using a personal computer with Expedata software (Sable Systems, Las Vegas, NV, USA).
Experimental protocol
All respirometry measurements took place during the day, the rest phase of nightjars and the time during which they naturally experience high T a. Prior to being placed inside the metabolic chamber, birds were given water (~3.0–4.0 ml) with a syringe to ensure that they were well hydrated. After being placed in the chamber, birds initially spent 30 min at T a ≈ 25–28 °C to habituate. Respirometry runs were separated into low (T a = 10, 15, 20 and 25 °C) and high (T a ≥ 30 °C) T a groups, with the experimental protocol varying depending on the Ta group. At low T a, nightjars were held at a T a set point for ~3 h before T a was changed. In addition, birds were only measured at two different T a values during the low T a runs (e.g., at 10 and 15 °C or at 20 and 25 °C). Thus, for low T a runs, birds were typically in the metabolic chamber for ~6 h. Air temperature changes during low T a trials occurred in 5 °C increments with the first T a set point and direction of T a change (i.e., increase or decrease) randomized (e.g., 10–15 °C or 15–10 °C). For measurements at high T a, we used a ramped T a profile starting at 30 °C with subsequent T a increases to 35, 38 and 40 °C. Above 40 °C, T a was increased in 2 °C increments. When chamber T a reached a stable value, birds were held at each T a set point for 10–15 min. Runs were terminated based on the same criteria used by Whitfield et al. (2015), and thus, the duration an individual was kept in the chamber (average ≈159 min) depended on their heat stress responses. Among species, nightjars lost on average 4.5 ± 2.3 g (6.6 ± 3.1% of M b) during high T a trials. The behavior of birds during each trial was continuously monitored using an infrared camera and light, allowing us to determine whether birds were calm or exhibiting signs of distress and attempts to escape (Whitfield et al. 2015). Upon removal from the chamber, birds were immediately weighed and then given water and held in front of a fan if necessary. Once T b decreased to normothermic levels (38–40 °C) birds were returned to the enclosure and then released at their site of capture that night.
Although our measurements of EWL, RMR and T b over short periods at each T a set point could be criticized as not representing steady-state physiological data, the ramped temperature protocol we used here is in many ways analogous to the sliding cold exposure method used in summit metabolism studies (Swanson et al. 1996); as is the case when exposing birds to very low temperatures for the purposes of measuring the upper limits of cold tolerance, it is simply not practical to expose birds to T a far above T b for extended periods. Moreover, our methods follow those of several recent studies (e.g., Smith et al. 2015; Whitfield et al. 2015; McKechnie et al. 2016a, b), and therefore, the data we present here are directly comparable to those presented by these authors.
Data analyses
Carbon dioxide and H2O traces were corrected for drift using the appropriate algorithms in Expedata. Resting values of \( \dot{V}_{{{\text{CO}}_{2} }} \), TEWL, and T b were taken as the average of the lowest, most stable 5-min period from CO2 traces at each T a set point. Data from birds that did not remain calm for 5 min at a T a set point were excluded from analyses. Excurrent flow rates from metabolic chambers were calculated using Eq. 9.3 of Lighton (2008). Rates of \( \dot{V}_{{{\text{CO}}_{2} }} \) and TEWL were calculated using Eqs. 9.5 and 9.6, respectively, of Lighton (2008). To convert ml H2O into mg H2O, we assumed 0.803 mg H2O ml−1 water vapor (Lighton 2008). We calculated RMR [Watts (W)] assuming 27.8 kJ l CO −12 and a respiratory exchange ratio (RER) of 0.71 (i.e., lipid metabolism; Withers 1992). Evaporative heat loss (W) was calculated assuming 2.40 J mg H2O−1 (Withers 1992).
All statistical analyses were conducted in R 3.2.1 (R Core Team 2015) with all values reported as mean ± standard deviation (SD). We fit broken-stick regression models using the ‘piecewise.linear’ function in the package SiZer (Sonderegger 2012) to identify inflection points in our T b, RMR, TEWL, and EHL/MHP response variables. Data were subsequently split based on inflection points, and we fitted linear mixed-effects models to the data using the packages lme4 (Bates et al. 2015) and nlme (Pinheiro et al. 2015) with T b, RMR, TEWL and EHL/MHP included as response variables and T a, sex, and M b as predictor variables. In addition, because we had data from two different field sites over both summer and winter, we included site and season as predictor variables in our analysis of the data for Freckled nightjars. One Freckled nightjar (hereafter: bird 5599) had abnormally high RMR values between T a ≈ 30–35 °C and 38–52 °C and the mixed-effects models at these T a ranges had Cook’s distances of 2.3 and 4.3, respectively, indicating that the data for this individual were highly influential on model parameter estimates (Quinn and Keough 2002). Thus, bird 5599 was ultimately excluded from the final analyses. In all models, individual identity was included as a random factor due to having repeated measurements on the same individual. Furthermore, because we collected data in three different periods spanning two summers for Rufous-cheeked nightjars and in two different periods over two summers for Freckled nightjars, we included capture period as a random factor in our models to account for any variation between periods. All models were initially run as maximal models with all predictors and their interactions included. The best models were selected based on Akaike Information Criterion adjusted for small sample sizes (AICc) using the ‘dredge’ function in the package MuMIn (Bartoń 2015). Models with AICc values differing by less than 2 (i.e., ΔAICc < 2) were treated as fitting the data equally well, and consequently, the most parsimonious model was selected as the minimal adequate model (Quinn and Keough 2002; Crawley 2007). Residuals of our minimal adequate models were graphically inspected to ensure they met assumptions of normality and homogeneity of variance. Occasionally, our data violated these assumptions due to outliers and we avoided transformations to conserve patterns in relationships and decided to use robust regression analyses for linear mixed models using the package robustlmm (Koller 2015). Because lme4 (Bates et al. 2015) does not inherently provide P values in its output (Bates 2006), we computed 95% confidence intervals (95% CI) for all fixed effects in our minimal adequate models using the function ‘confint.merMod’ in lme4 (Bates et al. 2015). We assumed a significance of less than 5% (P < 0.05) if the 95% CI did not include the value of zero (Faraway 2006; du Prel et al. 2009). For each fixed effect in the minimal adequate model, we report the estimate and accompanying standard error (SE) and 95% CI.
Results
Body mass
A total of 25 Rufous-cheeked nightjars were captured over the three study periods. The average M b of males and females the morning before measurements was 52.2 ± 4.5 and 53.9 ± 4.1 g, respectively. Prior to measurements, average M b for summer Namaqualand Freckled nightjars was 64.7 ± 6.3 g (n = 24) and average M b for summer Ezemvelo Freckled nightjars was 79.7 ± 4.4 g (n = 5), representing a significant between-population difference [95% CI = (−17.1, −5.1)]. Freckled nightjars at Ezemvelo were significantly (F 1,15 = 22.64, P < 0.001) heavier in winter (88.1 ± 2.8 g, n = 12) compared to summer.
Body temperature
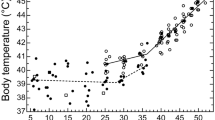
At T a ≤ 15 °C, Rufous-cheeked nightjar T b averaged 37.9 ± 0.7 °C (n = 5). At 20 ≤ T a ≤ 50 °C, the minimal adequate model only included T a [0.120 ± 0.008 °C °C−1, (0.104, 0.136); Fig. 1a]. Body temperature increased from 38.2 ± 1.0 °C (n = 9) at T a ≈ 20 °C to 41.7 ± 0.6 °C at T a ≈ 50 °C (n = 6). At T a ≥ 50 °C, T b stabilized at 41.6 ± 0.6 °C (n = 16, Fig. 1a).
