Abstract
This study was aimed at determining the role of the crustacean hyperglycemic hormone (CHH) in the physiological compensation to both saline and thermal stress, in the freshwater crayfish Cherax quadricarinatus. By determining the expression of the CHH gene in the eyestalk of juvenile crayfish, we found that maximal induction of CHH was induced at high salinity (10 g/L) and low temperature (20 °C). In order to investigate the role of CHH in the physiological compensation to such stressful conditions, recombinant CHH was supplied to stressed animals. CHH-injected crayfish showed increased hemolymphatic levels of glucose, in accordance with a significant utilization of glycogen reserves from the hepatopancreas. Furthermore, CHH administration allowed stressed animals to regulate hemolymphatic sodium and potassium at more constant levels than controls. Taken together, these results suggest a relevant role of CHH in increasing the energy available intended for processes involved in the physiological compensation of C. quadricarinatus to both saline and thermal stress.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Crustacean peptides have gained interest because of their involvement in the control of growth by molting, as well as in many metabolic processes and reproduction (Dircksen 2013). Crustacean hyperglycemic hormone (CHH) is a member of an eyestalk neuropeptide family, currently known as the CHH superfamily, which includes the molt-inhibiting hormone (MIH), the gonad-inhibiting or vitellogenin-inhibiting hormone (GIH/VIH), and the mandibular organ-inhibiting hormone (MOIH) (Webster et al. 2012). The members of this superfamily, based on their precursor and primary structures, are grouped into type-I (CHH) and type-II (MIH, MOIH, VIH/GIH) peptides (Lacombe et al. 1999). Although CHH is mainly synthesized and released by the X organ—sinus gland complex (XO-SG), some extra-eyestalk sources (such as pericardial organ, thoracic ganglion and hemocytes) have been reported (Kung et al. 2013; Webster et al. 2012; Fanjul-Moles 2006; Chung et al. 2010; Chung and Zmora 2008).
CHH is a pleiotropic neurohormone that plays a central role in metabolic processes, especially in regulation of blood sugar level, also exhibiting a secretagogue action on digestive enzymes (Keller and Sedlmeier 1988; Santos and Keller 1993a). Moreover, CHH is involved in the control of reproduction (de Kleijn et al. 1998; de Kleijn and Van Herp 1998), molting (Chung et al. 1999; Webster et al. 2000), lipid metabolism (Santos et al. 1997), and osmoregulation (Turner et al. 2013; Udomkit et al. 2004; Serrano et al. 2003; Spanings-Pierrot et al. 2000; Charmantier-Daures et al. 1994). Several studies have implicated this hormone in the physiological response of crustaceans to stressful conditions, a topic of great interest in crayfish aquaculture (Chang et al. 1999; Durand et al. 2000; Lorenzon et al. 1997, 2002; Santos et al. 2001). To this respect, emersion, handling and transport during commercial processes represent different stressors that require homeostatic regulation of several physiological variables.
After exposure to stressful episodes such as emersion, hypoxia, salinity or thermal stress, a rapid increase in CHH levels has been observed (Chang et al. 1998; Chung and Zmora 2008; Webster 1996) which leads to an elevation of glucose levels in hemolymph through mobilization of glycogen stores from both the hepatopancreas and muscle (Santos and Keller 1993b; Santos et al. 2001; Sedlmeier 1985). It is well known that the physiological compensation to stressors is an energy-demanding process requiring the animal to mobilize energy substrates to metabolically deal with stress (Barton and Schreck 1987; Vijayan et al. 1996, 1997). In order to overcome environmental fluctuations, the provision of a sufficient and sustained energy supply through both aerobic and anaerobic metabolic pathways is essential (Ho et al. 2002). In response to increased energy demands for physiological compensation to acute stress, changes in energy substrates, such as hemolymph glucose, lactate and lipids have been observed (Chittó et al. 2009; Chung and Zmora 2008; Da Silva and Kucharski 1992; Santos and Nery 1987; Yildiz et al. 2005).
Redclaw crayfish Cherax quadricarinatus (Von Martens) is a native species to the tropical region of Queensland, northern Australia, classified as a eurythermal, mesohaline species (Meade et al. 2002). Although this species can survive at a winter temperature as low as 10 °C, a reduced growth of both juvenile and adult crayfish has been reported below 22 °C (Jones 1997; Meade et al. 2002). Concerning salinity, although C. quadricarinatus is often maintained at a salinity of 25 g/L at the end of the grow-out period in farms (Jones 1997), or even when adults are able to regulate hemolymphatic ions up to 15 g/L of salinity (Prymaczok et al. 2008), survival of C. quadricarinatus juveniles is significantly reduced after 70 days of exposure to 5 g/L of salinity (Meade et al. 2002). In fact, the optimum for weight gain, survival, and growth efficiency has been observed within a narrower range of temperatures and salinities (Austin 1995; Jones 1997; Karplus et al. 1998; Meade et al. 2002; Nyström 2002). Since C. quadricarinatus is an attractive species to the aquaculture industry, most of the previous studies have primarily focused on the culture conditions and environmental requirements to optimize the growth of reared animals. However, few reports have focused on both the compensatory response to stressful conditions and the involvement of CHH in such response.
The present study was aimed at establishing CHH involvement in several physiological responses of C. quadricarinatus juvenile crayfish exposed to long-term saline and thermal stress, such as mobilization of energy reserves and ionic regulation. In order to allow the potential use of this hormone for aquaculture, recombinant CHH known to give a high yield and reproducibility was cloned.
Materials and methods
Animals
Advanced juveniles of C. quadricarinatus (5.45 ± 0.15 g of body weight) were purchased from a commercial hatchery (Pinzas Rojas S. R. L., Tucumán, Argentina). During at least 2 weeks of acclimation to laboratory conditions, the animals were kept in glass aquaria containing 15 L of dechlorinated tap water (hardness = 80 mg/L as CaCO3 equivalents, filtered through activated charcoal and cationic resin), at a temperature of 27 ± 1 °C (optimum value for the species) and photoperiod 14:10 (L:D); pH was maintained at 7.5 ± 0.5 with the addition of 0.1 N NaOH or HCl when necessary. During this period, the animals were fed three times a week with a commercial formulated diet (Tetra Color®, 47 % crude protein) and fresh leaves of Elodea sp, ad libitum.
