Abstract
In a seminal study published nearly 70 years ago, Scholander et al. (Biol Bull 99:259–271, 1950) employed Newton’s law of cooling to describe how metabolic rates (MR) in birds and mammals vary predictably with ambient temperature (T a). Here, we explore the theoretical consequences of Newton’s law of cooling and show that a thermoregulatory polygon provides an intuitively simple and yet useful description of thermoregulatory responses in endothermic organisms. This polygon encapsulates the region in which heat production and dissipation are in equilibrium and, therefore, the range of conditions in which thermoregulation is possible. Whereas the typical U-shaped curve describes the relationship between T a and MR at rest, thermoregulatory polygons expand this framework to incorporate the impact of activity, other behaviors and environmental conditions on thermoregulation and energy balance. We discuss how this framework can be employed to study the limits to effective thermoregulation and their ecological repercussions, allometric effects and residual variation in MR and thermal insulation, and how thermoregulatory requirements might constrain locomotor or reproductive performance (as proposed, for instance, by the heat dissipation limit theory). In many systems the limited empirical knowledge on how organismal traits may respond to environmental changes prevents physiological ecology from becoming a fully developed predictive science. In endotherms, however, we contend that the lack of theoretical developments that translate current physiological understanding into formal mechanistic models remains the main impediment to study the ecological and evolutionary repercussions of thermoregulation. In spite of the inherent limitations of Newton’s law of cooling as an oversimplified description of the mechanics of heat transfer, we argue that understanding how systems that obey this approximation work can be enlightening on conceptual grounds and relevant as an analytical and predictive tool to study ecological phenomena. As such, the proposed approach may constitute a powerful tool to study the impact of thermoregulatory constraints on variables related to fitness, such as survival and reproductive output, and help elucidating how species will be affected by ongoing climate change.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Endothermic animals such as birds and mammals can maintain body temperatures within a considerably narrow range when compared against the thermal extremes encountered in their habitats. This has shaped the ecology and evolution of these lineages, and a variety of patterns in nature reflect either directly or indirectly thermoregulatory constraints (Buckley et al. 2012). For example, species richness and distribution limits vary predictably with environmental temperature across birds and mammals (e.g., Root 1988; Kerr and Packer 1998; Jetz and Rahbek 2002; Pigot et al. 2010), scaling relationships remain a hot topic in ecology and comparative biology (Weibel et al. 2004; Rodriguez-Serrano and Bozinovic 2009; White et al. 2009; Sieg et al. 2009; Kolokotrones et al. 2010; Naya et al. 2013a, b; Bozinovic et al. 2014), the energetic basis of macroecological rules still puzzles ecologists and physiologists (Meiri and Dayan 2003; Millien et al. 2006) and countless comparative analyses support the notion of adaptive variation on thermoregulatory performance in response to climatic conditions (e.g., Scholander et al. 1950; Lovegrove 2000; Rezende et al. 2004; White et al. 2007; Swanson and Garland 2009; McNab 2009; Naya et al. 2013a). Thus, whereas the physiology underlying endothermic thermoregulation has been studied for over a century, its contribution to abundance, distribution and diversity patterns of birds and mammals remains the focus of extensive research.
However, research on the association between thermal physiology and ecology in endotherms is highly asymmetrical. Most work in the literature has focused on how different variables (e.g. body size or environmental temperature) affect physiological determinants of thermoregulatory performance, such as thermogenic capacity or thermal insulation. The enormous amount of empirical data resulting from this approach is evident, for instance, in recent reviews that have compiled measurements of basal metabolism for 533 avian (McNab 2009) and 695 mammalian species (Sieg et al. 2009), which correspond to roughly 5.1 and 12.6 % of all species in these groups, respectively (IUCN Red List 2014). Conversely, explicit attempts to quantify how thermoregulatory capacities might impact ecological variables, such as geographic distribution or activity patterns, encompass a very small fraction of studies on endotherm energetics (Root 1988; Repasky 1991; Canterbury 2002; Humphries et al. 2002). In addition, most research on the interplay between thermoregulation and spatial and temporal variation in climatic conditions (Lovegrove 2000; Nespolo et al. 2001), on the impact of thermoregulatory constraints on activity patterns (Bacigalupe et al. 2003; Rezende et al. 2003) or time and energy budgets (e.g., Goldstein 1988) and, ultimately, on fitness components, remains fundamentally descriptive. Even though the physiological responses involved in thermoregulation have been understood for decades and are general to virtually all endothermic species (see McNab 2012 and references therein), no single approach exists to predict how thermoregulatory capacities and their energy requirements in birds and mammals might impact different aspects of their ecology.
Here, we propose an expansion of Scholander et al. (1950) seminal application of Newton’s law of cooling to study the ecological and evolutionary repercussions of thermoregulation in birds and mammals (see also Gavrilov 2014). Whereas Scholander et al. (1950) and most subsequent studies employed this approach primarily to study heat balance in resting animals at cool temperatures (see McNab 2002a), the present work builds upon those by addressing whether Newton’s law of cooling can adequately describe thermoregulatory responses under more general conditions (i.e., at higher temperatures, during activity or reproduction). We take advantage of the relatively simple relationship described by Newton’s law of cooling and argue that, in spite of its inherent limitations as an oversimplified description of the mechanics of heat transfer (e.g., see Porter and Gates 1969; Strunk 1971; Bakken and Gates 1974; Porter et al. 2000), understanding how systems that obey this approximation work can be enlightening on conceptual grounds and relevant as an analytical and predictive tool. This is because this framework combines two fundamental currencies in ecological and evolutionary physiology, energy expenditure and ambient temperature, and confines the dimensional space in which endothermic organisms can thermoregulate into a limited region (Fig. 1).
Graphical representation of Newton’s law of cooling in an endotherm that maintains a constant body temperature T b by regulating its rates of heat production and heat loss. Heat production can increase or decrease within the range delimited by BMR and MMR, whereas heat loss can be modulated within the range set by C min and C max (i.e., maximum and minimum insulation), resulting in a delimited area in which thermoregulation is actually possible
Thermoregulatory polygons
Endotherms are able to maintain a relatively constant body temperature (T b) over a certain range of environmental temperatures (T a) through metabolic heat production. The typical relationship between metabolic rate and ambient temperature in resting endotherms is a complex U-shaped reaction norm that has been described in a multitude of species across many taxa (Fig. 2). At low temperatures, endothermic organisms produce heat to maintain high and constant T b, resulting in a negative relationship between metabolism and T a. Within the thermoneutral zone, which describes the range of T a where thermoregulation relies primarily on physical responses (e.g. changes in insulation and posture), metabolic rate reaches a minimum level (basal metabolic rate, BMR) and no longer changes as a function of T a. At T a above this zone, metabolic rate increases again as heat is actively dissipated by means of evaporative cooling and, in those species in which body temperature is allowed to rise, possibly due to Q 10 effects (Bartholomew 1982; Withers 1992). In this region, the relation between ambient temperature and metabolism is positive. The breakpoints that separate these three regions and set the boundaries of thermoneutrality are known as the lower and upper critical temperatures (T lc and T uc), respectively (Fig. 2).
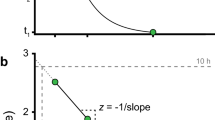
Thermoregulation in the fat mouse Steatomys pratensis. a Empirical measurements of MR and T b at different T a, connected by segments with slopes corresponding to C (Eq. 1), which can be modulated between extreme values C min and C max. b The relationship between MR and T a is generally represented as a U-shape curve, and the thermoneutral zone TNZ (shaded area) bound by a lower and an upper critical temperature describes the temperature range in which MR is minimal. c The thermoregulatory polygon represents the area in which S. pratensis can remain euthermic according to the observed ranges of MR, C and T b, and shows how the metabolic curve results from regulation of T b around a set point. Note that, if T b is not entirely constant and increases with T a (a), the regression between MR and T a below the TNZ is expected to underestimate C and overestimate T b (represented as C’ and T b ’ in b). Data from Perrin and Richardson (2005), original mass-specific estimates were multiplied by the reported mean body mass of 37.4 g (n = 17–65 individuals for each data point)
Newton’s law of cooling has been employed as a useful approximation to describe heat transfer at different T a (Scholander et al. 1950). Detailed descriptions of how the equation is obtained, how it relates to the real physics of heat exchange, and the principles behind indirect calorimetry (i.e., estimating heat production by measuring oxygen consumption) are available in some classic papers (Porter and Gates 1969; Bakken 1976) and in excellent introductory chapters of animal physiology textbooks (e.g. Withers 1992; Schmidt-Nielsen 1997; McNab 2002a; Angilletta 2009). For an endothermic organism in thermal balance, Newton’s law can be expressed as
where MR is metabolic heat production, and thermal conductance C and the gradient between body temperature T b and ambient temperature T a determine the rate of heat loss (note that thermal insulation, which is a more intuitive concept, is the reciprocal of C). The graphical interpretation of Newton’s law of cooling shows that a thermoregulatory polygon, enclosed within the range in which MR and C can be modulated, emerges from the regulation of T b around a set point (Fig. 2). Whereas the typical U-shaped curve describes metabolic responses at rest where energy expenditure is minimal, the polygon provides a more accurate representation of Newton’s law of cooling (which does not predict a positive relationship between MR and T a at temperatures above T uc, hence Q 10 effects and active heat dissipation must be taking place when this response is observed) and expands it to accommodate activity as well as other behaviors (Box 1).

Importantly, Newton’s law of cooling assumes thermal balance, but not necessarily a constant T b (i.e., T b can vary across different T a and yet the equality in Eq. 1 must hold). For a perfect thermoregulator at rest with an absolutely constant T b, extrapolation of the metabolic curve below thermoneutrality to MR = 0 should intersect the abscissa at T a = T b. However, for many organisms the regression approach extrapolates to T a > T b (McNab 1980, 2012; Schmidt-Nielsen 1997), as expected when T b increases with T a either because of detectable changes in core T b (Fig. 2) or alterations in the temperature gradient from core to skin (the assumption that core T b equals average T b over the organism’s body is implicit in the model; Bartholomew 1982). The latter can explain, for instance, why in previous analyses Newton’s law of cooling seemed to work better in small organisms and why, at low temperatures, intermediate and large species are apparently capable of decreasing C below estimates at the lower limit of thermoneutrality (McNab 2002b, 2012). Similarly, more labile T b in birds (Prinzinger et al. 1991; McKechnie and Lovegrove 2002) might explain why the metabolic curve extrapolates more often to T a > T b in this group in comparison to mammals (Schmidt-Nielsen 1997).