Body temperature (T b) as a function of air temperature (T a) in a Rufous-cheeked nightjars (Caprimulgus rufigena), b Freckled nightjars (Caprimulgus tristigma) from a mesic (Ezemvelo) and arid (Namaqualand) site and c Freckled nightjars from Ezemvelo during summer and winter. The regression line in a is a linear model fitted to T b versus T a. In b, the regression line represents the slope from a linear model fitted to T b versus T a. In c, regression lines represent the slopes from a linear model fitted to T b versus the interaction between T a and body mass (M b). The M b values used represent cross sections at the 10th, 50th, and 90th percentiles of the M b variable
At 10 ≤ T a ≤ 30 °C, T b of Freckled nightjars from Namaqualand averaged 38.7 ± 0.8 °C (n = 20, Fig. 1b). Body temperature in Namaqualand Freckled nightjars had a significant inflection point at T a = 32.4 °C (0.107, 0.263), above which T b increased with T a (Fig. 1b). Between T a ≈ 35 and 52 °C, there was no difference in T b between Namaqualand and summer Ezemvelo nightjars [0.659 ± 0.427 °C, (−0.279, 1.534)], with T b increasing at 0.140 ± 0.008 °C °C−1 [(0.124, 0.155), Fig. 1b]. For Namaqualand nightjars, T b increased from 38.8 °C (n = 10) at T a ≈ 35 °C to 40.7 °C at T a ≈ 52 °C (n = 2). Similarly, between T a ≈ 35 and 52 °C, summer Ezemvelo nightjar T b increased from 38.7 °C (n = 4) to 40.5 °C (n = 2).
Between T a ≈ 35 and 52 °C, the minimal adequate model explaining T b among Ezemvelo Freckled nightjars included T a (F 1,95 = 388.21, P < 0.0001), M b (F 1,15 = 0.56, P = 0.464) and the interaction between T a and M b (F 1,95 = 34.74, P < 0.0001; Fig. 1c). At 35 ≤ T a ≤ 52 °C, T b of Ezemvelo Freckled nightjars did not differ between summer and winter (F 1,15 = 2.31, P = 0.150; Fig. 1c), with mean winter T b = 40.1 ± 1.1 °C (n = 83) and summer T b = 39.5 ± 0.8 °C (n = 31).
Resting metabolic rate
At 10 ≤ T a ≤ 35 °C, the minimal adequate model for RMR in Rufous-cheeked nightjars only included T a [−0.013 ± 0.002 W °C−1, (−0.016, −0.010); Fig. 2a]. Resting metabolic rate decreased from 0.52 ± 0.1 W at T a ≈ 10 °C (n = 3) to 0.30 ± 0.06 W at T a ≈ 35 °C (n = 15). A significant inflection point in RMR occurred at T a = 35.8 °C (0.010, 0.020), which we interpret as the lower critical limit of thermoneutrality (T lc). At 38 ≤ T a ≤ 50 °C, the minimal adequate model only included sex (−0.133, −0.056), with females having a higher RMR (0.375 ± 0.051 W, n = 12) than males (0.284 ± 0.034 W, n = 70). At T a between 38 and 50 °C, RMR did not vary with T a [0.0009 ± 0.0006 W °C−1, (−0.0003, 0.002); Fig. 2a].
Resting metabolic rate (RMR) as a function of air temperature (T a) in a Rufous-cheeked nightjars (Caprimulgus rufigena), b Freckled nightjars (Caprimulgus tristigma) from a mesic (Ezemvelo) and arid (Namaqualand) site and c Freckled nightjars from Ezemvelo during summer and winter. In a, the regression lines between T a ≈ 10–35 °C and 38–50 °C are from two linear models fitted to RMR versus T a. In b, the regression line between T a ≈ 10–35 °C is from a linear model fitted to RMR versus T a in Namaqualand birds and the horizontal regression line is from a linear model fitted to RMR versus T a. In c, the regression line is from a linear model fitted to RMR versus T a. The outlier bird 5599 is marked with an X in b, c
For Namaqualand Freckled nightjars, the minimal adequate model for RMR at 10 ≤ T a ≤ 35 °C included T a [−0.020 ± 0.001 W °C−1, (−0.022, −0.018)] and M b [0.009 ± 0.002 W g−1, (0.005, 0.012)]. At T a ≈ 10 °C, RMR in Namaqualand Freckled nightjars was 0.79 W and decreased to 0.31 ± 0.04 W (n = 11) at T a ≈ 35 °C. An inflection point in RMR for Namaqualand Freckled nightjars occurred at T a = 35.1 °C (0.013, 0.025) and for summer Ezemvelo Freckled nightjars at T a = 36.6 °C [(0.009, 1.104); Fig. 2b], which we interpret as the T lc.
At 38 ≤ T a ≤ 52 °C, the minimal adequate model explaining variation in RMR between Ezemvelo and Namaqualand Freckled nightjars only included M b [0.005 ± 0.001 W g−1, (0.003, 0.008)]. Over the same T a range, RMR remained stable with increasing T a [0.0005 ± 0.0008 W °C−1, (−0.001, 0.002); Fig. 2b], averaging 0.37 ± 0.04 W (n = 27) in Ezemvelo Freckled nightjars and 0.30 ± 0.06 W (n = 81) in Namaqualand nightjars. When the data were analyzed using mass-specific values between T a ≈ 38 and 52 °C, T a [0.008 ± 0.011 mW g−1 °C−1, (−0.014, 0.030)] and site [0.095 ± 0.326 mW g−1, (−0.791, 0.676)] remained non-significant. Mass-specific RMR between T a ≈ 38 and 52 °C for Namaqualand nightjars was 4.48 ± 0.70 mW g−1 and for summer Ezemvelo nightjars 4.70 ± 0.42 mW g−1.
At 38 ≤ T a ≤ 48 °C, the null model emerged as the top model for RMR in summer and winter Ezemvelo Freckled nightjars. Over this T a range, RMR did not vary with T a [0.0009 ± 0.0007 W °C−1, (−0.0004, 0.002); Fig. 2c], nor did it differ between seasons [(−0.055, 0.022); Fig. 2c], with mean winter RMR = 0.35 ± 0.04 W (n = 61) and mean summer RMR = 0.37 ± 0.03 W (n = 24). Winter Ezemvelo birds displayed a shallow inflection point in RMR at T a = 49.5 °C (−0.004, 0.061), with RMR increasing to 0.41 ± 0.04 W (n = 6) at T a ≈ 52 °C (Fig. 2c). Mass-specific RMR between T a ≈ 38 and 48 °C was significantly higher in summer than winter among Ezemvelo birds (F 1,15 = 12.463, P = 0.003), with mass-specific RMR values of 4.68 ± 0.36 and 4.0 ± 0.44 mW g−1, respectively.