Effects of thermal and salinity stress on the levels of CHH gene expression
After the initial acclimation period, 60 intact C. quadricarinatus juveniles were isolated in 1.5 L glass containers filled with 1 L of freshwater or saline solution. Ten animals were assigned to each of the following experimental groups:
-
1)
Temperature = 27 °C, freshwater
-
2)
Temperature = 27 °C, salinity = 5 g/L
-
3)
Temperature = 27 °C, salinity = 10 g/L
-
4)
Temperature = 20 °C, freshwater
-
5)
Temperature = 20 °C, salinity = 5 g/L
-
6)
Temperature = 20 °C, salinity = 10 g/L
Saline solutions were achieved by adding artificial sea salts (Marine Mix, Germany) to freshwater at a gradual rate of 1 g/L per day. Temperature was changed from 27 to 20 °C (groups 4–6) at a rate of 0.5 °C per day, by placing the individual containers in a thermostat controlled incubator. Once the desired values of both temperature and salinity were attained in all groups (i.e., after 2 weeks), the animals were maintained at constant conditions for 30 days. Water temperature, salinity and pH were checked daily and maintained at a precision of ± 0.1 °C, ± 0.1 g/L and ± 0.01 pH units, respectively. During the entire experiment, the juveniles were fed three times a week (as mentioned for the initial acclimation period) and the water was replaced once a week.
At the end of the experiment, all animals were cold-anaesthetized and the eyestalks were quickly dissected and stored at −80 °C until RNA extraction. This RNA was used as substrate for semi-quantitative RT-PCR experiments specific for C. quadricarinatus CHH and β-actin, as stated out below.
Isolation of total RNA, RT-PCR and sequence analysis of eyestalk cDNA encoding CHH
Based on the sequence of C. quadricarinatus CHH cDNA reported by Shechter and colleagues (GenBank: DQ095778.1), we designed primers to specifically amplify C. quadricarinatus CHH cDNA by standard PCR procedures. After removal of the cuticle and non-neural tissues, four eyestalks from each experimental group were pooled. The tissue was homogenized in an all-glass homogenizer containing TRI REAGENT® (Molecular Research Center, Inc., USA) in order to run a semi-quantitative analysis. Total RNA was then extracted with TRI REAGENT® according to the manufacturer’s protocol. First-strand cDNA was synthesized from total RNA (2 μg) with 300 units of Moloney-murine-leukemia virus reverse transcriptase (Promega) for 1 h at 37 °C in a final volume of 20 μL, in the presence of a ribonuclease inhibitor (2 units, Promega) and an oligo-dT primer. PCR reactions were performed in 2.5 μL samples with 1 unit of a Taq DNA polymerase (Invitrogen), and 100 ng/μL of each primer in the appropriate reaction buffer containing 0.2 mM dNTPs and 1.5 mM MgCl2. CHH cDNA were amplified with the following gene-specific oligonucleotide primers; forward 5′-ATGAGCTCCAGGTGTTTGATCAGGCGTGT-3′ and reverse 5′-ATGGGCCCTAACATATTCGTCGACCACGTCT-3′. The PCR conditions involved the initial denaturing at 94 °C for 1 min, 30 or 35 cycles of denaturing at 94 °C for 45 s, annealing at 58 °C for 1 min and extension at 72 °C for 40 s, followed by a final extension at 72 °C for 10 min. PCR product was analyzed on 1.8 % agarose gel electrophoresis and visualized by SYBR® Safe DNA (invitrogen) staining under ultraviolet light.
β-actin, a constitutive gene that is not affected by the temperature or salinity changes, was used as housekeeping gene in the PCR experiments. RT-PCR experiments were replicated at least three times with reproducible results. For the sequence alignment of CHHs of different species, the NCBI GenBank database was used to search for homologous sequences of CHH cDNA sequence cloned. The alignments were done using Clustal W2 program.
Expression and purification of recombinant CHH
To generate the plasmid for bacterial expression of 6XHis-CHH, the CHH cDNA was isolated from a CHH encoding plasmid (generously provided by Prof. Sagi) and then subcloned into the pQE-30 vector (Qiagen). The vectors were sequenced by standard procedures.
An overnight culture of BL21(DE3)pLysS (Novagen) cells transformed with pGEX-CHH or pQE-CHH vector was diluted to a final OD600nm of 0.15 in LB medium containing ampicillin (100 μg/mL) and chloramphenicol (34 μg/mL). The culture was grown in agitation at 37 °C until an OD600 of 0.4 and then isopropyl-β-d-thiogalactoside (IPTG) was added to a final concentration of 1 mM for 4 h. After induction, bacterial cells were harvested by centrifugation at 4000g (4 °C) for 15 min, and, the pellet was resuspended in lysis buffer (50 mM NaH2PO4, 300 mM NaCl, 5 mM MgCl2, 10 mM Na2HPO4, 20 mM Imidazole, 10 % glycerol, 1 % NP-40, 1 mM β-mercaptoethanol, 1 mM PMSF, 5 % Protease Inhibitor Cocktail (Roche) at 4 °C, then 1 mL of lysozyme was added, and finally the cell lysates were incubated on ice for 30 min. Cell lysates were then sonicated and centrifuged at 10,000g (4 °C) for 30 min; the recombinant protein was purified by glutathione Sepharose 4B (Amersham) in the case of GST-CHH or to Ni–NTA resin (Qiagen) in the case of 6XHis-CHH. GST-CHH was eluted in buffer GST-E [PBS pH 8.0, 25 mM reduced glutathione, 10 % glycerol, 1 % NP-40, 1 mM β-mercaptoethanol, 1 mM PMSF, 5 % Protease Inhibitor Cocktail (Roche)]; and 6XHis-CHH was eluted in buffer HIS-E (10 mM Tris–HCl, pH 7.5, 100 mM NaCl, 250 mM imidazole, 10 % glycerol, 0.1 mM DTT, 1 mM PMSF) at room temperature. Fractions were dialyzed overnight against PBS and then analyzed by SDS-PAGE and Coomassie blue staining. The recombinant protein was purified under native conditions and therefore purification from inclusion bodies was not necessary. The recombinant (HIS-tagged) CHH concentration was determined by Bradford’s method (Bradford 1976) using the Bio-Rad Protein Assay kit.
