Abstract
Solute Carrier 15 (SLC15) family, alias H+-coupled oligopeptide cotransporter family, is a group of membrane transporters known for their role in the cellular uptake of di- and tripeptides (di/tripeptides) and peptide-like molecules. Of its members, SLC15A1 (PEPT1) chiefly mediates intestinal absorption of luminal di/tripeptides from dietary protein digestion, while SLC15A2 (PEPT2) mainly allows renal tubular reabsorption of di/tripeptides from ultrafiltration, SLC15A3 (PHT2) and SLC15A4 (PHT1) possibly interact with di/tripeptides and histidine in certain immune cells, and SLC15A5 has unknown function. Our understanding of this family in vertebrates has steadily increased, also due to the surge of genomic-to-functional information from ‘non-conventional’ animal models, livestock, poultry, and aquaculture fish species. Here, we review the literature on the SLC15 transporters in teleost fish with emphasis on SLC15A1 (PEPT1), one of the solute carriers better studied amongst teleost fish because of its relevance in animal nutrition. We report on the operativity of the transporter, the molecular diversity, and multiplicity of structural–functional solutions of the teleost fish orthologs with respect to higher vertebrates, its relevance at the intersection of the alimentary and osmoregulative functions of the gut, its response under various physiological states and dietary solicitations, and its possible involvement in examples of total body plasticity, such as growth and compensatory growth. By a comparative approach, we also review the few studies in teleost fish on SLC15A2 (PEPT2), SLC15A4 (PHT1), and SLC15A3 (PHT2). By representing the contribution of teleost fish to the knowledge of the physiology of di/tripeptide transport and transporters, we aim to fill the gap between higher and lower vertebrates.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
The SLC family series in teleost fish
With ~30,000 living species, teleost fish (infraclass Teleostei; Near et al. 2012b) represent the largest and most diverse group of vertebrates (Nelson 2006). Their biodiversity is largely due to a number of peculiarities in their genomes (reviewed by Volff 2005; Ravi and Venkatesh 2008); for example, a whole-genome duplication during the evolution of the Actinopterygian lineage led to hundreds of duplicate gene pairs that have been maintained in teleost fish genomes over 300–450 million years, thus providing a unique framework for the diversification of gene functions, the generation of diversity and the speciation process. Because of their enormous genomic variability, teleost fish are now considered very attractive models for studying a variety of biological questions, included those related to the evolution of genes, of their functions and/or of their regulatory control in the wider context of tissue, organ, system, and organism physiology (see Kassahn et al. 2009; Sato et al. 2009).
In line with the classification of the human genes for passive, ion-coupled transporters and exchangers in the Solute Carrier (SLC) gene series (Hediger et al. 2004, 2013), teleost fish genes for SLC-type transporters have preliminarily been collected after data mining in the GenBank database (Dec 2010–Mar 2011) (Verri et al. 2012). Despite the incompleteness of the annotation process and the limited amount of sequence information in this public database, SLC members were retrieved from ~50 teleost fish species, with zebrafish (Danio rerio) being the most represented organism (more than 300 genes found), followed by Atlantic salmon (Salmo salar), rainbow trout (Onchorhynchus mykiss), fugu (alias torafugu; Takifugu rubripes), mefugu (Takifugu obscurus), and Japanese eel (Anguilla japonica), and with other teleost fish models popular in genetics, developmental biology and genomics, such as medaka (Oryzias latipes), three-spined stickleback (Gasterosteus aculeatus), and spotted green pufferfish (Tetraodon nigroviridis), or economically relevant in aquaculture, such as European sea bass (Dicentrarchus labrax) and Atlantic cod (Gadus morhua), being less represented. As expected, several SLC genes were found in duplicate. Among the SLC members classified, only 41 had been analysed functionally, i.e., the cloned transporters had been tested for transport in heterologous expression systems, such as Xenopus laevis oocytes (for details, see Romero et al. 1998; Bossi et al. 2007; Markovich 2008). These belong—according to He et al. (2009) who assembled the SLC families in 12 functional clusters based on the nature of transported substrates—to the groups of the ‘inorganic cation/anion transporters’ (15 members), ‘urea, neurotransmitters and biogenic amines, ammonium, and choline transporters’ (10 members), ‘glucose and other sugars transporters’ (6 members), ‘metal ion transporters’ (6 members), ‘bile salts and organic anions transporters’ (2 members), and ‘amino-acid/oligopeptide transporters’ (2 members). The functional studies were mainly conducted with the aim to define the role(s) of the transporters in the adaptation of teleost fish at different salinities (cation, anion, urea, and ammonium transporters) or oxygen (glucose and other sugar transporters) levels, to establish how the uptake of biologically important or toxic metal ions (such as copper, iron, and cadmium) affects teleost fish development, or to assess the role of amino acid and oligopeptide transport in teleost fish nutrition and growth. Many transporters were cloned from more than one species to compare their function in different teleost fish backgrounds. Again, zebrafish was the most popular model in functional studies on SLC proteins; and, due to the good annotation of its genes and genome, it has been used to extract sequences to generate/recruit/design suitable molecular tools for functional analysis in both zebrafish itself and other teleost fish species. More recently (Jul 2013), 275 official genes encoding zebrafish SLC proteins (belonging to 51 families) have been retrieved from GenBank, with more than 50 of them having formally been designated as duplicate genes (Romano et al. 2014). Noteworthy, since several genome projects on species highly relevant in aquaculture have recently been or are to be completed, a significant surge of nucleotide/protein information on SLC-type transporters from the same as above or other teleost fish species is expected to be released soon.
In this review, we will focus on the Solute Carrier 15 (SLC15) family of the so-called H+-coupled oligopeptide transporters (for reviews, see Daniel and Kottra 2004; Smith et al. 2013b), with special emphasis on SLC15 family member A1 (SLC15A1) alias Peptide Transporter 1 (PEPT1), a H+-dependent transporter of di- and tripeptides (di/tripeptides) deeply studied in human and mammalian models, and to date representing one of the most well-characterised transporters also in teleost fish mainly due to its major role in protein absorption, nutrition, and possibly body growth. Because of the virtually complete lack of information, at least to our knowledge, on the putative role of PEPT1 as a nutrient sensor in teleost fish gut, updates on this topic will not be included in this discussion (nonetheless, for the most recent advances, excellent topical reviews are available; see Rønnestad et al. 2014; Zietek and Daniel 2015; Daniel and Zietek 2015). In addition, where available information will be provided for the other four members of the SLC15 family recognised to date among vertebrates, namely, SLC15 family member A2 (SLC15A2) alias Peptide Transporter 2 (PEPT2), SLC15 family member A3 (SLC15A3) alias Peptide/Histidine Transporter 2 (PHT2), SLC15 family member A4 (SLC15A4) alias Peptide/Histidine Transporter 1 (PHT1), and SLC15 family member A5 (SLC15A5), which have been much less studied than PEPT1 in teleost fish.
The H+-coupled oligopeptide transporter SLC15A1 (PEPT1) in teleost fish
In the next paragraphs, we report information on PEPT1, its (major) role(s) in gut physiology and localisation along (and outside) the gut, and conceptually organising the material around general topics: from molecular to cellular physiology, from tissue/organ physiology to the description of its potential role(s) and relevance at higher levels of biological organisation, and/or in more integrative, systemic, and applied frameworks. Whenever possible, we have made the teleost fish to meet the tetrapods data, thus aiming to provide a synopsis along the vertebrate series.
Major role of PEPT1 in teleost fish digestive/absorptive physiology
During the digestive process, hydrolysis of dietary proteins leads to high levels of di/tripeptides in the intestinal lumen, which is either further hydrolysed to their constituent amino acids or directly taken up in intact form into the intestinal epithelial cells. Following the apical influx, di/tripeptides are hydrolysed in the cytosol and the resulting amino acids cross the basolateral membrane using amino-acid-transporting systems. However, peptides not undergoing hydrolysis exit the cell by (a) not yet molecularly identified basolateral peptide transport system(s) (possibly responsible for both cell-to-blood efflux and blood-to-cell uptake; see Dyer et al. 1990; Thwaites et al. 1993a, b; Thamotharan et al. 1996a, b; Terada et al. 1999; Irie et al. 2004; Pieri et al. 2010; Berthelsen et al. 2013) and/or by other basolateral solute transporters that have been shown to allow transport of selected peptides (Daniel 2004).
At the apical membrane of the enterocytes, the transport of di/tripeptides is entirely mediated by PEPT1, which operates as a Na+-independent and H+-dependent transporter of a vast number of di/tripeptides, but not tetrapeptides or single free amino acids. Di/tripeptide transport is electrogenic and responds to both an inwardly directed transmembrane H+ gradient and a transmembrane internal negative electrical potential (Daniel 2004). Transport is enantio-selective and apparently involves a variable H+/substrate stoichiometry for uptake of neutral and charged peptides. PEPT1 is also responsible for the transport of orally active drugs, including β-lactam antibiotics, aminopeptidase and angiotensin-converting enzyme inhibitors, δ-aminolevulinic acid, and selected (e.g., antiviral) pro-drugs (Rubio-Aliaga and Daniel 2002, 2008; Brandsch 2013; Smith et al. 2013b), although simple interaction without transport also occurs for a variety of molecules (see Knütter et al. 2008; Brandsch 2009). Notably, formylmethionyl-leucyl-phenylalanine (fMLP), a major N-formylated peptide from bacteria in the human colonic lumen (Marasco et al. 1984; Chadwick et al. 1988) that contributes to intestinal inflammation and inflammatory bowel disease (IBD) progression (for review, see Adibi 2003), is another recognised substrate of PEPT1 (see Buyse et al. 2002b; Shi et al. 2006; Ingersoll et al. 2012; Wu and Smith 2013). In addition, PEPT1 mediates the transport of the bacterial proteoglycan-derived muramyl dipeptide (Vavricka et al. 2004; Ismair et al. 2006; Ma et al. 2015) and proinflammatory bacterial peptidoglycan-derived tripeptide l-Ala-γ-d-Glu-meso-diaminopimelic acid (Dalmasso et al. 2010).
In teleost fish, the evidence that intestinal di/tripeptide absorption occurs was first obtained by comparing in vivo the rates of intestinal absorption of Gly–Gly and Gly in rainbow trout (Bogé et al. 1981). However, the description of the causal mechanisms of di/tripeptide transport across plasma membranes was provided only 10 years later, when the transport of a dipeptide was shown in brush-border membrane (BBM) vesicles (BBMV) of the Mozambique tilapia (Oreochromis mossambicus) intestinal epithelial cells by testing uptake of Gly–Phe vs. Phe (Reshkin and Ahearn 1991). The indication that dipeptides are effectively co-transported with H+ was soon after provided showing Gly–Gly–dependent intravesicular acidification in the European eel (Anguilla anguilla) intestinal BBMV (Verri et al. 1992), which is acknowledged as the first experimental evidence of occurrence of the dipeptide-induced intracellular acidification event in the enterocyte (see Thwaites et al. 1993a, b, c). Since then, the existence of a carrier-mediated, H+-dependent transport of di/tripeptides in the BBM of fish enterocytes was confirmed in many teleost fish and detailed kinetics and substrate specificities of the transport activity were provided (see Maffia et al. 1997; Verri et al. 2000; reviewed by Verri et al. 2010). In 2003, the first peptide transporter from a teleost fish, i.e., the zebrafish PEPT1, was cloned and functionally characterised in X. laevis oocytes as a low-affinity/high-capacity system (Verri et al. 2003), with apparent substrate affinities in the 0.1–10 mM range depending on the structure, nature, and net charge at a certain pH of the tested peptides. Since then, cloning and functional characterisation of PEPT1 orthologs from other teleost fish has been achieved (Table 1). For three of them, i.e., the European sea bass, the Atlantic salmon, and the Antarctic icefish (Chionodraco hamatus), detailed kinetic analysis in X. laevis oocytes has been carried out, thus providing the basis for functional comparison among teleost fish orthologs, and among teleost fish and higher vertebrates (see the “Multiplicity of molecular structural–functional solutions in teleost fish PEPT1 proteins”). Notably, with increasing the number of sequences in the databanks, it was progressively evident that teleost fish PEPT1 is duplicated, as initially described in the Oriental weatherfish (alias Asian weatherloach) (Misgurnus anguillicaudatus) by Gonçalves and colleagues (Gonçalves et al. 2007), and thus, the concept that PEPT1A (alias SLC15A1A) and PEPT1B (alias SLC15A1B) genes occur in teleost fish genomes has fully emerged (for details, see also Romano et al. 2014). However, to date, transport data are available from PEPT1B transporters only (still named PEPT1 in many current papers) (Table 1), and the demonstration that PEPT1A-type proteins are functional is still lacking.
Organ/tissue distribution of PEPT1 in teleost fish
In mammals, PEPT1 is abundantly expressed in the epithelial cells from small intestine (duodenum, jejunum, and ileum) and kidney (proximal tubule S1 segments) and to a lesser extent in the pancreas, bile duct, and liver (for review, see Daniel 2004; Daniel and Kottra 2004; Gilbert et al. 2008b; Smith et al. 2013b). In general, no or little expression is observed in the colon, but it is unequivocally estimated that PEPT1 is expressed in the healthy distal colonic epithelium of mice, rats, and humans, where the protein is functional and contributes physiologically to electrolyte and water handling (Wuensch et al. 2013). Recently, functional expression of PEPT1 has also been observed in the nasal epithelium (Agu et al. 2011). In contrast to most species, ruminants express PEPT1 in the stomach (omasum and rumen) (Chen et al. 1999; Pan et al. 2001; Gilbert et al. 2008b). In birds, there is significant expression of PEPT1 in the ceca in addition to the small intestine and the kidney (Chen et al. 2002; Gilbert et al. 2008b). In elasmobranchs, recent data indicate PEPT1 expression in multiple components, i.e., esophagus, stomach, duodenum, scroll valve intestine, rectum, and pancreatic acinar cells, of the bonnethead shark (Sphyrna tiburo) digestive tract (Hart et al. 2016).
In teleost fish, PEPT1 is principally expressed in the intestine, and found to very low extent in other organs/tissues, including kidney, liver, spleen, and others (Table 2). The analysis of mRNA expression along the gut clearly indicates that PEPT1 is restricted to the intestine (Verri et al. 2010). However, patterns of expression greatly differ from species to species (Table 2). In fact, while in zebrafish, carps, weatherloaches (ord. Cypriniformes), and pufferfish (ord. Tetraodontiformes) PEPT1 seems to be confined to the most proximal portion(s) of the intestine, in cods (ord. Gadiformes) and killifish (alias mummichogs) (ord. Cyprinodontiformes) PEPT1 is almost uniformly distributed along the intestinal canal, most distal regions included. In between these extremes, salmons and trouts (ord. Salmoniformes) exhibit a steady decrease in PEPT1 expression passing from proximal-to-distal adjacent segments of the intestinal canal, while in sea basses, sea breams, and icefishes (ord. Perciformes), as well as turbots (ord. Pleuronectiformes), the proximal-to-distal drop of expression along the post-gastric alimentary canal seems steeper than in salmonids. This last assumption holds true also for tilapias (ord. Cichliformes). On the other side, in the Japanese eel larvae, PEPT1 expression in posterior intestine is much higher than in the preceding (anterior intestine) and following (rectum) segments. Whenever present, the pyloric ceca invariably expresses PEPT1 at very high levels, which suggests that this organ specialisation largely participates in the absorption of the di/tripeptides arising from dietary proteins. Interestingly, the spatio-temporal expression of PEPT1 intestinal mRNA largely varies during ontogeny (see Verri et al. 2003; Amberg et al. 2008; Ahn et al. 2013; Liu et al. 2013b, 2014), in response to nutritional states (such as food deprivation/refeeding; see Rønnestad et al. 2010; Hakim et al. 2009; Terova et al. 2009; Bucking and Schulte 2012; Koven and Schulte 2012; Liu et al. 2013a, b; reviewed by Verri et al. 2011), dietary challenges (see Gonçalves et al. 2007; Bakke et al. 2010; Ostaszewska et al. 2010a, b; Kwasek et al. 2012; Liu et al. 2013b; Kamalam et al. 2013; Ostaszewska et al. 2013; Terova et al. 2013), and/or environmental conditions (such as in freshwater/seawater adaptation; see Kalujnaia et al. 2007; Bucking and Schulte 2012), as well as under certain disease states (such as gut inflammation; see Frøystad-Saugen et al. 2009; Wang et al. 2013) (for a more detailed discussion on some of these dynamic aspects of PEPT1 expression, see the “Is there in teleost fish a role for PEPT1 in salt, water and/or acid–base homeostasis?” and “Is the intestinal transporter PEPT1 relevant for teleost fish growth?”).
After the first paper in which mRNA expression of PEPT1A and PEPT1B has contemporarily been detected in the mummichog (Fundulus heteroclitus macrolepidotus) (Bucking and Schulte 2012) and a more recent paper in which the first systematic comparative analysis of all the SLC15 family members in the same teleost fish background has been reported for the Nile tilapia (Oreochromis niloticus) (Huang et al. 2015), the earlier perception that PEPT1 mRNA expression data reflect the levels of expression of a single gene (implicitely meaning PEPT1B) has been replaced by the view that in teleost fish, two orthologous PEPT1 transporters may contemporarily be expressed and operate at the intestinal level (Table 2). As stated above, to date, functional analysis has not yet been performed for any known PEPT1A-type cloned transporters. However, the observation that, conversely to what was observed in the mummichog, in the Nile tilapia: (a) the expression of PEPT1A mRNA largely exceeds that of PEPT1B in the proximal intestine; (b) instead, the expression of PEPT1B mRNA exceeds that of PEPT1A in the mid intestine; and (c) thus, the PEPT1A-to-PEPT1B ratio inverts passing from proximal to mid intestine, suggests a role for PEPT1A in this species with respect to and in association with PEPT1B, with PEPT1A principally working in the proximal and PEPT1B in the mid part of the intestine. Since sequence differences (62–69% similarity at the protein level) exist between these two orthologs, it can be argued that they act synergistically to achieve optimal absorption of protein degradation products, but functions other than protein degradation products’ absorption cannot be excluded. In this respect, identification of similarities/differences in the transport function of the translated proteins is needed. Furthermore, these observations ask for a re-evaluation/re-assessment of the previous PEPT1 mRNA expression data in teleost fish in the new light of the specificity of the molecular tools used to perform the analyses, e.g., the primer set(s) for PCR analyses in the various species.
Another limit of the current expression studies on PEPT1-type transporters in teleost fish is the lack of adequate information at the protein level. Protein expression data are available for a few species, the first coming from a study in the orange-spotted grouper (Epinephelus coioides) using a commercial polyclonal rabbit anti-PEPT1 antibody (Yuen et al. 2007). In the proximal intestine of the juvenile orange-spotted grouper, PEPT1 protein is found constitutively expressed along the apical membrane of the intestinal mucosal cells. Expression is restricted to absorptive epithelial cells and it is more evident in enterocytes migrating towards the tips of the mucosal folds, whereas the mucus-secreting goblet cells are negative for PEPT1. Using the same commercial antibody, PEPT1 protein has been detected in rainbow trout (Ostaszewska et al. 2010b), common carp (Cyprinus carpio) (Ostaszewska et al. 2010a), and yellow perch (Perca flavescens) (Ostaszewska et al. 2013), with similar results as those in the orange-spotted grouper intestine. Thus, such as in higher vertebrates (Daniel 2004), the PEPT1 protein strongly associates with differentiated and mature absorptive enterocytes. More recently, a systematic depiction of PEPT1 protein expression at the intestinal level has been provided for the Japanese eel (Ahn et al. 2013), the spotted green pufferfish (Wang et al. 2013), and the red crucian carp (Carassius auratus) (Liu et al. 2014) using species-specific antibodies developed ad hoc. Such analyses virtually confirm the previous data on PEPT1 protein expression at the intestinal level obtained using the heterologous antibodies.
To recapitulate, in any fish species tested, PEPT1-type mRNA is predominantly expressed at the intestinal level, in the portion of the intestinal tract that is directly involved in digestion and absorption. Where competition for different functions occurs (e.g., in the Asian weatherloach that uses the hindgut as an accessory air-breathing organ), PEPT1 marks the portion(s) of the intestine committed to digestion and absorption. Expression is almost invariably restricted to the BBM of mature enterocytes, which makes PEPT1 a specific marker of terminal enterocyte differentiation (see Chen et al. 2009; Li et al. 2011a; Wang et al. 2013), although exceptions are observed, such as in the Japanese eel, where PEPT1 is also detected in the intracellular area of the epithelial cells of the intestinal tract, especially at the larval stage (see Ahn et al. 2013). Expression in other tissues is always much lower than in the post-gastric alimentary canal. The functional role of PEPT1 in such other tissues is not known in teleost fish yet. Noteworthy, disclosing the local expression of PEPT1A vs. PEPT1B along the intestinal tract of teleost fish as well as their distribution in the various tissues/organs of the many teleost fish models studied is now an obligatory step towards the full comprehension of the physiological role of such transporters. Finally, the enormous plasticity of PEPT1 expression at the intestinal level in teleost fish, as it results from the dynamic changes observed with ontogeny, dietary challenges, and disease states, parallels what was observed in the mammalian intestine (Daniel 2004; Daniel and Kottra 2004; Smith et al. 2013b), thus suiting the telost fish model for studies on the general aspects of mammalian (and human) biology, as it is the recent case of the development of a pufferfish model of intestinal inflammation finalised to the dissection of the molecular bases of IBD (Wang et al. 2013).
Multiplicity of molecular structural–functional solutions in teleost fish PEPT1 proteins
When the functional properties of the expressed transporters are detailed in terms of kinetic parameters, substrate specificities, and inhibition patterns, sensitivity to physicochemical parameters (pH, temperature, etc), teleost fish PEPT1 regularly exhibits an unexpected multiplicity of structural–functional solutions with respect to the higher vertebrate orthologs, which possibly reflects forms of physiological, ecological, and/or environmental adaptations. Some of the solutions found in PEPT1 transporters from a number of teleost fish species are reviewed below, and treated, whenever possible, in comparison with the human (Daniel and Kottra 2004; Smith et al. 2013b), mammalian (Daniel 2004), and avian (Gilbert et al. 2008a) counterparts.
pH dependence
In higher vertebrates, PEPT1 function has been studied in several mammalian and avian species, including human, rat, mouse, rabbit, sheep, pig, chicken, and turkey. In these species, transport via PEPT1 is reported to be pH dependent, with a slightly acidic extracellular pH (pH optimum 5.5–6.0) positively affecting the uptake of the transported substrate. In general, in mammalian PEPT1 proteins—that have been studied under a large variety of functional assays and experimental conditions—this effect correlates to a substantial increase of the apparent affinity for the substrate passing from alkaline to neutral-to-acidic external pH, with no considerable change in maximal transport rate; however, in human PEPT1, acidic pH increases both substrate apparent affinity and maximal transport rate (reviewed by, e.g., Daniel 2004; Daniel and Kottra 2004; Gilbert et al. 2008b; Smith et al. 2013b). After first zebrafish PEPT1 functional analysis (Verri et al. 2003), it was clear that many basic kinetic properties of the transporter were similar to those observed in higher vertebrates, e.g., the apparent affinity increases with decreasing pH. However, in contrast to higher vertebrates, zebrafish PEPT1 maximal transport rate steeply increased passing from acidic (6.5) to alkaline (8.5) extracellular pH. This evidence has been postulated to correlate to two feasible and complementary scenarios. On one hand, to the agastric state of the zebrafish (which is a freshwater teleost fish naturally inhabiting slow-moving or stagnant water bodies), and thus to the resulting prevalence of an alkaline pH (pH ≥ 7.5; see Nalbant et al. 1999; Verri et al. 2003; Wood et al. 2010) in the proximal intestinal lumen under normal physiological conditions, due to pancreas and gallbladder alkaline secretions not neutralised by stomach acidic secretions (for a discussion on the agastric status in teleost fish, see Horn et al. 2006; Day et al. 2011; Yúfera et al. 2012; Castro et al. 2013; for a recent review, see Grosell et al. 2011 and literature cited therein). On the other hand, to the most likely lack in the zebrafish enterocyte of the functional interaction between H+-dependent di/tripeptide cotransport and Na+/H+ exchange activities, which is opposite to what occurs in, e.g., the mammalian enterocyte with the duo PEPT1 and Na+/H+ exchanger 3 (NHE3), alias Solute Carrier 9 (SLC9) family member A3 (SLC9A3) (see Lucas et al. 1975; Kennedy et al. 2002; for review, see Thwaites and Anderson 2007), and the nematode Caenorhabditis elegans enterocyte with the duo Peptide transporter family 1 (PEPT-1) and Na+/H+ exchanger protein 2 (NHX-2) (see Spanier et al. 2009; Benner et al. 2011; for review, see Spanier 2014). In fact, in both systems, the functional cotransport/exchange interaction that exists at the apical membrane of the enterocyte locally generates an acidic microclimate and an inwardly directed pH gradient, which allows optimal uptake of di/tripeptides and concurrent prevention of extensive intracellular acidification. For a more detailed discussion on these functional interactions between di/tripeptide transporters and ion exchangers, see the “PEPT1 and ion exchangers, carbonic anhydrases and ion cotransporters”). The study on the PEPT1 of the European sea bass (that is, a primarily ocean-going teleost fish that sometimes enters brackish and fresh waters), which possesses a stomach, has confirmed the canonical increase in apparent substrate affinity at acidic pH, but—such as in mammals and not in the zebrafish—no significant effect of pH is found on the maximal transport rate of this transporter (Sangaletti et al. 2009). This similarity of the intestinal European sea bass transporters to the intestinal mammalian systems is further corroborated by the observation that the European sea bass Solute Carrier 6 (SLC6) family member A19 (SLC6A19, alias B0AT1), a neutral amino-acid transporter at the apical membrane of the enterocyte, displays a pH dependence that virtually mimics that of the mouse B0AT1 (Margheritis et al. 2013a). Interestingly, the PEPT1 of the Atlantic salmon (that is, a teleost fish following an anadromous migration pattern after years-long freshwater phases) exhibits only a slight increase in maximal transport rate passing from acidic to alkaline extracellular pH (Rønnestad et al. 2010), while the PEPT1 of the Antarctic icefish (which is a strictly seawater-confined teleost fish) displays virtually no pH dependence of the maximal transport rate (Rizzello et al. 2013), suggesting that in these two gastric teleost fish species, PEPT1 transporters conform more to the mammalian than the zebrafish paradigm. All together, these results—based on the comparative functional analysis of four teleost fish vs. higher vertebrate (mainly rabbit, but also human, rodent, pig, and bird) proteins—indicate a relevant molecular diversity among PEPT1 proteins, which result in relevant differences in certain measurable traits with a significant impact on the anatomical and physiological designs. This is the case of the ‘pH dependence of maximal transport rate’ trait, which in the zebrafish PEPT1, much departs from the ‘canonical’ function depicted for mammalian and the other teleost fish PEPT1 transporters tested so far.
Within this “pH dependence” framework, using a combination of advanced electrophysiological and biophysical protocols, Renna and colleagues (Renna et al. 2011b) have compared the same experimental setup and the transport kinetics of rabbit, European sea bass, and zebrafish PEPT1, proposing a unified model that explains their principal electrophysiological properties and different behaviours in terms of pH dependence. This model suggests a dual role for H+ in the operational mechanism of PEPT1. On the one hand, H+ are considered essential for neutralising the transporter during the inward substrate translocation and their release in the cytosol uncovers the net negative charge of the empty transporter, which then undergoes an energy-dissipating step to return to the outward-facing conformation (for a schematic description of the model, see Renna et al. 2011b). On the other hand, protonation of the transporter breaks the transport cycle and counteracts the boosting effects of external acidity on the turnover rate. A different balance between the two roles played by H+ may generate opposite effects on the maximal transport rate, as observed experimentally in the various vertebrate PEPT1 proteins kinetically analysed in detail (Mackenzie et al. 1996; Amasheh et al. 1997; Nussberger et al. 1997; Steel et al. 1997; Kottra and Daniel 2001; Kottra et al. 2002; Irie et al. 2005; Fujisawa et al. 2006; Sala-Rabanal et al. 2006; Sangaletti et al. 2009; Renna et al. 2011b). The existence of two apparently contrasting actions of external H+ does not negatively impact the overall efficiency of the substrate uptake, and the model can explain how PEPT1 sustains its function across different species and at the expected physiological pH conditions. Noteworthy, the model includes a transition state that may represent an H+ binding to an allosteric site (Renna et al. 2011b), which has been suggested to account for the smaller transport current observed at acidic pH in the zebrafish PEPT1 (Verri et al. 2003). However, in spite of the research performed for the last years, this putative allosteric site still remains elusive.
Temperature dependence
Adaptation of organisms to temperature requires proteins working at optimal thermodynamic conditions. This is especially true for membrane transporters, such as the intestinal PEPT1, that maintain nutrient homeostasis at both cellular and organism level. Like enzymes, the catalytic activity of membrane transporters depends on temperature (see Hazama et al. 1997; Beckman and Quick 2001; Binda et al. 2002; Karakossian et al. 2005; Takanaga et al. 2005; Bacconi et al. 2007; Mackenzie et al. 2007; Kukk et al. 2015). In such proteins, higher temperatures speed up all the reactions of the transport mechanism (i.e., the cycle of serial reactions), and with increasing temperature, an increase in transport rate, and thus in substrate uptake, is expected and effectively observed in many cases (see Beckman and Quick 2001; Hilgemann and Lu 1999; Wadiche and Kavanaugh 1998). Moreover, differences in kinetics are expected in orthologous transporters of species living at different temperatures; this is also observed for many transporters (see Maffia et al. 1996; Xue et al. 1999; Elias et al. 2001; Marshall et al. 2002; Maffia et al. 2003), such differences appearing strictly adaptive.
In PEPT1, the effects of temperature on the functional properties of the transporter have been investigated in detail using X. laevis oocytes in combination with electrophysiology and model substrates, such as the dipeptide Gly–Gln. When the rabbit (homeotherm with body temperature of 38–39 °C) PEPT1 is studied with respect to the zebrafish (tropical poikilotherm living at 18–24 °C) and the European sea bass (subtropical poikilotherm living at 8–24 °C) at a reference temperature (22 °C), marked differences are found in the transport kinetics of the proteins. In particular, at 22 °C, the two teleost fish proteins show similar kinetics, while the rabbit exhibits significantly slower kinetics in comparison with teleost fish. However, at values (30 °C) closer to the rabbit body temperature, the properties of the rabbit PEPT1 are more similar to those of the teleost fish (Bossi et al. 2012), suggesting that there may be no differences among species when the transporters work at their respective physiological conditions and that the differences in kinetics found in rabbit and teleost fish denote adaptive changes.
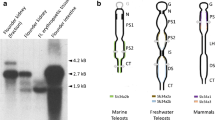
Needless to say, structural differences exist between mammalian and teleost fish proteins that may confer enhanced flexibility in the latter to compensate for the lower thermal kinetic energy available at lower temperatures. In general, in proteins, increased flexibility is achieved through single-site amino-acid substitutions in those protein regions that undergo large movements during the catalytic cycle (see Fields 2001; Somero 2004). However, in the PEPT1 of Chionodraco hamatus (Antarctic icefish; a polar poikilotherm living at −1.9 °C), a domain composed of one-to-six repeats of seven amino acids (VDMSRKS), placed as an extra stretch in the cytosolic COOH-terminal region of the transporter, and contributes in part but per se to cold adaptation (Rizzello et al. 2013). VDMSRKS is in a region not involved in transport activity, and, when transferred to the COOH terminus of rabbit (warm-adapted) PEPT1, it confers cold adaptation to the protein (Rizzello et al. 2013). To our knowledge, this strategy based on the inclusion of a new functional domain is unique among those known in proteins from psychrophilic vertebrates (see Feller and Gerday 2003; Somero 2003, 2004; Coppes Petricorena and Somero 2007; Pörtner et al. 2007; Beers and Jayasundara 2015). The domain is present not only in the Antarctic icefish (fam. Channichthyidae), but also found as VEMSRKS in another species of the sub-order Notothenioidei (notothenioids) (ord. Perciformes; for a recent classification of teleost fishes, see Near et al. 2012b; Betancur-R et al. 2013), i.e., the barbeled plunderfish Histiodraco velifer (fam. Artedidraconidae), as VEMSRKD in other two species of the same sub-order, i.e., Trematomus bernacchii (emerald rockcod) and T. pennellii (sharp-spined notothen) (fam. Nototheniidae) (Rizzello et al. 2013), and as LEMSRKS in a third member of Nototheniidae, i.e., Notothenia coriiceps (black rockcod alias Antarctic bullhead notothen), which genome has recently been sequenced (Shin et al. 2014) (Fig. 1). All these notothenioids live in the Antarctic waters. Conversely, the motif, and within it the central serine of a ‘putative’ (V/L)(D/E)MSRK(S/D) phosphorylation site (Antonia Rizzello and Alessandro Romano, preliminary results), is altered or fragmented in other Perciformes, such as Perca flavescens (yellow perch) (fam. Percidae), Epinephelus aeneus (white grouper) (fam. Serranidae), and Gasterosteus aculeatus (three-spined stickleback) (fam. Gasterosteidae), as well as in the neighbour species of the series Percomorpharia for which accurate PEPT1 COOH-terminus sequence annotations are available in the databanks, among which the European sea bass (fam. Moronidae) and Larimichthys crocea (large yellow croaker) (fam. Sciaenidae) (Fig. 1). Waiting for countercheck when PEPT1 sequences from cold temperate (sub-Antarctic and/or non-Antarctic) notothenioids will be available, these findings support the concept that the acquisition of the (V/L)(D/E)MSRK(S/D) domain dates back to at least the most recent common ancestor of the so-called ‘Antarctic clade’ (Near et al. 2012a), thus paralleling the most probable evolutionary origin of the antifreeze glycoproteins (AFGPs). In this context, the domain may represent a novel molecular marker of the evolutionary biological and eco-physiological diversification of the Antarctic fishes. In addition, since transferable to other proteins, the domain may represent a very useful molecular tool for future biotechnological applications.
Amino-acid sequence alignment of the COOH terminus of channichthyidae (the Antarctic icefish Chionodraco hamatus), artedidraconidae (the barbeled plunderfish Histiodraco velifer), nototheniidae (the black rockcod alias Antarctic bullhead nothoten Notothenia coriiceps, the emerald rockcod Trematomus bernacchii and the sharp-spined notothen Trematomus pennellii), percidae (the yellow perch Perca flavescens), serranidae (the white grouper Epinephelus aeneus), gasterosteidae (the three-spined stickleback Gasterosteus aculeatus), moronidae (the European sea bass Dicentrarchus labrax) and sciaenidae (the large yellow croaker Larimichthys crocea) PEPT1 proteins. For phylogenetic relationships among species, (see Near et al. 2012b; Betancur-R et al. 2013). Multiple sequence alignment was generated by ClustalW2 at http://www.ebi.ac.uk/Tools/msa/clustalw2/ using default parameters. TMD XII indicates the putative 12th transmembrane domain of the vertebrate PEPT1 proteins as predicted by TMHMM 2.0 (TMHMM Server v. 2.0 at www.cbs.dtu.dk/services/TMHMM-2.0/); the direct heptad repeats (VDMSRKS) close to the COOH-terminus of the Antarctic icefish are in bold and named D1-to-D6. Six, four, three, two, and one repeats have been found in PEPT1 sequences from different Antarctic icefish specimens (Rizzello et al. 2013); all of them represented in the alignment. Identical residues are highlighted in black and similar residues in gray (data generated at http://www.bioinformatics.org/sms2/color_align_cons.html; percentage of sequences that must agree for identity or similarity coloring to be added: 100%)
Species specificity of substrate uptake
It has long been known that teleost fish rely on a dietary supply of a well-balanced profile of essential (indispensable), non-essential (dispensable), and conditionally essential amino acids (for reviews, see Wilson 2002; Li et al. 2009). Among others (for a recent comprehensive review, see National Research Council 2011), the essential amino acids, lysine (Lys) and methionine (Met), are known to be highly growth limiting for teleost fish, and countless studies have shown that diets deficient in such amino acids may result in poor growth performances (reviewed among others by, e.g., Conceição et al. 2003, 2007; Glencross 2006; Kousoulaki et al. 2015). In particular, Lys is considered one of the most highly restrictive amino acids during preparation of fish feeds today (see Harris 1980; Tibaldi and Lanari 1991; Forster and Ogata 1998; Small and Soares 2000; Wang et al. 2005; Mai et al. 2006b; for recent reviews, see Nunes et al. 2014) and Lys deficiency invariably occurs when plant-based protein sources are used to replace fish meal (see Gaylord et al. 2004; Zhang et al. 2008; Deng et al. 2010; for recent reviews, see Conceição et al. 2012; Nunes et al. 2014; Kousoulaki et al. 2015). Lys availability is, e.g., reported to limit protein synthesis, protein accretion and growth, and in general to impair teleost fish metabolism (see Walton et al. 1984; Conceição et al. 2007; Espe et al. 2007; Li et al. 2014; reviewed among others by, e.g., Conceição et al. 2003, 2012; Ball et al. 2007). Analogously, Met is recognised by many authors as the most limiting amino acid in teleost fish diets (see Alam et al. 2000; Twibell et al. 2000; Ahmed et al. 2003; Luo et al. 2005; Mai et al. 2006a; Zhou et al. 2006; for recent reviews, see Conceição et al. 2012; Nunes et al. 2014), and like Lys, it is very critical in the preparation of feeds containing protein sources alternative for fish meal (see Ai and Xie 2005; Nguyen and Davis 2009; Sardar et al. 2009; Tulli et al. 2010; Figueiredo-Silva et al. 2015; for recent reviews, see Conceição et al. 2012; Nunes et al. 2014; Kousoulaki et al. 2015). Met availability is known to affect several biological functions, e.g., it greatly influences the biosynthetic pathways involving sulphur-based compounds, and more generally, it is known to impact teleost fish metabolism (see Walton et al. 1982; Kasper et al. 2000; Adibi and Khan 2011; Deng et al. 2011; Kuang et al. 2012; Duan et al. 2012; Ma et al. 2013; Wang et al. 2014a; reviewed among others by Conceição et al. 2012).
For their central role in fish nutrition, developing new strategies for the supplementation of essential and/or conditionally essential amino acids is considered crucial to date in an attempt to deal with the nutritional challenges posed by the need of feeding cultured fish with optimised artificial diets (Li et al. 2009). In this respect, being the route for the intake of di/tripeptides, PEPT1 has received considerable attention as a target for delivery of essential amino acids—Lys and Met in primis—in the form of di/tripeptides, and Lys- and, more recently, Met-containing di/tripeptides have been in the focus of advanced studies to elucidate the basic properties of their carrier-mediated transport. This with the confidence that information coming from the observed kinetics would have been beneficial to the formulation of new diets containing peptide hydrolysates or selected individual peptides rather than free amino acids (see Zambonino-Infante et al. 1997; Dabrowski et al. 2003, 2005; Aragão et al. 2004; Zhang et al. 2006; Rønnestad et al. 2007b) (for specific discussion on this subject, see the “Is the intestinal transporter PEPT1 relevant for teleost fish growth?”). The results of these efforts are summarised in Table 1, which reports the basic kinetic parameters—i.e., the maximal di/tripeptide-dependent inwardly directed transport current (I max) and the apparent concentration of di/tripeptide that yields one-half of I max (K 0.5)—as assessed for four teleost fish PEPT1 clones using the X. laevis oocytes’ heterologous expression system and the two-electrode voltage clamp (TEVC) electrophysiological setup. In teleost fish, the first evidence that Lys-containing peptides are transported by PEPT1 is from the initial observations in the zebrafish (namely, two Lys-containing dipeptides tested, with Lys–Gly resulting a better substrate than Gly–Lys; reference peptide: Gly–Gln; reference pH values: 7.5 and 8.5; for details, see Verri et al. 2010) and a more systematic analysis in the Atlantic salmon [namely, six Lys-containing dipeptides and a Lys-containing tripeptide tested, exhibiting the following relative scale of apparent binding affinity (expressed as K0.5 values): Lys–Pro ≈ Lys–Val > Ala–Lys > Lys–Pro-Val (good substrates) > Glu–Lys > Lys–Glu (intermediate substrates) ≫ Arg–Lys (non/poor substrate); reference peptide: Gly–Sar; reference pH: 6.5; for details, see Rønnestad et al. 2010]. Interestingly, the tripeptide Lys–Pro–Val, which in mammals exhibits strong anti-inflammatory effects at the intestinal mucosa after uptake via PEPT1 (Dalmasso et al. 2008a), is a good substrate of the Atlantic salmon transporter, which supports the notion that PEPT1 may play a role not only in fish nutrition, but also in the treatment of fish enteritis and associated inflammation (Rønnestad et al. 2010). In the same trial, other di/tripeptides containing amino acids other than Lys and known to be essential for the Atlantic salmon have been tested, showing that di/tripeptides containing phenylalanine (Phe–Phe, Phe–Tyr), leucine (Gly–Leu, Pro–Leu), histidine (β-Ala-His, alias carnosine), valine (Val–Pro–Pro), and isoleucine (Ile–Pro–Pro) are all transported, with Phe-containing di/tripeptides resulting by far the best among the di/tripeptides studied, and the others behaving as good-to-intermediate substrates. As expected (Vig et al. 2006), Pro–Gly is not a substrate of the Atlantic salmon PEPT1 (Rønnestad et al. 2010).