Modulation of T b is an intrinsic response in the thermoregulatory repertoire of birds and mammals (Boyles et al. 2011, 2013), and Newton’s law of cooling remains applicable to accommodate variation in T b during normothermia (Fig. 2) and heterothermia ranging from mild hypothermia to deep hibernation (Fig. 3). Accordingly, it has been employed to study thermoregulation and energy balance during torpor and hibernation (Hensaw 1968; Humphries et al. 2002), and to quantify the effects of reduced metabolic costs to maintain the thermal gradient T b − T a in conjunction with passive Q 10 effects of lowered T b on enzyme kinetics (Heldmaier and Ruf 1992). More specifically, Humphries et al. (2002) developed a bioenergetic model to compare the energy requirements to hibernate during the length of the winter against fat store estimates (Fig. 3), and predicted the range of hibernaculum temperatures in which the little brown bat Myotis lucifugus could successfully hibernate (see also Boyles and Brack 2009; Boyles and Willis 2010). Similar models may be employed to investigate under which conditions torpor may be favored by selection or to calculate the thermal niche during torpor or euthermia from first principles (e.g., Landry-Cuerrier et al. 2008).
Bioenergetic model to study torpor and hibernation in relation to thermoregulatory polygons. The thermal gradient is reduced following an active down regulation of MR and, at intermediate temperatures T b passively follows T a. As temperature keeps decreasing, T b eventually reaches a critical point and MR rises to prevent further hypothermia (Hainsworth and Wolf 1970; Heller and Colliver 1974; Heldmaier and Steinlechner 1981; Geiser and Baudinette 1987). The range of temperatures in which this strategy is energetically viable can be estimated by comparing rates of metabolic expenditure against available energy reserves. Modified from Humphries et al. (2002)
Thermoregulatory polygons inherently assume that organisms rely on the temperature gradient for effective thermoregulation and that there is a minimum and a maximum rate at which heat is dissipated (C min and C max, respectively, Fig. 2). These limits are not determined solely by the organism’s physiology because C reflects the combined action of morphological, physiological and environmental variables (Porter 2000). Nonetheless, more complex bioenergetic models taking into account the contribution of these different variables show that the relationship between heat loss and T a is quasi linear (e.g., Coyle and Porter 1986), supporting the linear approximation described in Eq. 1. According to Newton’s cooling law, thermal balance could not be maintained at temperatures approaching T b as the thermal gradient approaches zero (hence MR should tend to zero to satisfy Eq. 1), which is not true because animals also rely on evaporative cooling to dissipate heat. Heat loss due to evaporation E can be included in the model and results in a thermoregulatory polygon in which thermal equilibrium can be maintained at T a ≥ T b, effectively increasing the range of maximal temperatures that the organism may tolerate at the expense of water (Fig. 4). This expanded model captures why, at temperatures approaching or above T b, animals should remain inactive not only to minimize heat production but also C, which effectively reduces heat gain from the environment (Hinds and Calder 1973; Weathers and Schoenbaechler 1976; Tieleman and Williams 1999; Tieleman et al. 2002). Nonetheless, the contribution of evaporative cooling is almost always included in the estimation of C at cool and intermediate temperatures (but see, e.g., Hudson and Bernstein 1981; Tieleman and Williams 1999; Tieleman et al. 2002), and this works well for temperature gradients of a few degrees or more (McNab 1980; Schleucher and Withers 2001). Strategies to maintain a temperature gradient and reduce evaporative heat loss by behavioral and physiological means, such as vasoconstriction, changes in posture, the selection of suitable microhabitats and the elevation of the T b set point several degrees above normal levels during extreme heat or strenuous exercise (Heinrich 1977; Tieleman and Williams 1999; Walsberg 2000; Soobramoney et al. 2003), support the primary role of passive heat dissipation and the adequacy of the reduced model (Eq. 1) for the overwhelming majority of species and ecological scenarios in nature (but see Tieleman et al. 2002, 2003; McKechnie and Wolf 2010).
Newton’s law of cooling can be expanded to explicitly incorporate the contribution evaporative heat loss. a In the absence of evaporative heat loss, thermoregulation is only possible when Ta < T b − BMR/C max (Eq. 1). b The incorporation of evaporative heat loss E, which is subtracted from MR (Eq. 1), gives rise to thermoregulatory polygons in which T a can equal or surpass T b (in reality E increases concomitantly with T a at higher 0 temperatures, whereas here it was assumed to be constant for clarity). Heat tolerance increases with E and is maximized at temperatures above T b by dropping metabolic heat production MR and rates of heat gained from the environment to a minimum. Therefore, adopting C min is actually beneficial at temperatures above T b (for an empirical example, see Tieleman et al. 2002)
Boundaries to effective thermoregulation
The limits to MR, C and T b determine the area within which thermal balance is possible, and a variety of research questions on comparative energetics and their association with geographic distribution (Root 1988; Bozinovic and Rosenmann 1989; Rezende et al. 2004; Swanson and Garland 2009), physiological responses to acclimation and acclimatization (Bacigalupe et al. 2004a; Feist and White 1989) and the evolution of torpor and hibernation (Wang 1989; Humphries et al. 2002) revolve, in one way or another, around where these limits lie. For a euthermic animal at rest, BMR and maximum metabolic rates during cold exposure (MMR) set the metabolic limits and have been employed as standard measures in comparative analyses (Kleiber 1932; White and Seymour 2005; McNab 2009; Rezende et al. 2002, 2004). For most mammals and birds, however, the highest rates of heat production are attained during activity (Heinrich 1977), as suggested by measurements of maximum aerobic capacity measured during strenuous exercise \( \left( {\dot{V}{\text{O}}_{2} { \hbox{max} }} \right) \) (White and Seymour 2005; Wiersma et al. 2007a; Glazier 2008) and estimations of muscle efficiency (~70 % of the energy consumed by isolated muscles during contraction is lost as heat; Lichtwark and Wilson 2007). Consequently, these parameters can be employed to study thermal balance and temperature critical limits the short term.
With regards to heat dissipation, the expansion from the metabolic curve to thermoregulatory polygons essentially involves the incorporation of C max. The premise that a limit to heat dissipation exists is trivial from a theoretical perspective, but its estimation remains challenging because C reflects the interaction between morphological, physiological and environmental variables (Porter et al. 1994, 2000). Whereas C min is relatively straightforward to measure in quiescent animals minimizing their exposed surface area, C max varies as a function of posture, thermal conductivity of dry versus wet fur, forced convection during activity, and often includes evaporative heat loss as a confounding variable (Morrison et al. 1959; Conley 1985). Not surprisingly, C min has been estimated in a wide variety of monotremes (Schmidt-Nielsen et al. 1966; Dawson et al. 1978), birds, marsupials and placental mammals (reviewed in Bradley and Deavers 1980; Aschoff 1981; Schleucher and Withers 2001; Withers et al. 2006) while the variation in C max remains virtually unexplored (but see Gavrilov 2014; Speakman and Król 2010a; Speakman et al. 2014). Nonetheless, its relevance has been long recognized in exercise physiology, because the inability to dissipate heat at higher exercise intensity (i.e., higher MR) and/or ambient temperature (i.e., lower temperature differential) ultimately results in exhaustion due to unregulated hyperthermia (Heinrich 1977). More recently, heat dissipation has been proposed as a key process limiting energy expenditure and reproductive rates (Speakman and Król 2010a, b; Grémillet et al. 2012), hence C max may be a physiological parameter with important repercussions for life-history evolution.
Importantly, limits to effective thermoregulation will vary with time because physiological or behavioral responses that maximize thermotolerance in the short term may not be sustainable over extended periods (Fig. 5). For instance, MMR and \( \dot{V}{\text{O}}_{2} { \hbox{max} } \) constitute peak metabolic rates during an acute challenge, whereas the maximum sustainable MR (SusMR) has been proposed as a more adequate proxy of the metabolic ceiling (Peterson et al. 1990; Daan et al. 1990; Hammond and Diamond 1997; Bacigalupe and Bozinovic 2002; Speakman and Król 2010a) and has often been inferred from measurements of field metabolic rates (FMR) (Speakman 2000; Anderson and Jetz 2005). Contrasting temporal ranges partly explain why calculations of lower lethal temperatures from MMR and C min measurements result in surprisingly low estimates (e.g. −30 °C for a 5.7 g hummingbird, López-Calleja and Bozinovic 1995; see also Rosenmann and Morrison 1974; Rosenmann et al. 1975; Heldmaier et al. 1982; Bozinovic and Rosenmann 1989; Bozinovic et al. 1990; Holloway and Geiser 2001), and suggest that some small- to medium-sized arctic species can tolerate temperatures below the lowest record on earth with a small increase in metabolism (from 1.5 to 4 times BMR; Scholander et al. 1950; but see Peters 1983). Even though these estimates remain useful for comparative purposes, they have limited predictive power in realistic ecological settings, as comparisons of lower lethal temperatures estimated from MR and C min measurements against mortality data highlight (Hart 1962). Ultimately, ecologically relevant limits to thermoregulation are expected to be less extreme as the temporal range increases (Fig. 5) because animals must also maintain energy and water balance (e.g., the lower limits of MR and C over the course of many days would include bouts of activity and should be higher than BMR and C min, respectively). Estimating where these limits lie remains a major challenge, because (i) FMR measures do not necessarily constitute maximum sustainable levels of energy expenditure (Ricklefs et al. 1996), (ii) energy is allocated to multiple processes apart from heat production and (iii) heat generated as a byproduct of other activities is often employed for thermoregulatory purposes (i.e., heat substitution, see Humphries and Careau 2011).
Temporal effects on thermoregulatory performance. a The limits to MR and C are expected to be less extreme as longer temporal windows, as shown here for MR. b As a result, thermoregulatory polygons are expected to vary with the time scale involved. The association between MR and time in panel a was adapted from Peterson et al. 1990 (see also Piersma 2011), the outer polygon was calculated from allometry for a mammal weighing 100 g (Table 1) and T b = 37 °C and the inner polygon was drawn employing arbitrary parameters for illustrative purposes
Allometric effects
How body size affects thermoregulatory performance remains an important ecological question with implications for our understanding of patterns of geographic distribution (e.g., Bergmann’s rule), to estimate thermal niches (Kearney and Porter 2009; Porter and Kearney 2009) and the potential impact of rising temperatures on species energetics (e.g., Dillon et al. 2010 for ectothermic organisms) and tolerance limits (Khaliq et al. 2014). The limits to heat production and dissipation change predictably with body size, and many studies have quantified how BMR (Symonds and Elgar 2002; White and Seymour 2005; McNab 1988, 2009; Sieg et al. 2009; Clarke et al. 2010), FMR (Koteja 1991; Nagy et al. 1999; Speakman 2000; Nagy 2005; Anderson and Jetz 2005; Capellini et al. 2010; Hudson et al. 2013) and MMR (Bozinovic and Rosenmann 1989; Rezende et al. 2002, 2004; Swanson and Garland 2009; Weibel et al. 2004) vary with size, and the same is true for C min (Bradley and Deavers 1980; Aschoff 1981; Schleucher and Withers 2001; Withers et al. 2006). Conversely, little is known about T b (but see Clarke and Rothery 2008) or C max.
