Abstract
Exercise is very important for maintaining and increasing skeletal muscle mass, and is particularly important to prevent and care for sarcopenia and muscle disuse atrophy. However, the dose–response relationship between exercise quantity, duration/day, and overall duration and muscle mass is poorly understood. Therefore, we investigated the effect of exercise duration on skeletal muscle to reveal the relationship between exercise quantity and muscle hypertrophy in zebrafish forced to exercise. Adult male zebrafish were exercised 6 h/day for 4 weeks, 6 h/day for 2 weeks, or 3 h/day for 2 weeks. Flow velocity was adjusted to maximum velocity during continual swimming (initial 43 cm/s). High-speed consecutive photographs revealed that zebrafish mainly drove the caudal part. Additionally, X-ray micro computed tomography measurements indicated muscle hypertrophy of the mid-caudal half compared with the mid-cranial half part. The cross-sectional analysis of the mid-caudal half muscle revealed that skeletal muscle (red, white, or total) mass increased with increasing exercise quantity, whereas that of white muscle and total muscle increased only under the maximum exercise load condition of 6 h/day for 4 weeks. Additionally, the muscle fiver size distributions of exercised fish were larger than those from non-exercised fish. We revealed that exercise quantity, duration/day, and overall duration were correlated with skeletal muscle hypertrophy. The forced exercise model enabled us to investigate the relationship between exercise quantity and skeletal muscle mass. These results open up the possibility for further investigations on the effects of exercise on skeletal muscle in adult zebrafish.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Reduced skeletal muscle mass results in muscle weakness and a limited ability to support the body. Age-related sarcopenia and disuse muscle atrophy reduce exercise capacity as muscle mass decreases. Indeed, exercise is very important for maintaining and increasing skeletal muscle mass and is particularly important for preventing and treating sarcopenia and disuse muscle atrophy (Borst 2004; Melov et al. 2007; Reid and Fielding 2012; Bloomfield 1997). However, the dose–response relationship between exercise quantity (duration/day and overall duration) and muscle mass is poorly understood. We assume that the reason for this is a lack of appropriate, high-precision animal models.
Rodnick et al. (1989) studied the relationship between running distance (total work) and skeletal muscle mass during voluntary no-load wheel running for 6 weeks by juvenile Sprague–Dawley rats and reported hypertrophy of the soleus muscle but no dose response. Ishihara et al. (1998) showed a dose–response relationship between running distance (total work) and skeletal muscle mass (plantaris) during voluntary load-wheel running for 8 weeks by juvenile Sprague–Dawley rats. However, these studies did not investigate the effects of changing exercise duration and did not regulate exercise quantity because they studied voluntary wheel running. In contrast, treadmill studies in mice have regulated exercise quantity but have not examined the relationship between exercise quantity (duration/day and overall duration) and skeletal muscle mass (Kemi et al. 2002; Jeneson et al. 2007).
Zebrafish (Danio rerio) are vertebrates, and the structure and function of their organs and tissues are similar to those of humans. Zebrafish have recently been used as animal models in biomedical research (Lieschke and Currie 2007; Kishi et al. 2009; Best and Alderton 2008; Kishi 2014; Patton et al. 2014; Santoriello and Zon 2012) because of their advantages, such as ease of genetic manipulation and the low cost of breeding and testing. Studies on exercise and skeletal muscle in zebrafish began to increase in the early 2000s. For example, suppressing myostatin in zebrafish causes a double-muscle effect. Aging in zebrafish causes skeletal muscle atrophy, decreases physical ability (Gilbert et al. 2014), and increases the accumulation of oxidized protein in skeletal muscle (Kishi et al. 2003). It has also been found that exercise training in zebrafish enhances body growth (Palstra et al. 2010), leads to white muscle fiber hypertrophy (Palstra et al. 2014), and enhances expression of genes related to skeletal muscle production, myogenesis, and energy metabolism (Palstra et al. 2010; Palstra et al. 2014; McClelland et al. 2006). These reports suggest that skeletal muscle production, changes with aging, and the effects of exercise in zebrafish are similar to those in mammals. Furthermore, Lin (2012) and Hirata (2009) reported zebrafish as a useful animal model to study muscle diseases and drug discovery. Moreover, fish have been proposed to be an exceptional model for investigating vertebrate skeletal muscle function because their skeletal muscle is composed of red (slow) muscle and white (fast) muscle, as in mammals; but the muscle is separated into discrete red and white areas (i.e., zebrafish: Fig. 1), unlike in mammals (Rome 2005). Different muscle types or different muscle fiber types can be studied more easily. Therefore, zebrafish would be useful for studying the quantitative relationship between exercise and red/white skeletal muscle mass. However, previous zebrafish studies did not investigate the details of the relationship between exercise quantity and skeletal muscle mass. In addition, the mitochondrial gene expression response to exercise in zebrafish has been suggested to differ from that of mammals (Lemoine et al. 2010a); however, the expression pattern of genes involved in muscle catabolism and muscle cell proliferation and differentiation are unknown in zebrafish during acute exercise.
Zebrafish cross-sectional muscle diagram. a Hematoxylin–eosin (HE) stained cross-section of the caudal muscle (Fig. 2, line B). b Diagram of the cross-section. RM, red muscle fibers; WM white muscle fibers. All muscle area and muscle fiber size measurements were performed in the “analyzed area” within the dotted line square. Scale bars in a and b 500 µm
Here, we identified the area of skeletal muscle in zebrafish that generates the propulsive force for swimming by analyzing swimming with a high-speed camera and validated the area of skeletal muscle hypertrophy using X-ray micro computed tomography (CT) measurements. We investigated the effect of exercise duration/day and overall exercise duration on skeletal muscle to reveal the relationship between exercise quantity and skeletal muscle mass. We also investigated skeletal muscle gene expression levels during acute exercise training to confirm the muscle response to exercise intensity and the maximum velocity during continual swimming.