More recently, the kinetic properties of the European sea bass, zebrafish, and rabbit PEPT1 have systematically been studied by TEVC to assess the specificities of the uptake for a group of di/tripeptides containing Gly, Lys, and Met (namely, Lys–Lys, Met–Lys, Lys–Gly, Gly–Lys, Lys–Met, and Lys–Lys–Lys; reference peptide: Gly–Gln; reference pH: 7.5) in different PEPT1 orthologs under identical experimental conditions (for details, see Margheritis et al. 2013b). Interestingly, substantial species-specific differences are observed for the three PEPT1 clones in response to the series of di/tripeptides. In a first set of experiments, it has been found that with respect to Gly–Gln, the transport currents produced by the dipeptide couples Gly–Lys and Lys–Gly (on one hand) and Met–Lys and Lys–Met (on the other hand) rank differently in the sea bass and rabbit PEPT1 (with Lys–Gly > Gly–Gln > Gly–Lys and Lys–Met > Gly–Gln ≈ Met–Lys) and the zebrafish PEPT1 (with Gly–Gln > Lys–Gly > Gly–Lys and Gly–Gln ≈ Lys–Met ≈ Met–Lys). While the former scale also adapts to the Antarctic icefish PEPT1 (Rizzello et al. 2013), the latter is in accordance with the initial observations in zebrafish PEPT1 (Verri et al. 2010). Similarly, to what found with the pH dependence analysis (see the “pH dependence”), these direct substrate transport data indicate that the European sea bass performs more like the rabbit than the zebrafish PEPT1. In addition, in the European sea bass, all tested dipeptides produce similar overall transport activities independently of the charge position or amino-acid composition of the peptide, with the exception of Lys–Lys which is much less well transported (i.e., Lys–Met ≈ Met–Lys ≈ Gly–Gln ≈ Lys–Gly > Lys–Lys; Lys–Lys–Lys not transported), while in the zebrafish, Lys–Met and Met–Lys are better substrates in the di/tripeptide series tested (with Lys–Met ≈ Met–Lys > Gly–Gln > Lys–Gly > Lys–Lys; Lys–Lys–Lys not transported). Interestingly, Atlantic salmon PEPT1 conforms more to the European sea bass than the zebrafish model, since when tested in the Atlantic salmon PEPT1, Arg–Lys (that is structurally similar to Lys–Lys) is at the lowest level of the transport efficiency scale (Rønnestad et al. 2010). Finally, in the European sea bass and rabbit PEPT1, the transport currents elicited by Gly–Lys, Lys–Gly, Met–Lys, and Lys–Met are invariably independent on the external pH (pH 6.5 compared to pH 7.5), while in zebrafish, they are always pH dependent, with alkalinisation exerting an activation role (see also the “pH dependence”).
Interestingly, the definition of the peculiar features of the rabbit/sea bass vs. zebrafish transporter and their combination with the more general structural–functional information coming from both crystallographic (see Newstead et al. 2011; Newstead 2011; Solcan et al. 2012; Doki et al. 2013; Lyons et al. 2014; Parker et al. 2014; Fowler et al. 2015; Newstead 2015) and molecular modelling experiments (Meredith and Price 2006; Pedretti et al. 2008; Meredith 2009; Samsudin et al. 2016) has led to the identification of a natural amino-acid substitution (due to a C-to-T change in the nucleotide sequence) in the transmembrane domain VIII of a threonine (Thr) (in position 327 in rabbit and 330 in European sea bass) with an isoleucine (Ile) (in position 334 in zebrafish) that is relevant for the fine tuning of the characteristics of transport in the rabbit/sea bass vs. zebrafish PEPT1. This Thr is highly conserved in all vertebrates, teleost fish included, with the exception of the zebrafish and the other cyprinids (Fig. 2; see also Romano et al. 2014). As demonstrated by analysing the function of the mutated (Thr327Ile) rabbit transporter, which turns to work, such as a zebrafish PEPT1, when tested for Gly–Gln, Lys–Gly, and Lys–Met transports, such residue principally changes the way of interaction of the protein with di/tripeptides, showing variation in substrate selectivity and affinity and consequently in transport efficiency. Interestingly, the same Thr-to-Ile amino-acid change found in the cyprinid PEPT1 is present, but in PEPT1A instead of PEPT1 (Fig. 2), in three (the haplochromine lineage) of the five representative species from throughout the East African haplo-tilapiine lineage (which gave rise to all East African cichlid radiation; Brawand et al. 2014). Namely, five lineages—the Nile tilapia (Oreochromis niloticus), an ancestral lineage with low diversity, the lyretail cichlid (alias princess of Burundi) Neolamprologus brichardi (older radiation, Lake Tanganyika), the zebra mbuna Maylandia zebra (recent radiation, Lake Malawi), Pundamilia nyererei (very recent radiation, Lake Victoria), and Burton’s mouthbrooder Haplochromis burtoni (riverine species around Lake Tanganyika)—diverged primarily through geographical isolation, and three of them subsequently underwent adaptive radiations in the three largest lakes of Africa. While Thr is present in the riverine Nile tilapia and lyretail cichlid PEPT1A, Ile is present in the zebra mbuna-Burton’s mouthbrooder-P. nyererei PEPT1A leading to the hypothesis that in this fish group after gene duplication, the retained duplicated gene may have diverged in function through sub- or neofunctionalisation (for discussion, see also the “Organ/tissue distribution of PEPT1 in teleost fish”).
Amino-acid sequence alignment of PEPT1-type transporters in vertebrates (mammals-to-fish series). Multiple sequence alignment was generated by ClustalW2 at http://www.ebi.ac.uk/Tools/msa/clustalw2/ using default parameters. TMD VII, TMD VIII and TMD IX indicate the seventh, eighth, and nineth transmembrane domains as recently represented (Newstead 2015). The Thr-to-Ile amino-acid change discussed in the text (↑) occurs in TMD VIII in the same motif context in all cyprinid PepT1b and ancestor of haplochromine PepT1a sequences retrieved from GenBank (July 2015). Identical residues are highlighted in black and similar residues in gray (data generated at http://www.bioinformatics.org/sms2/color_align_cons.html; percentage of sequences that must agree for identity or similarity coloring to be added: 70%)
In an attempt to systematise the analysis of peptide transporters among diverse species, a comparative study has been published that evaluates if and how PEPT1 and PEPT2 transporters from mammalian (human, rat, and mouse), teleost fish (zebrafish), and nematode (C. elegans) models selectively transport (or vice versa are discriminated by) the fluorophore-conjugated dipeptides β-Ala- and d-Ala–Lys-N-7-amino-4-methylcoumarin-3-acetic acid (Kottra et al. 2013). Although preliminary, these findings indicate that there are measurable differences in terms of kinetics and/or substrate interaction/recognition parameters among mammalian, zebrafish, and C. elegans PEPT1 and PEPT2 transporters, with, e.g., the zebrafish PEPT1 transporter differing from the mammalian counterparts for its complete lack of interaction with the substrate d-Ala–Lys-N-7-amino-4-methylcoumarin-3-acetic acid. In a perspective, this selectivity based on the type of peptide transporter and species may be very helpful in better defining the structure–function determinants of the proteins of the SLC15 family, and an integration of this platform with the inclusion of peptide transporters from other reference (e.g., ovine, chicken, turkey, pig, and shark) animal models is worth to be achieved in the near future.
PEPT1 function at the intestinal epithelial cells: local operativity of the transporter
It has long been known that at the intestinal epithelium, PEPT1 responds directly to the presence of luminal substrates, which is most probably the simplest form of regulation, it is subjected to. Interaction can be either with substrates that are transported or with substrates that influence its activity without being transported. The studies on the local regulation of PEPT1 by luminal factors are still very limited in spite of the wide demand of knowledge on the subject for the possible implications in, e.g., animal and human nutrition, growth, and metabolism. In addition, at the intestinal level, PEPT1 operates in conjunction with many other membrane and non-membrane, transport, and non-transport proteins to optimally exert its major function of moving di/tripeptides across the apical membrane of the enterocyte. The description of the interactions that PEPT1 locally takes with luminal substrates and/or proteins and of the molecular and cellular mehanisms behind such interactions highly emphasises the very complex functional interplay that exists at the apical membrane of the intestinal cells among digestive, absorptive, acid–base balance, ionoregulatory, and osmoregulatory functions. Details on some of such functional relationships are illustrated in detail below. Due to the virtual lack of specific cell physiology studies in teleost fish, we often refer to mechanisms described in mammalian cells only.
PEPT1 and dietary protein, di/tripeptides, and free amino acids
Dietary protein as well as certain free amino acids and peptides are able to change PEPT1 expression in the small intestine and its maximal transport activity. In vivo feeding studies in rats and mice have first suggested this form of regulation for PEPT1 (for further discussion, see also the “PEPT1 and dietary protein levels”). In particular, it was early found that uptake of the dipeptide carnosine into everted intestinal sleeves of mice fed a high (72%) protein diet compared to a low (18%) protein diet was increased (30–70%) in the proximal regions of the gut (Ferraris et al. 1988). Moreover, in rats, a switch from a low-protein diet, comprising 4% casein, to a high-protein diet, containing 50% gelatine, produced increases in PEPT1 mRNA by 1.5–2-folds (Erickson et al. 1995). Later on, Shiraga et al. (1999) observed that in rats fed increasing quantities of dietary protein (i.e., 0, 5, 20, and 50% casein in the diet) for 3 days, the abundance of PEPT1 in the BBM increased proportionally to the protein intake (up to 2.2-fold at 50% casein vs. 0% casein) with concomitant increase in peptide transport activity (up to 2.2-fold at 50% casein vs. 0% casein). In addition, PEPT1 mRNA levels increased (up to 2.4-fold at 50% casein vs. 0% casein). Moreover, these authors observed that when administered in the diet both free amino acids (i.e., Phe among the tested amino acids) and dipeptides (i.e., Gly–Phe among the tested dipeptides) increased PEPT1 expression and maximal transport activity. Interestingly, they identified elements in the 5′ upstream region of the rat PEPT1 responsive to both dipeptides (namely, Gly–Sar, Gly–Phe, Lys–Phe, Phe–Val, and especially Asp–Lys) and free amino acids (namely, Lys, Arg, and especially Phe), suggesting involvement of an Activator Protein 1 (AP-1) binding site, an Amino-Acid Responsive Element (AARE)-like binding site identified next to an Activator Protein 2 (AP-2) binding sit, and an Octamer-binding protein (Oct) site for Oct1/Oct2 (for details, see Shiraga et al. 1999). Namely, AP-1 is a transcription factor associated with regulation of gene expression under amino-acid deprivation conditions (Pohjanpelto and Holtta 1990), while an AARE-like element similar to that found in PEPT1 promoter controls asparagine synthetase gene expression under essential amino-acid deprivation (Guerrini et al. 1993). Later on, these data were confirmed in the rodent model, since similar responsive elements were identified in the promoter region of the mouse PEPT1 (Fei et al. 2000). A set of in vitro experiments with cultured cells specifically expressing PEPT1 allowed evaluation of the effects of single specific substrates on the regulation of the transporter; e.g., the addition of Gly–Sar to the medium of Caco-2 cell cultures caused a significant increase in the expression of both human PEPT1 mRNA and protein, as well as an increase in transport activity (Thamotharan et al. 1998). Similar uptake experiments performed using Gly–Gln as a substrate confirmed the physiological relevance of these findings (Walker et al. 1998), suggesting that the substrates can per se alter PEPT1 function in the Caco-2 cells at the transcriptional and translational levels. All together, these findings show direct regulation of PEPT1 by its own transported substrates, which may have as such significant implications in the qualitative formulation of nutritional supplements. In this context, it has to be remarked that the substrate-induced upregulation of PEPT1 expression and function never exceeds 2–3-fold compared to the control and that it occurs at relatively short times (1–3 days after the start of the substrate supplementation), which suggests that upregulation involves local signals, and direct action on the enterocyte. A detailed analysis of the role of protein, amino acids, and di/tripeptides levels on PEPT1-mediated transport, gut physiology and ultimately body growth along the vertebrate scale, with major emphasis on teleost fish, is specifically provided below in this review (see the “Is the intestinal transporter PEPT1 relevant for teleost fish growth?”).
More recently, Mertl et al. (2008) proposed another form of regulation of PEPT1 by its substrates. They first demonstrated, in the heterologous X. laevis oocyte system, that a prolonged exposure to substrate of rabbit PEPT1 causes withdrawal of transporters from the plasma membrane. In particular, exposure of oocytes to Gly–Gln for 2 h results in a decrease in PEPT1-mediated maximal transport with no change of membrane capacitance, while exposure to substrate for 5 h decreases both transport and surface area by endocytotic removal of transporter proteins from the surface. Similar simultaneous decreases of current and surface area are also observed when endocytosis is stimulated by the activation of protein kinase C (PKC). Cytochalasin D inhibits all changes evoked by either dipeptide or PKC stimulation, whereas the PKC-selective inhibitor bisindolylmaleimide only affects PKC-stimulated endocytotic processes but not substrate-dependent withdrawal of PEPT1. Thus, membrane surface density of PEPT1 proteins seems to be controlled by the transport function of PEPT1, since prolonged substrate exposure, resulting in increased cytosolic amino acid and H+ concentrations and membrane depolarisation, triggers an as-yet-undefined intracellular signalling pathway that, via endocytosis, leads to time-dependent reversible subtraction of the transporters from the plasma membrane. This finding is particularly relevant, since PEPT1 may physiologically operate in a ‘reversed transport mode’ that under certain physiological conditions (i.e.. high di/tripeptide cytoplasmic concentration), it can translocate substrates from inside to outside the cell (Kottra and Daniel 2001; Kottra et al. 2002; Renna et al. 2011a). Therefore, the removal of the transporter from the membrane could have physiological meaning, since it would limit di/tripeptide cytoplasm-to-lumen outflow when very high concentrations of di/tripeptides are present in the enterocyte, a condition that invariably occurs with a dietary protein load after a meal and could represent a local brake to limit enterocyte overload.
PEPT1 and short-chain fatty acids
In mammals, the colonic lumen normally contains 100–150 mM total short-chain fatty acids (SCFAs) (see Wrong et al. 1965; Cummings et al. 1987). In mammals, the vast majority (95–99%) of the SCFAs produced in the colonic lumen are absorbed (see Cummings and Macfarlane 1991; Engelhardt et al. 1989), which invariably occur by means of both simple diffusion and carrier-mediated processes through, e.g., the monocarboxylic transporter 1 (MCT1), alias Solute Carrier 16 (SLC16) family member A1 (SLC16A1) (see, e.g., Buyse et al. 2002a), or the sodium/monocarboxylate transporter 1 (SMCT1), alias Solute Carrier 5 (SLC5) family member A8 (SLC5A8) (for a recent review, see Iwanaga and Kishimoto 2015). Notably, to date, there is considerable doubt about the apical localisation and hence the absorptive role of MCT1, whereas the role of SMCT1 as major apical membrane transporter of SCFAs in the colon is steadily emerging, in parallel with the sodium/monocarboxylate transporter 2 (SMCT2), alias SLC5 family member A12 (SLC5A12), in the small intestine (for review, see Iwanaga and Kishimoto 2015). Once in the cell, SCFAs are rapidly metabolised by colonocytes, and in this respect, these molecules remain the major respiratory fuels in the intestine. Actually, oxidation of SCFAs supplies 60–70% of the energy need in isolated colonocytes (Roediger and Millard 1996).
Butyrate is normally produced in the colonic lumen by bacterial fermentation of carbohydrates and dietary fibres (see Cummings 1981). As one of the three major SCFAs, the other two being acetate and propionate, butyrate represents the main intestinal fuel, even in the presence of other energetic substrates, such as glucose and glutamine (Clausen and Mortensen 1994). In addition to its function as the dominant energy source for colonocytes, butyrate also affects cellular proliferation, differentiation, and apoptosis (see McIntyre et al. 1993; Gamet et al. 1992; Ruemmele et al. 2003). Dalmasso et al. (2008b) first observed that butyrate treatment of human intestinal epithelial Caco2-BBE cells increases human PEPT1 promoter activity in a dose- and time-dependent manner, with maximal activity observed in cells treated with 5 mM butyrate for 24 h. Under this condition, human PEPT1 promoter activity, mRNA, and protein expression levels are all found to increase; accordingly, transport activity increases by ~2.5-fold. Molecular analyses reveal that the caudal-type homeobox 2 (CDX2), besides the cAMP response element-binding protein (CREB), is the most important transcription factor for butyrate-induced increase of human PEPT1 expression and activity in Caco2-BBE cells. Moreover, Caco2-BBE cells overexpressing CDX2 exhibit greater human PEPT1 expression level than wild-type cells. Finally, treatment of mice with 5 mM butyrate added to drinking water for 24 h increases colonic PEPT1 mRNA and protein expression levels, as well as it enhances PEPT1 transport activity in colonic apical membranes vesicles. Interestingly, in epithelial cells, butyrate is also found to potently stimulate the transcription of other membrane transporters, such as the rat Na+/H+ exchanger NHE3 (Kiela et al. 2001, 2007), the human γ-epithelial sodium channel (Zeissig et al. 2007), and the mouse sodium/monocarboxylate transporter 1 (SMCT1), alias Solute Carrier 5 (SLC5) family member A8 (SLC5A8) (Kakizaki et al. 2010), the last finding being relevant, since SLC5A8 transports butyrate itself, among many other monocarboxylates.
In farmed animals, butyrate has long been considered to be highly effective in increasing growth performance and intestinal integrity. This has been acknowledged, e.g., in piglets (see Gálfi and Bokori 1990; Biagi et al. 2007). In this respect, sodium buryrate has been used as a feed additive for pigs (see Manzanilla et al. 2006), as well as calves and cows (see Huhtanen et al. 1993; Ahring et al. 2001; Gorka et al. 2009), and today, it is assessed as a potential feed additive for cultured fish. Based on these findings, it has been argued that amino-acid absorption and growth may be improved in farmed animals, teleost fish included, by stimulating PEPT1 expression and activity via butyrate. Recent work in grass carp (Ctenopharyngodon idella) (Liu et al. 2013b), as well as in diploid red crucian carp (Carassius auratus), tetraploid fish obtained by inter-specific cross of female red crucian carp and male common carp, and triploid fish obtained by intercrossing of female red crucian carp and male allotetraploid fish (Liu et al. 2014), has specifically been conducted to support this proposition. In particular, both in vitro and in vivo butyrate treatments are found to significantly increase PEPT1 expression in the intestine of the grass carp in a dose- and time-dependent manner (0–9 mM and 7–28 days range tested) (Liu et al. 2013b). In addition, upregulation of PEPT1 expression by dietary butyrate (0–5 g/kg) has been observed in the triploid fish, a behaviour that parallels in this fish the increased expression of CDX2 (Liu et al. 2014).
PEPT1 monomers and PEPT1 multimers
Whether or not H+-dependent peptide transporters operate in monomeric form, or conversely, they interact to generate a multimeric (homotetrameric) complex at the apical membrane of the intestinal (and renal) epithelial cells, which is still an open question, supported in part by the early biochemical, biophysical, and functional (kinetic) evidences from isolated renal BBMs (e.g., Boll and Daniel 1995), from the rabbit PEPT1 overexpressed in X. laevis oocytes (e.g., Panitsas et al. 2006) and in part by recent preliminary structural evidences from PepTSo2, a bacterial peptide transporter from Shewanella oneidensis that has been reported to exist as a tetramer in detergents (e.g., Guettou et al. 2013). Taken together, these findings indicate that a higher level of organisation may be a feature of the peptide transporters and that the oligomeric state may play a functional role in the regulation of the transport in vivo. However, further studies are required to address this question and establish what the role of oligomerisation is in the membrane. In a perspective, a contribution to this debate could come from the analysis of the teleost fish PEPT1-type proteins, which in the forms of PEPT1A and PEPT1B might physiologically be co-expressed in the enterocyte, interacting cooperatively as heterotetramers for optimal di/tripeptide transport function. To extend the information on the possible functional role of the oligomerisation state, the two proteins might simultaneously be expressed in a heterologous (e.g., the X. laevis oocytes) system at variable monomer ratios to functionally establish if and how they may be part of the same multimeric structure.
PEPT1 and trypsin
Another finding in terms of functional relationships between di/tripeptide transporters and other proteins has recently been published and regards the occurrence of a physical interaction between PEPT1/PEPT2 proteins and trypsin (Beale et al. 2015). In particular, the large extracellular loop (named Extracellular Domain, ECD) invariably present in animal PEPT1/PEPT2 transporters, but not in bacterial, fungal or plant counterparts, has recently been crystallised and a pair of immunoglobulin(IG)-like domains connected in tandem and inserted between transmembrane domains 9 and 10 have been identified. These domains physically interact with trypsin. In particular, the crystallographic and biophysical analyses that revealed the specific interaction with trypsin led the authors to suggest a role in clustering a proteolytic activity to the site of peptide uptake across the membrane. Interestingly, due to the trypsin catalytic properties, it has been hypothesised that this functional coupling is finalised to the determination of high local concentration of basic amino acids (Lys and Arg) containing di/tripeptides (Beale et al. 2015). In this view, localisation of ECD within the PEPT1 structure would thus represent an adaptation to increase the concentration of Arg– and Lys-containing peptides, and thus improve the transport of such class of di/tripeptides into the cell. This finding opens discussions in gut digestive/absorptive physiology, since it establishes for the first time a direct material link between a specific protease and a protein degradation products transporter, with PEPT1 operating as part of a local network that involves both digestive and absorptive processes. Although preliminary, this study also highlights the modular nature of peptide transporters, possibly depicting another event of de novo insertion of a functional domain in the PEPT1 structure besides the VDMSRKS domain identified in the cytosolic COOH-terminal region of the Antarctic icefish PEPT1 (Rizzello et al. 2013). Intriguingly, the other three members of the SLC15 family, namely, SLC15A3 (PHT2), SLC15A4 (PHT1), and SLC15A5, all exhibit a large intracellular loop and a large extracellular loop—that are different than that present in PEPT1/PEPT2—which roles in the context of the protein activity still have to be examined.
PEPT1 and glucose transporters
Based on findings from intestinal perfusion studies performed in rats using substrates of PEPT1 and of the sodium/glucose cotransporter 1 (SGLT1), alias SLC5 family member A1 (SLC5A1), as well as a number of receptor ligands, the existence of an energy supply network for nutrients that involves both transporters and receptors and coordinates nutrient absorption in time and space has been proposed (Mace et al. 2009). According to this model, high luminal glucose concentrations would cause the incorporation of the facilitated glucose transporter 2 (GLUT2), alias Solute Carrier 2 (SLC2) family member A2 (SLC2A2), into the BBM of the enterocyte by recruitment from intracellular vesicles. This would allow bulk amounts of glucose to be absorbed from the lumen. In rats, GLUT2 trafficking would require the activity of SGLT1, membrane depolarisation, PKC βII activation and intracellular Ca2+ rising, with consequent fusion of the GLUT2-containing vesicles into the BBM. Intriguingly, the perfusion of rat small intestine with high glucose concentrations would also cause concomitant reduction of the BBM surface density of PEPT1 proteins, and thus of the di/tripeptide transport capacity. This complex interplay between GLUT2 and PEPT1 should be functional to preventing the hyperosmotic load of the enterocyte resulting from the simultaneous absorption of very large amounts of dietary sugars and peptides. Sweet taste and amino-acid receptors would operate as part of this complex network, thus contributing to the rapid regulation of the transport activity. At the moment, the existence of such a direct glucose-di/tripeptide network has been proposed essentially on the basis of data from the rat small intestine. More recently, an attempt in recapitulating in the mouse all the various aspects of the complex network defined in the rat model has failed (Röder et al. 2014). In addition, the human (see Santer et al. 2003; Ait-Omar et al. 2011; Gorboulev et al. 2012) and swine (see Moran et al. 2010) models seem to fit only partially or not at all into the proposed scheme, which emphasises the importance of including ‘species-specificity’ among the variables to consider to fully address the physiological relevance of this network.
PEPT1 and amino-acid transporters
As already mentioned (see the “Major role of PEPT1 in teleost fish digestive/absorptive physiology”), protein digestion products (i.e., the luminal load) are specifically transported from the intestinal lumen into the enterocyte (i.e., the cellular load) in the form of free amino acids, by means of a large variety of BBM amino-acid transporters, and in the form of di/tripeptides, by means of the BBM transporter PEPT1 only. While the former operates by transporting their amino-acid substrate(s) with high (<0.1 mM), medium (0.1 to 1 mM) and low (>1 mM) affinities (for review, see Bröer 2008), the latter operates by transporting its substrates with apparent relatively low affinity depending on the nature of the di/tripeptide, thus overlapping certain bulk absorbers of amino acids, e.g., SLC6A19 (alias B0AT1) and Solute Carrier 36 (SLC36) family member A1 (SLC36A1) (alias PAT1) that such as PEPT1 function in the millimolar range (for review, see Bröer 2008). Such a broad scope activity of PEPT1 is thought to be functional to managing with the large load of di/tripeptides generated by dietary protein digestion, as it occurs after a meal. In this respect, because of its kinetic properties, PEPT1 may cope with a relatively big load of dietary nitrogen in a relatively short time.
In this context, it is worth noting that a strict functional interplay exists between the many amino-acid transporters and the single di/tripeptide transporter and that uptake of free amino acid may indirectly be regulated by PEPT1 activity. In fact, since at the intestinal level, many amino-acid transporters serve as obligatory amino-acid exchangers (meaning that they mediate the simultaneous translocation of two amino acids across the membrane in opposite directions in a 1:1 stoichiometry), filling cells via PEPT1 with a variety of amino acids in the form of di/tripeptides that immediately undergo intracellular hydrolysis by means of cytoplasmic peptidases may be highly relevant for the net movement of amino acids from lumen to cell. This functional interaction was first demonstrated by Wenzel et al. (2001), who showed that uptake of dipeptides causes trans-stimulation of amino-acid uptake via the b0,+ system—i.e., the Solute Carrier 7 (SLC7) family member A9 (SLC7A9), alias b0,+ Amino-acid Transporter (b0,+AT), heterodimerically linked (by a disulphide bridge) to the Solute Carrier 3 (SLC3) family member A1 (SLC3A1), alias b0,+ amino-acid transporter related (rBAT)—that translocates among others the essential amino acids Lys and Arg (for a recent review on SLC3 and SLC7 families of amino-acid transporters, see Fotiadis et al. 2013). Interestingly, a direct relation between the PEPT1-mediated uptake of dipeptides and the trans-stimulated uptake of certain amino-acid-like drugs, such as gabapentin (which is used to treat a variety of central nervous system disorders, including seizure, neuropathic pain, and anxiety) through the transport system b0,+ has also been assessed (Nguyen et al. 2007). Further support to the existence of a functional interaction between amino-acid transporters and PEPT1 also emerges from recent studies conducted in mouse Cluster of Differentiation 98 heavy chain(CD98hc)-null Embryonic Stem(ES)-derived fibroblasts (de la Ballina et al. 2016). We discuss these findings also for their implications in the discussion on the role of PEPT1 in animal growth (see the “Is the intestinal transporter PEPT1 relevant for teleost fish growth?”). In mouse, ES-derived fibroblasts, CD98hc, alias SLC3 family member A2 (SLC3A2), alias 4F2 heavy chain (4F2hc) represent an essential part of the stress response network. In particular, three CD98hc-associated transporters—i.e., SLC7 family member A5 (SLC7A5), alias L-type amino-acid Transporter 1 (LAT1), SLC7 family member A11 (SLC7A11), alias x −c Transporter (xCT), and SLC7 family member A6 (SLC7A6), alias y +L-type amino-acid Transporter 2 (y+LAT2) that sustain operational amino-acid exchange activities converging to system L, system x −c , and system y+L, respectively—ensure that these cells have a balanced amino-acid content, which allows them to counterbalance oxidative stress (via CD98hc/xCT) and to fuel protein synthesis and concomitant cell proliferation (mainly via CD98hc/LAT1 and CD98hc/y+LAT2, but also via CD98hc/xCT) (for details on the substrate specificities of these heterodimers, see Fotiadis et al. 2013). In CD98hc-null ES-derived fibroblasts, all CD98hc-associated transporters fail to reach the plasma membrane, and thus, no amino-acid transport activities mediated by LAT1, xCT, and y+LAT2 occur, which result in a block of cell proliferation. In CD98hc-null cells, some experimental manoeuvres restore cell proliferation, e.g., β-mercaptoethanol (β-ME) supplementation that overcomes the absence of a functional x −c system is required to inhibit CD98hc-null cell death by ferroptosis. In such conditions, CD98hc-null cells exhibit: (a) reactive oxygen species accumulation; (b) intracellular amino-acid imbalance, i.e., increased levels of positively charged amino acids (Arg, Lys, and His), accumulation of neutral amino acids (Ala, Ser, Asn, Gln, and Met), and shortage of branched-chain (Val, Leu, and Ile) and aromatic (Phe and Tyr) amino acids; (c) modulation of CD98hc-independent amino-acid transporters and, notably, strong upregulation of PEPT1 expression; and (d) still limited cell proliferation. Interestingly, only an external supply of branched chain and aromatic amino acids in the form of dipeptides (which enter the cell via PEPT1) restores (rescues) cell proliferation in β-ME-treated CD98hc-null cells, suggesting that specific classes of dipeptides can compensate for the disrupted uptake of essential amino acids by CD98hc-dependent transport systems x −c , L, and y +L. Notably, the rescue effectiveness of branched chain and aromatic amino-acid-containing dipeptides in this experimental setup fits well with the substrate specificity ranks proposed for PEPT1-mediated transport (see Vig et al. 2006).
PEPT1 and intracellular peptidases
Kottra et al. (2009) first demonstrated that PEPT1 produces huge outward transport currents when oocytes are preloaded with hydrolysis-resistant dipeptides or when intracellular hydrolysis is prevented by the aminopeptidase inhibitor bestatin, substrate itself of PEPT1. Dipeptide preloading of oocytes also increases inward currents evoked by substrates provided on the extracellular side of the membrane, suggesting a faster turnover rate of PEPT1 in the presence of high substrate concentrations on the cytosolic side. Based on the knowledge that the intracellular amino-acid pool is dependent on the peptidase-driven breakdown rate of di/tripeptides, further research has been performed in vivo on the nematode C. elegans (reviewed by Spanier 2014), which expresses the intestinal high-capacity/low-affinity transporter PEPT-1 (Meissner et al. 2004). Interestingly, RNA interference gene silencing of two cytosolic peptidases, i.e., ZC416.6 (an ortholog of the mammalian bifunctional leukotriene A4 hydrolase/aminopeptidase, LTA4H) and R11H6.1/PES-9 (which is structurally related to the mammalian cytosolic dipeptidase CNDP2), is sufficient to reduce PEPT-1 expression and function (Benner et al. 2011). Since both mammalian peptidases LTA4H and CNDP2 are sensitive to bestatin (Davies et al. 2009) and amastatin (another aminopeptidase inhibitor) (Daniel and Adibi 1994), the impact of both compounds has been analysed on C. elegans PEPT-1 function. Both inhibitors reduce C. elegans PEPT-1 activity without changing its mRNA or protein abundance, thus indicating that both peptidases can modulate the intracellular amino-acid pool, which in turn affects the transport capacity of C. elegans PEPT-1. This mechanism is also conserved in mammals, since LTA4H and CNDP2 gene silencing by siRNA performed in Caco-2 cells results in a significantly reduced PEPT1 protein expression (Benner et al. 2011). All together, these results support the finding that the intracellular amino-acid pool, which is modulated by either changes in the extracellular peptide supply or a reduced intracellular peptide hydrolysis, is an evolutionarily conserved key regulator for the intestinal peptide transporter PEPT1. The adaptation of the capacity of PEPT1 in varying cytosolic substrate concentrations could be extremely relevant with respect to its role in providing bulk quantities of amino acids for growth, development, and other nutritional needs.
PEPT1 and ion exchangers, carbonic anhydrases, and ion cotransporters
As already indicated (see the “pH dependence”), it is fully recognised that at the apical membrane of the intestinal epithelial cells, a functional interaction may occur between H+-dependent di/tripeptide cotransporters and Na+/H+ exchangers, which is well documented for, e.g., the duos PEPT1/NHE3 in mammals and PEPT-1/NHX-2 in C. elegans. Analogously, the function of the H+-dependent di/tripeptide transporter PEPT2 is linked to the function of the Na+/H+ exchanger 1 (NHE1), alias SLC9 family member A1 (SLC9A1), and/or 2 (NHE2), alias SLC9 family member A2 (SLC9A2) (see Wada et al. 2005). However, at the moment, it is not yet known whether or not a direct material link exists between H+-dependent di/tripeptide transporters and Na+/H+ exchangers in the membrane. Multiple advantages originate from such a functional interaction. In fact, e.g., at the intestinal level, the Na+/H+ exchange activity allows the generation of the acidic microclimate at the luminal side of the apical membrane of the enterocyte and consequently of the inwardly directed pH gradient that optimises the PEPT1-mediated H+-dependent di/tripeptide uptake while preventing at the same time the intracellular acidification due to translocation of positive charges (H+ and/or positively charged di/tripeptides) from the luminal to the cytoplasmic side of the membrane (for a discussion on the functional role of Na+/H+ exchange in the generation of the acidic microclimate and inwardly directed pH gradient at the apical membrane, of its supporting role to the activity of di/tripeptide and many other H+-dependent solute transporters, and on the role of H+-dependent di/tripeptide cotransport in intracellular acidification in mammals and C. elegans, see Lucas et al. 1975; Thwaites et al. 1999; Kennedy et al. 2002; Thwaites et al. 2002; Spanier et al. 2009; Benner et al. 2011; for reviews, see Thwaites and Anderson 2007; Spanier 2014). In this respect, that PEPT1 functionally operates as an acid loader has been proven, since PEPT1 gene ablation (Pept1 −/− mouse) abolishes the effects of di/tripeptides on small intestinal fluid absorption, short-circuit current, and intracellular pH (Chen et al. 2010). Interestingly, this picture well-integrates with the observation—mainly based on the comparative analysis of gene function in wild-type vs. knockout mice—that in the mouse duodenal villous epithelium a Cl−/HCO3 − exchange activity, mediated by the Solute Carrier 26 (SLC26) family member A6 (SLC26A6, alias Pat-1), in conjunction with both cytosolic carbonic anhydrase (CA) II (CAII) and extracellular CA activities (CAIV, CAIX, and possibly CAXII and CAXIV; Stewart et al. 1999; Mizumori et al. 2006), contributes to the intracellular pH regulation during H+-dependent di/tripeptide absorption (Simpson et al. 2010), possibly by providing the enterocyte with a net HCO3 − import pathway (Simpson et al. 2010; see also Walker et al. 2011). Actually, under certain physiological conditions, Pat-1 kinetically exhibits a propensity to act as a base importer (i.e., Cl −out /HCO −3in ) (Simpson et al. 2007), and there are evidences suggestive of a model, at least in the mouse upper villous duodenal epithelium (for details, see Simpson et al. 2010), in which Pat-1 operates in concert with CA activity to import HCO3 − (Cl −out /HCO −3in exchange) thus counterbalancing cellular acidification. The contribution of Pat-1 activity in sustaining di/tripeptide absorption is considered small (15–20%), and this exchanger likely serves an auxiliary function to the greater intracellular pH regulation provided by the apical membrane Na+/H+ exchange. In this respect, Cl−/HCO3 − function of Pat-1 may represent a subsidiary process to regulate intracellular pH in the villous epithelium that is exposed to physiological extremes in pH, CO2, and ionic composition from gastric effluents.
Recently, a functional interaction between PEPT1 and the electrogenic Na+/HCO3 − Cotransporter 1 (NBCe1), alias Solute Carrier 4 (SLC4) family member A4 (SLC4A4) (for details on SLC4 transporters, see Romero et al. 2013) has also been shown (Yu et al. 2016). SLC4A4 is a basolateral membrane transporter strongly expressed in the small intestinal villous surface enterocytes (see Jakab et al. 2011), where PEPT1 is also located. SLC4A4 knockout mice develop jejunal failure, worsening of metabolic acidosis, and death in the third week of life, possibly due to high energy demand for hyperventilation, fluid secretory, and nutrient absorptive defects and relative scarcity of compensatory mechanisms. At the small intestinal level, in 2–3-week-old mice loss of SLC4A4 results in severely impaired Cl− and fluid (but not HCO3 −) secretory response, as well as in lack of active compensatory response during transport processes that load the surface enterocytes with acid, such as the luminal Cl−/HCO3 − exchange mediated by the SLC26 family member A3 (SLC26A3), alias Down-regulated in Adenoma (DRA), or the H+/dipeptide uptake mediated by PEPT1. This suggests that the electrogenic influx of HCO3 − via SLC4A4 allows maintenance of enterocyte anion homeostasis and pHi control. Its loss impairs small intestinal Cl− and fluid secretion as well as the neutralization of acid loads imposed on the enterocytes during nutrient (i.e., di/tripeptide) and electrolyte absorption (Yu et al. 2016).
Last but not least, in mammalian systems, PEPT1 plays a direct role in electrolyte and water handling, as it appears from the observation that PEPT1 protein functions in healthy distal colonic and rectal epithelium of mice, rats, and humans (Wuensch et al. 2013), and that, in particular, loss of the protein (as assessed in Pept1 −/− mice) results in a significant increase (10–15%) in water fecal loss.
To our knowledge, no similarly comprehensive and direct analysis on the potential relationships between H+-dependent di/tripeptide transporters and ion transporters is available so far for the teleost fish intestine despite the highly relevant implications such information would bear in a perspective, not only for understanding the basic kinetic properties of the various teleost fish PEPT1 proteins (see the “pH dependence”) but also for modelling the actual dynamic associations that subsist among digestion, absorption, acid–base regulation, ion regulation, and water regulation functions in the various teleost fish guts (see the “Is there in teleost fish a role of PEPT1 in salt, water and/or acid–base homeostasis?”).
Is there in teleost fish a role for PEPT1 in salt, water, and/or acid–base homeostasis?
The molecular and cellular mechanisms described in the previous ‘PEPT1 function at the intestinal epithelial cells: local operativity of the transporter’ section highly emphasises the complex local functional network that operates at the gastrointestinal epithelial cells between feeding, digestion, and assimilation of dietary degradation products, on one hand, and ion transport, osmotic water movements, and/or acid–base equilibrium processes, on the other hand. Disentangling this matter is a real challenge in aquatic animals, since they substantially differ in dietary habits, feeding strategies, behaviour, gut morphology and physiology, and digestive/absorptive specialisations (e.g., presence or absence of a stomach), in the frame of the extreme variety of the environments in which they live. In such a complex system of interactions, major issues emerge, in particular: (a) how (much) teleost fish living in freshwater or seawater habitats, or adapted to live at different salinities, and/or subjected to other environmental constraints (e.g., extreme acidic or alkaline waters) manage with the absorption of the intestinal luminal content that includes not only the elementary organic nutrients (sugars, amino acids, di/tripeptides, SCFAs, etc) but also the salts and water ingested with and/or present in the feedstuff; (b) how (much) the combined absorption of dietary nutrients, salts, and water impacts on osmoregulation, ion regulation, and acid–base balance processes at both local and organismal level (and vice versa); (c) how feeding, digestion, and absorption cooperate, influence and/or are influenced by other gastrointestinal functions. Many of these issues have received attention in the last few years with the publication of a number of excellent papers and comprehensive reviews (among many others, see Parks et al. 2008; Evans 2008a, b; Grosell et al. 2011; Laverty and Skadhauge 2012; Whittamore 2012; Sundell and Sundh 2012; Parker and Boron 2013; Larsen et al. 2014; Takei 2015; Kültz 2015, and literature cited therein).
In the next paragraphs, after briefly looking at the teleost fish gut as an osmoregulatory organ and at the composition of its content after a meal, we will focus on the current limited information on the possible involvement of PEPT1 in some of the questions discussed above. Complementary information on water, ion, and acid–base fluxes in teleost fish gut after a meal is provided as Supplementary Material.
Maintenance of osmotic homeostasis in teleost fish: role of the gut
Most teleost fish maintain the osmolality of their extracellular body fluids relatively constant at ~300 mosmol kg−1, independent of environmental salinity (for recent reviews, see Laverty and Skadhauge 2012; Kültz 2015, and literature cited therein). This control occurs in both stenohaline and euryhaline species, the latter normally experiencing large changes in environmental salinity during their life. To summarise, in freshwater-living teleosts active uptake of major ions, such as Na+ and Cl− occurs at the gill, with the kidney excreting high volumes of diluted urine to compensate for osmotic influx of water from the hypotonic environment. In these fish, the gut is almost invariably considered to play an ancillary role in ionoregulation and osmoregulation processes, although a significant role of the gastrointestinal tract in osmoregulation emerges with feeding, in relation to the uptake of dietary ions (for review, see Grosell et al. 2011, and literature cited therein) and prandial/postprandial drinking (see Smith 1930; Windell et al. 1969; Fuentes and Eddy 1997; Ruohonen et al. 1997; Kristiansen and Rankin 2001). In contrast, in seawater fish, the gills actively excrete excess Na+ and Cl− gained from the hypertonic environment, much of this load originating from the gastrointestinal tract since marine fish drink seawater to replace osmotic water loss across the gills and body surface. In seawater teleosts, the gastrointestinal tract is essential for ion and water balance with respect to freshwater fish, since it mediates many physiological processes, among which desalinisation of ingested water, solute-coupled fluid absorption, and HCO3 − secretion, which typically lead to more vigorous water uptake via concurrent luminal CaCO3 precipitation (see Hirano and Mayer-Gostan 1976; Parmelee and Renfro 1983; Kurita et al. 2008; Whittamore et al. 2010; Faggio et al. 2011; Esbaugh and Grosell 2014; for review, see Wilson et al. 2002; Grosell 2006, 2011). At the gastrointestinal level, to support the osmoregulatory function teleost fish also regulate divalent ion transport (such as Ca2+, Mg2+ and SO4 2−) and water permeability (for an immediate summary, see Kültz 2015, and literature cited therein).
