Abstract
Many of the far-reaching impacts of climate change on ecosystem function will be due to alterations in species interactions. However, our understanding of the effects of temperature on the dynamics of interactions between species is largely inadequate. Inducible defences persist in prey populations because defensive traits increase survival in the presence of predators but are costly when they are absent. Large-scale changes in the thermal climate are likely to alter the costs or benefits of these defences for ectotherms, whose physiological processes are driven by environmental temperature. A shift in costs of defensive traits would affect not only predator–prey interactions, but also the strength of selection for inducible defences in natural populations. We investigate the effect of temperature on the costs of behavioural defences in larvae of the marine toad, Rhinella marinus. Larvae were reared in the presence or absence of predator cues at both 25 and 30 °C. When exposed to predation cues, larvae reduced activity and spent less time feeding. Exposure to predation cues also reduced metabolic rate, presumably as a by-product of reducing activity levels. Larvae exposed to predation cues also grew more slowly, were smaller at metamorphosis and were poorer jumpers after metamorphosis—three traits associated with fitness in post-metamorphic anurans. We found that the costs of behavioural defences, in terms of larval growth, post-metamorphic size and jumping performance, were exacerbated at cooler temperatures. The thermal sensitivity of costs associated with defensive traits may explain geographic variation in plasticity of defensive traits in other species and suggests that changes in environmental temperature associated with climate change may affect predator–prey interactions in subtle ways not previously considered.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Global increases in temperature will produce substantial changes in the abundance and distribution of organisms (Graham and Grimm 1990; Hughes 2000; McCarty 2001; McLaughlin et al. 2002). Although our ability to predict the impacts of both changes in abundance and distribution has dramatically improved with recent advances in modelling, we still have an inadequate understanding of the likely impacts of temperature on biological interactions (Urban 2015). In fact, it is probable that many of the far-reaching impacts of climate change on ecosystem function will be due to such alterations in species interactions (Urban 2015). For example, climate-induced changes in predator populations are likely to carry over to their prey because of the close link between their ecologies. This means that prey species may be affected both directly via changing temperatures as well as indirectly via modifications to predator populations. Understanding the influences of temperature on predator–prey dynamics is critical to improving our ability to predict future impacts of climate change on ecosystem function.
Many species can modify their behaviour, morphology or life history to increase their chances of survival when living alongside predators: by reducing detection by, or encounters with, predators (Juliano and Reminger 1992; Sih 1992; Turner 1996; van Uitregt et al. 2011; Werner and Anholt 1993), or by increasing their capacity for escape (Havel and Dodson 1984; Nilsson et al. 1995; Wilson et al. 2005). However, defensive traits typically bear substantial ecological and/or physiological costs to the individual, making them too expensive to maintain when predators are absent. The evolutionary maintenance of inducible defences is, therefore, governed by a functional trade-off between the benefits of defensive and non-defensive phenotypes against their costs in each different environment (Callahan et al. 2008; DeWitt 1998; DeWitt and Sheiner 2004; Hammill et al. 2008). For example, prey species that reduce activity levels to avoid detection by predators (Juliano and Reminger 1992; Lima 1998; Skelly and Werner 1990; Stoks et al. 2005; van Uitregt et al. 2011) constrain their ability to forage and accrue energy for growth, development, and reproduction. This simple cost/benefit trade-off drives selection for alternate phenotypic optima across predator-present and predator-absent environments. Changes in the thermal environment may intensify the costs of defensive traits, particularly for ectothermic prey species, whose metabolic requirements vary with temperature. For example, behavioural defences that limit foraging time (e.g., hiding) are likely to come at a greater cost for ectotherms in warmer conditions because of increased basal metabolic requirements. In this scenario, individuals have a greater need to forage, and any defensive behaviours that limit their ability to do so are expected to slow growth and development.
Anurans typically exhibit defensive traits only during larval development, making the effects of thermal and predator plasticity particularly important (McCollum and Van Buskirk 1996; Van Buskirk 2000). At warm temperatures, tadpoles have high metabolic demands (Niehaus et al. 2011) and must feed more frequently. This means that behavioural traits that impede feeding (i.e., hiding from predators) should be costly to growth and development, and could affect important life history traits such as size and timing of metamorphosis (Fraker 2008; McCollum and Van Buskirk 1996; Van Buskirk 2000). However, both growth and development are generally accelerated at warmer temperatures, shortening the larval period and exposure time to predation risk. The net accrual of costs at metamorphosis then should be reduced at warmer temperatures. van Uitregt et al. (2011) demonstrate this phenomenon in larvae of the mosquito, Aedes notoscriptus, where larvae metamorphosed earlier and at smaller sizes when exposed to predator cues with the effects being greater at cooler temperatures.
