Abstract
Ectothermic animals adapted to different environmental temperatures are hypothesized to have biological membranes with different chemical and physical properties such that membrane properties are optimized for their particular thermal environments. To test this hypothesis we analyzed the composition of phospholipid fatty acids (PLFAs) in seven different populations of Enchytraeus albidus originating from different thermal environments. The seven populations differ markedly in origin (polar-temperate) and are also characterized by marked difference in cold tolerance. The dominant PLFAs of E. albidus were C20:5, C20:4 and C20:2 (53–61 % of total PLFA) followed by C18:0, C20:1 and C22:2 (17–20 % of total PLFA). As hypothesized the PLFA composition varied significantly between populations and molar percentage of several of the PLFAs (particularly C18:2) correlated with the lower lethal temperature (LT50) of the seven populations. Unsaturation ratio (UFA/SFA) and average PLFA chain length also correlated significantly with LT50, such that cold sensitive populations had a shorter chain length and a lower UFA/SFA compared to cold tolerant populations. Reconstituted membranes of the least and most cold tolerant populations were used to compare membranes’ physical properties by fluorescence anisotropy and bending rigidity. Measurements of anisotropy did not show any overall difference between populations with different cold tolerance. This could be interpreted as if E. albidus populations have achieved a similar “optimal” fluidity of the membrane with a somewhat different PLFA composition. Our study suggests that membrane lipid composition could be important for the cold tolerance of E. albidus; however, these differences are not easily differentiated in the measurements of the membranes’ physical properties. Other parameters such as accumulation of glucose for cryoprotection and energy supply may also be important components of enchytraeid freeze tolerance.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The cell membrane functions as a selective barrier between the intra- and extra-cellular compartments. In fully functioning cells, membranes are in a liquid–crystalline phase (Hazel 1995; Hazel and Williams 1990). When biological membranes are cooled sufficiently they gradually undergo a transition from the liquid–crystalline phase to gel phase whereby the membrane may lose some of its selective properties (Hazel 1995; Hazel and Williams 1990). Such a phase transition may, for example, induce the clustering of integral membrane proteins (James and Branton 1973) or lead to dissipation of transmembrane ion gradients (Singer 1981). The composition of membrane phospholipids is highly important for the temperature at which the phase transition occurs, and it is often found that cold adaptation or cold acclimation is associated with changes in the membrane phospholipid composition (Hazel 1989; Kostal 2010). This phenomenon is known as “homeoviscous adaptation” (HVA), and in ectothermic organisms it helps to preserve the appropriate physico-chemical properties of the membrane during temperature changes (Cossins 1977a, b; Hazel and Williams 1990; Kostal 2010; Sinensky 1974). HVA is efficient mainly due to biochemical changes of the membrane including phospholipid head-groups, cholesterol content and the composition of the phospholipid fatty acyl side-chains (PLFAs) (Cossins and Bowler 1987; Hazel 1995; Hazel and Williams 1990; Murray et al. 2007). Although HVA should ensure optimal membrane properties at the temperature at which the organism is acclimated, there are some studies suggesting adaptive differences in membrane composition that could be related to population differences in tolerance to acute cold exposures (Holmstrup et al. 2007; Ohtsu et al. 1998). Cold adapted populations may have a different membrane composition than warm adapted populations even if they are maintained at the same temperature. In this context HVA can be seen as a dynamic response that ensures both optimal conditions at the temperature that the animal is acclimated, but also affects the reaction norm if the animal is exposed acutely to a different temperature.
It is well-known that alterations of membrane lipids occur during cold acclimation in ectothermic organisms (Cossins and Bowler 1987; Hayward et al. 2007). The responses to low temperatures typically include changes in membrane lipid composition that are associated with a desaturation (increase of unsaturated fatty acids), shortening of the average fatty acid chain length, head group restructuring and changes of the cholesterol-to-phospholipid ratio (reviewed by: Hazel 1989; Kostal 2010). These changes contribute differently to the preservation of the fluid membrane and also to the reduction in phase transition temperature (Hazel 1995; Hazel and Williams 1990; Kostal 2010). More unsaturated PLFAs increase membrane fluidity, which partly is due to the lower melting temperature of unsaturated PLFAs compared to saturated PLFAs; and similarly short PLFAs will have a lower melting temperature compared to longer PLFAs (Huang et al. 1997; Kostal 2010). Moreover, phospholipids with phosphatidylethanolamine (PE) as head group have a higher phase transition temperature and thereby create a less fluid membrane compared to membranes with high proportions of phosphatidylcholine phospholipids (PC) (Huang et al. 1996, 1997). Finally, cholesterol changes the physiological and functional properties of the membrane, however, its impact depends on the phase state of the membrane (Hazel 1989; Kostal 2010). Overall, the behavior of mixtures is relatively complex, and sometimes it is difficult to predict fluidity based on simple assumptions.