Teleost fish gut luminal content after a meal
Most of the functions of the gastrointestinal tract closely depend on the primary goal of any animal, that of assimilating nutrients from food. On their hand, dietary proteins, carbohydrates, nucleic acids, and lipids, via digestion, largely contribute to the generation, after a meal, of a highly hyperosmotic state in the luminal compartment (see Kuzmina et al. 2008), which causes in parallel large addition of water of both exogenous (environment) and/or endogenous (body) origin into the gastrointestinal tract to counterbalance this condition (for review, see Grosell et al. 2011, and literature cited therein). Furthermore, dietary proteins, carbohydrates, nucleic acids, and lipids, via absorption of their degradation products, intimately participate in the transepithelial transport of ions and water at the gastrointestinal level. Analogously, ion gradients (principally Na+ and H+ gradients) strongly support the transepithelial transport of both organic and inorganic solutes, mostly when such solutes are present in the intestinal lumen at lower concentrations. In addition, organic nutrients, such as amino acids, di/tripeptides, glucose, SCFAs, etc, may by themselves be in the favourable condition of generating robust solute concentration gradients (e.g., when they are highly concentrated in the intestinal lumen as after a meal and/or when they accumulate into the cytoplasm after they have crossed the apical membrane of the enterocyte), which is functional to their own transport and/or to the transport of other solutes. Alteration of any of these physiological mechanisms may result in various pathophysiological conditions and/or disease states (malabsorption, diarrhea, etc).
In teleost fish, the role of the diet and of its components and its reciprocal impact on ion, osmotic, and/or acid–base regulation processes is largely underestimated to date, given that the largest part of the studies has so far been carried out on fasted animals. However, it is easy to conceive that at the gastrointestinal level, and in particular after a big meal, the ingested food, as it results from the complexity of the digestive processes (for reviews, see Cyrino et al. 2008; Grosell et al. 2011; Ray et al. 2012, and literature cited therein), invariably leads to the first instance to a massive increase in luminal osmolality, which is a measure of all the dissolved solutes; not just the salts but also all the organic compounds, e.g., sugars, amino acids, small peptides, nucleosides, etc, that are present in the luminal compartment during/after digestion (for reviews, see Laverty and Skadhauge 2012; Kültz 2015, and literature cited therein). In the context of this review, it is worthy of note that in the jejunal contents of animals or humans, the major fraction of protein degradation products after a meal consists of peptides with 3-to-6 amino-acid residues, which corresponds to a concentration of 120–145 mM, while the total concentration of all amino acids ranges between 30 and 60 mM (see Adibi and Mercer 1973; for review, see Daniel 2004). Thus, in aquatic organisms, the luminal content in the gut of a feeding animal is a very complex combination of digested organic products and the amount of salts contained in the water and/or food ingested with the feed ration, with further obligatory contributions deriving from osmotic water movements, secretions (gastric juice in gastric species, bile salts, mucus, pancreatic juice, etc), cast-off epithelium, microbiota and microbiota-related metabolites, and so on. Notably, any time an aquatic organism feeds, a meal load also results in a consistent salt load in terms of amount of major salts ingested with a normal feed ration; this salt load may vary significantly and be considerably high—when, for example, commercial diets and/or salt-rich invertebrates, such as Artemia salina, are supplied, as it happens in aquaculture feeding procedures. This is considered potentially beneficial to freshwater teleost fish, but potentially threatening for seawater fish (see Grosell et al. 2011, and literature cited therein). In fact, while in freshwater teleost fish, the amount of major salts ingested via a normal ration and absorbed via the gastrointestinal tract may far exceed that absorbed from the water by the gills, as first demonstrated by Smith and colleagues (Smith et al. 1989), in seawater teleosts salts and water absorption from food can heavily overlap ion and water absorption from drinking (see Grosell et al. 2011, and literature cited therein). Analogously, any time an aquatic organism consumes a dry diet—as it happens in modern aquaculture with fish regularly feeding extremely concentrated dry pelleted diets—a meal load may represent a considerable physiological stress, since the gastrointestinal tract is evolutionarily adapted to cope with the large amounts of water found in natural prey items (for review, see Buddington et al. 1997). This eventually results in longer retention times of chyme and/or increased addition of water of both exogenous and endogenous origin to the lumen of the gastrointestinal tract during and after feeding (see Windell et al. 1969; Ruohonen et al. 1997; Kristiansen and Rankin 2001; Bucking and Wood 2006; Harter et al. 2013, 2015; for review, see Jobling 1986). In such a composite scenario, a sort of roadmap has been planned by expert animal comparative and integrative physiologists in the last few years to assess the links among feeding, osmoregulation, ion regulation, and acid–base processes in teleost fish, and based on a series of seminal papers, investigations on the mutual roles of osmotic pressure, environmental salinity, and/or dietary salt load as major challenges in fish feeding have started. Part of this information is summarised in Supplementary Material.
PEPT1 and salt, water, and acid–base homeostasis in teleost fish
To our knowledge, only a limited number of studies have examined the impact of changes in environmental salinity on the expression of PEPT1. In one study, using a microarray approach to screen osmoregulatory tissues for changes in gene expression followed by quantitative validation of the differentially expressed genes by Northern blotting, Kalujnaia et al. (2007) have shown that expression of PEPT1 in the intestine of the freshwater-acclimated European eel decreases by up to 70% upon acclimation to seawater, according to the following time course: 50% by 2 days, 50% by 7 days, and up to 70% by long-term (5 months) acclimation. Based on sequence similarity analyses, it is likely that the transporter detected in this study represents a PEPT1A-type form (see Bucking and Schulte 2012; Tiziano Verri, personal observations). Notably, the fish used were migratory ‘silver’ European eels captured in eel traps, and they were kept unfed for the whole experimental salinity-transfer period (November–April), since eels normally fast over the winter months (Nyman 1972) and also they do not eat at water temperatures lower than 10–11 °C (Gousset 1990). Thus, it is possible that the observed decrease in gene expression reflects the starvation state instead of the freshwater-acclimated state. In another study, the expression analysis performed on mummichog intestinal PEPT1 mRNA extracted from both freshwater- and seawater-acclimated fish fed daily to satiation with commercial fish flakes has revealed that both PEPT1A and PEPT1B are detectable in freshwater-acclimated fish, while only PEPT1B is detectable in seawater-acclimated fish. The mRNA levels of PEPT1A and PEPT1B in freshwater-acclimated fish are uniformly distributed along the intestinal tract; similarly, PEPT1B in the anterior intestine is not significantly different with respect to that in the posterior intestine in seawater-acclimated fish. On the functional side, the uptake of the hydrolysable dipeptide alanyl–alanine (Ala–Ala), as measured in vitro by using gut sac preparations, has revealed no significant differences in transport rates at the luminal pH of 7.0 in freshwater- vs. seawater-acclimated fish. However, increasing the luminal pH to 8.0 results in a significant decrease in the overall dipeptide transport in freshwater- vs. seawater-acclimated fish, while it results in no significant changes in Ala–Ala uptake rate in seawater-acclimated fish (Bucking and Schulte 2012). In their elegant experiment that compared the transcriptome of the anterior and posterior intestinal sections of freshwater- and saltwater-adapted fish, Ronkin and colleagues have recently examined the salinity adaptation of two closely related species: the high salinity-tolerant Mozambique tilapia and the less salinity-tolerant Nile tilapia (Ronkin et al. 2015). This analysis revealed high similarity in gene expression response to salinity change between species in the posterior intestine and large differences in the anterior intestine. Notably, in the anterior intestine, tens of genes were found upregulated in seawater in one species and downregulated in the other (e.g., 47 upregulated in the Nile tilapia and downregulated in the Mozambique tilapia, and 21 showing the reverse pattern), a high proportion being transporters and ion channels. Among them is also PEPT1A, upregulated in seawater/downregulated in freshwater in the Mozambique tilapia and upregulated in freshwater/downregulated in seawater in the Nile tilapia. The results of this study point out a group of genes that differ in their salinity-dependent regulation pattern (showing a sort of ‘reversed salinity-dependent expression’) in the anterior intestine and may play a role in the differential salinity tolerance of these two sister species. More recently, as a result of an RNA-Seq-based transcriptomic analysis performed on gill, kidney, and intestine, PEPT1-type mRNAs have been identified among the ‘fluctuating’ genes potentially involved in osmoregulation and response to salinity stress in the striped catfish (Pangasianodon hypophthalmus), a salinity tolerant and air-breathing siluriform (see Nguyen et al. 2016). All together, these findings call for systematic investigations to validate function(s) and expression pattern(s) of the PEPT1-type genes and to elucidate their potential role(s) in salinity adaptation in various teleost fish species. Similar studies on other nutrient transporters are ongoing (see Nitzan et al. 2016).
Diets supplemented with salt (NaCl) are increasingly more widely used in the fish farming industry, since dietary salt is assumed to play a positive role in several aspects of teleost fish biology, growth, and feeding efficiency included (for review, see Salman 2009). However, the effects of dietary salt supplementation on intestinal physiological components and functions (e.g., chyme composition, nutrient, and micronutrient absorption, digestibility, etc) generally appears limited (see Nakajima and Sugiura 2016, and discussion therein), except perhaps under certain special circumstances (e.g., in low Na+ water and in certain groups of fish). Again, to our knowledge, there is very little information on the relationships between dietary salt administration and PEPT1 expression at the intestinal level. Very recently, with the aim to investigate the effects of diets (in the form of extruded pellets) containing decreasing fish meal inclusion levels and the effects of the addition of salt (NaCl) to the diet on growth performances, feed conversion ratio and intestinal mRNA transcript levels in freshwater-adapted European sea bass, a detailed spatial quantification of B0AT1 (alias SLC6A19) and PEPT1 (namely, PEPT1B) mRNA copies has been achieved along the intestinal tract (Rimoldi et al. 2015). Interestingly, while B0AT1 mRNA levels in the anterior and posterior intestine of freshwater-adapted European sea bass fed for 6 weeks were not modulated by dietary protein sources and salt supplementation, the inclusion of salt (3% NaCl) in a diet containing a low (10%) fish meal level significantly upregulated PEPT1 mRNA levels in the posterior intestine. In parallel, fish growth correlated with fish meal content in the diets, and the addition of the salt to the diet containing 10% fish meal improved feed intake, specific growth rate, and feed conversion ratio (Rimoldi et al. 2015).
All together, these data, through preliminary, not only account for a possible, very complex interplay between alimentary and osmoregulative functions in teleost fish gut, but also highlight the need to develop a standard in the experimental approaches, protocols, and procedures finalised to the study of such concurrent processes, mainly due to the large number of variables that have to be considered together. In addition, certain morphological, e.g., the absence of a stomach, and environmental, e.g., salinity, constraints may significantly impart species-specific rules in teleost fish gut physiology. Possible advantages for feeding, digestive, and absorptive activities remain to be explored, but it is a matter of fact that in teleost fish, some nutrient transporters, such as PEPT1, have evolved species-specific adaptation to some local conditions, such as pH, via structural changes in the protein (see the “pH dependence” paragraph above). Combining all the pieces of the puzzle to complete the portrait is now necessary to approach teleost fish gut function at the higher level of organ and system physiology.
Is the intestinal transporter PEPT1 relevant for teleost fish growth?
In vertebrates, PEPT1 acts during ontogeny, participates in nutritional stress response by reacting to, e.g., short-term fasting and fasting/refeeding manoeuvres, and it is possibly involved in some examples of total body plasticity, such as compensatory growth. In addition, diets of different composition may influence PEPT1 gene expression, sometimes very effectively. Many of these aspects are detailed in the next paragraphs with a focus on teleost fish and in comparison, whenever possible, to the information available from higher vertebrates.
PEPT1 expression during ontogeny
Early ontogenetic development of the gut occurs via obligatory morphogenesis and cell differentiation events during fetal development. In this period, the intestine prepares itself for postnatal life when it gets the complete control of nutrient absorption (see Puchal and Buddington 1992; for review, see Pacha 2000). Notably, prenatal expression of nutrient transporters occurs parallel with gut morpho-functional development (see Guandalini and Rubino 1982; Shen et al. 2001; for review, see Pacha 2000). In mammals, there are at least two critical phases of intestinal development: (a) at birth, when the animal shifts diet from amniotic fluid to mother’s milk and (b) at weaning, when it shifts diet from milk to a solid diet (for reviews, see Pacha 2000; Karasov et al. 2011; Karasov and Douglas 2013, and literature cited therein). At birth, the intestine becomes the site of nutrient assimilation, and the animal begins to consume a high-protein milk diet; at weaning, it changes to the adult diet, predominantly consisting of carbohydrates. Notably, dietary changes during the prenatal, suckling or weaning period may have irreversible effects on nutrient transport, and these effects are carried over until adulthood (see Karasov et al. 1985; for review, see Pacha 2000). Critical phases of intestinal development, e.g., hatching, first feeding, etc, are distinguishable also in lower vertebrates, such as birds and teleost fish (for discussion, see the paragraph below).
In mammals, the perinatal/postnatal period is central for peptide transport and PEPT1 expression (for review, see Adibi 2003). As earlier seen in rabbit and guinea pig (Himukai et al. 1980; Guandalini and Rubino 1982), a burst of peptide transport activity occurs in the intestine around birth, which is sustained by PEPT1 change of expression during this ontogenic phase. Notably, it was initially observed that the levels of PEPT1 mRNA greatly increase (~3-fold) in 4-day-old rats with respect to rats at birth, and decrease with time reaching the adult levels by 28 days after birth (Miyamoto et al. 1996). By checking rat intestinal PEPT1 mRNA and protein levels at regular intervals from 17 days before to 75 days after birth, Shen et al. (2001) found that expression levels in duodenum, jejunum and ileum rapidly increase at birth, reach high values by 3-to-5 days after birth (mRNA and protein level: 100%), and then rapidly fall down by 14 days after birth (mRNA level: 11–13%; protein level: 30–40%); a transient burst (mRNA level: 25%, with an ileal spike up to 58%; protein level: 59–88%) follows at weaning (24 days after birth), before PEPT1 expression reaches a plateau (mRNA level: 25%; protein level 70%) at adulthood (75 days after birth). A transient increase in PEPT1 expression is also present in the colon during the first days after birth in contrast to the lack of expression in adult rats; i.e., colonic PEPT1 mRNA and protein are high 1-to-5 days after birth (mRNA and protein level: 100%), drop to very low levels 7 days after birth and then they are not detectable (Shen et al. 2001). PEPT1 protein expression was also determined in rat duodenum at day 18 of gestation, birth, weaning (21 days after birth) and adulthood (Hussain et al. 2002). In this study, expression was strongest at birth, while at weaning and adulthood, it was similar but stronger than at day 18 of gestation; no peak was observed at weaning. Conversely, in a study aimed at determining the proximal-to-distal distribution of PEPT1 in rat intestine 4-to-50 days after birth, no expression changes were reported during this time period (Rome et al. 2002). In summary, these papers in rats have provided the early molecular observations on peptide transport function during mammalian development, indicating induction of expression at birth (with milk or its components, suckling, etc as possible inducers) and/or at weaning (with solid diet or its components, sub-optimal nutrition, etc as possible inducers), and representing the basis for applied physiology studies in livestock and poultry (for review, see Gilbert et al. 2008b).
In ruminants, PEPT1 expression has been analysed in rumen, omasum, duodenum, jejunum, and ileum in lambs at 2, 4, and 8 weeks after birth (Poole et al. 2003). No changes of expression were observed in this study, possibly because analysis was performed in lambs at 2 weeks, i.e., after earlier changes at birth had occurred already, and at 8 weeks, i.e., lambs had not yet been weaned. Lambs allowed to both nurse and access a creep diet at birth showed decreased PEPT1 mRNA levels in the rumen with respect to lambs allowed to nurse but not to access the diet, which has been interpreted as due to the limited contact of the rumen epithelium—after the obstruction caused by the ingested diet—with luminal ‘inducers of expression’ contained in the milk.
In swine, studies in Tibetan piglets (Wang et al. 2009) showed PEPT1 mRNA expression in duodenum, proximal and distal jejunum and ileum, which continuously increased (up to ~3-fold) from early days to the middle (0-to-14 days after birth) and gradually decreased (~1.5-fold vs. early days) from the middle to the end (21-to-35 days after birth) of the suckling period (for details on the regional variation, see Wang et al. 2009). Analogously, in postnatal Yucatan miniature pigs, PEPT1 function and mRNA expression at 1, 2, 3 (suckling), and 6 weeks (post-weaning) after birth indicated significant peptide transport activity in the small intestine during the first week of suckling and with diet transition following weaning. Moreover, while no significant differences were found at the mRNA level along the different sections of the small intestine, colonic PEPT1 mRNA in post-weaned pigs decreased by ~90% with respect to suckling pigs (Nosworthy et al. 2013). In addition, a large decrease (more than 60%) in PEPT1 mRNA intestinal expression was found within the first 48 h after birth, further supporting the significance of PEPT1 in the newborn pigs (D’Inca et al. 2011). In this context, it is worth noting that significant effects of maternal over- and undernutrition can be observed on intestinal morphology, enzyme activity, and gene expression of nutrient transporters, PEPT1 included, in newborn and weaned pigs (Cao et al. 2014), with overnutrition enhancing intestinal function via up-regulation of digestive enzyme activities and gene expression of transporters in both newborn and weaning piglets, and undernutrition impairing fetal intestinal development.
In birds, the pre- and postnatal changes in gut function support the animal need to adjust the shift from a lipid-rich yolk diet inside the egg to a carbohydrate- and protein-based diet post-hatch (for review, see Gilbert et al. 2008b; Karasov et al. 2011; Karasov and Douglas 2013). Although with a different embryology with respect to mammals, birds show similar regulation of PEPT1 to prepare the intestine for immediate uptake of nutrients at hatch. In turkey, a 3.3-fold increase in intestinal PEPT1 mRNA expression was observed from 5 days before hatch to day of hatch (Van et al. 2005), which was later confirmed by microarray analysis (de Oliveira et al. 2009) and more classical molecular biology approaches (Weintraut et al. 2016), that also indicated greater PEPT1 expression in females than males. In chicken, a ~50-fold increase in intestinal PEPT1 mRNA was found passing from 18 days before hatch to day of hatch, with a peak of expression immediately before hatch (Chen et al. 2005). After hatch, there was an increase (~3-fold vs. hatch) in PEPT1 mRNA abundance with time (as measured up to 35 days post-hatch). PEPT1 expression was analysed, with amino acid and sugar transporters, in the small intestine of two genetically selected lines of broiler chicks from 18 days before hatch to 14 days post-hatch (Gilbert et al. 2007). For the membrane transporters studied, mRNA levels increased with age with strong induction from 18 days before hatch to day of hatch. PEPT1 expression, in one of the lines, further increased with age with greatest (~3-fold vs. day of hatch) expression 14 days post-hatch, while in the other, there was a decrease 7 days post-hatch to the values measured at birth followed by a new increase (~2.5-fold vs. day of hatch) 14 days post-hatch, which suggests that broilers show the ability for peptide absorption to increase with maturity, although with some genetically-driven specificities. Out of the transporter genes evaluated, only PEPT1 was influenced by the genetic line, since there was a ~2-fold difference in PEPT1 mRNA expression in the two lines. In broilers, a constant temporal expression pattern was described in the gut in the 15-to-20 day range of embryonic development (Miska et al. 2014, 2015). Interestingly, the expression profile of 162 SLC genes was evaluated in broilers on days 18 and 20 before hatch and days 1, 3, 7, and 14 post-hatch by Affimetrix technology. In this study, many intestinal transporters were upregulated between 18 and 14 days before hatch, PEPT1 included (~8-fold change for the day of hatch to embryonic day 18 ratio; and ~2-fold change for the day 14 post-hatch to day of hatch ratio) (Li et al. 2008). The developmental expression described for broilers intestinal PEPT1 mRNA was also confirmed in Leghorn chicken by Zwarycz and Wong (2013), who found an increase (up to more than 50-fold depending on the small intestinal segment) from 18 days before hatch to a peak of expression 1 day post-hatch followed by a decrease 3 days post-hatch and a second increase that continued up to 14 days post-hatch (for further details, see Zwarycz and Wong 2013). Interestingly, in the domestic pigeon (Columba livia), PEPT1 mRNA expression also showed a significant increase (~5-fold) in expression in duodenum, jejunum and ileum passing from 16 days before hatch to day of hatch, which was followed by a constant increase in expression (~16-fold at 14 days post-hatch) (Dong et al. 2012; Gao et al. 2016). In this context, it has to be noted that during the embryonic stages of birds, there is a significant shift of absorbing function from the yolk syncytial membrane (YSM) to the small intestine. In chicks, PEPT1 gene expression in the YSM increased (~4-fold) until day 15 and then decreased by the same extent until day 21 of embryonic development, whereas expression in the intestine increased (~10-fold) from day 15 to day 21 (see Speier et al. 2012); a finding that parallels what was observed in the domestic pigeon (see Dong et al. 2012; Chen et al. 2016; Gao et al. 2016).
In teleost fish, a major ontogenetic change occurs when the source of nutrients and energy necessary to continue larval development passes from the yolk reserves to the ingested food, which mainly consists of protein and fat in carnivores but is higher in carbohydrates in omnivores and herbivores (see Karasov et al. 2011; Karasov and Douglas 2013, and literature cited therein). Initially, a functional gastric region may be absent in teleost fish gut (see Grosell et al. 2011; Karasov et al. 2011; Karasov and Douglas 2013) and, as described also for mammals, it is possible that pinocytosis plus intracellular digestion operates as a major early mechanism of nutrient absorption (see Önal and Langdon 2008; Yang et al. 2010; see also Karasov and Hume 1997), followed later on by expression of gastric H+ pump and pepsinogen for specific protein digestion (Darias et al. 2007; see also Grosell et al. 2011). Yet, the agastric condition is often maintained in adult teleost fish, which occurs in a larger than expected number of teleost fish (for review, see Grosell et al. 2011, and literature cited therein). In teleost fish, PEPT1 expression data during ontogeny are scarce. PEPT1 gene expression during ontogeny was first observed in the zebrafish, an agastric fish (Verri et al. 2003). PEPT1 mRNA levels were analysed for the first 7 days of embryonic development. Faint PEPT1 mRNA expression was detected 2 days post-fertilisation, but expression slightly increased 3 days and reached maximal values 4–7 days post-fertilisation. Studies of PEPT1 mRNA expression by in situ hybridisation revealed specific labelling 4–5 day post-fertilisation, but not 3 day post-fertilisation or at earlier stages of embryonic development. In situ analysis confirmed that zebrafish PEPT1 is abundantly expressed in the intestinal tube starting 4 day post-fertilisation; then, high levels of transcript were present in the epithelium of the proximal intestine (also known as ‘the intestinal bulb’), with no mRNA detected in the distal intestine. Therefore, such as in higher vertebrates, zebrafish PEPT1 expression seems to precede the functional maturation of the gut, which is completed by 5 day post-fertilisation thus making the fish ready to perform first feeding and digestion of external food. Five days post-fertilisation the larva starts seeking external food, although a remnant of the yolk is still present. Together, these data support the idea that proximal intestine is the site of most efficient di/tripeptide absorption in zebrafish, with PEPT1 expressed at high levels before the animal starts to rely on external food. Further studies conducted in another cyprinid, i.e., the grass carp (C. idella), and covering pre- and post-hatching stages, showed a highly dynamic pattern of expression during development (Liu et al. 2013b). Pre-hatch, PEPT1 mRNA reached maximal levels at the gastrula stage, followed by a significant decrease in PEPT1 mRNA at the organ stage and by a second increase until the hatching stage. This rise continued post-hatch up to 1 day after hatching. Then, expression quickly declined 2 days after hatching. Afterwards, mRNA expression increased again reaching maximum values at 7 days after hatching. Finally, gene expression stayed constant from 14 to 34 days post-hatch. With respect to blastocyst, increases never exceeded 2.0–2.2-folds, while decreases went down to 0.2–0.5-folds. Notably, the pre-hatch developmental expression shown in this study paralleled that described in detail in another cyprinid, namely, the red crucian carp (Liu et al. 2014). In this case, transcripts were first detected as maternal mRNAs in both fertilised and unfertilised eggs and the expression levels significantly increased (up to ~5-fold vs. egg) to the multi-cell stage and then decreased through the gastrula to the muscle contraction stage (down to ~0.5-fold vs. egg). A small increase was observed after the heart beat stage, which increased (up to ~2-fold) until the hatching stage. Such analysis was not extended post-hatch. In another study conducted in the Atlantic cod (Amberg et al. 2008), a gastric teleost fish, PEPT1 mRNA expression was detected at day of hatching and its levels steadily increased, following the onset of exogenous feeding, for the first 3 week post-hatching (up to ~7-fold at 22 day post-hatch). Analogously, PEPT1 mRNA expression was also analysed post-hatch in the Japanese eel, another gastric teleost fish (Ahn et al. 2013). Analyses in Japanese eel larvae conducted from 0 to 12 days post-hatch revealed that PEPT1 mRNA expression significantly increased (up to ~8-fold) between 5 and 7 day post-hatching. PEPT1 expression started to increase at 6 day post-hatching, i.e., the time point that coincided with the time larvae started feeding. This expression pattern paralleled that of trypsinogen, which in Japanese eel larvae is detected in the gastric region that also includes the pancreas. Notably, both PEPT1 and trypsinogen mRNA levels, as measured from 6 day post-hatching onwards, were transiently affected by the feeding status, since their levels from 9 to 11 day post-hatching were significantly higher (~2-fold) in the non-feeding with respect to the feeding group, then decreasing to comparable values 12 days post-hatching in both groups. This temporal stimulation of PEPT1 and trypsinogen in non-feeding larvae—interpreted as a compensatory response for malnutrition (Ahn et al. 2013)—suggests the existence of an early regulatory system for di/tripeptide absorption in response to the nutritional status that operates aside the standard ontogenic program. Recently, novel mRNA expression data have been provided for the development of the larval digestive system of gilthead sea bream (Sparus aurata) and European sea bass, two commonly farmed fish species (Cordero et al. 2016). The expression levels of some nutrition- and immune-relevant genes, PEPT1 included, were analysed from egg to 73 day post-fertilisation, with results pointing to a linear increase of PEPT1 gene expression for the first week in gilthead sea bream and for the first two weeks in sea bass development, followed by a plateau in both species lasting up to 73 day post-fertilisation.
In summary, during ontogeny, a general increase in PEPT1 gene expression virtually occurs in all vertebrates. This conclusion comes from experiments mainly conducted in rodents, livestock and poultry, with more limited information from teleost fish. No data are available from humans; i.e., there is only one report to date, at least to our knowledge, specifically dealing with PEPT1 expression in the intestine of humans in the pediatric age range (see Mooij et al. 2016; for a review, see Boudry et al. 2010). PEPT1 gene expression has been studied mostly at the mRNA level, with little data available at the protein level. In general, results indicate a general increase of PEPT1 expression during ontogeny with occurrence of some significant bursts usually observable in relatively short phases of the animal life, such as at birth, hatching, first feeding, weaning, etc, which are generally accompanied by dramatic changes in dietary style, mode of food intake, quantity and quality of food that enters the gut, malnutrition or sub-optimal nutrition, etc. Whether or not a correlation exists between PEPT1 gene expression during development and ‘critical windows’, i.e., specific periods of susceptibility or vulnerability to external challenges (for a recent review, see Mueller et al. 2015), is a question that needs further and specific investigation. In this context, it has to be noted that PEPT1 gene expression occurs within the general framework of the organisation/re-organisation of the gastrointestinal structure and function during development. For instance, during the late embryogenesis, enterocytes are immature; but after hatch enterocytes mature and villi elongate to increase surface area for absorption, as observed and specifically detailed, e.g., in chicks (see Geyra et al. 2001; Uni et al. 2003). In this context, greater expression of PEPT1 may be functional to efficiently use the increased peptide load from the exogenous feedstuff. In addition, it has to be noted that expression of PEPT1 may also increase simply due to the structural changes in the intestine, since enterocyte numbers increase with maturity. Thus, expression of PEPT1 in a tissue may change due to an increased number of enterocytes rather than to a significant increase in PEPT1 expression per enterocyte (as discussed by, e.g., Zwarycz and Wong 2013). However, although with major limits, the existence of (a) dynamic response(s) that occur(s) in short time lapses suggest the co-presence of a large variety of forms of regulation of the expression of the transporter, which may involve local, blood and/or nervous factors (for detailed reviews on these topics, see Zabielski 2007; Mourad and Saadé 2011; Spanier 2014).
PEPT1 and circadian rhythms
Since the first studies in rodents, it was recognised that at the intestinal level, PEPT1 expression and activity can be altered by the diurnal rhythm (see Pan et al. 2002, 2003, 2004). In rats, that are nocturnal and consume food mainly during the dark phase, intestinal PEPT1 expression was found to increase at night, paralleling their normal feeding behaviour. In particular, in rats maintained in a 12 h light/dark cycle with light from 08:00 to 20:00, there was a significant variation of both Gly–Sar uptake (~2-fold), as measured by intestinal loop and everted intestine preparations, and PEPT1 protein and mRNA levels (~3.5-fold), with a maximum at 20:00 and minimum at 8:00 (Pan et al. 2002). In fed rats, PEPT1 protein level was significantly higher at 20:00 than at 8:00, whereas fasting for 2–4 days resulted in the disappearance of the differences of PEPT1 protein levels between 20:00 and 8:00. In addition, intestinal absorption of ceftibuten, an oral antibiotic and also substrate of PEPT1, was also greater at 20:00 than at 8:00 in fed rats, but not different in rats fasted for 4 days. In contrast to protein expression and function, after 4 days of fasting PEPT1 mRNA levels maintained the circadian rhythm (Pan et al. 2003). Interestingly, when the diurnal rhythm was examined in rats under various feeding conditions—i.e., fed, food deprived for 1–4 days, food deprived for 4 days and then refed for 1–2 days, and fed during the daytime (9:00–15:00) for 10 days—it came out that (a) refeeding for 2 days after 4 days of food deprivation brought the diurnal variation in PEPT1 protein expression to normal rhythm; (b) eating food during the daytime (9:00–15:00) shifted the peaks of PEPT1 mRNA and protein expressions from the dark phase to the light phase, these findings suggesting that food intake, more (or rather) than the light cycle, can drive the diurnal rhythm of PEPT1 expression in rats. Later, Saito et al. (2008) found in rats that D site of albumin promoter (albumin D box) binding protein (DBP), a clock-controlled gene, regulates the circadian oscillation of PEPT1 expression during normal and restricted feeding conditions in contrast to other transcription factors, such as Sp1 transcription factor (SP1), CDX2, and peroxisome proliferator activated receptor alpha (PPARα) that are known to contribute to the basal, intestine-specific, and fasting-induced expression of PEPT1, respectively (for details, see the “PEPT1 and feed deprivation” paragraph below; for review, see Terada and Inui 2007).
It has recently been found that in mouse, maintained in a 12 h light/dark cycle with light from 00:00 to 12:00, intestinal expression of PEPT1 oscillates during the daily feeding cycle, since bile acids regulate the oscillation of PEPT1 expression by modulating PPARα activity (Okamura et al. 2014). Like rats, mice are nocturnal. According to Okamura et al. (2014), PPARα activates the intestinal expression of PEPT1 mRNA during the light period, so that PEPT1 protein levels have a peak before the start of the dark phase. After food intake, the bile acids accumulated in the intestinal epithelial cells interfering with recruitment of the co-transcriptional activator CREB-binding protein (CREBBP, alias p300) on the promoter region of PEPT1 gene, thus suppressing PPARα-mediated transactivation of PEPT1. Suppression of PPARα-mediated transactivation by bile acids causes an oscillation in the intestinal expression of PEPT1 during the daily feeding cycle, which leads to changes in the intestinal absorption rhythm. Thus, due to the nature of its components, a molecular clock-independent mechanism also seems to operate at the intestinal level of the mouse by which bile acid-regulated PPARα activity controls the expression of PEPT1. Moreover, Qandeel and colleagues analysed the diurnal vs. segmental expression and function of PEPT1 in rat small intestine, observing diurnal variations in mRNA, protein, and transport function in duodenum and jejunum, but not in ileum (Qandeel et al. 2009b). Mucosal levels of mRNA and protein, as well as Gly–Sar uptake by everted sleeves, were determined at 09:00, 15:00, 21:00, and 03:00 in rats maintained in a 12 h light/dark cycle with light from 06:00 to 18:00. Under these conditions, PEPT1 mRNA and Gly–Sar uptake vary diurnally in duodenum and jejunum (with a peak at 15:00), but not in ileum. However, protein levels vary minimally, with virtually no changes in duodenum and very limited increases in jejunum and ileum with peaks at 21:00 and 03:00. Notably, maximal Gly–Sar uptake is observed in jejunum; analysis of the kinetic parameters showing that differences in Gly–Sar uptake are due to changes in maximal velocity. As suggested by the authors, the simultaneity between peaks of PEPT1 mRNA and transport function would involve an anticipatory mechanism that induces transcription of new mRNA, and a possible recruitment of pre-formed protein from cytoplasmic stores to the apical membrane to further increase uptake. Interestingly, Qandeel and colleagues also reported that under the above experimental conditions complete abdominal vagotomy abolishes diurnal variations in gene expression and transport function of PEPT1 (Qandeel et al. 2009a). In particular, vagotomy seems to mediate, at least in part, the diurnal variations of PEPT1 protein expression and transport function, but not to regulate or modulate the diurnal rhythms of PEPT1 mRNA expression. Going into the details of the neural regulation of PEPT1 function is beyond the aims of this review; but for a detailed analysis of the neural regulation of PEPT1, comprehensive reviews have been published (see Zabielski 2007; Mourad and Saadé 2011; Spanier 2014).
Information in teleost fish on this type of regulation is limited to cyprinids (grass carp and zebrafish). In particular, results by Liu et al. (2013b) in the grass carp show that intestinal PEPT1 mRNA levels have a diurnal (~3-fold) variation, with PEPT1 expression low during the day and high during the night, in this way largely resembling what was observed in rodents (see Pan et al. 2002). These experiments were conducted on fish maintained in a 12 h light/dark cycle with light from 06:00 to 18:00; and mRNA levels were measured every 3 h. Notably, when analysed in foregut, midgut and hindgut, PEPT1 mRNA expression profiles differ in the three segments, with the hindgut fairly departing from the foregut and midgut with respect to the time mRNA expression peaks at night; in fact, while in the foregut the peak of expression is observed from 21:00 to 06:00, in the midgut, it is restricted from 03:00 to 06:00 and in the hindgut to 03:00 only (Liu et al. 2013b). To date, it is not known whether such daily variations are the result of a clock-dependent or clock-independent process, or both, and further studies are required to address this point in teleost fish, so that information can be used for, e.g., better diet composition and feeding management in aquaculture. In this respect, a contribution to the question comes from the zebrafish model, since it has recently been reported that in zebrafish larvae PEPT1B seems to be clock-regulated (Laranjeiro and Whitmore 2014). In particular, larvae maintained in a 12 h light/dark cycle were collected at four time points—i.e. zeitgeber time (ZT) 3 (light), 9 (light), 15 (dark) and 21 (dark)—per day between 4 and 7 day post-fertilisation. Three intestinal-specific genes included in the study, i.e., fatty acid-binding protein 2 (FABP2), alias intestinal-type fatty acid-binding protein (IFABP), which is involved in fatty acid absorption and metabolism, caudal-type homeobox 1b (CDX1B), a transcription factor essential for the regulation of proliferation and differentiation of various zebrafish intestinal cell lineages and functionally equivalent to mammalian CDX2 (Flores et al. 2008; Chen et al. 2009; Hu et al. 2013), and PEPT1B itself, showed circadian rhythmicity on the light:dark cycle. Notably, the peak of PEPT1B expression (2.0–2.5-fold with respect to the lowest expression during the 24 h period) followed (21ZT) that of CDX1B (15ZF), and the peak of CDX1B followed (3ZF) that of FABP2 (Laranjeiro and Whitmore 2014). The above-described clock-controlled expression of intestinal absorption genes suggests that in zebrafish larvae, intestinal function is already highly programmed and circadian regulated, a phenomenon known to occur in the adult mammalian intestine (see Pacha and Sumova 2013). In addition, the transcription factor CDX1B is likely to provide a direct link during zebrafish larval development between circadian clock, cell cycle and cell differentiation.
PEPT1 and feed deprivation
The quantity of food available in the gut—and thus, a large variety of sub-optimal nutritional conditions, such as underfeeding, malnourishment, brief fasting, prolonged fasting, starvation, etc—strongly affects gastrointestinal physiology at all levels of gut morpho-functional organisation (for critical reviews and books, see, among many others Chappell et al. 2003; Barboza and Hume 2006; Wang et al. 2006; Shaw et al. 2012; McCue 2010, 2012, and literature cited therein). Depending on the time and mode of effective food deprivation, significant changes can be observed, ranging progressively from simple variation of gene expression (e.g., under a food limitation condition applied for short times) to dramatic whole organ (re-)organisation (e.g., under a food limitation condition applied for long times). The morpho-functional alterations are evident in the gut with respect to other organs, basically due to the relatively high metabolic costs of maintaining the high turnover rate of gut tissues (see McNurlan and Garlick 1981; McMillan and Houlihan 1989; Croom et al. 1999); and both ectotherms (see Secor and Diamond 2000; Starck and Beese 2002; Cramp and Franklin 2003; Perez-Velasquez et al. 2003; Secor 2003; Naya and Bozinovic 2006) and endotherms (see Hume and Biebach 1996; Battley et al. 2000; Ferraris and Carey 2000; Bauchinger et al. 2005) almost invariably reduce the mass of their gastrointestinal tissues to reduce the energetic demand of this organ during starvation. With this in mind, it is natural that PEPT1 is influenced/conditioned by and/or subjected to alterations virtually due to all food limitation/deprivation states.
Going into the details of the various feed deprivation conditions is beyond the scope of this review. In addition, we are aware of many questions that arise when the studies on the physiology of malnourishment/fasting/starvation/etc are compared, as mainly due to (a) the little uniformity in the terminology used for the description of the phenomena; (b) the variety of definitons and experimental approaches used to exemplify the various states of feed restriction; (c) the species-specificity of the response because of the variable metabolic architectures of the animal models studied; (d) the influence of the wild vs. laboratory environment; and (e) other factors. However, with the limits of the following descriptions, we will try to integrate in one section all the information on PEPT1 and feed deprivation in vertebrates, with emphasis on teleost fish, describing the results obtained passing from short- to long-term deprivation states and taking into account that the vast majority of the papers published deal with relatively short-term deprivation studies in higher vertebrates, namely, mammals and birds, and relatively long-term deprivation studies in lower vertebrates, namely, teleost fish.
It has been known for decades that in mammals, PEPT1 responds to feed deprivation, in part but not only because of its late expression in the mature enterocyte. After early studies in human volunteers (Vazquez et al. 1985), studies conducted in rats have led to the observation that short-term starvation increases PEPT1 mRNA and protein expression (see Thamotharan et al. 1999; Ogihara et al. 1999; Ihara et al. 2000). In particular, PEPT1 mRNA and protein were found to increase ~3-fold, and the rate of peptide transport to increase ~2-fold (with a measurable effect of the maximal transport rate of the transporter) after a brief (only 1 day) fasting (Thamotharan et al. 1999). A significant increase in PEPT1 protein abundance was also observed (from the tip of the villus to its base) in the enterocytes of rats fasted for 4 days, a more prolonged fasting condition (Ogihara et al. 1999). In another study in rats, animals starved for 4 days, semistarved (50% amount of control) for 10 days, or given total parenteral nutrition (TPN) for 10 days, respectively, exhibited a ~1.8-fold, ~1.6-fold, and ~1.6-fold increase of PEPT1 at both mRNA and protein level when compared to control (free feeding) rats; in contrast, SGLT1 expression did not show any significant changes. Upregulation of PEPT1 occurred in spite of the decreased mucosal weight observed in starved and TPN rats (down to ~0.4-fold and ~0.5-fold, respectively); and thus, the increased expression of PEPT1 mRNA and protein was interpreted as a functional mechanism to compensate for the reduced mucosal surface area during fasting with an increase in the enterocytic population of PEPT1 transporters (Ihara et al. 2000; for review, see also Adibi 2003; Daniel 2004; Gilbert et al. 2008b, and literature cited therein). Next, by analysing the longitudinal pattern of expression in rat small intestine, Naruhashi and colleagues observed that PEPT1 mRNA level was low in the upper region and gradually increased passing from the upper to lower regions in fed animals. In this study, fasting rats for 2 days induced PEPT1 mRNA expression, and such induction was more prominent (up to ~3-fold in the more proximal segments) in the upper region of the small intestine. Moreover, PEPT1 transport capacity closely paralleled PEPT1 mRNA expression along the small intestine in both fed and fasted conditions (Naruhashi et al. 2002). Other studies confirmed and extended the preliminary findings on PEPT1 in fasting states. In particular, Howard et al. (2004) examined the effects of TPN (intended as an experimental manoeuvre to completely remove intestinal luminal nutrition) and of the following administration (by blood infusion) of the intestinotrophic hormone glucagon-like peptide 2 (GLP-2) on the mRNA expression of PEPT1 and nine apical and basolateral amino-acid transporters in rat small intestine. Compared to orally fed rats, TPN for 7 days upregulated (~1.7-fold) PEPT1 mRNA in the distal small intestine (ileum), whereas proximal (duodenal) mRNA was unchanged. Analogously, removal of luminal nutrition increased the expression of the apical transporters Solute Carrier 1 (SLC1) family member A5 (SLC1A5, alias ASCT2), SLC1 family member A1 (SLC1A1, alias EAAC1), and SLC3A1 (alias rBAT) in the ileum, while it decreased the expression of the basolateral transporters SLC1 family member A4 (SLC1A4, alias ASCT1), Solute Carrier 38 (SLC38) family member A2 (SLC38A2, alias SAT2/SNAT2) and of the basolateral/apical transporter SLC6 family member A9 (SLC6A9, alias GLYT1) in the duodenum. Conversely, SLC7 family member A1 (alias CAT1), Solute Carrier 36 (SLC36) family member A1 (alias PAT1) and SLC38 family member A5 (alias SN2/SNAT5) mRNA abundances remained unaffected by the treatment. Notably, administration of GLP-2, which is a trophic factor that maintains cellular protein synthesis during luminal starvation, reversed the effect of TPN on the mRNA expression of both PEPT1 and the amino-acid transporters tested. Taken together, the results by Howard and colleagues likely support the view of Ferraris and Diamond (1989), who previously described the regulation of nutrient transporters as a way to match uptake capacity to requirements without wasting energy on unnecessary transporters. Further elucidation on the functional response of PEPT1 in the fasting state derived from experiments in rats in which the three different phases of response to food deprivation (i.e., phase I, phase II, and phase III) had accurately been determined in parallel to PEPT1 expression and localisation at the intestinal epithelium level (Habold et al. 2007). Briefly, such as in the other mammals and birds, rats exhibit three distinct levels of energy depletion during prolonged fasting that designate three sequential phases. Phase I that lasts only a few hours after fasting has started and is a rapid period of adaptation characterised by flushing of the gut content, increasing mobilisation of fat stores and lowering of protein use. Phase II that lasts 5–6 days starting immediately after phase I and is a long phase of economy during which most of the energy expenditure is derived from lipids, whereas body proteins are spared. Phase III that starts immediately after phase II and is characterised by an increase in protein use and by a progressive exhaustion of the fat stores; during this phase, a change in animal behaviour also promotes food foraging, which anticipates a phase of lethal depletion of energy (for a thorough description of fasting phases I, II, and III, see Wang et al. 2006). Namely, in the context of a larger fasting/refeeding experimental scheme (for further details, see the “PEPT1 and compensatory growth”), PEPT1 expression and localisation were studied in normally fed, phase II and phase III fasted rats, and they were found to increase strongly in phase II and especially in phase III fasted animals (i.e., phase III > phase II) with respect to normally fed animals. Notably, after a short phase I that lasted only one day, these rats were in phase II from day 2 to day 6 and entered phase III from day 7 onwards (Habold et al. 2007; see also Habold et al. 2004, 2005). These results are particularly relevant also, because they allowed condensation of all the data on PEPT1 and fasting published until then into a more schematic and comprehensive picture. Later on, Ma and colleagues observed that in mice as little as 16 h of fasting were enough to cause significant (~2-fold) upregulation of PEPT1 protein expression in duodenal, jenunal and ileal segments and consequent increase of the in vivo oral absorption of Gly–Sar, so that systemic exposure and peak plasma concentrations of this dipeptide were ~1.4- and ~1.7-fold higher, respectively, in fasted vs. fed animals (Ma et al. 2012). This functional adaptation was effective, since it was fully abolished in PEPT1 knockout mice (Hu et al. 2008; Ma et al. 2012). Notably, the results reported in rodents have been confirmed in birds. In particular, the expression levels of PEPT1 were analysed in broiler chicks subjected to a 24 h feed restriction (feed withdrawn), and a ~2-fold increase in PEPT1 expression was observed after with respect to before feed restriction (Madsen and Wong 2011; see below in this paragraph for further details on this study).