Evaporative water loss
Between T a ≈ 10 and 38 °C, the minimal adequate model for TEWL in Rufous-cheeked nightjars only included T a [0.007 ± 0.001 g H2O h−1 °C−1, (0.005, 0.009); Fig. 3a]. At T a ≈ 10 °C, TEWL averaged 0.10 ± 0.04 g H2O h−1 (n = 3) and increased to 0.28 ± 0.05 g H2O h−1 at T a ≈ 38 °C (n = 13). A significant inflection point in TEWL occurred at T a = 39.5 °C (0.088, 0.119), above which TEWL increased rapidly (Fig. 3a). Between T a ≈ 40 and 56 °C, the minimal adequate model for TEWL only included T a [0.099 g H2O h−1 °C−1, (0.093, 0.105); Fig. 3a], with TEWL reaching 2.49 ± 0.24 g H2O h−1 (n = 2) at T a ≈ 56 °C.
Total evaporative water loss (TEWL) as a function of air temperature (T a) in a Rufous-cheeked nightjars (Caprimulgus rufigena), b Freckled nightjars (Caprimulgus tristigma) from a mesic (Ezemvelo) and arid (Namaqualand) site and c Freckled nightjars from Ezemvelo during summer and winter. In a, the regression lines between T a ≈ 10–38 °C and 40–56 °C are from two linear models fitted to TEWL versus T a. In b, regression lines are from a linear model fitted to TEWL versus the interaction between site and T a. In c, regression lines represent the common regression slope from a linear model fitted to TEWL versus season with T a as a covariate
Total evaporative water loss for Freckled nightjars from Namaqualand remained low and stable at 10 ≤ T a ≤ 25 °C, averaging 0.14 ± 0.03 g H2O h−1 (n = 9). Significant inflection points in TEWL occurred at T a = 38.3 °C (0.074, 0.110) for Namaqualand nightjars and at T a = 37.0 °C (0.091, 0.183) for summer Ezemvelo nightjars, above which TEWL increased rapidly (Fig. 3b).
The minimal adequate model explaining summer TEWL at 40 ≤ T a ≤ 52 °C in Ezemvelo and Namaqualand Freckled nightjars included T a [0.142 ± 0.010 g H2O h−1 °C−1, (0.123, 0.163)], site [1.747 ± 0.523 g H2O h−1, (0.742, 2.802)] and the interaction between T a and site [−0.049 ± 0.011 g H2O h−1 °C−1, (−0.072, −0.026); Fig. 3b]. The slope of the interaction between T a and site for Namaqualand birds was 0.093 g H2O h−1 °C−1 and for Ezemvelo birds was 0.142 g H2O h−1 °C−1. At T a = 40–52 °C, average summer TEWL was lower in Namaqualand nightjars (0.91 ± 0.38 g H2O h−1, n = 73) compared to Ezemvelo nightjars (1.31 ± 0.55 g H2O h−1, n = 23). For Namaqualand Freckled nightjars, TEWL = 0.13 g H2O h−1 at 10 °C, increasing to 1.78 ± 0.27 g H2O h−1 (n = 2) at 52 °C. For summer Ezemvelo Freckled nightjars, TEWL = 0.42 ± 0.13 g H2O h−1 (n = 4) at T a ≈ 30 °C, increasing to 2.32 g H2O h−1 at 56 °C. Between 40 ≤ T a ≤ 52 °C, the minimal adequate model for mass-specific TEWL included T a (1.488 ± 0.072 mg H2O g−1 h−1 °C−1, (1.349, 1.636)] and site (−5.164, −1.096), with mass-specific TEWL averaging 13.51 ± 5.44 mg H2O g−1 h−1 in Namaqualand birds and 16.41 ± 6.85 mg H2O g−1 h−1 in summer Ezemvelo birds.
Among Ezemvelo Freckled nightjars, TEWL at T a < 40 °C remained low and stable in both winter and summer (Fig. 3c). There was a significant inflection point in winter TEWL at T a = 39.9 °C (0.078, 0.151), above which TEWL increased rapidly (Fig. 3c). The minimal adequate model for TEWL among Ezemvelo Freckled nightjars at T a ≈ 40–52 °C included T a (0.119 ± 0.004 g H2O h−1 °C−1, F 1,67 = 781.515, P < 0.0001) and season (F 1,15 = 24.665, P < 0.001). Mean TEWL was higher in summer (1.31 ± 0.55 g H2O h−1, n = 23) than winter (0.98 ± 0.48 g H2O h−1, n = 62, Fig. 3c). Winter TEWL = 0.35 ± 0.10 g H2O h−1 at T a ≈ 35 °C (n = 10) and increased to 1.90 ± 0.22 g H2O h−1 at 52 °C (n = 6). The minimal adequate model for mass-specific TEWL between T a ≈ 40 and 52 °C included T a (F 1,66 = 811.814, P < 0.0001), season (F 1,15 = 42.272, P < 0.0001) and the interaction between T a and season (F 1,66 = 14.552, P < 0.001). Mass-specific TEWL averaged 11.21 ± 5.60 mg H2O g−1 h−1 for winter birds and 16.41 ± 6.85 mg H2O g−1 h−1 in summer birds between T a ≈ 40–52 °C.
Ratio of evaporative heat loss to metabolic heat production
In Rufous-cheeked nightjars, at T a below thermoneutrality EHL/MHP increased significantly and linearly with T a [0.020 ± 0.002 °C−1, (0.016, 0.023); Fig. 4a] from 0.13 ± 0.07 at 10 °C (n = 3) to 0.63 ± 0.10 at 38 °C (n = 13). The slope of EHL/MHP versus T a changed significantly at T a = 39.2 °C (0.176, 0.234), corresponding to rapid increases in TEWL. Between T a ≈ 40 and 56 °C, the minimal adequate model for EHL/MHP only included T a [0.214 ± 0.009 °C−1, (0.196, 0.233); Fig. 4a]. At T a ≥ 44 °C, all birds were dissipating more than 100% of MHP through evaporation. At T a ≈ 56 °C, mean EHL/MHP = 5.15 ± 1.2 (n = 2; Fig. 4a).
The ratio of evaporative heat loss to metabolic heat production (EHL/MHP) as a function of air temperature (T a) in a Rufous-cheeked nightjars (Caprimulgus rufigena), b Freckled nightjars (Caprimulgus tristigma) from a mesic (Ezemvelo) and arid (Namaqualand) site and c Freckled nightjars from Ezemvelo during summer and winter. In a, the regression lines are from two linear models fitted to EHL/MHP versus T a between T a ≈ 10–38 °C and 40–56 °C. In b, regression lines represent the common slope from a linear model fitted to EHL/MHP versus site with T a as a covariate. In c, regression lines are from a linear model fitted to EHL/MHP versus the interaction between season and T a
In Freckled nightjars at our Namaqualand study site, EHL/MHP remained low between T a ≈ 10 and 25 °C, with EHL/MHP = 0.11 at T a ≈ 10 °C and 0.21 ± 0.02 at T a ≈ 25 °C (n = 2). Rapid increases in EHL/MHP occurred above T a = 38.5 °C (0.165, 0.269) and above T a = 36.8 °C (0.138, 0.286) in Namaqualand and summer Ezemzelo Freckled nightjars, respectively. At 40 ≤ T a ≤ 52 °C, the minimal adequate model for EHL/MHP included T a [0.230 ± 0.008 °C−1, (0.213, 0.246)] and site [−0.535 ± 0.190, (−0.907, −0.163)], with Ezemvelo nightjars having higher EHL/MHP values (Fig. 4b). In Namaqualand Freckled nightjars, maximum EHL/MHP was 4.5 ± 0.5 at T a ≈ 52 °C (n = 2).