Earlier studies of membrane adaptation in relation to cold tolerance of terrestrial invertebrates are most often based on correlating the lipid composition with the cold tolerance using HVA as explanatory paradigm, but without directly measuring the HVA parameters (Bahrndorff et al. 2007; Bennett et al. 1997; Holmstrup et al. 2007; Kostal and Simek 1998; Ohtsu et al. 1998; Overgaard et al. 2009; van Dooremalen and Ellers 2010). Historically only few studies linked membrane composition and thermal adaptations to physical properties of biological membranes of fish (see for example: Cossins 1977a; Cossins et al. 1977; Hazel 1989), however, during the last years a number of papers have also measured the physical properties of membranes (including membrane fluidity) and linked these to composition and thermal adaptation in terrestrial invertebrates (Bouvrais et al. 2013; Kostal 2010; Los and Murata 2004; Rais et al. 2010; Rozsypal et al. 2014; Waagner et al. 2013a). Most studies considering the physical properties have used fluorescence anisotropy (polarization) to characterize membrane physical properties in ectothermic animals (Lentz 1989; Litman and Barenholz 1982; Rozsypal et al. 2014). Simply put, fluorescence anisotropy is a measure of the orientation freedom experienced by a fluorescent molecule embedded in the membrane with more “fluid” membranes having a lower degree of anisotropy. An alternative measure of the membrane’s physical properties is, the “bending rigidity” of membranes, which can be obtained using the technique of Giant Vesicle Fluctuation Analysis. This is a parameter describing the stiffness of the membrane bilayer (Bouvrais 2012; Henriksen et al. 2004), and different levels of stiffness have been linked to variance in low temperature tolerance of springtails and enchytraeids (Holmstrup et al. 2014; Waagner et al. 2013a). The concept of HVA was developed to explain the compensatory behavior of functional membranes in hydrated cells. For freeze-tolerant organisms the situation might be slightly different due to the extensive freezing-induced dehydration of cells. However, a few studies show that HVA is also applicable to freeze-tolerant species (Holmstrup et al. 2014; Kostal et al. 2003).
The terrestrial oligochaete Enchytraeus albidus is a freeze-tolerant species (i.e. it tolerates internal ice formation) and it has a broad geographical distribution. Populations originating from different environmental conditions (from temperate to arctic environments) are all freeze tolerant, but have markedly different cold tolerance when examined in common garden experiments (Fisker et al. 2014a, b; Patrício Silva et al. 2013; Slotsbo et al. 2008). As other freeze-tolerant oligochaete species, E. albidus mobilize high concentrations of glucose during freezing, which acts as a cryoprotectant and as a fuel for metabolism during overwintering (Fisker et al. 2014b; Slotsbo et al. 2008). However, other physiological adaptations to low temperature, including for example, adaptive adjustments of membrane lipid composition, are possibly also important for freeze tolerance. In the present study we therefore employed a common garden approach (populations originating from different environments were transplanted in a common laboratory environment) to test the hypotheses that (a) differences in cold tolerance is related to differences in PLFA composition (as a molar percentage of individual PLFAs, chain length and unsaturation, respectively, at a given temperature), and (b) the more cold tolerant worms have the same membrane fluidity when cold acclimated at 5 °C as less cold tolerant worms even though there are observed differences in PLFA composition. To evaluate if membrane PLFA composition was related to cold tolerance we investigated seven geographically distinct populations that differed considerably in their cold tolerance. Further, the two populations that differed most with respect to cold tolerance were used to relate the cold tolerance and PLFA composition to differences in membrane fluidity parameters.
Materials and methods
Phospholipid fatty acid extraction
PLFAs were extracted from enchytraeids that originated from seven different populations (Table 1). All populations had been maintained in a laboratory culture for at least two generations at 5 °C before they were sampled. Upon sampling, worms were placed in 2 mL Eppendorf vials and snap frozen in liquid nitrogen. Samples were freeze dried for 24 h and stored at −80 °C before extraction of phospholipids (PL) used to evaluate membrane PLFA composition or measurements of fluorescence anisotropy and bending rigidity of reconstituted membranes. To extract phospholipids a phosphate buffer (0.75 mL, 50 mM, pH 7.4) and a steel ball were added to the vials and the worms were crushed with a TissueLyser II (Qiagen, Copenhagen, Denmark) at 30 Hz for 30 s after which another 0.75 mL phosphate buffer was added. The samples were then transferred to 12 mL glass centrifuge tubes and crude lipids were extracted by adding 3 mL methanol and 1.5 mL chloroform. The tubes were mixed for 1 min and incubated at room temperature for 3 h after which another 1.5 mL chloroform and 1.5 mL phosphate buffer was added. Samples were mixed again and then left overnight at room temperature after which samples were centrifuged at 1,600g for 10 min ensuring separation in water (hydrophilic) and organic (hydrophobic) phases. The water phase was discarded, and the organic phase was transferred to clean glass tubes. One-third was transferred to a tube and kept at −80 °C for later analysis of cholesterol. The remaining organic phase was evaporated under a stream of N2. Phospholipids were separated from other lipids using solid-phase silica columns (100 mg; Bond Elute, Agilent Technologies, Santa Clara, CA, USA). The columns were conditioned with 1.5 mL chloroform and the samples were added by re-dissolving them in 3 × 300 µL chloroform. Using vacuum, the samples were slowly filtered through the columns. A volume of 1.5 mL chloroform was pulled through the columns to elute neutral lipids (mainly triglycerides) followed by 6 mL acetone to elute lipids of intermediate polarity. These samples were discarded and not considered in the present study. Polar lipids (mainly phospholipids) were then eluted into glass vials by washing the silica columns with 1.5 mL methanol. Samples used for measurements of fluorescence anisotropy and bending rigidity were not extracted further than this step. Samples used for PLFA analysis were evaporated under N2 after which phospholipids were trans-esterified by a mild alkaline methanolysis (Dowling et al. 1986; Waagner et al. 2013b) and dried again under N2. Finally the fatty acid methyl esters (FAME) were re-dissolved in heptane for further analysis.
Membrane composition and cholesterol analysis
The FAME composition was analyzed by gas chromatography mass spectrometry (Shimadzu GCMS-QP2010 Plus) equipped with an autosampler (Columbia, MD, USA) using the same procedure as described in Waagner et al. (2013b). The mol % distributions were calculated for each PLFA together with a number of generalized indexes such as average number of C atoms in the fatty acid chains (length), UFA/SFA ratio (Σ unsaturated fatty acids/Σ saturated fatty acids), mol % poly unsaturated fatty acids (PUFA), and the degree of unsaturation (calculated as Σ(% monoenes + 2 × % dienes + 3 × % trienes…)/100) (Kates 1986).