Tadpoles of marine toads exhibit behavioural aversion, increase refuge use and reduce activity in the presence of chemical predation cues, presumably to limit their detection by, or encounters with, predators (Hagman and Shine 2008, 2009b, c). When exposed to chemical predation cues as larvae, R. marinus metamorphose at a smaller size (Hagman and Shine 2008, 2009a, c), which is generally construed as a cost of the behavioural response due to reduced feeding. Based on studies of other anurans, smaller body sizes at metamorphosis and slower development rates are costly because they delay the time until first reproduction, increase desiccation risk, and decrease locomotor performance, fecundity and even survival (Capellan and Nicieza 2007; Chelgren et al. 2006; Child et al. 2009; Ficetola and de Bernardi 2006). The present study investigates the effect of temperature on the induction and costs of behavioural defences in the marine toad, Rhinella marinus. We predict that temperature could affect behavioural defences in R. marinus and their costs in two ways: (1) Warmer temperatures may exacerbate the costs of reduced activity by increasing the basal metabolic requirements and their need to feed or (2) Warmer temperatures may ameliorate the costs of reduced activity because accelerated development reduces the time exposed to predation and accrual of costs of defensive behaviours.
Methods
Rhinella marinus embryos from at least ten different clutches were collected from several ephemeral pools near Cairns in Northern Queensland, Australia. Embryos were estimated to be approximately 2 days post fertilisation at the time of collection, based on personal observation of embryonic development of spawn collected in Brisbane, Queensland. Embryos were immediately transported to the laboratory at The University of Queensland in Brisbane and left in the laboratory to settle over night.
Larvae from all clutches were mixed and randomly assigned to different treatments. Larvae were reared through metamorphosis in either control or treatment (exposure to predation cues) conditions under 25 or 30 °C thermal regimes. One hundred and sixty larvae were randomly allocated among the four groups of temperature and treatment combinations. Each larva was reared in an individual plastic container (12 × 17 × 7 cm deep) set within temperature-controlled water baths (73 × 51 × 16 cm deep) with 10 replicate containers in each water bath and eight water baths in each temperature regime. Each replicate container was filled with approximately 700 mL of dechlorinated aged tap water with a 1 cm gravel base. Water baths were placed on shelves within a single room with a water bath of each temperature on every shelf. Water temperatures were maintained at 25.0 ± 0.5 and 30.0 ± 0.5 °C using thermostatically control aquarium heaters (AquarWorld) with aeration provided to promote circulation and uniformity of water temperature across all replicates in each water bath. Temperature in each water bath was monitored using iButton data loggers (DS1922L3F50, Maxim, Sunnyvale, CA, USA).
Predation cues were produced in a solution by macerating surplus R. marinus larvae (Gosner stage 25; Gosner 1960) in water and adding it to the containers of the predator treatment group. Tadpoles of a cumulative mass of 4 g were macerated using scissors and a mortar and pestle, in 150 mL of water taken from their holding tank. The solution was then filtered through 5 cm of compact cotton wool to remove particulates. One millilitre of this solution was added to each replicate container of the treatment tadpoles each day throughout their development, with fresh cues prepared daily. Control individuals received 1 mL of water taken from the holding tank of the surplus tadpoles each day to control for any effect that olfactory cues from living conspecifics might have. Tadpoles were fed boiled spinach daily with water changes occurring weekly.
Activity and feeding behaviour of each larva was quantified by census method, where the observer took an instantaneous assessment of the behavioural status of each individual every 20 min for 4 h, on day 3, 4, 5, 10 and 12. Larval behaviour was classed as either (1) inactive: motionless on base; (2) active: swimming or foraging around rocks with tail beating; or (3) feeding: with obvious movement of mouthparts on the boiled spinach provided as food, usually with tail beating. In our analysis larvae were considered active when feeding, as this movement would also make the larvae conspicuous to predators. Data from each day were cumulative so that each individual had a measured proportion of time in each activity class throughout larval development.