As first shown by Shimakura and colleagues the increased intestinal expression of PEPT1 in a short-term fasting state in rodents depends on PPARα, one of the members of a family of nuclear receptors activated by fatty acid ligands (Shimakura et al. 2006a). In particular, in PPARα knockout mice the induced expression of PEPT1 after 48 h fasting was fully abolished, whereas in wild-type mice, there was a significant increase in both PPARα and PEPT1 expressions. When a PPARα ligand (i.e., WY-14643) was orally gavaged to fed rats for 5 days, expression of PEPT1 mRNA increased, and when Caco-2 cells were treated with the same ligand, both PEPT1 expression and Gly–Sar uptake increased. Although in this work, the functional response element or other regulatory region of the promoter was not determined, Shimakura and colleagues suggested that PEPT1 is either directly regulated by PPARα through binding to a regulatory region or that PPARα induces expression of transcription factors, such as the basal SP1 (Shimakura et al. 2005) or the intestine-specific CDX2 (Shimakura et al. 2006b), which are also found to be upregulated in response to fasting (Shimakura et al. 2006a). Interestingly, the results reported in rodents have been confirmed in a study conducted on broiler chicks in which the expression levels of PEPT1 and PPARα have been analysed after animals were subjected to a 24 h feed restriction (feed withdrawn) (Madsen and Wong 2011). As mentioned above, in this study, a ~2-fold increase in PEPT1 expression is observed in fasted chicks (~1.5-fold in duodenum, ~2.5-fold in jejunum and ~2.4-fold in ileum), while in parallel, PPARα increases by ~2.5-fold (~2.2-fold in duodenum, ~2.9-fold in jejunum and ~2.5-fold in ileum). Such as in mammals, also in birds PEPT1 mRNA upregulation occurs in spite of the dramatic changes in the morphology of the small intestinal mucosa, an aspect that in this group of animals has been studied in detail (see Uni et al. 1998; Fischer da Silva et al. 2007).
In teleost fish, experimental data on the effects of food deprivation on PEPT1 physiology are more fragmentary than in higher vertebrates, while, at least to our knowledge, no data are available in reptiles and amphibians. Gonçalves et al. (2007) first observed that 4 week feed deprivation led to downregulation (to 0.4–0.5-folds) of PEPT1A mRNA levels in the foregut and to disappearance of the mRNA signal in the hindgut of the agastric Asian weatherloach; no data were provided on PEPT1B in this study (Gonçalves et al. 2007). Later on, Terova and colleagues reported that 5 week feed deprivation of the gastric European sea bass resulted in ~70% reduction of intestinal PEPT1B mRNA levels with respect to control fish fed ad libitum (Terova et al. 2009). This tendency to reduction of intestinal PEPT1B expression (~35% reduction) could already be observed 4 days after the start of the feed deprivation protocol. Rønnestad et al. (2010) reported that 6 day feed deprivation downregulated intestinal PEPT1B expression in juvenile Atlantic salmon, another gastric teleost fish. In particular, 6 day feed deprivation (feed deprived group) resulted in a ~70% reduction of PEPT1B mRNA levels with respect to the control (fed group). In a recent study by Huang and colleagues in the Nile tilapia to assess the response of the various members of the SLC15 family to waterborne heavy metal exposure (Huang et al. 2015), the intestinal mRNA levels of PEPT1A and PEPT1B were comparatively measured in fasted vs. fed teleost fish. In particular, after 1 week feed deprivation, intestinal PEPT1A mRNA levels were significantly reduced by ~50% in fasted with respect to fed animals, whereas PEPT1B mRNA levels were only reduced by ~15% only. In another paper, Tian and colleagues systematically addressed the molecular activities in terms of expression of endocrine-, amino acid and peptide transporter-, and metabolic enzyme-related genes in 35 day old mixed sex zebrafish fed (0 h) and fasted for 384 h (i.e., 16 days), with fish sampled at 0, 3, 6, 12, 24, 48, 96, 192, and 384 h after feeding (Tian et al. 2015). This way, these authors covered both short- and long-term fasting conditions. Although with the limits of the experiment, performed on the whole fish instead of the intestine, a comparative set of mRNA expression data was produced, and in particular, the expression of amino acid vs. peptide transporters was evaluated. While PEPT1 expression exponentially decreased with time (down to ~0.3-fold at 384 h), there was a significant decrease of expression (down to ~0.2-fold at 12 h) followed by a peak (up to ~1.9-fold at 24 h) and a second downregulation event (down to ~0.1-fold at 384 h) for the neutral amino-acid transporter B0AT1. Analogously, the neutral and cationic amino-acid transporter ATB0,+ (alias SLC6 family member A14, SLC6A14) exhibited a decrease of expression (~0.4-fold at 6 h) followed by a dramatic peak (up to ~3.5-fold at 24 h) and a second downregulation (to ~0.5-fold at 192–384 h), while the high-affinity/low-capacity peptide transporter PEPT2 showed a decrease (~0.7-fold at 6 h) followed by a peak (~2.0-fold at 96 h) and a second decrease (~0.6-fold at 384 h). In addition, the neutral amino-acid transporter ASCT2 (alias SLC1A5) exhibited an initial upregulation (~1.6-fold at 3–6 h) followed by a rapid decrease (down to ~0.4-fold at 24–384 h). Although with the limits said, these data suggest the existence of a differential expression of genes known to be intestinally expressed and involved in amino-acid absorption in spite of the drop of gut mass and the overall (re-)organisation of gut anatomy and morphology that occurs during fasting. Noteworthy, the downregulation vs. upregulation of PEPT1 vs. B0AT1, both known to be functionally expressed along the fish gut and both kinetically characterised in great detail (see also Margheritis et al. 2013a; Rimoldi et al. 2015), suggests that in spite of the morpho-functional changes that occur in the gut with fasting, dynamic and active changes in gene expression take place to allow the fish to face optimally a state of nutritional limitation. Further and specific studies are required to fully address this point.
In summary, that the intestinal PEPT1 mRNA and protein expression is reduced under a condition of feed deprivation (feed withdrawn) seems to be a common theme in fish, and it emerges as a hallmark of a state of undernutrition and as an adaptive response that contrasts with the experimental evidences from higher vertebrates (mammals and birds), in which an upregulation of PEPT1 mRNA and protein levels is invariably reported in malnourished animals (see the cases discussed above; for review, see Nielsen and Brodin 2003; Daniel 2004; Gilbert et al. 2008b). It remains to integrate such findings on teleost fish PEPT1 in the teleost fish growth scheme taking into account that significant differences exist between endotherms (mammals and birds) and ectotherms (reptiles, amphibians and fishes) and that teleost fish largely fit into the ectotherm model (for recent review, see Zaldúa and Naya 2014). Moreover, it has to be clarified how teleost fish PEPT1 operates in the various phases of fasting. If they exist in teleost fish, such phases of fasting most probably span a much more extended time frame (i.e., at least one order of magnitude higher than in mammals and birds) (see Bar 2014; for reviews, see Wang et al. 2006; Zaldúa and Naya 2014, and literature cited therein). That PEPT1 dynamically cooperates, in a larger and more integrated network of adaptive programs/strategies but with major differences between ectotherms and endotherms, to adapt to food limitation/deprivation states, is a concept to develop systematically.
PEPT1 and compensatory growth
‘Compensatory growth’ and ‘catch-up growth’ are expressions frequently used as synonims to indicate the faster than optimal growth that occurs following a period of dietary restriction during the development of many animals. The former expression often refers to a faster than usual growth rate, while the latter implies attainment of a control size (see Hector and Nakagawa 2012). Compensatory growth is a widespread phenomenon present in both invertebrates and vertebrates, and in vertebrates, it is virtually recognised in all groups: humans, other mammals, birds, reptiles, amphibians, but most extensively fish (for reviews, see Ali et al. 2003; Dmitriew 2011; Won and Borski 2013, and literature cited therein). Many relevant specificities can be emphasised from vertebrate group to vertebrate group, with differences found between, e.g., endotherms (mammals and birds) and ectotherms (reptiles, amphibians and fish), determinate growers (again mammals and birds) and indeterminate growers (again reptiles, amphibians and fish), etc, which makes comparative analysis of this biological phenomenon across vertebrates not easy to unravel.
Although experiments of fasting/refeeding have been performed (e.g., in the rat; see Pan et al. 2003, 2004), to our knowledge, specific data available on PEPT1 expression/function in the context of a strict compensatory growth protocol are practically absent in higher vertebrates, mainly due to the lack of application of specific study designs to assess the effects on animal growth. Conversely, data from teleost fish models clearly indicate that PEPT1 responds to fasting and refeeding when the animals are subjected to protocols/procedures/manoeuvres of compensatory growth (such as in the so-called ‘fasting/refeeding paradigm’, which is widely used to investigate the molecular mechanisms underlying the transition from a catabolic to an anabolic state in fish; see Chauvigné et al. 2003; Montserrat et al. 2007a, b; Terova et al. 2007). The first evidence of the involvement of PEPT1 in compensatory growth in teleost fish comes from experiments performed in the European sea bass, a temperate organism, which is an excellent model for muscle growth studies as it exhibits an extensive juvenile (post-metamorphosis) muscle growth that contributes to its large adult size. This is advantageous in compensatory growth studies, since it allows easy determination of the compensatory effect(s) in terms of growth. Indeed, in most teleost fish, growth is indeterminate and the major part of absorbed nutrients is invested in accreting the skeletal (white) muscle (Mommsen 2001), which forms 40–60% of the body mass in most adult fish. As it accounts for more than half of the fish body mass, and as it exhibits the highest growth rate efficiency than any other tissues (Houlihan et al. 1995), skeletal muscle represents per se an excellent overall measure of fish growth. Combined with this is the fact that the natural life cycle of many teleost fish species, the European sea bass included, comprises natural fasting periods as a consequence of variable temporal (e.g., seasonal) and spatial (e.g., areal) food availability in the environment. In this respect, fish are well adapted to survive for long periods without food, and metabolic depression seems to be an important strategy in response to periods of food scarcity (Cook et al. 2000; O’Connor et al. 2000). When food levels are restored, growth can increase over and above normal rates, following the compensatory growth plan (MacKenzie et al. 1998; Ali et al. 2003).
In the European sea bass, the nutritional status influences the expression of key growth-related genes (for a comprehensive review on genes involved, see Won and Borski 2013) that encode for proteins that in both endocrine and autocrine/paracrine fashion promote compensatory growth induced by refeeding (as assessed by applying a fasting/refeeding paradigm in which fish are kept unfed and monitored for 35 days, namely, at 4 and 35 day time points, and then re-alimented and monitored for 21 days, namely, at 4, 10, and 21 day time points; see Terova et al. 2006, 2007). In particular, it was found that the nutritional status influences the expression of myostatin in muscle and insulin-like growth factors I and II in liver and muscle, and that such regulatory responses highly correlate with changes in fish body weight and shape (for details, see Verri et al. 2011, and literature cited therein). At the gastrointestinal level, the stomach responds to fasting by upregulating ghrelin and downregulating gastricsin (alias pepsinogen C) expression as part of an integrated response to food availability (see Verri et al. 2011, and literature cited therein; for further comparison, see also Tian et al. 2015). In concert, PEPT1 expression in the proximal intestine decreases (down to ~0.3-fold) during fasting and increases (up to ~1.5-fold) during refeeding (Terova et al. 2009; Verri et al. 2011), suggesting that PEPT1 operates in direct support of teleost fish (muscle) growth. Instead, in the European sea bass Hakim et al. (2009), who measured PEPT1 expression at 0 (fed control), 3, 7, 14, and 21 days fasting period, observed, within a general decline of PEPT1 mRNA expression during the experimental period, a significant burst (~3-fold increase) of PEPT1 mRNA levels at 7 days fasting before the gene expression was down-regulated again, which contrasts to what was observed by Terova et al. (2009). This apparent contradiction can, however, be reconciled by the recent findings obtained in mummichogs subjected to a fasting/refeeding protocol in which fasting was administred for 21 days, with time points at 1, 3, 5, 7, and 21 days, and refeeding for 7 days, with time points at 1 and 7 days (Bucking and Schulte 2012). In this experiment, a more complex scheme emerged in which PEPT1 mRNA expression increased with an early burst (~1.5-fold vs. pre-fasted fish) of expression at 3 days of fasting, which was followed by a dramatic decrease at 7 and mostly (down to ~0.2-fold vs. pre-fasted fish) 21 day fasting. Upon resumption of feeding, PEPT1 was upregulated compared to pre-fasted controls and after 7 days of re-feeding expression was again above (~1.5-fold) control values. Interestingly, such time course occurred similarly for both PEPT1B and PEPT1A, which were tested separately and independently in this experiment. Further verification to the time course described in the other teleost fish come from experiments conducted in zebrafish subjected to a fasting/refeeding paradigm in which fish were maintained unfed and monitored for 5 days at the 1, 2, 3, and 5 day time points, and then re-alimented and monitored for 4 days, at the 1 h and 1, 2, and 4 day time points (Koven and Schulte 2012). With respect to the regular feeding condition (control), PEPT1 mRNA expression steadily decreased (with no early burst observed) during the fasting period and then drastically increased over the control values immediately after the refeeding started and up to the monitored refeeding period when the increase was eightfold higher than in fish fed prior to fasting. This effect on PEPT1 was concerted with the dynamic response observed for the mRNA products of other key genes, e.g., the satiety hormones cholecystokinin, gastrin-releasing peptide and ghrelin. As one of the latest findings, in the zebrafish within a general decrease of expression (see the “PEPT1 and feed deprivation”) a small peak of PEPT1 mRNA expression, although not significant, can be observed at 4 days of fasting (Tian et al. 2015).
With their complete analysis of the Nile tilapia SLC15 transporters expression pattern under different experimental settings, Huang et al. (2015) tested SLC15 transporters, namely, PEPT1A (alias SLC15A1A), PEPT1B (alias SLC15A1B), PEPT2 (alias SLC15A2), PHT1 (alias SLC15A4), PHT2 (alias SLC15A3), and SLC15A5 for proximal intestinal mRNA expression under a simplified fasting-refeeding protocol consisting of 7 day fasting followed by 7 day refeeding. The mRNA levels of SLC15A1A, SLC15A1B, SLC15A2, and SLC15A5 in the proximal intestine were all downregulated after fasting for one week (by ~50, ~10, ~70, and ~60%, respectively) and recovered after refeeding the fish for 1 week (all were back to the control value with the exception of SLC15A1B that exhibited a ~2.5-fold increase in mRNA expression with respect to the control). Conversely, the fasting-refeeding expression pattern of SLC15A4 was different from the other members, since the mRNA transcript of SLC15A4 significantly increased with respect to the control in spite of the fasting-refeeding state.
Taken together these data suggest that the time course of PEPT1 expression during a fasting/refeeding protocol proceeds according to a more complex scheme than as previously thought, in which a short-term burst may possibly occur early during the fasting period within a general decrease of expression that is increasingly more evident at long-term. Soon after the refeeding starts PEPT1 expression dramatically increases and almost invariably exceeds the control value measured in the fed animal. Thus, based on the results coming from numerous experiments in different species and with numerous time points, we propose that the short-term increase measured in the European sea bass (at 7 days), in the mummichog (at 3 days), and possibly in the zebrafish (at 4 days) may be a short-term physiological response as previously described in mammals and birds (see Ogihara et al. 1999; Thamotharan et al. 1999; Ihara et al. 2000; Naruhashi et al. 2002; Shimakura et al. 2006a; Habold et al. 2007; Gilbert et al. 2008b). Then, a decrease occurs which is mostly related to more dramatic morpho-functional changes that occur in the gut. Moreover, such findings on PEPT1—taken in the frame of compensatory growth protocols—suggest that PEPT1 plays an active role in animal feeding by operating, at the gastrointestinal level, as part of an integrated response network to food availability that directly supports body weight. These findings also document a correlation between the expression of an intestinal transporter, such as PEPT1, which is primarily involved in the low-affinity/high-capacity uptake of dietary protein degradation products, and animal growth in a vertebrate model. In teleost fish, PEPT1 may contribute to provide in the form of di/tripeptides the necessary amount of amino acids to sustain net protein synthesis, and thus body growth.
In a perspective, complementary information on how PEPT1 may dynamically respond to fasting-refeeding solicitations can come from the application of experimental schemes other than those described above, i.e., relative long times of fasting and then continuous refeeding, by which the transcript/protein abundance is assayed over several days or weeks. Conversely, the relative long fasting could be followed by a single satiating meal treatment (see Valente et al. 2012) and the biological response analysed at shorter times, i.e., over hours or days (post-prandial), as recently assessed for a number of amino-acid transporters in the Chinese perch (Siniperca chuatsi) (Wu et al. 2016).
PEPT1 and dietary protein levels
The effects of variation in dietary protein quantity/quality are known for more than one century when the experimental manipulations of dietary protein first started in laboratory and farm models. All together, the data collected indicate that variation in absolute protein quantity changes the feeding behaviour since high-protein diets suppress food intake, moderately low-protein diets increase food intake, and very low-protein diets (i.e., the insufficient protein levels) suppress food intake and block growth. However, also variation in absolute protein quality (i.e., the balance of amino acids) significantly influences food intake since many species exhibit a surprising ability to detect and then avoid imbalanced diets (for comprehensive reviews on these subjects, see Morrison et al. 2011; Morrison and Laeger 2015).
As already mentioned, in rat intestine mRNA expression and transport rate of PEPT1 increase (1.5–2.0-folds) when animals receive a high-protein (50%) diet as compared to a low-protein (4%) diet (Erickson et al. 1995). This finding first indicated that PEPT1 is affected by different levels of dietary protein, an observation followed by many other experimental data, mainly from rodents (see Daniel 2004; Gilbert et al. 2008b; see also the “PEPT1 and dietary protein, di/tripeptides and free amino acids”). More recently, studies conducted in PEPT1 knockout mice (see Nässl et al. 2011a, b) have led to the notion that PEPT1, at least in the adult rodent, is not required for regular amino-acid nutrition; however, PEPT1 plays a role during conditions of high-protein loads. Indeed, Pept1 −/− mice are viable; and apparently they do not exhibit any obvious impairments in reproduction, body weight or other biometric and biochemical parameters when fed a standard (i.e., a high-carbohydrate containing) diet (see Hu et al. 2008). In addition, they are reported to grow at similar rates as wild-type mice when fed a standard diet (see Hu et al. 2008; Nässl et al. 2011b; Kolodziejczak et al. 2013), although significant differences (reduction) in growth rates are observed when growth is monitored for longer (than 7–8 weeks) times (see Zhang et al. 2016; Hannelore Daniel, personal communication). Yet, the plasma amino-acid levels of Pept1 −/− mice increase with respect to wild type, which suggests altered systemic amino-acid handling. In addition, administration of an acute high-protein (vs. low protein) load via gastric gavage causes significant alteration of the plasma concentrations of many (up to ~15) amino acids (with proline showing the largest concentration difference) (see Chen et al. 2010; Nässl et al. 2011a). Nässl and colleagues also demonstrated that, with respect to Pept1 −/− mice fed ad libitum a medium-protein (control) diet (with 21% of energy available as protein), Pept1 −/− mice fed a high-protein diet (with 45% energy available as protein) considerably reduce food intake, which is followed by a marked (and significant) loss of body weight, while Pept1 −/− mice fed a low-protein diet (with only 8% of energy available as protein) do not show any effects. This phenomenon is time-dependent, since Pept1 −/− mice exhibit a reduced consumption of food over the first 4 days but then they gradually increase food consumption and by 18 days they have the same intake rate as wild-type animals; but, Pept1 −/− mice fail to show any significant weight gain over the entire feeding period, thus indicating reduced assimilation of intestinal energy (Nässl et al. 2011b). Based on these data, it could be argued that since high-protein diets increase satiety and reduce, at least transiently, food intake, which associates with a retarded weight gain (for review see, Westerterp-Plantenga et al. 2009; Morrison et al. 2011; Daniel and Zietek 2015; Morrison and Laeger 2015), and since PEPT1 is not largely expressed in the brain, it is possible that (at least in mice) PEPT1 is part of a gut-brain signalling axis, through which protein-rich diets are sensed in the gut (for review, see Daniel and Zietek 2015). In this context, it is worth noting the highly remarkable response of Pept1 −/− mice fed ad libitum a high-fat diet (with 45% energy available as fat), since these animals seem protected from high-fat diet-induced obesity (Kolodziejczak et al. 2013; for comparison, see also Do et al. 2014). As said, Pept1 −/− mice show no obvious differences in gross phenotype with respect to wild-type animals when fed on a standard (high-carbohydrate) diet. Conversely, when fed a high-fat diet to induce obesity they show markedly reduced weight gain and reduced body fat stores, and appear protected from hyperglycemia and hyperinsulinemia. In addition, they exhibit reduced caloric intake, no changes in energy expenditure, but increased energy content in feces (Kolodziejczak et al. 2013). When analysed in detail, Pept1 −/− mice show increased cecal biomass when fed on both high-carbohydrate and high-fat diets, which suggests limited capacity in digesting and/or absorbing the dietary constituents in the small intestine. Yet, metabolite profiling of the caecal contents shows consistent differences of the two diets, since high levels and broad assortment of sugars are present in Pept1 −/− mice fed on the high-carbohydrate diet whereas animals fed the high-fat diet exhibit high levels of free fatty acids and absence of sugars. Notably, in Pept1 −/− mice fed the high-fat diet, the analysis of intestinal morphology reveals the lack of adaptation of the upper small intestinal mucosa to the trophic effects of the diet, in terms of, e.g., increased villus length and increased surface area (Kolodziejczak et al. 2013). Thus, while exhibiting minimal effects of body weight gain, PEPT1 deficient mice seem to have very complex phenotypes when they are challenged by altered diets. They generally appear less flexible in adapting to diet changes leading to different phenotypes on diets rich in carbohydrates, protein or fat.
In rodents, not only protein quantity, but also protein quality may affect PEPT1 mRNA and protein levels, as exemplified by the recent paper of Li et al. (2016), who reported for the first time the impact of casein, soy and meat proteins (at a recommended intake of 20%) on intestinal PEPT1 expression (and mucosal structure) of young rats fed for 7 days with casein (control), soy, red (beef and pork) and white (chicken and fish) meat proteins. Interestingly, possibly due to the fact that different sources of dietary proteins—e.g. dairy, plant and meat proteins—significantly differ in amino-acid composition, the experiments showed that with respect to casein protein diet: a) PEPT1 mRNA and protein levels in duodenum had a positive response (increase) to soy protein diet and a negative response (decrease) to meat protein diets and b) PEPT1 mRNA and protein levels in jejunum had the same response (no change) to soy protein and beef protein diet and a negative response (decrease) to pork, chicken and fish protein diets (Li et al. 2016; for comparison see also Song et al. 2016)
Due to the influence of dietary protein on its expression and function, in the last decade, expectations of PEPT1 have arisen from the livestock and poultry industry. In fact, dietary protein is costly, and even fractional improvements in its utilisation have the commercial potential to save money and the ecological potential to reduce nitrogen excretion into the environment. In this respect, studies have started on the regulation of dietary protein on PEPT1 function in farmed species. In poultry, the effect of different dietary protein levels on PEPT1 expression and its possible association with body growth has been analysed in chickens fed 12, 18 and 24% crude protein (CP) at food intake restricted to that consumed by birds fed the 12% CP diet (Chen et al. 2005). In this feeding setting, greater levels of CP in the diet could be associated with greater PEPT1 expression during the first 35 day post-hatch. In particular, during the time course (0, 1, 3, 5, 7, 10, 14 21, 28, and 35 day post-hatch) chickens fed 12% CP exhibited a decrease in PEPT1 mRNA abundance throughout the experiment (down to 0.5-fold at 35 vs. 0 days post-hatch) in duodenum, jejunum and ileum, whereas chickens fed the restricted 18 and 24% CP showed an increase (up to 2.0-fold at 35 vs. 0 day post-hatch) in the three sections of the small intestine. While PEPT1 mRNA levels were the lowest in chickens fed the low-protein (12% CP) diet, intermediate for chickens fed the medium-protein (18% CP) diet, and the highest in chickens fed the high-protein (24% CP) diet, the body weight of the chicken fed the 12% CP diet was lower (632 g at 35 days) than that of the chicken fed the 18% CP diet (862 g at 35 days) and this lower than that of the chicken fed the 24% CP diet (949 g at 35 days). In another set of experiments, the same authors observed that in chickens fed the 24% CP diet ad libitum (40% greater intake), PEPT1 mRNA abundance is much lower than that of the 18 and 24% CP feed-restricted groups, and close to that of chickens fed the 12% CP diet; in parallel, the body weight of the chicken fed the 12% CP diet is lower (504 g at 35 days) than that of the chicken fed the 18% CP diet (746 g at 35 days) and this lower than that of the chicken fed the 24% CP diet (877 g at 35 days), while the body weight of the animals fed ad libitum the 24% CP diet is the highest (1854 g at 35 days) (Chen et al. 2005). All together, these results suggest that under the above experimental feeding designs, upregulation of PEPT1 expression is most probably not due to the increase in CP but instead to the feed restriction.
In poultry, dietary protein quality, as revealed by the balance of amino acids required for maximal growth and protein synthesis and the limiting amino acids present in the meal (for specific facts in poultry, see Fisher et al. 1959), also correlates to PEPT1 expression (Gilbert et al. 2008a). In particular, chickens fed a diet containing a low-quality protein (corn gluten meal) for the first 2 week post-hatch show decreased expression of PEPT1 from day 3 to day 7 (down to ~0.6-fold vs. day 3) followed by increased expression to day 14 (up to ~1.3-fold vs. day 3); however, in restricted chickens that consumed an equal amount of the same diet substituted with a greater quality protein (soybean meal), PEPT1 mRNA levels continuously rise with age from day 3 to day 7 (up to ~1.5-fold vs. day 3) and from day 7 to day 14 post-hatch (up to ~1.5-fold vs. day 3), and chickens are heavier (soybean meal restricted chicken body weight: 97 g at day 14; corn gluten meal fed chicken body weight: 66 g at day 14) (Gilbert et al. 2008a). This finding has been read as while in chickens consuming the soybean meal continuous upregulation of PEPT1 serves as a mechanism to maximise assimilation of amino acids from a well-balanced mixture, in chickens consuming the corn gluten meal the effect is more complex due to the imbalanced diet. Again, in chickens fed the soybean meal ad libitum (~3-fold greater intake than the other groups), PEPT1 mRNA levels are systematically lower than that of the soybean meal restricted animals, and lower-to-equal to that of chickens fed the corn gluten meal; in parallel, the body weight of the animals fed the soybean meal ad libitum is much higher (200 g at 14 days) than that of the other two groups (97 and 66 g at 14 days, respectively) (Gilbert et al. 2008a).
All together, these observations in mammals and birds suggest that both abundance and lack of substrates can affect PEPT1. Upregulation by a high-protein diet may represent a mechanism to take advantage of the abundant resources, whereas upregulation in response to a lack of substrates may represent a compensatory mechanism to scavenge amino acids in the lumen. The extent of the response, in terms of change of PEPT1 expression and activity, also depends on other factors, such as time interval for a particular dietary manipulation, availability of transported substrates, amino-acid composition (also in terms of concentrations of free and peptide-bound amino acids), and presence of other components in the lumen (i.e., sugars, vitamins, minerals, fats, etc) that can affect/influence/change digestive and absorptive dynamics. Moreover, the extent of the response involving PEPT1 can depend on the actual animal physiological status. For example, young growing animals have a relatively high-protein requirement, and many results from several species indicate that young animals select for a higher percent protein compared to older individuals (see Bradford and Gous 1991; Forbes and Shariatmadari 1994; White et al. 2000; Jean et al. 2002). In addition, animal lines that are bred for high muscle growth tend to choose diets higher in protein compared to slower growing lines (see Forbes and Shariatmadari 1994). Moreover, chronic administration of growth hormone increases muscle mass, reduces body fat, and produces an increased selection for dietary protein (see Roberts et al. 1995; Phositlimpagul et al. 2002). Under all these (and other) physiological states, no data are virtually available on the possible role/involvement of PEPT1 and new studies are required to fully address the role of this transporter in the animal growth dynamics.
In teleost fish, such as in livestock and poultry, interest on diet formulations and compositions has emerged parallel to the development of aquaculture and aquafeeds and to the economical implications related to the need of reducing costs while maintaining an adequate amount of good quantity/quality protein in fish feeds (for comprehensive reviews, see Hardy 1996; El-Sayed 1999; Watanabe 2002; Drew et al. 2007; Gatlin et al. 2007; Glencloss et al. 2007; Drakeford and Pascoe 2008; Lim et al. 2008, Sales 2009, and literature cited therein; see also the “Species-specificity of substrate uptake” paragraph above, and literature cited therein). Going into the question of diets, feed composition, ingredients, ingredient substitutions, fish meal replacement, etc is far beyond the aims of this review. Yet, to add to the evidences from the mammalian and avian models, an attempt has been made to summarise the data available from those studies addressed to the identification of possible relationships among PEPT1, diets and growth in teleost fish. In this context, as already said, teleost fish represent excellent models to study the effects of protein administration on body growth, because of the direct correlation that exists between dietary protein availability and accretion of the body (i.e., skeletal muscle) mass, and because of the acknowledged role of PEPT1 as a major intestinal route to absorb the bulk of digested protein needed to sustain body growth (see, e.g., the “PEPT1 and compensatory growth” paragraph above, and literature cited therein). Studying teleost fish in the ‘vertebrate’ context is even more relevant if one considers that (a) teleost fish require higher protein levels (up to 50% and more) than mammals and birds; (b) in a complete meal, i.e. a meal that supplies all the ingredients (protein, carbohydrates, fats, vitamins and minerals) necessary for optimal fish growth and health, the required protein levels may be (more than) double than the required fat and carbohydrate levels (i.e., 18–50% protein vs. 10–25% lipid vs. 15–20% carbohydrate); (c) teleost fish, especially when reared in high densities, require a high-quality, nutritionally complete, balanced diet to grow rapidly and remain healthy (see National Research Council 2011, and literature cited therein). In general, protein requirements are lower for herbivorous and omnivorous than carnivorous fish, and higher for fish reared in high than low-density aquaculture systems. In addition, protein requirements are higher for smaller fish; and as fish grow larger, their protein requirements usually decrease. In addition, protein requirements can vary with rearing environment, water temperature and water quality, as well as with the genetic background and feeding rates of the fish. As a general rule, proteins are used by the fish for their growth if adequate levels of fats and carbohydrates are present in the diet; if not, proteins are used for energy and life support rather than growth (see National Research Council 2011, and literature cited therein). Thus, in cultured teleost fish, while influencing growth, body size, body composition, etc, the right choice of the dietary source may significantly contribute, in a wider context, to animal welfare (for review, see among others Conceição et al. 2012).
PEPT1 and protein degradation products (hydrolysates, di/tripeptides, amino acids)
In the last decade, an increasing number of researchers, many involved in teleost fish biology, physiology and nutrition, has been focusing and discussing on the role that protein degradation products (from protein hydrolysates to oligopeptides to di/tripeptides to free amino acids) instead of intact protein can potentially have on animal growth; and, in this context, on the role that the di/tripeptide intestinal transport via PEPT1 can eventually play in the uptake of dietary amino acids coming from such protein degradation products. This attention mainly derived from the observation that teleost fish can efficiently use dietary di/tripeptides for development, growth, and metabolism and, consequently, that balanced peptide-based diets or peptide rather than amino-acid supplementation could have been beneficial to solving the nutritional inadequacy problems of formulated feeds for many cultured fish species (for reviews, see Dabrowski et al. 2010; Conceição et al. 2012; and literature cited therein). In higher vertebrates, similar studies are available on both mammalian and bird models, but they apper more oriented to the evaluation of the effects that protein hydrolysates, peptides and/or selected amino acids can have locally on various selected gastrointestinal function(s) (among many others, see Darcel et al. 2005; Gilbert et al. 2010; Liou et al. 2011; Nakajima et al. 2012; Diakogiannaki et al. 2013; Ito et al. 2016) than to the systematic evaluation of their effects on animal growth (among many others, see Ouellet et al. 1997; Vente-Spreeuwenberg et al. 2004; Li et al. 2011b; Chen et al. 2013; Opheim et al. 2016).
With this in mind, in the following paragraphs, we aim to summarise the state of the art on the experiments that have been conducted in the last few years to support the concept of the existence of a direct correlation between di/tripeptides and teleost fish growth and briefly provide the findings of the various studies as tables (for details, see Tables 3, 4, and 5). In these tables, data on protein hydrolysates, synthetic di/tripeptides (single or in combination) and/or synthetic amino-acid supplementations are considered. Note that while the number of papers published on di/tripeptides (single or in combination) and fish growth is very limited, and the results of all the relevant papers have been listed in Tables 3 and 4, dozens of papers have been published on the use of protein hydrolysates of various origin for better fish growth (among many others, see Oliva-Teles et al. 1999; Refstie et al. 2004; Hevrøy et al. 2005; Espe et al. 2006; Ostaszewska et al. 2008b; Chotikachinda et al. 2013; Smith et al. 2013a; Xu et al. 2016b; for recent reviews, see Chalamaiah et al. 2012; Jayathilakan et al. 2012; Martinez-Alvarez et al. 2015). Notably, since the early studies on the subject, it was clear that protein hydrolysates may contain large fractions of free amino acids, with molecular weight (MW) lower than 200 Da, and small peptides, i.e. di/tripeptides, with MW between 200 and 500 Da, and larger oligopeptides, with MW between 500 and 2500 Da (among many others, see Zambonino-Infante et al. 1997; Kotzamanis et al. 2007; Gisbert et al. 2012; Skalli et al. 2014). The results of only a limited number of papers specifically dealing with the effects of size-fractionated protein hydrolysates has been listed in Table 5. For a more extended and detailed analysis of the effects of protein-, peptide- and free amino-acid-based diets in fish nutrition, refer to Rønnestad et al. (2007b) and to Dabrowski et al. (2010).
In the first instance, experimental trials involving single dipeptide-containing diets have been conducted to establish whether or not a correlation between animal growth and inclusion of a selected dipeptide in a diet could be established. Overall, these studies have been performed feeding a given teleost fish species, e.g., common carp, Japanese flounder (Paralichthys olivaceus), rainbow trout, red sea bream (Pagrus major), yellow perch, at different developmental stages, e.g., larvae, alevins, juveniles, for various time intervals, e.g., from 4 to 9 weeks, and testing various synthetic dipeptides, e.g., Lys–Gly, Leu-Gly, carnosine, Met-Met, Phe-Phe, as single dipeptide included into a formulated diet. Growth has been assessed in terms of changes in body mass, while PEPT1 expression has in case been evaluated at the mRNA (and eventually protein) level (for details see Table 4). In spite of the absolutely large differences among the studies, some general conclusions can be drawn from these experiments, i.e., the supplementation of amino acids in the form of single synthetic dipeptide in a formulated diet seems to have beneficial influence on fish growth, since the dipeptide acts at least at the same level and often more effectively than its constituent amino acids supplemented in free form. In addition, both dipeptide- and constituent free amino-acid-based diets invariably allow fish to grow better with respect to amino-acid-deficient diets, i.e., a diet lacking of the constituent amino acids or a restricted diet, and very often at the same level as a standard complete commercial diet does (with the dipeptide-based diet occasionally performing even better than standard diet). When analysed at the mRNA and/or protein level, PEPT1 expression is found at least equal and more often increased in fish fed the dipeptide- with respect to fish fed the amino-acid-based diet. In addition, fish fed both dipeptide- and free amino-acid-based diets almost invariably show higher PEPT1 expression with respect to fish fed diets devoid of the dipeptide or the amino acids. In addition, PEPT1 expression is found either increased or equal in fish fed the dipeptide- or amino-acid-based diets with respect to fish fed the standard complete commercial diet (with PEPT1 expression being invariably higher in fish fed the dipeptide-based diet than fish fed the standard diet). Also because of the very limited number of experiments, how much these differences in growth and PEPT1 expression truly depend on the (form of amino acid) supplementation or to intrinsic (amino acid) imbalance/deficiency of the various tested diets is not easy to assess and in this respect more extended studies are needed.
In the second instance, experimental trials involving diets containing a mix of dipeptides have been conducted to establish whether or not a correlation between animal growth and inclusion of a mix of dipeptides in a given diet could be established. Overall, these studies have been performed feeding a given teleost fish species, e.g., silver bream (Vimba vimba), common carp, rainbow trout, South American pacu (Piaractus mesopotamicus), at different developmental stages, e.g., larvae, alevins, juveniles, for various time intervals, e.g., from 2 to 6 weeks, and testing various mixes of dipeptides included into a formulated diet. Growth has generally been assessed in terms of changes in body mass, while PEPT1 expression has never been evaluated (for details see Table 5). With respect to those testing a single synthetic dipeptide, these trials involving a mix of synthetic dipeptides are more homogenous in terms of experimental design and results obtained are more coherent somehow. In particular, the supplementation of amino acids in the form of a mix of dipeptides in a formulated diet appears to positively affect fish growth since a mix of dipeptides acts on weight gain more effectively than a mix of free amino acids. Notably, while a diet in which the mix of dipeptides replaces the intact protein portion never allows fish to grow as much as a standard complete diet does, partial (50%) replacement of the protein portion with the mix of synthetic dipeptides (1:1 ratio) results in very similar growth rates as a standard complete diet. However, this diet never matches the performance of a complete intact protein-based diet and/or live food (Artemia nauplii). Unfortunately, these experiments lack of any PEPT1 expression analyses so that no conclusion can be drawn in terms of possible involvement of PEPT1 in fish growth under such a type of dipeptide supplementation.
In the third instance, experimental trials involving size-fractionated protein hydrolysates have been conducted to establish whether or not a correlation between animal growth and inclusion of size-fractionated protein hydrolysates in a given diet could be established. Overall, these studies have been performed feeding a teleost fish species, e.g., Atlantic cod, Japanese flounder, large yellow croaker, rainbow trout, turbot (Scophthalmus maximus), at different developmental stages, e.g., larvae, juveniles, adults, for various time intervals, e.g., from 4 to 13 weeks, and testing various size-fractionated protein hydrolysates, e.g., fractions with MW from lower than 100 to higher than 20,000 Da, included into a formulated diet. Growth has generally been assessed in terms of changes in body mass, while PEPT1 expression has occasionally been evaluated at the mRNA level (for details, see Table 5). With respect to the experiments conducted with the synthetic dipeptides, these trials involving size-fractionated protein hydrolysates are very homogenous in terms of experimental design and results obtained are highly comparable, revealing that fractions containing small MW compounds included in formulated diets result very important for optimal fish growth, since they positively affect growth, as well as feed utilisation, protein retention, gastrointestinal functions, etc. Notably, overall PEPT1 expression is not significantly affected by the inclusion of protein hydrolysate fractions. However, a change of the relative levels of PEPT1 expression along the gut are observed in the presence of different fractions (see Bakke et al. 2010), suggesting that PEPT1 may be variably recruited along the whole intestine, including the most distal part, in response to changes in the luminal protein source content.