To quantify the range of variation in C at rest, we compiled measurements of MR and T b at different T a from the literature, the only criteria for inclusion being that all values were reported in the same study and that measurements encompassed a wide range of T a. We calculated C for the different T a (Eq. 1) and selected the lowest value as C min and the highest C obtained at T a < T b as C max (Fig. 2). Because C max can be inflated due to evaporative heat loss as T b − T a decreases, abnormally high values observed at very small temperature differentials of 2.7 ± 0.6 °C (± SD) were removed following visual inspection (N = 5 spp), and an estimate at a lower T a was selected as C max instead. The resulting dataset encompasses 46 avian species with a body mass range from 5.5 to 1500 g and 43 mammalian species weighing between 4.0 and 11,400 g (Fig. 6). Allometric relations for C min closely resemble results by Aschoff (1981) with a more extensive dataset, suggesting that values compiled here are representative of the variation in C observed in both lineages.
The association between body size and lower and upper limits reported for a metabolic rate MR, b thermal conductance C and c body temperature T b in euthermic mammals. Data in white and gray correspond to extreme values observed during exposure to cold and warm conditions, respectively (see text for details). Continuous lines represent relations obtained by regular linear regressions (Table 1) and the dotted lines depict allometric relations for BMR (Sieg et al. 2009) and C min (Aschoff 1981) for comparative purposes. The histograms describe how the ratio or the difference between maximum and minimum values observed for these variables is distributed. Results for birds are qualitatively identical (see Supplementary Material)
Scaling relationships for C min and C max indicate that the relationship between these variables remains relatively constant across body sizes (Table 1). As expected, the residual variation around the regression was higher in C max than in C min (Fig. 6), reflecting the difficulty to estimate C max precisely because some values may be either submaximal or inflated due to evaporative heat loss. Accordingly, C max remains significantly correlated with the temperature differential T b − T a in the pooled dataset (P < 0.001), which is not the case for C min (P = 0.88). Nonetheless, these estimations provide a rough idea of how much C can be modulated in resting animals within a metabolic chamber: C max adjusted for a temperature differential of 2 °C is 3.9 times higher than C min in birds, as estimated by the median of the distribution (95 % of the values fall within 3.0 and 5.2 times C min), and 4.1 times in mammals (95 % between 2.8 and 5.4 times C min). Estimates of C max reported by Gavrilov (2014) for passerines and non-passerines, corresponding to roughly four times C min, are in close agreement with the range of values found here. Even though structural constraints in hair density and length across different ranges of body sizes might also affect the general relationship between C min and C max, the ratio between these variables was not significantly associated with body mass in either birds or mammals (P > 0.6 in both cases).
To address whether variation in thermoregulatory polygons with body size predicts allometric effects in other thermal descriptors, such as the range of thermoneutrality and its lower and upper limits (T lc and T uc) (Morrison 1960; Calder and King 1972), we complemented the dataset built for C with measurements of MR data from the literature (for rodents, Rezende et al. 2004; for birds, Dawson and Carey 1976; Hinds et al. 1993; Cooper 2002; Rezende et al. 2002; Vezina et al. 2006; Wiersma et al. 2007a, b; Olson 2009) (Fig. 6). The close correspondence between theoretical predictions based on the scaling of BMR, C min and C max (Table 1) and empirical estimates for placental mammals supports this prediction and confirms the long recognized trend (Scholander et al. 1950; Morrison 1960; Calder and King 1972; Gardner et al. 2011) that larger species exhibit lower T lc and T uc, and a broader range of thermoneutrality than small species (Fig. 7). Even though quantitative predictions should change with different scaling parameters, as a general rule, the thermal niche of larger species is expected to shift and/or expand toward cooler temperatures because the scaling exponents for MR are larger than C and T a = T b − MR/C (Fig. 8). For example, Morrison (1960) suggested that T lc should decrease in proportion to mass0.25 based on an exponent of 0.75 for BMR and 0.5 for C min, whereas the analysis described here suggests a decrease proportional to mass0.16 (Fig. 7). Certainly, these allometric effects may vary across taxa (Sieg et al. 2009; White et al. 2009), and might partly account for macroecological trends such as Bergmann’s rule (Ashton et al. 2000; Meiri and Dayan 2003; Millien et al. 2006; Ramirez et al. 2008), body size evolution in mammals (Smith et al. 2010) and size distribution differences between terrestrial and aquatic lineages (Smith and Lyons 2011). In this context, thermoregulatory polygons may be employed to model how the complex relationship between body size, thermoregulatory performance, activity and energy balance (see also Wunder 1975) may give rise to different ecological and evolutionary scenarios.
Body size effects on a upper critical thermal limits (T uc), b lower critical thermal limits (T lc) and c the temperature range of the thermoneutral zone (TNZ) can be predicted with the proposed framework from T b, BMR, C min and C max. Empirical data for mammals were obtained from Riek and Geiser (2013) and predicted values were calculated from allometry assuming a T b = 37 °C. We employed the allometric relation described by Sieg et al. (2009) for BMR and the equations obtained in this study for C min and C max (Table 1). The shaded area depicts the prediction interval associated with the standard error of the allometric exponent of C min and C max
Body size effects on a thermoregulatory polygons and b the metabolic scope for other activities, based on the scaling of MR and C reported for mammals (Table 1, Fig. 5) and assuming that T b = 37 °C. Body size has a major impact on energy expenditure and cold tolerance, whereas effects on heat tolerance are not as pronounced. Additionally, based on the allometric estimates employed here, the temperature in which aerobic scope is maximal tends to decrease with body size
Residual variation
After removing the effects of body size, the residual variation in MR and C was significantly correlated with ecological variables such as temperature (Scholander et al. 1950; Rezende et al. 2004; Swanson and Garland 2009; Naya et al. 2013b), aridity (McNab and Morrison 1963; Lovegrove et al. 1991) and diet (McNab 1986). However, most comparative analyses have focused on a single physiological parameter, effectively ignoring multivariate selection and the concerted evolution of multiple characters involved in thermoregulation (but see Rezende et al. 2004; Lovegrove 2005; Naya et al. 2013b). This is unfortunate because upper and lower limits of heat production and dissipation may increase or decrease in concert if they share a common physiological and/or genetic basis (Fig. 9). Because animals must either conserve or dissipate heat to regulate T b, the boundaries of thermoregulatory polygons should emerge as a compromise to multiple selective pressures acting on different aspects of performance, rather than optimal limits evolving independently of each other. Seasonal plastic responses in thermoregulatory performance, which involve concomitant changes in MR and C (Rosenmann et al. 1975; Heldmaier et al. 1982; Feist and White 1989; Piersma et al. 1995; Nespolo et al. 2001; Lovegrove 2005; Swanson and Liknes 2006), support this interpretation and are difficult to explain in the absence of physiological constraints. For example, increased maintenance costs of thermogenically active tissues have been invoked to explain the higher BMR observed during the cold season in many endothermic species because the adaptive significance of higher BMR per se remains unclear (Dawson and O’Connor 1996; Williams and Tieleman 2000; Liknes et al. 2002; Swanson 2010). Interestingly, increased winter BMR is typical of species from high latitudes whereas their counterparts from subtropical latitudes exhibit stable or reduced BMR (Smit and McKechnie 2010). A similar pattern emerges in comparative analyses on the association between BMR and MMR, which suggests that their residual variation is generally correlated across species from temperate zones (Duttenhoffer and Swanson 1996; Rezende et al. 2002, 2004), which is not the case in the tropics (Wiersma et al. 2007a).
Residual variation in metabolic rate and thermal conductance, after removing the effects of body size, and its effect on thermoregulatory polygons. a Residual variation in BMR and MMR is positively correlated in both birds and mammals. b The same pattern is true for the residual variation of C min and C max (see main text). c Increased MR and lower C within the range described here result in important differences in thermal tolerance and energy budgets, as show schematically with two polygons calculated for a 100 g mammal assuming a T b = 37 °C and including a residual variation in log10-transformed data of ±0.1 and. This difference of 0.2 log10 units implies that MR and C of the ‘cold-adapted’ species corresponds to, respectively, 1.58 and 0.63 times that of its ‘warm-adapted’ counterpart
Conversely, for C virtually no information on the association between upper and lower limits is available. Even though postural changes, vasoconstriction and evaporative water loss can be employed to modulate C, insulation afforded by the fur and feather covering imposes a physical restriction that should give rise to a close association between C min and C max. In the dataset compiled here, the residual variation in these variables controlling for body mass and the temperature differential for C max (see above) are highly correlated in both birds (r 44 = 0.673, P = 2.9 × 10−7) and mammals (r 41 = 0.736, P = 1.8 × 10−8) and support this prediction (Fig. 9). Similarly, Gavrilov (2014) reported a nearly constant ratio between these variables across passerines and non-passerines, which is inherently indicative of an association between these variables. Taken together, these analyses indicate that upper and lower limits of heat production on the one hand, and heat dissipation on the other hand, are often correlated and may evolve in concert (Fig. 9). Nonetheless, there is a need not only for more comparative studies to evaluate this hypothesis, but also intraspecific ones that might evaluate the potential joint evolutionary trajectories of both traits.