For winter Ezemvelo Freckled nightjars, EHL/MHP increased rapidly above T a = 39.8 °C [(0.118, 0.248); Fig. 4c]. The minimal adequate model for EHL/MHP between T a ≈ 40 and 52 °C included season (F 1,15 = 24.568, P < 0.001), T a (F 1,66 = 922.255, P < 0.0001) and the interaction between season and T a (F 1,66 = 12.597, P < 0.001; Fig. 4c). The slope of the interaction between T a and season for winter Ezemvelo birds was 0.184 °C−1 and for summer Ezemvelo birds was 0.240 °C−1.
Discussion
Rufous-cheeked nightjars and Freckled nightjars exhibited a pronounced capacity to tolerate high heat loads and were able to maintain T b far below T a. Moreover, even at the highest T a to which they were exposed, all individuals of both species maintained T b below ~42.5 °C. At T a values above 37.0–39.9 °C, TEWL increased rapidly with increasing T a, whereas RMR remained stable, resulting in efficient evaporative cooling (i.e., large increases in EHL with small increases in MHP at a given T a). The maximum evaporative cooling capacity of both species exceeds that of most birds investigated to date. In the following discussion, we compare the thermoregulatory patterns and evaporative cooling efficiency at high T a in the two nightjar species we studied with data from other caprimulgids. We subsequently place our results in a broader context and compare the physiological responses of our study species at high T a to those of other avian taxa studied under similar conditions.
Body temperature
Both nightjar species displayed relatively labile T b that was positively related to T a. Qualitatively similar patterns of Tb lability have been documented in Common Nighthawks (Chordeiles minor; Lasiewski and Dawson 1964), Spotted nightjars (Dawson and Fisher 1969) and Poorwills (Phalaenoptilus nuttallii; Lasiewski and Seymour 1972). Increases in Tb at moderate to high T a appears common among birds and has been observed in phylogenetically diverse taxa (Weathers and Schoenbaechler 1976; Weathers 1981; Tieleman and Williams 1999). Increases in T b at high T a should enhance a bird’s water economy in hot environments through a more favorable T b − T a gradient, thereby increasing the potential for dry heat loss when T a < T b and decreasing the potential for dry heat gain when T a > T b (Tieleman and Williams 1999). In addition, heat stored internally during the day can be dissipated later through non-evaporative pathways when T a is conducive (Weathers and Schoenbaechler 1976; Weathers 1981; Tieleman and Williams 1999). For example, through heat stored in body tissue, Rufous-cheeked nightjars could have saved a maximum of 128 mg H2O at T a ≈ 50 °C by allowing T b to increase 1.7 °C relative to T b at T a ≈ 38 °C. Similarly, Namaqualand Freckled nightjars could have saved 139 mg H2O at T a ≈ 50 °C by allowing T b to increase 1.5 °C relative to T b at T a ≈ 38 °C. Thus, for nocturnal species like nightjars, where during the day prolonged periods of high rates of evaporative water loss are common, a labile T b would presumably contribute to the conservation of body water and avoidance of dehydration.
Both Rufous-cheeked nightjars and Freckled nightjars increased T b as T a increased within the TNZ without concomitant increases in metabolic heat production, a phenomenon previously reported for a number of avian species (Weathers and Schoenbaechler 1976; Weathers 1981; Tieleman and Williams 1999). However, given the thermal sensitivity of biochemical reaction rates, one might expect an increase in metabolic rate with T b due to a Q 10 effect. For example, assuming a Q 10 of 2.5, we would have expected mean RMR in Rufous-cheeked nightjars to increase from 0.299 W at T a = 38 °C to 0.350 W at T a = 50 °C when mean T b increased from 40 to 41.7 °C. Yet, at T a = 50 °C, mean RMR = 0.296 W. Traditionally, Q 10 has been used to describe relationships between metabolic rate and temperature in ectotherms and some authors have argued whether Q 10 effects should be applied to endotherms (Snyder and Nestler 1990; Heldmaier and Ruf 1992), or even whole-animal energy metabolism in general (Chaui-Berlinck et al. 2002, 2004). Perhaps, avian species are actively suppressing metabolism as T b increases within the TNZ (e.g., hyperthermia-induced hypometabolism; Lovegrove et al. 2014), explaining the apparent lack of a Q 10 effect. To the best of our knowledge, however, no exact physiological mechanisms have been presented to explain this phenomenon.
Although both species increased T b with T a, absolute limits of T b remained low compared to that of other avian taxa under similar conditions of heat stress. For example, at T a ≈ 48 °C, average T b in both nightjars was 39.9–41.0 °C (Table 1), compared to 43.0–44.7 °C in three southern African ploceid passerines (Whitfield et al. 2015). In Spinifex Pigeons (Geophaps plumifera), T b increased to 43.4 °C at T a ≈ 47–51 °C (Withers and Williams 1990), whereas over the same T a range, the mean T b of Ezemvelo Freckled nightjars was only 40.8 °C. Relatively small increases in T b are apparently common among caprimulgids when exposed to extreme T a, highlighting their capacity to defend large T a − T b gradients (Dawson and Fisher 1969; Lasiewski and Seymour 1972). The ability of caprimulgids to maintain these large thermal gradients is likely primarily attributable to the energetically efficient mechanism of gular flutter (Bartholomew et al. 1962). Presumably, this allows nightjars to nest on the bare ground where T e is high, but predation risk to incubating adults is reduced (Amat and Masero 2004).
Resting metabolic rate
A distinct TNZ was not evident for either species. Lower critical limits of thermoneutrality occurred at T a ≈ 35–37 °C. There was some indication for upper critical limits of thermoneutrality (T uc) at T a ≈ 50 °C, although the inflection points were not significant. Similar T lc values have been reported for Poorwills (T lc ≈ 35 °C; Bartholomew et al. 1962), Common Nighthawks (T lc ≈ 35 °C; Lasiewski and Dawson 1964) and Spotted nightjars (T lc ≈ 32 °C; Dawson and Fisher 1969). Dawson and Fisher (1969) attributed a high T lc in Spotted nightjars to a low basal metabolic rate, a phenotype common among caprimulgids (Lane et al. 2004). Several species of columbid birds (Marder and Arieli 1988; Withers and Williams 1990; McKechnie et al. 2016b) and houbara bustards (Tieleman et al. 2002b) have shown similar patterns of either a very high T uc or no discernible T uc, a pattern that contrasts with the large increases in RMR at T a > T b observed in many other avian taxa (Trost 1972; Weathers 1997; Williams 1999; Tieleman et al. 2002a; Whitfield et al. 2015; McKechnie et al. 2016a). Both caprimulgids and columbids implement gular fluttering as a mechanism for evaporative heat dissipation, whereas many avian taxa (particularly passerines), only use panting to unload heat (Lasiewski and Bartholomew 1966; Bartholomew et al. 1968; Dawson 1982). The movement of the thorax associated with panting produces a larger muscular cost than that of gular fluttering, resulting in elevated metabolic rates and increased endogenous heat production (Whittow 1976). Lasiewski et al. (1966), for example, observed Zebra Finches (Taeniopygia castonatis) at T a ≈ 43.5 °C incapable of dissipating all of their metabolic heat resulting in an EHL/MHP of 0.96. Consequently, gular fluttering aids in limiting endogenous heat production thus allowing for more efficient evaporative cooling.