The resting metabolic rate of all larvae was measured on days 8 and 9 of the experiment with equal numbers of each temperature and treatment combination measured across days. Larvae were sealed in individual 5 mL glass vials with an integrated oxygen sensor spot (W-In-SP-PSt3-NAU-D5-YOP, Presens, Regensburg, Germany) attached to the bottom surface inside the vial. Vials were filled with water from the container that each larvae was reared and then placed on a multi-channel oxygen meter (SDR SensorDish® Reader, PResens, Resenburg, Germany) that measured the oxygen concentration (% air-saturation) at 2 min intervals for 1.5 h using optical fluorescence-based oxygen respirometry (see Köster et al. (2008) for details). Respirometry measurements were performed in a dark temperature-controlled cabinet to ensure animals settled quickly and were under resting conditions. Temperature was maintained at 25.0 ± 0.5 °C for 25 °C treatment group and 30.0 ± 0.5 °C for the 30 °C treatment group. Following respirometry, larvae were blotted dry on damp paper towel and mass was recorded using an analytical balance. Larvae were then returned to their respective containers to continue experimental treatment through to metamorphosis.
The rate of oxygen consumption (\({\dot{\text{V}}\text{o}}_{2}\), mL O2 h−1) of larvae as a proxy for metabolic rate was calculated by fitting a linear regression to the data obtained from the oxygen meter (% air-saturation on time), and using the equation:
where m t = slope derived from the trial with the larva (% air-saturation h−1), m c = mean slope derived from the controls (% air- saturation h−1), (note that the difference between m t and m c is divided by 100 to convert the percentage of oxygen in the media to a fraction). V v = volume of respirometry vial (L), 0.025 L. V t = volume of larva, larva mass (kg)/density of muscle (1.06 kg L−1; Alexander 1982). β o 2 = O2 capacitance of media (mL L−1), 5.80 mL L−1 for air-saturated freshwater at 25 °C (Riley and Chester 1971), 5.29 mL L−1 for air-saturated freshwater at 30 °C (Riley and Chester 1971).
When the forelimbs of a larva emerged (Gosner stage 40) the majority of water was drained from their container and a perforated lid affixed to prevent escape. Larvae were then returned to the water bath to complete metamorphosis at their rearing temperature. Upon full resorption of the tail (Gosner stage 42) the container was removed from the water bath and placed in a room at 25 °C and left overnight. The following day, jumping performance and mass of the metamorph was measured at 25 °C in a controlled temperature room. Jumping performance was measured by filming a minimum of three jumps of each metamorph. Jumps were elicited on a cloth surface by gently touching the urostyle with forceps, and filmed from above using a high-speed digital camera (Casio Ex-FH25, CASIO computer Co. Ltd, Tokyo, Japan). Footage was played back and jump distances measured using the video analysis software Tracker (Cabrillo college, California, USA) with the longest jump distance taken as a measure of maximum jumping performance. Mass was measured using an analytical balance after blotting the metamorph dry with paper towel. Metamorphs were then anaesthetised by chilling and digital images of ventral surface taken. Images were then analysed using imaging software, ImageJ (Bethesda, Maryland, USA) to measure (1) snout-urostyle length, (2) femur length and (3) tibiofibular length. Metamorphs were then euthanised by freezing.
Data analysis
Activity and feeding data were arcsine square-root transformed for normality. Activity data were analysed using a two-way ANOVA with temperature and treatment as predictors. Feeding data were analysed using ANCOVA with temperature and treatment as categorical predictors and activity as a covariate. Larval masses (days 8 and 9) were analysed using two-way ANOVA with temperature and treatment as predictors. A two-way ANCOVA was used to analyse larval metabolic rate data with temperature and treatment as categorical predictors and larval mass included as a covariate. The effects of exposure to predation cues and temperature on time to metamorphosis were determined using parametric survival analysis with a logistic error distribution used as it returned the lowest residual deviance. Comparisons among treatments in mass at metamorphosis were analysed using a two-way ANCOVA with treatment and temperature as categorical predictors and proportion of time feeding as a covariate. Snout-vent length, femur length and tibiofibular length were collapsed into a single descriptor of size taken from the first component of a principal component analysis (pc1). The pc1 was then used to compare among treatments with an ANCOVA with treatment and temperature as categorical predictors. Maximum jump distance was analysed using a two-way ANCOVA with treatment and temperature as categorical predictors and incorporating size (pc1) as a continuous predictor. All analyses were done in the R programming environment and all models with covariates underwent stepwise model simplification (R Development Core Team 2008 ; Crawley 2007).