Studies focusing on a single physiological parameter also neglect that equivalent rates of heat production and loss are necessary to maintain a constant T b (Eq. 1), hence MR and C are expected to respond in a coordinated fashion to changes in the thermal environment. In a comparative analysis encompassing 127 rodent species, Naya et al. (2013b) found highly significant covariation between BMR and C min after removing size effects (see also McNab and Morrison 1963; Lovegrove et al. 1991; Bozinovic et al. 1999; McNab 1995; Lovegrove 2005), and that interspecific differences in this coordinated response are mainly associated with the mean annual temperatures that species they encounter. Their analyses conclusively show that MR and C evolve as a coordinated system but, contrary to their suggestion, also demonstrate that compensation is not entirely perfect across species from different thermal environments. Perfect compensation occurs when variation in MR and C is exactly proportional (i.e., 1:1 relation), and involves vertical shifts in thermoregulatory polygons without any changes on the temperature axis because the ratio MR/C remains constant (and therefore T b − T a remains unchanged; Eq. 1). The fact that Naya et al. (2013b) describe a negative association between residual C min and annual mean temperatures and a 1:1.099 relation between C min and BMR (see their Fig. 1) indicates that the thermoneutral zone of species from cold regions are displaced toward lower temperatures, as originally described by Scholander et al. (1950). Nonetheless, perfect compensation remains a very useful concept because it encapsulates the physiological conditions under which energy budgets can be modulated without any impact on the range of thermal tolerance and, consequently, energy savings associated with different thermoregulatory strategies (e.g., evolving higher MR vs. higher insulation) can be readily estimated across the entire thermal range of the polygon.
Taken together, the association between upper and lower limits of heat production and dissipation and the covariation between MR and C suggest that the suite of physiological responses required for efficient thermoregulation is somewhat constrained. Under which conditions these constraints might contribute to the evolution of different thermoregulatory strategies remain virtually unexplored (but see Rezende et al. 2002, 2004; Lovegrove 2005; Naya et al. 2013b; Careau 2013), and yet they may impact how different mammals and birds might respond to climate change and to the predicted increase in temperature variability on a daily and seasonal basis. Addressing these questions requires a more holistic approach focusing on the concerted evolution of subordinate traits underlying thermoregulatory performance. Consequently, parameters describing the thermoregulatory polygon of a given species (e.g. BMR, MMR, C min and C max) should be consistently measured and reported in the same study, and analyses should address how the boundaries of the polygon respond in tandem to thermal challenges and how they correlate with different climate predictors.
Activity and reproduction
Both activity and reproduction require energy and inevitably produce heat as a byproduct. Therefore, a trade-off between these processes and thermoregulation is expected due to energy allocation when it is cold and heat dissipation when it is warm, with potentially important repercussions for population dynamics and geographic ranges across environmental thermal gradients (Hall et al. 1992). Considerable attention has been given to the extrinsic and intrinsic factors that might limit energy acquisition and expenditure, such as food availability and the capacity to digest and assimilate food (Konarzewski and Diamond 1994; Hammond and Diamond 1997; Speakman 2000; Bacigalupe and Bozinovic 2002), whereas only recently has the putative role of heat dissipation been recognized (but see Greenwood and Wheeler 1985). As a consequence of the heat dissipation limit theory (Speakman and Król 2010a, b), an increasing number of studies are currently assessing how heat dissipation might impact the ecology and evolution of endothermic lineages (e.g., Voigt and Lewanzik 2011; Greenberg et al. 2012; Kurnath and Dearing 2013; Larose et al. 2013; Zub et al. 2013; Okrouhlík et al. 2015).
The aerobic scope available for activity and reproduction across different temperatures can be readily calculated as the difference between the upper and lower boundaries of the thermoregulatory polygon (Fig. 8). Consequently, the scope for activity and reproduction is maximized at intermediate temperatures and decreases toward the thermal extremes, where thermoregulatory constraints may impair these functions. Importantly, bursts of activity involve high-energy turnover rates for brief periods of time, and reproduction and parental care encompass increased energy costs usually distributed across weeks, months or even years; therefore, the time frame in which these processes occur cannot be neglected during the calculation of aerobic scope (Fig. 5). Additionally, this approach assumes that the heat generated through activity or reproduction is not employed for thermoregulation at low temperatures, whereas actually the possibility of heat substitution should result in an aerobic scope that is not necessarily reduced at lower T a because a fraction of the energy required for these activities is also employed for thermogenesis (Humphries and Careau 2011).
According to resource allocation theory, thermoregulatory costs required for maintenance are expected to be largely additive to productive costs necessary for growth or reproduction (Ricklefs 1974; Weiner 1992; Rauw 2009), which is supported by empirical studies combining multiple stressors, such as the costs of lactation and cold exposure in mammals (Hammond et al. 1994; Rogowitz 1996, 1998; Hammond and Kristan 2000; Johnson and Speakman 2001; Zhang and Wang 2007; Simons et al. 2011). Several mammalian species exhibit a decrease in thermogenic capacity and its subjacent physiological machinery during lactation (reviewed in Speakman 2008), suggesting that reproduction costs impinge not only on increased energy demands but also on a reduction on the metabolic ceiling. Therefore, thermoregulatory requirements during cold seasons may partly account, in conjunction with decreased productivity and food availability, for seasonal patterns of reproduction in species from temperate regions (Burger 1949; Masman et al. 1986; Weathers and Sullivan 1993; Berteaux 1998; Fournier et al. 1999) and geographical variation in breeding seasons (Bronson 1985). Conversely, partial heat substitution during locomotion seems to be widespread across birds and mammals (Chai et al. 1998; Bruinzeel and Piersma 1998; Chappell and Hammond 2004; Chappell et al. 2004; Lovvorn 2007; Humphries and Careau 2011; but see Wunder 1970; Hart 1971), with important repercussions for activity patterns at different temperatures (Chappell et al. 2004), energy budgets in free-ranging animals (Webster and Weathers 1990; Weathers and Sullivan 1993) and during long-distance migrations (Bruinzeel and Piersma 1998). In principle, perfect substitution should result in activity at low temperatures being virtually free of energy costs (Humphries and Careau 2011 and references therein). Nevertheless, this argument neglects the fact that postural changes and forced convection during locomotion inevitably jeopardize insulation (Box 1), and thus thermal balance. Everything else being equal, an increase in C must be compensated by a proportional increase in MR to maintain T b (Eq. 1), which suggests that remaining active at moderate or low temperatures imposes a thermoregulatory cost that can be mitigated but never fully circumvented with heat substitution. This subject has received little consideration in the literature (but see Zerba and Walsberg 1992; Zerba et al. 1999), and has been neglected in theoretical analyses that employ C min to study the association between thermoregulation and energy budgets (e.g., Root 1988; Humphries and Careau 2011).
At environmental temperatures above the optimal in which aerobic scope is maximized, heat production during activity and/or reproduction may exceed maximum rates of heat dissipation and thus the organism might face death by unregulated hyperthermia (Fig. 8b). This is in accordance with empirical studies showing that sustained activity at moderate to high temperatures almost irrevocably results in hyperthermia (Torre-Bueno 1976; Heinrich 1977; Hudson and Bernstein 1981; Hirth et al. 1987; Nybo 2008) and/or reduced maximum aerobic performance and endurance as T a increases (Galloway and Maughan 1997; Chappell et al. 2004). The impact of elevated T b on activity and locomotor performance has long been recognized and, not surprisingly, many terrestrial birds and mammals show bimodal patterns of activity to avoid high mid-day temperatures and minimize the risk of hyperthermia (Owen-Smith 1988; Wauters 2000; Bacigalupe et al. 2003; Maloney et al. 2005). Nonetheless, the putative role of heat dissipation in the ecology and evolution of these groups remains to be fully explored (Piersma 2011). For example, nocturnality has been hypothesized to be predominant among bats to prevent overheating during flight (e.g., Thomas and Suthers 1972; Speakman et al. 1994; Voigt and Lewanzik 2011) and the evolution of sexual size dimorphism across birds and mammals, with females being generally smaller in both groups, has been proposed as result of increased susceptibility to hyperthermia (Greenwood and Wheeler 1985). From an evolutionary perspective, the capacity to dissipate heat is expected to be under strong directional selection because of the direct impact of transient hyperthermia on fertility, conception rates and embryo survival (e.g., Gwazdaukas et al. 1981; Webb 1987; Setchell et al. 1988; Karaca et al. 2002; Hansen 2009). Additionally, heat dissipation may constrain increased energy turnover rates and impose a limit to the number and/or size of offspring (Fig. 10), with potentially important repercussions to the evolution of life histories across different ranges of body size or geographic distribution (Speakman and Król 2010a, b).
The heat dissipation limit theory postulates that the organism’s capability to dissipate heat sets the limit to sustainable metabolic rates. a The theory predicts that the ceiling on energy expenditure should be lower at high T a, which is supported by empirical measurements in lactating voles (Microtus arvalis) with different litter sizes. The relationship between MR and litter size was obtained from measurements of food intake in Simons et al. (2011) (their Fig. 6) scaled to a daily energy expenditure of 91.8 and 59.3 kJ/day recorded in their study for dams with a mean litter size of 3.5 pups at 21 and 30 °C, respectively. b Observed limits can be readily explained with the polygon framework proposed here. Upper and lower estimates of energy expenditure coincide with the polygon limits expected from allometry for a 25 g mammal (Table 1) with a T b = 37 °C, illustrating how the proposed framework might predict the limits to heat dissipation and their putative impact on the evolution of life history in endothermic organisms (see Speakman and Król 2010b)
Allometric analyses suggest that larger mammalian species have a reduced scope to increase MR relative to BMR (Speakman and Król 2010a) and the ratio between FMR and BMR indeed decreases with body size (Westerterp and Speakman 2008), which has led to the proposition that the lower fecundity of larger species ultimately reflects a physiological constraint resulting from their lower surface to volume ratio (Speakman and Król 2010b). Even though the impact of heat on activity and reproductive performance has been long recognized in large animals (e.g., in the livestock literature; Renaudeau et al. 2003; Odongo et al. 2006), the heat dissipation limit theory postulates that this limitation is general across different ranges of body size (Speakman and Król 2010a; Piersma 2011). Accordingly, many insects are endothermic during flight and can maintain T b elevated several degrees above T a, which demonstrates that even small ectotherms cannot readily dissipate the heat generated during strenuous activity (Heinrich 1977). This is expected on theoretical grounds because, irrespective of body size, heat dissipation rates must eventually reach a limit that is conveyed by C max in the proposed framework. When the combination of metabolic and environmental heat load surpasses this limit, animals are expected to overheat or attempt to maintain constant T b at the expense of water balance (Fig. 4). Consequently, thermoregulatory polygons provide a theoretical venue to studying both the energy costs of activity and reproduction as well as their impact on thermoregulatory performance. Relying primarily on knowledge of activity and reproductive costs and their impact on overall MR, this approach can be employed to address how temporal and geographical variation in environmental temperatures might translate into different selective pressures acting on thermoregulation and, indirectly, on patterns of activity and reproductive output (Fig. 10).