In addition to gular fluttering, cutaneous evaporative water loss (CEWL) appears to be an efficient mechanism for dissipating heat at high T a and low humidities, allowing for large increases in evaporative water loss with small or nonexistent increases in RMR (Marder and Arieli 1988; McKechnie and Wolf 2004; McKechnie et al. 2016b). Marder and Arieli (1988), for example, observed heat-acclimated Rock Pigeons (Columba livia) defending normothermic T b at T a ≈ 60 °C without any discernible increases in RMR and suggested that this species’ TNZ may extend up to T a ≈ 65 °C. In addition, McKechnie et al. (2016b) found that four species of austral columbids exposed to T a above the TNZ showed only small increases in RMR, with one species, the Namaqua Dove (Oena capensis), initiating panting/gular fluttering only at T a ≈ 55 °C. The contribution of CEWL to TEWL is variable among avian taxa (Dawson 1982) and apparently influenced by habitat (Marder et al. 2003). To the best of our knowledge, only one study has measured CEWL in a caprimulgid (Lasiewski et al. 1971). These authors found that at T a ≈ 35 °C CEWL represented 51% of TEWL in a single Poorwill, suggesting that the contribution of CEWL to evaporative cooling in caprimulgids is substantial and comparable to other birds (Wolf and Walsberg 1996), at least at moderate T a. If CEWL remains substantial at T a > T b, this will contribute, in concert with gular fluttering, to the low RMR values observed in nightjars during periods of high evaporative water loss.
Among Freckled nightjars at Ezemvelo, mass-specific RMR between T a ≈ 38 and 48 °C was significantly greater (by 16%) during summer compared to winter. The seasonal metabolic acclimatization we documented in Freckled nightjars, whereby RMR at thermoneutrality is lower in winter than in summer, is similar to the pattern reported for some other southern Hemisphere birds (Smit and McKechnie 2010), although a more recent analysis found that the direction of seasonal metabolic adjustments is more variable in subtropical species (McKechnie et al. 2015). Smit and McKechnie (2010) argued that the downregulation of metabolism during winter in some southern hemisphere birds is primarily influenced by demands for energy and/or water conservation, and not cold tolerance as the case for temperate-zone species, although the functional significance of these seasonal adjustments remains unclear (McKechnie et al. 2015). Doucette and Geiser (2008) documented a lack of seasonal variation in metabolic rate of Australian Owlet-nightjars (Aegotheles cristatus; Aegothelidae). To the best of our knowledge, however, our study is the first to quantify seasonal adjustments in the metabolic rate of a member of the Caprimulgidae.
Evaporative water loss
In both Rufous-cheeked nightjars and Freckled nightjars, TEWL increased sharply above T a = 37–40 °C. The magnitude of fractional increases in TEWL in our two study species was similar to that observed in other caprimulgids. Lasiewski and Dawson (1964) observed a fivefold increase in TEWL between T a ≈ 35 and 44 °C in Common Nighthawks, whereas Dawson and Fisher (1969) reported a 20-fold increase in TEWL of Spotted nightjars at T a ≈ 56.5 °C relative to values at T a ≈ 23–25 °C.
At T a ≈ 25 °C, TEWL of Rufous-cheeked nightjars was equivalent to 3.8 g H2O day−1 and in Namaqualand Freckled nightjars 3.2 g H2O day−1, 258 and 208%, respectively, of the values predicted by Williams (1996) for arid-zone species. Similarly, TEWL of Common Nighthawks at T a ≈ 25 °C exceeded allometric predictions by 25% (Lasiewski and Dawson 1964). These comparatively high rates of TEWL may be due to the fact that 25 °C is below the T lc in these species, and RMR is hence greater than thermoneutral values (Lasiewski and Dawson 1964). At T a ≥ 40 °C, the rates of increase in TEWL (i.e., the slopes of mass-specific TEWL versus T a) were 92, 78, 106, and 84% of those predicted allometrically by McKechnie and Wolf (2010) in Rufous-cheeked nightjars, Namaqualand Freckled nightjars, summer Ezemvelo and winter Ezemvelo Freckled nightjars, respectively. Lasiewski and Dawson (1964) observed a rate of increase in TEWL of 1.4 mg H2O g−1 h−1 °C−1 between T a ≈ 35 and 44 °C in Common Nighthawks (M b ~ 72.5 g), 82% of that predicted allometrically by McKechnie and Wolf (2010). With the exception of Ezevmelo Freckled nightjars in summer, a general pattern of low fractional increases in TEWL (i.e., shallower slopes of TEWL versus T a) seems to emerge among the caprimulgids investigated to date, suggesting that at high T a, nightjars may have lower TEWL rates than expected based on body size, consistent with the notion of highly efficient evaporative cooling in this taxon.
Both whole-animal and mass-specific summer TEWL at T a ≥ 40 °C were lower in Namaqualand Freckled nightjars compared to Ezemvelo conspecifics, supporting our prediction that birds from a more arid site have lower TEWL once M b is considered. Moreover, the mass-specific rate of increase in TEWL was shallower in Namaqualand compared to Ezemvelo nightjars, despite the former birds being smaller than the latter, and hence contrasting with expectations from allometric scaling (McKechnie and Wolf 2010). Several studies have indicated that birds inhabiting hot, arid environments have evolved reduced TEWL rates compared to mesic counterparts (Dawson 1982; Williams 1996; Williams and Tieleman 2005). Tieleman et al. (2002a) investigated four species of lark, two mesic and two arid, and found that TEWL was 27% lower in arid compared to mesic species. Furthermore, fractional increases in TEWL at T a ≈ 40 °C relative to T a ≈ 25 °C were smaller in the arid-zone species (Tieleman et al. 2002a). Our comparison of TEWL in two populations of Freckled nightjars is hence consistent with the notion of rates of TEWL in arid-zone birds generally being lower than those of their mesic counterparts.
Although M b was significantly lower during summer, Ezemvelo nightjars had greater TEWL at any given T a during summer compared to winter, contrary to our prediction of a lower TEWL in summer-acclimatized individuals. Weathers and Caccamise (1978) investigated seasonal changes in the thermal physiology of Monk Parakeets (Myiopsitta monachus) and found that TEWL at T a ≈ 20–30 °C was lower in winter, a result the authors related to reduced water availability in winter. Several workers have found that birds can significantly augment CEWL through changes in water vapor diffusion resistance via alterations in the composition of lipids in the stratum corneum (Haugen et al. 2003; Muñoz-Garcia et al. 2008; Williams et al. 2012). Arad et al. (1987), for example, observed an overall reduction in TEWL in dehydrated pigeons at T a ≈ 45 °C due to reductions in CEWL. Therefore, if CEWL represents a large percentage of TEWL in nightjars, as suggested by Lasiewski et al. (1971), phenotypic flexibility in resistance to water vapor diffusion during the dry season would likely have adaptive value.