Materials and methods
Animals
Adult male zebrafish (D. rerio) were offspring of fish purchased from a local pet supplier (Meito-Suien Co., Ltd., Remix, Nagoya, Japan). All fish were kept and raised at approximately 28 °C under a 14-h light and 10-h dark cycle, and water quality conditions were maintained according to The Zebrafish Book (Westerfield 2007). The fish were concurrently spawned from the same parent in each experiment described below and were 6–7 months of age.
Exercise setup
The exercise setup for the zebrafish was a 320 L Brett-style recirculating swim flume, which was modified from the Personal-Tank PT-70S (West Japan Fluid Engineering Laboratory Co., Sasebo, Japan). This setup has three swim courses (all 70-cm long, 10-cm wide, and 30-cm high) for exercise and a non-flow bypass tank (1.7 L) as a non-exercise control. Each swim course or non-flow bypass tank has a maximum capacity of ten fish. Photoperiod and water were maintained under breeding conditions.
Experimental design
Experiment 1
Fish were weighed under anesthesia with 0.0075 % (w/v) tricaine (Sigma-Aldrich, St. Louis, MO, USA) and allocated into two groups (n = 12/group) with similar body weights. One group (exercised group) was transferred to the swim courses, and the other group (non-exercised group) was transferred to the non-flow bypass tanks. The exercised group was exercised 6 h/day, 5 days/week for 4 weeks. Swimming form was studied using a high-speed digital camera EX-ZR1000 (Casio Computer Co., Tokyo Japan) from the dorsal side at 240 fps to observe propulsive movement. Flow velocity was adjusted for the maximum velocity at which the fish could swim continuously (initial 43 cm/s) and was readjusted once/week. All fish were fed a full diet twice daily. The diet was composed of 60 % Otohime B2 (Marubeni Nisshin Feed Co., Tokyo, Japan) and 40 % gluten (Wako Pure Chemical Industries, Osaka, Japan) (Zang et al. 2011). The day after the final exercise day, the fish were euthanized with excess anesthetic; their body weight was measured; and they were gutted by laparotomy. A cross-sectional area of the skeletal muscle on each side was estimated using X-ray micro-computed tomography (CT). Then, the sample was preserved in 10 % neutral buffered formalin solution (Wako Pure Chemical Industries).
Experiments 2 and 3
Fish were allocated into two groups (n = 8/group) with similar body weights. One group (exercised group) was transferred to the swim course, and the other group (non-exercised group) was transferred to the non-flow bypass tank. The exercised group was exercised 6 h/day, 5 days/week for 2 weeks during experiment 2 and for 3 h/day, 5 days/week for 2 weeks during experiment 3. Flow velocity was adjusted to the maximum velocity at which the fish could swimming continuously (43 cm/s) and was readjusted once/week. All fish were fed the full diet twice daily, as in experiment 1. The day after the final exercise day, the fish were euthanized with excess anesthetic, their body weight was measured, and they were preserved in 10 % neutral buffered formalin solution.
Experiment 4
Twenty fish were divided randomly into exercised and non-exercised groups (n = 10/group). After the fish in the exercised group swam at 21.5 cm/s for 15 min (acclimation), swimming speed was increased to 43 cm/s (maximum velocity at which the fish could swim continuously) for 2 h 45 min. Fish in the non-exercised group were transferred to the non-flow bypass tank. All fish were euthanized with excess anesthetic and iced immediately after training. Whole bodies were preserved in RNAlater (Sigma-Aldrich) after laparotomy and stored at 4 °C until the gene expression analysis.
Skeletal muscle area measurement by X-ray micro-CT
Dissected zebrafish were fixed in a stretched position on a sample holder. The X-ray micro-CT scan was performed with an in vivo System R_mCT 3D micro-CT scanner (Rigaku Corp., Tokyo, Japan). The settings were: voltage 90 kV, current 100 µA, magnification 4×, slice thickness (scanning width) 50 µm, and shooting time 17 s. The images were viewed and reconstructed with i-View type R software (J. Morita Mfg., Kyoto, Japan). The CT images were visualized and analyzed using dedicated CT Atlas Metabolic Analysis ver. 2.03 software (Rigaku). The Hounsfield unit (HU) value of the muscle, which is a high moisture tissue, was considered to be near the HU of water (HU = 0) and between the HU of fat tissue (−350.0 to −145.0, according to the manufacturer’s instructions) and the HU of bone (>224.6 to detect the bone signal). X-ray micro-CT can measure the volume of total tissue, fat tissue, or bone within the measurement area based on the difference in X-ray transmittance among tissues. Therefore, we measured of skeletal muscle volume using X-ray micro-CT because the samples consisted mainly of muscle, fat, and bone. Skeletal muscle cross-sectional area was measured on the axial section containing the third abdominal vertebra as the mid-cranial half muscle (Fig. 2, line A) and the axial section containing the posterior end of the anal fin junction as the mid-cranial half muscle (Fig. 2, line B), which were searched in the 3D X-ray micro-CT data.
Cross-sectional position for X-ray micro computed tomography (CT) and histological analysis. Cross-section at lines A mid-cranial half muscle and B mid-caudal half muscle in zebrafish were measured and analyzed as cranial skeletal muscle and caudal skeletal muscle, respectively, by micro-CT. Histological analysis was performed at line B
Skeletal muscle morphometrics analysis
The morphometrics analysis was done by the same person, who was blinded to the study design. Red muscle, white muscle, and these muscle fibers were observed in hematoxylin–eosin (HE)-stained cross-sections of the caudal half muscle (Fig. 2, line B) using a BZ-9000 (Keyence Co., Osaka, Japan) microscope. The cross-sectional area of the muscle or muscle fiber was measured using the manual area definition and BZ-II (Keyence) image analysis software. All skeletal muscle area and muscle fiber size measurements were made in the whole epaxial quadrant (Fig. 1b). The total numbers of fibers analyzed in each muscle sample were 106–272 (red muscle) and 441–886 (white muscle).