Concluding remarks
Contrasting with the typical U-shaped curve described in textbooks, thermoregulatory polygons acknowledge that activity, behavior and environmental conditions such as wind or rain alter the relationship between T a and MR (Box 1). Taking into consideration the limits to heat production and dissipation, thermoregulatory polygons describe the set of conditions under which thermal balance is possible and thus, permit a better understanding of the potential impact of thermoregulatory constraints on traits relevant to fitness such as activity levels and reproductive performance. As a caveat, this approach remains an approximation and conveniently ignores processes such as radiation (Porter and Gates 1969) or that animals are not always in thermal equilibrium (Tieleman and Williams 1999). Limitations and reservations about the use of Newton’s law of cooling have been given by several authors (Strunk 1971; Calder and King 1972; Bakken and Gates 1974), all of which essentially emphasize that the relationship it describes and parameter C are not explicitly associated with any single process of heat transfer and may obscure the contribution of relevant physiological responses (the most important one being evaporative cooling). Consequently, the adequacy of its underlying assumptions should always be considered in light of the model system being studied and the research question (e.g. whereas solar radiation may be of little importance on studies comparing species thermoregulatory performance across a wide range of environments, it may dramatically alter species thermoregulatory behavior on a daily basis). Nonetheless, the general constraint imposed by thermal balance remains true regardless of the difficulties on estimating empirically where the limits to heat production and dissipation lie, or the number of physiological factors that might be ultimately involved. In spite of the inherent limitations of Newton’s law of cooling as an oversimplified description of the mechanics of heat transfer (e.g., see Strunk 1971; Bakken and Gates 1974; Porter et al. 2000), understanding how this general model behaves can be enlightening on conceptual grounds and relevant as an analytical and predictive tool to study ecological phenomena.
In this context, substantial progress toward understanding how different organisms cope with their thermal environment is likely to be made by combining the relative contribution of residual variation in MR and C with allometric effects (see above). For example, Root (1988) suggested that thermoregulatory constraints ultimately explain northern distribution limits of many North American avian species during winter employing Newton’s cooling law. Repasky (1991) refuted this hypothesis, arguing that body size gradients would be expected because metabolism increases with size and no gradients in body size were detected in this dataset. However, a lack of association between northern distributions limits and body size does not exclude the possibility of thermal constraints because species of similar size may differ in physiological capacities and in thermal tolerance. Accordingly, the best model explaining avian distribution boundaries in this dataset included both allometric effects and residual variation in MR and C (Canterbury 2002), and latitudinal gradients in avian body size have been detected more recently in larger datasets (Ramirez et al. 2008; Olson et al. 2009). Similarly, on a temporal scale, declining body size has been proposed as a major response to ongoing climate change (Gardner et al. 2011), and thermoregulatory polygons may be employed to study from a theoretical perspective how changes in average and extreme temperatures might drive the evolution of body size in endothermic lineages and broad scale geographic trends (e.g., Smith et al. 2010; Smith and Lyons 2011; Teplitsky and Millien 2014).
Whereas in many systems the limited knowledge on how organismal traits may respond to environmental changes prevents physiological ecology from becoming a fully developed predictive science (see Violle et al. 2014), ultimately we contend that the lack of theoretical developments that translate current physiological understanding into formal mechanistic models remains the main impediment to study the ecological and evolutionary repercussions of thermoregulation in endotherms (see also Angilletta 2009). From an ecological perspective, thermoregulatory polygons describe how the thermal niche and energy expenditure of different endothermic organisms vary as a function of physiological parameters T b, MR and C (Eq. 1). Even though several models are available to estimate the niche breadth of endothermic species (Porter and Gates 1969; Porter et al. 2000; Humphries et al. 2002; Porter and Kearney 2009), the approach described here has three primary advantages that make it particularly compelling: it is intuitive, extraordinarily simple and the physiological variables involved can be easily measured. While the time and effort involved in constructing and validating new models constitute a major problem (Kearney and Porter 2009), Newton’s law of cooling has been used extensively and several parameters have already been measured in a multitude of species. The adaptive potential of different descriptors of the polygon, as well as correlated responses to selection, can be easily studied with complementary approaches, such as selection experiments (Konarzewski et al. 2005), repeatability and heritability analyses (e.g. Chappell et al. 1995; Bacigalupe et al. 2004b; Nespolo et al. 2005; Szafranska et al. 2007) and estimations of phenotypic selection for thermotolerance and/or energy expenditure in the field (e.g. Hayes and O’Connor 1999; Boratynski et al. 2010; Fletcher et al. 2015). Furthermore, polygons can be combined with microclimate data (Kearney et al. 2014a, b) to predict with increasing accuracy how changes in thermal regimes might affect different organisms. Overall, we consider that the proposed approach may constitute a powerful analytical tool to study the impact of thermoregulatory constraints on variables related to fitness such as energy balance and reproductive output, and help elucidating how species will be affected by ongoing climate change as other mechanistic models (Deutsch et al. 2008; Huey et al. 2009; Angilletta 2009; Dillon et al. 2010).
References
Anderson KJ, Jetz W (2005) The broad-scale ecology of energy expenditure of endotherms. Ecol Lett 8:310–318
Angilletta MJ (2009) Thermal adaptation: a theoretical and empirical synthesis. Oxford University Press, NY
Aschoff J (1981) Thermal conductance in mammals and birds: its dependence in body size and circadian phase. Comp Biochem Physiol 69A:611–619
Ashton KG, Tracy MC, de Queiroz A (2000) Is Bergmann’s rule valid for mammals? Am Nat 156:390–415
Bacigalupe LD, Bozinovic F (2002) Design, limitations and sustained metabolic rate: lessons from small mammals. J Exp Biol 205:2963–2970
Bacigalupe LD, Rezende EL, Kenagy GK, Bozinovic F (2003) Activity and space use by degus: a trade-off between thermal conditions and food availability? J Mamm 84:311–318
Bacigalupe LD, Nespolo RF, Opazo JC, Bozinovic F (2004a) Phenotypic flexibility in a novel thermal environment: phylogenetic inertia in thermogenic capacity and evolutionary adaptation in organ size. Physiol Biochem Zool 77:805–815
Bacigalupe LD, Nespolo RF, Bustamante DM, Bozinovic F (2004b) The quantitative genetics of sustained energy budget in a wild mouse. Evolution 58:421–429
Bakken GS (1976) A heat transfer analysis of animals: unifying concepts and the application of metabolic chamber data to field ecology. J Theor Biol 60:337–384
Bakken GS, Gates DM (1974) Notes on “heat loss from a Newtonian animal”. J Theor Biol 45:283–292
Bartholomew GA (1982) Body temperature and energy metabolism. In: Gordon MS (ed) Animal physiology. MacMillan, NY, pp 333–406
Berteaux D (1998) Testing energy expenditure hypotheses: reallocation versus increased demand in Microtus pennsylvanicus. Acta Theriol 43:13–21
Boratynski Z, Koskela E, Oksanen TA (2010) Sex-specific selection on energy metabolism: selection coefficients for winter survival. J Evol Biol 23:1969–1978
Boyles JG, Brack V (2009) Modeling survival rates of hibernating mammals with individual-based models of energy expenditure. J Mamm 90:9–16
Boyles JG, Willis CKR (2010) Could localized warm areas inside cold caves reduce mortality of hibernating bats affected by white-nose syndrome? Front Ecol Environ 8:92–98
Boyles JG, Smit B, McKechnie AE (2011) A new comparative metric for estimating heterothermy in endotherms. Physiol Biochem Zool 84:115–123
Boyles JG, Thompson AB, McKechnie AE, Malan E, Humphries MM, Careau V (2013) A global heterothermic continuum in mammals. Global Ecol Biogeogr 22:1029–1039
Bozinovic F, Rosenmann M (1989) Maximum metabolic rate of rodents: physiological and ecological consequences on distributional limits. Funct Ecol 3:173–181
Bozinovic F, Novoa FF, Veloso C (1990) Seasonal changes in energy expenditure and digestive tract of Abrothrix andinus (Cricetidae) in the Andes Range. Physiol Zool 63:1216–1231
Bozinovic F, Lagos JA, Marquet PA (1999) Geographic energetics of the Andean mouse, Abrothrix andinus. J Mamm 80:205–209
Bozinovic F, Ferri-Yañez F, Naya H, Araujo M, Naya D (2014) Thermal tolerances in rodents: species that evolved in cold climates exhibit a wider thermoneutral zone. Evol Ecol Res 16:143–152
Bradley SR, Deavers DR (1980) A re-examination of the relationship between thermal conductance and body weight in mammals. Comp Biochem Physiol 65A:465–476
Bronson FH (1985) Mammalian reproduction: an ecological perspective. Biol Reprod 32:1–26
Bruinzeel LW, Piersma T (1998) Cost reduction in the cold: heat generated by terrestrial locomotion partly substitutes for thermoregulation costs in Knot Calidris canutus. Ibis 140:323–328
Buckley LB, Hurlbert AH, Jetz W (2012) Broad-scale ecological implications of ectothermy and endothermy in changing environments. Global Ecol Biogeogr 21:873–885