It is worth underlining that, due to the complexity and variety of feeding selections, procedures and protocols and to the implicit lack of general standardisation associated to feeding a diet—that changes from species to species and depending on fish biology, developmental (st)age, rearing conditions, environmental conditions, etc—it is intrinsically difficult to compare the results reported in this research field. In this respect, a systematic meta-analytic approach, with the aim of providing a quantitative synthesis of the data available from the scientific literature, would be meaningful, given that new tools are gradually starting to be offered to allow evaluation and integration of the results for given groups of studies, included those manifesting apparently contradictory results (among many others, see Sales 2009, 2011; Hua and Bureau 2012; Collins et al. 2013; Benstead et al. 2014, and literature cited therein).
The other H+-coupled oligopeptide transporters in teleost fish
Besides SLC15A1 (PEPT1), four other members of the SLC15 family have been recognised in higher vertebrates to date, namely SLC15A2 (alias PEPT2), SLC15A3 (alias PHT2), SLC15A4 (alias PHT1), and SLC15A5, which differ considerably one another for spatio-temporal expression, substrate affinity and biological function(s) (for reviews, see Fredriksson et al. 2008; Höglund et al. 2011; Sreedharan et al. 2011; Smith et al. 2013b). All but SLC15A3 are found in teleost fish genomes. However, at least to our knowledge, none of them is present in duplicated form in these genomes. In the following paragraphs, synthetic sketches of these members of the SLC family in teleost fish will be drawn, in comparison with information from higher vertebrates.
SLC15A2 (PEPT2)
As for PEPT1, PEPT2 properties have been described in detail in mammalian systems (see Rubio-Aliaga and Daniel 2002; Daniel and Rubio-Aliaga 2003; Rubio-Aliaga and Daniel 2008; Kamal et al. 2008; Smith et al. 2013b). With respect to PEPT1, PEPT2 is a high-affinity/low-capacity system, with apparent affinity constants of 0.005–0.5 mM depending on the substrate, and it exerts its function in both epithelial (where it is naturally located on the apical membrane) and non-epithelial cells. In mammals, PEPT2 is primarily expressed in kidney (epithelial cells of the proximal tubule S2 and S3 segments), peripheral nervous system (satellite glial cells), central nervous system (astrocytes, ependymal and subependymal cells, epithelial cells of the choroid plexus), lung (type II pneumocytes, tracheal and bronchial epithelial cells), lactating mammary gland (epithelial cells of the terminal duct and glandules as well as the main segmental ducts). Expression of PEPT2 mRNA has also been reported in spleen, colon, and pancreas (for reviews, see Rubio-Aliaga and Daniel 2002, 2003; Smith et al. 2013b, and literature cited therein), and more recently skin (Kudo et al. 2016). Notably, in the gastrointestinal tract PEPT2 has been found specifically expressed in glial cells and tissue-resident macrophages in the neuromuscular layer of the enteric nervous system (Rühl et al. 2005). In birds, a single study, at the best of our knowledge, conducted in embryonic and posthatch chicks indicates that PEPT2 is expressed in kidney and brain, with much lower expression in the gastrointestinal tract (proventriculus, duodenum, jejunum, ileum, ceca, large intestine), lung, heart, bursa of Fabricius, and liver (Zwarycz and Wong 2013).
In contrast to mammals and birds, information on PEPT2 in lower vertebrates, elasmobranchs (Hart et al. 2016) and teleost fish included, is limited. Electrophysiological (TEVC) analysis after cRNA injection in X. laevis oocytes has been performed for zebrafish PEPT2, suggesting that this transporter is a high-affinity/low-capacity transporter (K 0.5 for Gly–Gln of 0.018 mM at −120 mV and pH 7.5) (Romano et al. 2006). Zebrafish PEPT2 mRNA is mainly detected in adult brain, kidney, gut, as well as gill, skeletal muscle and spleen although at low extent. Interestingly, otic vesicle, the embryonic structure that develops into the auditory/vestibular organ, homolog to the higher vertebrate inner ear, specifically expresses PEPT2 (Romano et al. 2006). In the zebrafish, in a recent expression survey involving 25 among endocrine-, amino acid and peptide-transporter, and metabolic enzyme-related genes, PEPT2 has been monitored in 35 days old fish during fasting period of 16 days (Tian et al. 2015; for further details see also the “PEPT1 and feed deprivation” and “PEPT1 and compensatory growth” paragraphs above); in particular, expression of PEPT2 mRNA extracted from whole fish was found to decrease (down to ~0.2-fold) after 6 h fasting, to increase (up to ~2-fold) between 12 h and 8 days after the start of the fasting procedure, and to decrease again (down to ~0.5-fold) at the end of the experiment (16 days fasting). In another cyprinid, i.e. the common carp, low levels of PEPT2 mRNA expression have also been observed in the intestine, similar to zebrafish (Ostaszewska et al. 2010a). In this study, although limited, expression data have been generated in the presence of different diets, suggesting a possible effect of a selected dipeptide, i.e. Lys–Gly, on the intestinal expression of the transporter (Ostaszewska et al. 2010a; see also Table 3). In the Nile tilapia, PEPT2 mRNA has been detected in the liver, gill, brain, muscle, spleen, intestine, kidney, and stomach, with highest expression in intestine, stomach and kidney; in particular, at the intestinal level, significantly higher expression of PEPT2 mRNA has been measured in the mid intestine vs. distal intestine, with minimal expression in the proximal intestine (Huang et al. 2015). In line with the above presented observations, after a recent expression survey involving 13 among peptide, neutral and cationic amino-acid transporter genes, PEPT2 has directly been monitored along the intestine of the juvenile turbot, with observed PEPT2 mRNA levels exponentially increasing passing from the middle intestine to the distal intestine to the rectum (where the abundance of PEPT2 transcripts was several hundred-fold higher than that observed in the stomach, pyloric ceca and proximal intestine; Xu et al. 2016a). It could be argued that like in the kidney tubule, where PEPT1 (the low-affinity/high-capacity transporter) and PEPT2 (the high-affinity/low-capacity transporter) work in concert one downstream of the other to efficiently reabsorb peptide-bound amino acids from the tubular fluid (see Smith et al. 2013b), a similar regional compartimentalisation of peptide transporters may occur in the intestinal canal of the teleost fish (Bisesi et al. 2015; Tiziano Verri, unpublished data) to make the complete absorption of the di/tripeptides of dietary origin effective. However, at the moment a transepithelial PEPT2-mediated absorption of dietary protein degradation products at the intestinal level, although reasonable in theory, is not fully supported by proper functional data and in the light of the current knowledge on the cellular (cell-type) distribution of this transporter along the intestine, and detailed studies are needed to address this point.
SLC15A4 (PHT1), SLC15A3 (PHT2), and SLC15A5
SLC15A4 (PHT1), originally cloned from a rat brain cDNA library (Yamashita et al. 1997) and then from the human placental trophoblast BeWo cell line (Bhardwaj et al. 2006), is considered a high-affinity transporter for histidine (His), with a substrate affinity of 0.017 mM measured in the X. laevis oocytes expression system using radiolabeled substrate, and for His-containing dipeptides such as carnosine (Yamashita et al. 1997; Bhardwaj et al. 2006). However, certain molecules, such as valacyclovir (i.e., the valine substituted amino acid prodrug of acyclovir), have also been shown to be transported by PHT1 (Bhardwaj et al. 2006), while others are thought to interact somehow with the transporter, as reported for a limited number of di/tripeptides shown to inhibit PHT1-mediated His uptake (Bhardwaj et al. 2006; Hu et al. 2014). To our knowledge, no functional data are available from vertebrates other than mammals, teleost fish included.
Initial tissue expression studies reported that PHT1 is abundantly expressed in brain, eye, lung, gastrointestinal tract and placenta (Yamashita et al. 1997; Herrera-Ruiz et al. 2001; Bhardwaj et al. 2006). In particular, Herrera-Ruiz et al. (2001) observed PHT1 mRNA in rat stomach, small intestine, ceca, and colon, and in human heart, kidney, leukocytes, liver, lung, ovary, pancreas, placenta, prostate, skeletal muscle, small intestine, spleen, testis, and thymus. Such broad distribution of PHT1 expression in mammals is very similar to what was observed in birds (Zwarycz and Wong 2013), where PHT1 is detected in proventriculus, duodenum, jejunum, ileum, ceca, large intestine, brain, heart, bursa of Fabricius, lung, kidney, and liver of embryonic and posthatch chicks, and where its expression increases in liver, brain, large intestine, and jejunum posthatch, suggesting an increase in expression that is parallel to the maturation of the tissue (Zwarycz and Wong 2013). In teleost fish, PHT1 expression has been analysed in the Nile tilapia and found in stomach, intestine (with proximal intestine > distal intestine > mid intestine), brain, kidney, gill, liver, spleen and muscle (Huang et al. 2015). In a preliminary screening, PHT1 has been detected in a wide range of zebrafish tissues, namely gills, brain, heart, liver, intestine, muscle, eye, ovary, pancreas and kidney (Barca 2007; Pisani 2012). To date, the notion of a widespread distribution of PHT1 across organism tissues is overcome by the observation that PHT1 is predominatly transcribed in the immune system. In particular, PHT1 is expressed in several types of immune cells, among which dendritic cells, activated macrophages and B cells (Sasawatari et al. 2011), and this supports the observations about the wide range of tissue distribution in mammals, birds and fish. Such link to the immune system also explains why PHT1 may play a fundamental role in certain disorders such as diabetes, inflammatory bowel disease, systemic lupus erythematosus and colitis as already widely recognised (among many others, see Takeuchi et al. 2008; Han et al. 2009; Lee et al. 2009; Sasawatari et al. 2011; see also Soga et al. 2014).
To date, PHT1 is acknowledged to operate in the double physiological role of transporter of histidine and peptides (a) across the cell membrane and (b) from the endosome/lysosome to the cytosol (Sasawatari et al. 2011), although nuclear localisation has been reported in human nasal epithelial cells (Agu et al. 2011). However, its functional relevance as endosome/lysosome operating transporter is steadily emerging. In fact, while the first reports located PHT1 at the cell surface of intestinal cells, mainly due to the observation that cells transiently transfected with PHT1 are able to transport His and carnosine (see Bhardwaj et al. 2006), recent data on the functional role(s) of PHT1 in nervous and immune systems pathophysiology has brought attention to the intracellular compartments (see Sasawatari et al. 2011; Hu et al. 2014). There, PHT1 principally transports substrates from inside the endosome/lysosome to the cytosol, and in immune cells (plasmacytoid dendritic cells and B cells), it particularly mediates the transport of specific substrates, such as some ligands of the Nucleotide-binding Oligomerization Domain-containing protein 1 (Lee et al. 2009). In this respect, its pH-dependence, with a higher uptake of substrates observed at acidic than neutral pH, would help it to function optimally in the course of the endosomal acidification and lysosomal maturation.
SLC15A3 (PHT2), such as PHT1, has originally been cloned from a rat brain cDNA library (Sakata et al. 2001), and when reconstituted in liposomes it has been shown to mediate the transport of His, carnosine and certain other dipeptides, such as His-Leu, in a H+-dependent manner (Sakata et al. 2001). The PHT2 protein is expressed intracellularly, and in particular it is localised at the lysosomal membrane level (Sakata et al. 2001). In rat, PHT2 mRNA is abundantly expressed in lung, spleen and thymus, and faintly detected in brain, liver, adrenal gland and heart (Sakata et al. 2001). PHT2 expression is also found in, e.g., human and rodent skeletal muscle (Everaert et al. 2013), rat thyroid (Romano et al. 2010), bovine and human retina (Ocheltree et al. 2003), human nasal cells (Agu et al. 2011), and possibly mouse prostate (Sun et al. 2013a). Noteworthy, PHT2 is highly expressed in macrophages, as specifically observed in macrophages isolated from mouse and human spleen (Sun et al. 2013b) or generated from blood monocytes (Moreau et al. 2011), which suggests, in parallel with the intracellular localisation data, that a possible role for PHT2 in these cells is to mediate the transport of cellular protein digestion products out from lysosomes. In this respect it is worth noting that PHT2 upregulation occurs in macrophages under lipopolysaccharide (LPS) stimulation (Wang et al. 2014b).
To our knowledge, no information on PHT2 is available from vertebrates other than mammals. This is due, as for birds, reptiles and amphibians, to the lack of specific literature or, as for teleost fish, to the absence of the SLC15A3 gene in their genomes as it results from sequence databanks consulting (see also Huang et al. 2015).
SLC15A5 is a gene very recently reported, which is present in vertebrate genomes (see Fredriksson et al. 2008; Höglund et al. 2011; Sreedharan et al. 2011). However, at least to our knowledge, no functional studies have been published so far involving this transporter. In human, SLC15A5 expression has been reported in the pituitary, liver, thymus and spleen (Sreedharan et al. 2011). On the other hand, in the Nile tilapia SLC15A5 has been detected in the stomach, intestine (with proximal intestine ≫ mid intestine), muscle and liver (Huang et al. 2015). Preliminary expression data from our laboratory comparing human, rodent and zebrafish tissues indicate that SLC15A5 is expressed in human testicle, rat brain and testicle, and zebrafish ovary, liver, pancreas and gills (Barca 2007; Pisani 2012). The different tissue distribution observed in fish with respect to mammals might be suggestive of a different (compensatory) role of SLC15A5 in genomes devoid of SLC15A3. However, further studies are required to address this point.
Conclusions and perspectives
The systematic use of animal models, the increasing number of commercial species grown for human consumption and the new attitude to complement biological information on humans with data from vertebrate and invertebrate ‘non-conventional’ organisms make comparative, developmental integrative, environmental and ecological physiology trendy disciplines, that will meet translational and systems biology soon (for a recent review, see Mueller et al. 2015, and literature cited therein). In this context, due to their phylogenetic position, teleost fish represent major reference models and will play a central role.
For peptide transporters, and especially for PEPT1, at a molecular level of investigation the application of the comparative approach shows that teleost fish proteins can exhibit unusual properties with respect to the mammalian counterparts, which calls for an unexpeced functional plasticity of this class of proteins, suggests new molecular structure/function paradigms, and helps to understand, e.g., how they molecularly adapt to match the nutrient requirements of a species, and how they functionally (co)operate to support ecological diversification (see Karasov et al. 2011; Karasov and Douglas 2013). From a practical perspective, the detailed functional analysis of the teleost fish proteins can also help to define the optimal sets of peptide (and non-peptide) substrates taken up via PEPT1, which can generate a positive impact in teleost fish nutrition and feed production (see Dabrowski et al. 2010). The information from teleost fish models can also help to inform on how a human transporter works, e.g., the ‘human-to-fish’ analysis of vertebrate sequences can contribute to delineate the relevance of single conserved residues and their role in the definition of a molecular phenotype, also in the context of certain natural variations (single nucleotide polymorphisms) that occur in human PEPT1 (see Saito et al. 2002; Bruzzoni-Giovanelli et al. 2015; Zhang et al. 2004; Anderle et al. 2006; Zucchelli et al. 2009; for review see Zaïr et al. 2008). The comparative approach on PEPT1 can also be of support for producing improved foods for human consumption and/or for designing novel functional foods; this also considering the relevance that peptide-based diets can have in clinical applications, such as enteral nutrition (for meta-analyses and reviews, see Braunschweig et al. 2001; Gramlich et al. 2004; Kudsk 2007; de Aguilar-Nascimento et al. 2010).
While revising the literature from mammals to fish, we have focused in this paper on the functional responses—mainly in terms of variation of gene expression—of the SLC15 transporters, with emphasis on PEPT1, in relation to various physiological conditions. Papers dealing with pathophysiological conditions have intentionally been excluded, although variations do occur under infections, surgeries, drug exposure, etc (see Daniel 2004; Daniel and Kottra 2004; Gilbert et al. 2008b; Smith et al. 2013b). At such cellular/tissue level of investigation, it emerges that, e.g., changes in PEPT1 expression at both mRNA and protein level for most of the dietary challenges and alimentary conditions tested almost never exceed 2–3-fold the control, which indicates that the cellular/tissue response in terms of variation of PEPT1 expression can be rather limited if compared to other structural–functional changes that occur in the digestive tract, at cellular (e.g., changes in the height of microvilli), tissue (e.g., changes in the number of enterocytes), organ (e.g., changes in the number of villi), etc level of organisation. At the same time, the role of PEPT1 can acquire stronger meaning if its activity is seen as part of a highly concerted action that includes all the membrane players (transporters, receptors, etc) specifically expressed by the intestinal epithelium. In this respect, comprehensive, systematic analyses of the whole membrane transport protein network (the so-called ‘transportome’) expressed along the digestive tract have been performed, leading to the detection/localisation of a large number of SLC genes along the rostrocaudal axis of the digestive tract in many vertebrates (among many others, see Terada et al. 2005; Hilgendorf et al. 2007; Kim et al. 2007; Meier et al. 2007; Wang et al. 2010; Cedernaes et al. 2011; Haller et al. 2012; Drozdzik et al. 2014; Teerapornpuntakit et al. 2014; Pérez-Torras et al. 2016). In this context, PEPT1 emerges as a highly specific marker of gut function since it is invariably expressed in those districts of the digestive tract where absorption of the bulk of protein degradation products occurs first.
At a more integrative level of investigation, one of the questions raised in this review is whether or not in teleost fish intestinal peptide transport and transporters—since PEPT1 is the major route for the absorption of protein degradation products after a meal—play any roles in and/or somehow functionally contribute to salt, water and/or acid–base homeostastic processes and vice versa. The picture that emerges is fragmentary for what regards the recognised functional interactions between di/tripeptides, on one side, and ion and water absorptive processes, on the other, but it indicates that functional links exist and that they can be more or less robust depending on teleost species and/or specific morpho-functional background considered (gastric vs. agastric condition, seawater vs. freshwater environment, carnivorous vs. herbivorous vs. omnivorous alimentary habit, etc). A better understanding of the intestinal processes of nutrient, ion and water absorption and their integration within the frame of the organism dynamics will help clarify the fate of an ingested meal in a teleost fish and to elucidate the physiological events that occur afterwards.
Another question at the integrative level of investigation raised in the review regards the option that a link exists between absorption of dietary protein degradation products via PEPT1 and animal growth, and that animal growth can somehow be influenced by dietary components transported by PEPT1. In this context, a large number of papers amongst those reviewed pointed to farmed animals, from livestock and poultry to cultured fish. In such studies, biometric analyses (weight, length, BMI, condition factor, etc) are carefully conducted on large numbers of individuals, often in conjunction with molecular, biochemical and/or physiological analyses aimed at establishing (a) correlation(s) to any growth indexes. The studies on farmed animals well integrate the results from more conventional models, such as mice, C. elegans, etc—knockout and transgenic organisms included—that are typically used to investigate the mechanisms of animal growth response (see Meissner et al. 2004; Spanier et al. 2009; Benner et al. 2011; Kolodziejczak et al. 2013). All of them suggest a link between PEPT1 and animal growth. Many of the studies on farmed animals have typically been conducted on individuals during phases of ‘linear growth’, a typical trait of indeterminate growers, such as telost fish, but also an underestimated trait of determinate growers, such as higher vertebrates, humans included. In a perspective, it would be worth focusing on PEPT1 and the ‘linear growth’ trait during specific phases of human lifespan, such as the first year of life and the teenage years, when linear increases of growth seem to occur (among many others, see Adair et al. 2013; Küpers et al. 2015; de Beer et al. 2015; Araújo de França et al. 2016).
While in teleost fish the research on PEPT1 transporters is rather well established to date, it has to be noted that the studies regarding the other members of the SLC15 family in these organisms are still at their infancy, although the few results available for PEPT2 (see Romano et al. 2006), PHT1 and PHT2 (see Huang et al. 2015) highly substantiate the notion that teleost fish models can be of support and/or inspiration for developing original concepts in epithelial (e.g., intestinal, renal, hearing) and non-epithelial (e.g.,muscular, nervous, immune system) physiology.
In conclusion, we have reviewed the vast majority of the literature on SLC15 family members in teleost fish models, with major emphasis on PEPT1, one of the most studied nutrients transport systems in teleost fish. We have also underlined the contribution of such studies to the physiology of the di/tripeptide transport across membranes and compared them to the more structured studies performed in higher vertebrates. Novel and original hints (e.g., performing comparative -omics analyses across vertebrates, deepening the human phenotype by exploiting new evo-devo conceptual schemes, developing preclinical models of disease, etc) on this research can come from such a comparative approach.
References
Adair LS, Fall CH, Osmond C, Stein AD, Martorell R, Ramirez-Zea M, Sachdev HS, Dahly DL, Bas I, Norris SA, Micklesfield L, Hallal P, Victora CG, COHORTS group, (2013) Associations of linear growth and relative weight gain during early life with adult health and human capital in countries of low and middle income: findings from five birth cohort studies. Lancet 382:525–534. doi:10.1016/S0140-6736(13)60103-8
Adibi SA (2003) Regulation of expression of the intestinal oligopeptide transporter (Pept-1) in health and disease. Am J Physiol Gastrointest Liver Physiol 285:G779–G788. doi:10.1152/ajpgi.00056.2003
Adibi SF, Khan MA (2011) Total sulphur amino acid requirement and cystine replacement value for fingerling rohu, Labeo rohita: effects on growth, nutrient retention and body composition. Aquacult Nutr 17:E583–E594. doi:10.1111/j.1365-2095.2010.00799.x
Adibi SA, Mercer DW (1973) Protein digestion in human intestine as reflected in luminal, mucosal, and plasma amino acid concentrations after meals. J Clin Invest 52:1586–1594. doi:10.1172/JCI107335
Agu R, Cowley E, Shao D, Macdonald C, Kirkpatrick D, Renton K, Massoud E (2011) Proton-coupled oligopeptide transporter (POT) family expression in human nasal epithelium and their drug transport potential. Mol Pharm 8:664–672. doi:10.1021/mp100234z
Ahmed I, Khan MA, Jafri AK (2003) Dietary methionine requirement of fingerling Indian major carp, Cirrhinus mrigala (Hamilton). Aquacult Int 11:449–462. doi:10.1023/B:AQUI.0000004181.89420.a2
Ahn H, Yamada Y, Okamura A, Tsukamoto K, Kaneko T, Watanabe S (2013) Intestinal expression of peptide transporter 1 (PEPT1) at different life stages of Japanese eel, Anguilla japonica. Comp Biochem Physiol B: Biochem Mol Biol 166:157–164. doi:10.1016/j.cbpb.2013.08.005
Ahring BK, Ibrahim AA, Mladenovska Z (2001) Effect of temperature increase from 55 to 65 °C on performance and microbial population dynamics of an anaerobic reactor treating cattle manure. Water Res 35:2446–2452. doi:10.1016/S0043-1354(00)00526-1
Ai Q, Xie X (2005) Effects of replacement of fish meal by soybean meal and supplementation of methionine in fish meal/soybean meal-based diets on growth performance of the southern catfish Silurus meridionalis. J World Aquacult Soc 36:498–507. doi:10.1111/j.1749-7345.2005.tb00397.x
Ait-Omar A, Monteiro-Sepulveda M, Poitou C, Le Gall M, Cotillard A, Gilet J, Garbin K, Houllier A, Château D, Lacombe A, Veyrie N, Hugol D, Tordjman J, Magnan C, Serradas P, Clément K, Leturque A, Brot-Laroche E (2011) GLUT2 accumulation in enterocyte apical and intracellular membranes: a study in morbidly obese human subjects and ob/ob and high fat-fed mice. Diabetes 60:2598–2607. doi:10.2337/db10-1740
Aksnes A, Hope B, Høstmark Ø, Albrektsen S (2006a) Inclusion of size fractionated fish hydrolysate in high plant protein diets for Atlantic cod, Gadus morhua. Aquaculture 261:1102–1110. doi:10.1016/j.aquaculture.2006.07.038
Aksnes A, Hope B, Jönsson E, Björnsson Albrektsen S (2006b) Size-fractionated fish hydrolysate as feed ingredient for rainbow trout (Oncorhynchus mykiss) fed high plant protein diets: I: Growth, growth regulation and feed utilization. Aquaculture 261:305–317. doi:10.1016/j.aquaculture.2006.07.025
Alam MS, Teshima SI, Ishikawa M, Koshio S (2000) Methionine requirement of juvenile Japanese flounder Paralichthys olivaceus. J World Aquacult Soc 31:618–626. doi:10.1111/j.1749-7345.2000.tb00911.x
Ali M, Nicieza A, Wootton RJ (2003) Compensatory growth in fishes: a response to growth depression. Fish Fish 4:147–190. doi:10.1046/j.1467-2979.2003.00120.x
Amasheh S, Wenzel U, Boll M, Dorn D, Weber WM, Clauss W, Daniel H (1997) Transport of charged dipeptides by the intestinal H+/peptide symporter PepT1 expressed in Xenopus laevis oocytes. J Membr Biol 155:247–256. doi:10.1007/s002329900177
Amberg JJ, Myr C, Kamisaka Y, Jordal AE, Rust MB, Hardy RW, Koedijk R, Rønnestad I (2008) Expression of the oligopeptide transporter, PepT1, in larval Atlantic cod (Gadus morhua). Comp Biochem Physiol B: Biochem Mol Biol 150:177–182. doi:10.1016/j.cbpb.2008.02.011
Anderle P, Nielsen CU, Pinsonneault J, Krog PL, Brodin B, Sadée W (2006) Genetic variants of the human dipeptide transporter PEPT1. J Pharmacol Exp Ther 316:636–646. doi:10.1124/jpet.105.094615
Aragão C, Conceição LEC, Martins D, Rønnestad I, Gomes E, Dinis MT (2004) A balanced dietary amino acid profile improves amino acid retention in post-larval Senegalese sole (Solea senegalensis). Aquaculture 233:293–304. doi:10.1016/j.aquaculture.2003.08.007
Araújo de França GV, De Lucia Rolfe E, Horta BL, Gigante DP, Yudkin JS, Ong KK, Victora CG (2016) Associations of birth weight, linear growth and relative weight gain throughout life with abdominal fat depots in adulthood: the 1982 Pelotas (Brazil) birth cohort study. Int J Obes (Lond) 40:14–21. doi:10.1038/ijo.2015.192
Bacconi A, Ravera S, Virkki LV, Murer H, Forster IC (2007) Temperature dependence of steady-state and presteady-state kinetics of a type Iib Na+/Pi cotransporter. J Membr Biol 215:81–92. doi:10.1007/s00232-007-9008-1
Bakke S, Jordal AE, Gómez-Requeni P, Verri T, Kousoulaki K, Aksnes A, Rønnestad I (2010) Dietary protein hydrolysates and free amino acids affect the spatial expression of peptide transporter PepT1 in the digestive tract of Atlantic cod (Gadus morhua). Comp Biochem Physiol B: Biochem Mol Biol 156:48–55. doi:10.1016/j.cbpb.2010.02.002
Ball RO, Urschel KL, Pencharz PB (2007) Nutritional consequences of interspecies differences in arginine and lysine metabolism. J Nutr 137:1626S–1641S
Bar N (2014) Physiological and hormonal changes during prolonged starvation in fish. Can J Fish Aquat Sci 71:1447–1458. doi:10.1139/cjfas-2013-0175
Barboza PS, Hume ID (2006) Physiology of intermittent feeding: integrating responses of vertebrates to nutritional deficit and excess. Physiol Biochem Zool 79:250–264. doi:10.1086/499984
Barca A (2007) Caratterizzazione dei geni SLC15 della serie PHT: splicing alternativo e ruolo del Nonsense-Mediated mRNA Decay (NMD). Dissertation, University of Salento
Battley PF, Piersma T, Dietz MW, Tang S, Dekinga A, Hulsman K (2000) Empirical evidence for differential organ reductions during trans-oceanic bird flight. Proc Biol Sci 267:191–195. doi:10.1098/rspb.2000.0986
Bauchinger U, Wohlmann A, Biebach H (2005) Flexible remodeling of organ size during spring migration of the garden warbler (Sylvia borin). Zool (Jena) 108:97–106. doi:10.1016/j.zool.2005.03.003
Beale JH, Parker JL, Samsudin F, Barrett AL, Senan A, Bird LE, Scott D, Owens RJ, Sansom MS, Tucker SJ, Meredith D, Fowler PW, Newstead S (2015) Crystal structures of the extracellular domain from PepT1 and PepT2 provide novel insights into mammalian peptide transport. Structure 23:1889–1899. doi:10.1016/j.str.2015.07.016
Beckman ML, Quick MW (2001) Substrates and temperature differentiate ion flux from serotonin flux in a serotonin transporter. Neuropharmacology 40:526–535. doi:10.1016/S0028-3908(00)00191-X
Beers JM, Jayasundara N (2015) Antarctic notothenioid fish: what are the future consequences of ‘losses’ and ‘gains’ acquired during long-term evolution at cold and stable temperatures? J Exp Biol 218:1834–1845. doi:10.1242/jeb.116129
Benner J, Daniel H, Spanier B (2011) A glutathione peroxidase, intracellular peptidases and the TOR complexes regulate peptide transporter PEPT-1 in C. elegans. PLoS One 6:e25624. doi:10.1371/journal.pone.0025624
Benstead JP, Hood JM, Whelan NV, Kendrick MR, Nelson D, Hanninen AF, Demi LM (2014) Coupling of dietary phosphorus and growth across diverse fish taxa: a meta-analysis of experimental aquaculture studies. Ecology 95:2768–2777. doi:10.1890/13-1859.1
Berthelsen R, Nielsen CU, Brodin B (2013) Basolateral glycylsarcosine (Gly–Sar) transport in Caco-2 cell monolayers is pH dependent. J Pharm Pharmacol 65:970–979. doi:10.1111/jphp.12061
Betancur-R R, Broughton RE, Wiley EO, Carpenter K, López JA, Li C, Holcroft NI, Arcila D, Sanciangco M, Cureton Ii JC, Zhang F, Buser T, Campbell MA, Ballesteros JA, Roa-Varon A, Willis S, Borden WC, Rowley T, Reneau PC, Hough DJ, Lu G, Grande T, Arratia G, Ortí G (2013) The tree of life and a new classification of bony fishes. PLoS Curr 5. doi:10.1371/currents.tol.53ba26640df0ccaee75bb165c8c26288
Bhardwaj RK, Herrera-Ruiz D, Eltoukhy N, Saad M, Knipp GT (2006) The functional evaluation of human peptide/histidine transporter 1 (hPHT1) in transiently transfected COS-7 cells. Eur J Pharm Sci 27:533–542. doi:10.1016/j.ejps.2005.09.014
Biagi G, Piva A, Moschini M, Vezzali E, Roth FX (2007) Performance, intestinal microflora, and wall morphology of weanling pigs fed sodium butyrate. J Anim Sci 85:1184–1191. doi:10.2527/jas.2006-378
Binda F, Bossi E, Giovannardi S, Forlani G, Peres A (2002) Temperature effects on the presteady-state and transport-associated currents of GABA cotransporter rGAT1. FEBS Lett 512:303–307. doi:10.1016/S0014-5793(02)02271-8
Bisesi JH, Ngo T, Ponnavolu S, Liu K, Lavelle CM, Afrooz ARMN, Saleh NB, Ferguson PL, Denslow ND, Sabo-Attwood T (2015) Examination of single-walled carbon nanotubes uptake and toxicity from dietary exposure: tracking movement and impacts in the gastrointestinal system. Nanomaterials 5:1066–1086. doi:10.3390/nano5021066
Bogé G, Rigal A, Peres G (1981) Rates of in vivo intestinal absorption of glycine and glycylglycine by rainbow trout (Salmo gairdneri R.). Comp Biochem Physiol A Physiol 69:455–459. doi:10.1016/0300-9629(81)93004-8
Boll M, Daniel H (1995) Target size analysis of the peptide/H+-symporter in kidney brush-border membranes. Biochim Biophys Acta 1233:145–152. doi:10.1016/0005-2736(94)00245-K
Bossi E, Fabbrini MS, Ceriotti A (2007) Exogenous protein expression in Xenopus oocytes: basic procedures. Methods Mol Biol 375:107–131. doi:10.1007/978-1-59745-388-2_6
Bossi E, Cherubino F, Margheritis E, Oyadeyi AS, Vollero A, Peres A (2012) Temperature effects on the kinetic properties of the rabbit intestinal oligopeptide cotransporter PepT1. Pflugers Arch 464:183–191. doi:10.1007/s00424-012-1125-8
Boudry G, David ES, Douard V, Monteiro IM, Le Huërou-Luron I, Ferraris RP (2010) Role of intestinal transporters in neonatal nutrition: carbohydrates, proteins, lipids, minerals, and vitamins. J Pediatr Gastroenterol Nutr 51:380–401. doi:10.1097/MPG.0b013e3181eb5ad6
Bradford MMV, Gous RM (1991) The response of growing pigs to a choice of diets differing in protein content. Anim Sci 52:185–192. doi:10.1017/S0003356100005821
Brandsch M (2009) Transport of drugs by proton-coupled peptide transporters: pearls and pitfalls. Expert Opin Drug Metab Toxicol 5:887–905. doi:10.1517/17425250903042292
Brandsch M (2013) Drug transport via the intestinal peptide transporter PepT1. Curr Opin Pharmacol 13:881–887. doi:10.1016/j.coph.2013.08.004
Braunschweig CL, Levy P, Sheean PM, Wang X (2001) Enteral compared to parenteral nutrition: a meta-analysis. Am J Clin Nutr 74:534–542
Brawand D, Wagner CE, Li YI, Malinsky M, Keller I, Fan S, Simakov O, Ng AY, Lim ZW, Bezault E, Turner-Maier J, Johnson J, Alcazar R, Noh HJ, Russell P, Aken B, Alföldi J, Amemiya C, Azzouzi N, Baroiller JF, Barloy-Hubler F, Berlin A, Bloomquist R, Carleton KL, Conte MA, D’Cotta H, Eshel O, Gaffney L, Galibert F, Gante HF, Gnerre S, Greuter L, Guyon R, Haddad NS, Haerty W, Harris RM, Hofmann HA, Hourlier T, Hulata G, Jaffe DB, Lara M, Lee AP, MacCallum I, Mwaiko S, Nikaido M, Nishihara H, Ozouf-Costaz C, Penman DJ, Przybylski D, Rakotomanga M, Renn SC, Ribeiro FJ, Ron M, Salzburger W, Sanchez-Pulido L, Santos ME, Searle S, Sharpe T, Swofford R, Tan FJ, Williams L, Young S, Yin S, Okada N, Kocher TD, Miska EA, Lander ES, Venkatesh B, Fernald RD, Meyer A, Ponting CP, Streelman JT, Lindblad-Toh K, Seehausen O, Di Palma F (2014) The genomic substrate for adaptive radiation in African cichlid fish. Nature 513:375–381. doi:10.1038/nature13726
Bröer S (2008) Amino acid transport across mammalian intestinal and renal epithelia. Physiol Rev 88:249–286. doi:10.1152/physrev.00018.2006
Bruzzoni-Giovanelli H, González JR, Sigaux F, Villoutreix BO, Cayuela JM, Guilhot J, Preudhomme C, Guilhot F, Poyet JL, Rousselot P (2015) Genetic polymorphisms associated with increased risk of developing chronic myelogenous leukemia. Oncotarget 6:36269–36277. doi:10.18632/oncotarget.5915
Bucking C, Schulte PM (2012) Environmental and nutritional regulation of expression and function of two peptide transporter (PepT1) isoforms in a euryhaline teleost. Comp Biochem Physiol A Mol Integr Physiol 161:379–387. doi:10.1016/j.cbpa.2011.12.008
Bucking C, Wood CM (2006) Water dynamics in the digestive tract of the freshwater rainbow trout during the processing of a single meal. J Exp Biol 209:1883–1893. doi:10.1242/jeb.02205
Buddington RK, Krogdahl A, Bakke-Mckellep AM (1997) The intestines of carnivorous fish: structure and functions and the relations with diet. Acta Physiol Scand 638:67–80
Buyse M, Tsocas A, Walker F, Merlin D (2002a) Bado A (2002b) PepT1-mediated fMLP transport induces intestinal inflammation in vivo. Am J Physiol Cell Physiol 283(6):C1795–C1800. doi:10.1152/ajpcell.00186.2002
Buyse M, Sitaraman SV, Liu X, Bado A, Merlin D (2002b) Luminal leptin enhances CD147/MCT-1-mediated uptake of butyrate in the human intestinal cell line Caco2-BBE. J Biol Chem 277:28182–28190. doi:10.1074/jbc.M203281200