Gene expression analysis
Skeletal muscle tissues were collected from the caudal half of the muscle and placed in RNAlater to extract the RNA. Total RNA was extracted using the RNeasy lipid tissue mini kit (Qiagen K.K., Tokyo, Japan) according to the manufacturer’s instructions. cDNA was synthesized using the High-Capacity RNA-to-cDNA Kit (Applied Biosystems, Foster City, CA, USA).
Quantitative real-time polymerase chain reaction (PCR) was performed on the cDNA samples using the TaqMan Fast Universal PCR Master Mix (Applied Biosystems) or the Fast SYBR Green Master Mix (Applied Biosystems) and the ABI Prism 7500 Fast Real-Time PCR System (Applied Biosystems) in accordance with the manufacturer’s instructions. TaqMan gene expression assays included: eukaryotic translation elongation factor 1 alpha 1, like 1 [EF1α: Dr03432748_m1 (GenBank: L47669)], nuclear respiratory factor 1 [NRF1: Dr03074214_m1 (GenBank: AL590150)], citrate synthase [CS: Dr03434061_m1 (GenBank: CR381531)], myogenin [Dr03138081_m1 (GenBank: AF202639)], myogenic differentiation 1 [MyoD: Dr03138243_g1 (GenBank: BC114261)], F-box protein 32 [atrogin1: Dr03151496_m1 (GenBank: BC052112)], and tripartite motif containing 63 [MuRF1: Dr03193823_s1 (GenBank: BC071428)]. The SYBR Green primer sets were: peroxisome proliferator-activated receptor gamma and coactivator 1 alpha [PGC1α (GenBank: AY998087), forward primer (5′–3′): TGAGGAAAATGAGGCCAACT, reverse primer (3′–5′): AGCTTCTTCAGCAGGGAAGG]. Baseline and threshold were set manually in accordance with the manufacturer’s instructions. Relative mRNA expression levels were determined using EF1α as an endogenous standard.
Statistical analyses
All data are presented as means ± standard errors unless otherwise indicated. The normality of all data was tested using the Kolmogorov–Smirnov test (with Lilliefors’ correction) for the histogram analysis of muscle fiber cross-sectional area or the Shapiro–Wilk test for all other data. Comparisons of the muscle cross-sectional area results in experiment 1, the mean cross-sectional muscle fiber area in experiment 1, and the histogram analysis of the muscle fiber cross-sectional area in experiments 1–3 between the non-exercised and exercised groups were analyzed using the Mann–Whitney U test because the data were not normal. All other normal data were analyzed with Student’s t test. The increase in the skeletal muscle ratio to test the dose-dependency between exercise quantity and skeletal muscle hypertrophy was analyzed using the Jonckheere–Terpstra test for trends. Statistical analyses were performed using IBM SPSS Statistics ver. 23 (IBM Corp., Armonk, NY, USA). A p value <0.05 was considered significant.
Results
Water flow, food consumption, and body weight
Water flow during all exercise training was increased 10 % each week, except week 4 in experiment 1 (Table 1). All fish were able to complete the exercise training. Food consumption of the exercised training group increased by 45 % during experiment 1, by 23 % during experiment 2, and by 14 % during experiment 3 compared with that in non-exercised fish, although no difference in body weight was detected between the exercised and non-exercised groups (Table 2).
Identifying the maximum skeletal muscle driving area during swimming
We defined the driving area as the maximum bent position of the body, and considered that this area generated most of the propulsive force for swimming. The high-speed sequential photography revealed that zebrafish drove mainly from their caudal half muscle compared with the cranial half when swimming quickly (Fig. 3). The ratio of the mid-caudal half skeletal muscle cross-sectional area (Fig. 2, line B) to the mid-cranial half skeletal muscle cross-sectional area (Fig. 2, line A) was higher in exercised fish than that in non-exercised fish (Fig. 4).
Consecutive high-speed photographs of zebrafish during fast swimming. All photographs were taken with a high-speed digital camera (240 frames/s) from the dorsal side. a–f Consecutive photographs during half of a tail beat cycle (20.8 ms). g A + F overlay. The body part indicated by the two-headed arrow contracts and bends the body and generates most of the propulsive force
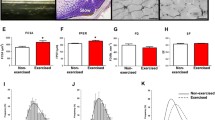
Muscle hypertrophy in zebrafish after swimming exercise. The ratio of caudal skeletal muscle cross-sectional area (Fig. 2, line B) to cranial skeletal muscle cross-sectional area (Fig. 2, line A) was analyzed by X-ray micro- computed tomography. Values are means ± standard errors. Significant values between non-exercised and exercised groups: *p < 0.05
Effect of exercise term and duration on skeletal muscle area and fiber size
Red, white, and total muscle cross-sectional areas measured at the ends of experiments 1–3 are shown in Table 2. Red muscle cross-sectional areas of exercise-trained fish in experiments 1–3 were larger than those in non-exercised fish. White and total muscle cross-sectional areas in experiment 1 were larger than those in non-exercised fish.