Ratio of evaporative heat loss to metabolic heat production
Caprimulgids have long been recognized as having a pronounced capacity for evaporative cooling (Bartholomew et al. 1962; Lasiewski and Dawson 1964; Dawson and Fisher 1969; Dawson 1982) and our data for two southern African species are consistent with this notion. The efficiency of evaporative cooling in nightjars becomes apparent when compared to that of other species for which data exist. For example, at T a ≈ 48 °C, Whitfield et al. (2015) recorded average EHL/MHP ratios of 1.41, 1.46 and 1.61 in Scaly-feathered Weavers (Sporopipes squamifrons), White-browed sparrow-Weavers (Plocepasser mahali), and Sociable Weavers (Philetairus socius), respectively. In comparison, the lowest average EHL/MHP value we recorded at T a ≈ 48 °C was 2.47 (Table 1). Bartholomew et al. (1962) found Poorwills had an EHL/MHP ratio that was 1.8 times greater than Dawson’s (1958) measurements for cardinals at T a ≈ 40 °C. The low resting metabolic rates of nightjars (Lane et al. 2004), coupled with the energetically efficient mechanism of gular fluttering, appears to allow this group of birds to rapidly dissipate heat evaporatively with only small or no increases in metabolism. In male Rufous-cheeked nightjars, for instance, between T a ≈ 38 and 56 °C RMR increased by just 15%, whereas TEWL increased by 845%, allowing these birds to maintain a mean T b equivalent to 14.4 °C below T a. Lasiewski (1969) documented Poorwills increasing their oxygen consumption by 13% and TEWL by 750% at T a ≈ 47 °C relative to T a ≈ 35 °C. At T a ≈ 56 °C, Rufous-cheeked nightjars were dissipating approximately 515% of their metabolic heat production. This value exceeds those previously reported for species that have also exhibited efficient cooling capacities (Dawson and Fisher 1969; Marder and Arieli 1988). Smith et al. (2015), for example, measured an EHL/MHP of 3.08 at T a = 56 °C and an EHL/MHP of 3.69 at T a = 58 °C in Mourning doves (Zenaida macroura) and White-winged doves (Zenaida asiatica), respectively. McKechnie et al. (2016b) measured an EHL/MHP ratio of 4.70 at T a = 60 °C in Namaqua doves (Oena capensis). Therefore, to the best of our knowledge, the EHL/MHP of 5.15 measured here for Rufous-cheeked nightjars is the highest avian value yet reported.
The maximum EHL/MHP ratios estimated here were calculated on an assumed RER = 0.71, corresponding to lipid metabolism. However, if RMR is recalculated assuming an RER = 1.0 (i.e., a thermal equivalence of 21.4 J ml−1 CO2; Withers 1992), corresponding to carbohydrate metabolism, estimated RMR is 23% lower. Consequently, maximum EHL/MHP ratios increase by 30%, with maximum evaporative cooling capacities of 7.8, 6.4, 5.6, and 4.4 in Rufous-cheeked nightjars, Namaqualand Freckled nightjars, summer Ezemvelo Freckled nightjars and winter Ezemvelo Freckled nightjars, respectively. This uncertainty highlights an unfortunate and largely unavoidable limitation of our approach to measuring heat tolerance at high T a. The high flow rates required to keep chamber humidity low prevent accurate recordings of oxygen consumption and consequently RER. However, given that nightjars feed on the wing and must be hand-fed in captivity, we feel confident that our birds were post-absorptive during measurements and likely metabolizing lipids.
At 40 ≤ T a ≤ 52 °C, summer Ezemvelo Freckled nightjars were more efficient at dissipating metabolic heat compared to Namaqualand conspecifics, contrary to our prediction that evaporative cooling should be more efficient in Namaqualand birds. This result is likely due to TEWL being lower in Namaqualand compared to summer Ezemvelo Freckled nightjars. Conversely, Freckled nightjars at Ezemvelo had a greater EHL/MHP ratio during summer compared to winter. Since RMR between T a ≈ 40–52 °C remained relatively stable across seasons, the variation in the efficiency of evaporative cooling likely reflects differences in TEWL rates rather than metabolic variation.
Conclusions
Caprimulgids’ capacity for efficient evaporative cooling appears to arise from rates of evaporative water loss that are quantitatively similar to those of other birds, combined with metabolic rates that remain at minimal resting levels even when T e is far above T b. Low resting metabolic heat production, together with the lack of a discernible T uc, limits nightjars’ endogenous heat production, minimizing their total heat load and allowing them to rapidly dissipate heat gained from the environment. In addition, fractional rates of increase in TEWL for the two nightjar species studied here were generally lower than expected for their size. Nevertheless, at high T a, nightjars are still forced to evaporate substantial amounts of water for extended periods, raising questions as to the hydration status of these birds at sunset and how this may affect drinking patterns and prey selection.
Nightjars inhabit some of the hottest environments on the planet. For instance, several species reside in extremely hot deserts and semi-desert habitats [e.g., Common Poorwill; Egyptian Nightjar (Caprimulgus aegyptius); Nubian Nightjar (Caprimulgus nubicus); Sykes’s Nightjar (Caprimulgus mahrattensis); Cleere and Nurney 1998]. A nocturnal lifestyle coupled with roosting and nesting in sites exposed to full solar radiation exposes nightjars to some of the highest T e experienced by any endotherm. Moreover, their nocturnal habits mean that they must often endure very high T e for extended periods between sunrise and sunset, especially in summer with lengthy daylight periods. Yet, nightjars appear reluctant to use pronounced hyperthermia, especially when compared to other species exposed to high T a (Lasiewski and Seymour 1972; Whitfield et al. 2015; Noakes et al. 2016). Incubating in such conditions places additional thermoregulatory pressures on these birds, because it forces incubating adults to maintain a T b within a narrow range well below T a. Such thermally challenging conditions have undoubtedly exerted strong selective pressures on nightjars to evolve physiological traits that provide the basis for pronounced heat tolerance.