Burger JW (1949) A review of experimental investigations on seasonal reproduction in birds. Wilson Bull 61:211–230
Calder WA, King JR (1972) Body weight and the energetics of temperature regulation: a re-examination. J Exp Biol 56:775–780
Canterbury G (2002) Metabolic adaptation and climatic constraints on winter bird distribution. Ecology 83:946–957
Capellini I, Venditti C, Barton RA (2010) Phylogeny and metabolic scaling in mammals. Ecology 91:2783–2793
Careau V (2013) Basal metabolic rate, maximum thermogenic capacity and aerobic scope in rodents: interaction between environmental temperature and torpor use. Biol Lett 9:20121104
Chai P, Chang AC, Dudley R (1998) Flight thermogenesis and energy conservation in hovering hummingbirds. J Exp Biol 201:963–968
Chappell MA, Hammond KA (2004) Maximal aerobic performance of deer mice in combined cold and exercise challenges. J Comp Physiol B 174:41–48
Chappell MA, Bachman GC, Odell JP (1995) Repeatability of maximal aerobic performance in Belding’s Ground Squirrels, Spermophilus beldingi. Funct Ecol 9:498–504
Chappell MA, Garland T, Rezende EL, Gomes FR (2004) Voluntary running in deer mice: speed, distance, energy costs and temperature effects. J Exp Biol 207:3839–3854
Clarke A, Rothery P (2008) Scaling of body temperature in mammals and birds. Funct Ecol 22:58–67
Clarke A, Rothery P, Isaac NJ (2010) Scaling of basal metabolic rate with body mass and temperature in mammals. J Anim Ecol 79:610–619
Conley KE (1985) Evaporative water loss: thermoregulatory requirements and measurements in the deer mouse and white rabbit. J Comp Physiol B 155:433–436
Conley KE, Porter WP (1986) Heat loss from deer mice (Peromyscus): evaluation of seasonal limits to thermoregulation. J Exp Biol 126:249–269
Cooper SJ (2002) Seasonal metabolic acclimatization in Mountain Chickadees and Juniper Titmice. Physiol Biochem Zool 75:386–395
Daan S, Masman D, Grownewold A (1990) Avian basal metabolic rates: their association with body composition and energy expenditure in nature. Am J Physiol 259:R333–R340
Dawson WR, Carey C (1976) Seasonal acclimatization to temperature in Cardueline Finches. J Comp Physiol 112:317–333
Dawson WR, O’Connor TP (1996) Energetic features of avian thermoregulatory response. In: Carey C (ed) Avian Energetics and Nutritional Ecology. Chapman & Hall, NY, pp 85–124
Dawson TJ, Fanning D, Bergin TJ (1978) Metabolism and temperature regulation in the New Guines monotreme Zaglossus bruijni. Aust Zool 20:99–104
Deutsch CA, Tewksbury JJ, Huey RB, Sheldon KS, Ghalambor CK, Haak DC, Martin PR (2008) Impacts of climate warming on terrestrial ectotherms across latitude. Proc Natl Acad Sci USA 105:6668–6672
Dillon ME, Wang G, Huey RB (2010) Global metabolic impacts of recent climate warming. Nature 467:704–707
Duttenhoffer MA, Swanson DL (1996) Relationship of basal to summit metabolic rate in passerine birds and the aerobic capacity model for the evolution of endothermy. Physiol Zool 69:1232–1254
Feist DD, White RG (1989) Terrestrial mammals in cold. In: Wang LCH (ed) Advances in comparative and environmental physiology 4. Springer, Berlin, pp 328–360
Fletcher QE, Speakman JR, Boutin S, Lane JE, McAdam AG, Gorrell JC, Coltman DW, Humphries MM (2015) Daily energy expenditure during lactation is strongly selected in a free-living mammal. Funct Ecol 29:195–208
Fournier F, Thomas DW, Garland T (1999) A test of two hypotheses explaining the seasonality of reproduction in temperate mammals. Funct Ecol 13:523–529
Galloway SDR, Maughan RJ (1997) Effects of ambient temperature on the capacity to perform prolonged cycle exercise in man. Med Sci Sports Exerc 29:1240–1249
Gardner JL, Peters A, Kearney MR, Joseph L, Heinsohn R (2011) Declining body size: a third universal response to warming? Trends Ecol Evol 26:285–291
Gavrilov VM (2014) Ecological and scaling analysis of the energy expenditure of rest, activity, flight, and evaporative water loss in passeriformes and non-passeriformes in relation to seasonal migrations and to the occupation of boreal stations in high and moderate latitudes. Quat Rev Biol 89:107–150
Geiser F, Baudinette RV (1987) Seasonality of torpor and thermoregulation in three dasyurid marsupials. J Comp Physiol B 157:335–344
Glazier DS (2008) Effects of metabolic level on the body size scaling of metabolic rate in birds and mammals. Proc R Soc B 275:1405–1410
Goldstein DL (1988) Estimates of daily energy expenditure in birds: the time-energy budget as an integrator of laboratory and field studies. Am Zool 28:829–844
Greenberg R, Cadena V, Danner RM, Tattersal G (2012) Heat loss may explain bill size differences between birds occupying different habitats. PLoS One 7:e40933
Greenwood PJ, Wheeler P (1985) The evolution of sexual size dimorphism in birds and mammals: a ‘hot blooded’ hypothesis. In: Greenwood PJ, Harvey PH, Slatkin M (eds) Evolution: essays in honour of John Maynard Smith. Cambridge University Press, Cambridge, pp 287–299
Grémillet D, Meslin L, Lescroel A (2012) Heat dissipation limit theory and the evolution of avian functional traits in a warming world. Funct Ecol 26:1001–1006
Gwazdaukas FC, Lineweaver JA, Vinson WE (1981) Rates of conception by artificial insemination of dairy cattle. J Dairy Sci 64:358–362
Hainsworth FR, Wolf LL (1970) Regulation of oxygen consumption and body temperature during torpor in a humming bird, Eulampis jugularis. Science 168:368–369
Hall CAS, Stanford JA, Hauer FR (1992) The distribution and abundance of organisms as a consequence of energy balances along multiple environmental gradients. Oikos 65:377–390
Hammond KA, Diamond J (1997) Maximal sustained energy budgets in humans and animals. Nature 386:457–462
Hammond KA, Kristan DM (2000) Responses to lactation and cold exposure by Deer Mice (Peromyscus maniculatus). Physiol Biochem Zool 73:547–556
Hammond KA, Konarzewski M, Torres R, Diamond JM (1994) Metabolic ceilings under a combination of peak energy demands. Physiol Zool 68:1479–1506
Hansen PJ (2009) Effects of heat stress on mammalian reproduction. Phil Trans R Soc B 364:3341–3350
Hart JS (1962) Seasonal acclimatization in four species of small wild birds. Physiol Zool 35:224–236
Hart JS (1971) Rodents. In: Whittow CG (ed) Comparative physiology of thermoregulation. Academic Press, NY, pp 1–149
Hayes JP, O’Connor CS (1999) Natural selection on thermogenic capacity of high altitude deer mice. Evolution 53:1280–1287
Heinrich B (1977) Why some animals have evolved to regulate a high body temperature? Am Nat 111:623–640
Heldmaier G, Ruf T (1992) Body temperature and metabolic rate during natural hypothermia in endotherms. J Comp Physiol B 162:696–706
Heldmaier G, Steinlechner S (1981) Seasonal pattern and energetics of short daily torpor in the Djungarian hamster, Phodopus sungorus. Oecologia 48:265–270
Heldmaier G, Steinlechner S, Rafael J (1982) Nonshivering thermogenesis and cold resistance during seasonal acclimatization in the Djungarian Hamster. J Comp Physiol B 149:1–9
Heller HC, Colliver GW (1974) CNS regulation of body tem perature during hibernation. Am J Physiol 227:583–589
Hensaw RE (1968) Thermoregulation during hibernation: application of Newton’s law of cooling. J Theor Biol 20:79–90
Hinds DS, Calder WA (1973) Temperature regulation of the Pyrrhuloxia and the Arizona cardinal. Physiol Zool 46:55–71
Hinds D, Baudinette RV, Macmillen RE, Halpern EA (1993) Maximum metabolism and the aerobic factorial scope of endotherms. J Exp Biol 182:41–56
Hirth K-D, Biesel W, Nachtigall W (1987) Pigeon flight in a wind tunnel III: regulation of body temperature. J Comp Physiol B 157:111–116
Holloway JC, Geiser F (2001) Seasonal changes in the thermoenergetics of the marsupial sugar glider, Petaurus breviceps. J Comp Physiol B 171:643–650
Hudson DM, Bernstein MH (1981) Temperature regulation and heat balance in flying White-Necked Ravens, Corvus cryptoleucus. J Exp Biol 90:267–281
Hudson LN, Isaac NJB, Reuman DC (2013) The relationship between body mass and field metabolic rate among individual birds and mammals. J Anim Ecol 82:1009–1020
Huey RB, Deutsch CA, Tewksbury JJ, Vitt LJ, Hertz PE, Alvarez-Perez HJ, Garland T (2009) Why tropical forest lizards are vulnerable to climate warming. Proc R Soc B 276:1939–1948
Humphries MM, Careau V (2011) Heat for nothing or activity for free? Evidence and implications of activity-thermoregulatory heat substitution. Integr Comp Biol 51:419–431
Humphries MM, Thomas DW, Speakman JR (2002) Climate-mediated energetic constraints on the distribution of hibernating mammals. Nature 418:313–316
Humphries MM, Boutin S, Thomas DW, Ryan JD, Selman C, McAdam AG, Berteaux D, Speakman JR (2005) Expenditure freeze: the metabolic response of small mammals to cold. Ecol Lett 8:1326–1333
Jetz W, Rahbek C (2002) Geographic range size and determinants of avian species richness. Science 297:1548–1551
Johnson MS, Speakman JR (2001) Limits to sustained energy intake V: effect of cold-exposure during lactation in Mus musculus. J Exp Biol 204:1967–1977
Karaca AG, Parker HM, McDaniel CD (2002) Elevated body temperature directly contributes to heat stress infertility of broiler breeder males. Poultry Sci 81:1892–1897
Kearney M, Porter WP (2009) Mechanistic niche modelling: combining physiological and spatial data to predict species’ ranges. Ecol Lett 12:334–350
Kearney M, Shamakhy A, Tingley R, Karoly DJ, Hoffmann AA, Briggs PR, Porter WP (2014a) Microclimate modelling at macro scales: a test of a general microclimate model integrated with gridded continental-scale soil and weather data. Methods Ecol Evol 5:273–286
Kearney M, Isaac AP, Porter WP (2014b) microclim: Global estimates of hourly microclimate based on long-term monthly climate averages. Sci Data 1:140006
Kerr J, Packer L (1998) The impact of climate change on mammal diversity in Canada. Environ Monit Assess 49:263–270
Khaliq I, Hof C, Prinziger R, Bohning-Gaese K, Pfenninger M (2014) Global variation in thermal tolerances and vulnerability of endotherms to climate change. Proc R Soc B 281:20141097
