Abstract
The physiological role of leptin in fish is not fully elucidated. In the present study, the involvement of the leptin system in lipid deposition and mobilization in rainbow trout during feeding and 1, 2 and 4 weeks of fasting was investigated in two lines of rainbow trout with different muscle and visceral adiposity: a fat line (FL) with high total energy reserves, high muscle adiposity, but low visceral adiposity and a lean line (LL) with lower total energy reserves and lower muscle adiposity, but higher visceral adiposity. During 4 weeks of fasting, muscle lipids decreased by 63 % in the FL fish, while no such energy mobilization from muscle occurred in the LL fish. On the other hand, lipid stores in liver and visceral adipose tissue was utilized to a similar extent by the two fish lines during fasting. Under normal feeding conditions, plasma leptin levels were higher in the LL than the FL fish, suggesting a possible contribution of visceral adipocytes to plasma leptin levels. Plasma leptin-binding protein levels did not differ between the lines and were not affected by fasting. After 4 weeks of fasting, the long leptin receptor and the leptin-binding protein isoforms 1 and 3 muscle expression increased in the LL fish, as well as hepatic expression of leptin A1 and the two binding protein isoforms. These responses were not seen in the FL fish. The data suggest that the Lep system in rainbow trout is involved in regulation of energy stores and their mobilization.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Animals store ingested energy primarily as triglycerides in adipose tissues. Teleost fish have several species-specific strategies on where lipids are stored, and the amount of energy stored often varies with life stage as well as season, especially in temperate and sub-Arctic species such as the salmonids. Salmonids deposit significant amounts of lipids in the musculature, in adipocytes within the myosepta as well as in intracellular lipid droplets in myocytes (Weil et al. 2013), and muscle lipid content can reach 15–20 % (Brækkan 1959; Davidson et al. 2014). In salmonids, lipids are also deposited in visceral and subcutaneous stores. On the other hand, the salmonid liver is relatively small and hepatic energy stores of salmonids are thus relatively limited compared with many other teleost species with much larger livers (Weil et al. 2013).
Functional energy homeostasis must include a feedback system through which signaling of the amount of energy stored and the rate of energy spent will allow the appropriate central regulation of appetite and foraging behavior. Kennedy (1953) formulated a lipostat hypothesis in which information on peripheral lipid deposits is conveyed to the hypothalamus by unknown signaling substances such as circulating metabolites, affecting appetite and food intake in such a way as to allow young rats to maintain constant adiposity. Following the discovery of the hormone leptin (Lep) which is secreted from mammalian adipose tissues (MacDougald et al. 1995), Lep was rapidly hailed as being the adipose-to-brain signaling link in the lipostatic control of constant body weight in both rodents and humans (Ahima 2000; Park and Ahima 2015; Sawchenko 1998). However, in other mammals, different relationships between Lep and energy homeostasis have been noted. In hibernating raccoon dog (Nyctereutes procyonoides), plasma Lep levels are high as their body weight decreases (Nieminen et al. 2000), and in striped mice (Rhabdomys pumilio) plasma Lep levels are high during the dry season and low during the feeding season (Schradin et al. 2014).
In salmonids, there is a correlation between increased adiposity and decreased food intake during feed content manipulation (Johansen et al. 2002), food deprivation (Jobling and Miglavs 1993; Metcalfe and Thorpe 1992) and food restriction (Silverstein et al. 1999), supporting a lipostatic regulatory system. However, for most temperate to sub-Arctic fish species including salmonids, as indeed for many mammalian species, maximal fitness is not reached through keeping constant body weight and adiposity over their seasonal life cycles. Rather, for these species, long-term survival is secured by a significant, seasonal increase in adiposity when food is available, followed by long periods of sustained energy mobilization during winter (Jørgensen et al. 1997; McCue 2010; Nelson 1980; Sheridan 1994).
Lep is a 16 kDa peptide hormone belonging to the cytokine superfamily (Madej et al. 1995), and research on Lep endocrinology in teleosts was set in motion by the pioneering identification of the lep gene in the pufferfish (Takifugu rubripes) in 2005 (Kurokawa et al. 2005). Lep has an anorexigenic function in teleost fish (Aguilar et al. 2010, 2011; de Pedro et al. 2006; Huising et al. 2006; Li et al. 2010; Murashita et al. 2008; Volkoff et al. 2003; Won et al. 2012;), similar to its action in mammals (Munzberg and Morrison 2015), even if inhibition of food intake in response to Lep treatment has not been observed in all teleosts studied (Baker et al. 2000; Londraville and Duvall 2002). Several fish species have two Lep paralogs, LepA and LepB, such as medaka (Oryzias latipes; Kurokawa and Murashita 2009), orange-spotted grouper (Epinephelus coioides; Zhang et al. 2013) and zebrafish (Danio rerio; Gorissen et al. 2009), whereas salmonids such as Atlantic salmon (Salmo salar) and rainbow trout (Oncorhynchus mykiss) have four paralogs: Lep-A1 and Lep-A2 as well as Lep-B1 and Lep-B2 (Angotzi et al. 2013; Rønnestad et al. 2010).
Gene expression analysis suggests that the liver is the major production site of Lep in fish, irrespective of species and the relative importance of the liver as an energy-storing organ (Gorissen et al. 2009; Huising et al. 2006; Rønnestad et al. 2010; Tinoco et al. 2012; Won et al. 2012). However, data on salmonids also indicate low level lep gene expression in adipose tissues (Murashita et al. 2008; Rønnestad et al. 2010; Salmerón et al. 2015a), and Lep release from adipocytes has been demonstrated in vitro (Salmerón et al. 2015a).
Studies on plasma Lep levels and tissue lep gene expression in fish indicate species diversity in how the Lep system responds to changes in nutritional conditions, as well as a phylogenetic diversity of Lep action among vertebrate classes (Londraville et al. 2014). Plasma Lep levels increase in response to fasting or food restriction in Atlantic salmon (Johnsen et al. 2011; Trombley et al. 2012), fine flounder (Paralichthys adspersus; Fuentes et al. 2012, 2013) and rainbow trout (Kling et al. 2009; Johansson and Björnsson 2015), whereas plasma Lep decreases in the green sunfish (Lepomis cyanellus; Johnson et al. 2000) and burbot (Lota lota; Nieminen et al. 2003). Fasting decreases lep expression in the liver of striped bass (Morone saxatilis; Won et al. 2012) and proximal intestine of red-bellied piranha (Pygocentrus nattereri; Volkoff 2015), whereas it does not affect hepatic lep expression in common carp (Cyprinus carpio; Huising et al. 2006), hepatic or hypothalamic lep expression in goldfish (Carassius auratus; Tinoco et al. 2012), visceral adipose lep expression in zebrafish (Oka et al. 2010) or hepatic lepa expression in zebrafish (Gorissen et al. 2009). However, hepatic lepa expression in orange-spotted grouper (Zhang et al. 2013) and hepatic lepb expression in zebrafish (Gorissen et al. 2009) increase during fasting.
The functional (long form) of the Lep receptor (LepRL) as well as a truncated Lep receptor (LepRT) has been characterized in rainbow trout. Even though only the LepRL mediates the ligand binding of Lep through intracellular pathways, both receptor forms have similar affinity for Lep. Thus, the LepRT is suggested to modulate Lep signaling at the tissue level and to be involved in central regulation of food intake (Gong and Björnsson 2014). Besides, three short forms of the Lep receptor (LepRS1, S2, and S3) have been identified in the rainbow trout (Gong et al. 2013a). These isoforms consist of the extracellular receptor domain only, and thus have the ligand-binding properties as the full-length LepRL, while they lack the transcellular domain needed for membrane anchoring. Thus, they are released into circulation as plasma Lep-binding proteins (LepBP) and can affect plasma half-life of the peptide hormone and modulate the physiological actions of Lep, both centrally and peripherally (Gong et al. 2013a).