As mentioned above one-third of the organic phase of the crude lipid extract was used for cholesterol analysis. The organic phase was evaporated under a stream of nitrogen and then silylated by adding 900 µL pyridine, 90 µL hexamethyldisilizane, and 10 µL chlorotrimethylsilane (all Sigma Aldrich, Brøndby, Denmark). Silylation took place under 2 h of incubation period while the samples were held in darkness at room temperature. After this the samples were centrifuged and the supernatant transferred to autosampling vials for GCMS analysis. A Shimadzu GCMS-QP2010 (Shimadzu, Kyoto, Japan) with autosampler and a factorFourTM capillary column VF-5 ms (length 30 m, inner diameter 0.25 mm, film thickness of 0.25 µm; Varian, Netherlands) was used to perform the cholesterol analysis. The autosampler was operating in split mode with a split ratio of 5 and the injection volume was 2.0 µL. Helium was used as carrier gas with a total flow of 7.2 mL min−1 and a column flow at 0.70 mL min−1. Injection temperature was 250 °C and the oven was programed as follows: initial temperature of 50 °C was held for 2 min, then increased to 230.0 °C with a rate of 20.0 °C min−1, finally it was increased to 280 °C with a rate of 8.0 °C min−1 where it was held for 5 min. Electron ionization mode at 70 eV was used for the mass spectrometer. The ion source temperature was 210.0 °C and the interface temperature 250.0 °C. Identification of cholesterol was based on external standards (Avanti lipids). GCMS Solution software (Shimadzu, Kyoto, Japan) was used for identification of peaks and calculation of concentration was based on specific standard curves.
Bending rigidity
Giant Unilamellar Vesicles (GUVs) were produced using whole body membrane E. albidus phospholipids (PL) extracts obtained as described above. Samples of ~100 mg dry tissue produced about 1 mg dry mass phospholipids. Since cholesterol was removed from the phospholipid fraction during the extraction procedure, a quantity of cholesterol was introduced as a chloroform cholesterol solution (Avanti lipids). Cholesterol was added to get cholesterol:PL molar ratio of ~20 % (see Bouvrais et al. (2013) for further details). The solvent (methanol/chloroform) of cholesterol:PL solution was removed using rotary evaporator ensuring that the dried lipid sample formed a film on the glass surface. The dried lipid film was subsequently hydrated using MilliQ water (Millipore, Bedford MA, USA) to obtain multilamellar vesicles (MLVs) after gentle agitation. Small unilamellar vesicles (SUVs) were produced using sonication (Misonix sonicator 3000), which lasted 30 min with the power fixed at 3 W successively on for 10 s and off for 5 s to prevent heating of the sample. The sonicated dispersion was then centrifuged and filtered using a 0.2 mm filter (sterile cellulose acetate membrane) to remove metal particles released by the tip of the sonicator. Finally, Giant Unilamellar Vesicles were prepared using the electroformation method following the protocol described by Pott et al. (2008). SUVs deposits at a lipid concentration of about 0.1 mg mL−1 were made on the electrodes. The water from the deposits was partially evaporated during 3–4 h under reduced pressure by introducing the electrodes in a desiccator and during this step the electrodes were protected from light to prevent lipid damages. Then, the electrodes were immersed in pure water previously introduced in a glass cell. An electric field was applied to the electrodes using a waveform generator (Agilent 33120A 15 MHz function). Vesicles with a diameter between 10 and 50 µm were visible on the electrodes after a few hours.
After having been detached from the electrodes at the end of the electroformation protocol, vesicles were observed directly in the electroformation cuvette, which was placed in a custom-made temperature-controlled chamber set at 20 °C. Vesicles were visualized using a phase contrast microscope (Axiovert S100 Zeiss, Göttingen, Germany), equipped with an x40/0.60 objective (440865 LD Achroplan), so that the vesicle two-dimensional contour could be seen in the focal plane of the objective. A CCD Camera (SONY SSCDC50AP) was used to record a series of 15,000 pictures at a rate of 25 frames s−1 with a video integration time of 4 ms. The video image sequences of the GUV thermal fluctuations were analyzed as described previously by Mitov et al. (1992). Information regarding custom-made software to perform contour extraction, contour cleaning and fluctuations analysis procedures are found in Mitov et al. (1992). The bending rigidity was determined by a precise analysis of the statistical distribution of vesicle contours based on a simple Fourier decomposition of the angular correlation function as described previously (Meleard et al. 2011). The bending elastic modulus, κ, represented an average of measurements amongst a population of 13 and 17 vesicles made using E. albidus lipid extracts coming from Germany and Svalbard, respectively.
Fluorescence anisotropy
Approximately 15 mg enchytraeid dry mass (expected to contain 0.15 mg of phospholipids) were used per sample of which one-third was used for the final measurements. Small unilamellar vesicles (SUVs) were made in several steps. First, the methanol was evaporated and then 750 µL of chloroform was added. Cholesterol was added with the chloroform to an approximate final cholesterol:PL molar ratio of ~20 %. The chloroform was evaporated under nitrogen flow while slowly rolling the glass vial (to distribute the lipids in an even coating on the glass wall). Samples were placed in a 40 °C water bath for 20 min under constant nitrogen flow and subsequently placed in a desiccator under vacuum for 1 h at room temperature to further evaporate all solvents. Volumes of 7.5 mL milliQ water were added to 0.15 mg PL to have a final concentration of 0.05 mg PL in 2.5 mL milliQ water. After the addition of water, the vials were flushed with nitrogen and vortexed, wrapped in tin foil and placed in a shaking 40 °C water bath for 1 h to redissolve the phospholipids. Every 15 min the samples were vortexed for approximately 20 s leading to formation of large multilamellar vesicles. To finally form the SUVs, the samples were sonicated in a bath-type sonicator for 1 h (45 kHz 80 W VWR sonicator, temperature did not exceed 45 °C). One-third of each sample were used for measurements (containing 2.5 mL milliQ and ~0.05 mg PL), and 0.125 µL of DPH fluorescence dye [5 mM DPH (1,6-Diphenyl-1,3,5-hexatriene dissolved in N,N-Dimethylformamide)]. All samples were wrapped in tin foil, flushed with nitrogen and gently mixed where after they were left over night in the dark at room temperature. Fluorescence anisotropy was measured using a Perkin-Elmer MPF-44A fluorescence spectrophotometer with 5 nm spectral band widths (slit widths). Excitation was done at 360 nm and emission was measured at 430 nm. Fluorescence measurements were carried out over the temperature range: 4–10–15–20–25 ± 0.3 °C. Vesicle preparations were allowed to equilibrate for 15 min at each temperature before measurement and each preparation was measured in triplicate at each temperature with a 20 s interval. Anisotropy values were calculated as described by Litman and Barenholz (1982). Pure milliQ water was used as a blank and all measurements were corrected for this measurement. Further, the measurements of the samples were corrected with a calculated G-factor (Litman and Barenholz 1982).