The skeletal muscle ratios (red, white, and total) increased in each experiment as calculated from muscle cross-sectional area of individual fish in the exercised group divided by mean muscle cross-sectional area in the non-exercised group (Fig. 5). The red, white, and total muscle ratios increased in response to increased exercise quantity, low: experiment 3 (2 weeks, 3 h), mid: experiment 2 (2 weeks, 6 h), high: experiment 1 (4 weeks, 6 h). Mean cross-sectional red and white muscle fiber sizes in experiments 1–3 are shown in Fig. 6. In experiment 1, mean cross-sectional red muscle fiber sizes were 554.7 ± 24.5 and 705.0 ± 31.6 μm2 in non-exercised and exercised fish (Fig. 6a), respectively, whereas white muscle fiber sizes were 1543.0 ± 28.6 and 1661.2 ± 40.3 μm2 in non-exercised and exercised fish, respectively (Fig. 6b). Both red and white muscle fiber sizes from exercised fish were larger than those from non-exercised fish. In experiment 2, mean cross-sectional red muscle fiber sizes were 478.4 ± 23.5 and 593.7 ± 16.2 μm2 in non-exercised and exercised fish, respectively (Fig. 6c), whereas white muscle fiber sizes were 1532.8 ± 60.6 and 1544.7 ± 59.2 μm2 in non-exercised and exercised fish, respectively (Fig. 6d). Red muscle fibers from exercised fish were larger than those from non-exercised fish. In experiment 3, mean cross-sectional red muscle fibers sizes were 496.5 ± 18.9 and 604.7 ± 17.5 μm2 in non-exercised and exercised fish, respectively (Fig. 6e), whereas white muscle fiber sizes were 1451.4 ± 36.2 and 1502.5 ± 51.9 μm2 in non-exercised and exercised fish, respectively (Fig. 6f) Only red muscle fibers from exercised fish were larger than those from non-exercised fish. The cross-sectional red and white muscle fiber size distributions in experiments 1–3 are shown in Fig. 7. The muscle fiber size distributions of exercised fish, other than white muscle in experiment 2 (Fig. 7d), were larger than those from non-exercised fish.
Effects of changing exercise quantity on increases in the skeletal muscle ratio. The skeletal muscle mass ratio of each muscle type increased with each increase in exercise quantity; 2 weeks, 3 h/day in experiment 3; 2 weeks, 6 h/day in experiment 2; and 4 weeks 6 h/day in experiment 1. The ratio values are means ± standard deviations. Increasing trend in the muscle ratio with the increase in exercise quantity was detected by the Jonckheere–Terpstra test for a trend in red muscle (p < 0.01), white muscle (p < 0.01), and total muscle (p < 0.01)
Morphometric analysis of mean muscle fiber area in zebrafish after swimming exercise. a Mean, red muscle fiber cross-sectional area in the non-exercised and exercised groups in experiment 1. b Mean, white muscle fiber cross-sectional area in experiment 1. c Mean, red muscle fiber cross-sectional area in experiment 2. d Mean, white muscle fiber cross-sectional area in experiment 2. e Mean, red muscle fiber cross-sectional area in experiment 3. f Mean, white muscle fiber cross-sectional area (right side) in experiment 3. All muscle fiber cross-sectional values are means ± standard errors. Significant values between non-exercised and exercised groups: **p < 0.01, and *p < 0.05
Histogram of the morphometric analysis results of zebrafish muscle fibers after swimming exercise. a Histogram of red muscle fiber cross-sectional area in the non-exercised and exercised groups for experiment 1. b Histogram of white muscle fiber cross-sectional area in experiment 1. c Histogram of red muscle fiber cross-sectional area in experiment 2. d Histogram of white muscle fiber cross-sectional area in experiment 2. e Histogram of red muscle fiber cross-sectional area in experiment 3. f Histogram of white muscle fiber cross-sectional area in experiment 3. All muscle fiber cross-sectional values are means ± standard errors. Transit distribution of muscle fibers, except white muscle fibers in experiment 2 (d), to a large size was detected by the Mann–Whitney U test. Red muscle fiber (p < 0.001) and white muscle fiber (p < 0.001) in experiment 1; red muscle fiber (p < 0.01) in experiment 2; red muscle fiber (p < 0.001) and white muscle fiber (p < 0.001) in experiment 3
Effect of exercise training on gene expression in skeletal muscle
The acute mRNA expression response was assessed in genes encoding a regulator of mitochondrial biogenesis, function, and activity (PGC1α, NRF1, and CS); a muscle differentiation regulator (MyoD); a muscle development regulator (myogenin); and muscle-specific ubiquitin ligases (atrogin1 and MuRF1) during the single exercise examination in experiment 4 (Fig. 8). All mRNA levels in exercised fish were significantly higher than those in non-exercised fish.
Effects of a single exercise on skeletal muscle gene expression. Peroxisome proliferator-activated receptor gamma and coactivator 1 alpha (PGC1α), nuclear respiratory factor 1 (NRF1), citrate synthase (CS), myogenic differentiation 1 (MyoD), myogenin, atrogin1, and MuRF1 mRNA expression levels in the caudal skeletal muscle half after acute exercise in experiment 4. Expression of each mRNA was normalized against corresponding expression in the non-exercised group. Student’s t test was used for the PGC1α, NRF1, MyoD, myogenin, and atrogin1 expression levels by because the data were normal. The Mann–Whitney U test was used to test CS and MuRF1 expression levels because the data were not normal. Values are means ± standard errors. Significant values between non-exercised and exercised groups: ***p < 0.001, **p < 0.01, *p < 0.05 by Student’s t test; +++ p < 0.001, + p < 0.05 by Mann–Whitney U test
Discussion
Observing the swimming form of zebrafish showed that they drove the caudal-half of the skeletal muscle to generate high speeds during swimming. The X-ray micro-CT measurements revealed muscle hypertrophy of the mid-caudal half part compared with that of the mid-cranial half part. These findings show that skeletal muscle in the caudal half hypertrophies as exercise duration per day and overall duration increases over time in exercised fish (experiments 1–3) compared with that in non-exercised fish (Fig. 5). Experiments 1–3 revealed skeletal muscle hypertrophy with increasing duration/day and term, which was considered dose-dependent exercise quantity. White muscle also hypertrophied with more exercise and increased significantly with duration/day and term in this study. Additionally, the gene expression patterns observed during a single exercise bout suggested that exercise intensity in this study was sufficient to affect skeletal muscle. Therefore, we demonstrated for the first time in zebrafish that exercise quantity dose-dependently increased skeletal muscle hypertrophy. Skeletal muscle hypertrophy is an adaptation to the quantity of exercise that also occurs during human exercise training (Konopka and Harber 2014). No forced exercise study has shown that increasing exercise quantity increases skeletal muscle mass within 4 weeks. Therefore, we suggest that zebrafish is a promising model to investigate the effect of exercise on skeletal muscle and to develop technologies, medicine, and foods that will help individuals maintain and increase skeletal muscle mass.