A breeding program with muscle adiposity as a selection criterion has over seven generations established two divergent rainbow trout lines, with high or low muscle lipid content. Studies on third and fourth generation fish indicate that glucose and lipid metabolism differs between the two fish lines (Kamalam et al. 2012; Kolditz et al. 2008a; Skiba-Cassy et al. 2009), but insulin induces comparable changes in glucose and lipid metabolism in both lines (Jin et al. 2014).
The aim of the current study was to use these energetically divergent rainbow trout lines to elucidate if and how the Lep system is involved in regulating energy homeostasis in rainbow trout, in particular in lipid deposition and mobilization. This was done by comparing the Lep system profiles, including plasma Lep and LepBP levels, as well as peripheral tissue expression of various LepR isoforms. The responses to changes in nutritional status were also evaluated and compared during a 4-week fasting of these fish.
Materials and methods
Fish and experimental conditions
Two lines of rainbow trout with divergent muscle adiposity were used and compared in the current study. The lines have been selected for breeding for high or low muscle adiposity (Quillet et al. 2005) for seven generations and are designated as fat-line (FL) and lean-line (LL) fish, respectively. In brief, the selection process has been based on the individual relative fat index of the fish when they are around 200–300 g in body weight (BW), obtained using microwave transmittance technology (Distell Fish Fatmeter, http://www.distell.com). In comparison with other commercially bred rainbow trout lines, it appears that the FL fish have similar muscle adiposity, in the range of 6–18 % (Akhan et al. 2010; Davidson et al. 2014; Suarez et al. 2014), while the LL fish have relatively low muscle adiposity, more reminiscent of wild steelhead (rainbow) trout (Penney and Moffitt 2014, 2015). The fish were bred at the Pisciculture Expérimentale INRA des Monts d’Arrée (PEIMA-INRA) facility in Brittany, France, where the study was carried out.
At the start of the experiment, on 15 April 2014, 50 fish were stocked into each of 16 circular, outdoor tanks, each with water volume of 1.8 m3 and water flow of 3 m3 h−1 and oxygen levels >6.0 mg L−1. Eight tanks were stocked with FL fish and eight tanks with LL fish (Fig. 1). At the time of stocking, the average BW of the FL and LL fish was 238 ± 3 and 262 ± 3 g, respectively. When fed, the fish received “Le Gouessant” size 5 pellets (aqua.legouessant.com) by automatic feeders five times per day. The daily ration was recalculated once a week based on size and temperature and increased from about 1.16 to 1.25 % BW day−1 over the course of the experiment. The fish experienced natural, ambient light and water conditions, and during the experiment, from mid-April to early June, the average daily water temperature rose gradually from 10.6 to 13.5 °C.
Schematic drawing of the experimental design. Fifty lean-line (LL) or fat-line (FL) rainbow trout (Oncorhynchus mykiss) were placed in each of the 16 experimental tanks. Following a 3-week acclimation period, four experimental treatments were initiated 1 day apart over a 4-day period, allowing for sampling of one experimental treatment daily over a corresponding 4-day period (June 3–7, 2014) at the end of the 4-week experimental period
Experimental design
As outlined in Fig. 1, the fish were acclimated to the environmental conditions and fed for 3 weeks, after which four different feeding regimes were initiated, each including duplicate tanks of FL as well as LL fish. Within the different feeding regimes, the fish were subjected to 0 (0w = fully fed treatment), 1 (1w), 2 (2w) and 4 weeks (4w) of fasting, respectively. It is important to note that the fasting was initiated in a stepwise fashion, 4, 2, and 1 weeks prior to the near-simultaneous sampling of all treatment groups. Although not shown in the schematic drawing (Fig. 1), the experimental regimes were initiated 1 day apart to allow the terminal sampling from each experimental treatment to be carried out 1 day apart, maintaining a 28-day experimental treatment period for all groups.
Sampling
Fish
During the terminal sampling, 10 fish were sampled from each tank, resulting in 20 FL fish and 20 LL fish being sampled from each of the 0w, 1w, 2w and 4w fasting treatments. Each day, fish were netted from the four tanks of a given treatment in a sequence, alternating between FF and LF tanks. From each tank, three, three and then four fish were netted together. When netted, the fish were immediately placed in a lethal dose (160 mg l−1) of isoeugenol (ScanAqua). When ventilation movement ceased, the fish were measured for body weight (BW) and body (fork) length (BL), after which 3–5 ml of blood was drawn from the caudal vessels using a heparinized syringe. Thereafter, the fish were decapitated and various tissues sampled (see below).
Blood
The blood was immediately placed on ice and centrifuged for 5 min at 6 °C and 800g within 15 min of sampling. The obtained plasma was then divided into several aliquots and frozen at −20 °C.
Liver and muscle
The liver was removed, weighed and a piece the size of a thumbnail was collected. Three longitudinal pieces of dorsal, “white” muscle, roughly 3–5 mm wide, 15–20 mm long and 10 mm at the thickest part, were cut from the area proximal to the dorsal fin on the right side of the fish and the skin was removed. Liver and muscle samples were frozen and kept in liquid nitrogen, as were the plasma samples, until shipped on dry ice to Gothenburg, where they were kept at −80 °C until analyses.
Gastrointestinal weight and food content
The gastrointestinal tract was dissected from the esophagus to the anus together with associated visceral fat and weighed before and after all food was removed.
Gonads
In fish showing early gonadal growth, gonads were dissected out and weighed.
Muscle lipid content
Lipids were extracted from 70 to 120 mg of muscle following the protocol Folch et al. (1957). The tissue was homogenized for 20 s in 6 ml chloroform–methanol (1:2) in a glass tube, chloroform was added to bring the solution to 1:1 (chloroform–methanol), homogenized for 15 s more and subsequently centrifuged at 2095×g for 10 min at +4 °C. The supernatant was moved to a clean glass tube, the remaining tissue was re-extracted with 4 ml fresh chloroform–methanol (1:1), homogenized (15 s), centrifuged as before and the supernatant added to that previously collected. The supernatant was then mixed with 4.1 ml 0.88 % KCl solution, shaken thoroughly and stored at 4 °C overnight. The day after, the chloroform-lipid phase was removed with a 10 ml Hamilton syringe and transferred to a clean pre-weighed 10 ml glass tube. The glass tube was kept at 40 °C in a heating block and the chloroform was evaporated with N2 gas for approximately 3 h. The tube was re-weighed to obtain the lipid weight and lipid content calculated as % of the muscle wet weight.
Rainbow trout leptin radioimmunoassay
For determining the plasma Lep levels, a radioimmunoassay (RIA) homologous for both Atlantic salmon and rainbow trout (Kling et al. 2009) was used. Briefly, a 14 aa-long Lep fragment sequentially identical between Atlantic salmon and rainbow trout (0.05 µg µl−1) was iodinated with 2 µl 125I (7.4 MBq) and chloramine T as a reducing reagent. The synthesized Lep fragment used for iodination and standards represents amino acid residues 110–123 of rainbow trout Lep-A1 (Accession No. AB354909) and has a 71 % sequence identity with rainbow trout Lep-A2 (Accession No. JX123129; Angotzi et al. 2013). Thus, while the RIA has been established to measure Lep-A1, possible cross-reactivity with Lep-A2 cannot be excluded.