Kleiber M (1932) Body size and metabolism. Hilgardia 6:315–353
Kolokotrones T, Savage V, Deeds EJ, Fontana W (2010) Curvature in metabolic scaling. Nature 464:753–756
Konarzewski M, Diamond J (1994) Peak sustained metabolic rate and its individual variation in cold-stressed mice. Physiol Zool 67:1186–1212
Konarzewski M, Ksiazek A, Lapo IB (2005) Artificial selection on metabolic rates and related traits in rodents. Integr Comp Biol 45:416–425
Koteja P (1991) On the relation between basal and field metabolic rates in birds and mammals. Funct Ecol 5:56–64
Kurnath P, Dearing MD (2013) Warmer ambient temperatures depress liver function in a mammalian herbivore. Biol Lett 9:20130562
Landry-Cuerrier M, Murno D, Thomas DW, Humprhies MM (2008) Climate and resource determinants of fundamental and realized niches of hibernating chipmunks. Ecology 89:3306–3316
Larose J, Boulay P, Sigal RJ, Wright HE, Kenny GP (2013) Age-related decrements in heat dissipation during physical activity occur as early as the age of 40. PLoS One 8:e83148
Lichtwark GA, Wilson AM (2007) Is Achilles tendon compliance optimised for maximum muscle efficiency during locomotion? J Biochem 40:1768–1775
Liknes ET, Scott SM, Swanson DM (2002) Seasonal acclimatization in the American Goldfinch revisited: to what extent do metabolic rates vary seasonally? Condor 104:548–557
López-Calleja MJ, Bozinovic F (1995) Maximum metabolic rate, thermal insulation and aerobic scope in a small-sized Chilean hummingbird (Sephanoides sephaniodes). Auk 112:1034–1036
Lovegrove BG (2000) The zoogeography of mammalian basal metabolic rate. Am Nat 156:201–219
Lovegrove BG (2005) Seasonal thermoregulatory responses in mammals. J Comp Physiol B 175:231–247
Lovegrove BG, Heldmaier G, Knight M (1991) Seasonal and circadian energetic patterns in an arboreal rodent, Thallomys paedulcus, and a burrow-dwelling rodent, Aethomys namaquensis, from the Kalahari desert. J Therm Biol 16:199–209
Lovvorn JR (2007) Thermal substitution and aerobic efficiency: measuring and predicting effects of heat balance on endotherm diving energetics. Phil Trans R Soc B 362:2079–2093
Maloney SK, Moss G, Cartmell T, Mitchell D (2005) Alteration in diel activity patterns as a thermoregulatory strategy in black wildebeest (Connochaetes gnou). J Comp Physiol A 191:1055–1064
Masman D, Gordijn M, Daan S, Dijkstra C (1986) Ecological energetics of the kestrel: field estimates of energy intake throughout the year. Ardea 74:24–39
McKechnie AE, Lovegrove BG (2002) Avian facultative hypothermic responses: a review. Condor 104:705–724
McKechnie AE, Wolf BO (2010) Climate change increases the likelihood of catastrophic avian mortality events during extreme heat waves. Biol Lett 6:253–256
McNab BK (1980) On estimating thermal conductance in endotherms. Physiol Zool 53:145–156
McNab BK (1986) The influence of food habits on the energetics of eutherian mammals. Ecol Monogr 56:1–19
McNab BK (1988) Complications inherent in scaling the basal rate of metabolism in mammals. Quat Rev Biol 63:25–54
McNab BK (1995) Energy expenditure and conservation in frugivorous and mixed-diet carnivorans. J Mammal 76:206–222
McNab BK (2002a) The physiological ecology of vertebrates: a view from energetics. Cornell University Press, NY
McNab BK (2002b) Short-term energy conservation in endotherms in relation to body mass, habits, and environment. J Therm Biol 27:459–466
McNab BK (2009) Ecological factors affect the level and scaling of avian BMR. Comp Biochem Physiol 152:22–45
McNab (2012) Extreme measures: the ecological energetics of birds and mammals. Univ Chicago Press, Chicago
McNab BK, Morrison P (1963) Body temperature and metabolism in subspecies of Peromyscus from arid and mesic environments. Ecol Monograph 33:63–82
Meiri S, Dayan T (2003) On the validity of Bergmann’s rule. J Biogeogr 30:331–351
Millien V, Sk Lyons, Olson L, Smith FA, Wilson AB, Yom-Tov Y (2006) Ecotypic variation in the context of global climate change: revisiting the rules. Ecol Lett 9:853–869
Morrison P (1960) Some interrelations between weight and hibernation function. Bull Mus Comp Zool Harv 124:75–90
Morrison P, Ryser FA, Dawe AR (1959) Studies on the physiologyof the masked shrew Sorex cinereus. Physiol Zool 32:256–271
Nagy KA (2005) Field metabolic rate and body size. J Exp Biol 208:1621–1625
Nagy KA, Girard I, Brown TK (1999) Energetics of free-ranging mammals, repriles and birds. Annu Rev Nutr 19:247–277
Naya DE, Spangerberg L, Naya H, Bozinovic F (2013a) How does evolutionary variation in basal metabolic rates arise? A statistical assessment and a mechanistic model. Evolution 67:1463–1476
Naya DE, Spangerberg L, Naya H, Bozinovic F (2013b) Thermal conductance and basal metabolic rate are part of a coordinated system for heat transfer regulation. Proc R Soc B 280:20131629
Nespolo RF, Bacigalupe LD, Rezende EL, Bozinovic F (2001) When non-shivering thermogenesis equals maximun metabolic rate: thermal acclimation and phenotypic plasticity of fossorial Spalacopus cyanus (Rodentia). Physiol Biochem Zool 74:325–332
Nespolo RF, Bustamante DM, Bacigalupe LD, Bozinovic F (2005) Quantitative genetics of bioenergetics and growth-related traits in the wild mammal, Phyllotis darwini. Evolution 59:1829–1837
Nybo L (2008) Hyperthermia and fatigue. J Appl Physiol 104:871–878
Odongo NE, AlZahal O, Lindinger MI, Duffield TF, Valdes EV, Terrell SP, McBride BW (2006) Effects of mild heat stress and grain challenge on acid-base balance and rumen tissue histology in lambs. J Anim Sci 84:447–455
Okrouhlík J, Burda H, Kunc P, Knížková I, Šumbera R (2015) Surprisingly low risk of overheating during digging in two subterranean rodents. Physiol Behav 138:236–241
Olson JR (2009) Metabolic performance and distribution in Black-Capped (Poecile atricapillus) and Carolina Chickadees (P. carolinensis). Ph.D. dissertation, Ohio State University. xvi + 141 pp
Olson VA, Davies RG, Orme CDL, Thomas GH, Meiri S, Blackburn TM, Gaston KJ, Owens IPF, Bennett PM (2009) Global biogeography and ecology of body size in birds. Ecol Lett 12:249–259
Owen-Smith RN (1988) Megaherbivores: the influence of very large body size on ecology. Cambridge University Press, Cambridge
Perrin MR, Richardson EJ (2005) Metabolic rate, maximum metabolism, and advantages of torpor in the fat mouse Steatomys pratensis natalensis. J Therm Biol 30:603–610
Peters RH (1983) The ecological implications of body size. Cambridge University Press, New York
Peterson CC, Nagy KH, Diamond J (1990) Sustained metabolic scope. Proc Natl Acad Sci USA 87:2324–2328
Piersma T (2011) Why marathon migrants get away with high metabolic ceilings: towards an ecology of physiological restraint. J Exp Biol 214:295–302
Piersma T, Cadée N, Daan S (1995) Seasonality in basal metabolic rate and thermal conductance in a long-distance migrant shorebird, the knot (Calidris canutus). J Comp Physiol B 165:37–45
Pigot AL, Owens IPF, Orme CDL (2010) The environmental limits to geographic range expansion in birds. Ecol Lett 13:705–715
Porter WP, Gates DM (1969) Thermodynamic equilibria of animals with environment. Ecol Monographs 39:227–244
Porter WP, Kearney M (2009) Size, shape, and the thermal niche of endotherms. Proc Natl Acad Sci USA 106:19666–19672
Porter WP, Munger JC, Stewart WE, Budaraju S, Jaeger J (1994) Endotherm energetics: from a scalable individual-based model to ecological applications. Austral J Zool 42:125–162
Porter WP, Budaraju S, Stewart WE, Ramankutty N (2000) Calculating climate effects on birds and mammals: impacts on biodiversity, conservation, population parameters, and global community structure. Am Zool 40:597–630
Prinzinger R, Prebmar A, Schleucher E (1991) Body temperature in birds. Comp Biochem Physiol 99A:499–506
Ramirez L, Diniz-Filho JAF, Hawkins BA (2008) Partitioning phylogenetic and adaptive components of the geographical body-size pattern of New World birds. Global Ecol Biogeogr 17:100–110
Rauw WM (2009) Resource allocation theory applied to farm animal production. CABI Publishing, Wallingford
Renaudeau D, Noblet J, Dourmad JY (2003) Effect of ambient tempera- ture on mammary gland metabolism in lactating sows. J Anim Sci 81:217–231
Repasky RR (1991) Temperature and the northern distributions of wintering birds. Ecology 72:2274–2285
Rezende EL, Swanson DL, Novoa FF, Bozinovic F (2002) Passerines versus nonpasserines: so far, no statistical differences in avian energetics. J Exp Biol 205:101–107
Rezende El, Cortés A, Bacigalupe LD, Nespolo RF, Bozinovic F (2003) Ambient temperature limits above-ground activity of the subterranean rodent Spalacopus cyanus. J Arid Envir 55:63–74
Rezende EL, Bozinovic F, Garland T (2004) Climatic adaptation and the evolution of maximum and basal rates of metabolism in rodents. Evolution 58:1361–1374
Ricklefs RE (1974) Energetics of reproduction in birds. In: Payntery RA (ed) Avian energetics. Publ Nuttall Omithol 15, pp 152–292
Ricklefs RE, Konarzewski M, Daan S (1996) The relationship between basal metabolic rate and daily energy expenditure in birds and mammals. Am Nat 147:1047–1071
Riek A, Geiser F (2013) Allometry of thermal variables in mammals: consequences of body size and phylogeny. Biol Rev 88:564–572
Rodrigues-Serrano E, Bozinovic F (2009) Interplay between global patterns of environmental temperature and variation in nonshivering thermogenesis of rodent species across large spatial scales. Global Change Biol 15:2116–2122
Rogowitz GL (1996) Trade-offs in energy allocation during lactation. Am Zool 36:197–204
Rogowitz GL (1998) Limits to milk flow and energy allocation during lactation of the hispid cotton rat (Sigmodon hispidus). Physiol Zool 71:312–320
Root T (1988) Energy constraints on avian distributions and abundances. Ecology 69:330–339
Rosenmann M, Morrison P (1974) Maximum oxygen consumption and heat loss facilitation in small homeotherms by He-O2. Am J Physiol 226:490–495
Rosenmann M, Morrison P, Feist D (1975) Seasonal changes in the metabolic capacity of Red-backed Voles. Physiol Zool 48:303–310
Schleucher E, Withers PC (2001) Re-evaluation of the allometry of wet thermal conductance for birds. Comp Physiol Biochem A 129:821–827