In this study, we determined that the caudal half skeletal muscle contracted and bent the body to generate the main propulsive force for swimming, which was analyzed with a high-speed camera to ensure that exercise quantity was dose-dependently associated with skeletal muscle hypertrophy. Hypertrophy of skeletal muscle is a response to a new load and the need for increased poster output to maintain position against increased flow in the case of fish. In mammals, the soleus and plantaris muscles respond to exercise and are often used to investigate the relationship between exercise quantity and skeletal muscle mass because they drive walking and running. Most mammalian skeletal muscle studies isolated muscle and weighed each part. However, no study has isolated and weighed fish skeletal muscle because it has a different structure. Meulen et al. (2006) measured skeletal muscle cross-sectional area to study skeletal muscle hypertrophy during exercise in juvenile zebrafish. In their report, exercise quantity-dependent skeletal muscle hypertrophy was not shown clearly because growth rate in juvenile zebrafish is affected by skeletal muscle hypertrophy. Other zebrafish studies measured body weight (Palstra et al. 2010) or skeletal muscle fiber size (Palstra et al. 2014) to investigate the relationship between exercise and skeletal muscle mass. Accordingly, it is unclear how much exercise causes dose-dependent skeletal muscle hypertrophy. We determined the skeletal muscle area driving high-speed swimming. Each fish has a respective swimming mode, as the suffix “-form” (e.g., thunniform) refers to the type of movement and not to the body form. For example, the mode is classified based on the type of muscle used, such as caudal muscle or the trunk (e.g., anguilliform, subcarangiform, carangiform, thunniform, or ostraciiform) to the type using the dorsal, anal, or pectoral fin (e.g., amiiform, balistiform, tetraodontiform, gymnotiform, rajiform, diodontiform, or labriform) for propulsive movement (Lindsey 1979). Although zebrafish are assumed to be the caudal type, such as anguilliform, subcarangiform, carangiform, thunniform, or ostraciiform, there was little detailed knowledge of zebrafish swimming mode until now. Therefore, we observed high-speed consecutive photographs of zebrafish swimming (Fig. 3) and the appearance (Fig. 2) of zebrafish with reference to Lindsey (1979), and considered that zebrafish are carangiforms because they have major characteristics of carangiforms, such as wavelength/body length >1.0; wavelength visible on body <0.5; amplitude/body length: undulations confined to posterior 1/3; body shape: mass concentrated anteriorly and peduncle quite narrow; and causal fin: posterior margin notched. The observations of the other two fish were the same. In addition, all fish swimming in this study displayed the same swimming mode. Rome et al. (1993) reported that most of the power for swimming originates from the caudal muscle of scup (Stenotomus chrysops), whose swimming form seems to be subcarangiform or carangiform. Accordingly, we considered that zebrafish drive their caudal muscle during swimming. However, it is difficult to identify the specific part of the skeletal muscle in the caudal area that drives swimming the most in zebrafish because the bead of fast swimming fish was incompletely constant, and the part of the body that bends the most does not necessarily correspond to the skeletal muscle generating most of the propulsive force. Accordingly, we assigned the area of the axial section containing the posterior end of the anal fin junction to the mid-caudal half of the muscle mass and the area of the axial section containing the third abdominal vertebra to the mid-cranial half of the muscle mass (Fig. 2) and compared these two parts using X-ray micro-CT. The results indicated that the mid-caudal half of the muscle hypertrophied in response to exercise training more than that of the mid-cranial half of the muscle (Fig. 4). In carp, which are closely related to zebrafish, the red muscle fibers run parallel to the body axis just under the skin, and the white muscle fibers (approximately 25 % as long as red fibers) run in a helical fashion with respect to the spine (Rome 2002). These features suggest that the bending part directly contact the red muscle and that the part around the bending white muscle are the major drivers. Thus, hypertrophy of the caudal half of the muscle was considered reasonable. The morphometrics analysis of the mid-caudal half of muscle cross-sectional area revealed that the mid-caudal half of the muscle hypertrophied in response to exercise training (Table 2). The specific assignment of the axial section containing the posterior end of the anal fin junction as mid-caudal half of the muscle was considered to contribute largely to investigate muscle hypertrophy response to the quantity of exercise in detail.