Determination of biological activity of recombinant CHH in vivo
Five crayfish were assigned to each of the following experimental groups:
-
Control = animals injected with vehicle (PBS)
-
CHH50 = animals injected with recombinant CHH (herein rCHH) at a concentration of 50 pmol/crayfish
-
CHH100 = animals injected with recombinant CHH at a concentration of 100 pmol/crayfish
-
ESE = animals injected with two equivalents of crude eyestalk extract (positive control)
In all cases, the injected volume of recombinant CHH, ESE or vehicle was 50 μL, administered at the base of the third pair or pereiopods (pre-branchial sinus), by means of a 1 mL syringe provided with a 30 G needle. To obtain the eyestalk extract (ESE group) bilateral eyestalk ablation of C. quadricarinatus juveniles was performed 48 h prior to the start of the experiment. Animals in intermolt were anaesthetized on ice for 1 min, and then eyestalks were carefully cut and then pulled out at their basal articular membrane by fine tweezers, cauterizing the wound. Animals were then immediately returned to the tank and left to recover. Batches of 20 eyestalks were quickly deep frozen at −20 °C and stored until required for processing, i.e., each batch of 20 frozen eyestalks was homogenized in 500 μL of cold PBS and then centrifuged for 10 min at 14,000 rpm and 4 °C. The clear supernatant was taken for injection.
At 0.5, 1 and 2 h after injection, 50 μL of hemolymph were withdrawn from the pericardial sinus of each animal, by means of a sterile 1 mL syringe provided with a 27 G needle. An additional group of five animals were used to determine the initial glucose level (0 h). Glucose hemolymphatic content was quantified by means of the glucose oxidase method (commercial kit from Wiener Lab., Argentina).
Effect of recombinant CHH on energy mobilization and ionic regulation of stressed animals
Eight crayfish were randomly assigned to each of the following groups:
-
1)
Animals maintained in freshwater and injected with vehicle (PBS)
-
2)
Animals maintained at a salinity of 10 g/L and injected with vehicle
-
3)
Animals maintained in freshwater and injected with rCHH
-
4)
Animals maintained at a salinity of 10 g/L and injected with rCHH
All crayfish were maintained at 20 °C throughout the 30 day experiment, in similar glass containers and under the same acclimation regime to both temperature and salinity used previously. The animals were injected twice a week with 50 μL of rCHH, at a concentration of 50 pmol/crayfish, as stated above.
Mortality was recorded throughout the experiment. At the end of the experiment, a sample of hemolymph (100–200 μL) was taken from the base of the 4th or 5th pereiopod of each animal, by using a sterile 1 mL syringe provided with a 27 G needle, and frozen at −20 °C for subsequent analysis of ions and glucose. Immediately after hemolymph withdrawal, crayfish were anesthetized in an ice-cold bath, and both the hepatopancreas and abdominal muscle were quickly dissected, frozen, and stored at −80 °C for final analysis of glycogen.
Glycogen was extracted from tissues after precipitation with Na2SO4, following the method described by Van Handel (Van Handel 1965), and then hydrolyzed to glucose with HCl, followed by neutralization with Na2CO3 (Geary et al. 1981). Glycogen levels in hepatopancreas and muscle (as glucose equivalents), as well as glucose hemolymphatic levels, were then determined by the glucose oxidase method, (Wiener Lab., Argentina). Sodium and potassium hemolymphatic levels were measured by means of a flame photometer (Crudo Caamaño SRL, Argentina), after appropriate dilution of the samples.
Statistical analysis
A repeated measure one-way ANOVA was used to compared hemolymphatic glucose levels over time (Sokal and Rohlf 1981). Box’s M test and Mauchley’s test were used for checking homogeneity and sphericity of covariance, respectively. When pertinent, Tukey test was used for multiple comparisons of means. The Fisher exact test (Sokal and Rohlf 1981) was used to compare the survival rate among groups. The remaining variables measured were analyzed by a two-way ANOVA (taking type of injection and salinity as factors), followed by the post hoc Tukey test for multiple comparisons (Sokal and Rohlf 1981). Lilliefors and Levene tests were used for checking normality and homogeneity of variance, respectively. A 5 % significance level was always considered (Sokal and Rohlf 1981).
Results
Effects of thermal and salinity stress on CHH gene expression and cDNA encoding
Concerning the effect of both thermal and salinity stress on CHH expression, we found a approximately threefold up-regulation of CHH mRNA when animals were exposed for 30 days to either high salinities (Fig. 1a, lanes 2 and 3 vs. 1, and lane 6 vs. 4) or low temperatures, (Fig. 1a, lane 4 vs. 1). However, while at 27 °C, both assayed salinities produced a high CHH expression, at 20 °C CHH mRNA up-regulation only increased at the salinity of 10 g/L (Fig. 1a, lane 6 vs. 4), but not at 5 g/L (Fig. 1a, lane 5 vs. 4), suggesting that the changes in CHH levels due to high salinity strongly depend on the temperature.