Statistical analyses
Population differences of fatty acid composition were analyzed using Principal Component Analysis (PCA) of the molar percentage distributions of individual PLFAs. We used the covariance matrix that is based on mean-centered variables since it is an appropriate matrix when variables are measured in the same units and differences in variance between variables make an important contribution to interpretation. Differences in the first and second Principal Component (PC1 and PC2) between populations were tested using Pearson product-moment correlations to find relations between PCs and cold tolerance of populations [LT50: lower lethal temperature causing 50 % mortality was used (Fisker et al. 2014b)]. Spearman Rank Order Correlation was used for testing relations between LT50 and calculated PLFA variables: chain length of FAs, unsaturation ratio, PUFA and degree of unsaturation. Further we also tested the correlation (Spearman Rank Order Correlation) between LT50 and PLFAs or LT50 and mol % of PLFAs divided into number of double bonds to discover which PLFAs that are abundant in high proportions and thereby vary in relation to the cold tolerance of populations.
Fluorescence anisotropy data were analyzed by a two-way ANOVA testing the effect of temperature and population. Further, one-way ANOVA was used for testing effect of population on bending rigidity data. Post hoc Tukey’s test was used for pairwise comparisons.
Results
Membrane composition
Twenty different fatty acids were identified in the PLFA fractions of the seven populations (Table 2). The PLFAs C18:0, C20:1, C20:2, C20:4 and C20:5 were dominating and amounted to about 70 % of the total PLFAs. Especially PLFAs that contained 4 and 5 double bonds were abundant, whereas FAs with few double bonds were less abundant. The cholesterol proportion did not vary significantly between populations.
The first two principal components (PC1 and PC2) found by PCA accounted for a large part of the total variation in the molar percentage of PLFAs (74 %) among populations (Fig. 1a) and subsequent PCs were therefore not considered further. The less cold tolerant populations (Germany, Bergen and Kullen) are grouped in the lower right quadrat (shown in gray circle), meaning that they had a high PC1 score and a low PC2 score, while more cold tolerant populations (Hólar, Mosfellsbær, Nuuk and Svalbard) had a high PC2 and a low PC1 score, grouping them above the plot diagonal line (dashed in Fig. 1a). The loading values (Fig. 1b) showed that PLFAs such as C20:3 and C20:5 were more abundant in the less cold tolerant populations (high PC1 and low PC2 values), whereas C18:2, C20:4, C22:2 and C22:3 were found in higher percentages in cold tolerant populations (low PC1 and high PC2 values). Further, both PC1 and PC2 were found to correlate significantly to LT50 values of the respective populations (Fig. 2a, b). There was a positive correlation between PC1 and LT50 whereas the correlation between PC2 and LT50 was negative.
Results of multivariate principal component analysis of the differences between molar percentages of the phospholipid fatty acids (PLFA) extracted from seven different Enchytraeus albidus populations. a The scores from the first and second principal components are indicated and the percentage variation explained by each component is stated in parentheses (mean ± SE, N = 5–6). The gray circle shows the less cold tolerant populations and the dashed line separates the more cold tolerant populations in the left corner. b Component loading values. PLFAs that were more abundant in the less cold tolerant populations are marked with gray dots, whereas PLFAs that were found in higher percentages in cold tolerant populations are marked with black dots. White dots indicate PLFAs that were equally abundant in all populations with regard to cold tolerance
The molar percentages of seven PLFAs (including C15:0, C20:5 and C22:5) correlated positively with LT50 values, meaning that populations with high molar percentages of these specific PLFAs have a low cold tolerance (Table 3, top part). Further, the molar percentages of PLFAs with zero and five double bonds correlated positively with LT50. However, there was a negative correlation between PLFAs with two and four double bonds and LT50, thus molar percentages of PLFAs with this number of double bonds are high in populations with a high cold tolerance (Table 3, middle part). Five PLFAs (including C18:2, C20:4 and C22:2) correlated negatively with LT50. Cold tolerance (LT50) correlated significantly with PLFA chain length and unsaturation ratio (UFA/SFA) (Fig. 3a, b; Table 3, bottom part), such that populations with a high cold tolerance had long PLFA chain lengths and a high unsaturation ratio. No correlation was, however, found between LT50 and degree of unsaturation or LT50 and PUFA (Table 3, bottom part).
Correlations between calculated phospholipid fatty acid (PLFA) variables and lower lethal temperature causing 50 % mortality (LT50) of seven different Enchytraeus albidus populations (mean ± SE, N = 5–6). a Average number of C atoms of the PLFA, b UFA/SFA is the proportion of unsaturated fatty acids divided by the proportion of saturated fatty acids. P values and correlation coefficients (R) are indicated on each plot
Membrane fluidity
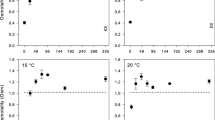
Fluorescence anisotropy revealed a significant effect of temperature as expected, but no difference between populations (Fig. 4; two-way ANOVA; temperature: F 4,39 = 376.8, P < 0.001; population: NS) and further no interaction was found between temperature and population. These results therefore merely show that the membrane becomes more fluid with increasing temperature since anisotropy of the membrane decreased with increasing temperature. The bending rigidity of GUVs formed from phospholipids extracted from E. albidus originating from Germany and Svalbard revealed a significant difference between the two populations (Fig. 5; one-way ANOVA; F 1,21 = 17.2, P < 0.001). This indicated that the population from Germany had a more fluid membrane than the Svalbard population.