Abbreviations
- EHL:
-
Evaporative heat loss
- M b :
-
Body mass
- MHP:
-
Metabolic heat production
- RMR:
-
Resting metabolic rate
- T a :
-
Air temperature
- T b :
-
Body temperature
- T e :
-
Operative temperature
- TEWL:
-
Total evaporative water loss
- T lc :
-
Lower critical limit of thermoneutrality
- T uc :
-
Upper critical limit of thermoneutrality
References
Amat JA, Masero JA (2004) Predation risk on incubating adults constrains the choice of thermally favourable nest sites in a plover. Anim Behav 67:293–300
Arad Z, Gavrieli-Levin I, Eylath U, Marder J (1987) Effect of dehydration on cutaneous water evaporation in heat-exposed pigeons (Columba livia). Physiol Zool 60:623–630
Bartholomew GA, Cade TJ (1963) The water economy of land birds. Auk 80:504–539
Bartholomew GA, Dawson WR (1979) Thermoregulatory behavior during incubation in heermann’s gulls. Physiol Zool 52:422–437
Bartholomew GA, Hudson JW, Howell TR (1962) Body temperature, oxygen consumption, evaporative water loss and heart rate in the poor-will. Condor 64:117–125
Bartholomew GA, Lasiewski RC, Crawford EC Jr (1968) Patterns of panting and gular flutter in cormorants, pelicans, owls, and doves. Condor 70:31–34
Bartoń K (2015) MuMIn: multi-model inference. R package version 1.15.1. http://CRAN.R-project.org/package=MuMIn
Bates D (2006) [R] lmer, p-values and all that. https://stat.ethz.ch/pipermail/r-help/2006-May/094765. Accessed 10 July 2015
Bates D, Maechler M, Bolker B, Walker S (2015) lme4: linear mixed-effects models using eigen and S4. R package version 1.1-9. https://CRAN.R-project.org/package=lme4
Boyles JG, Smit B, McKechnie AE (2012) Variation in body temperature is related to ambient temperature but not experimental manipulation of insulation in two small endotherms with different thermoregulatory patterns. J Zool 287:224–232
Brigham RM, McKechnie AE, Doucette LI, Geiser F (2012) Heterothermy in caprimulgid birds: a review of inter- and intraspecific variation in free-ranging populations. In: Ruf T, Bieber C, Arnold W, Millesi E (eds) Living in a seasonal world: thermoregulatory and metabolic adaptation. Springer, Berlin, Heidelberg, pp 175–187
Calder WA Jr, Schmidt-Nielsen K (1966) Evaporative cooling and respiratory alkalosis in the pigeon. Proc Natl Acad Sci USA 55:750–756
Chaui-Berlinck JG, Monteiro LHA, Navas CA, Bicudo JEPW (2002) Temperature effects on energy metabolism: a dynamic system analysis. Proc Roy Soc Lond B Biol 269:15–19
Chaui-Berlinck JG, Navas CA, Monteiro LHA, Bicudo JEPW (2004) Temperature effects on a whole metabolic reaction cannot be inferred from its components. Proc Roy Soc Lond B Biol 271:1415–1419
Cleere N, Nurney D (1998) Nightjars: a guide to the nightjars, nighthawks, and their relatives. Yale University Press, New Haven
Core Team R (2015) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Cowles RB, Dawson WR (1951) A cooling mechanism of the Texas nighthawk. Condor 53:19–22
Crawley MJ (2007) The R book. Wiley, West Sussex
Cunningham SJ, Martin RO, Hojem CL, Hockey PAR (2013) Temperatures in excess of critical thresholds threaten nestling growth and survival in a rapidly-warming arid savanna: a study of common fiscals. PLoS One 8(9):e74613
Dawson WR (1958) Relation of oxygen consumption and evaporative water loss to temperature in the cardinal. Physiol Zool 31:37–48
Dawson WR (1982) Evaporative losses of water by birds. Comp Biochem Phys A 71:495–509
Dawson WR, Fisher CD (1969) Responses to temperature by the spotted nightjar (Eurostopodus guttatus). Condor 71:49–53
Dexter RW (1956) Further banding and nesting studies of the eastern nighthawk. Bird-Banding 27:9–16
Doucette LI, Geiser F (2008) Seasonal variation in thermal energetics of the Australian owlet-nightjar (Aegotheles cristatus). Comp Biochem Phys A 151:615–620
du Plessis KL, Martin RO, Hockey PAR, Cunningham SJ, Ridley AR (2012) The costs of keeping cool in a warming world: implications of high temperatures for foraging, thermoregulation and body condition of an arid-zone bird. Glob Change Biol 18:3063–3070
du Prel JB, Hommel G, Röhrig B, Blettner M (2009) Confidence interval or p-value? Dtsch Arztebl Int 106:335–339
Faraway JJ (2006) Extending the linear model with R: generalized linear, mixed effects and nonparametric regression models. Chapman & Hall/CRC, Boca Raton
Ganey JL (2004) Thermal regimes of Mexican Spotted Owl nest stands. Southwest Nat 49:478–486
Grant GS (1982) Avian incubation: egg temperature, nest humidity, and behavioral thermoregulation in a hot environment. Ornithol Monogr 30:1–75
Haugen MJ, Tieleman BI, Williams JB (2003) Phenotypic flexibility in cutaneous water loss and lipids of the stratum corneum. J Exp Biol 206:3581–3588
Heldmaier G, Ruf T (1992) Body temperature and metabolic rate during natural hypothermia in endotherms. J Comp Physiol B 162:696–706
Ingels J, Ribot JH, de Jong BHJ (1984) Vulnerability of eggs and young of the blackish nightjar (Caprimulgus nigrescens) in Suriname. Auk 101:388–391
Koller M (2015) robustlmm: robust linear mixed effects models. R package version 1.7-6. http://CRAN.R-project.org/package=robustlmm
Lane JE, Swanson DL, Brigham RM, McKechnie AE (2004) Physiological responses to temperature by whip-poor-wills: more evidence for the evolution of low metabolic rates in caprimulgiformes. Condor 106:921–925
Lasiewski RC (1969) Physiological responses to heat stress in the poorwill. Am J Physiol 217:1504–1509
Lasiewski RC, Bartholomew GA (1966) Evaporative cooling in the Poor-will and the Tawny Frogmouth. Condor 68:253–262
Lasiewski RC, Dawson WR (1964) Physiological responses to temperature in the common nighthawk. Condor 66:477–490
Lasiewski RC, Seymour RS (1972) Thermoregulatory responses to heat stress in four species of birds weighing approximately 40 grams. Physiol Zool 45:106–118
Lasiewski RC, Acosta AL, Bernstein MH (1966) Evaporative water loss in birds-I. Characteristics of the open flow method of determination, and their relation to estimates of thermoregulatory ability. Comp Biochem Physiol 19:445–457
Lasiewski RC, Bernstein MH, Ohmart RD (1971) Cutaneous water loss in the roadrunner and poor-will. Condor 73:470–472
Lighton JRB (2008) Measuring metabolic rates: a manual for scientists. Oxford University Press, Oxford
Ligon JD (1968) The biology of the Elf Owl, (Micrathene whitneyi). Miscellaneous Publications Museum of Zoology, University of Michigan No. 136
Ligon JD (1969) Some aspects of temperature relations in small owls. Auk 86:458–472
Lovegrove BG, Canale C, Levesque D, Fluch G, Řeháková-Petrů M, Ruf T (2014) Are tropical small mammals physiologically vulnerable to arrhenius effects and climate change? Physiol Biochem Zool 87:30–45. doi:10.1086/673313
Marder J, Arieli Y (1988) Heat balance of acclimated pigeons (Columba livia) exposed to temperatures up to 60 °C Ta. Comp Biochem Physiol A 91:165–170
Marder J, Withers PC, Philpot RG (2003) Patterns of cutaneous water evaporation by Australian pigeons. Isr J Zool 49:111–129
McKechnie AE, Wolf BO (2004) Partitioning of evaporative water loss in white-winged doves: plasticity in response to short-term thermal acclimation. J Exp Biol 207:203–210
McKechnie AE, Wolf BO (2010) Climate change increases the likelihood of catastrophic avian mortality events during extreme heat waves. Biol Lett 6:253–256
McKechnie AE, Noakes MJ, Smit B (2015) Global patterns of seasonal acclimatization in avian resting metabolic rates. J Ornithol 156:367–376. doi:10.1007/s10336-015-1186-5