a Levels of CHH mRNA expression in eyestalks of crayfish exposed to thermal and salinity stress. Upper panel relative expression indicated as ratio of OD CHH × 1000/OD β-actin (OD optic density measured by Image J). Data shown correspond to the average of three independent RT-PCRs made with four eyestalks taken from each treatment. Lower panel RT-PCR performed to assess CHH or β-actin expression in the eyestalks after a 30-day exposure to 0, 5 or 10 g/L salinity, at both 27 and 20 °C temperature; b RT-PCR to amplify the cDNA of CHH from muscle, eyestalk and hepatopancreas. −RT negative control corresponding to CHH specific RT-PCR using eyestalk RNA (retrotranscriptase was omitted in the RT-PCR). M DNA ladder, bp base pairs; c protein sequence alignments of CHHs from C. quadricarinatus and other crustacean species. Numbers on the right margin represent amino acids positions. Amino acids: “*”: identical. “:”: conserved substitutions. “.”: semi-conserved substitution. Cystine residues are boxed. Presumably, the isolated PCR band corresponds to a partial cDNA fragment which encodes a shorter protein lacking six amino acids in the C-terminal end
Despite the PCR products corresponding to CHH, cDNA migrates as a single band of ≈200 pb in agarose gels, which is the expected size for C. quadricarinatus CHH, the specificity of the primers used was further evaluated by two different approaches; first a RT-PCR was conducted using total RNA isolated from muscle and hepatopancreas, two tissues where CHH is not normally expressed. As expected for CHH specific primers, and in contrast to eyestalk, no PCR product was obtained when RNA isolated from muscle or hepatopancreas was used in the RT-PCR experiments (Fig. 1b). Primers specificity was also tested by sequencing the obtained PCR product. Sequence analysis revealed that the obtained PCR product corresponds to the previously reported partial CHH sequence (Shechter et al.; GenBank: DQ095778.1, data not shown). Multiple alignments of the amino acidic sequence of CHH from the C. quadricarinatus (DQ095778.1), C. destructor A (P83485), C. destructor B (P83486), Astacus leptodactylus (AAS45406.1), Procambarus clarkii (AAB33097.1) and Orconectes limosus (228307) are shown in Fig. 1c. The percentages of similarity in the amino acids sequence between CHH of C. quadricarinatus, Cherax destructor A, C. destructor B, Astacus leptodactylus, Procambarus clarkii and Orconectes limosus were 80,95, 86, 83 and 82 %, respectively.
Production of biologically active of recombinant CHH
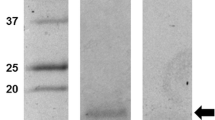
The purity of rCHH was determined by SDS-PAGE and coomassie brilliant blue staining. Since rCHH eluted as a single protein of an expected molecular weight of 8–9 kDa with no major contaminant proteins (Fig. 2, fraction #1), we used this fraction for further experiments; rCHH concentration in fraction #1 was estimated in 20 ng/μL.
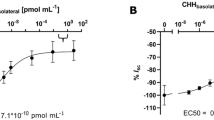
We found a significant (p < 0.05) increase of hemolymphatic glucose levels in rCHH-injected animals (averaging 15 ± 0.36 mg/100 mL), compared to the vehicle-injected group (6.67 ± 0.44 mg/100 mL), when 50 pmol of rCHH was administrated for 30 min (Fig. 3). Similar results were obtained when 100 pmol of rCHH was used suggesting that the lowest dose of rCHH used in this assay (50 pmol/crayfish) is enough to trigger the maximum hyperglycemic response to this recombinant hormone in vivo. Additionally, the injection of crude eyestalk extract led to a significant (p < 0.05) hyperglycemic response, stronger than rCHH, reaching a maximum of glucose of 37.89 ± 1.09 mg/100 mL after 2 h of injection (Fig. 3).
Biological activity of rCHH. Juveniles of C. quadricarinatus were injected with vehicle (control), 50 pmol of rCqCHH (CHH50), 100 pmol of rCqCHH (CHH100) or two eyestalk equivalent (ESE). N = 5 in all cases. Mean values ± standard error are indicated. * Significant differences (p < 0.05) between CHH injected and control groups, at every time. ** Significant differences (p < 0.05) between ESE and the remaining groups, at every time. + significant differences (p < 0.05) between different times after injection (2 vs. 0.5 and 1 h)
Effect of rCHH injection on energy mobilization and ionic regulation of stressed animals
After 30 days of exposure to the different assayed combinations of temperature and salinity, no significant differences (p > 0.05) in mortality (averaging 9.4 %) were observed among treatments. As shown in Fig. 4a, glycemia of rCHH-injected animals was higher as compared to controls, at any salinity assayed. Although not significant (p > 0.05), the increase of glucose levels driven by rCHH injection was ~80 % and 40 % in animals kept on freshwater and 10 g/L, respectively, with respect to animals injected with vehicle. On the other hand, a significant increase (p < 0.05) of glucose levels was seen upon saline treatment in relation to the freshwater group, regardless of the injection received (Fig. 4a). Concerning the hepatopancreas, glycogen levels found in rCHH-injected groups were lower than those of the control (vehicle) group, although the differences were significant (p < 0.05) only at 10 g/L of salinity (Fig. 4b). In both hepatopancreas and muscle, glycogen content of control crayfish maintained at 10 g/L was significant (p > 0.05) higher than those maintained in freshwater. In muscle, rCHH administration led to a significantly increase of glycogen levels (p < 0.05), but only in animals maintained in freshwater (Fig. 4c).
Levels of glucose and glycogen of C. quadricarinatus juveniles injected with rCHH. a hemolymphatic glucose levels, b and c glycogen levels in hepatopancreas and abdominal muscle, respectively. Mean values ± standard error, as well as the number of animals measured (N), are indicated. * Significant differences (p < 0.05) between treatments, for each salinity. + significant differences (p < 0.05) between salinities, for each treatment
A significant (p < 0.05) increase in the hemolymph concentration of both Na+ and K+ was observed at 10 g/l compared to freshwater, but only for control crayfish. Animals injected with rCHH always showed an ion concentration similar to the control crayfish maintained in freshwater. Hence, at 10 g/L the hemolymph concentration of both Na+ and K+ measured in rCHH-injected animals was significantly (p < 0.05) lower than that of controls (Fig. 5a, b).