Discussion
Variance in membrane composition between populations
The ability to survive freezing is likely to be of importance for E. albidus. Although all populations of E. albidus are freeze tolerant, the ability to tolerate freezing may be particularly important for populations in arctic areas that encounter winter temperatures low enough to cause freezing of body fluids over long periods. A suite of physiological traits is important for survival of freezing including accumulation of cryoprotectants (in E. albidus glucose is accumulated) protecting against freeze-induced cellular dehydration, alterations in cellular metabolism due to freezing, and rapid regulation of cell-volume by transport of water and solutes across the cell membrane (Calderon et al. 2009; Fisker et al. 2014b; Philip et al. 2008; Tanghe et al. 2005). Appropriate membrane fluidity is also likely important for survival at low temperature because a stiffened membrane may not allow proper functionality of integral proteins responsible for transport of water, ions and solutes across the membrane (Los and Murata 2004; Stubbs and Smith 1984). “Homeoviscous adaptation” (HVA) is thought to preserve appropriate fluidity by altering the chemical composition of membranes, and this is probably also an integrated part of adaptation to freeze tolerance. Thus, we hypothesized that differences in PLFAs of the seven populations (as molar percentage of individual PLFAs, length and unsaturation) would be related to cold tolerance of the individual populations.
Previous studies on Drosophila species and cold acclimation of the springtail Megaphorura arctica have shown that the length of PLFAs and degree of unsaturation decreased in species/populations with higher cold tolerance or during cold acclimation (Bahrndorff et al. 2007; Ohtsu et al. 1998). However, other studies show different and contradicting results in relation to phospholipid composition and cold tolerance (Bennett et al. 1997; Kostal et al. 2003; Michaud and Denlinger 2006; Ohtsu et al. 1999; Pruitt and Lu 2008; Reynolds et al. 2014). For example, Ohtsu et al. (1999) did not find any relationship between unsaturated proportion of PLFAs and cold acclimation in Drosophila flies, and Kostal et al. (2003) found an increased PLFA chain length with cold acclimation in Chymomyza costata. Thus, correlative evidence suggests that short unsaturated fatty acids are associated with cold tolerance (Kostal 2010), but the patterns may be more complex, particularly when considering that many of the studies on invertebrates are using whole animal homogenates and therefore may mask or ignore putative organ specific responses (Tomcala et al. 2006). In the present study, E. albidus populations with high cold tolerance had a high UFA/SFA index and a long chain length compared to less cold tolerant populations (Table 3, bottom part). Earlier results like these have been suggested to be due to different adaptive responses of the organisms (Holmstrup et al. 2007; Kostal 2010; Reynolds et al. 2014), meaning that the unsaturation and chain length can be varied to obtain the same optimal membrane fluidity. This seems to be the case for the E. albidus populations, whereas parameters as PUFA and degree of unsaturation did not correlate with cold tolerance, and might therefore not be as important for cold tolerance as unsaturation ratio and PLFA chain length.
In other species of ectothermic animals there has been a particular focus on linoleic acid (C18:2) in relation to cold adaptation. Thus, for some invertebrates C18:2 is important for cold tolerance in both chill-sensitive species and freeze-tolerant species (Holmstrup et al. 2007; Kostal et al. 2003; Overgaard et al. 2008; Tomcala et al. 2006). In accordance with these previous studies, C18:2 proved to correlate with cold tolerance across the seven E. albidus populations (LT50; R = −0.581, Table 3). The negative correlation points to some role of this PLFA, where higher abundance of C18:2 is associated with higher cold tolerance. However, the relative proportion of C18:2 was low and differences in this PLFA might not be as important as other PLFAs which make up larger molar percentages (i.e. C20:5 (accounts for around 20 %) R = 0.571, Table 3). General characteristics of PLFAs, such as presence of two double bonds, substantially reduce the transition temperature from liquid crystalline to gel phase (Kostal 2010). This is congruent with the significant negative correlation found in the present study between the sum of all PLFAs with two double bonds and LT50 (Table 3, middle part). We also found a negative correlation between LT50 and PLFAs with four double bonds suggesting that these PLFAs might also be beneficial for cold tolerance, while saturated PLFAs and PLFAs with five double bonds were positively correlated to LT50 (i.e. negatively correlated to cold tolerance).
Membrane fluidity
The dynamic nature or fluidity of the phospholipid bilayers is an important and fundamental property. Lipid composition gives an indication of physical properties of the membrane, however, changes in PLFA composition only provide indirect evidence for changes in fluidity and these measurements should therefore preferably be supplemented with physical measurements of fluidity. For example, it is known that changes in desaturation, shortening of the average fatty acid chain length and head group restructuring contribute differently to the preservation of the fluid membrane (Hazel 1995; Hazel and Williams 1990; Kostal 2010). Physical measurements of membrane fluidity can be used as a check on several aspects of the membranes’ state. Membrane order and rates of molecular motion are in practice two fundamentally different kinds of information given by physical methods employed to estimate membrane fluidity (Hazel 1989; Seelig and Seelig 1977; Stubbs and Smith 1984). Further, no single biophysical technique is responsive to all aspects of membrane dynamics (Hazel 1989). One of the most used methods to determine membrane fluidity is the measurement of fluorescence anisotropy of the DPH probe (Hazel 1989; Shinitzky and Barenholz 1978; Stubbs and Smith 1984). Another method is Vesicle Fluctuation Analysis, which describes the bending rigidity of the membrane bilayer (Bouvrais 2012; Henriksen et al. 2004). The fluorescence anisotropy describes how the DPH is embedded in the membrane (Jahnig 1979), whereas bending rigidity is based on direct observation of the membrane flexibility through its thermal fluctuations, which is related (indirectly) to membrane fluidity (Dimova et al. 2006).