Our results demonstrate that forced exercise in zebrafish increased skeletal muscle mass dose dependently with the quantity of exercise in short-term. Several rodent studies have also shown a relationship between exercise quantity and skeletal muscle hypertrophy (Rodnick et al. 1989; Ishihara et al. 1998; Brown et al. 1992; McMahon et al. 2014). However, these studies were performed using a voluntary exercise wheel. In contrast, the zebrafish model has advantages of their innate behavior of swimming against water flow and the ability to undergo forced exercise without penalty or reward, as in many fish (McClelland 2012). A relatively heavily loaded exercise, which includes moving through the high density space of water, was forced uniformly on all exercised fish. In addition, zebrafish are diurnal, like humans. Zebrafish have a simple skeletal structure, and the caudal half of the zebrafish skeletal muscle generates most of the propulsive force. These advantages over rodents suggest that the zebrafish exercise model, in which exercise quantity can be accurately controlled and skeletal muscle can be easily analyzed with minimum influence (McClelland 2012), can be used, to accurately investigate the relationship between exercise quantity and skeletal muscle mass.
Our results reveal skeletal muscle hypertrophy without an increase in body weight, although previous reports showed body growth with a suggestion of skeletal muscle hypertrophy by using larval or juvenile zebrafish (Meulen et al. 2006; Palstra et al. 2010). This study used mature adult zebrafish, which grow very little (Biga and Goetz 2006) or they were fed only enough to exercise. We are considering this topic for a future study under strict control of nutritional composition and intake.
The muscle fiber data (Fig. 6) show that hypertrophy of the muscle fibers increased muscle mass, as in mammals. In contrast, the red muscle hypertrophy was suggested after a minimal exercise (experiment 3) compared with that of white muscle (Table 2; Fig. 5). Red muscle is more active during sustainable swimming, but not during the escape response, in salmon or carp (Rome 2005), whose swimming mode is similar to that of zebrafish. The exercise pattern in this study was sustainable swimming; thus, zebrafish red muscle was considered activated and hypertrophied more than those of white muscle. Munoz et al. (1994) reported that voluntary wheel exercise by juvenile rats begins to increase (90 % red muscle fibers) soleus muscle mass 1 week after starting the experiment and began to increase plantaris (90 % white muscle fibers) muscle mass 2 weeks after starting the experiment. In our study, red muscle mass increased when exercise quantity was low, and white muscle mass increased as exercise quantity increased (Table 2; Fig. 5). Ishihara et al. (1991) reported that 6.5 weeks of voluntary wheel exercise by juvenile rats causes hypertrophy of the soleus and plantaris and increases fiber type composition and fiber area of fast-twitch oxidative glycolytic fibers, which are endurance-related white muscle fibers in the plantaris. The exercise conditions in our study increased endurance-related white muscle (data not shown), referred to as “pink muscle” in fish (Syme 2005). Thus, the exercise conditions in our study were equal to those of mammalian endurance exercise studies performed by Munoz et al. (1994) and Ishihara et al. (1991). The muscle fiber results indicated that the white muscle fiber size distribution did not change in experiment 2, even though the fibers became larger in experiments 1 and 3. Although the reason is unclear, it seems that insufficient caloric intake caused catabolism of white muscle, which was less activated than red muscle. The increase in the feed intake ratio by exercise in experiment 2 (23 %) was lower than that in experiment 1 (45 %), despite that the exercise quantity for the first and second week duration was nearly equal between the two experiments.
PGC1α responds to exercise and growth of mitochondria, in part, through direct interactions with NRF1 in animals (Konopka and Harber 2014; Uguccioni et al. 2010; Lira et al. 2010; LeMoine et al. 2010b; Pilegaard et al. 2003), although Lemoine et al. (2010a) reported that the PGC1α gene expression level does not increase after acute exercise, but its putative target gene (the mitochondrial enzyme CS) increases 24 h after acute exercise. They discussed differences in PGC1α function between zebrafish and mammals. In contrast, acute exercise in our study increased PGC1α, NRF1, and CS gene expression levels. Our exercise condition (42 cm/s) was harder than the condition in the study by Lemoine et al. (2010b) (9 cm/s). Accordingly, zebrafish PGC1α may be less sensitive than that of mammals and may respond to high-intensity exercise, as shown in our study. Additionally, the muscle-specific gene expression pattern, which is related to muscle catabolism and muscle cell proliferation and differentiation, suggested a muscle remodeling response to exercise. Expression of atrogin1 and MuRF1, which are muscle-specific ubiquitin ligases, increased after acute exercise. Increased atrogin1 and MuRF1 expression levels have also been reported in humans after acute exercise (Pasiakos et al. 2010). MyoD and myogenin gene expression, which facilitate muscle proliferation, differentiation, and regeneration, increased after acute exercise. These genes, particularly myogenin, increase in human skeletal muscle after acute resistance exercise (Yang et al. 2005). These genes could be responding to the amino acid supply for energy and muscle remodeling during and after exercise. Further study (e.g., using microarray, such as Palstra et al. 2014, or RNA-seq on red or white muscle) is needed to analyze the responses of skeletal muscle in detail. However, the gene expression patterns we observed suggest that exercise training affects muscle remodeling and the properties of mitochondria.
Our results demonstrate exercise quantity-dependent skeletal muscle hypertrophy for the first time in a forced exercise study. This is a significant outcome because the rodent model is not useful for investigating this relationship. The character of the skeletal muscle response in zebrafish, as shown in this study, may also contribute to investigations of motor neurons or neuromuscular junctions using the mutant, transgenic, or knockout zebrafish lines (Santoriello and Zon 2012; Daikoku et al. 2015; Kishi 2014).
Conclusions
The forced zebrafish exercise model allowed us to investigate the relationship between exercise quantity and skeletal muscle mass. We revealed that skeletal muscle hypertrophied with increasing exercise quantity, duration/day, and overall duration. Analyses of caudal half muscle, which was strongly activated during fast swimming, open the possibility of investigations on the effects of exercise on skeletal muscle. The results from this study demonstrate that zebrafish is a promising model to investigate the effect of exercise on skeletal muscle mass.