Effect of rCHH injection on ionic regulation of stressed crayfish. a, b Hemolymphatic sodium and potassium concentrations, respectively. Mean values ± standard error, as well as the number of animals measured (N), are indicated. * Significant differences (p < 0.05) between treatments, for each salinity. + significant differences (p < 0.05) between salinities, for each treatment
Discussion
The CHH/MIH/GIH family members are multifunctional peptides involved in the regulation of key biological processes of crustaceans, often displaying overlapping activities. Although several attempts have been done to assign a specific physiological function to each peptide, many aspects of their physiological functions still remain unknown (Böcking et al. 2002). In the current study, the involvement of CHH in the chronic stress caused by both temperature and salinity was studied in a representative species of decapods crustacean, the crayfish C. quadricarinatus.
To date, several gene sequences of crayfish CHH have been reported. The CHH cDNA fragment (200pb) from C. quadricarinatus isolated by Shechter and colleagues (GenBank: DQ095778.1), has been used in this study to estimate CHH mRNA levels by PCR. A discrete PCR band, corresponding to C. quadricarinatus mRNA, was obtained when total RNA isolated from eyestalk was used in RT-PCR experiments. Presumably, the isolated PCR band corresponds to a partial cDNA fragment which encodes a shorter protein lacking six amino acids in the C-terminal end; this region has been hypothesized to be important in species specificity but not necessarily crucial to hyperglycemic function (Lacombe et al. 1999; Mettulio et al. 2004). Multiple alignments of the protein encoded by the CHH cDNA of C. quadricarinatus showed high degree of amino acid similarity with proteins from others crustacean species. Moreover, rCHH contains critical structural elements such as the six cysteine residues that are characteristic of CHH family proteins (Keller 1992).
Although CHH secretion from the eyestalk in response to several stressors is well documented (Chang et al. 1998; Chung and Webster 2006; Kuo and Yang 1999; Webster 1996), the effect of stress on CHH synthesis at the eyestalk remains unanswered. Our results showed that CHH mRNA levels were increased by both temperature and salinity. In accordance with our results, a recent study made on Litopenaeus vannamei found that either hypo- or hyper-salinity can increase the CHH mRNA levels in eyestalks, although they returned to control group values after 48 h of salinity challenge (Liu et al. 2014).
Our current results, together with those from previous published studies (Prymaczok et al. 2012), lead us to conclude that juveniles of C. quadricarinatus are strongly affected by a temperature as low as 20 °C, especially when combined with a salinity of 10 g/L. Furthermore, the CHH expression profile described in this study suggests that CHH plays a role in the compensatory response to this kind of stress. Moreover, the CHH increment seen after 30 days of exposure to stressful conditions, suggests a long-term action of this hormone under such conditions.
Several reports have been shown that CHH itself is able to regulate both ion and water balance (Liu et al. 2014; Turner et al. 2013; Serrano et al. 2003; Spanings-Pierrot et al. 2000). However, a weak effect of salinity on CHH gene expression at the eyestalks has been reported (Lago-Lestón et al. 2007, in shrimps). It has been suggested that the CHH isoform expressed at the pericardial organs could be specifically aimed at regulating both ionic and osmotic status, as well as the acid–base balance (Jeon et al. 2012). On the other hand, a significant increase of CHH levels in hemolymph by effect of thermal stress has been reported by several authors (Chang et al. 1998; Kuo and Yang 1999; Reddy and Sainath 2009; Lago-Lestón et al. 2007).
Through rCHH administration, we confirmed that CHH is involved in the stress response of crayfish and found that the hormone is able to improve the performance of stressed crayfish. For this purpose, we produced the recombinant hormone using CHH cDNA from C. quadricarinatus eyestalks, the main organ of CHH synthesis. The hyperglycemic activity of recombinant hormone was evaluated by performing a dose response test in eyestalk ablated animals. Since the injection of any rCHH dose produced a similar and significant glucose increment, we concluded that the lowest dose used was sufficient to trigger the highest response. In fact, the hemolymphatic rCHH concentration just after injecting the lowest dose, expected to be in the micromolar order, was higher than the hemolymphatic CHH levels reported for several crustacean species under stress conditions (Chang et al. 1998; Stentiford et al. 2001; Webster 1996). The hyperglycemic response obtained by injecting 50 pmol of rCHH was similar to that obtained by Katayama et al. (2002) in Marsupenaeus japonicus and by Gu et al. (2000) in Metapenaeus ensis, using an effective dose of hormones two folds higher.
An important role in stabilizing CHH conformation is probably played by the six cysteines present in all the neuropeptides of the CHH/MIH/GIH family. These cysteine residues establish three disulfide bridges present in all crustacean species studied up to date (Katayama et al. 2002; Kawakami et al. 2000; Kegel et al. 1989). For instance, the substitution of a cysteine residue with a serine results in the total loss of hyperglycemic activity of the recombinant CHH, due to the formation of a single disulfide bridge (Mettulio et al. 2004). As previously mentioned, the rCHH produced by us contains the six cysteine residues, aiding to the proper folding of the recombinant hormone.
However, the hyperglycemic response triggered by rCHH was lower as compared with the ESE response. The cause of this difference could be attributed to the absence of posttranslational modifications in the recombinant protein and/or presence of other factors in the eyestalk extract that might contribute to the hyperglycemic activity. The lack of in vivo amidation at the C-terminus of the rCHH could be also involved. Although the amidation is essential in providing full biological functionality to CHH, the non-amidated peptide retained a lower, but still significant, potency (Katayama et al. 2002; Mosco et al. 2008; Nagai et al. 2009).
The increase of hemolymphatic glucose levels has been mentioned to be a secondary response and a useful tool to assess stress in crustaceans (Gu et al. 2000). As established in several works, the hyperglycemia triggered by CHH is the main response for numerous decapods species to a variety of physiological challenges, such as Callinectes sapidus (Santos and Colares 1990), Neohelice granulata (Schmitt and Santos 1993), Carcinus maenas, O. limosus (Santos and Keller 1993c), Gecarcoidea natalis and Cardisoma hirtipes (Adamczewska and Morris 1996). Salinity has been previously reported as a factor that elevates glycemia in several crustacean species (Chung and Webster 2006; Da Silva and Kucharski 1992; Prymaczok et al. 2008, 2012; Santos and Nery 1987; Yildiz et al. 2005).