Rainbow trout leptin-binding protein radioimmunoassay
A rainbow trout- and Atlantic salmon (Salmo salar)-specific RIA was used to measure the plasma leptin-binding protein (LepBP) levels (Gong et al. 2013a). A recombinant LepR395/604-GST peptide, composed of amino acids 395–604 of the rainbow trout LepR, was used for standards and iodinated using the chloramine T method. Anti-LepBP (http://www.Agrisera.com) specific against LepR395/604-GST was used as primary antibody.
Quantitative real-time PCR
The total RNA was extracted from muscle and adipose tissue by TRI Reagent (Sigma Aldrich, St. Louis, MO, USA) and from liver with an RNeasy Plus Mini kit (Qiagen, Hilden, Germany). The RNA concentrations were quantified with a NanoDrop 2000c spectrophotometer (Thermo Scientific, Saveen Werner AB, Malmö, Sweden). The first-strand cDNA was synthesized with 1 μg total RNA and iScript™ cDNA synthesis reagents (with a mix of oligo (dT) and random hexamer primers) in a total volume of 20 μl (Bio-Rad Laboratories, Hercules, CA, USA) according to the manufacturer’s manuals. The genomic DNA was eliminated from the RNA preparation with TRI reagent and RNeasy Plus Mini Kit. To control potential genomic DNA contamination, 1 μg of total RNA and cDNA synthesis reagents without iScript reverse transcriptase was used as the negative control, and few qPCR products (<1 % of qPCR products from the tested cDNA samples) were amplified from the control. The mRNA levels of the genes of the Lep paralog A1 (lepa1), the long-form LepR (leprl) and the two LepBPs (leprs1 and leprs3) were quantified by qPCR, using 0.2–0.5 μl of the cDNA synthesized from the 1 μg total RNA, SSo Advanced™ Universal SYBR Green Supermix (Bio-Rad Laboratories, Hercules, CA, USA) and primers (their nucleotide sequences are listed in Table 1) in a total volume of 10 μl. Data were collected by a CFX Connect Thermal Cycler (Bio-RAD Laboratories, Hercules, CA, USA). The qPCR efficiencies for various assays are 95–110 % for ef1α, 102–120 % for lepa1, 97–105 % for leprl, 103–115 % leprs1 and 97–105 % for leprs3. The relative gene expression was calculated using the elongation factor 1α gene (ef1α) as a reference gene, according to the Pfaffl (2001) method.
Calculations and statistics
The reported body weight (BW) of the fish is the wet weight after food has been removed from the GI tract. Condition factor (CF) was calculated as BW × BL−3 × 100, liver somatic index (LSI) as liver weight × BW−1 × 100, gonadal somatic index (GSI) as gonad weight × BW−1 × 100, and visceral somatic index (VSI; in this study, the viscera and thus the VSI did not include heart, liver or gallbladder) as the weight of the gastrointestinal tract with visceral adipose tissue attached, but food removed × BW−1 × 100. Muscle lipid content was calculated as extracted lipid weight × muscle wet weight−1 × 100. All data are given as mean ± SEM. Data were tested for normal distribution using a Shapiro–Wilk test. Normally distributed parameters (BL, BW, CF, LSI, VSI, plasma LepBP levels, relative gene expression levels of lepa1, leprl, leprs1 and leprs3 in muscle, liver and adipose tissue) were analyzed individually with two-way ANOVAs (fish line and treatment as independent factors), followed by Tukey’s HSD post hoc test with Bonferroni adjustments, while those failing the normality test (plasma Lep levels) were square root transformed before being analyzed as described above. Statistical analysis was carried out using IBM SPSS Statistics 20.0.0.1 (IBM, Chicago, USA).
Ethical permit
The experiments were carried out in accordance with the European Communities Council Directive of 24 November 1986 (86/609/EEC), under the official license 29-036 of Labbé Laurent. The PEIMA-INRA research facility is further approved for animal experimentation through license C29-277-02.
Results
Strain differences between the fully fed (0w) fish
Body characteristics
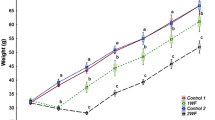
At the time of sampling (June 3), the 0w LL fish (31.9 ± 0.4 cm and 488 ± 33 g) were on average 11 % longer and 33 % heavier than the FL fish (28.8 ± 0.5 cm and 366 ± 17 g), but both strains had similar CF of about 1.5 and LSI of 1.2. The LL fish had higher (15 %) VSI compared with the FL, while the FL fish had 50 % higher muscle lipid content (Fig. 2). Based on the average weight of all fish stocked for the experiment, and the final weight of the 20 0w fish sampled for each strain, the specific growth rates over the 49-day period, from stocking to sampling, was 1.28 and 0.89 % day−1 for the LL and FL fish, respectively. No difference in gastrointestinal feed content between strains was noted (data not shown).
a Body (fork) length, b body weight, c condition factor (CF), d liver somatic index (LSI), e visceral somatic index (VSI) and f muscle lipid content in fat-line (FL; gray bars) and lean-line (LL; black bars) rainbow trout (Oncorhynchus mykiss), under feeding conditions (0w) and after 1, 2 and 4 weeks of fasting (1w, 2w and 4w), at the time of sampling on June 3–7, 2014. Data are shown as mean ± SEM (n = 20, except in f n = 10–11). Different letters indicate significant differences within a group (lower-case for LL, upper-case for FL). *p < 0.05 and # p < 0.01 denote significant difference between LL and FL
Plasma leptin and leptin-binding protein levels
Plasma Lep levels were higher (p < 0.01) in the LL fish (Fig. 3a), while plasma LepBP levels were similar in fish of the two strains (Fig. 3b).
a Plasma leptin, and b plasma leptin-binding protein (LepBP) levels in fat-line (FL; gray bars) and lean-line (LL; black bars) rainbow trout (Oncorhynchus mykiss), under feeding conditions (0w) and after 1, 2 and 4 weeks of fasting (1w, 2w and 4w). Data are shown as mean ± SEM (leptin n = 17–20, LepBP n = 11–20). Different letters indicate significant differences within a group (lowercase for LL, uppercase for FL). *p < 0.05 and # p < 0.01 denote significant differences between LL and FL
Gene expression
None of the genes examined differed in the expression between strains in the fully fed fish (Figs. 4, 5, 6).