Results
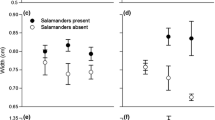
Larvae of R. marinus reared with predator cues were less active than control individuals (Fig. 1a; F 1,130 = 45.6; P < 0.001). Predator-exposed individuals were 44 % less active at 25 °C and 35 % less active at 30 °C than control larvae. Larvae reared at 30 °C were more active than those at 25 °C (Fig. 1a; F 1,130 = 31.7; P < 0.001), but the effect of exposure to predator cues was unaffected by temperature (Fig. 1a; F 1,130 = 0.03; P = 0.85). Larvae exposed to predation cues also spent less time feeding than control individuals (Fig. 1b; F 1,127 = 44.9; P < 0.001), and spent 50 % less time feeding than control individuals at 25 °C and 44 % less time feeding at 30 °C. Overall, larvae reared at 30 °C spent more time feeding than individuals reared at 25 °C (Fig. 1b; F 1,127 = 37.6; P < 0.001). The proportion of time each larva spent active was also a significant predictor of the amount of time they spent feeding (Fig. 1b; F 1,127 = 42.3; P < 0.001).
Proportion of time larval Rhinella marinus spent, a active and b feeding when reared in the presence of chemical predation cues (predator) compared to control conditions. Larvae exposed to predation cues spent less time active and feeding (P < 0.001) as did those reared at 25 °C (P < 0.001). However, the interaction between temperature and exposure to predation cues was not significant
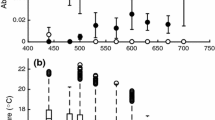
Mass of the larvae (days 8 & 9) was affected by temperature treatment, with those reared at 25 °C smaller than those reared at 30 °C (Fig. 2a; F 1,130 = 16.02; P < 0.001). Although predator treatment did not affect the mass of the larvae (Fig. 2; F 1,130 = 2.3; P = 0.13), the interaction between treatment and temperature was significant (Fig. 2; F 1,130 = 4.2; P = 0.04) suggesting that the effect of exposure to predation cues was significant at 25 °C but not at 30 °C. Metabolic rate was higher in individuals reared and tested at 30 °C than those reared and tested at 25 °C (Fig. 3; F 1,128 = 39.1; P < 0.001). Importantly, those larvae reared in predation cues had lower metabolic rates than control individuals (Fig. 3; F 1,128 = 4.5; P = 0.04) even when accounting for differences in mass between the groups (F 1,128 = 43.0; P < 0.001).
Larval mass of Rhinella marinus on day 8 and 9 of larval development when reared in control conditions compared to those exposed to predation cues (predator) at both 25 and 30 °C. While exposure to predator treatment had no effect on mass (F 1,130 = 2.3; P = 0.13), larvae reared at the 25 °C were smaller (F 1,130 = 16.02; P < 0.001); and the interaction between temperature and exposure to predation cues was significant (F 1,130 = 4.2; P = 0.04)
The relationship between mass and oxygen consumption of larval Rhinella marinus on days 8 and 9 of larval development when reared in control conditions and exposed to predation cues (predator) at both 25 °C (left panel) and 30 °C (right panel). Individuals reared and tested at 30 °C had higher metabolic rates than those reared and tested at 25 °C (F 1,128 = 39.1; P < 0.001). Larvae reared in predation cues had lower metabolic rates than control individuals (F 1,128 = 4.5; P = 0.04) even when accounting for the differences in mass between the groups (F 1,128 = 43.0; P < 0.001). However, the interaction between temperature and exposure to predation cues was not significant
There was no significant effect of temperature or predator treatment on survival to metamorphosis with 87.5 and 95 % surviving in control and treatment conditions, respectively, at 25 °C, and 90 and 85 % survival in control and treatment at 30 °C. While there was no significant effect of exposure to predation cues on the time to metamorphosis (z = 0.1; P = 0.90), those reared at 30 °C metamorphosed around 5–6 days earlier than those at 25 °C (z = −10.4; P < 0.001). Post-metamorphic mass was smaller in individuals exposed to predation cues as larvae (Fig. 4a; F 1,127 = 36.6; P < 0.001) and smaller