In the current study, hemolymph glucose levels increased as response to saline water, in both control and rCHH treated crayfish, with respect to the same treatments maintained in freshwater. Accordingly, at 10 g/L salinity, the injected CHH produced a significant decrease in hepatopancreatic glycogen, compared to control, which was not observed in freshwater. Concerning muscle glycogen, CHH-injected crayfish showed a significantly higher level than control when they were maintained in freshwater, but no differences were observed in saline-acclimated animals. This later result suggests a possible increased utilization of muscle glycogen in crayfish acclimated to high salinity, by effect of CHH or any other hormone. Taken together, the results concerning glycogen mobilization basically exhibit about a higher responsiveness to CHH in the crayfish previously acclimated to high salinity; this apparent sensitization could be explaining by an augmented expression of CHH receptors in some tissues (i.e., hepatopancreas and muscle).
Crayfish species are commonly exposed to both hypo-osmotic and hypo-ionic environments, therefore being subjected to a constant ion loss and water gain by diffusion across the body surface. Without compensation, osmotic and ionic imbalances would strongly affect metabolic integrity, leading to death. As reported previously, juveniles of C. quadricarinatus exert a hyper-ionic regulation at salinities below 15 g/L (Prymaczok et al. 2008). However, although juveniles were able to survive at salinity of 10 g/L, their ionic regulatory capacity was impaired at such salinity, particularly in combination with a sub-optimal temperature such as 20 °C (Prymaczok et al. 2012; current study). Accordingly, CHH mRNA and protein levels were increased, both at low temperature and increased salinity, indicating an endogenous triggering of CHH under these stressful conditions. Moreover, rCHH injected juveniles were able to maintain, when submitted to different saline media, a better constancy than control animals in both Na+ and K+ hemolymphatic levels, suggesting a possible role of CHH in osmo- and ionic regulation, likewise suggested by previous studies (Chung and Webster 2006; Webster et al. 2012). A recent study showed that when Discoplax celeste is acclimated to increasingly saline water regimes, the circulating levels of CHH in the hemolymph increased (Turner et al. 2013). CHH has been also described as a hormone needed to facilitate both water and ion uptake during molting (Chung et al. 1999; Webster et al. 2000).
In summary, a clear expression of endogenous CHH under conditions of both temperature and salinity stress was demonstrated. The injection of recombinant CHH stressed crayfish produced an increased mobilization of glycogen stores, being associated as well to an apparent better ionic regulation. This study contributes to a better understanding of the mechanisms involved in the response to changes in environmental variables and, eventually, to the development of novel technologies intended to improve growing of crustaceans under unfavorable conditions.
References
Adamczewska AM, Morris S (1996) The respiratory gas transport, acid-base state, ion and metabolite status of the Christmas Island Blue Crab, Cardisoma hirtipes (Dana) assessed in situ with respect to immersion. Physiol Zool 69:67–92
Austin CM (1995) Effect of temperature and salinity on the survival and growth of juvenile redclaw (Cherax quadricarinatus). Freshwater Crayfish 10:419–426
Barton BA, Schreck CB (1987) Metabolic cost of acute physical stress in juvenile steelhead. Trans Am Fish Soc 116:257–263
Böcking D, Dircksen H, Keller R (2002) The crustacean neuropeptides of the CHH/MIH/GIH family: structures and biological activities. In: Wiese K (ed) The crustacean nervous system. Springer, Berlin, pp 84–97
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Chang ES, Keller R, Chang SA (1998) Quantification of crustacean hyperglycemic hormone by ELISA in hemolymph of the lobster, Homarus americanus, following various stresses. Gen Comp Endocrinol 111:359–366
Chang ES, Chang SA, Keller R, Reddy PS, Snyder MJ, Spees JL (1999) Quantification of stress in lobsters: crustacean hyperglycemic hormone, stress proteins, and gene expression. Am Zool 39:487–495
Charmantier-Daures M, Charmantier G, Janssen KP, Aiken DE, van Herp F (1994) Involvement of eyestalk factors in the neuroendocrine control of osmoregulation in adult American lobster Homarus americanus. Gen Comp Endocrinol 94:281–293
Chittó ALF, Schein V, Etges R, Kucharski LC, Silva RSMD (2009) Effects of photoperiod on gluconeogenic activity and total lipid concentration in organs of crabs, Neohelice granulata, challenged by salinity changes. Invertebr Biol 128:261–268
Chung JS, Webster SG (2006) Binding sites of crustacean hyperglycemic hormone and its second messengers on gills and hindgut of the green shore crab, Carcinus maenas: a possible osmoregulatory role. Gen Comp Endocrinol 147:206–213
Chung JS, Zmora N (2008) Functional studies of crustacean hyperglycemic hormones (CHHs) of the blue crab, Callinectes sapidus—the expression and release of CHH in eyestalk and pericardial organ in response to environmental stress. FEBS J 275:693–704
Chung JS, Dircksen H, Webster SG (1999) A remarkable, precisely timed release of hyperglycemic hormone from endocrine cells in the gut is associated with ecdysis in the crab Carcinus maenas. Proc Natl Acad Sci 96:13103–13107
Chung JS, Zmora N, Katayama H, Tsutsui N (2010) Crustacean hyperglycemic hormone (CHH) neuropeptides family: functions, titer, and binding to target tissues. Gen Comp Endocrinol 166(2010):447–454