Gene expression of LepR genes in rainbow trout (Oncorhynchus mykiss) muscles. Quantitative relative expression of a leprl, b leprs1 and c leprs3, normalized by ef1α in lean line (LL; black bars) and fat-line (FL; gray bars) fish fed (0w) or fasted for 1, 2 or 4 weeks (1w, 2w and 4w). Data are shown as mean ± SEM (n = 7–10). Different letters indicates significant differences within a group (lowercase for LL, uppercase for FL). *p < 0.05 and # p < 0.01 signifies significant difference between LL and FL
Quantitative relative gene expression of hepatic lepa1 (a), leprl (b), leprs1 (c), and leprs3 (d), normalized by ef1α, in fat-line (FL; black bars) and lean-line (LL; gray bars) rainbow trout (Oncorhynchus mykiss), under feeding conditions (0w) and after 1, 2 and 4 weeks of fasting (1w, 2w and 4w). Data are shown as mean ± SEM (n = 7–10). Different letters indicate significant differences within a group (lowercase for LL, uppercase for FL). *p < 0.05 and # p < 0.01 denote significant differences between LL and FL
Quantitative relative gene expression of lepa1 (a), leprl (b), leprs1 (c) and leprs3 (d) in visceral adipose tissue, normalized by ef1α, in fat-line (FL; black bars) and lean-line (LL; gray bars) rainbow trout (Oncorhynchus mykiss), under feeding conditions (0w) and after 1, 2 and 4 weeks of fasting (1w, 2w and 4w). Data are shown as mean ± SEM (n = 7–11). Different letters indicate significant differences within a group (lowercase for LL, uppercase for FL). *p < 0.05 and # p < 0.01 denote significant differences between LL and FL
Sex and maturation
90 % of the fish sampled were immature, with no visible gonadal growth, and no attempt was made to determine the sex of these fish. However, early gonadal growth was noted for a number of females. For the LL fish, two females with mean gonadosomatic index (GSI) of 0.15 were observed during sampling of the 0w group. For the FL fish, 14 females with mean GSI of 0.31 were observed, 6, 5, 1 and 2 fish in the 0w, 1w, 2w and 4w samplings, respectively. Statistical analysis (not shown) did not find these individuals to be significantly different from the majority of the fish sampled. Thus, they were included in the full analysis of the experimental results.
Effects of fasting
Body characteristics
Both the LL and FL fish decreased in BL and BW (Fig. 2a, b), LL fish after 1 week while FL fish showed a later decrease of BL at 4w and BW at 2w. The CF was differentially affected by fasting; CF decreased in the LL fish, but not the LL fish (Fig. 2c). The LSI profiles (Fig. 2d) did not differ between strains; they decreased (p < 0.01) rapidly during the first week of fasting (24 and 31 % decrease for the LL and FL fish, respectively) after which longer fasting did not reduce the LSI any further. VSI decreased in both strains with the length of fasting periods (Fig. 2e); however, the LL fish always had higher VSI than the FL fish. The muscle lipid contents in the two strains (Fig. 2f) were differentially affected by fasting. Muscle lipid content was not affected by fasting in the LL fish, while in the FL fish it decreased with the length of the fasting period, reaching similar levels as those of the LL fish after the 4-week fasting period.
Plasma leptin and leptin-binding protein levels
There was no significant change in plasma Lep levels in the FL fish over the 4-week fasting period (Fig. 3a). However, plasma Lep levels in the LL fish decreased gradually with increasing length of fasting, and were similar to the FL plasma Lep levels after the 4-week fasting period. Plasma LepBP levels did not change between fish lines or length of fasting (Fig. 3b).
Gene expression
In muscle, lepa1 expression levels were too low to be accurately quantified by qPCR; thus, data are not shown. leprl expression increased gradually with the length of fasting in both strains and after 4 weeks of fasting was significantly different from fish fasted for shorter periods of time in the LL fish and those fasted for 1w in FL fish (Fig. 4a). The leprs1 and leprs3 expression significantly increased after the 4-week fasting period in the LL fish, but not in the FL fish (Fig. 4b, c). Strain differences were seen after 4 weeks of fasting in leprl, leprs1 and leprs3 expressions, with higher expression levels in the LL fish compared with the FL fish (p < 0.05) (Fig. 4a–c).
Hepatic lepa1 expression increased in the LL fish after the 4-week fasting period and was significantly higher than the expression in fish that had fasted for only 2 weeks (Fig. 5a). This increase was not seen in the FL fish. The hepatic leprl expression did not change during fasting in any of the two fish lines (Fig. 5b). The hepatic leprs1 and leprs3 expressions did not change for the first 2 weeks of fasting in any of the two fish lines. However, both short receptor form expressions increased significantly in the LL strain after the 4-weeks fasting period compared to shorter periods of fasting, but only the leprs1 did so in the FL fish (Fig. 5c, d). Strain differences were seen in lepa1 and leprs3 expression levels after the 4-week fasting period, showing higher expression levels in the LL fish than FL fish (Fig. 5a, d).
In adipose tissue, lepa1, leprl, leprs1 and leprs3 were neither differently expressed after different fasting periods nor were there any significant differences in expression between the two fish lines (Fig. 6a–d).
Discussion
Differences between the rainbow trout lines under feeding conditions
In the current study, the initial larger body size of the LL fish indicates that they have grown faster than the FL fish, agreeing with studies on earlier generations of the two lines (Kamalam et al. 2012; Kolditz et al. 2008a; Quillet et al. 2007; Skiba-Cassy et al. 2009). As no differences in gastrointestinal feed content were found, it appears that the difference in growth rate is primarily due to a greater feed conversion efficiency of the LL fish, which is in agreement with conclusions drawn from studies on the third generation of these breeding-selected lines (Kolditz et al. 2008b).
The feeding FL fish had about double the muscle adiposity of the LL fish. This was expected as muscle adiposity has been used as the main breeding selection criterion to create the two lines of rainbow trout, and significant differences in muscle adiposity have already been noted in earlier generations (Kamalam et al. 2012; Kolditz et al. 2008a, b; Quillet et al. 2005; Skiba-Cassy et al. 2009). Assuming that muscle is 56 % of body weight (Jezierska et al. 1982) for both rainbow trout lines, the FL fish would have about ~22 g kg−1 more muscle fat than the LL fish. The LL fish, on the other hand, have higher VSI than the FL fish, and thus appear to compensate for their lower muscle energy reserves with higher visceral adiposity. This demonstrates a marked differentiation in energy deposition routes between the two lines, as noted in earlier generations (Kamalam et al. 2012; Kolditz et al. 2008a, b; Skiba-Cassy et al. 2009). Assuming that the difference in VSI between the lines is due to fat deposition, the LL fish have ~14 g kg−1 more visceral fat than the FL fish, indicating that the compensation is only partial and that the FL fish have greater overall energy stores. As the FL and LL fish were found to have similar total body fat content after three generations of selection (Kolditz et al. 2008b), it appears that the two rainbow trout lines at INRA-PEIMA are now diverging not only in terms of muscle adiposity, but also in total body lipid reserves.
Under feeding conditions, plasma Lep levels are higher in the LL fish than the FL fish, demonstrating that breeding selection aimed at differentiated muscle adiposity also leads to changes in plasma Lep levels. It is currently not known which tissues contribute to the circulating Lep levels in fish. Although the liver is likely to contribute substantially to the plasma Lep levels, based on the strong, hepatic lep expression of all teleost species examined (Frøiland et al. 2010; Gong et al. 2013b; Gorissen et al. 2009; Huising et al. 2006; Kurokawa and Murashita 2009; Kurokawa et al. 2005; Li et al. 2010; Murashita et al. 2008; Rønnestad et al. 2010), other tissues may also contribute, such as adipose tissue (Salmerón et al. 2015a), and gastrointestinal tract (Ettore et al. 2012; Russo et al. 2011; Volkoff 2015). Visceral fat, the main lipid reserve of the LL fish, consists primarily of adipocytes, while muscle fat, the main lipid reserve of the FL fish, is to a large extent in the form of lipid droplets within myocytes (Sheridan 1994). Visceral adipocytes from rainbow trout have been demonstrated to secrete Lep in vitro (Salmerón et al. 2015a). Together, these data suggest that visceral fat, rather than muscle fat, may contribute to circulating Lep levels in rainbow trout. During early sexual maturation of male Atlantic salmon, Lep has negative correlation with total body lipid content (Trombley et al. 2012) as with mesenteric fat index in immature rainbow trout (Salmerón et al. 2015a), whereas Lep has positive correlation with mesenteric somatic index in GH-treated rainbow trout (Kling et al. 2012). Also, plasma Lep levels often increase during fasting/food-restriction conditions when fat reserves are being mobilized and metabolized (Johansson and Björnsson 2015; Kling et al. 2009; Rønnestad et al. 2010; Salmerón et al. 2015a). Together, these data indicate that other tissues contribute significantly to plasma Lep levels, and/or that Lep secretion is not correlated to the size of the secreting tissues, but is differentially regulated during various environmental and physiological conditions.