Cai ZN, Li WJ, Mai KS, Xu W, Zhang YJ, Ai QH (2015) Effects of dietary size-fractionated fish hydrolysates on growth, activities of digestive enzymes and aminotransferases and expression of some protein metabolism related genes in large yellow croaker (Larimichthys crocea) larvae. Aquaculture 440:40–47. doi:10.1016/j.aquaculture.2015.01.026
Cao M, Che L, Wang J, Yang M, Su G, Fang Z, Lin Y, Xu S, Wu D (2014) Effects of maternal over- and undernutrition on intestinal morphology, enzyme activity, and gene expression of nutrient transporters in newborn and weaned pigs. Nutrition 30:1442–1447. doi:10.1016/j.nut.2014.04.016
Castro LF, Gonçalves O, Mazan S, Tay BH, Venkatesh B, Wilson JM (2013) Recurrent gene loss correlates with the evolution of stomach phenotypes in gnathostome history. Proc Biol Sci 281:20132669. doi:10.1098/rspb.2013.2669
Cedernaes J, Olszewski PK, Almén MS, Stephansson O, Levine AS, Fredriksson R, Nylander O, Schiöth HB (2011) Comprehensive analysis of localization of 78 solute carrier genes throughout the subsections of the rat gastrointestinal tract. Biochem Biophys Res Commun 411:702–707. doi:10.1016/j.bbrc.2011.07.005
Chadwick VS, Mellor DM, Myers DB, Selden AC, Keshavarzian A, Broom MF, Hobson CH (1988) Production of peptides inducing chemotaxis and lysosomal enzyme release in human neutrophils by intestinal bacteria in vitro and in vivo. Scand J Gastroenterol 23:121–128. doi:10.3109/00365528809093861
Chalamaiah M, Dinesh Kumar B, Hemalatha R, Jyothirmayi T (2012) Fish protein hydrolysates: proximate composition, amino acid composition, antioxidant activities and applications: a review. Food Chem 135:3020–3038. doi:10.1016/j.foodchem.2012.06.100
Chappell VL, Thompson MD, Jeschke MG, Chung DH, Thompson JC, Wolf SE (2003) Effects of incremental starvation on gut mucosa. Dig Dis Sci 48:765–769. doi:10.1023/A:1022849112100
Chauvigné F, Gabillard JC, Weil C, Rescan PY (2003) Effect of refeeding on IGFI, IGFII, IGF receptors, FGF2, FGF6, and myostatin mRNA expression in rainbow trout myotomal muscle. Gen Comp Endocrinol 132:209–215. doi:10.1016/S0016-6480(03)00081-9
Chen H, Wong EA, Webb KE Jr (1999) Tissue distribution of a peptide transporter mRNA in sheep, dairy cows, pigs, and chickens. J Anim Sci 77:1277–1283
Chen H, Pan YX, Wong EA, Bloomquist JR, Webb KE Jr (2002) Molecular cloning and functional characterization of a chicken intestinal peptide transporter (cPepT1) in Xenopus oocytes and Chinese hamster ovary cells. J Nutr 132:387–393
Chen H, Pan Y, Wong EA, Webb KE Jr (2005) Dietary protein level and stage of development affect expression of an intestinal peptide transporter (cPepT1) in chickens. J Nutr 135:193–198
Chen YH, Lu YF, Ko TY, Tsai MY, Lin CY, Lin CC, Hwang SP (2009) Zebrafish cdx1b regulates differentiation of various intestinal cell lineages. Dev Dyn 238:1021–1032. doi:10.1002/dvdy.21908
Chen M, Singh A, Xiao F, Dringenberg U, Wang J, Engelhardt R, Yeruva S, Rubio-Aliaga I, Nässl AM, Kottra G, Daniel H, Seidler U (2010) Gene ablation for PEPT1 in mice abolishes the effects of dipeptides on small intestinal fluid absorption, short-circuit current, and intracellular pH. Am J Physiol Gastrointest Liver Physiol 299:G265–G274. doi:10.1152/ajpgi.00055.2010
Chen Q, Zhang H, Zheng Y, Shan A, Bi Z (2013) Effects of enzymatically hydrolyzed blood cells on growth performance and intestinal characteristics of newly weaned piglets. Livest Sci 157:514–519. doi:10.1016/j.livsci.2013.09.004
Chen MX, Li XG, Yan HC, Wang XQ, Gao CQ (2016) Effect of egg weight on composition, embryonic growth, and expression of amino acid transporter genes in yolk sac membranes and small intestines of the domestic pigeon (Columba livia). Poult Sci 95:1425–1432. doi:10.3382/ps/pew044
Chotikachinda R, Tantikitti C, Benjakul S, Rustad T, Kumarnsit E (2013) Production of protein hydrolysates from skipjack tuna (Katsuwonus pelamis) viscera as feeding attractants for Asian seabass (Lates calcarifer). Aquacult Nutr 19:773–784. doi:10.1111/anu.12024
Clausen MR, Mortensen PB (1994) Kinetic studies on the metabolism of short-chain fatty acids and glucose by isolated rat colonocytes. Gastroenterology 106:423–432
Collins SA, Overland M, Skrede A, Drew MD (2013) Effect of plant protein sources on growth rate in salmonids: meta-analysis of dietary inclusion of soybean, pea and canola/rapeseed meals and protein concentrates. Aquaculture 400:85–100. doi:10.1016/j.aquaculture.2013.03.006
Conceição LEC, Grasdalen H, Rønnestad I (2003) Amino acid requirements of fish larvae and post-larvae: new tools and recent findings. Aquaculture 227:221–232. doi:10.1016/S0044-8486(03)00505-2
Conceição LEC, Ribeiro L, Engrola S, Aragão C, Morais S, Lacuisse M, Soares F, Dinis MT (2007) Nutritional physiology during development of Senegalese sole (Solea senegalensis). Aquaculture 268:64–81. doi:10.1016/j.aquaculture.2007.04.030
Conceição LE, Aragão C, Dias J, Costas B, Terova G, Martins C, Tort L (2012) Dietary nitrogen and fish welfare. Fish Physiol Biochem 38:119–141. doi:10.1007/s10695-011-9592-y
Cook JT, Sutterlin AM, McNiven MA (2000) Effect of food deprivation on oxygen consumption and body composition of growth-enhanced transgenic Atlantic salmon (Salmo salar). Aquaculture 188:47–63. doi:10.1016/S0044-8486(00)00333-1
Coppes Petricorena ZL, Somero GN (2007) Biochemical adaptations of notothenioid fishes: comparisons between cold temperate South American and New Zealand species and Antarctic species. Comp Biochem Physiol A Mol Integr Physiol 147:799–807. doi:10.1016/j.cbpa.2006.09.028
Cordero H, Guzmán-Villanueva LT, Chaves-Pozo E, Arizcun M, Ascencio-Valle F, Cuesta A, Esteban MA (2016) Comparative ontogenetic development of two marine teleosts, gilthead seabream and European sea bass: New insights into nutrition and immunity. Dev Comp Immunol 65:1–7. doi:10.1016/j.dci.2016.06.011
Cramp RL, Franklin CE (2003) Is re-feeding efficiency compromised by prolonged starvation during aestivation in the green striped burrowing frog, Cyclorana alboguttata? J Exp Zool A Comp Exp Biol 300:126–132. doi:10.1002/jez.a.10272
Croom WJ, Brake J, Coles BA, Havenstein GB, Christensen VL, McBride BW, Peebles ED, Taylor IL (1999) Is intestinal absorption capacity rate-limiting for performance in poultry? J Appl Poult Res 8:242–252. doi:10.1093/japr/8.2.242
Cummings JH (1981) Short chain fatty acids in the human colon. Gut 22:763–779. doi:10.1136/gut.22.9.763
Cummings JH, Macfarlane GT (1991) The control and consequences of bacterial fermentation in the human colon. J Appl Bacteriol 70:443–459. doi:10.1111/j.1365-2672.1991.tb02739.x
Cummings JH, Pomare EW, Branch WJ, Naylor CP, Macfarlane GT (1987) Short chain fatty acids in human large intestine, portal, hepatic and venous blood. Gut 28:1221–1227. doi:10.1136/gut.28.10.1221
Cyrino JEP, Bureau DP, Kapoor BG (2008) Feeding and digestive functions of fishes. Science Publisher, Enfield
D’Inca R, Gras-Le Guen C, Che L, Sangild PT, Le Huërou-Luron I (2011) Intrauterine growth restriction delays feeding-induced gut adaptation in term newborn pigs. Neonatology 99:208–216. doi:10.1159/000314919
Dabrowski K, Lee KJ, Rinchard J (2003) The smallest vertebrate, teleost fish, can utilize synthetic dipeptide-based diets. J Nutr 133:4225–4229
Dabrowski K, Terjesen BF, Zhang Y, Phang JM, Lee KJ (2005) A concept of dietary dipeptides: a step to resolve the problem of amino acid availability in the early life of vertebrates. J Exp Biol 208:2885–2894. doi:10.1242/jeb.01689
Dabrowski K, Zhang YF, Kwasek K, Hliwa P, Ostaszewska T (2010) Effects of protein-, peptide- and free amino acid-based diets in fish nutrition. Aquacult Res 41:668–683. doi:10.1111/j.1365-2109.2010.02490.x
Dalmasso G, Charrier-Hisamuddin L, Nguyen HT, Yan Y, Sitaraman S, Merlin D (2008a) PepT1-mediated tripeptide KPV uptake reduces intestinal inflammation. Gastroenterology 134:166–178. doi:10.1053/j.gastro.2007.10.026
Dalmasso G, Nguyen HTT, Yan Y, Charrier-Hisamuddin L, Sitaraman SV, Merlin D (2008b) Butyrate transcriptionally enhances peptide transporter PepT1 expression and activity. PLoS One 3(6):e2476. doi:10.1371/journal.pone.0002476
Dalmasso G, Nguyen HT, Charrier-Hisamuddin L, Yan Y, Laroui H, Demoulin B, Sitaraman SV, Merlin D (2010) PepT1 mediates transport of the proinflammatory bacterial tripeptide l-Ala-γ-d-Glu-meso-DAP in intestinal epithelial cells. Am J Physiol Gastrointest Liver Physiol 299:G687–G696. doi:10.1152/ajpgi.00527.2009
Daniel H (2004) Molecular and integrative physiology of intestinal peptide transport. Annu Rev Physiol 66:361–384. doi:10.1146/annurev.physiol.66.032102.144149
Daniel H, Adibi SA (1994) Functional separation of dipeptide transport and hydrolysis in kidney brush border membrane vesicles. FASEB J 8:753–759
Daniel H, Kottra G (2004) The proton oligopeptide transporter family SLC15 in physiology and pharmacology. Pflugers Arch 447:610–618. doi:10.1007/s00424-003-1101-4
Daniel H, Rubio-Aliaga I (2003) An update on renal peptide transporters. Am J Physiol Renal Physiol 284:F885–F892. doi:10.1152/ajprenal.00123.2002
Daniel H, Zietek T (2015) Taste and move: glucose and peptide transporters in the gastrointestinal tract. Exp Physiol 100:1441–1450. doi:10.1113/EP085029
Darcel NP, Liou AP, Tomé D, Raybould HE (2005) Activation of vagal afferents in the rat duodenum by protein digests requires PepT1. J Nutr 135:1491–1495
Darias MJ, Murray HM, Gallant JW, Douglas SE, Yúfera M, Martínez-Rodríguez G (2007) Ontogeny of pepsinogen and gastric proton pump expression in red porgy (Pagrus pagrus): determination of stomach functionality. Aquaculture 270:369–378. doi:10.1016/j.aquaculture.2007.04.045
Davies DR, Mamat B, Magnusson OT, Christensen J, Haraldsson MH, Mishra R, Pease B, Hansen E, Singh J, Zembower D, Kim H, Kiselyov AS, Burgin AB, Gurney ME, Stewart LJ (2009) Discovery of leukotriene A4 hydrolase inhibitors using metabolomics biased fragment crystallography. J Med Chem 52:4694–4715. doi:10.1021/jm900259h
Day RD, German DP, Manjakasy JM, Farr I, Hansen MJ, Tibbetts IR (2011) Enzymatic digestion in stomachless fishes: how a simple gut accommodates both herbivory and carnivory. J Comp Physiol B 181:603–613. doi:10.1007/s00360-010-0546-y
de Aguilar-Nascimento JE, Dock-Nascimento DB, Bragagnolo R (2010) Role of enteral nutrition and pharmaconutrients in conditions of splanchnic hypoperfusion. Nutrition 26:354–358. doi:10.1016/j.nut.2009.08.021
de Beer M, Vrijkotte TG, Fall CH, van Eijsden M, Osmond C, Gemke RJ (2015) Associations of infant feeding and timing of linear growth and relative weight gain during early life with childhood body composition. Int J Obes (Lond) 39:586–592. doi:10.1038/ijo.2014.200
de la Ballina LR, Cano-Crespo S, González-Muñoz E, Bial S, Estrach S, Cailleteau L, Tissot F, Daniel H, Zorzano A, Ginsberg MH, Palacín M, Féral CC (2016) Amino acid transport associated to Cluster of Differentiation 98 heavy chain (CD98hc) iIs at the cross-road of oxidative stress and amino acid availability. J Biol Chem 291:9700–9711. doi:10.1074/jbc.M115.704254
de Oliveira JE, Druyan S, Uni Z, Ashwell CM, Ferket PR (2009) Prehatch intestinal maturation of turkey embryos demonstrated through gene expression patterns. Poult Sci 88:2600–2609. doi:10.3382/ps.2008-00548
Deng DF, Dominy W, Ju ZY, Koshio S, Murashige R, Wilson RP (2010) Dietary lysine requirement of juvenile Pacific threadfin (Polydactylus sexfilis). Aquaculture 308:44–48. doi:10.1016/j.aquaculture.2010.07.041
Deng J, Kong L, An Q, Bi B, Tao L, Zhang X (2011) Effect of dietary pH adjustment on the utilization of supplemental methionine and lysine by juvenile common carp, Cyprinus carpio. J World Aquac Soc 42:696–704. doi:10.1111/j.1749-7345.2011.00514.x
Diakogiannaki E, Pais R, Tolhurst G, Parker HE, Horscroft J, Rauscher B, Zietek T, Daniel H, Gribble FM, Reimann F (2013) Oligopeptides stimulate glucagon-like peptide-1 secretion in mice through proton-coupled uptake and the calcium-sensing receptor. Diabetologia 56:2688–2696. doi:10.1007/s00125-013-3037-3
Dmitriew CM (2011) The evolution of growth trajectories: what limits growth rate? Biol Rev Camb Philos Soc 86:97–116. doi:10.1111/j.1469-185X.2010.00136.x
Do TT, Hindlet P, Waligora-Dupriet AJ, Kapel N, Neveux N, Mignon V, Deloménie C, Farinotti R, Fève B, Buyse M (2014) Disturbed intestinal nitrogen homeostasis in a mouse model of high-fat diet-induced obesity and glucose intolerance. Am J Physiol Endocrinol Metab 306:E668–E680. doi:10.1152/ajpendo.00437.2013
Doki S, Kato HE, Solcan N, Iwaki M, Koyama M, Hattori M, Iwase N, Tsukazaki T, Sugita Y, Kandori H, Newstead S, Ishitani R, Nureki O (2013) Structural basis for dynamic mechanism of proton-coupled symport by the peptide transporter POT. Proc Natl Acad Sci USA 110:11343–11348. doi:10.1073/pnas.1301079110
Dong XY, Wang YM, Yuan C, Zou XT (2012) The ontogeny of nutrient transporter and digestive enzyme gene expression in domestic pigeon (Columba livia) intestine and yolk sac membrane during pre- and posthatch development. Poult Sci 91:1974–1982. doi:10.3382/ps.2012-02164
Drakeford B, Pascoe S (2008) The substitutability of fishmeal and fish oil in diets for salmon and trout: a meta-analysis. Aquac Econ Manag 12:155–175. doi:10.1080/13657300802306079
Drew MD, Borgeson TL, Thiessen DL (2007) A review of processing of feed ingredients to enhance diet digestibility in finfish. Anim Feed Sci Tech 138:118–136. doi:10.1016/j.anifeedsci.2007.06.019
Drozdzik M, Gröer C, Penski J, Lapczuk J, Ostrowski M, Lai Y, Prasad B, Unadkat JD, Siegmund W, Oswald S (2014) Protein abundance of clinically relevant multidrug transporters along the entire length of the human intestine. Mol Pharm 11:3547–3555. doi:10.1021/mp500330y
Duan Y, Zhu X, Han D, Yang Y, Xie S (2012) Dietary choline requirement in slight methionine-deficient diet for juvenile gibel carp (Carassius auratus gibelio). Aquacult Nutr 18:620–627. doi:10.1111/j.1365-2095.2011.00930.x
Dyer J, Beechey RB, Gorvel JP, Smith RT, Wootton R, Shirazi-Beechey SP (1990) Glycyl-l-proline transport in rabbit enterocyte basolateral-membrane vesicles. Biochem J 269:565–571. doi:10.1042/bj2690565
Elias CL, Xue XH, Marshall CR, Omelchenko A, Hryshko LV, Tibbits GF (2001) Temperature dependence of cloned mammalian and salmonid cardiac Na+/Ca2+ exchanger isoforms. Am J Physiol Cell Physiol 281:C993–C1000
El-Sayed AFM (1999) Alternate dietary protein sources for farmed tilapia, Oreochromis spp. Aquaculture 179:149–168. doi:10.1016/S0044-8486(99)00159-3
Engelhardt WV, Luciano L, Reale E, Gros G, Rechkemmer G (1989) Transport of SCFA across the large intestinal epithelium of guinea pig. Acta Vet Scand Suppl 86:103–106
Erickson RH, Gum JR Jr, Lindstrom MM, McKean D, Kim YS (1995) Regional expression and dietary regulation of rat small intestinal peptide and amino acid transporter mRNAs. Biochem Biophys Res Commun 216:249–257. doi:10.1006/bbrc.1995.2617
Esbaugh AJ, Grosell M (2014) Esophageal desalination is mediated by Na+, H+ exchanger-2 in the gulf toadfish (Opsanus beta). Comp Biochem Physiol A Mol Integr Physiol 171:57–63. doi:10.1016/j.cbpa.2014.02.012
Espe M, Lemme A, Petri A, El-Mowafi A (2006) Can Atlantic salmon (Salmo salar) grow on diets devoid of fism meal? Aquaculture 255:255–262. doi:10.1016/j.aquaculture.2005.12.030
Espe M, Lemme A, Petri A, El-Mowafi A (2007) Assessment of lysine requirement for maximal protein accretion in Atlantic salmon using plant protein diets. Aquaculture 263:168–178. doi:10.1016/j.aquaculture.2006.10.018
Evans DH (2008a) Osmotic and ionic regulation: cells and animals. CRC Press, Boca Raton
Evans DH (2008b) Teleost fish osmoregulation: what have we learned since August Krogh, Homer Smith, and Ancel Keys. Am J Physiol Regul Integr Comp Physiol 295:R704–R713. doi:10.1152/ajpregu.90337.2008
Everaert I, De Naeyer H, Taes Y, Derave W (2013) Gene expression of carnosine-related enzymes and transporters in skeletal muscle. Eur J Appl Physiol 113:1169–1179. doi:10.1007/s00421-012-2540-4
Faggio C, Torre A, Lando G, Sabatino G, Trischitta F (2011) Carbonate precipitates and bicarbonate secretion in the intestine of sea bass, Dicentrarchus labrax. J Comp Physiol B 181:517–525. doi:10.1007/s00360-010-0538-y
Fei YJ, Sugawara M, Liu JC, Li HW, Ganapathy V, Ganapathy M, Leibach F (2000) cDNA structure, genomic organization, and promoter analysis of the mouse intestinal peptide transporter PEPT1. Biochim Biophys Acta 1492:145–154. doi:10.1016/S0167-4781(00)00101-9
Feller G, Gerday C (2003) Psychrophilic enzymes: hot topics in cold adaptation. Nat Rev Microbiol 1:200–208. doi:10.1038/nrmicro773
Ferraris RP, Carey HV (2000) Intestinal transport during fasting and malnutrition. Annu Rev Nutr 20:195–219. doi:10.1146/annurev.nutr.20.1.195
Ferraris RP, Diamond JM (1989) Specific regulation of intestinal nutrient transporters by their dietary substrates. Annu Rev Physiol 51:125–141. doi:10.1146/annurev.ph.51.030189.001013
Ferraris RP, Diamond J, Kwan WW (1988) Dietary regulation of intestinal transport of the dipeptide carnosine. Am J Physiol 255:G143–G150
Fields PA (2001) Review: protein function at thermal extremes: balancing stability and flexibility. Comp Biochem Physiol A: Mol Integr Physiol 129:417–431. doi:10.1016/S1095-6433(00)00359-7
Figueiredo-Silva C, Lemme A, Sangsue D, Kiriratnikom S (2015) Effect of DL-methionine supplementation on the success of almost total replacement of fish meal with soybean meal in diets for hybrid tilapia (Oreochromis niloticus x Oreochromis mossambicus). Aquacult Nutr 21:234–241. doi:10.1111/anu.12150
Fischer da Silva AV, Maiorka A, Borges SA, Santin E, Boleli IC, Macari M (2007) Surface area of the tip of the enterocytes in small intestine mucosa of broilers submitted to early feed restriction and supplemented with glutamine. Int J Poult Sci 6:31–35. doi:10.3923/ijps.2007.31.35
Fisher H, Griminger P, Leveille GA (1959) Protein depletion and amino acid requirement in the growing chick. J Nutr 69:117–123
Flores MV, Hall CJ, Davidson AJ, Singh PP, Mahagaonkar AA, Zon LI, Crosier KE, Crosier PS (2008) Intestinal differentiation in zebrafish requires Cdx1b, a functional equivalent of mammalian Cdx2. Gastroenterology 135:1665–1675. doi:10.1053/j.gastro.2008.07.024
Forbes JM, Shariatmadari F (1994) Diet selection for protein by poultry. Worlds Poult Sci J 50:7–24. doi:10.1079/WPS19940002
Forster I, Ogata HY (1998) Lysine requirement of juvenile Japanese flounder Paralichthys olivaceus and juvenile red sea bream Pagrus major. Aquaculture 161:131–142. doi:10.1016/S0044-8486(97)00263-9
Fotiadis D, Kanai Y, Palacín M (2013) The SLC3 and SLC7 families of amino acid transporters. Mol Aspects Med 34:139–158. doi:10.1016/j.mam.2012.10.007
Fowler PW, Orwick-Rydmark M, Radestock S, Solcan N, Dijkman PM, Lyons JA, Kwok J, Caffrey M, Watts A, Forrest LR, Newstead S (2015) Gating topology of the proton-coupled oligopeptide symporters. Structure 23:290–301. doi:10.1016/j.str.2014.12.012
Fredriksson R, Nordström KJ, Stephansson O, Hägglund MG, Schiöth HB (2008) The solute carrier (SLC) complement of the human genome: phylogenetic classification reveals four major families. FEBS Lett 582:3811–3816. doi:10.1016/j.febslet.2008.10.016
Frøystad-Saugen MK, Lilleeng E, Bakke-McKellep AM, Vekterud K, Valen EC, Hemre GI, Krogdahl A (2009) Dietary intestinal gene expression in Atlantic salmon (Salmo salar L.) fed genetically modified maize. Aquacult Nutr 15:104–115. doi:10.1111/j.1365-2095.2008.00572.x
Fuentes J, Eddy FB (1997) Drinking in marine, euryhaline and freshwater teleost fish. In: Hazon N, Eddy FB, Flik G (eds) Ionic regulation in animals. Spring-Verlag, New York, pp 135–149
Fujisawa Y, Tateoka R, Nara T, Kamo N, Taira T, Miyauchi S (2006) The extracellular pH dependency of transport activity by human oligopeptide transporter 1 (hPEPT1) expressed stably in Chinese hamster ovary (CHO) cells: a reason for the bell-shaped activity versus pH. Biol Pharm Bull 29:997–1005. doi:10.1248/bpb.29.997
Gálfi P, Bokori J (1990) Feeding trial in pigs with a diet containing sodium n-butyrate. Acta Vet Hung 38:3–17
Gamet L, Daviaud D, Denis-Pouxviel C, Remesy C, Murat JC (1992) Effects of short-chain fatty acids on growth and differentiation of the human colon-cancer cell line HT29. Int J Cancer 52:286–289. doi:10.1002/ijc.2910520222
Gao CQ, Yang JX, Chen MX, Yan HC, Wang XQ (2016) Growth curves and age-related changes in carcass characteristics, organs, serum parameters, and intestinal transporter gene expression in domestic pigeon (Columba livia). Poult Sci 95:867–877. doi:10.3382/ps/pev443
Gatlin DM, Barrows FT, Brown P, Dabrowski K, Gaylord TG, Hardy RW, Herman E, Hu G, Krogdahl A, Nelson R, Overturf K, Rust M, Sealey W, Skonberg D, Souza JE, Stone D, Wilson R, Wurtele E (2007) Expanding the utilization of sustainable plant products in aquafeeds: a review. Aquac Res 38:551–579. doi:10.1111/j.1365-2109.2007.01704.x
Gaylord TG, Rawles SD, Gatlin DM (2004) Amino acid availability from animal, blended, and plant feedstuffs for hybrid striped bass (Morone chrysops × M. saxatilis). Aquacult Nutr 10:345–352. doi:10.1111/j.1365-2095.2004.00310.x
Geyra A, Uni Z, Sklan D (2001) Enterocyte dynamics and mucosal development in the posthatch chick. Poult Sci 80:776–782. doi:10.1093/ps/80.6.776
Gilbert ER, Li H, Emmerson DA, Webb KE Jr, Wong EA (2007) Developmental regulation of nutrient transporter and enzyme mRNA abundance in the small intestine of broilers. Poult Sci 86:1739–1753. doi:10.1093/ps/86.8.1739
Gilbert ER, Li H, Emmerson DA, Webb KE Jr, Wong EA (2008a) Dietary protein quality and feed restriction influence abundance of nutrient transporter mRNA in the small intestine of broiler chicks. J Nutr 138:262–271
Gilbert ER, Wong EA, Webb KE Jr (2008b) Board-invited review: peptide absorption and utilization: Implications for animal nutrition and health. J Anim Sci 86:2135–2155. doi:10.2527/jas.2007-0826
Gilbert ER, Li H, Emmerson DA, Webb KE Jr, Wong EA (2010) Dietary protein composition influences abundance of peptide and amino acid transporter messenger ribonucleic acid in the small intestine of 2 lines of broiler chicks. Poult Sci 89:1663–1676. doi:10.3382/ps.2010-00801
Gisbert E, Skalli A, Fernandez I, Kotzamanis Y, Zambonino-Infante JL, Fabregat R (2012) Protein hydrolysates from yeast and pig blood as alternative raw materials in microdiets for gilthead sea bream (Sparus aurata) larvae. Aquaculture 338:96–104. doi:10.1016/j.aquaculture.2012.01.007
Glencloss BD, Booth M, Allan GL (2007) A feed is only as good as its ingredients: a review of ingredient evaluation strategies for aquaculture feeds. Aquac Nutr 13:17–34. doi:10.1111/j.1365-2095.2007.00450.x
Glencross B (2006) The nutritional management of barramundi, Lates calcarifer – a review. Aquacult Nutr 12:291–309. doi:10.1111/j.1365-2095.2006.00410.x
Gonçalves AF, Castro LF, Pereira-Wilson C, Coimbra J, Wilson JM (2007) Is there a compromise between nutrient uptake and gas exchange in the gut of Misgurnus anguillicaudatus, an intestinal air-breathing fish? Comp Biochem Physiol Part D Genomics Proteomics 2:345–355. doi:10.1016/j.cbd.2007.08.002
Gorboulev V, Schürmann A, Vallon V, Kipp H, Jaschke A, Klessen D, Friedrich A, Scherneck S, Rieg T, Cunard R, Veyhl-Wichmann M, Srinivasan A, Balen D, Breljak D, Rexhepaj R, Parker HE, Gribble FM, Reimann F, Lang F, Wiese S, Sabolic I, Sendtner M, Koepsell H (2012) Na+-d-glucose cotransporter SGLT1 is pivotal for intestinal glucose absorption and glucose-dependent incretin secretion. Diabetes 61:187–196. doi:10.2337/db11-1029
Gorka P, Kowalski ZM, Pietrzak P, Kotunia A, Kiljanczyk R, Flaga J, Holst JJ, Guilloteau P, Zabielski R (2009) Effect of sodium butyrate supplementation in milk replacer and starter diet on rumen development in calves. J Physiol Pharmacol 60:47–53
Gousset B (1990) European eel (Anguilla anguilla L.) farming technologies in Europe and in Japan: application of a comparative analysis. Aquaculture 87:209–235. doi:10.1016/0044-8486(90)90060-Z
Gramlich L, Kichian K, Pinilla J, Rodych NJ, Dhaliwal R, Heyland DK (2004) Does enteral nutrition compared to parenteral nutrition result in better outcomes in critically ill adult patients? A systematic review of the literature. Nutrition 20:843–848. doi:10.1016/j.nut.2004.06.003
Grosell M (2006) Intestinal anion exchange in marine fish osmoregulation. J Exp Biol 209:2813–2827. doi:10.1242/jeb.02345
Grosell M (2011) Intestinal anion exchange in marine teleosts is involved in osmoregulation and contributes to the oceanic inorganic carbon cycle. Acta Physiol 202:421–434. doi:10.1111/j.1748-1716.2010.02241.x
Grosell M, Farrell AP, Brauner CJ (2011) The multifunctional gut of fish. Academic Press, London
Guandalini S, Rubino A (1982) Development of dipeptide transport in the intestinal mucosa of rabbits. Pediatr Res 16:99–103. doi:10.1203/00006450-198202000-00004
Guerrini L, Gong SS, Mangasarian K, Basilico C (1993) Cis- and trans-acting elements involved in amino acid regulation of asparagine synthetase gene expression. Mol Cell Biol 13:3202–3212. doi:10.1128/MCB.13.6.3202
Guettou F, Quistgaard EM, Trésaugues L, Moberg P, Jegerschöld C, Zhu L, Jong AJ, Nordlund P, Löw C (2013) Structural insights into substrate recognition in proton-dependent oligopeptide transporters. EMBO Rep 14:804–810. doi:10.1038/embor.2013.107
Habold C, Chevalier C, Dunel-Erb S, Foltzer-Jourdainne C, Le Maho Y, Lignot JH (2004) Effects of fasting and refeeding on jejunal morphology and cellular activity in rats in relation to depletion of body stores. Scand J Gastroenterol 39:531–539. doi:10.1080/00365520410004514
Habold C, Foltzer-Jourdainne C, Le Maho Y, Lignot JH, Oudart H (2005) Intestinal gluconeogenesis and glucose transport according to body fuel availability in rats. J Physiol 566:575–586. doi:10.1113/jphysiol.2005.085217
Habold C, Reichardt F, Foltzer-Jourdainne C, Lignot JH (2007) Morphological changes of the rat intestinal lining in relation to body stores depletion during fasting and refeeding. Pflugers Arch 455:323–332. doi:10.1007/s00424-007-0289-0
Hakim Y, Harpaz S, Uni Z (2009) Expression of brush border enzymes and transporters in the intestine of European sea bass (Dicentrarchus labrax) following food deprivation. Aquaculture 290:110–115. doi:10.1016/j.aquaculture.2009.02.008
Haller S, Schuler F, Lazic SE, Bachir-Cherif D, Krämer SD, Parrott NJ, Steiner G, Belli S (2012) Expression profiles of metabolic enzymes and drug transporters in the liver and along the intestine of beagle dogs. Drug Metab Dispos 40:1603–1610. doi:10.1124/dmd.112.045443
Han JW, Zheng HF, Cui Y, Sun LD, Ye DQ, Hu Z, Xu JH, Cai ZM, Huang W, Zhao GP, Xie HF, Fang H, Lu QJ, Xu JH, Li XP, Pan YF, Deng DQ, Zeng FQ, Ye ZZ, Zhang XY, Wang QW, Hao F, Ma L, Zuo XB, Zhou FS, Du WH, Cheng YL, Yang JQ, Shen SK, Li J, Sheng YJ, Zuo XX, Zhu WF, Gao F, Zhang PL, Guo Q, Li B, Gao M, Xiao FL, Quan C, Zhang C, Zhang Z, Zhu KJ, Li Y, Hu DY, Lu WS, Huang JL, Liu SX, Li H, Ren YQ, Wang ZX, Yang CJ, Wang PG, Zhou WM, Lv YM, Zhang AP, Zhang SQ, Lin D, Li Y, Low HQ, Shen M, Zhai ZF, Wang Y, Zhang FY, Yang S, Liu JJ, Zhang XJ (2009) Genome-wide association study in a Chinese Han population identifies nine new susceptibility loci for systemic lupus erythematosus. Nat Genet 41:1234–1237. doi:10.1038/ng.472
Hardy RW (1996) Alternate protein sources for salmon and trout diets. Anim Feed Sci Tech 59:71–80. doi:10.1016/0377-8401(95)00888-8
Harris LE (1980) Diet stuffs. In: Pillay TVR (ed) Fish Diet Technology. UNDP/FAO, Rome, pp 111–168
Hart HR, Evans AN, Gelsleichter J, Ahearn GA (2016) Molecular identification and functional characteristics of peptide transporters in the bonnethead shark (Sphyrna tiburo). J Comp Physiol B 186:855–866. doi:10.1007/s00360-016-0999-8
Harter TS, Verreth JA, Heinsbroek LT, Schrama JW (2013) Isoenergetic replacement of fat by starch in diets for African catfish (Clarias gariepinus): effect on water fluxes in the gastro intestinal tract. PLoS ONE 8:e55245. doi:10.1371/journal.pone.0055245
Harter TS, Heinsbroek LTN, Schrama JW (2015) The source of dietary non-protein energy affects in vivo protein digestion in African catfish (Clarias gariepinus). Aquacult Nutr 21:569–577. doi:10.1111/anu.12185
Hazama A, Loo DD, Wright EM (1997) Presteady-state currents of the rabbit Na+/glucose cotransporter (SGLT1). J Membr Biol 155:175–186. doi:10.1007/s002329900169
He L, Vasiliou K, Nebert DW (2009) Analysis and update of the human solute carrier (SLC) gene superfamily. Hum Genomics 3:195–206. doi:10.1186/1479-7364-3-2-195
Hector KL, Nakagawa S (2012) Quantitative analysis of compensatory and catch-up growth in diverse taxa. J Anim Ecol 81:583–593. doi:10.1111/j.1365-2656.2011.01942.x
Hediger MA, Romero MF, Peng JB, Rolfs A, Takanaga H, Bruford EA (2004) The ABCs of solute carriers: physiological, pathological and therapeutic implications of human membrane transport proteins. Pflugers Arch 447:465–468. doi:10.1007/s00424-003-1192-y
Hediger MA, Clémençon B, Burrier RE, Bruford EA (2013) The ABCs of membrane transporters in health and disease (SLC series): introduction. Mol Aspects Med 34:95–107. doi:10.1016/j.mam.2012.12.009
Herrera-Ruiz D, Wang Q, Gudmundsson OS, Cook TJ, Smith RL, Faria TN, Knipp GT (2001) Spatial expression patterns of peptide transporters in the human and rat gastrointestinal tracts, Caco-2 in vitro cell culture model, and multiple human tissues. AAPS PharmSci. 3:E9. doi:10.1208/ps030109
Hevrøy EM, Espe M, Waagbø R, Sandnes K, Ruud M, Hemre GI (2005) Nutrient utilization in Atlentic salmon (Salmo salar L.) fed increased levels of fish protein hydrolysate during a period of fast growth. Aquacult Nutr 11:301–313. doi:10.1111/j.1365-2095.2005.00357.x
Hilgemann DW, Lu CC (1999) GAT1 (GABA:Na+:Cl−) cotransport function. Database reconstruction with an alternating access model. J Gen Physiol 114:459–475. doi:10.1085/jgp.114.3.459
Hilgendorf C, Ahlin G, Seithel A, Artursson P, Ungell AL, Karlsson J (2007) Expression of thirty-six drug transporter genes in human intestine, liver, kidney, and organotypic cell lines. Drug Metab Dispos 35:1333–1340. doi:10.1124/dmd.107.014902
Himukai M, Konno T, Hoshi T (1980) Age-dependent change in intestinal absorption of dipeptides and their constituent amino acids in the guinea pig. Pediatr Res 14:1272–1275. doi:10.1203/00006450-198011000-00024
Hirano T, Mayer-Gostan N (1976) Eel esophagus as an osmoregulatory organ. Proc Natl Acad Sci USA 73:1348–1350. doi:10.1073/pnas.73.4.1348
Höglund PJ, Nordström KJ, Schiöth HB, Fredriksson R (2011) The solute carrier families have a remarkably long evolutionary history with the majority of the human families present before divergence of Bilaterian species. Mol Biol Evol 28:1531–1541. doi:10.1093/molbev/msq350
Horn MH, Gawlicka AK, German DP, Logothetis EA, Cavanagh JW, Boyle KS (2006) Structure and function of the stomachless digestive system in three related species of the New World silverside fishes (Atherinopsidae) representing herbivory, omnivory, and carnivory. Mar Biol 149:1237–1245. doi:10.1007/s00227-006-0281-9
Houlihan DF, Carter CG, McCarthy ID (1995) Protein synthesis in fish. In: Hochachka PW, Mommsen TP (eds) Biochemistry and molecular biology of fishes, vol 4., Metabolic biochemistryElsevier Biomedical, Amsterdam, pp 191–200
Howard A, Goodlad RA, Walters JR, Ford D, Hirst BH (2004) Increased expression of specific intestinal amino acid and peptide transporter mRNA in rats fed by TPN is reversed by GLP-2. J Nutr 134:2957–2964
Hu Y, Smith DE, Ma K, Jappar D, Thomas W, Hillgren KM (2008) Targeted disruption of peptide transporter Pept1 gene in mice significantly reduces dipeptide absorption in intestine. Mol Pharm 5:1122–1130. doi:10.1021/mp8001655
Hu B, Chen H, Liu X, Zhang C, Cole GJ, Lee JA, Chen X (2013) Transgenic overexpression of cdx1b induces metaplastic changes of gene expression in zebrafish esophageal squamous epithelium. Zebrafish 10:218–227. doi:10.1089/zeb.2012.0784
Hu Y, Xie Y, Keep RF, Smith DE (2014) Divergent developmental expression and function of the proton-coupled oligopeptide transporters PepT2 and PhT1 in regional brain slices of mouse and rat. J Neurochem 129:955–965. doi:10.1111/jnc.12687
Hua K, Bureau DP (2012) Exploring the possibility of quantifying the effects of plant protein ingredients in fish feeds using meta-analysis and nutritional model simulation-based approaches. Aquaculture 356:284–301. doi:10.1016/j.aquaculture.2012.05.003
Huang Q, Vera Delgado JM, Seni Pinoargote OD, Llaguno RA (2015) Molecular evolution of the Slc15 family and its response to waterborne copper and mercury exposure in tilapia. Aquat Toxicol 163:140–147. doi:10.1016/j.aquatox.2015.04.011
Huhtanen P, Miettinen H, Ylinen M (1993) Effect of increasing ruminal butyrate on milk yield and blood constituents in dairy cows fed a grass silage-based diet. J Dairy Sci 76:1114–1124. doi:10.3168/jds.S0022-0302(93)77440-8
Hume ID, Biebach H (1996) Digestive tract function in the long-distance migratory garden warbler, Sylvia borin. J Comp Physiol B 166:388–395. doi:10.1007/BF02336922
Hussain I, Kellett GL, Affleck J, Shepherd EJ, Boyd CAR (2002) Expression and cellular distribution during development of the peptide transporter (PepT1) in the small intestinal epithelium of the rat. Cell Tissue Res 307:139–142. doi:10.1007/s00441-001-0473-z
Ihara T, Tsujikawa T, Fujiyama Y, Bamba T (2000) Regulation of PEPT1 peptide transporter expression in the rat small intestine under malnourished conditions. Digestion 61:59–67. doi:10.1159/000007736
Ingersoll SA, Ayyadurai S, Charania MA, Laroui H, Yan Y, Merlin D (2012) The role and pathophysiological relevance of membrane transporter PepT1 in intestinal inflammation and inflammatory bowel disease. Am J Physiol Gastrointest Liver Physiol 302:G484–G492. doi:10.1152/ajpgi.00477.2011
Irie M, Terada T, Okuda M, Inui K (2004) Efflux properties of basolateral peptide transporter in human intestinal cell line Caco-2. Pflugers Arch 449:186–194. doi:10.1007/s00424-004-1326-x
Irie M, Terada T, Katsura T, Matsuoka S, Inui K (2005) Computational modelling of H+-coupled peptide transport via human PEPT1. J Physiol 565:429–439. doi:10.1113/jphysiol.2005.084582
Ismair MG, Vavricka SR, Kullak-Ublick GA, Fried M, Mengin-Lecreulx D, Girardin SE (2006) hPepT1 selectively transports muramyl dipeptide but not Nod1-activating muramyl peptides. Can J Physiol Pharmacol 84:1313–1319. doi:10.1139/y06-076
Ito K, Yamaguchi M, Noma T, Yamaji T, Itoh H, Oda M (2016) Whey protein hydrolysates enhance water absorption in the perfused small intestine of anesthetized rats. Biosci Biotechnol Biochem 80:1587–1593. doi:10.1080/09168451.2016.1166931
Iwanaga T, Kishimoto A (2015) Cellular distributions of monocarboxylate transporters: a review. Biomed Res 36:279–301. doi:10.2220/biomedres.36.279
Jakab RL, Collaco AM, Ameen NA (2011) Physiological relevance of cell-specific distribution patterns of CFTR, NKCC1, NBCe1, and NHE3 along the crypt-villus axis in the intestine. Am J Physiol Gastrointest Liver Physiol 300:G82–G98. doi:10.1152/ajpgi.00245.2010
Jayathilakan K, Sultana K, Radhakrishna K, Bawa AS (2012) Utilization of byproducts and waste materials from meat, poultry and fish processing industries: a review. J Food Sci Technol 49:278–293. doi:10.1007/s13197-011-0290-7
Jean C, Fromentin G, Tomé D, Larue-Achagiotis C (2002) Wistar rats allowed to self-select macronutrients from weaning to maturity choose a high-protein, high-lipid diet. Physiol Behav 76:65–73. doi:10.1016/S0031-9384(02)00676-5
Jobling M (1986) Gastrointestinal overload: a problem with formulated feeds? Aquaculture 51:257–263. doi:10.1016/0044-8486(86)90317-0
Kakizaki F, Aoki K, Miyoshi H, Carrasco N, Aoki M, Taketo MM (2010) CDX transcription factors positively regulate expression of solute carrier family 5, member 8 in the colonic epithelium. Gastroenterology 138:627–635. doi:10.1053/j.gastro.2009.10.047
Kalujnaia S, McWilliam IS, Zaguinaiko VA, Feilen AL, Nicholson J, Hazon N, Cutler CP (2007) Cramb G (2007) Transcriptomic approach to the study of osmoregulation in the European eel Anguilla anguilla. Physiol Genomics 31:385–401. doi:10.1152/physiolgenomics.00059.2007
Kamal MA, Keep RF, Smith DE (2008) Role and relevance of PEPT2 in drug disposition, dynamics, and toxicity. Drug Metab Pharmacokinet 23:236–242. doi:10.2133/dmpk.23.236
Kamalam BS, Panserat S, Aguirre P, Geurden I, Fontagné-Dicharry S, Médale F (2013) Selection for high muscle fat in rainbow trout induces potentially higher chylomicron synthesis and PUFA biosynthesis in the intestine. Comp Biochem Physiol A Mol Integr Physiol 164:417–427. doi:10.1016/j.cbpa.2012.11.020