Da Silva RSM, Kucharski LC (1992) Effect of hyposmotic stress on the carbohydrate metabolism of crabs maintained on high protein or carbohydrate-rich diet. Comp Biochem Physiol 101A:631–634
de Kleijn DP, Van Herp F (1998) Involvement of the hyperglycemic neurohormone family in the control of reproduction in decapod crustaceans. Invert Reprod Dev 33:263–272
de Kleijn DP, Janssen KP, Waddy SL, Hegeman R, Lai WY, Martens GJ, Van Herp F (1998) Expression of the crustacean hyperglycaemic hormones and the gonad-inhibiting hormone during the reproductive cycle of the female American lobster Homarus americanus. J Endocrinol 156:291–298
Dircksen H (2013) Crustacean bioactive peptides. In: Kastin A (ed) Handbook of biologically active peptides, 2nd edn. Academic Press, Baton Rouge, pp 209–221
Durand F, Devillers N, Lallier FH, Regnault M (2000) Nitrogen excretion and change in blood components during emersion of the subtidal spider crab Maia Squinado (L.). Comp Biochem Physiol 127A:259–271
Fanjul-Moles ML (2006) Biochemical and functional aspects of crustacean hyperglycemic hormone in decapod crustaceans: review and update. Comp Biochem Physiol 142C:390–400
Geary N, Langhans W, Scharrer E (1981) Metabolic concomitants of glucagon-induced suppression of feeding in the rat. Am J Physiol 241:R330–R335
Gu PL, Yu KL, Chan SM (2000) Molecular characterization of an additional shrimp hyperglycemic hormone: cDNA cloning, gene organization, expression and biological assay of recombinant proteins. FEBS Lett 472:122–128
Ho PY, Kuo CH, Kuo CM, Chen YN (2002) Physiological responses of spotted grouper juveniles, Epinephelus coioides, under acute thermal shock. J Fisheries Soc Taiwan 29:275–285
Jeon JM, Kim BK, Lee JH, Kim HJ, Kang CK, Mykles DL, Kim HW (2012) Two type I crustacean hyperglycemic hormone (CHH) genes in Morotoge shrimp (Pandalopsis japonica): cloning and expression of eyestalk and pericardial organ isoforms produced by alternative splicing and a novel type I CHH with predicted structure shared with type II CHH peptides. Comp Biochem Physiol 162B:88–89
Jones CM (1997) The biology and aquaculture potential of the tropical freshwater crayfish Cherax quadricarinatus. Dept of Primary Ind, Brisbane
Karplus I, Zoran M, Milstein A, Harpaz S, Eran Y, Joseph D, Sagi A (1998) Culture of the Australian red-claw crayfish (Cherax quadricarinatus) in Israel III. Survival in earthen ponds under ambient winter temperatures. Aquaculture 166:259–267
Katayama H, Ohira T, Aida K, Nagasawa H (2002) Significance of a carboxyl-terminal amide moiety in the folding and biological activity of crustacean hyperglycemic hormone. Peptides 23:1537–1546
Kawakami T, Toda C, Akaji K, Nishimura T, Nakatsuji T, Ueno K, Sonobe M, Sonobe H, Aimoto S (2000) Synthesis of a molt-inhibiting hormone of the American crayfish, Procambarus clarkii, and determination of the location of its disulfide linkages. J Biochem 128:455–461
Kegel G, Reichwein B, Weese S, Gaus G, Peter-Katalinic J, Keller R (1989) Amino acid sequence of the crustacean hyperglycemic hormone (CHH) from the shore crab, Carcinus maenas. FEBS Lett 255:10–14
Keller R (1992) Crustacean neuropeptides: structures, functions and comparative aspects. Experientia 48:439–448
Keller R, Sedlmeier D (1988) A metabolic hormone in crustaceans: The hyperglycemic neuropeptide. In: Epple A, Scanes CG, Stetson MH (eds) Progress in comparative endocrinology. Wiley-Liss, New York, pp 265–271
Kung PC, Wu SH, Nagaraju PC, Tsai WS, Lee CY (2013) Crustacean hyperglycemic hormone precursor transcripts in the hemocytes of the crayfish Procambarus clarkii: novel sequence characteristics relating to gene splicing pattern and transcript stability. Gen Comp Endocrinol 186:80–84
Kuo CM, Yang YH (1999) Hyperglycemic responses to cold shock in the freshwater giant prawn Macrobrachium rosenbergii. J Comp Physiol 169B:49–54
Lacombe C, Greve P, Martin G (1999) Overview on the sub-grouping of the crustacean hyperglycemic hormone family. Neuropeptides 33:71–80
Lago-Lestón A, Ponce E, Muñoz ME (2007) Cloning and expression of hyperglycemic (CHH) and molt-inhibiting (MIH) hormones mRNAs from the eyestalk of shrimps of Litopenaeus vannamei grown in different temperature and salinity conditions. Aquaculture 270:343–357
Liu M, Pan L, Li L, Zheng D (2014) Molecular cloning, characterization and recombinant expression of crustacean hyperglycemic hormone in white shrimp Litopenaeus vannamei. Peptides 53:115–124
Lorenzon S, Giulianini PG, Ferrero EA (1997) Lipopolysaccharide-induced hyperglycemia is mediated by CHH release in crustaceans. Gen Comp Endocrinol 108:395–405
Lorenzon S, Pasqual P, Ferrero EA (2002) Different bacterial lipopolysaccharides as toxicants and stressors in the shrimp Palaemon elegans. Fish Shellfish Immunol 13:27–45
Meade ME, Doeller JE, Kraus DW, Watts SA (2002) Effects of temperature and salinity on weight gain, oxygen consumption rate, and growth efficiency in juvenile red-claw crayfish Cherax quadricarinatus. J World Aq Soc 33:188–198
Mettulio R, Giulianini PG, Ferrero EA, Lorenzon S, Edomi P (2004) Functional analysis of crustacean hyperglycemic hormone by in vivo assay with wild-type and mutant recombinant proteins. Regul Pept 119:189–197
Mosco A, Edomi P, Guarnaccia C, Lorenzon S, Pongor S, Ferrero EA, Giulianini PG (2008) Functional aspects of CHH C-terminal amidation in crayfish species. Regul Pept 147:88–95
Nagai C, Asazuma H, Nagata S, Ohira T, Nagasawa H (2009) A convenient method for preparation of biologically active recombinant CHH of the kuruma prawn, Marsupenaeus japonicus, using the bacterial expression system. Peptides 30:507–517