The difference in plasma Lep levels between the FL and LL fish is not reflected in corresponding differences in lepa1 expression in hepatic or adipose tissues. Such lack of correlation between lep gene expression and plasma Lep levels has been noted in several studies on salmonids (Frøiland et al. 2012; Rønnestad et al. 2010; Trombley et al. 2012). This may, e.g., be due to changes in hormonal turnover rates, as half-life of proteins can vary from minutes to days, whereas mRNA degradation rates fall within a much tighter range of hours (Vogel and Marcotte 2012). Ligand binding to receptors as well as to binding proteins will affect plasma half-life of peptide hormones. The present data, however, do not indicate this as the reason for the different plasma Lep levels, as no differences between the lines are noted in the gene expression of the various LepR isoforms, or in the plasma LepBP levels.
While most fish in the current study were immature, early gonadal growth (GSI of 0.13–0.45 %) was noted in few females, mostly of the FL strain. As gonadal development is mainly supported by visceral and muscle fat (Aksnes et al. 1986; Manor et al. 2012; Nassour and Léger 1989), it has been suggested that muscle lipid is mobilized during early gonadal growth and a depletion of visceral adiposity is not necessary (Aussanasuwannakul et al. 2011). It is thus possible that the earlier initiation of female maturation in the FL fish is linked to their higher muscle lipid content and/or the larger total body lipid stores.
Effects of fasting
Experimentally imposed fasting, also defined as starvation (McCue 2010), has progressive, physiological and behavioral effects on the animals, with the initial period usually being that of energy depletion (Bines 1999). The initial response to fasting of both rainbow trout lines appears to be rapid hepatic energy mobilization over the first week as indicated by decreased LSI, well in line with other studies on fasting rainbow trout, showing early, rapid decline in LSI (Jezierska et al. 1982; Milne et al. 1979; Takashima et al. 1971). Concomitant with this and continuing throughout the 4-week fasting period, both the LL and FL fish appear to mobilize visceral lipid depots at a fairly steady rate and to a similar extent, with VSI decreasing by ~30 % in both strains. In contrast, there is a striking difference in how muscle lipid stores are mobilized by the two lines. While the FL fish mobilize significant amount of muscle lipids, decreasing muscle lipid content by 63 % over the 4-week fasting period, the LL fish do not mobilize significant amounts of muscle lipids. The energy mobilization by the LL fish appears similar as seen during a 4-week fasting of smaller (mean BW 90 g) rainbow trout with epaxial muscle lipid content of 2.7 %, where lipids were chiefly mobilized from viscera and only marginally from the muscle (Jezierska et al. 1982). Overall, the current data indicate that the FL fish mobilize more lipid energy than the LL fish during fasting. However, analysis of oxygen consumption and/or metabolic enzyme activities would be needed to elucidate if this is due to higher metabolic rate of the FL fish. Starvation leads to suppressed metabolism to conserve energy (Gingerich et al. 2010; McCue 2010). Although the mechanisms have not been fully elucidated in fish, they include biochemical changes such as decreased aerobic and anaerobic enzymatic activities (Navarro and Gutiérrez 1995), and behavioral/physical changes such as decreased competitive foraging activity, leading to decreased oxygen consumption and routine metabolism (Alsop and Wood 1997; Brett and Zala 1975). The current study thus suggests that the FL fish do not suppress their metabolism as early and/or as much as the LL fish, possibly due to their initially greater energy reserves. Thus, while muscle lipids are rapidly mobilized in the FL fish, they are spared in the LL fish. Muscle lipid content of wild, anadromous steelhead trout decreases from 5 % at the early stages of spawning migration to 2–3 % at later stages (Penney and Moffitt 2015). Together, the current and previous data indicate that muscle lipids can be readily utilized when needed, if the lipid reserves are relatively large from the onset, but when fish have less muscle lipids stored (2–4 %), energy is primarily mobilized from visceral stores. Possibly, the mechanism for the “sparing” of muscle lipids is related to the action of Lep in the LL fish, as the expression of the LepR genes, leprl, leprs1 and leprs3, is increased only in the LL fish after a 4-week fasting period. The upregulation of the two LepBPs (leprs1 and leprs3) may act locally as dominant negatives (Gong and Björnsson 2014), obstructing Lep from activating some of its signaling pathways. In the current study, plasma Lep levels are only slightly affected by fasting, with a gradual decrease of Lep levels in the LL fish over the first 2 weeks, but not affected at all in the FL fish. The response differs markedly from the fasting-induced increase in plasma Lep levels previously observed for this species (Johansson and Björnsson 2015; Kling et al. 2009; Salmerón et al. 2015a). Similar to the limited changes in plasma Lep levels during fasting, plasma LepBP levels were not affected in either fish line, but previously, plasma LepBP levels have been found to decrease after 3-week fasting of rainbow trout (Gong et al. 2013a). While this demonstrates a previously unknown plasticity in the fasting-induced Lep response, the underlying reasons for the discrepancy can only be speculated upon. The earlier studies were carried out under laboratory conditions of constant photoperiod and temperature, whereas the current study was carried out under ambient increasing photoperiod and temperature. The previously observed fasting-related increases in plasma Lep levels, while decreasing in plasma LepBP levels, have been suggested to be a part of an energy-sparing strategy where appetite is suppressed during periods of low food availability. However, the current study was carried out during a period of rapid spring–summer growth phase, perhaps eliciting a different appetite-regulating response to withdrawal of food. This would be in line with data indicating seasonal changes in Lep endocrinology in salmonids (Frøiland et al. 2010; Trombley et al. 2012).
The upregulation of hepatic lepa1 expression, while there is no change in the leprl expression, in the LL fish after 4-week fasting is a similar gene expression pattern as in Atlantic salmon after 7 weeks of restricted feeding (Trombley et al. 2012). There are differential tissue leprl expression responses to the 4-week fasting in the present study, with increased expression in the muscle, but no expression changes in the liver and adipose tissue. Similar differential tissue expression profiles of leprl have been observed in Atlantic salmon during feed restriction (Trombley et al. 2014). As LepRL is the only fully functional receptor form which can activate intracellular Lep signaling, the increased leprl expression may lead to tissue sensitization, allowing increased Lep stimulation of glucose and/or lipid metabolism in muscle after 4-week fasting (Bjørbæk and Kahn 2004), likely related to activation of the AMP-activated protein kinase pathway and decrease of target of rapamycin activation (Fuentes et al. 2013). Notably, the increase in leprl gene expression after 4-week fasting in the LL fish is paralleled with increased gene expression of the two LepBP genes, leprs1 and leprs3. The different gene expression profiles of the various Lep receptor isoforms between the two fish lines in response to fasting suggest their differential muscle Lep endocrinology, likely partly playing a role in their differential metabolism regulation. Strain difference is also seen in hepatic lepa1 and leprs3 expression to 4-week fasting, while no change in leprs is observed. The increase in hepatic lepa1 could be a response to diminishing energy stores, leading to increased endocrine and/or paracrine Lep levels. As leprs3 encodes a major LepBP and has higher gene expression in liver than leprs1 and leprs2 encoding the other two LepBPs (Gong et al. 2013a), the increased leprs3 expression may relate to the high lepa1 expression to bind hepatic Lep before the hormone is release into the circulation. In rainbow trout, the only tissue in which Lep action has been studied at the cellular level is adipose tissue, where Lep suppresses the fatty acid transporter-1 expression, which should lead to decreased fatty acid uptake and storage (Salmerón et al. 2015b).