Abbreviations
- CT:
-
Computed tomography
- HU:
-
Hounsfield unit
- EF1α:
-
Eukaryotic translation elongation factor 1 alpha 1, like 1
- NRF1:
-
Nuclear respiratory factor 1
- CS:
-
Citrate synthase
- MyoD:
-
Myogenic differentiation 1
- MuRF1:
-
Tripartite motif containing 63
- PGC1α:
-
Peroxisome proliferator-activated receptor gamma, and coactivator 1 alpha
References
Best JD, Alderton WK (2008) Zebrafish: an in vivo model for the study of neurological diseases. Neuropsychiatr Dis Treat 4:567–576
Biga PR, Goetz FW (2006) Zebrafish and giant danio as models for muscle growth: Determinate vs. indeterminate growth as determined by morphometric analysis. Am J Physiol Regul Integr Comp Physiol 291:R1327-R1337
Bloomfield SA (1997) Changes in musculoskeletal structure and function with prolonged bed rest. Med Sci Sports Exerc 29:197–206
Borst SE (2004) Interventions for sarcopenia and muscle weakness in older people. Age Ageing 33:548–555. doi:10.1093/ageing/afh201
Brown M, Ross TP, Holloszy JO (1992) Effects of ageing and exercise on soleus and extensor digitorum longus muscles of female rats. Mech Ageing Dev 63:69–77
Daikoku E, Saito M, Ono F (2015) Zebrafish mutants of the neuromuscular junction: swimming in the gene pool. J Physiol Sci 65:217–221
Gilbert MJ, Zerulla TC, Tierney KB (2014) Zebrafish (Danio rerio) as a model for the study of aging and exercise: physical ability and trainability decrease with age. Exp Gerontol 50:106–113. doi:10.1016/j.exger.2013.11.013
Hirata H (2009) Zebrafish muscular disease models towards drug discovery. Expert Opin Drug Discov 4:507-513. doi:10.1517/17460440902835483
Ishihara A, Inoue N, Katsuta S (1991) The relationship of voluntary running to fibre type composition, fibre area and capillary supply in rat soleus and plantaris muscles. Eur J Appl Physiol Occup Physiol 62:211–215
Ishihara A, Roy RR, Ohira Y, Ibata Y, Edgerton VR (1998) Hypertrophy of rat plantaris muscle fibers after voluntary running with increasing loads. J Appl Physiol 84:2183–2189
Jeneson JA, de Snoo MW, Verlinden NA, Joosten BJ, Doornenbal A, Schot A, Everts ME (2007) Treadmill but not wheel running improves fatigue resistance of isolated extensor digitorum longus muscle in mice. Acta Physiol (Oxf) 190:151–161. doi:10.1111/j.1748-1716.2007.01680.x
Kemi OJ, Loennechen JP, Wisloff U, Ellingsen O (2002) Intensity-controlled treadmill running in mice: cardiac and skeletal muscle hypertrophy. J Appl Physiol 93:1301–1309. doi:10.1152/japplphysiol.00231.2002
Kishi S (2014) Using zebrafish models to explore genetic and epigenetic impacts on evolutionary developmental origins of aging. Transl Res 163:123–135. doi:10.1016/j.trsl.2013.10.004
Kishi S, Uchiyama J, Baughman AM, Goto T, Lin MC, Tsai SB (2003) The zebrafish as a vertebrate model of functional aging and very gradual senescence. Exp Gerontol 38:777–786
Kishi S, Slack BE, Uchiyama J, Zhdanova IV (2009) Zebrafish as a genetic model in biological and behavioral gerontology: where development meets aging in vertebrates–a mini-review. Gerontology 55:430–441. doi:10.1159/000228892
Konopka AR, Harber MP (2014) Skeletal muscle hypertrophy after aerobic exercise training. Exerc Sport Sci Rev 42:53–61. doi:10.1249/jes.0000000000000007
Lemoine CM, Craig PM, Dhekney K, Kim JJ, McClelland GB (2010a) Temporal and spatial patterns of gene expression in skeletal muscles in response to swim training in adult zebrafish (Danio rerio). J Comp Physiol B 180:151–160. doi:10.1007/s00360-009-0398-5
Lemoine CM, Lougheed SC, Moyes CD (2010b) Modular evolution of PGC-1α in vertebrates. J Mol Evol 70:492–505. doi:10.1007/s00239-010-9347-x
Lieschke GJ, Currie PD (2007) Animal models of human disease: zebrafish swim into view. Nat Rev Genet 8:353–367. doi:10.1038/nrg2091
Lin YY (2012) Muscle diseases in the zebrafish. Neuromuscul Disord 22:673–684. doi:10.1016/j.nmd.2012.04.007
Lindsey CC (1979) 1 Form, function, and locomotory habits in fish. Fish Physiol 7:1–100. doi:10.1016/S1546-5098(08)60163-6
Lira VA, Benton CR, Yan Z, Bonen A (2010) PGC-1alpha regulation by exercise training and its influences on muscle function and insulin sensitivity. Am J Physiol Endocrinol Metab 299:E145–E161. doi:10.1152/ajpendo.00755.2009
McClelland GB (2012) Muscle remodeling and the exercise physiology of fish. Exerc Sport Sci Rev 40:165–173. doi:10.1097/JES.0b013e3182571e2c
McClelland GB, Craig PM, Dhekney K, Dipardo S (2006) Temperature- and exercise-induced gene expression and metabolic enzyme changes in skeletal muscle of adult zebrafish (Danio rerio). J Physiol 577(Pt 2):739–751. doi:10.1113/jphysiol.2006.119032
McMahon CD, Chai R, Radley-Crabb HG, Watson T, Matthews KG, Sheard PW, Soffe Z, Grounds MD, Shavlakadze T (2014) Lifelong exercise and locally produced insulin-like growth factor-1 (IGF-1) have a modest influence on reducing age-related muscle wasting in mice. Scand J Med Sci Sports 24:e423–e435. doi:10.1111/sms.12200