in those reared at 25 °C than those reared at 30 °C (Fig. 4a; F 1,127 = 12.3; P < 0.001). The proportion of time that individuals spent feeding was also a significant predictor of mass at metamorphosis (F 1,127 = 4.4; P = 0.04). However, the interaction between treatment and temperature was not significant (F 1,127 = 2.137; P = 0.14). The size (pc1) of metamorphs was smaller for individuals reared in predation cues (Fig. 4b; F 1,127 = 32.3; P < 0.001), and smaller at 25 °C than at 30 °C (Fig. 4b; F 1,127 = 30.7; P < 0.001). This effect of exposure to predation cues on size (pc1) was greater at 25 °C than at 30 °C (Fig. 4b; F 1,127 = 4.4; P = 0.04). Larvae reared in predation cues were also poorer jumpers than those reared in control conditions (Fig. 4c; F 1,129 = 28.4; P < 0.001) with the same true for those reared at 25 °C compared to those at 30 °C (Fig. 4c; F 1,129 = 24.5; P < 0.001). Furthermore, the greater effect of exposure to predation cues at 25 °C than at 30 °C approached significance (Fig. 4c; F 1,129 = 3.9; P = 0.051). Incorporating size (pc1) as a covariate into the analysis shows that individuals exposed to predation cues are poorer jumpers (F 1,126 = 37.7; P < 0.001), and those reared at 25 °C are poorer jumpers than those reared at 30 °C (F 1,126 = 43.2; P < 0.001). While there was no significant interaction between temperature and exposure to predation cues on jumping performance (F 1,126 = 0.1; P = 0.74), the reduction in jump distance due to exposure to predation cues was 16.4 % at 25 °C compared to only 7.7 at 30 °C.
a Post-metamorphic mass, b size (pc1 of a principal component analysis of snout-urostyle, femur and tibiofibular lengths) and c maximum jump distances of Rhinella marinus when reared as larvae in the presence (predator) and absence (control) of chemical predation cues at both 25 and 30 °C. (N.B. pc1 was standardised by adding the most negative pc1 value to all values so that the minimum number was 0 and all others were positive). The rearing temperature and exposure to predation cues had a significant effect on all three metamorph traits. The interaction between the two was only significant for size (b; F 1,127 = 4.4; P = 0.04) but did also approach significance for jumping performance (F 1,129 = 3.9; P = 0.051)
Discussion
Little is known of how abiotic factors influence the costs and benefits of inducible defences in prey. Here, we show that larval R. marinus reduce activity in the presence of predation cues that subsequently reduces their overall foraging time and leads to slower growth, smaller metamorphic size and poorer jumping performance. Furthermore, some of these effects of exposure to predation cues were greater at cooler temperatures. Such data indicate that for at least some traits, temperature can alter the costs of inducible behavioural defences and may subsequently alter the outcome of predator/prey interactions.
Reducing activity is a common strategy utilised by prey to increase their chances of survival in high predation environments (Juliano and Reminger 1992; Lima 1998; Skelly and Werner 1990; Stoks et al. 2005; van Uitregt et al. 2011). By limiting activity, prey can reduce their conspicuousness to visual predators and/or their encounter rate with ambush predators. Rhinella marinus larvae in our experiment reduced activity by approximately 44 % at 25 °C and by 35 % at 30 °C. Previous work on this species also found that they can avoid regions where predation cues are present in the environment, and increase refuge use in the presence of such cues (Hagman and Shine 2008; Hagman and Shine 2009b, c). Empirical evidence of survival benefits of such behavioural responses is abundant for prey from many taxa including amphibian larvae (Juliano and Reminger 1992; Lima 1998; Skelly and Werner 1990; Stoks et al. 2005; van Uitregt et al. 2011). While no such data exist explicitly for R. marinus, it is likely that they share similar predators to those for which reduced activity carries a survival benefit.