The current study suggest that after 4-week fasting, Lep-induction of metabolic pathways may decrease in the liver and muscle by the increased presence of Lep receptor isoforms in these tissues, while continuing to inhibit fatty acid uptake and storage in adipose tissue. Further, the partly differentiated expression of Lep receptor isoforms in the two fish lines indicates that the LL fish may be earlier affected by fasting than the FL fish.
In conclusion, the current study strengthens the view that the Lep system in rainbow trout is under a complex regulatory control which needs to be further elucidated, both in terms of sources of Lep secretion and how the control mechanisms may change in relation to physiological status and/or life cycle trajectory.
The differentiated plasma Lep levels in the two rainbow trout stains, together with their differentiated lipid reserves, both in terms of size and tissue distribution, and how these energy reserves are subsequently mobilized during fasting, raise the possibility that in a highly domesticated species as the rainbow trout, a physiological condition akin to metabolic syndrome and/or leptin resistance can occur. It is also likely that vertebrates such as the salmonids, which in nature have a limited growing season with high food intake and increased adiposity, have regulatory mechanisms which can override the anorexigenic function of the Lep system, as this would be beneficiary for long-term survival for species where maximal fitness is not reached through maintaining a constant body weight over the seasonal life cycle. Understanding how seasonal/environmental conditions impact on the Lep system in fish in the wild is an important future step in the further elucidation of Lep function in fish.
References
Aguilar AJ, Conde-Sieira M, Polakof S, Miguez JM, Soengas JL (2010) Central leptin treatment modulates brain glucosensing function and peripheral energy metabolism of rainbow trout. Peptides 31:1044–1054
Aguilar AJ, Conde-Sieira M, Lopez-Patino MA, Miguez JM, Soengas JL (2011) In vitro leptin treatment of rainbow trout hypothalamus and hindbrain affects glucosensing and gene expression of neuropeptides involved in food intake regulation. Peptides 32:232–240
Ahima RS (2000) Leptin and the neuroendocrinology of fasting. Front Horm Res 26:42–56
Akhan S, Okumus I, Sonay FD, Kocak N (2010) Growth, slaughter yield and proximate composition of Rainbow Trout (Oncorhynchus mykiss) raised under commercial farming condition in Black Sea. Kafkas Univ Vet Fak 16:S291–S296
Aksnes A, Gjerde B, Roald SO (1986) Biological, chemical and organoleptic changes during maturation of farmed Atlantic salmon, (Salmo salar). Aquaculture 53:7–20
Alsop DH, Wood CM (1997) The interactive effects of feeding and exercise on oxygen consumption, swimming performance and protein usage in juvenile rainbow trout (Oncorhynchus mykiss). J Exp Biol 200:2337–2346
Angotzi AR, Stefansson SO, Nilsen TO, Rathore RM, Rønnestad I (2013) Molecular cloning and genomic characterization of novel leptin-like genes in salmonids provide new insight into the evolution of the leptin gene family. Gen Comp Endocrinol 187:48–59
Aussanasuwannakul A, Kenney PB, Weber GM, Yao JB, Slider SD, Manor ML, Salem M (2011) Effect of sexual maturation on growth, fillet composition, and texture of female rainbow trout (Oncorhynchus mykiss) on a high nutritional plane. Aquaculture 317:79–88
Baker DM, Larsen DA, Swanson P, Dickhoff WW (2000) Long-term peripheral treatment of immature coho salmon (Oncorhynchus kisutch) with human leptin has no clear physiologic effect. Gen Comp Endocrinol 118:134–138
Bines J (1999) Starvation and fasting. In: Sadler MJ, Strain JJ, Caballero B (eds) Encylopedia of human nutrition, vol 3. Academic Press, New York, pp 1779–1786
Bjørbæk C, Kahn BB (2004) Leptin signaling in the central nervous system and the periphery. In: Means AR (ed) Recent progress in hormone research, vol 59. The Endocrine Society Press, Maryland, pp 305–331
Brækkan OR (1959) A comparative study of vitamins in the trunk muscles of fishes. Fiskeridirektoratets skrifter/serie teknologiske undersøkelser 3:8
Brett JR, Zala CA (1975) Daily pattern of nitrogen excretion and oxygen-consumption of sockeye salmon (Oncorhynchus nerka) under controlled conditions. J Fish Res Board Can 32:2479–2486
Davidson JW, Kenney PB, Manor M, Good CM, Aussanasuwannakul A, Turk PJ, Welsh C, Summerfelt ST (2014) Growth performance, fillet quality and reproductive maturity of rainbow trout (Oncorhynchus mykiss) cultured to 5 kilograms within freshwater recirculating systems. Aquacult Res Dev 5:238
de Pedro N, Martinez-Alvarez R, Delgado MJ (2006) Acute and chronic leptin reduces food intake and body weight in goldfish (Carassius auratus). J Endocrinol 188:513–520
Ettore V, Finizia R, Elena C, Giovanni T, David F, de Paolo G, Marina P (2012) Immunohistochemical and immunological detection of ghrelin and leptin in rainbow trout (Oncorhynchus mykiss) and murray cod (Maccullochella peelii peelii) as affected by different dietary fatty acids. Microsc Res Tech 75:771–780
Folch J, Lees M, Stanley GHS (1957) A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem 226:497–509
Frøiland E, Murashita K, Jørgensen EH, Kurokawa T (2010) Leptin and ghrelin in anadromous Arctic charr: cloning and change in expressions during a seasonal feeding cycle. Gen Comp Endocrinol 165:136–143
Frøiland E, Jobling M, Björnsson BTh, Kling P, Ravuri CS, Jorgensen EH (2012) Seasonal appetite regulation in the anadromous Arctic charr: evidence for a role of adiposity in the regulation of appetite but not for leptin in signalling adiposity. Gen Comp Endocrinol 178:330–337
Fuentes EN, Kling P, Einarsdottir IE, Alvarez M, Valdes JA, Molina A, Björnsson BTh (2012) Plasma leptin and growth hormone levels in the fine flounder (Paralichthys adspersus) increase gradually during fasting and decline rapidly after refeeding. Gen Comp Endocrinol 177:120–127
Fuentes EN, Safian D, Einarsdottir IE, Valdes JA, Elorza AA, Molina A, Björnsson BTh (2013) Nutritional status modulates plasma leptin, AMPK and TOR activation, and mitochondrial biogenesis: implications for cell metabolism and growth in skeletal muscle of the fine flounder. Gen Comp Endocrinol 186:172–180
Gingerich AJ, Philipp DP, Suski CD (2010) Effects of nutritional status on metabolic rate, exercise and recovery in a freshwater fish. J Comp Physiol B 180:371–384
Gong N, Björnsson BTh (2014) Leptin signaling in the rainbow trout central nervous system is modulated by a truncated leptin receptor isoform. Endocrinology 155:2445–2455
Gong N, Einarsdottir IE, Johansson M, Björnsson BTh (2013a) Alternative splice variants of the rainbow trout leptin receptor encode multiple circulating leptin-binding proteins. Endocrinology 154:2331–2340