The measurements of fluorescence anisotropy showed no difference between worms from a temperate and an arctic population, however, there was an effect of temperature as expected. HVA cannot occur in fixed membrane systems, therefore, an increase in temperature will obviously increase membrane fluidity and vice versa. In comparison, we found a significant difference between the membrane bending rigidity of the Germany and Svalbard populations. The difference in bending rigidity might originate from the significant differences found in PLFA composition between these two populations. An earlier study has shown that bending rigidity estimated by Vesicle Fluctuation Analysis is highly sensitive to PUFA and chain length, such that higher unsaturation entails a less rigid membrane (Rawicz et al. 2000). This sensitivity to PUFA proportions is likely what we detected in the bending rigidity measurements, since the difference in proportional PUFA content is relatively large between the Germany and Svalbard populations (Germany 77.4 %, Svalbard 73.5 %; t test P value = 0.015). The sensitivity of anisotropy in relation to unsaturation of PLFAs has been debated (Hazel 1989; Stubbs et al. 1981; Stubbs and Smith 1984), and this might be why no population difference in anisotropy was detected despite relatively large differences in PLFA compositions. Nevertheless, we found that the Svalbard population had a more rigid membrane compared to the Germany population, when measured at 20 °C by the bending rigidity method. Furthermore, when using the more common method of anisotropy, our hypothesis was confirmed since we found no difference in membrane fluidity between the two populations over a broad range of temperatures suggesting that the populations had adapted their membranes to have the most “optimal” membrane fluidity despite some differences in PLFA composition. Another interpretation could be that the differences in PLFA composition are somehow related to the membranes’ ability to cope with freezing of extra-cellular water as such, without any relations to the overall fluidity. However, the current study has no data to support or falsify this suggestion.
Although the present study used several independent measures of membrane properties to investigate the putative adaptive responses, we caution that the results may be biased due to the nature of the sampling protocol. Firstly, the small size of the enchytraeids complicates selection of tissue specific samples, and secondly our chemical analysis did not include analysis of head-groups. Collectively, such measures could provide a further level of detail, which may be easier to obtain using larger experimental animals. Nonetheless we found clear correlation of some PLFAs as C18:2, C20:4, C22:2 and C22:3, and also unsaturation ratio (UFA/SFA) and PLFA chain length with cold tolerance in E. albidus populations. This supports other studies and also indirectly the HVA theory. However, physical measurements of membrane fluidity did not show any direct relation to cold tolerance. A similar finding by Thurmond et al. (1994) showed that the lipid composition varied in the microorganism Acholeplasma laidlawii when exposed to different growth media, however this was not manifested in a change in the physical measurements of membrane fluidity [measured as order parameters calculated from 2H nuclear magnetic resonance (NMR) spectra]. We suggest that this direct relation is absent due to the HVA where the membrane is optimized to function at a specific temperature. Therefore, different E. albidus populations are achieved at a similar “optimal” fluidity of the membrane with somewhat different composition, but whether this different composition favors the more cold tolerant populations during freezing needs to be investigated further. Low temperature exposure in E. albidus also involves accumulation of glucose to function as a cryoprotectant but also as a fuel during prolonged freezing (Fisker et al. 2014b). Our study therefore suggests that E. albidus populations that differ in cold tolerance also differ in membrane composition; however, the accumulation of glucose, which functions as cryoprotectant and fuel, is also important during freezing. The methods we have employed here may open up possibilities for studying the effects of glucose on the physico-chemical properties of reconstituted membranes to validate the putative role of glucose as an agent that might affect the fluidity of the membranes of ectothermic animals.
In conclusion, our studies on E. albidus originating from contrasting environments highlight, that differences in chemical composition of membranes may not necessarily result in predicted physical properties of reconstituted membranes. Furthermore, it could be interesting to investigate how the membrane composition and fluidity change in response to temperature acclimation.