Reducing activity as a behavioural defence is assumed to be costly because it decreases foraging time and ultimately slows growth and development and in some cases, fitness of adults (Beketov and Liess 2007; Sih 1992; Turner 1997; Werner and Anholt 1993). We showed that larval R. marinus spend less time feeding in the presence of chemical predation cues. The slower growth and smaller mass and size at metamorphosis of those from predator environments are presumably symptomatic of reduced feeding rates. In this experiment, the significant effect of larval foraging time on mass at metamorphosis strengthens the link between behavioural defences and their costs. Similar intuitive evidence exists for numerous prey taxa that reduce activity in the presence of predators and are purported to represent a fitness cost (McPeek et al. 2001; Steiner 2007; van Uitregt et al. 2011). In anurans, a smaller size at metamorphosis can delay the time until first reproduction, increase desiccation risk, and decrease locomotor performance, fecundity and even survival (Capellan and Nicieza 2007; Chelgren et al. 2006; Child et al. 2009; Ficetola and de Bernardi 2006). We also found that metamorphs of R. marinus that were reared in predation cues had poorer jumping abilities, even when the influence of size was included as a covariate. Poorer jumping performance is likely to reduce both prey capture and predator avoidance capabilities. We suggest that the demonstrated effects here on post-metamorphic size and locomotor performance of R. marinus when exposed to predation cues as larvae represent fitness costs.
Empirical data and theoretical predictions of the effects of exposure to predation cues on the metabolic rate of prey are limited (Alton et al. 2011; McPeek 2004; Steiner and Van Buskirk 2009). Short-term exposure to predation cues is generally associated with an increase in metabolic rate (Steiner and Van Buskirk 2009). However, Steiner and Van Buskirk (2009) show that metabolic rate is unchanged in Rana temporaria larvae when chronically exposed to predation cues. Alton et al. (2011) also found that larvae of the striped marsh frog (Limnodynastes peronii) had no change in metabolic rate when exposed to predation cues. In our experiment, larval R. marinus exposed to predation cues had lower metabolic rates than those from control environments. Larval R. marinus do not exhibit the morphological defences that are common in other anurans that are thought to be energetically costly to produce and maintain. Therefore, a reduced rate of oxygen consumption in larval R. marinus in the presence of predation cues is intuitive, given the consistent reduction in activity. In our experiment, oxygen consumption was measured in the water in which they were reared (control or treatment), which are, therefore, likely to induce behavioural defences during respirometry. This reduction in metabolism could partially offset the energy deficit suffered from reduced feeding.
The effects of exposure to predation cues on larval mass (at days 8 and 9) and post metamorphic size were greater at 25 °C than at 30 °C. Although the effects of exposure to predation cues on jumping performance were not statistically significant between temperature treatments, the magnitude of the change was greater at 25 °C than 30 °C, which is suggestive of a more pronounced response at the lower temperature. We predicted that if greater costs for predator induced defences were exhibited at cooler temperatures, then this would be due to delayed metamorphosis and a greater net accumulation of costs. However, even though the lower larval masses of predator induced larvae was more pronounced at 25 °C, this was not due to any extended exposure to predation cues at the colder temperature, as all larvae were measured at the same time point. What is most surprising is that there was no significant reduction in larval mass at 30 °C, despite a 44 % reduction in the proportion of time larvae spent feeding. It seems that the growth/predation risk trade-off during early larval development, while obviously present at 25 °C, is negated at 30 °C. We can only speculate that such data can be explained by changes in metabolic or digestive physiology between thermal regimes. In our data, we show that while reducing feeding when exposed to predation cues, larval R. marinus partially offset this energy deficit by reducing energy expenditure on activity. It is plausible that this energy saving carried greater weight at 30 °C, than at 25 °C, offsetting the entire energy deficit from reduced feeding. Alternatively, feeding efficiency as well as absorption and/or assimilation rates during digestion could be up-regulated at the warmer temperature and offset the energy deficit when reducing feeding in the presence of predation cues.