McKechnie AE, Smit B, Whitfield MC, Noakes MJ, Talbot WA, Garcia M, Gerson AR, Wolf BO (2016a) Avian thermoregulation in the heat: evaporative cooling capacity in an archetypal desert specialist, Burchell’s sandgrouse (Pterocles burchelli). J Exp Biol 219:2137–2144
McKechnie AE, Whitfield MC, Smit B, Gerson AR, Smith EK, Talbot WA, McWhorter TJ, Wolf BO (2016b) Avian thermoregulation in the heat: efficient evaporative cooling allows for extreme heat tolerance in four southern Hemisphere columbids. J Exp Biol 219:2145–2155
McNab BK (2006) The relationship among flow rate, chamber volume and calculated rate of metabolism in vertebrate respirometry. Comp Biochem Phys A 145:287–294
Muñoz-Garcia A, Cox RM, Williams JB (2008) Phenotypic flexibility in cutaneous water loss and lipids of the stratum corneum in house sparrows (Passer domesticus) following acclimation to high and low humidity. Physiol Biochem Zool 81:87–96
Noakes MJ, Wolf BO, McKechnie AE (2016) Seasonal and geographical variation in heat tolerance and evaporative cooling capacity in a passerine bird. J Exp Biol. doi:10.1242/jeb.132001
Pinheiro J, Bates D, DebRoy S, Sarkar D, R Core Team (2015) nlme: linear and nonlinear mixed effects models. R package version 3.1-121. http://CRAN.R-project.org/package=nlme
Porter WP, Gates DM (1969) Thermodynamic equilibria of animals with environment. Ecol Monogr 39:227–244
Quinn GP, Keough MJ (2002) Experimental design and data analysis for biologists. Cambridge University Press, Cambridge
Smit B, McKechnie AE (2010) Avian seasonal metabolic variation in a subtropical desert: basal metabolic rates are lower in winter than in summer. Funct Ecol 24:330–339
Smit B, Boyles JG, Brigham RM, McKechnie AE (2011) Torpor in dark times: patterns of heterothermy are associated with the lunar cycle in a nocturnal bird. J Biol Rhythm 26:241–248
Smith EK, O’Neill J, Gerson AR, Wolf BO (2015) Avian thermoregulation in the heat: resting metabolism, evaporative cooling and heat tolerance in Sonoran Desert doves and quail. J Exp Biol 218:3636–3646
Snyder GK, Nestler JR (1990) Relationships between body temperature, thermal conductance, Q10 and energy metabolism during daily torpor and hibernation in rodents. J Comp Physiol B 159:667–675
Sonderegger D (2012) SiZer: significant zero crossings. R package version 0.1-4. http://CRAN.R-project.org/package=SiZer
Spottiswoode CN, Jackson HD (2005a) Rufous-cheeked nightjar. In: Hockey PAR, Dean WRJ, Ryan PG (eds) Roberts birds of southern Africa, 7th edn. The Trustees of the John Voelcker Bird Book Fund, Cape Town, pp 271–272
Spottiswoode CN, Jackson HD (2005b) Freckled nightjar. In: Hockey PAR, Dean WRJ, Ryan PG (eds) Roberts birds of southern Africa, 7th edn. The Trustees of the John Voelcker Bird Book Fund, Cape Town, pp 266–268
Steyn P (1971) Notes on the breeding biology of the freckled nightjar. Ostrich 42:179–188
Swanson DL, Drymalski MW, Brown JR (1996) Sliding vs static cold exposure and the measurement of summit metabolism in birds. J Therm Biol 21:221–226
Tattersall GJ, Sinclair BJ, Withers PC, Fields PA, Seebacher F, Cooper CE, Maloney SK (2012) Coping with thermal challenges: physiological adaptations to environmental temperatures. Compr Physiol 2:2151–2202
Tieleman BI, Williams JB (1999) The role of hyperthermia in the water economy of desert birds. Physiol Biochem Zool 72:87–100
Tieleman BI, Williams JB, Buschur ME (2002a) Physiological adjustments to arid and mesic environments in larks (Alaudidae). Physiol Biochem Zool 75:305–313
Tieleman BI, Williams JB, LaCroix F, Paillat P (2002b) Physiological responses of houbara bustards to high ambient temperatures. J Exp Biol 205:503–511
Trost CH (1972) Adaptations of horned larks (Eremophila alpestris) to hot environments. Auk 89:506–527
Walsberg GE, Voss-Roberts KA (1983) Incubation in desert-nesting doves: mechanisms for egg cooling. Physiol Zool 56:88–93
Weathers WW (1981) Physiological thermoregulation in heat-stressed birds: consequences of body size. Physiol Zool 54:345–361
Weathers WW (1997) Energetics and thermoregulation by small passerines of the humid, lowland tropics. Auk 114:341–353
Weathers WW, Caccamise DF (1978) Seasonal acclimatization to temperature in monk parakeets. Oecologia 35:173–183
Weathers WW, Schoenbaechler DC (1976) Regulation of body temperature in the budgerygah, Melopsittacus undulatus. Aust J Zool 24:39–47
Weller MW (1958) Observations on the incubation behavior of a common nighthawk. Auk 75:48–59
Whitfield MC, Smit B, McKechnie AE, Wolf BO (2015) Avian thermoregulation in the heat: scaling of heat tolerance and evaporative cooling capacity in three southern African arid-zone passerines. J Exp Biol 218:1705–1714
Whittow GC (1976) Regulation of body temperature. In: Sturkie PD (ed) Avian physiology, 3rd edn. Springer, New York, pp 146–173
Williams JB (1996) A phylogenetic perspective of evaporative water loss in birds. Auk 113:457–472
Williams JB (1999) Heat production and evaporative water loss of dune larks from the Namib desert. Condor 101:432–438
Williams JB, Tieleman BI (2005) Physiological adaptation in desert birds. Bioscience 55:416–425
Williams JB, Muñoz-Garcia A, Champagne A (2012) Climate change and cutaneous water loss of birds. J Exp Biol 215:1053–1060
Withers PC (1977) Respiration, metabolism, and heat exchange of euthermic and torpid poorwills and hummingbirds. Physiol Zool 50:43–52
Withers PC (1992) Comparative animal physiology. Saunders College Publishing, Orlando
Withers PC, Williams JB (1990) Metabolic and respiratory physiology of an arid-adapted Australian bird, the spinifex pigeon. Condor 92:961–969
Wolf BO, Walsberg GE (1996) Respiratory and cutaneous evaporative water loss at high environmental temperatures in a small bird. J Exp Biol 199:451–457
Acknowledgements
We thank Duncan MacFadyen and E. Oppenheimer & Son for allowing us to conduct research on their properties, Pieter and Verencia Benade for their hospitality and access to their farm. Cathy Bester, Ryno Kemp and Pieter Erasmus provided valuable assistance in the field. Lastly, we thank Bruce Woodroffe and Awesome Tools (Cape Town, South Africa) for discounted lighting equipment. This paper was improved through the helpful comments of two anonymous reviewers. Funding was provided by the DST-NRF Center of Excellence at the Percy FitzPatrick Institute and University of Pretoria. This material is based on work supported by the National Science Foundation under IOS-1122228 to B.O.W. Any opinions, findings and conclusions or recommendations expressed in this material are those of the author(s) and do not necessarily reflect the views of the National Science Foundation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
All procedures performed in studies involving animals were in accordance with the ethical standards of the institution or practice at which the studies were conducted. This study was approved by the University of Pretoria Animal Ethics Committee (Project EC068-13) and complies with current South African laws.
Additional information
Communicated by I. D. Hume.
Rights and permissions
About this article
Cite this article
O’Connor, R.S., Wolf, B.O., Brigham, R.M. et al. Avian thermoregulation in the heat: efficient evaporative cooling in two southern African nightjars. J Comp Physiol B 187, 477–491 (2017). https://doi.org/10.1007/s00360-016-1047-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00360-016-1047-4