References
Bahrndorff S, Petersen SO, Loeschcke V, Overgaard J, Holmstrup M (2007) Differences in cold and drought tolerance of high arctic and sub-arctic populations of Megaphorura arctica Tullberg 1876 (Onychiuridae : collembola). Cryobiology 55(3):315–323
Bennett VA, Pruitt NL, Lee RE (1997) Seasonal changes in fatty acid composition associated with cold-hardening in third instar larvae of Eurosta solidaginis. J Comp Physiol B Biochem Syst Environ Physiol 167(4):249–255
Bouvrais H (2012) Bending rigidities of lipid bilayers: their determination and main inputs in biophysical studies. In: Iglic A (ed) Advances in Planar Lipid Bilayers and Liposomes, vol 15. Advances in Planar Lipid Bilayers and Liposomes. pp 1–75
Bouvrais H, Holmstrup M, Westh P, Ipsen JH (2013) Analysis of the shape fluctuations of reconstituted membranes using GUVs made from lipid extracts of invertebrates. Biol Open 2(4):373–378
Calderon S, Holmstrup M, Westh P, Overgaard J (2009) Dual roles of glucose in the freeze-tolerant earthworm Dendrobaena octaedra: cryoprotection and fuel for metabolism. J Exp Biol 212(6):859–866
Cossins AR (1977a) Adaptation of biological-membranes to temperature—effect of temperature-acclimation of goldfish upon viscosity of synaptosomal membranes. Biochim Biophys Acta 470(3):395–411
Cossins AR (1977b) Evolutionary and seasonal adaptation of membranes to temperature. Biochem Soc Trans 5(1):106–107
Cossins AR, Bowler K (1987) Temperature biology of animals. Chapman and Hall, New York
Cossins AR, Friedlander MJ, Prosser CL (1977) Correlations between behavioral temperature adaptations of goldfish and viscosity and fatty-acid composition of their synaptic-membranes. J Comp Physiol 120(2):109–121
Dimova R, Aranda S, Bezlyepkina N, Nikolov V, Riske KA, Lipowsky R (2006) A practical guide to giant vesicles. Probing the membrane nanoregime via optical microscopy. J Phys Cond Matt 18(28): S1151–S1176
Dowling NJE, Widdel F, White DC (1986) Phospholipid ester-linked fatty-acid biomarkers of acetate-oxidizing sulfate-reducers and other sulfide-forming bacteria. J Gen Microbiol 132:1815–1825
Fisker KV, Holmstrup M, Malte H, Overgaard J (2014a) Effect of repeated freeze-thaw cycles on geographically different populations of the freeze tolerant worm Enchytraeus albidus (Oligochaeta). J Exp Biol 217:3843–3852
Fisker KV, Overgaard J, Sørensen JG, Slotsbo S, Holmstrup M (2014b) Roles of carbohydrate reserves for local adaptation to low temperatures in the freeze tolerant oligochaete Enchytraeus albidus. J Comp Physiol B Biochem Syst Environ Physiol 184(2):167–177
Hayward SAL, Murray P, Gracey AY, Cossins AR (2007) Beyond the lipid hypothesis: mechanisms underlying phenotypic plasticity in inducible cold tolerance. In: Csermely P, Vigh L (eds) Molecular aspects of the stress response: Chaperones, membranes and networks. Landers Bioscience, Austin, pp 132–142
Hazel JR (1989) Cold adaptation in ectotherms: regulation of membrane function and cellular metabolism. In: Wang LCH (ed) Animal adaptation to cold. Springer, New York, pp 1–50
Hazel JR (1995) Thermal adaptation in biological-membranes—is homeoviscous adaptation the explanation. Annu Rev Physiol 57:19–42
Hazel JR, Williams EE (1990) The role of alterations in membrane lipid-composition in enabling physiological adaptation of organisms to their physical-environment. Prog Lipid Res 29(3):167–227
Henriksen J, Rowat AC, Ipsen JH (2004) Vesicle fluctuation analysis of the effects of sterols on membrane bending rigidity. Eur Biophys J Biophys Lett 33(8):732–741
Holmstrup M, Sørensen LI, Bindesbøl AM, Hedlund K (2007) Cold acclimation and lipid composition in the earthworm Dendrobaena octaedra. Comp Biochem Phys Mol Integr Physiol 147(4):911–919
Holmstrup M, Bouvrais H, Westh P, Wang C, Slotsbo S, Waagner D, Enggrob K, Ipsen JH (2014) Lipophilic contaminants influence cold tolerance of invertebrates through changes in cell membrane fluidity. Environ Sci Technol 48(16):9797–9803
Huang CH, Li S, Lin HN, Wang GQ (1996) On the bilayer phase transition temperatures for monoenoic phosphatidylcholines and phosphatidylethanolamines and the interconversion between them. Arch Biochem Biophys 334(1):135–142
Huang CH, Lin HN, Li SS, Wang GQ (1997) Influence of the positions of cis double bonds in the sn-2-acyl chain of phosphatidylethanolamine on the bilayer’s melting behavior. J Biol Chem 272(35):21917–21926
Jahnig F (1979) Structural order of lipids and proteins in membranes—evaluation of fluorescence anisotropy data. Proc Natl Acad Sci USA 76(12):6361–6365
James R, Branton D (1973) Lipid-dependent and temperature-dependent structural changes in Acholeplasma-laidlawii cell-membranes. Biochim Biophys Acta 323(3):378–390
Kates M (1986) Techniques of Lipodology. Elsevier, Amsterdam
Kostal V (2010) Cell structural modifications in insects at low temperatures. In: Denlinger DL, Lee RE (eds) Low temperature biology of insects. Cambridge University Press, Cambridge, pp 116–140
Kostal V, Simek P (1998) Changes in fatty acid composition of phospholipids and triacylglycerols after cold-acclimation of an aestivating insect prepupa. J Comp Physiol B Biochem Syst Environ Physiol 168(6):453–460
Kostal V, Berkova P, Simek P (2003) Remodelling of membrane phospholipids during transition to diapause and cold-acclimation in the larvae of Chymomyza costata (Drosophilidae). Comp Biochem Physiol Biochem Mol Biol 135(3):407–419
Lentz BR (1989) Membrane fluidity as detected by diphenylhexatriene probes. Chem Phys Lipids 50(3–4):171–190
Litman BJ, Barenholz Y (1982) Fluorescent probe: diphenylhexatriene. Methods Enzymol 81:678–685
Los DA, Murata N (2004) Membrane fluidity and its roles in the perception of environmental signals. Biochimica Et Biophysica Acta-Biomembranes 1666(1–2):142–157