We show that the costs of behavioural defences exhibited by larval R. marinus are greater at cooler temperatures. For populations occurring at cooler climates we would expect selection for maintenance of inducible defences to be weaker as the associated costs of inducing the defences would be higher. This assumes the benefits of behavioural defences (increased survival) are unchanged at cooler temperatures. However, this is not likely to be true. In the case of ectothermic predators, both hunger and capacity to capture prey will likely be reduced at cooler temperatures. In this scenario, the costs of defences would be high and the benefits minimal. In contrast, at warmer temperatures the benefits of inducible defenses would be high and the costs low, indicating that the maintenance of inducible defences would be favoured. In any case, such thermal dependence of costs and benefits of inducible defences may play a role in driving the geographic variation in inducible defences that have been detected in other studies (Laurila et al. 2006, 2008) and suggests that large-scale changes in climate may alter predator–prey interactions in subtle and unpredictable ways. It is clear that the physiological processes involved in inducible defences and the manifestation of their costs are complex. Our limited knowledge of the physiology of inducible defences undermines our understanding of how they persist in natural populations and the likely impact of environmental variation on their dynamics.
References
Alexander RM (1982) Locomotion of animals. Blackie, Glasgow
Alton LA, White CR, Wilson RS, Franklin CE (2011) The energetic cost of exposure to UV radiation for tadpoles is greater when they live with predators. Funct Ecol 26:94–103
Beketov MA, Liess M (2007) Predation risk perception and food scarcity induce alterations of life-cycle traits of the mosquito Culex pipens. Ecol Entomol 32:405–410
Callahan HS, Maughan H, Steiner UK (2008) Phenotypic plasticity, costs of phenotypes, and costs of plasticity toward an integrative view. Year Evolut Biol 1133:44–66
Capellan E, Nicieza AG (2007) Trade-offs across life stages: does predator-induced hatching plasticity reduce anuran post-metamorphic performance? Evol Ecol 21:445–458
Chelgren ND, Rosenberg DK, Heppel SS, Gitelman AI (2006) Carryover aquatic effects on survival of metamorphic frogs during pond emigration. Ecol Appl 16:250–261
Child T, Phillips BL, Shine R (2009) Does desiccation risk drive the distribution of juvenile cane toads (Bufo marinus) in tropical Australia? J Trop Ecol 25:193–200
Crawley MJ (2007) The R book. Wiley, West Sussex
Dewitt TJ (1998) Costs and limits of phenotypic plasticity: tests with predator-induced morphology and life history in a freshwater snail. J Evol Biol 11:465–480
Dewitt TJ, Sheiner SM (2004) Phenotypic plasticity: functional and conceptual approaches. Oxford University Press, Oxford
Ficetola GF, De Bernardi F (2006) Trade-off between larval development rate and post metamorphic traits in the frog Rana latastei. Evol Ecol 20:143–158
Fraker ME (2008) The effect of hunger on the strength and duration of the antipredator behavioural response of green frog (Rana clamitans) tadpoles. Behav Ecol Sociobiol 62:1202–1205
Gosner KL (1960) A simplified table for staging anuran embryos and larvae with notes on identification. Herpetologica 16:183–190
Graham RW, Grimm EC (1990) Effects of global climate change on the patterns of terrestrial biological communities. Trends Ecol Evol 5:289–292
Hagman M, Shine R (2008) Understanding the toad code: behavioural responses of cane toad (Chaunus marinus) larvae and metamorphs to chemical cues. Austral Ecol 33:37–44
Hagman M, Shine R (2009a) Factors influencing responses to alarm pheromone by larvae of invasive cane toads, Bufo marinus. J Chem Ecol 35:265–271
Hagman M, Shine R (2009b) Larval alarm pheromones as a potential control for invasive cane toads (Bufo marinus) in tropical Australia. Chemoecology 19:211–217
Hagman M, Hayes RA, Capon RJ, Shine R (2009) Alarm cues experienced by cane toad tadpoles affect post-metamorphic morphology and chemical defences. Funct Ecol 23:126–132
Hammill E, Rogers A, Beckerman AP (2008) Costs, benefits and the evolution of inducible defences: a case study with Daphnia pulex. J Evol Biol 21:705–715