Melov S, Tarnopolsky MA, Beckman K, Felkey K, Hubbard A (2007) Resistance exercise reverses aging in human skeletal muscle. PLoS One 2:e465. doi:10.1371/journal.pone.0000465
Munoz KA, Aannestad A, Tischler ME, Henriksen EJ (1994) Skeletal muscle protein content and synthesis after voluntary running and subsequent unweighting. Metabolism 43:994–999
National Research Council Committee for the Update of the Guide for the C, Use of Laboratory A (2011) Guide for the care and use of laboratory animals, 8th edn. National Academies Press, Washington
Palstra AP, Tudorache C, Rovira M, Brittijn SA, Burgerhout E, van den Thillart GEEJM, Spaink HP, Planas JV (2010) Establishing zebrafish as a novel exercise model: swimming economy, swimming-enhanced growth and muscle growth marker gene expression. PLoS One 5:e14483. doi:10.1371/journal.pone.0014483
Palstra AP, Rovira M, Rizo-Roca D, Torrella JR, Spaink HP, Planas JV (2014) Swimming-induced exercise promotes hypertrophy and vascularization of fast skeletal muscle fibres and activation of myogenic and angiogenic transcriptional programs in adult zebrafish. BMC Genom 15:1136. doi:10.1186/1471-2164-15-1136
Pasiakos SM, McClung HL, McClung JP, Urso ML, Pikosky MA, Cloutier GJ, Fielding RA, Young AJ (2010) Molecular responses to moderate endurance exercise in skeletal muscle. Int J Sport Nutr Exerc Metab 20:282–290
Patton EE, Dhillon P, Amatruda JF, Ramakrishnan L (2014) Spotlight on zebrafish: translational impact. Dis Model Mech 7:731–733. doi:10.1242/dmm.017004
Pilegaard H, Saltin B, Neufer PD (2003) Exercise induces transient transcriptional activation of the PGC-1α gene in human skeletal muscle. J Physiol 546:851–858. doi:10.1113/jphysiol.2002.034850
Reid KF, Fielding RA (2012) Skeletal muscle power: a critical determinant of physical functioning in older adults. Exerc Sport Sci Rev 40:4–12. doi:10.1097/JES.0b013e31823b5f13
Rodnick KJ, Reaven GM, Haskell WL, Sims CR, Mondon CE (1989) Variations in running activity and enzymatic adaptations in voluntary running rats. J Appl Physiol 66:1250–1257
Rome LC (2002) The design of vertebrate muscular systems: comparative and integrative approaches. Clin Orthop Relat Res 403(suppl):S59–S76
Rome LC (2005) Principles of actuation in the muscular system of fish. IEEE J Ocean Eng 30:630–646. doi:10.1109/JOE.2005.852346
Rome LC, Swank D, Corda D (1993) How fish power swimming. Science 261(5119):340–343
Santoriello C, Zon LI (2012) Hooked! Modeling human disease in zebrafish. J Clin Invest 122:2337–2343. doi:10.1172/jci60434
Syme DA (2005) Functional properties of skeletal muscle. Fish Physiol 23:179–240. doi:10.1016/S1546-5098(05)23006-6
Uguccioni G, D’Souza D, Hood DA (2010) Regulation of PPARgamma coactivator-1alpha function and expression in muscle: effect of exercise. PPAR Res. doi:10.1155/2010/937123
van der Meulen T, Schipper H, van den Boogaart JG, Huising MO, Kranenbarg S, van Leeuwen JL (2006) Endurance exercise differentially stimulates heart and axial muscle development in zebrafish (Danio rerio). Am J Physiol Regul Integr Comp Physiol 291:R1040–R1048. doi:10.1152/ajpregu.00116.2006
Westerfield M (2007) The zebrafish book: a guide for the laboratory use of zebrafish (Danio rerio), 5th edn. M. Westerfield
Yang Y, Creer A, Jemiolo B, Trappe S (2005) Time course of myogenic and metabolic gene expression in response to acute exercise in human skeletal muscle. J Appl Physiol 98:1745–1752. doi:10.1152/japplphysiol.01185.2004
Zang L, Morikane D, Shimada Y, Tanaka T, Nishimura N (2011) A novel protocol for the oral administration of test chemicals to adult zebrafish. Zebrafish 8:203–210. doi:10.1089/zeb.2011.0726
Acknowledgments
The authors thank Ms. Mari Tsutsumi for maintaining the zebrafish colony, Ms. Sayaka Koike for measuring muscle fiber sizes, and Dr. Yoshihiko Baba, West Japan Fluid Engineering Laboratory Co., Ltd., for creating the zebrafish exercise setup.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
No author has any competing interest to declare.
Ethical approval
All experiments in this study conformed to the regulations approved by the Animal Care and Experimentation Facility Committee of Kao Corporation and with those outlined in The Zebrafish Book (Westerfield 2007) and the Guide for the Care and use of Laboratory Animals 8th edition (National Research Council Committee for the Update of the Guide for the and Use of Laboratory 2011).
Additional information
Communicated by G. Heldmaier.
Rights and permissions
About this article
Cite this article
Hasumura, T., Meguro, S. Exercise quantity-dependent muscle hypertrophy in adult zebrafish (Danio rerio). J Comp Physiol B 186, 603–614 (2016). https://doi.org/10.1007/s00360-016-0977-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00360-016-0977-1