Schmidt-Nielsen K (1997) Animal physiology: adaptation and environment. Cambridge University Press, Cambridge
Schmidt-Nielsen K, Dawson TJ, Crawford EC (1966) Temperature regulation in the echidna (Tachyglossus aculeatus). J Cell Physiol 67:63–71
Scholander PF, Hock R, Walters V, Johnson F, Irving L (1950) Heat regulation in some arctic and tropical mammals and birds. Biol Bull 99:259–271
Sears MW, Hayes JP, O´Connor CSO, Geluso K, Sedinger JS (2006) Individual variation in thermogenic capacity affects above-ground activity of high-altitude deer mice. Funct Ecol 20:97–104
Setchell BP, D’Occhio MJ, Hall MJ, Laurie MS, Tucker MJ, Zupp JL (1988) Is embryonic mortality increased in normal female rats mated to subfertile males? J Reprod Fert 82:567–574
Sieg A, O’Connor MP, McNair JN, Grant BW, Agosta SJ, Dunham AE (2009) Mammalian metabolic allometry: do intraspecific variation, phylogeny, and regression models matter? Am Nat 174:720–733
Simons MJP, Reimert I, van der Vinne V, Hambly C, Vaanholt LM, Speakman JR, Gerkema MP (2011) Ambient temperature shapes reproductive output during pregnancy and lactation in the common vole (Microtus arvalis): a test of the heat dissipation limit theory. J Exp Biol 214:38–49
Smit B, McKechnie AE (2010) Avian seasonal metabolic variation in a subtropical desert: basal metabolic rates are lower in winter than in summer. Funct Ecol 24:330–339
Smith FA, Lyons SK (2011) How big should a mammal be? A macroecological look at mammalian body size over space and time. Phil Trans R Soc B 366:2364–2378
Smith FA, Boyer AG, Brown JH, Costa DP, Dayan T, Ernest SKM, Evans AR, Fortelius M, Gittleman JL, Hamilton MJ, Harding LE, Lintulaakso K, Lyons SK, McCain C, Okie JG, Saarinen JJ, Sibly RM, Stephens PR, Theodor J, Uhen MD (2010) The evolution of maximum body size of terrestrial mammals. Science 330:1216–1219
Soobramoney S, Downs CT, Adams NJ (2003) Physiological variability in the Fiscal Shrike Lanius collaris along an altitudinal gradient in South Africa. J Therm Biol 28:581–594
Speakman JR (2000) The cost of living: field metabolic rates of small mammals. Adv Ecol Res 30:177–297
Speakman JR (2008) The physiological costs of reproduction in small mammals. Phyl Trans R Soc B 363:375–398
Speakman JR, Król E (2010a) Maximal heat dissipation capacity and hyperthermia risk: neglected key factors in the ecology of endotherms. J Anim Ecol 79:726–746
Speakman JR, Król E (2010b) The heat dissipation limit theory and evolution of life histories in endotherms: time to dispose of the disposable soma theory? Integr Comp Biol 5:793–807
Speakman JR, Hays CG, Webb PI (1994) Is hyperthermia a constraint on the diurnal activity of bats? J Theor Biol 171:325–341
Speakman JR, Al-Jothery AH, Król E, Hawkins J, Chetoui A, Saint-Lambert A, Gamo Y, Shaw SC, Valencak T, Bünger L, Hill W, Vaanholt L, Hambly C (2014) Limits to sustained energy intake. XXII. Reproductive performance of two selected mouse lines with different thermal conductance. J Exp Biol 217:3718–3732
Strunk TH (1971) Heat loss from a Newtonian animal. J Theor Biol 33:35–61
Swanson DL (2010) Seasonal metabolic variation in birds: functional and mechanistic correlates. In: Thompson CF (ed) Current Ornithology, vol 17. Springer, New York, pp 75–129
Swanson DL, Garland T (2009) The evolution of high summit metabolism and cold tolerance in birds and its impact on present-day distributions. Evolution 63:184–194
Swanson DL, Liknes ET (2006) A comparative analysis of thermogenic capacity and cold tolerance in small birds. J Exp Biol 209:466–474
Symonds MRE, Elgar MA (2002) Phylogeny affects estimation of metabolic scaling in mammals. Evolution 56:2330–2333
Szafranska PA, Zub K, Konarzewski M (2007) Long-term repeatability of body mass and resting metabolic rate in free-living weasels, Mustela nivalis. Funct Ecol 21:731–737
Teplitsky C, Millien V (2014) Climate warming and Bergmann’s rule through time: is there any evidence? Evol Appl 7:156–168
Thomas SP, Suthers RA (1972) The physiology and energetics of bat fligh. J Exp Biol 57:317–335
Tieleman BI, Williams JB (1999) The role of hyperthermia in the water economy of desert birds. Physiol Biochem Zool 72:87–100
Tieleman BI, Williams JB, LaCroix F, Paillat P (2002) Physiological responses of Houbara bustards to high ambient temperatures. J Exp Biol 205:503–511
Tieleman BI, Williams JB, Bloomer P (2003) Adaptation of metabolism and evaporative water loss along an aridity gradient. Proc R Soc Lond B 270:207–214
Torre-Bueno JR (1976) Temperature regulation and heat dissipation during flight in birds. J Exp Biol 65:471–482
Vezina F, Jalvingh KM, Dekinga A, Piersma T (2006) Acclimation to different thermal conditions in a northerly wintering shorebird is driven by body mass-related changes in organ size. J Exp Biol 209:3141–3154
Violle C, Reich PB, Pacala SW, Enquist BJ, Kattje J (2014) The emergence and promise of functional biogeography. Proc Natl Acad Sci USA 38:13690–13696
Voigt CC, Lewanzik D (2011) Trapped in the darkness of the night: thermal and energetic constraints of daylight flight in bats. Proc R Soc B 278:2311–2317
Walsberg GE (2000) Small mammals in hot deserts: some generalizations revisited. Bioscience 50:109–120
Wang LCH (1989) Ecological, physiological, and biochemical aspects of torpor in mammals and birds. In: Wang LCH (ed) Comparative and Environmental Physiology 4. Springer, Berlin, pp 361–402
Wanner SP, Costa KA, Soares ADN, Cardoso VN, Coimbra CC (2014) Physical exercise-induced changes in the core body temperature of mice depend more on ambient temperature than on exercise protocol or intensity. Int J Biometeor 58:1077–1085
Wauters LA (2000) Squirrels: medium-sized granivores in woodland habitats. In: Halle S, Stenseth NC (eds) Activity patterns in small mammals: an ecological approach. Springer, Berlin, pp 131–144
Weathers W, Schoenbaechler C (1976) Contribution of gular flutter to evaporative cooling in Japanese quail. J Appl Physiol 40:521–524
Weathers WW, Sullivan KA (1993) Seasonal patterns of time and energy allocation by birds. Physiol Zool 66:511–536
Webb DR (1987) Thermal tolerance of avian embryos: a review. Condor 89:874–898
Webster MD, Weathers WW (1990) Heat produced as a by-product of foraging activity contributes to thermoregu- lation by verdins, Auriparus flaviceps. Physiol Zool 63:777–794
Weibel ER, Bacigalupe LD, Schmitt B, Hoppeler H (2004) Allometric scaling of maximal metabolic rate in mammals: muscle aerobic capacity as determinant factor. Resp Physiol Neurobiol 140:115–132
Weinert D, Waterhouse J (1998) Diurnally changing effects of locomotor activity on body temperature in laboratory mice. Physiol Behav 63:837–843
Westerterp KR, Speakman JR (2008) Physical activity energy expenditure has not declined since the 1980s and matches energy expenditures of wild mammals. Int J Obesity 32:1256–1263
White CR, Seymour RS (2005) Allometric scaling of mammalian metabolism. J Exp Biol 208:1611–1619
White CR, Blackburn TM, Marin GR, Butler PJ (2007) Basal metabolic rate of birds is associated with habitat temperature and precipitation, not primary productivity. Proc R Soc B 274:287–293
White CR, Blackburn TM, Seymour RS (2009) Phylogenetically informed analysis of the allometry of mammalian basal metabolic rate supports neither geometric nor quarter-power scaling. Evolution 63:2658–2667
Wiener J (1992) Physiological limits to sustainable energy budgets in birds and mammals: ecological implications. Trends Ecol Evol 7:384–388
Wiersma P, Chappell MA, Williams JB (2007a) Cold- and exercise-induced peak metabolic rates in tropical birds. Proc Natl Acad Sci USA 104:20866–20871
Wiersma P, Muñoz-Garcia Waler A, Williams JB (2007b) Tropical birds have a slow pace of life. Proc Natl Acad Sci USA 104:9340–9345
Williams JB, Tieleman BI (2000) Flexibility in basal metabolic rate and evaporative water loss among hoopoe larks exposed to different environmental temperatures. J Exp Biol 203:3153–3159
Withers PC (1992) Comparative animal physiology. Saunders, Fort Worth
Withers PC, Cooper CE, Larcombe AN (2006) Environmental correlates of physiological variables in marsupials. Physiol Biochem Zool 79:437–453
Wunder BA (1970) Energetics of running activity in Merriam’s chipmunk, Eutamias merriami. Comp Biochem Physiol 33:821–836
Wunder BA (1975) A model for estimating metabolic rate of active or resting mammals. J Theor Biol 49:345–354
Zerba E, Walsberg GE (1992) Exercise-generated heat contributes to thermoregulation by Gambel’s quail in the cold. J Exp Biol 171:409–422
Zerba E, Dana AN, Lucia MA (1999) The influence of wind and locomotor activity on surface temperature and energy expenditure of the eastern house finch (Carpodacus mexicanus) during cold stress. Physiol Biochem Zool 72:265–276
Zhang X-Y, Wang D-H (2007) Thermogenesis, food intake and serum leptin in cold-exposed lactating Brandt’s voles Lasiopodomys brandtii. J Exp Biol 210:512–521
Zub K, Fletcher QE, Szafranska PA, Konarzewski M (2013) Male weasels decrease activity and energy expenditure in response to high ambient temperatures. PLoS One 8:e72646
Zub K, Szafrańska PA, Konarzewski M, Redman P, Speakman JR (2009) Trade-offs between activity and thermoregulation in a small carnivore, the least weasel Mustela nivalis. Proc R Soc B 276:1921–1927
Acknowledgments
We thank L. E. Castañeda, M. Santos, F. Bozinovic and D. L. Swanson for discussions at different stages of the conception of this manuscript and comments on previous drafts, and Ian Hume and four anonymous reviewers for their extensive comments on the original submission. LDB was supported by a FONDECYT grant 1120461.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by I. D. Hume.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Rezende, E.L., Bacigalupe, L.D. Thermoregulation in endotherms: physiological principles and ecological consequences. J Comp Physiol B 185, 709–727 (2015). https://doi.org/10.1007/s00360-015-0909-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00360-015-0909-5