Havel JE, Dodson SI (1984) Chaoborus predation on typical and spined morphs of Daphnia pulex: behavioural observations. Limnol Oceanogr 29:487–494
Hughes L (2000) Biological consequences of global warming: is the signal already apparent. Trends Ecol Evol 15:56–61
Juliano SA, Reminger L (1992) The relationship between vulnerability to predation and behavior of larval treehole mosquitos —geographic and ontogenic differences. Oikos 63:465–476
Köster M, Krause C, Parrenhöfer GA (2008) Time-series measurements of oxygen consumption of copepod nauplii. Mar Ecol Prog Ser 353:157–164
Laurila A, Pakkasmaa S, Merila J (2006) Population divergence in growth rate and antipredator defences in Rana arvalis. Oecologia 147:585–595
Laurila A, Lindgren B, Laugen AT (2008) Antipredator defenses along a latitudinal gradient in Rana temporaria. Ecology 89:1399–1413
Lima SL (1998) Nonlethal effects in the ecology of predator-prey interactions—what are the ecological effects of anti-predator decision-making? Bioscience 48:25–34
Mccarty JP (2001) Ecological conseqences of recent climate change. Conserv Biol 15:320–331
McCollum SA, Van Buskirk J (1996) Costs and benefits of a predator-induced polyphenism in the Grey Treefrog Hyla Chrysoscelis. Evolution 50:583–593
Mclaughlin JF, Hellmann JJ, Boggs CL, Ehrlich PR (2002) Climate change hastens population extinctions. Proc Natl Acad Sci USA 99:6070–6074
Mcpeek MA (2004) The growth/predation risk trade-off: so what is the mechanism? Am Nat 163:E88–E111
Mcpeek MA, Grace M, Richardson JML (2001) Physiological and behavioural responses to predators shape the growth/predation risk trade-off in damselflies. Ecology 82:1535–1545
Niehaus AC, Wilson RS, Seebacher F, Franklin CE (2011) Striped marsh frog (Limnodynastes peronii) tadpoles do not acclimate metabolic performance to thermal variability. J Exp Biol 214:1965–1970
Nilsson PA, Bronmark C, Pettersson LB (1995) Benefits of a predator-induced morphology in crucian carp. Oecologia 104:291–296
R Development Core Team (2008) R: a language and environment for statistical computing. R: foundation for statistical computing, Vienna, Austria
Riley JP, Chester R (1971) Introduction fo marine chemistry. Academic Pres, London
Sih A (1992) Prey uncertainty and the balancing of antipredator and feeding needs. Am Nat 139:1052–1069
Skelly DK, Werner EE (1990) Behavioral and life-historical responses of larval american toads to an odonate predator. Ecology 71:2313–2322
Steiner UK (2007) Investment in defense and cost of predator-induced defense along a resource gradient. Oecologia 152:201–210
Steiner UK, Van Buskirk J (2009) Predator-induced changes in metabolism cannot explain the growth/predation risk tradeoff. PLoS One 4:e6160
Stoks R, De Block M, Mcpeek MA (2005) Alternative growth and energy storage responses to mortality threats in damselflies. Ecol Lett 8:1307–1316
Turner AM (1996) Freshwater snails alter habitat use in response to predation. Anim Behav 51:747–756
Turner AM (1997) Contrasting short-term and long-term effects of predation risk on consumer habitat use and resources. Behav Ecol 8:120–125
Urban MC (2015) Accelerating extinction risk from climate change. Science 348:571–573. doi:10.1126/science.aaa4984
Van Buskirk J (2000) The costs of an inducible defense in anuran larvae. Ecology 81:2813–2821
van Uitregt VO, Hurst T, Wilson RS (2011) Reduced size and starvation resistance in adult mosquitoes, Aedes notoscriptus exposed to predation cues as larvae. J Anim Ecol. doi:10.1111/j.1365-2656.2011.01880.x
Werner EE, Anholt BR (1993) Ecological consequences of the trade-off between growth and mortality-rates mediated by foraging activity. American Naturalist 142:242–272
Wilson RS, Kraft PG, Van Damme R (2005) Predator-specific changes in the morphology and swimming performance of larval Rana lessonae. Funct Ecol 19:238–244
Acknowledgements
This study was supported by the ARC Discovery Project DP120101215.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by I. D. Hume.
Rights and permissions
About this article
Cite this article
van Uitregt, V.O., Alton, L.A., Heiniger, J. et al. Warmer temperatures reduce the costs of inducible defences in the marine toad, Rhinella marinus . J Comp Physiol B 186, 123–130 (2016). https://doi.org/10.1007/s00360-015-0938-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00360-015-0938-0