Kamaszewski M, Prasek M, Ostaszewska T, Dabrowski K (2014) The influence of feeding diets containing wheat gluten supplemented with dipeptides or free amino acids on structure and development of the skeletal muscle of carp (Cyprinus carpio). Aquacult Int 22:259–271. doi:10.007/s10499-013-9683-0
Karakossian MH, Spencer SR, Gomez AQ, Padilla OR, Sacher A, Loo DD, Nelson N, Eskandari S (2005) Novel properties of a mouse gamma-aminobutyric acid transporter (GAT4). J Membr Biol 203:65–82. doi:10.1007/s00235-004-0732-5
Karasov WH, Douglas AE (2013) Comparative digestive physiology. Compr Physiol 3:741–783. doi:10.1002/cphy.c110054
Karasov WH, Hume ID (1997) Vertebrate gastrointestinal system. In: Dantzler W (ed) Handbook of comparative physiology. Am Physiol Soc, Bethesda, pp 409–480
Karasov WH, Solberg DH, Chang SD, Hughes M, Stein ED, Diamond JM (1985) Is intestinal transport of sugars and amino acids subject to critical-period programming? Am J Physiol 249:G770–G785
Karasov WH, Martínez del Rio C, Caviedes-Vidal E (2011) Ecological physiology of diet and digestive systems. Annu Rev Physiol 73:69–93. doi:10.1146/annurev-physiol-012110-142152
Kasper CS, White MR, Brown PB (2000) Choline is required by tilapia when methionine is not in excess. J Nutr 130:238–242
Kassahn KS, Dang VT, Wilkins SJ, Perkins AC, Ragan MA (2009) Evolution of gene function and regulatory control after whole-genome duplication: comparative analyses in vertebrates. Genome Res 19:1404–1418. doi:10.1101/gr.086827.108
Kennedy DJ, Leibach FH, Ganapathy V, Thwaites DT (2002) Optimal absorptive transport of the dipeptide glycylsarcosine is dependent on functional Na+/H+ exchange activity. Pflugers Arch 445:139–146. doi:10.1007/s00424-002-0910-1
Kiela PR, Hines ER, Collins JF, Ghishan FK (2001) Regulation of the rat NHE3 gene promoter by sodium butyrate. Am J Physiol Gastrointest Liver Physiol 281:G947–G956
Kiela PR, Kuscuoglu N, Midura AJ, Midura-Kiela MT, Larmonier CB, Lipko M, Ghishan FK (2007) Molecular mechanism of rat NHE3 gene promoter regulation by sodium butyrate. Am J Physiol Cell Physiol 293:C64–C74. doi:10.1152/ajpcell.00277.2006
Kim SS, Lee KJ (2013) Comparison of leucine requirement in olive flounder (Paralichthys olivaceus) by free or synthetic dipeptide forms of leucine. Anim Feed Sci Technol 183:195–201. doi:10.1016/j.anifeedsci.2013.05.008
Kim HR, Park SW, Cho HJ, Chae KA, Sung JM, Kim JS, Landowski CP, Sun D, Abd El-Aty AM, Amidon GL, Shin HC (2007) Comparative gene expression profiles of intestinal transporters in mice, rats and humans. Pharmacol Res 56:224–236. doi:10.1016/j.phrs.2007.06.005
Kim SS, Rahimnejad S, Song JW, Lee KJ (2012) Comparison of growth performance and whole-body amino acid composition in red seabream (Pagrus major) fed free or dipeptide form of phenylalanine. Asian-Aust J Anim Sci 25:1138–1144. doi:10.5713/ajas.2012.12054
Knütter I, Wollesky C, Kottra G, Hahn MG, Fischer W, Zebisch K, Neubert RH, Daniel H, Brandsch M (2008) Transport of angiotensin-converting enzyme inhibitors by H+/peptide transporters revisited. J Pharmacol Exp Ther 327:432–441. doi:10.1124/jpet.108.143339
Kolodziejczak D, Spanier B, Pais R, Kraiczy J, Stelzl T, Gedrich K, Scherling C, Zietek T, Daniel H (2013) Mice lacking the intestinal peptide transporter display reduced energy intake and a subtle maldigestion/malabsorption that protects them from diet-induced obesity. Am J Physiol Gastrointest Liver Physiol 304:G897–G907. doi:10.1152/ajpgi.00160.2012
Kottra G, Daniel H (2001) Bidirectional electrogenic transport of peptides by the proton-coupled carrier PEPT1 in Xenopus laevis oocytes: its asymmetry and symmetry. J Physiol 536:495–503. doi:10.1111/j.1469-7793.2001.0495c.xd
Kottra G, Stamfort A, Daniel H (2002) PEPT1 as a paradigm for membrane carriers that mediate electrogenic bidirectional transport of anionic, cationic, and neutral substrates. J Biol Chem 277:32683–32691. doi:10.1074/jbc.M204192200
Kottra G, Frey I, Daniel H (2009) Inhibition of intracellular dipeptide hydrolysis uncovers large outward transport currents of the peptide transporter PEPT1 in Xenopus oocytes. Pflugers Arch 457:809–820. doi:10.1007/s00424-008-0562-x
Kottra G, Spanier B, Verri T, Daniel H (2013) Peptide transporter isoforms are discriminated by the fluorophore-conjugated dipeptides β-Ala- and d-Ala–Lys-N-7-amino-4-methylcoumarin-3-acetic acid. Physiol Rep 1:e00165. doi:10.1002/phy2.165
Kotzamanis YP, Gisbert E, Gatesoupe FJ, Zambonino-Infante J, Cahu C (2007) effects of different dietary levels of fish protein hydrolysates on growth, digestive enzymes, gut microbiota, and resistance to Vibrio anguillarum in European sea bass (Dicentrarchus labrax) larvae. Comp Biochem Physiol A Mol Integr Physiol 147:205–214. doi:10.1016/j.cbpa.2006.12.037
Kousoulaki K, Saether BS, Albrektsen S, Noble C (2015) Review on European sea bass (Dicentrarchus labrax, Linnaeus, 1758) nutrition and feed management: a practical guide for optimizing feed formulation and farming protocols. Aquacult Nutr 21:129–151. doi:10.1111/anu.12233
Koven W, Schulte P (2012) The effect of fasting and refeeding on mRNA expression of PepT1 and gastrointestinal hormones regulating digestion and food intake in zebrafish (Danio rerio). Fish Physiol Biochem 38:1565–1575. doi:10.1007/s10695-012-9649-6
Kristiansen HR, Rankin JC (2001) Discrimination between endogeneous and exogeneus water sources in juvenile rainbow trout fed extruded dry feed. Aquat Living Resour 14:359–366. doi:10.1016/S0990-7440(01)01131-7
Kuang SY, Xiao WW, Feng L, Liu Y, Jiang J, Jiang WD, Hu K, Li SH, Tang L, Zhou XQ (2012) Effects of graded levels of dietary methionine hydroxy analogue on immune response and antioxidant status of immune organs in juvenile Jian carp (Cyprinus carpio var. Jian). Fish Shellfish Immunol 32:629–636. doi:10.1016/j.fsi.2011.12.012
Kudo M, Katayoshi T, Kobayashi-Nakamura K, Akagawa M, Tsuji-Naito K (2016) H+/peptide transporter (PEPT2) is expressed in human epidermal keratinocytes and is involved in skin oligopeptide transport. Biochem Biophys Res Commun 475:335–341. doi:10.1016/j.bbrc.2016.05.093
Kudsk KA (2007) Beneficial effect of enteral feeding. Gastrointest Endoscopy Clin North Am 17:647–662. doi:10.1016/j.giec.2007.07.003
Kukk S, Stepanov V, Järv J (2015) Thermal stability of dopamine transporters. J Membr Biol 248:775–781. doi:10.1007/s00232-015-9794-9
Kültz D (2015) Physiological mechanisms used by fish to cope with salinity stress. J Exp Biol 218:1907–1914. doi:10.1242/jeb.118695
Küpers LK, L’Abée C, Bocca G, Stolk RP, Sauer PJ, Corpeleijn E (2015) Determinants of weight gain during the first two years of life: the GECKO Drenthe birth cohort. PLoS One 10:e0133326. doi:10.1371/journal.pone.0133326
Kurita Y, Nakada T, Kato A, Doi H, Mistry AC, Chang MH, Romero MF, Hirose S (2008) Identification of intestinal bicarbonate transporters involved in formation of carbonate precipitates to stimulate water absorption in marine teleost fish. Am J Physiol Regul Integr Comp Physiol 294:R1402-R1412. doi: 10.1152/ajpregu.00759.2007
Kuzmina VV, Zhivaev NG, Botjazhova OA (2008) The biochemical composition of chyme in fish species with different diets. Inland Water Biol 1:282–286. doi:10.1134/S1995082908030127
Kwasek K, Zhang Y, Hliwa P, Gomulka P, Ostaszewska T, Dabrowski K (2009) Free amino acids as indicators of nutritional status of silver bream (Vimba vimba), when using commercial and purified diets. Comp Biochem Physiol A Mol Integr Physiol 153:113–119. doi:10.1016/j.cbpa.2009.01.003
Kwasek K, Zhang Y, Dabrowski K (2010) Utilization of dipeptide/protein based diets in larval and juvenile Koi carp: post-prandial free amino acid levels. J Anim Physiol Anim Nutr 94:35–43. doi:10.1111/j.1439-0396.2008.00877.x
Kwasek K, Terova G, Wojno M, Dabrowski K, Wick M (2012) The effect of dietary dipeptide lysine-glycine on growth, muscle proteins, and intestine PepT1 gene expression in juvenile yellow perch. Rev Fish Biol Fisheries 22:797–812. doi:10.1007/s11160-012-9266-6
Laranjeiro R, Whitmore D (2014) Transcription factors involved in retinogenesis are co-opted by the circadian clock following photoreceptor differentiation. Development 141:2644–2656. doi:10.1242/dev.104380
Larsen EH, Deaton LE, Onken H, O’Donnell M, Grosell M, Dantzler WH, Weihrauch D (2014) Osmoregulation and excretion. Compr Physiol 4:405–573. doi:10.1002/cphy.c130004
Laverty G, Skadhauge E (2012) Adaptation of teleosts to very high salinity. Comp Biochem Physiol A Mol Integr Physiol 163:1–6. doi:10.1016/j.cbpa.2012.05.203
Lee J, Tattoli I, Wojtal KA, Vavricka SR, Philpott DJ, Girardin SE (2009) pH-dependent internalization of muramyl peptides from early endosomes enables Nod1 and Nod2 signaling. J Biol Chem 284:23818–23829. doi:10.1074/jbc.M109.033670
Li H, Gilbert ER, Zhang Y, Crasta O, Emmerson D, Webb KE Jr, Wong EA (2008) Expression profiling of the solute carrier gene family in chicken intestine from the late embryonic to early post-hatch stages. Anim Genet 39:407–424. doi:10.1111/j.1365-2052.2008.01744.x
Li P, Mai K, Trushenski J, Wu G (2009) New developments in fish amino acid nutrition: towards functional and environmentally oriented aquafeeds. Amino Acids 37:43–53. doi:10.1007/s00726-008-0171-1
Li IC, Chan CT, Lu YF, Wu YT, Chen YC, Li GB, Lin CY, Hwang SP (2011a) Zebrafish krüppel-like factor 4a represses intestinal cell proliferation and promotes differentiation of intestinal cell lineages. PLoS ONE 6:e20974. doi:10.1371/journal.pone.0020974
Li X, Xiong H, Yang K, Peng D, Li W, Yin Y, Liu J (2011b) Effects of rice dreg protein and its hydrolysate on growth performance and small intestine morphology of early-weaned rats. J Sci Food Agric 91:687–693. doi:10.1002/jsfa.4237
Li XY, Tang L, Hu K, Liu Y, Jiang WD, Jiang J, Wu P, Chen GF, Li SH, Kuang SY, Feng L, Zhou XQ (2014) Effect of dietary lysine on growth, intestinal enzymes activities and antioxidant status of sub-adult grass carp (Ctenopharyngodon idella). Fish Physiol Biochem 40:659–671. doi:10.1007/s10695-013-9874-7
Li M, Li C, Song S, Zhao F, Xu X, Zhou G (2016) Meat proteins had different effects on oligopeptide transporter PEPT1 in the small intestine of young rats. Int J Food Sci Nutr. doi:10.1080/09637486.2016.1210574
Lim CE, Webster CD, Lee CS (2008) Alternative protein sources in aquaculture diets. Haworth Press, New York
Liou AP, Chavez DI, Espero E, Hao S, Wank SA, Raybould HE (2011) Protein hydrolysate-induced cholecystokinin secretion from enteroendocrine cells is indirectly mediated by the intestinal oligopeptide transporter PepT1. Am J Physiol Gastrointest Liver Physiol 300:G895–G902. doi:10.1152/ajpgi.00521.2010
Liu L, Li C, Su B, Beck BH, Peatman E (2013a) Short-term feed deprivation alters immune status of surface mucosa in channel catfish (Ictalurus punctatus). PLoS ONE 8:e74581. doi:10.1371/journal.pone.0074581
Liu Z, Zhou Y, Feng J, Lu S, Zhao Q, Zhang J (2013b) Characterization of oligopeptide transporter (PepT1) in grass carp (Ctenopharyngodon idella). Comp Biochem Physiol B: Biochem Mol Biol 164:194–200. doi:10.1016/j.cbpb.2012.11.008
Liu Z, Zhou Y, Liu S, Zhao Q, Feng J, Lu S, Xiong G, Xie D, Zhang J, Liu Y (2014) Characterization and dietary regulation of oligopeptide transporter (PepT1) in different ploidy fishes. Peptides 52:149–156. doi:10.1016/j.peptides.2013.12.017
Lucas ML, Schneider W, Haberich FJ, Blair JA (1975) Direct measurement by pH-microelectrode of the pH microclimate in rat proximal jejunum. Proc R Soc Lond B Biol Sci 192:39–48. doi:10.1098/rspb.1975.0150
Luo Z, Liu Y, Mai K, Tian L, Yang H, Liu D (2005) Dietary l-methionine requirement of juvenile grouper Epinephelus coioides at a constant dietary cystine level. Aquaculture 249:409–418. doi:10.1016/j.aquaculture.2005.04.030
Lyons JA, Parker JL, Solcan N, Brinth A, Li D, Shah ST, Caffrey M, Newstead S (2014) Structural basis for polyspecificity in the POT family of proton-coupled oligopeptide transporters. EMBO Rep 15:886–893. doi:10.15252/embr.201338403
Ma K, Hu Y, Smith DE (2012) Influence of fed-fasted state on intestinal PEPT1 expression and in vivo pharmacokinetics of glycylsarcosine in wild-type and Pept1 knockout mice. Pharm Res 29:535–545. doi:10.1007/s11095-011-0580-9
Ma R, Hou H, Mai K, Bharadwaj AS, Cao H, Ji F, Zhang W (2013) Comparative study on the effects of l-methionine or 2-hydroxy-4-(methylthio) butanoic acid as dietary methionine source on growth performance and anti-oxidative responses of turbot (Psetta maxima). Aquaculture 412:136–143. doi:10.1016/j.aquaculture.2013.07.021
Ma G, Shi B, Liu J, Zhang H, YinTao Z, Lou X, Liang D, Hou Y, Wan S, Yang W (2015) Nod2-Rip2 signaling contributes to intestinal injury induced by muramyl dipeptide via oligopeptide transporter in rats. Dig Dis Sci 60:3264–3270. doi:10.1007/s10620-015-3762-1
Mace OJ, Lister N, Morgan E, Shepherd E, Affleck J, Helliwell P, Bronk JR, Kellett GL, Meredith D, Boyd R, Pieri M, Bailey PD, Pettcrew R, Foley D (2009) An energy supply network of nutrient absorption coordinated by calcium and T1R taste receptors in rat small intestine. J Physiol 587:195–210. doi:10.1113/jphysiol.2008.159616
Mackenzie B, Loo DDF, Fei YJ, Liu W, Ganapathy V, Leibach FH, Wright EM (1996) Mechanisms of the human intestinal H+-coupled oligopeptide transporter hPEPT1. J Biol Chem 271:5430–5437. doi:10.1074/jbc.271.10.5430
MacKenzie DS, Van Putte CM, Leiner KA (1998) Nutrient regulation of endocrine function in fish. Aquaculture 161:3–25. doi:10.1016/S0044-8486(97)00253-6
Mackenzie B, Takanaga H, Hubert N, Rolfs A, Hediger MA (2007) Functional properties of multiple isoforms of human divalent metal-ion transporter 1 (DMT1). Biochem J 403:59–69. doi:10.1042/BJ20061290
Madsen SL, Wong EA (2011) Expression of the chicken peptide transporter 1 and the peroxisome proliferator-activated receptor α following feed restriction and subsequent refeeding. Poult Sci 90:2295–2300. doi:10.3382/ps.2010-01173
Maffia M, Acierno R, Cillo E, Storelli C (1996) Na+-d-glucose cotransport by intestinal BBMVs of the Antarctic fish Trematomus bernacchii. Am J Physiol 271:R1576–R1583
Maffia M, Verri T, Danieli A, Thamotharan M, Pastore M, Ahearn GA, Storelli C (1997) H+-glycyl-l-proline cotransport in brush-border membrane vesicles of eel (Anguilla anguilla) intestine. Am J Physiol 272:R217–R225
Maffia M, Rizzello A, Acierno R, Verri T, Rollo M, Danieli A, Döring F, Daniel H, Storelli C (2003) Characterisation of intestinal peptide transporter of the Antarctic haemoglobinless teleost Chionodraco hamatus. J Exp Biol 206:705–714. doi:10.1242/jeb.00145
Mai K, Wan J, Ai Q, Xu W, Liufu Z, Zhang L, Zhang C, Li H (2006a) Dietary methionine requirement of large yellow croaker, Pseudosciaena crocea R. Aquaculture 253:564–572. doi:10.1016/j.aquaculture.2005.08.010
Mai K, Zhang L, Ai Q, Duan Q, Zhang C, Li H, Wan J, Liufu Z (2006b) Dietary lysine requirement of juvenile Japanese seabass, Lateolabrax japonicus. Aquaculture 258:535–542. doi:10.1016/j.aquaculture.2006.04.043
Mamauag REP, Gao J, Nguyen BT, Ragaza JA, Koshio S, Ishikawa M, Yokoyama S (2012) Supplementations of dl-methionine and methionine dipeptide in diets are effective for the development and growth of larvae and juvenile red sea bream, Pagrus major. J World Aquacult Soc 43:362–374. doi:10.1111/j.1749-7345.2012.00563.x
Manzanilla EG, Nofrarías M, Anguita M, Castillo M, Perez JF, Martín-Orúe SM, Kamel C, Gasa J (2006) Effects of butyrate, avilamycin, and a plant extract combination on the intestinal equilibrium of early-weaned pigs. J Anim Sci 84:2743–2751. doi:10.2527/jas.2005-509
Marasco WA, Phan SH, Krutzsch H, Showell HJ, Feltner DE, Nairn R, Becker EL, Ward PA (1984) Purification and identification of formyl-methionyl-leucy l-phenylalanine as the major peptide neutrophil chemotactic factor produced by Escherichia coli. J Biol Chem 259:5430–5439
Margheritis E, Terova G, Cinquetti R, Peres A, Bossi E (2013a) Functional properties of a newly cloned fish ortholog of the neutral amino acid transporter B0AT1 (SLC6A19). Comp Biochem Physiol A: Mol Integr Physiol 166:285–292. doi:10.1016/j.cbpa.2013.06.027
Margheritis E, Terova G, Oyadeyi AS, Renna MD, Cinquetti R, Peres A, Bossi E (2013b) Characterization of the transport of lysine-containing dipeptides by PepT1 orthologs expressed in Xenopus laevis oocytes. Comp Biochem Physiol A Mol Integr Physiol 164:520–528. doi:10.1016/j.cbpa.2012.12.016
Markovich D (2008) Expression cloning and radiotracer uptakes in Xenopus laevis oocytes. Nat Protoc 3:1975–1980. doi:10.1038/nprot.2008.151
Marshall C, Elias C, Xue XH, Le HD, Omelchenko A, Hryshko LV, Tibbits GF (2002) Determinants of cardiac Na+/Ca2+ exchanger temperature dependence: NH2-terminal transmembrane segments. Am J Physiol Cell Physiol 283:C512–C520. doi:10.1152/ajpcell.00558.2001
Martinez-Alvarez O, Chamorro S, Brenes A (2015) Protein hydrolysates from animal processing by-products as a source of bioactive molecules with interest in animal feeding: a review. Food Res Int 73:204–212. doi:10.1016/j.foodres.2015.04.005
McCue MD (2010) Starvation physiology: reviewing the different strategies animals use to survive a common challenge. Comp Biochem Physiol A: Mol Integr Physiol 156:1–18. doi:10.1016/j.cbpa.2010.01.002
McCue MD (2012) Comparative physiology of fasting, starvation and food limitation. Spinger-Verlag, Berlin Heidelberg
McIntyre A, Gibson PR, Young GP (1993) Butyrate production from dietary fibre and protection against large bowel cancer in a rat model. Gut 34:386–391. doi:10.1136/gut.34.3.386
McMillan DN, Houlihan DF (1989) Short-term responses of protein synthesis to re-feeding in rainbow trout. Aquaculture 79:37–46. doi:10.1016/0044-8486(89)90443-2
McNurlan MA, Garlick PJ (1981) Protein synthesis in liver and small intestine in protein deprivation and diabetes. Am J Physiol 241:E238–E245
Meier Y, Eloranta JJ, Darimont J, Ismair MG, Hiller C, Fried M, Kullak-Ublick GA, Vavricka SR (2007) Regional distribution of solute carrier mRNA expression along the human intestinal tract. Drug Metab Dispos 35:590–594. doi:10.1124/dmd.106.013342
Meissner B, Boll M, Daniel H, Baumeister R (2004) Deletion of the intestinal peptide transporter affects insulin and TOR signalling in Caenorhabditis elegans. J Biol Chem 279:36739–36745. doi:10.1074/jbc.M403415200
Meredith D (2009) Review. The mammalian proton-coupled peptide cotransporter PepT1: sitting on the transporter-channel fence? Philos Trans R Soc Lond B Biol Sci 364:203–207. doi:10.1098/rstb.2008.0139
Meredith D, Price RA (2006) Molecular modelling of PepT1: towards a structure. J Membr Biol 213:79–88. doi:10.1007/s00232-006-0876-6
Mertl M, Daniel H, Kottra G (2008) Substrate-induced changes in the density of peptide transporter PEPT1 expressed in Xenopus oocytes. Am J Physiol Cell Physiol 295:C1332–C1343. doi:10.1152/ajpcell.00241.2008
Miska KB, Fetterer RH, Wong EA (2014) The mRNA expression of amino acid transporters, aminopeptidase N, and the di- and tri-peptide transporter PepT1 in the embryo of the domesticated chicken (Gallus gallus) shows developmental regulation. Poult Sci 93:2262–2270. doi:10.3382/ps.2014-03983
Miska KB, Fetterer RH, Wong EA (2015) mRNA expression of amino acid transporters, aminopeptidase, and the di- and tri-peptide transporter PepT1 in the intestine and liver of posthatch broiler chicks. Poult Sci 94:1323–1332. doi:10.3382/ps/pev059
Miyamoto K, Shiraga T, Morita K, Yamamoto H, Haga H, Taketani Y, Tamai I, Sai Y, Tsuji A, Takeda E (1996) Sequence, tissue distribution and developmental changes in rat intestinal oligopeptide transporter. Biochim Biophys Acta 1305:34–38. doi:10.1016/0167-4781(95)00208-1
Mizumori M, Meyerowitz J, Takeuchi T, Lim S, Lee P, Supuran CT, Guth PH, Engel E, Kaunitz JD, Akiba Y (2006) Epithelial carbonic anhydrases facilitate PCO2 and pH regulation in rat duodenal mucosa. J Physiol 573:827–842. doi:10.1113/jphysiol.2006.107581
Mommsen TP (2001) Paradigms of growth in fish. Comp Biochem Physiol B Biochem Mol Biol 129:207–219. doi:10.1016/S1096-4959(01)00312-8
Montserrat N, Gabillard JC, Capilla E, Navarro MI, Gutiérrez J (2007a) Role of insulin, insulin-like growth factors, and muscle regulatory factors in the compensatory growth of the trout (Oncorhynchus mykiss). Gen Comp Endocrinol 150(3):462–472. doi:10.1016/j.ygcen.2006.11.009
Montserrat N, Gómez-Requeni P, Bellini G, Capilla E, Pérez-Sánchez J, Navarro I, Gutiérrez J (2007b) Distinct role of insulin and IGF-I and its receptors in white skeletal muscle during compensatory growth of gilthead sea bream (Sparus aurata). Aquaculture 267:188–198. doi:10.1016/j.aquaculture.2007.04.024
Mooij MG, de Koning BE, Lindenbergh-Kortleve DJ, Simons-Oosterhuis Y, van Groen BD, Tibboel D, Samsom JN, de Wildt SN (2016) Human intestinal PEPT1 transporter expression and localization in preterm and term infants. Drug Metab Dispos 44:1014–1019. doi:10.1124/dmd.115.068809
Moran AW, Al-Rammahi MA, Arora DK, Batchelor DJ, Coulter EA, Ionescu C, Bravo D, Shirazi-Beechey SP (2010) Expression of Na+/glucose co-transporter 1 (SGLT1) in the intestine of piglets weaned to different concentrations of dietary carbohydrate. Br J Nutr 104:647–655. doi:10.1017/S0007114510000954
Moreau A, Le Vee M, Jouan E, Parmentier Y, Fardel O (2011) Drug transporter expression in human macrophages. Fundam Clin Pharmacol 25:743–752. doi:10.1111/j.1472-8206.2010.00913.x
Morrison CD, Laeger T (2015) Protein-dependent regulation of feeding and metabolism. Trends Endocrinol Metab 26:256–262. doi:10.1016/j.tem.2015.02.008
Morrison CD, Reed SD, Henagan TM (2011) Homeostatic regulation of protein intake: in search of a mechanism. Am J Physiol Regul Integr Comp Physiol 302:R917–R928. doi:10.1152/ajpregu.00609.2011
Mourad FH, Saadé NE (2011) Neural regulation of intestinal nutrient absorption. Prog Neurobiol 95:149–162. doi:10.1016/j.pneurobio.2011.07.010
Mueller CA, Eme J, Burggren WW, Roghair RD, Rundle SD (2015) Challenges and opportunities in developmental integrative physiology. Comp Biochem Physiol A: Mol Integr Physiol 184:113–124. doi:10.1016/j.cbpa.2015.02.013
Nakajima M, Sugiura S (2016) Effects of dietary NaCl on the in vivo apparent absorption of dietary nutrients determined in rainbow trout (Oncorhynchus mykiss). Aquaculture 460:1–7. doi:10.1016/j.aquaculture.2016.04.003
Nakajima S, Hira T, Hara H (2012) Calcium-sensing receptor mediates dietary peptide-induced CCK secretion in enteroendocrine STC-1 cells. Mol Nutr Food Res 56:753–760. doi:10.1002/mnfr.201100666
Nalbant P, Boehmer C, Dehmelt L, Wehner F, Werner A (1999) Functional characterization of a Na+-phosphate cotransporter (NaPi-II) from zebrafish and identification of related transcripts. J Physiol 520:79–89. doi:10.1111/j.1469-7793.1999.00079.x
Naruhashi K, Sai Y, Tamai I, Suzuki N, Tsuji A (2002) PepT1 mRNA expression is induced by starvation and its level correlates with absorptive transport of cefadroxil longitudinally in the rat intestine. Pharm Res 19:1417–1423. doi:10.1023/A:1020436028194
Nässl AM, Rubio-Aliaga I, Fenselau H, Marth MK, Kottra G, Daniel H (2011a) Amino acid absorption and homeostasis in mice lacking the intestinal peptide transporter PEPT1. Am J Physiol Gastrointest Liver Physiol 301:G128–G137. doi:10.1152/ajpgi.00017.2011
Nässl AM, Rubio-Aliaga I, Sailer M, Daniel H (2011b) The intestinal peptide transporter PEPT1 is involved in food intake regulation in mice fed a high-protein diet. PLoS ONE 6:e26407. doi:10.1371/journal.pone.0026407
National Research Council (2011) Nutrient requirements of fish and shrimp. The National Academic Press, Washington
Naya DE, Bozinovic F (2006) The role of ecological interactions on the physiological flexibility of lizards. Funct Ecol 20:601–608. doi:10.1111/j.1365-2435.2006.01137.x
Near TJ, Dornburg A, Kuhn KL, Eastman JT, Pennington JN, Patarnello T, Zane L, Fernández DA, Jones CD (2012a) Ancient climate change, antifreeze, and the evolutionary diversification of Antarctic fishes. Proc Natl Acad Sci USA 109:3434–3439. doi:10.1073/pnas.1115169109
Near TJ, Eytan RI, Dornburg A, Kuhn KL, Moore JA, Davis MP, Wainwright PC, Friedman M, Smith WL (2012b) Resolution of ray-finned fish phylogeny and timing of diversification. Proc Natl Acad Sci USA 109:13698–13703. doi:10.1073/pnas.1206625109
Nelson JS (2006) Fishes of the world. John Wiley and Sons, New York
Newstead S (2011) Towards a structural understanding of drug and peptide transport within the proton-dependent oligopeptide transporter (POT) family. Biochem Soc Trans 39:1353–1358. doi:10.1042/BST0391353
Newstead S (2015) Molecular insights into proton coupled peptide transport in the PTR family of oligopeptide transporters. Biochim Biophys Acta 1850:488–499. doi:10.1016/j.bbagen.2014.05.011
Newstead S, Drew D, Cameron AD, Postis VL, Xia X, Fowler PW, Ingram JC, Carpenter EP, Sansom MS, McPherson MJ, Baldwin SA, Iwata S (2011) Crystal structure of a prokaryotic homologue of the mammalian oligopeptide-proton symporters, PepT1 and PepT2. EMBO J 30:417–426. doi:10.1038/emboj.2010.309
Nguyen TN, Davis DA (2009) Methionine requirement in practical diets of juvenile Nile tilapia, Oreochromis niloticus. J World Aquacult Soc 40:410–416. doi:10.1111/j.1749-7345.2009.00261.x
Nguyen TV, Smith DE, Fleisher D (2007) PEPT1 enhances the uptake of gabapentin via trans-stimulation of b0,+ exchange. Pharm Res 24:353–360. doi:10.1007/s11095-006-9155-6
Nguyen TV, Jung H, Nguyen TM, Hurwood D, Mather P (2016) Evaluation of potential candidate genes involved in salinity tolerance in striped catfish (Pangasianodon hypophthalmus) using an RNA-Seq approach. Mar Genomics 25:75–88. doi:10.1016/j.margen.2015.11.010
Nie GX, Yan X, Wang JL, Ming H, Wang B, Zheng JL, Li XJ, Kong XH (2012) Peptide transporter Pept1 in Cyprinus carpio L’.s intestine: cDNA cloning and sequence analysis. Turk J Biochem 37:204–211. doi:10.5505/tjb.2012.95914
Nielsen CU, Brodin B (2003) Di/tri-peptide transporters as drug delivery targets: regulation of transport under physiological and patho-physiological conditions. Curr Drug Targets 4:373–388. doi:10.2174/1389450033491028
Nitzan T, Rozenberg P, Cnaani A (2016) Differential expression of amino-acid transporters along the intestine of Mozambique tilapia (Oreochromis mossambicus) and the effect of water salinity and time after feeding. Aquaculture. doi:10.1016/j.aquaculture.2016.01.020
Nosworthy MG, Bertolo RF, Brunton JA (2013) Ontogeny of dipeptide uptake and peptide transporter 1 (PepT1) expression along the gastrointestinal tract in the neonatal Yucatan miniature pig. Br J Nutr 110:275–281. doi:10.1017/S0007114512005041
Nunes AJP, Sà MVC, Browdy CL, Vazquez-Anon M (2014) Practical supplementation of shrimp and fish feeds with crystalline amino acids. Aquaculture 431:20–27. doi:10.1016/j.aquaculture.2014.04.003
Nussberger S, Steel A, Trotti D, Romero MF, Boron WF, Hediger MA (1997) Symmetry of H+ binding to the intra- and extracellular side of the H+-coupled oligopeptide cotransporter PepT1. J Biol Chem 272:7777–7785. doi:10.1074/jbc.272.12.7777
Nyman L (1972) Some effects of temperature on eel (Anguilla) behaviour. Inst Freshwater Res 52:90–102
O’Connor KI, Taylor AC, Metcalfe NB (2000) The stability of standard metabolic rate during a period of food deprivation in juvenile Atlantic salmon. J Fish Biol 57:41–51. doi:10.1111/j.1095-8649.2000.tb00774.x
Ocheltree SM, Keep RF, Shen H, Yang D, Hughes BA, Smith DE (2003) Preliminary investigation into the expression of proton-coupled oligopeptide transporters in neural retina and retinal pigment epithelium (RPE): lack of functional activity in RPE plasma membranes. Pharm Res 20:1364–1372. doi:10.1023/A:1025741723724
Ogihara H, Suzuki T, Nagamachi Y, Inui K, Takata K (1999) Peptide transporter in the rat small intestine: ultrastructural localization and the effect of starvation and administration of amino acids. Histochem J 31:169–174. doi:10.1023/A:1003515413550
Okamura A, Koyanagi S, Dilxiat A, Kusunose N, Chen JJ, Matsunaga N, Shibata S, Ohdo S (2014) Bile acid-regulated peroxisome proliferator-activated receptor-α (PPARα) activity underlies circadian expression of intestinal peptide absorption transporter PepT1/Slc15a1. J Biol Chem 289:25296–25305. doi:10.1074/jbc.M114.577023
Oliva-Teles A, Cerqueira AL, Gonçalves P (1999) The utilization of diets containing high levels of fish protein hydrolysate by turbot (Scophthalmus maximus) juveniles. Aquaculture 179:195–201. doi:10.1016/S0044-8486(99)00162-3
Önal U, Langdon C (2008) Çelik I (2008) Ontogeny of the digestive tract of larval percula clownfish, Amphiprion percula (Lacépède 1802): a histological perspective. Aquac Res 39:1077–1086. doi:10.1111/j.1365-2109.2008.01968.x
Opheim M, Sterten H, Øverland M, Kjos NP (2016) Atlantic salmon (Salmo salar) protein hydrolysate: effect on growth performance and intestinal morphometry in broiler chickens. Livest Sci 187:138–145. doi:10.1016/j.livsci.2016.03.005
Ostaszewska T, Dabrowski K, Hliwa P, Gomolka P, Kwasek K (2008a) Nutritional regulation of intestine morphology in larval cyprinid fish, silver bream (Vimba vimba). Aquacult Nutr 39:1268–1278. doi:10.1111/j.1365-2109.2008.01989.x
Ostaszewska T, Dabrowski K, Wegner A, Krawiec M (2008b) The effects of feeding on muscle growth dynamics and the proliferation of myogenic progenitor cells during pike perch development (Sander lucioperca). J World Aquacult Soc 39:184–195. doi:10.1111/j.1749-7345.2008.00151.x
Ostaszewska T, Dabrowski K, Kamaszewski M, Grochowski P, Verri T, Rzepkowska M, Wolnicki J (2010a) The effect of plant protein-based diet supplemented with dipeptide or free amino acids on digestive tract morphology and PepT1 and PepT2 expressions in common carp (Cyprinus carpio L.). Comp Biochem Physiol A Mol Integr Physiol 157:158–169. doi:10.1016/j.cbpa.2010.06.162
Ostaszewska T, Kamaszewski M, Grochowski P, Dabrowski K, Verri T, Aksakal E, Szatkowska I, Nowak Z, Dobosz S (2010b) The effect of peptide absorption on PepT1 gene expression and digestive system hormones in rainbow trout (Oncorhynchus mykiss). Comp Biochem Physiol A: Mol Integr Physiol 155:107–114. doi:10.1016/j.cbpa.2009.10.017
Ostaszewska T, Dabrowski K, Kamaszewski M, Kwasek K, Grodzik M, Bierla J (2013) The effect of dipeptide, Lys–Gly, supplemented diets on digestive tract histology in juvenile yellow perch (Perca flavescens). Aquacult Nutr 19:100–109. doi:10.1111/j.1365-2095.2012.00948.x
Ouellet DR, Seoane JR, Veira DM, Proulx JG (1997) Effects of supplementation with fish meal or fish protein hydrolysate on growth, nutrient digestibility and rumen fermentation of growing cattle fed grass silage. Anim Feed Sci Technol 68:307–326. doi:10.1016/S0377-8401(97)00035-7
Pacha J (2000) Development of intestinal transport function in mammals. Physiol Rev 80:1633–1667
Pacha J, Sumova A (2013) Circadian regulation of epithelial functions in the intestine. Acta Physiol (Oxf) 208:11–24. doi:10.1111/apha.12090
Pan YX, Wong EA, Bloomquist JR, Webb KE Jr (2001) Expression of a cloned ovine gastrointestinal peptide transporter (oPepT1) in Xenopus oocytes induces uptake of oligopeptides in vitro. J Nutr 131:1264–1270
Pan X, Terada T, Irie M, Saito H, Inui KI (2002) Diurnal rhythm of H+-peptide cotransporter in rat small intestine. Am J Physiol Gastrointest Liver Physiol 283:G57–G64. doi:10.1152/ajpgi.00545.2001
Pan X, Terada T, Okuda M, Inui KI (2003) Altered diurnal rhythm of intestinal peptide transporter by fasting and its effects on the pharmacokinetics of ceftibuten. J Pharmacol Exp Ther 307:626–632. doi:10.1124/jpet.103.055939
Pan X, Terada T, Okuda M, Inui KI (2004) The diurnal rhythm of the intestinal transporters SGLT1 and PEPT1 is regulated by the feeding conditions in the rat. J Nutr 134:2211–2215
Panitsas KE, Boyd CA, Meredith D (2006) Evidence that the rabbit proton-peptide co-transporter PepT1 is a multimer when expressed in Xenopus laevis oocytes. Pflugers Arch 452:53–63. doi:10.1007/s00424-005-0002-0
Parker MD, Boron WF (2013) The divergence, actions, roles, and relatives of sodium-coupled bicarbonate transporters. Physiol Rev 93:803–959. doi:10.1152/physrev.00023.2012
Parker JL, Mindell JA, Newstead S (2014) Thermodynamic evidence for a dual transport mechanism in a POT peptide transporter. Elife 3:e04273. doi:10.7554/eLife.04273
Parks SK, Tresguerres M, Goss GG (2008) Theoretical considerations underlying Na+ uptake mechanisms in freshwater fishes. Comp Biochem Physiol C: Toxicol Pharmacol 148:411–418. doi:10.1016/j.cbpc.2008.03.002
Parmelee JT, Renfro JL (1983) Esophageal desalination of seawater in flounder: role of active sodium transport. Am J Physiol 245:R888–R893
Pedretti A, De Luca L, Marconi C, Negrisoli G, Aldini G, Vistoli G (2008) Modeling of the intestinal peptide transporter hPepT1 and analysis of its transport capacities by docking and pharmacophore mapping. ChemMedChem 3:1913–1921. doi:10.1002/cmdc.200800184
Pérez-Torras S, Iglesias I, Llopis M, Lozano JJ, Antolín M, Guarner F, Pastor-Anglada M (2016) Transportome profiling identifies profound alterations in Crohn’s Disease partially restored by commensal bacteria. J Crohns Colitis 10:850–859. doi:10.1093/ecco-jcc/jjw042
Perez-Velasquez M, Gonzalez-Felix M, Lawrence AL, Gatlin DM (2003) Changes in lipid class and fatty acid composition of adult male Litopenaeus vannamei (Boone) in response to culture temperature and food deprivation. Aquacult Res 34:1205–1213. doi:10.1046/j.1365-2109.2003.00931.x
Phositlimpagul A, Edwards GL, Azain MJ (2002) Hepatic vagotomy disrupts somatotropin-induced protein selection. Physiol Behav 75:193–200. doi:10.1016/S0031-9384(01)00643-6
Pieri M, Christian HC, Wilkins RJ, Boyd CA, Meredith D (2010) The apical (hPepT1) and basolateral peptide transport systems of Caco-2 cells are regulated by AMP-activated protein kinase. Am J Physiol Gastrointest Liver Physiol 299:G136–G143. doi:10.1152/ajpgi.00014.2010
Pisani P (2012) Caratterizzazione di un trasportatore funzionalmente orfano della famiglia genica SLC15: il trasportatore SLC15A4(PHT1) (peptide/histidine transporter 1). Dissertation, University of Salento
Pohjanpelto P, Holtta E (1990) Deprivation of a single amino acid induces protein synthesis-dependent increase in c-jun, c-myc and ornithine decarboxylase mRNAs in Chinese hamster ovary cells. Mol Cell Biol 10:5814–5821. doi:10.1128/MCB.10.11.5814
Poole CA, Wong EA, McElroy AP, Veit HP, Webb KE Jr (2003) Ontogenesis of peptide transport and morphological changes in the ovine gastrointestinal tract. Small Rum Res 50:163–176. doi:10.1016/S0921-4488(03)00103-2
Pörtner HO, Peck L, Somero G (2007) Thermal limits and adaptation in marine Antarctic ectotherms: an integrative view. Phil Trans R Soc B Biol Sci 362:2233–2258. doi:10.1098/rstb.2006.1947
Puchal AA, Buddington RK (1992) Postnatal development of monosaccharide transport in pig intestine. Am J Physiol Gastrointest Liver Physiol 262:G895–G902
Qandeel HG, Alonso F, Hernandez DJ, Duenes JA, Zheng Y, Scow JS, Sarr MG (2009a) Role of vagal innervation in diurnal rhythm of intestinal peptide transporter 1 (PEPT1). J Gastrointest Surg 13:1976–1985. doi:10.1007/s11605-009-0984-6
Qandeel HG, Duenes JA, Zheng Y, Sarr MG (2009b) Diurnal expression and function of peptide transporter 1 (PEPT1). J Surg Res 156:123–128. doi:10.1016/j.jss.2009.03.052
Rahimnejad S, Lee KJ (2014) Comparison of free and dipeptide lysine utilization in diets for juvenile olive flounder Paralichthys olivaceus. Fish Aquat Sci 17:433–439. doi:10.5657/FAS.2014.0433
Ravi V, Venkatesh B (2008) Rapidly evolving fish genomes and teleost diversity. Curr Opin Genet Dev 18:544–550. doi:10.1016/j.gde.2008.11.001
Ray AK, Ghosh K, Ringø E (2012) Enzyme-producing bacteria isolated from fish gut: a review. Aquac Nut 18:465–492. doi:10.1111/j.1365-2095.2012.00943.x
Refstie S, Olli JJ, Standal H (2004) Feed intake, growth, and protein utilization by post-smolt Atlantic salmon (Salmo salar) in response to graded levels of fish protein hydrolysate in the diet. Aquaculture 239:331–349. doi:10.1016/j.aquaculture.2004.06.015
Renna MD, Oyadeyi AS, Bossi E, Kottra G, Peres A (2011a) Functional and structural determinants of reverse operation in the pH-dependent oligopeptide transporter PepT1. Cell Mol Life Sci 68:2961–2975. doi:10.1007/s00018-010-0604-3
Renna MD, Sangaletti R, Bossi E, Cherubino F, Kottra G, Peres A (2011b) Unified modeling of the mammalian and fish proton-dependent oligopeptide transporter PepT1. Channels 5:89–99. doi:10.4161/chan.5.1.13505
Reshkin SJ, Ahearn GA (1991) Intestinal glycyl-L-phenylalanine and L-phenylalanine transport in a euryhaline teleost. Am J Physiol 260:R563–R569