Gong Y, Luo Z, Zhu QL, Zheng JL, Tan XY, Chen QL, Lin YC, Lu RH (2013b) Characterization and tissue distribution of leptin, leptin receptor and leptin receptor overlapping transcript genes in yellow catfish (Pelteobagrus fulvidraco). Gen Comp Endocrinol 182:1–6
Gorissen M, Bernier NJ, Nabuurs SB, Flik G, Huising MO (2009) Two divergent leptin paralogues in zebrafish (Danio rerio) that originate early in teleostean evolution. J Endocrinol 201:329–339
Huising MO, Geven EJ, Kruiswijk CP, Nabuurs SB, Stolte EH, Spanings FA, Verburg-van Kemenade BM, Flik G (2006) Increased leptin expression in common carp (Cyprinus carpio) after food intake but not after fasting or feeding to satiation. Endocrinology 147:5786–5797
Jezierska B, Hazel JR, Gerking SD (1982) Lipid mobilization during starvation in the rainbow trout (Salmo gairdneri Richardson), with attention to fatty acids. J Fish Biol 21:681–692
Jin J, Panserat S, Kamalam BS, Aguirre P, Veron V, Medale F (2014) Insulin regulates lipid and glucose metabolism similarly in two lines of rainbow trout divergently selected for muscle fat content. Gen Comp Endocrinol 204:49–59
Jobling M, Miglavs I (1993) The size of lipid depots—a factor contributing to the control of food-intake in Arctic charr (Salvelinus alpinus). J Fish Biol 43:487–489
Johansen SJS, Ekli M, Jobling M (2002) Is there lipostatic regulation of feed intake in Atlantic salmon (Salmo salar L.)? Aquacult Res 33:515–524
Johansson M, Björnsson BTh (2015) Elevated plasma leptin levels of fasted rainbow trout decrease rapidly in response to feed intake. Gen Comp Endocrinol 214:24–29
Johnsen CA, Hagen O, Adler M, Jonsson E, Kling P, Bickerdike R, Solberg C, Björnsson BTh, Bendiksen EA (2011) Effects of feed, feeding regime and growth rate on flesh quality, connective plasma hormones in farmed Atlantic salmon (Salmo salar L.). Aquaculture 318:343–354
Johnson RM, Johnson TM, Londraville RL (2000) Evidence for leptin expression in fishes. J Exp Zool 286:718–724
Jørgensen EH, Johansen SJS, Jobling M (1997) Seasonal patterns of growth, lipid deposition and lipid depletion in anadromus Arctic charr. J Fish Biol 51:312–326
Kamalam BS, Medale F, Kaushik S, Polakof S, Skiba-Cassy S, Panserat S (2012) Regulation of metabolism by dietary carbohydrates in two lines of rainbow trout divergently selected for muscle fat content. J Exp Biol 215:2567–2578
Kennedy GC (1953) The role of depot fat in the hypothalamic control of food intake in the rat. Proc R Soc Lond B 140:578–596
Kling P, Rønnestad I, Stefansson SO, Murashita K, Kurokawa T, Björnsson BTh (2009) A homologous salmonid leptin radioimmunoassay indicates elevated plasma leptin levels during fasting of rainbow trout. Gen Comp Endocrinol 162:307–312
Kling P, Jonsson E, Nilsen TO, Einarsdottir IE, Rønnestad I, Stefansson SO, Björnsson BTh (2012) The role of growth hormone in growth, lipid homeostasis, energy utilization and partitioning in rainbow trout: interactions with leptin, ghrelin and insulin-like growth factor I. Gen Comp Endocrinol 175:153–162
Kolditz C, Borthaire M, Richard N, Corraze G, Panserat S, Vachot C, Lefevre F, Medale F (2008a) Liver and muscle metabolic changes induced by dietary energy content and genetic selection in rainbow trout (Oncorhynchus mykiss). Am J Physiol 294:R1154–R1164
Kolditz CI, Paboeuf G, Borthaire M, Esquerre D, SanCristobal M, Lefevre F, Medale F (2008b) Changes induced by dietary energy intake and divergent selection for muscle fat content in rainbow trout (Oncorhynchus mykiss), assessed by transcriptome and proteome analysis of the liver. BMC Genom 9
Kurokawa T, Murashita K (2009) Genomic characterization of multiple leptin genes and a leptin receptor gene in the Japanese medaka (Oryzias latipes). Gen Comp Endocrinol 161:229–237
Kurokawa T, Uji S, Suzuki T (2005) Identification of cDNA coding for a homologue to mammalian leptin from pufferfish (Takifugu rubripes). Peptides 26:745–750
Li GG, Liang XF, Xie Q, Li G, Yu Y, Lai K (2010) Gene structure, recombinant expression and functional characterization of grass carp leptin. Gen Comp Endocrinol 166:117–127
Londraville RL, Duvall CS (2002) Murine leptin injections increase intracellular fatty acid-binding protein in green sunfish (Lepomis cyanellus). Gen Comp Endocrinol 129:56–62
Londraville RL, Macotela Y, Duff RJ, Easterling MR, Liu Q, Crespi EJ (2014) Comparative endocrinology of leptin: assessing function in a phylogenic context. Gen Comp Endocrinol 203:146–157
MacDougald OA, Hwang CS, Fan H, Lane MD (1995) Regulated expression of the obese gene product (leptin) in white adipose tissue and 3T3-L1 adipocytes. Proc Nat Acad Sci USA 92:9034–9037
Madej T, Boguski MS, Bryant SH (1995) Threading analysis suggests that the obese gene product may be a helical cytokine. FEBS Lett 373:13–18
Manor ML, Weber GM, Salem M, Yao JB, Aussanasuwannakul A, Kenney PB (2012) Effect of sexual maturation and triploidy on chemical composition and fatty acid content of energy stores in female rainbow trout (Oncorhynchus mykiss). Aquaculture 364:312–321
McCue MD (2010) Starvation physiology: reviewing the different strategies animals use to survive a common challenge. Comp Biochem Phys A 156(1):1–18
Metcalfe NB, Thorpe JE (1992) Anorexia and defended energy levels in over-wintering juvenile salmon. J Anim Ecol 61:175–181
Milne RS, Leatherland JF, Holub BJ (1979) Changes in plasma thyroxine, triiodothyronine and cortisol associated with starvation in rainbow trout (Salmo gairdneri). Environ Biol Fish 4:185–190
Munzberg H, Morrison CD (2015) Structure, production and signaling of leptin. Metabolism 64:13–23
Murashita K, Uji S, Yamamoto T, Rønnestad I, Kurokawa T (2008) Production of recombinant leptin and its effects on food intake in rainbow trout (Oncorhynchus mykiss). Comp Biochem Physiol B 150:377–384
Nassour I, Léger CL (1989) Deposition and mobilisation of body fat during sexual maturation in female trout (Salmo gairdneri Richardson). Aquat Living Resour 2:153–159
Navarro I, Gutiérrez J (1995) Fasting and stravation. In: Hochachka PW, Mommsen TP (eds) Biochemistry and molecular biology of fishes, vol 4. Elsevier Science BV, Amsterdam, pp 393–434
Nelson RA (1980) Protein and fat metabolism in hibernating bears. Fed Proc 39:2955–2958
Nieminen P, Hyvarinen H, Kakela R, Asikainen J (2000) Plasma leptin and thyroxine of mink (Mustela vison) vary with gender, diet and subchronic exposure to PCBs. Comp Biochem Physiol A 127:515–522
Nieminen P, Mustonen AM, Hyvarinen H (2003) Fasting reduces plasma leptin- and ghrelin-immunoreactive peptide concentrations of the burbot (Lota lota) at 2 degrees C but not at 10 degrees C. Zool Sci 20:1109–1115