Nyström P (2002) Ecology. In: Holdrich DM (ed) Biology of freshwater crayfish. Blackwell Science, New York, pp 192–235
Prymaczok NC, Medesani DA, Rodríguez EM (2008) Levels of ions and organic metabolites in the adult freshwater crayfish, Cherax quadricarinatus, exposed to different salinities. Mar Freshwat Behav Physiol 48:121–130
Prymaczok NC, Chaulet A, Medesani DA, Rodríguez EM (2012) Survival, growth, and physiological responses of advanced juveniles freshwater crayfish (Cherax quadricarinatus), reared at low temperature and high salinities. Aquaculture 334–337:176–181
Reddy PS, Sainath SB (2009) Hyperglycemic hormone in freshwater prawn Macrobrachium rosenbergii: purification from eyestalk nervous tissue and quantification by ELISA in hemolymph following various stresses. Aquaculture 286:290–295
Santos EA, Colares EP (1990) Blood glucose changes in the blue crab Callinectes sapidus Rathbun on transfer from sea water to air. Braz J Med Biol Res 23:333–335
Santos EA, Keller R (1993a) Crustacean hyperglycemic hormone (CHH) and the regulation of carbohydrate metabolism: current perspective. Comp Biochem Physiol 106A:405–411
Santos EA, Keller R (1993b) Regulation of circulating levels of the crustacean hyperglycemic hormone-evidence of a dual feedback control system. J Comp Physiol 163A:374–379
Santos EA, Keller R (1993c) Effect of exposure to atmospheric air on blood glucose and lactate concentrations in two crustacean species: a role of the crustacean hyperglycemic hormone (CHH). Comp Biochem Physiol 106:343–347
Santos EA, Nery LEM (1987) Blood glucose regulation in an estuarine crab, Chasmagnathus granulata (Dana 1851), exposed to different salinities. Comp Biochem Physiol 87A:1033–1035
Santos EA, Nery LE, Keller R, Goncalves AA (1997) Evidence for the involvement of the crustacean hyperglycemic hormone in the regulation of lipid metabolism. Physiol Zool 70:415–420
Santos EA, Keller R, Rodriguez E, Lopez L (2001) Effects of serotonin and fluoxetine on blood glucose regulation in two decapod species. Braz J Med Biol Res 34:75–80
Schmitt ASC, Santos EA (1993) Lipid and carbohydrate metabolism of the intertidal crab Chasmagnathus granulata Dana 1851 (Crustacea: Decapoda) during emersion. Comp Biochem Physiol 106A:329–336
Sedlmeier D (1985) Mode of action of the crustacean hyperglycemic hormone. Am Zool 25:223–232
Serrano L, Blanvillain G, Soyez D, Charmantier G, Grousset E, Aujoulat F, Spanings-Pierrot C (2003) Putative involvement of crustacean hyperglycemic hormone isoforms in the neuroendocrine mediation of osmoregulation in the crayfish Astacus leptodactylus. J Exp Biol 206:979–988
Sokal RR, Rohlf FJ (1981) Biometry, 2nd edn. Freeman, New York
Spanings-Pierrot C, Soyez D, Van Herp F, Gompel M, Skaret G, Grousset E, Charmantier G (2000) Involvement of crustacean hyperglycemic hormone in the control of gill ion transport in the crab Pachygrapsus marmoratus. Gen Comp Endocrinol 119:340–350
Stentiford GD, Chang ES, Chang SA, Neil DM (2001) Carbohydrate dynamics and the crustacean hyperglycemic hormone (CHH): effects of parasitic infection in Norway lobsters (Nephrops norvegicus). Gen Comp Endocrinol 121:13–22
Turner LM, Webster SG, Morris S (2013) Roles of crustacean hyperglycaemic hormone in ionic and metabolic homeostasis in the Christmas Island blue crab, Discoplax celeste. J Exp Biol 216:1191–1201
Udomkit A, Treerattrakool S, Panyim S (2004) Crustacean hyperglycemic hormones of Penaeus monodon: cloning, production of active recombinant hormones and their expression in various shrimp tissues. J Exp Mar Biol Ecol 298:79–91
Van Handel E (1965) Estimation of glycogen in small amount of tissue. Anal Biochem 11:256–265
Vijayan M, Mommsen T, Eacute GI, Met H, Moon T (1996) Metabolic effects of cortisol treatment in a marine teleost, the sea raven. J Exp Biol 199:1509–1514
Vijayan MM, Pereira C, Graul EG, Lwama GK (1997) Metabolic responses to confinement stress in tilapia: the role of cortisol. Comp Biochem Physiol 116C:89–95
Webster S (1996) Measurement of crustacean hyperglycaemic hormone levels in the edible crab Cancer pagurus during emersion stress. J Exp Biol 199:1579–1585
Webster SG, Dircksen H, Chung JS (2000) Endocrine cells in the gut of the shore crab Carcinus maenas immunoreactive to crustacean hyperglycaemic hormone and its precursor-related peptide. Cell Tissue Res 300:193–205
Webster SG, Keller R, Dircksen H (2012) The CHH-superfamily of multifunctional peptide hormones controlling crustacean metabolism, osmoregulation, moulting, and reproduction. Gen Comp Endocrinol 175:217–233
Yildiz HY, Köksal G, Benli AC (2005) Physiological responses of the crayfish, Astacus leptodactylus, to saline water. Crustaceana 77:1271–1276
Acknowledgments
This work was supported by grants from both the University of Buenos Aires UBACYT (2012–2015 program, code 20020110100044) and CONICET (PIP 2010–2012, code 100884). The authors wish to thank Dr. Prof. Amir Sagi for his generous gift of the CHH cDNA, Dr. Juan Gerez for his valuable help with the analysis of CHH expression, and Amir Dyzenchauz for revising the English style.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by H. V. Carey.
Rights and permissions
About this article
Cite this article
Prymaczok, N.C., Pasqualino, V.M., Viau, V.E. et al. Involvement of the crustacean hyperglycemic hormone (CHH) in the physiological compensation of the freshwater crayfish Cherax quadricarinatus to low temperature and high salinity stress. J Comp Physiol B 186, 181–191 (2016). https://doi.org/10.1007/s00360-015-0954-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00360-015-0954-0