Rimoldi S, Bossi E, Harpaz S, Cattaneo AG, Bernardini G, Saroglia M, Terova G (2015) Intestinal B0AT1 (SLC6A19) and PEPT1 (SLC15A1) mRNA levels in European sea bass (Dicentrarchus labrax) reared in fresh water and fed fish and plant protein sources. J Nutr Sci 4:e21. doi:10.1017/jns.2015.9
Rizzello A, Romano A, Kottra G, Acierno R, Storelli C, Verri T, Daniel H, Maffia M (2013) Protein cold adaptation strategy via a unique seven-amino acid domain in the icefish (Chionodraco hamatus) PEPT1 transporter. Proc Natl Acad Sci USA 110:7068–7073. doi:10.1073/pnas.1220417110
Roberts TJ, Azain MJ, White BD, Martin RJ (1995) Rats treated with somatotropin select diets higher in protein. J Nutr 125:2669–2678
Röder PV, Geillinger KE, Zietek TS, Thorens B, Koepsell H, Daniel H (2014) The role of SGLT1 and GLUT2 in intestinal glucose transport and sensing. PLoS ONE 9:e89977. doi:10.1371/journal.pone.0089977
Roediger WE, Millard S (1996) Colonocyte metabolism. Gut 38:792–793. doi:10.1136/gut.38.5.792-a
Romano A, Kottra G, Barca A, Tiso N, Maffia M, Argenton F, Daniel H, Storelli C, Verri T (2006) High-affinity peptide transporter PEPT2 (SLC15A2) of the zebrafish Danio rerio: functional properties, genomic organization, and expression analysis. Physiol Genomics 24:207–217. doi:10.1152/physiolgenomics.00227.2005
Romano A, Barca A, Kottra G, Daniel H, Storelli C, Verri T (2010) Functional expression of SLC15 peptide transporters in rat thyroid follicular cells. Mol Cell Endocrinol 315:174–181. doi:10.1016/j.mce.2009.11.002
Romano A, Barca A, Storelli C, Verri T (2014) Teleost fish models in membrane transport research: the PEPT1(SLC15A1) H+-oligopeptide transporter as a case study. J Physiol 592:881–897. doi:10.1113/jphysiol.2013.259622
Rome S, Barbot L, Windsor E, Kapel N, Tricottet V, Huneau JF, Reynes M, Gobert JG, Tomé D (2002) The regionalization of PepT1, NBAT and EAAC1 transporters in the small intestine of rats are unchanged from birth to adulthood. J Nutr 132:1009–1011
Romero M, Kanai Y, Gunshin H, Hediger M (1998) Expression cloning using Xenopus laevis oocytes. Methods Enzymol 296:17–52. doi:10.1016/S0076-6879(98)96004-9
Romero MF, Chen AP, Parker MD, Boron WF (2013) The SLC4 family of bicarbonate (HCO3 −) transporters. Mol Aspects Med 34:159–182. doi:10.1016/j.mam.2012.10.008
Ronkin D, Seroussi E, Nitzan T, Doron-Faigenboim A, Cnaani A (2015) Intestinal transcriptome analysis revealed differential salinity adaptation between two tilapiine species. Comp Biochem Physiol Part D Genomics Proteomics 13:35–43. doi:10.1016/j.cbd.2015.01.003
Rønnestad I, Gavaia PJ, Viegas CS, Verri T, Romano A, Nilsen TO, Jordal AE, Kamisaka Y, Cancela ML (2007a) Oligopeptide transporter PepT1 in Atlantic cod (Gadus morhua L.): cloning, tissue expression and comparative aspects. J Exp Biol 210:3883–3896. doi:10.1242/jeb.007898
Rønnestad I, Kamisaka Y, Conceição LEC, Morais S, Tonheim SK (2007b) Digestive physiology of marine fish larvae: hormonal control and processing capacity for proteins, peptides and amino acids. Aquaculture 268:82–97. doi:10.1016/j.aquaculture.2007.04.031
Rønnestad I, Murashita K, Kottra G, Jordal AE, Narawane S, Jolly C, Daniel H, Verri T (2010) Molecular cloning and functional expression of Atlantic salmon peptide transporter 1 in Xenopus oocytes reveals efficient intestinal uptake of lysine-containing and other bioactive di- and tripeptides in teleost fish. J Nutr 140:893–900. doi:10.3945/jn.109.118240
Rønnestad I, Akiba Y, Kaji I, Kaunitz JD (2014) Duodenal luminal nutrient sensing. Curr Opin Pharmacol 19:67–75. doi:10.1016/j.coph.2014.07.010
Rubio-Aliaga I, Daniel H (2002) Mammalian peptide transporters as targets for drug delivery. Trends Pharmacol Sci 23:434–440. doi:10.1016/S0165-6147(02)02072-2
Rubio-Aliaga I, Daniel H (2008) Peptide transporters and their roles in physiological processes and drug disposition. Xenobiotica 38:1022–1042. doi:10.1080/00498250701875254
Ruemmele FM, Schwartz S, Seidman EG, Dionne S, Levy E, Lentze MJ (2003) Butyrate induced Caco-2 cell apoptosis is mediated via the mitochondrial pathway. Gut 52:94–100. doi:10.1136/gut.52.1.94
Rühl A, Hoppe S, Frey I, Daniel H, Schemann M (2005) Functional expression of the peptide transporter PEPT2 in the mammalian enteric nervous system. J Comp Neurol 490:1–11. doi:10.1002/cne.20617
Ruohonen K, Grove DJ, McIlroy JT (1997) The amount of food ingested in a single meal by rainbow trout offered chopped herring, dry and wet diets. J Fish Biol 51:93–105. doi:10.1111/j.1095-8649.1997.tb02516.x
Saito S, Iida A, Sekine A, Ogawa C, Kawauchi S, Higuchi S, Nakamura Y (2002) Catalog of 238 variations among six human genes encoding solute carriers (hSLCs) in the Japanese population. J Hum Genet 47:576–584. doi:10.1007/s100380200088
Saito H, Terada T, Shimakura J, Katsura T, Inui K (2008) Regulatory mechanism governing the diurnal rhythm of intestinal H+/peptide cotransporter 1 (PEPT1). Am J Physiol Gastrointest Liver Physiol 295:G395–G402. doi:10.1152/ajpgi.90317.2008
Sakata K, Yamashita T, Maeda M, Moriyama Y, Shimada S, Tohyama M (2001) Cloning of a lymphatic peptide/histidine transporter. Biochem J 356:53–60. doi:10.1042/bj3560053
Sala-Rabanal M, Loo DDF, Hirayama BA, Turk E, Wright EM (2006) Molecular interactions between dipeptides, drugs and the human intestinal H+/oligopeptide cotransporter, hPEPT1. J Physiol 574:149–166. doi:10.1113/jphysiol.2006.107904
Sales J (2009) The effect of fish meal replacement by soyabean products on fish growth: a meta-analysis. Br J Nutr 102:1709–1722. doi:10.1017/S0007114509991279
Sales J (2011) First feeding of freshwater fish larvae with live feed versus compound diets: a meta-analysis. Aquacult Int 19:1217–1228. doi:10.1007/s10499-011-9424-1
Salman NA (2009) Effect of dietary salt on feeding, digestion, growth and osmoregulation in teleost fish. In: Handy RD, Bury NR, Flik G (eds) Essential reviews in experimental biology, vol 1., Osmoregulation and ion transport: integrating physiological, molecular and environmental aspectsSociety for Experimental Biology Press, London, pp 109–150
Samsudin F, Parker JL, Sansom MSP, Newstead S, Fowler PW (2016) Accurate prediction of ligand affinities for a proton-dependent oligopeptide transporter. Cell Chem Biol 23:299–309. doi:10.1016/j.chembiol.2015.11.015
Sangaletti R, Terova G, Peres A, Bossi E, Corà S, Saroglia M (2009) Functional expression of the oligopeptide transporter PepT1 from the sea bass (Dicentrarchus labrax). Pflugers Arch 459:47–54. doi:10.1007/s00424-009-0700-0
Santer R, Hillebrand G, Steinmann B, Schaub J (2003) Intestinal glucose transport: evidence for a membrane traffic-based pathway in humans. Gastroenterology 124:34–39. doi:10.1053/gast.2003.50009
Sardar P, Abid M, Randhawa HS, Prabhakar SK (2009) Effect of dietary lysine and methionine supplementation on growth, nutrient utilization, carcass compositions and haemato-biochemical status in Indian Major Carp, Rohu (Labeo rohita H.) fed soy protein-based diet. Aquacult Nutr 15:339–346. doi:10.1111/j.1365-2095.2008.00598.x
Sasawatari S, Okamura T, Kasumi E, Tanaka-Furuyama K, Yanobu-Takanashi R, Shirasawa S, Kato N, Toyama-Sorimachi N (2011) The solute carrier family 15A4 regulates TLR9 and NOD1 functions in the innate immune system and promotes colitis in mice. Gastroenterology 140:1513–1525. doi:10.1053/j.gastro.2011.01.041
Sato Y, Hashiguchi Y, Nishida M (2009) Temporal pattern of loss/persistence of duplicate genes involved in signal transduction and metabolic pathways after teleost-specific genome duplication. BMC Evol Biol 9:127. doi:10.1186/1471-2148-9-127
Secor SM (2003) Gastric function and its contribution to the postprandial metabolic response of the Burmese python Python molurus. J Exp Biol 206:1621–1630. doi:10.1242/jeb.00300
Secor SM, Diamond J (2000) Evolution of regulatory responses to feeding in snakes. Physiol Biochem Zool 73:123–141. doi:10.1086/316734
Shaw D, Gohil K, Basson MD (2012) Intestinal mucosal atrophy and adaptation. World J Gastroenterol 18:6357–6375. doi:10.3748/wjg.v18.i44.6357
Shen H, Smith D, Brosius F III (2001) Developmental expression of PepT1 and PepT2 in rat small intestine, colon, and kidney. Pediatr Res 49:789–795. doi:10.1203/00006450-200106000-00013
Shi B, Song D, Xue H, Li N, Li J (2006) PepT1 mediates colon damage by transporting fMLP in rats with bowel resection. J Surg Res 136:38–44. doi:10.1016/j.jss.2006.05.025
Shimakura J, Terada T, Katsura T, Inui K (2005) Characterization of the human peptide transporter PEPT1 promoter: sp1 functions as a basal transcriptional regulator of human PEPT1. Am J Physiol Gastrointest Liver Physiol 289:G471–G477. doi:10.1152/ajpgi.00025.2005
Shimakura J, Terada T, Saito H, Katsura T, Inui K (2006a) Induction of intestinal peptide transporter 1 expression during fasting is mediated by peroxisome proliferators-activated receptor alpha. Am J Physiol Gastrointest Liver Physiol 291:G851–G856. doi:10.1152/ajpgi.00171.2006
Shimakura J, Terada T, Shimada Y, Katsura T, Inui K (2006b) The transcription factor Cdx2 regulates the intestine-specific expression of human peptide transporter 1 through functional interaction with Sp1. Biochem Pharmacol 71:1581–1588. doi:10.1016/j.bcp.2006.03.001
Shin SC, Ahn DH, Kim SJ, Pyo CW, Lee H, Kim MK, Lee J, Lee JE, Detrich HW, Postlethwait JH, Edwards D, Lee SG, Lee JH, Park H (2014) The genome sequence of the Antarctic bullhead notothen reveals evolutionary adaptations to a cold environment. Genome Biol 15:468. doi:10.1186/s13059-014-0468-1
Shiraga T, Miyamoto K, Tanaka H, Yamamoto H, Taketani Y, Morita K, Tamai I, Tsuji A, Takeda E (1999) Cellular and molecular mechanisms of dietary regulation on rat intestinal H+/peptide transporter PepT1. Gastroenterology 116:354–362. doi:10.1016/S0016-5085(99)70132-0
Simpson JE, Schweinfest CW, Shull GE, Gawenis LR, Walker NM, Boyle KT, Soleimani M, Clarke LL (2007) PAT-1 (Slc26a6) is the predominant apical membrane Cl−/HCO3 − exchanger in the upper villous epithelium of the murine duodenum. Am J Physiol Gastrointest Liver Physiol 292:G1079–G1088. doi:10.1152/ajpgi.00354.2006
Simpson JE, Walker NM, Supuran CT, Soleimani M, Clarke LL (2010) Putative anion transporter-1 (Pat-1, Slc26a6) contributes to intracellular pH regulation during H+-dipeptide transport in duodenal villous epithelium. Am J Physiol Gastrointest Liver Physiol 298:G683–G691. doi:10.1152/ajpgi.00293.2009
Skalli A, Zambonino-Infante JL, Kotzamanis Y, Fabregat R, Gisbert E (2014) Peptide molecular weight distribution of soluble protein fraction affects growth performance and quality in European sea bass (Dicentrarchus labrax) larvae. Aquacult Nutr 20:118–131. doi:10.1111/anu.12058
Small BC, Soares JH Jr (2000) Quantitative dietary lysine requirement of juvenile striped bass Morone saxatilis. Aquacult Nutr 6:207–212. doi:10.1046/j.1365-2095.2000.00140.x
Smith HS (1930) The absorption and excretion of water and salts by marine teleosts. Am J Physiol 93:480–505
Smith NF, Talbot C, Eddy FB (1989) Dietary salt intake and its relevance to ionic regulation in freshwater salmonids. J Fish Biol 35:749–753. doi:10.1111/j.1095-8649.1989.tb03026.x
Smith DE, Clémençon B, Hediger MA (2013a) Proton-coupled oligopeptide transporter family SLC15: physiological, pharmacological and pathological implications. Mol Aspects Med 34:323–336. doi:10.1016/j.mam.2012.11.003
Smith DL Jr, Barry RJ, Powell ML, Nagy TR, D’Abramo LR, Watts SA (2013b) Dietary protein source influence on body size and composition in growing zebrafish. Zebrafish 10:439–446. doi:10.1089/zeb.2012.0864
Snyder GS, Gaylord TG, Barrows FT, Overturf K, Cain KD, Hill RA, Hardy RW (2012) Effects of carnosine supplementation to an all-plant protein diet for rainbow trout (Oncorhynchus mykiss). Aquaculture 338:72–81. doi:10.1016/j.aquaculture.2011.12.042
Soga M, Ohashi A, Taniguchi M, Matsui T, Tsuda T (2014) The di-peptide Trp-His activates AMP-activated protein kinase and enhances glucose uptake independently of insulin in L6 myotubes. FEBS Open Bio 4:898–904. doi:10.1016/j.fob.2014.10.008
Solcan N, Kwok J, Fowler PW, Cameron AD, Drew D, Iwata S, Newstead S (2012) Alternating access mechanism in the POT family of oligopeptide transporters. EMBO J 31:3411–3421. doi:10.1038/emboj.2012.157
Somero GN (2003) Protein adaptations to temperature and pressure: complementary roles of adaptive changes in amino acid sequence and internal milieu. Comp Biochem Physiol B Biochem Mol Biol 136:577–591. doi:10.1016/S1096-4959(03)00215-X
Somero GN (2004) Adaptation of enzymes to temperature: searching for basic “strategies”. Comp Biochem Physiol B: Biochem Mol Biol 139:321–333. doi:10.1016/j.cbpc.2004.05.003
Song S, Hooiveld GJ, Li M, Zhao F, Zhang W, Xu X, Muller M, Li C, Zhou G (2016) Dietary soy and meat proteins induce distinct physiological and gene expression changes in rats. Sci Rep 6:20036. doi:10.1038/srep20036
Spanier B (2014) Transcriptional and functional regulation of the intestinal peptide transporter PEPT1. J Physiol 592:871–879. doi:10.1113/jphysiol.2013.258889
Spanier B, Lasch K, Marsch S, Benner J, Liao W, Hu H, Kienberger H, Eisenreich W, Daniel H (2009) How the intestinal peptide transporter PEPT-1 contributes to an obesity phenotype in Caenorhabditits elegans. PLoS ONE 4:e6279. doi:10.1371/journal.pone.0006279
Speier JS, Yadgary L, Uni Z, Wong EA (2012) Gene expression of nutrient transporters and digestive enzymes in the yolk sac membrane and small intestine of the developing embryonic chick. Poult Sci 91:1941–1949. doi:10.3382/ps.2011-02092
Sreedharan S, Stephansson O, Schiöth HB, Fredriksson R (2011) Long evolutionary conservation and considerable tissue specificity of several atypical solute carrier transporters. Gene 478:11–18. doi:10.1016/j.gene.2010.10.011
Starck JM, Beese K (2002) Structural flexibility of the small intestine and liver of garter snakes in response to feeding and fasting. J Exp Biol 205:1377–1388
Steel A, Nussberger S, Romero MF, Boron WF, Boyd CA, Hediger MA (1997) Stoichiometry and pH dependence of the rabbit proton-dependent oligopeptide transporter PepT1. J Physiol 498:563–569. doi:10.1113/jphysiol.1997.sp021883
Stewart AK, Boyd CA, Vaughan-Jones RD (1999) A novel role for carbonic anhydrase: cytoplasmic pH gradient dissipation in mouse small intestinal enterocytes. J Physiol 516:209–217. doi:10.1111/j.1469-7793.1999.209aa.x
Sun D, Tan F, Fang D, Wang Y, Zeng S, Jiang H (2013a) Expression of proton-coupled oligopeptide transporter (POTs) in prostate of mice and patients with benign prostatic hyperplasia (BPH) and prostate cancer (PCa). Prostate 73:287–295. doi:10.1002/pros.22568
Sun D, Wang Y, Tan F, Fang D, Hu Y, Smith DE, Jiang H (2013b) Functional and molecular expression of the proton-coupled oligopeptide transporters in spleen and macrophages from mouse and human. Mol Pharm 10:1409–1416. doi:10.1021/mp300700p
Sundell KS, Sundh H (2012) Intestinal fluid absorption in anadromous salmonids: importance of tight junctions and aquaporins. Front Physiol 3:388. doi:10.3389/fphys.2012.00388
Takanaga H, Mackenzie B, Peng JB, Hediger MA (2005) Characterization of a branched-chain amino-acid transporter SBAT1 (SLC6A15) that is expressed in human brain. Biochem Biophys Res Commun 337:892–900. doi:10.1016/j.bbrc.2005.09.128
Takei Y (2015) From aquatic to terrestrial life: evolution of the mechanisms for water acquisition. Zoolog Sci 32:1–7. doi:10.2108/zs140142
Takeuchi F, Ochiai Y, Serizawa M, Yanai K, Kuzuya N, Kajio H, Honjo S, Takeda N, Kaburagi Y, Yasuda K, Shirasawa S, Sasazuki T, Kato N (2008) Search for type 2 diabetes susceptibility genes on chromosomes 1q, 3q and 12q. J Hum Genet 53:314–324. doi:10.1007/s10038-008-0254-6
Teerapornpuntakit J, Klanchui A, Karoonuthaisiri N, Wongdee K, Charoenphandhu N (2014) Expression of transcripts related to intestinal ion and nutrient absorption in pregnant and lactating rats as determined by custom-designed cDNA microarray. Mol Cell Biochem 391:103–116. doi:10.1007/s11010-014-1992-8
Terada T, Inui K (2007) Gene expression and regulation of drug transporters in the intestine and kidney. Biochem Pharmacol 73:440–449. doi:10.1016/j.bcp.2006.10.010
Terada T, Sawada K, Saito H, Hashimoto Y, Inui K (1999) Functional characteristics of basolateral peptide transporter in the human intestinal cell line Caco-2. Am J Physiol 276:G1435–G1441
Terada T, Shimada Y, Pan X, Kishimoto K, Sakurai T, Doi R, Onodera H, Katsura T, Imamura M, Inui K (2005) Expression profiles of various transporters for oligopeptides, amino acids and organic ions along the human digestive tract. Biochem Pharmacol 70:1756-1763. doi: 10.1016/j.bcp.2005.09.027
Terjesen BF, Lee KJ, Zhang Y, Failla M, Dabrowski K (2006) Optimization of dipeptide-protein mixtures in experimental diet formulations for rainbow trout (Oncorhynchus mykiss) alevins. Aquaculture 254:517–525. doi:10.1016/j.aquaculture.2005.11.013
Terova G, Bernadini G, Binelli G, Gornati R, Saroglia M (2006) cDNA encoding sequences for myostatin and FGF6 in sea bass (Dicentrarchus labrax L.) and the effect of fasting and refeeding on their abundance levels. Domest Anim Endocrinol 30:304–319. doi:10.1016/j.domaniend.2005.08.003
Terova G, Rimoldi S, Chini V, Gornati R, Bernardini G, Saroglia M (2007) Cloning and expression analysis of insulin-like growth factor I and II in liver and muscle of sea bass (Dicentrarchus labrax L.) during long-term fasting and refeeding. J Fish Biol 70B:219–233. doi:10.1111/j.1095-8649.2007.01402.x
Terova G, Corà S, Verri T, Rimoldi S, Bernardini G, Saroglia M (2009) Impact of feed availability on PepT1 mRNA expression levels in sea bass (Dicentrarchus labrax). Aquaculture 294:288–299. doi:10.1016/j.aquaculture.2009.06.014
Terova G, Robaina L, Izquierdo M, Cattaneo A, Molinari S, Bernardini G, Saroglia M (2013) PepT1 mRNA expression levels in sea bream (Sparus aurata) fed different plant protein sources. SpringerPlus 2:17. doi:10.1186/2193-1801-2-17
Tesser MB, Terjesen BF, Zhang Y, Portella MC, Dabrowski K (2005) Free- and peptide-based dietary arginine supplementation for the South American fish pacu (Piaractus mesopotamicus). Aquacult Nutr 11:443–453. doi:10.1111/j.1365-2095.2005.00373.x
Thamotharan M, Gomme G, Zonno V, Maffia M, Storelli C, Ahearn GA (1996a) Electrogenic, proton-coupled, peptide (glycylsarcosine) transport in herbivorous and carnivorous teleosts. Am J Physiol 270:R939–R947
Thamotharan M, Zonno V, Storelli C, Ahearn GA (1996b) Basolateral dipeptide transport by the intestine of the teleost Oreochromis mossambicus. Am J Physiol 270:R948–R954
Thamotharan M, Bawani S, Zhou X, Adibi S (1998) Mechanism of dipeptide stimulation of its own transport in a human intestinal cell line. Proc Assoc Am Physicians 110:361–368
Thamotharan M, Bawani SZ, Zhou X, Adibi SA (1999) Functional and molecular expression of intestinal oligopeptide transporter (PEPT-1) after a brief fast. Metabolism 6:681–684. doi:10.1016/S0026-0495(99)90164-6
Thwaites DT, Anderson CM (2007) H+-coupled nutrient, micronutrient and drug transporters in the mammalian small intestine. Exp Physiol 92:603–619. doi:10.1113/expphysiol.2005.029959
Thwaites DT, Brown CD, Hirst BH, Simmons NL (1993a) H+-coupled dipeptide (glycylsarcosine) transport across apical and basal borders of human intestinal Caco-2 cell monolayers display distinctive characteristics. Biochim Biophys Acta 1151:237–245. doi:10.1016/0005-2736(93)90108-C
Thwaites DT, Brown CD, Hirst BH, Simmons NL (1993b) Transepithelial glycylsarcosine transport in intestinal Caco-2 cells mediated by expression of H+-coupled carriers at both apical and basal membranes. J Biol Chem 268:7640–7642
Thwaites DT, Hirst BH, Simmons NL (1993c) Direct assessment of dipeptide/H+ symport in intact human intestinal (Caco-2) epithelium: a novel method utilising continuous intracellular pH measurement. Biochem Biophys Res Commun 194:432–438. doi:10.1006/bbrc.1993.1838
Thwaites DT, Ford D, Glanville M, Simmons NL (1999) H+/solute-induced intracellular acidification leads to selective activation of apical Na+/H+ exchange in human intestinal epithelial cells. J Clin Invest 104:629–635. doi:10.1172/JCI7192
Thwaites DT, Kennedy DJ, Raldua D, Anderson CM, Mendoza ME, Bladen CL, Simmons NL (2002) H+/dipeptide absorption across the human intestinal epithelium is controlled indirectly via a functional Na+/H+ exchanger. Gastroenterology 122:1322–1333. doi:10.1053/gast.2002.32992
Tian J, He G, Mai K, Liu C (2015) Effects of postprandial starvation on mRNA expression of endocrine-, amino acid and peptide transporter-, and metabolic enzyme-related genes in zebrafish (Danio rerio). Fish Physiol Biochem 41:773–787. doi:10.1007/s10695-015-0045-x
Tibaldi E, Lanari D (1991) Optimal dietary lysine levels for growth and protein utilisation of fingerlings seabass (Dicentrarchus labrax L.) fed semipurified diets. Aquaculture 95:297–304. doi:10.1016/0044-8486(91)90095-O
Tulli F, Messina M, Calligaris M, Tibaldi E (2010) Response of European sea bass (Dicentrarchus labrax) to graded levels of methionine (total sulfur amino acids) in soya protein-based semi-purified diets. Br J Nutr 104:664–673. doi:10.1017/S0007114510001029
Twibell RG, Wilson KA, Brown PB (2000) Dietary sulphur amino acid requirement of juvenile yellow perch fed the maximum cystine replacement value for methionine. J Nutr 130:612–616
Uni Z, Ganot S, Sklan D (1998) Posthatch development of mucosal function in the broiler small intestine. Poult Sci 77:75–82. doi:10.1093/ps/77.1.75
Uni Z, Tako E, Gal-Garber O, Sklan D (2003) Morphological, molecular, and functional changes in the chicken small intestine of the late-term embryo. Poult Sci 82:1747–1754. doi:10.1093/ps/82.11.1747
Valente LM, Bower NI, Johnston IA (2012) Postprandial expression of growth-related genes in Atlantic salmon (Salmo salar L.) juveniles fasted for 1 week and fed a single meal to satiation. Br J Nutr 108:2148–2157. doi:10.1017/S0007114512000396
Van L, Pan YX, Bloomquist JR, Webb KE Jr, Wong EA (2005) Developmental regulation of a turkey intestinal peptide transporter (PepT1). Poult Sci 84:75–82. doi:10.1093/ps/84.1.75
Vavricka SR, Musch MW, Chang JE, Nakagawa Y, Phanvijhitsiri K, Waypa TS, Merlin D, Schneewind O, Chang EB (2004) hPepT1 transports muramyl dipeptide, activating NF-kappaB and stimulating IL-8 secretion in human colonic Caco2/bbe cells. Gastroenterology 127:1401–1409. doi:10.1053/j.gastro.2004.07.024
Vazquez JA, Morse EL, Adibi SA (1985) Effect of starvation on amino acid and peptide transport and peptide hydrolysis in humans. Am J Physiol 249:G563–G566
Vente-Spreeuwenberg MAM, Verdonk JMAJ, Koninkx JFJG, Beynen AC, Verstegen MWA (2004) Dietary protein hydrolysates vs. the intact proteins do not enhance mucosal integrity and growth performance in weaned piglets. Livest Prod Sci 85:151–164. doi:10.1016/S0301-6226(03)00132-5
Verri T, Maffia M, Storelli C (1992) H+/glycyl-glycine cotransport in eel intestinal brush-border membrane vesicles: studies with the pH-sensitive dye acridine orange. Biochim Biophys Acta 1110:123–126. doi:10.1016/0005-2736(92)90303-4
Verri T, Maffia M, Danieli A, Herget M, Wenzel U, Daniel H, Storelli C (2000) Characterisation of the H+/peptide cotransporter of eel intestinal brush-border membranes. J Exp Biol 203:2991–3001
Verri T, Kottra G, Romano A, Tiso N, Peric M, Maffia M, Boll M, Argenton F, Daniel H, Storelli C (2003) Molecular and functional characterisation of the zebrafish (Danio rerio) PEPT1-type peptide transporter. FEBS Lett 549:115–122. doi:10.1016/S0014-5793(03)00759-2
Verri T, Romano A, Barca A, Kottra G, Daniel H, Storelli C (2010) Transport of di- and tripeptides in teleost fish intestine. Aquacult Res 41:641–653. doi:10.1111/j.1365-2109.2009.02270.x
Verri T, Terova G, Dabrowski K, Saroglia M (2011) Peptide transport and animal growth: the fish paradigm. Biol Lett 7:597–600. doi:10.1098/rsbl.2010.1164
Verri T, Terova G, Romano A, Barca A, Pisani P, Storelli C, Saroglia M (2012) The SoLute Carrier (SLC) family series in teleost fish. In: Saroglia M, Liu Z (eds) Functional genomics in aquaculture. Wiley, Oxford, pp 219–320
Vig BS, Stouch TR, Timoszyk JK, Quan Y, Wall DA, Smith RL, Faria TN (2006) Human PEPT1 pharmacophore distinguishes between dipeptide transport and binding. J Med Chem 49:3636–3644. doi:10.1021/jm0511029
Volff JN (2005) Genome evolution and biodiversity in teleost fish. Heredity 94:280–294. doi:10.1038/sj.hdy.6800635
Wada M, Miyakawa S, Shimada A, Okada N, Yamamoto A, Fujita T (2005) Functional linkage of H+/peptide transporter PEPT2 and Na+/H+ exchanger in primary cultures of astrocytes from mouse cerebral cortex. Brain Res 1044:33–41. doi:10.1016/j.brainres.2005.02.064
Wadiche JI, Kavanaugh MP (1998) Macroscopic and microscopic properties of a cloned glutamate transporter/chloride channel. J Neurosci 18:7650–7661
Walker D, Thwaites DT, Simmons NL, Gilbert HJ, Hirst BH (1998) Substrate upregulation of the human small intestinal peptide transporter, hPepT1. J Physiol 507:697–706. doi:10.1111/j.1469-7793.1998.697bs.x
Walker NM, Simpson JE, Hoover EE, Brazill JM, Schweinfest CW, Soleimani M, Clarke LL (2011) Functional activity of Pat-1 (Slc26a6) Cl−/HCO3 − exchange in the lower villus epithelium of murine duodenum. Acta Physiol 201:21–31. doi:10.1111/j.1748-1716.2010.02210.x
Walton MJ, Cowey CB, Adron JW (1982) Methionine metabolism in rainbow trout fed diets of differing methionine and cysteine content. J Nutr 112:1525–1535
Walton MJ, Cowey C, Adron JW (1984) The effect of dietary lysine levels on growth and metabolism of rainbow trout (Salmo gairdneri). Br J Nutr 52:115–122. doi:10.1079/BJN19840077
Wang S, Liu YH, Tian LX, Xie MQ, Yang HJ, Wang Y, Liang GY (2005) Quantitative dietary lysine requirement of juvenile grass carp Ctenopharyngodon idella. Aquaculture 249:419–429. doi:10.1016/j.aquaculture.2005.04.005
Wang T, Hung CC, Randall DJ (2006) The comparative physiology of food deprivation: from feast to famine. Annu Rev Physiol 68:223–251. doi:10.1146/annurev.physiol.68.040104.105739
Wang W, Shi C, Zhang J, Gu W, Li T, Gen M, Chu W, Huang R, Liu Y, Hou Y, Li P, Yin Y (2009) Molecular cloning, distribution and ontogenic expression of the oligopeptide transporter PepT1 mRNA in Tibetan suckling piglets. Amino Acids 37:593–601. doi:10.1007/s00726-008-0178-7
Wang Z, Du J, Lam SH, Mathavan S, Matsudaira P, Gong Z (2010) Morphological and molecular evidence for functional organization along the rostrocaudal axis of the adult zebrafish intestine. BMC Genom 11:392. doi:10.1186/1471-2164-11-392
Wang P, Lu YQ, Wen Y, Yu DY, Ge L, Dong WR, Xiang LX, Shao JZ (2013) IL-16 induces intestinal inflammation via PepT1 upregulation in a pufferfish model: new insights into the molecular mechanism of inflammatory bowel disease. J Immunol 191:1413–1427. doi:10.4049/jimmunol.1202598
Wang Q, He G, Wang X, Mai K, Xu W, Zhou H (2014a) Dietary sulphur amino acid modulations of taurine biosynthesis in juvenile turbot (Psetta maxima). Aquaculture 422:141–145. doi:10.1016/j.aquaculture.2013.12.014
Wang Y, Sun D, Song F, Hu Y, Smith DE, Jiang H (2014b) Expression and regulation of the proton-coupled oligopeptide transporter PhT2 by LPS in macrophages and mouse spleen. Mol Pharm 11:1880–1888. doi:10.1021/mp500014r
Watanabe T (2002) Strategies for further development of aquatic feeds. Fisheries Sci 68:242–252. doi:10.1046/j.1444-2906.2002.00418.x
Wei Y, Liang M, Mu Y, Zheng K, Xu H (2015) The effect of ultrafiltered fish protein hydrolysate level on growth performance, protein digestibility and mRNA expression of PepT1 in juvenile turbot (Scophthalmus maximus L.). Aquacult Nutr. doi:10.1111/anu.12319
Weintraut ML, Kim S, Dalloul RA, Wong EA (2016) Expression of small intestinal nutrient transporters in embryonic and posthatch turkeys. Poult Sci 95:90–98. doi:10.3382/ps/pev310
Wenzel U, Meissner B, Döring F, Daniel H (2001) PEPT1-mediated uptake of dipeptides enhances the intestinal absorption of amino acids via transport system b0,+. J Cell Physiol 186:251–259. doi:10.1002/1097-4652(200102)186:2<251:AID-JCP1027>3.0.CO;2-F
Westerterp-Plantenga MS, Nieuwenhuizen A, Tomé D, Soenen S, Westerterp KR (2009) Dietary protein, weight loss, and weight maintenance. Annu Rev Nutr 29:21–41. doi:10.1146/annurev-nutr-080508-141056
White BD, Porter MH, Martin RJ (2000) Effects of age on the feeding response to moderately low dietary protein in rats. Physiol Behav 68:673–681. doi:10.1016/S0031-9384(99)00229-2
Whittamore JM (2012) Osmoregulation and epithelial water transport: lessons from the intestine of marine teleost fish. J Comp Physiol B 182:1–39. doi:10.1007/s00360-011-0601-3
Whittamore JM, Cooper CA, Wilson RW (2010) HCO3 − secretion and CaCO3 precipitation play major roles in intestinal water absorption in marine teleost fish in vivo. Am J Physiol Regul Integr Comp Physiol 298:R877–R886. doi:10.1152/ajpregu.00545.2009
Wilson RP (2002) Amino acid and proteins. In: Halver JE, Hardy RW (eds) Fish Nutrition. Academic Press, London, pp 162–164
Wilson RW, Wilson JM, Grosell M (2002) Intestinal bicarbonate secretion by marine teleost fish: why and how? Biochim Biophys Acta 1566:182–193. doi:10.1016/S0005-2736(02)00600-4
Windell JT, Norris DO, Kitchell JF, Norris JS (1969) Digestive response of rainbow trout, Salmo gairdneri, to pellet diets. J Fish Res Board Can 26:1801–1812. doi:10.1139/f69-164
Won ET, Borski RJ (2013) Endocrine regulation of compensatory growth in fish. Front Endocrinol (Lausanne) 4:74. doi:10.3389/fendo.2013.00074
Wood CM, Bucking C, Grosell M (2010) Acid-base responses to feeding and intestinal Cl− uptake in freshwater- and seawater-acclimated killifish, Fundulus heteroclitus, an agastric euryhaline teleost. J Exp Biol 213:2681–2692. doi:10.1242/jeb.039164
Wrong O, Metcalfe-Gibson A, Morrison RB, Ng ST, Howard AV (1965) In vivo dialysis of faeces as a method of stool analysis. I. Technique and results in normal subjects. Clin Sci 28:357–375
Wu SP, Smith DE (2013) Impact of intestinal PepT1 on the kinetics and dynamics of N-formyl-methionyl-leucyl-phenylalanine, a bacterially-produced chemotactic peptide. Mol Pharm 10:677–684. doi:10.1021/mp300477w
Wu P, Li Y, Cheng J, Chen L, Zeng M, Wu Y, Wang J, Zhang J, Chu W (2016) Transcriptome analysis and postprandial expression of amino acid transporter genes in the fast muscles and gut of Chinese perch (Siniperca chuatsi). PLoS One 11:e0159533. doi:10.1371/journal.pone.0159533
Wuensch T, Schulz S, Ullrich S, Lill N, Stelzl T, Rubio-Aliaga I, Loh G, Chamaillard M, Haller D, Daniel H (2013) The peptide transporter PEPT1 is expressed in distal colon in rodents and humans and contributes to water absorption. Am J Physiol Gastrointest Liver Physiol 305:G66–G73. doi:10.1152/ajpgi.00491.2012
Xu D, He G, Mai K, Zhou H, Song F (2016a) Expression pattern of peptide and amino acid transporter genes in digestive tract of juvenile turbot (Scophthalmus maximus L.). J Ocean Univ China. 15:334–340. doi:10.1007/s11802-016-2768-4
Xu H, Mu Y, Zhang Y, Li J, Liang M, Zheng K, Wei Y (2016b) Graded levels of fish protein hydrolysate in high plant diets for turbot (Scophthalmus maximus): effects on growth performance and lipid accumulation. Aquaculture 454:140–147. doi:10.1016/j.aquaculture.2015.12.006
Xue XH, Hryshko LV, Nicoll DA, Philipson KD, Tibbits GF (1999) Cloning, expression, and characterization of the trout cardiac Na+/Ca2+ exchanger. Am J Physiol 277:C693–C700
Yamashita T, Shimada S, Guo W, Sato K, Kohmura E, Hayakawa T, Takagi T, Tohyama M (1997) Cloning and functional expression of a brain peptide/histidine transporter. J Biol Chem 272:10205–10211. doi:10.1074/jbc.272.15.10205
Yang RB, Xie CX, Fan QX, Gao C, Fang LB (2010) Ontogeny of the digestive tract in yellow catfish Pelteobagrus fulvidraco larvae. Aquaculture 302:112–123. doi:10.1016/j.aquaculture.2010.02.020
Yu Q, Liu X, Liu Y, Riederer B, Li T, Tian DA, Tuo B, Shull G, Seidler U (2016) Defective small intestinal anion secretion, dipeptide absorption, and intestinal failure in suckling NBCe1-deficient mice. Pflugers Arch. doi:10.1007/s00424-016-1836-3
Yuen BB, Wong CK, Woo NY, Au DW (2007) Induction and recovery of morphofunctional changes in the intestine of juvenile carnivorous fish (Epinephelus coioides) upon exposure to foodborne benzo[a]pyrene. Aquat Toxicol 82:181–194. doi:10.1016/j.aquatox.2007.02.010
Yúfera M, Moyano FJ, Astola A, Pousão-Ferreira P, Martínez-Rodríguez G (2012) Acidic digestion in a teleost: postprandial and circadian pattern of gastric pH, pepsin activity, and pepsinogen and proton pump mRNAs expression. PLoS ONE 7:e33687. doi:10.1371/journal.pone.0033687
Zabielski R (2007) Hormonal and neural regulation of intestinal function in pigs. Livest Sci 108:32–40. doi:10.1016/j.livsci.2007.01.022
Zaïr ZM, Eloranta JJ, Stieger B, Kullak-Ublick GA (2008) Pharmacogenetics of OATP (SLC21/SLCO), OAT and OCT (SLC22) and PEPT (SLC15) transporters in the intestine, liver and kidney. Pharmacogenomics 9:597–624. doi:10.2217/14622416.9.5.597
Zaldúa N, Naya DE (2014) Digestive flexibility during fasting in fish: a review. Comp Biochem Physiol A: Mol Integr Physiol 169:7–14. doi:10.1016/j.cbpa.2013.12.006
Zambonino-Infante JL, Cahu CL, Peres A (1997) Partial substitution of di- and tripeptides for native proteins in sea bass diet improves Dicentrarchus labrax larval development. J Nutr 127:608–614
Zeissig S, Fromm A, Mankertz J, Weiske J, Zeitz M, Fromm M, Schulzke JD (2007) Butyrate induces intestinal sodium absorption via Sp3-mediated transcriptional up-regulation of epithelial sodium channels. Gastroenterology 132:236–248. doi:10.1053/j.gastro.2006.10.033
Zhang EY, Fu DJ, Pak YA, Stewart T, Mukhopadhyay N, Wrighton SA, Hillgren KM (2004) Genetic polymorphisms in human proton-dependent dipeptide transporter PEPT1: implications for the functional role of Pro586. J Pharmacol Exp Ther 310:437–445. doi:10.1124/jpet.104.065912
Zhang Y, Dabrowski K, Hliwa P, Gomulka P (2006) Indispensable amino acid concentrations decrease in tissues of stomachless fish, common carp in response to free amino acid- or peptide-based diets. Amino Acids 31:165–172. doi:10.1007/s00726-006-0345-7
Zhang C, Ai Q, Mai K, Tan B, Li H, Zhang L (2008) Dietary lysine requirement of large yellow croaker, Pseudosciaena crocea R. Aquaculture 283:123–127. doi:10.1016/j.aquaculture.2008.06.035
Zhang Y, Viennois E, Zhang M, Xiao B, Han MK, Walter L, Garg P, Merlin D (2016) PepT1 expression helps maintain intestinal homeostasis by mediating the differential expression of miRNAs along the crypt-villus axis. Sci Rep 6:27119. doi:10.1038/srep27119
Zheng K, Liang M, Yao H, Wang J, Chang Q (2012) Effect of dietary fish protein hydrolysate on growth, feed utilization and IGF-I levels of Japanese flounder (Paralichthys olivaceus). Aquacult Nutr 18:297–303. doi:10.1111/j.1365-2095.2011.00896.x
Zheng K, Liang M, Yao H, Wang J, Chang Q (2013a) Effect of size-fractionated fish protein hydrolysate on growth and feed utilization of turbot (Scophthalmus maximus L.). Aquacult Res 44:895–902. doi:10.1111/j.1365-2109.2012.03094.x
Zheng K, Xu T, Qian C, Liang M, Wang X (2013b) Effect of low molecular weight fish protein hydrolysate on growth performance and IGF-I expression in Japanese flounder (Paralichthys olivaceus) fed high plant protein diets. Aquacult Nutr 20:372–380. doi:10.1111/anu.12090
Zhou QC, Wu ZH, Tan BP, Chi SY, Yang QH (2006) Optimal dietary methionine requirement for juvenile cobia (Rachycentron canadum). Aquaculture 258:551–557. doi:10.1016/j.aquaculture.2006.03.035
Zietek T, Daniel H (2015) Intestinal nutrient sensing and blood glucose control. Curr Opin Clin Nutr Metab Care 18:381–388. doi:10.1097/MCO.0000000000000187
Zucchelli M, Torkvist L, Bresso F, Halfvarson J, Hellquist A, Anedda F, Assadi G, Lindgren GB, Svanfeldt M, Janson M, Noble CL, Pettersson S, Lappalainen M, Paavola-Sakki P, Halme L, Färkkilä M, Turunen U, Satsangi J, Kontula K, Löfberg R, Kere J, D’Amato M (2009) PepT1 oligopeptide transporter (SLC15A1) gene polymorphism in inflammatory bowel disease. Inflamm Bowel Dis 15:1562–1569. doi:10.1002/ibd.20963
Zwarycz B, Wong EA (2013) Expression of the peptide transporters PepT1, PepT2, and PHT1 in the embryonic and posthatch chick. Poult Sci 92:1314–1321. doi:10.3382/ps.2012-02826
Acknowledgements
We wish to express our deep gratitude to Prof. Ian Hume for the invitation to write this review. This work was mainly supported by Grants from the University of Salento (Fondi ex-60%).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by I. D. Hume.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Verri, T., Barca, A., Pisani, P. et al. Di- and tripeptide transport in vertebrates: the contribution of teleost fish models. J Comp Physiol B 187, 395–462 (2017). https://doi.org/10.1007/s00360-016-1044-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00360-016-1044-7