Oka T, Nishimura Y, Zang L, Hirano M, Shimada Y, Wang Z, Umemoto N, Kuroyanagi J, Nishimura N, Tanaka T (2010) Diet-induced obesity in zebrafish shares common pathophysiological pathways with mammalian obesity. BMC Physiol 10:21
Park HK, Ahima RS (2015) Physiology of leptin: energy homeostasis, neuroendocrine function and metabolism. Metabolism 64:24–34
Penney ZL, Moffitt CM (2014) Proximate composition and energy density of stream-maturing adult steelhead during upstream migration, sexual maturity, and kelt emigration. Trans Am Fish Soc 143:399–413
Penney ZL, Moffitt CM (2015) Fatty-acid profiles of white muscle and liver in stream-maturing steelhead trout (Oncorhynchus mykiss) from early migration to kelt emigration. J Fish Biol 86:105–120
Pfaffl MW (2001) A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 29:2002–2007
Quillet E, Le Guillou S, Aubin J, Fauconneau B (2005) Two-way selection for muscle lipid content in pan-size rainbow trout (Oncorhynchus mykiss). Aquaculture 245:49–61
Quillet E, Le Guillou S, Aubin J, Labbe L, Fauconneau B, Medale F (2007) Response of a lean muscle and a fat muscle rainbow trout (Oncorhynchus mykiss) line on growth, nutrient utilization, body composition and carcass traits when fed two different diets. Aquaculture 269:220–231
Rønnestad I, Nilsen TO, Murashita K, Angotzi AR, Gamst Moen AG, Stefansson SO, Kling P, Björnsson BTh, Kurokawa T (2010) Leptin and leptin receptor genes in Atlantic salmon: cloning, phylogeny, tissue distribution and expression correlated to long-term feeding status. Gen Comp Endocrinol 168:55–70
Russo F, De Girolamo P, Neglia S, Gargiulo A, Arcamone N, Gargiulo G, Varricchio E (2011) Immunohistochemical and immunochemical characterization of the distribution of leptin-like proteins in the gastroenteric tract of two teleosts (Dicentrarchus labrax and Carassius auratus L.) with different feeding habits. Microsc Res Tech 74:714–719
Salmerón C, Johansson M, Angotzi AR, Rønnestad I, Jonsson E, Björnsson BT, Gutierrez J, Navarro I, Capilla E (2015a) Effects of nutritional status on plasma leptin levels and in vitro regulation of adipocyte leptin expression and secretion in rainbow trout. Gen Comp Endocrinol 210:114–123
Salmerón C, Johansson M, Asaad M, Angotzi AR, Rønnestad I, Stefansson SO, Jönsson E, Björnsson BTh, Gutiérrez J, Navarro I, Capilla E (2015b) Roles of leptin and ghrelin in adipogenesis and lipid metabolism of rainbow trout adipocytes in vitro. Comp Biochem Phys B 188:40–48
Sawchenko PE (1998) Toward a new neurobiology of energy balance, appetite, and obesity: the anatomists weigh in. J Comp Neurol 402:435–441
Schradin C, Raynaud J, Arrive M, Blanc S (2014) Leptin levels in free ranging striped mice (Rhabdomys pumilio) increase when food decreases: the ecological leptin hypothesis. Gen Comp Endocr 206:139–145
Sheridan MA (1994) Regulation of lipid metabolism in poikilothermic vertebrates. Comp Biochem Phys B 107:495–508
Silverstein JT, Shearer KD, Dickhoff WW, Plisetskaya EM (1999) Regulation of nutrient intake and energy balance in salmon. Aquaculture 177:161–169
Skiba-Cassy S, Lansard M, Panserat S, Medale F (2009) Rainbow trout genetically selected for greater muscle fat content display increased activation of liver TOR signaling and lipogenic gene expression. Am J Physiol 297:R1421–R1429
Suarez MD, Garcia-Gallego M, Trenzado CE, Guil-Guerrero JL, Furne M, Domezaine A, Alba I, Sanz A (2014) Influence of dietary lipids and culture density on rainbow trout (Oncorhynchus mykiss) flesh composition and quality parameter. Aquacult Eng 63:16–24
Takashima F, Hibiya T, Watanabe T, Hara T (1971) Endocrinological studies on lipid metabolism in rainbow trout—1. Differences in lipid content of plasma, liver and visceral adipose tissue between sexually immature and mature females. Bull Jpn Soc Sci Fish 37:307–311
Tinoco AB, Nisembaum LG, Isorna E, Delgado MJ, de Pedro N (2012) Leptins and leptin receptor expression in the goldfish (Carassius auratus). Regulation by food intake and fasting/overfeeding conditions. Peptides 34:329–335
Trombley S, Maugars G, Kling P, Björnsson BTh, Schmitz M (2012) Effects of long-term restricted feeding on plasma leptin, hepatic leptin expression and leptin receptor expression in juvenile Atlantic salmon (Salmo salar L.). Gen Comp Endocrinol 175:92–99
Trombley S, Mustafa A, Schmitz M (2014) Regulation of the seasonal leptin and leptin receptor expression profile during early sexual maturation and feed restriction in male Atlantic salmon (Salmo salar L.) parr. Gen Comp Endocrinol 204:60–70
Vogel C, Marcotte EM (2012) Insights into the regulation of protein abundance from proteomic and transcriptomic analyses. Nat Rev Genetics 13:227–232
Volkoff H (2015) Cloning, tissue distribution and effects of fasting on mRNA expression levels of leptin and ghrelin in red-bellied piranha (Pygocentrus nattereri). Gen Comp Endocrinol 217–218:20–27
Volkoff H, Eykelbosh AJ, Peter RE (2003) Role of leptin in the control of feeding of goldfish (Carassius auratus): interactions with cholecystokinin, neuropeptide Y and orexin A, and modulation by fasting. Brain Res 972:90–109
Weil C, Lefevre F, Bugeon J (2013) Characteristics and metabolism of different adipose tissues in fish. Rev Fish Biol Fish 23:157–173
Won ET, Baltzegar DA, Picha ME, Borski RJ (2012) Cloning and characterization of leptin in a Perciform fish, the striped bass (Morone saxatilis): control of feeding and regulation by nutritional state. Gen Comp Endocrinol 178:98–107
Zhang H, Chen H, Zhang Y, Li S, Lu D, Meng Z, Liu X, Lin H (2013) Molecular cloning, characterization and expression profiles of multiple leptin genes and a leptin receptor gene in orange-spotted grouper (Epinephelus coioides). Gen Comp Endocrinol 181:295–305
Acknowledgments
We thank the staff at the PEIMA-INRA aquaculture research facility, especially Thierry Kerneïs and Labbé Laurent, for help with the experimental setup, fish care and sampling, and Linda Hasselberg-Frank for assistance with the molecular analysis. The study was carried out under the Transnational Access Grant 0083/05/01/10/A of the European Union AQUAEXCEL infrastructure. The research was also financed by the Swedish Research Council for Environment, Agricultural Sciences and Spatial Planning (FORMAS), Grants 229-2009-298 and 223-2011-1356.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by H. V. Carey.
Rights and permissions
About this article
Cite this article
Johansson, M., Morgenroth, D., Einarsdottir, I.E. et al. Energy stores, lipid mobilization and leptin endocrinology of rainbow trout. J Comp Physiol B 186, 759–773 (2016). https://doi.org/10.1007/s00360-016-0988-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00360-016-0988-y