Meleard P, Pott T, Bouvrais H, Ipsen JH (2011) Advantages of statistical analysis of giant vesicle flickering for bending elasticity measurements. Eur Phys J E 34(10)
Michaud MR, Denlinger DL (2006) Oleic acid is elevated in cell membranes during rapid cold-hardening and pupal diapause in the flesh fly Sarcophaga crassipalpis. J Insect Physiol 52(10):1073–1082
Mitov MD, Faucon JF, Méléard P, Bivas I, Bothorel P (1992) Thermal fluctuations of membranes. In: Gokel GW (ed) Advances In Supramolecular Chemistry. Jai Press, Greenwich, pp 93–139
Murray P, Hayward SAL, Govan GG, Gracey AY, Cossins AR (2007) An explicit test of the phospholipid saturation hypothesis of acquired cold tolerance in Caenorhabditis elegans. Proc Natl Acad Sci USA 104(13):5489–5494
Ohtsu T, Kimura MT, Katagiri C (1998) How Drosophila species acquire cold tolerance—Qualitative changes of phospholipids. Eur J Biochem 252(3):608–611
Ohtsu T, Katagiri C, Kimura MT (1999) Biochemical aspects of climatic adaptations in Drosophila curviceps, d-immigrans, and d-albomicans (Diptera : Drosophilidae). Environ Entomol 28(6):968–972
Overgaard J, Tomcala A, Sorensen JG, Holmstrup M, Krogh PH, Simek P, Kostal V (2008) Effects of acclimation temperature on thermal tolerance and membrane phospholipid composition in the fruit fly Drosophila melanogaster. J Insect Physiol 54(3):619–629
Overgaard J, Tollarova M, Hedlund K, Petersen SO, Holmstrup M (2009) Seasonal changes in lipid composition and glycogen storage associated with freeze-tolerance of the earthworm, Dendrobaena octaedra. J Comp Physiol B Biochem Syst Environ Physiol 179(5):569–577
Patrício Silva AL, Holmstrup M, Kostal V, Amorim MJB (2013) Soil salinity increases survival of freezing in the enchytraeid Enchytraeus albidus. J Exp Biol 216:2732–2740
Philip BN, Yi S-X, Elnitsky MA, Lee RE Jr (2008) Aquaporins play a role in desiccation and freeze tolerance in larvae of the goldenrod gall fly Eurosta solidaginis. J Exp Biol 211(7):1114–1119
Pott T, Bouvrais H, Méléard P (2008) Giant unilamellar vesicle formation under physiologically relevant conditions. Chem Phys Lipids 154(2):115–119
Pruitt NL, Lu C (2008) Seasonal changes in phospholipid class and class-specific fatty acid composition associated with the onset of freeze tolerance in third-instar larvae of Eurosta solidaginis. Physiol Biochem Zool 81(2):226–234
Rais A, Miller N, Stillman JH (2010) No evidence for homeoviscous adaptation in intertidal snails: analysis of membrane fluidity during thermal acclimation, thermal acclimatization, and across thermal microhabitats. Mar Biol 157(11):2407–2414
Rawicz W, Olbrich KC, McIntosh T, Needham D, Evans E (2000) Effect of chain length and unsaturation on elasticity of lipid bilayers. Biophys J 79(1):328–339
Reynolds AM, Lee RE, Costanzo JP (2014) Membrane adaptation in phospholipids and cholesterol in the widely distributed, freeze-tolerant wood frog, Rana sylvatica. J Comp Physiol B Biochem Syst Environ Physiol 184:371–383
Rozsypal J, Koštál V, Berková P, Zahradníčková H, Šimek P (2014) Seasonal changes in the composition of storage and membrane lipids in overwintering larvae of the codling moth, Cydia pomonella. J Therm Biol 45:124–133
Seelig A, Seelig J (1977) Effect of a single cis double-bond on structure of a phospholipid bilayer. Biochemistry 16(1):45–50
Shinitzky M, Barenholz Y (1978) Fluidity parameters of lipid regions determined by fluorescence polarization. Biochim Biophys Acta 515(4):367–394
Sinensky M (1974) Homeoviscous adaptation—homeostatic process that regulates viscosity of membrane lipids in Escherichia-coli. Proc Natl Acad Sci U S A 71(2):522–525
Singer M (1981) Permeability of phosphatidylcholine and phosphatidylethanolamine bilayers. Chem Phys Lipids 28(3):253–267
Slotsbo S, Maraldo K, Malmendal A, Nielsen NC, Holmstrup M (2008) Freeze tolerance and accumulation of cryoprotectants in the enchytraeid Enchytraeus albidus (Oligochaeta) from Greenland and Europe. Cryobiology 57(3):286–291
Stubbs CD, Smith AD (1984) The modification of mammalian membrane poly-unsaturated fatty-acid composition in relation to membrane fluidity and function. Biochim Biophys Acta 779(1):89–137
Stubbs CD, Kouyama T, Kinosita K, Ikegami A (1981) Effect of double-bonds on the dynamic properties of the hydrocarbon region of lecithin bilayers. Biochemistry 20(15):4257–4262
Tanghe A, Carbrey JM, Agre P, Thevelein JM, Van Dijck P (2005) Aquaporin expression and freeze tolerance in Candida albicans. Appl Environ Microbiol 71(10):6434–6437
Thurmond RL, Niemi AR, Lindblom G, Wieslander A, Rilfors L (1994) Membrane thickness and molecular ordering in Acholeplasma-laidlawii strain-a studied by h-2 NMR-spectroscopy. Biochemistry 33(45):13178–13188
Tomcala A, Tollarova M, Overgaard J, Simek P, Kostal V (2006) Seasonal acquisition of chill tolerance and restructuring of membrane glycerophospholipids in an overwintering insect: triggering by low temperature, desiccation and diapause progression. J Exp Biol 209(20):4102–4114
van Dooremalen C, Ellers J (2010) A moderate change in temperature induces changes in fatty acid composition of storage and membrane lipids in a soil arthropod. J Insect Physiol 56(2):178–184
Waagner D, Bouvrais H, Ipsen JH, Holmstrup M (2013a) Linking membrane physical properties and low temperature tolerance in arthropods. Cryobiology 67(3):383–385
Waagner D, Holmstrup M, Bayley M, Sørensen JG (2013b) Induced cold-tolerance mechanisms depend on duration of acclimation in the chill-sensitive Folsomia candida (Collembola). J Exp Biol 216(11):1991–2000
Acknowledgments
We thank K. L. Enggrob for assistance with measurements of cholesterol and PLFA. This study was supported by the Danish Research Council (MH), Sapere Aude DFF-Starting grants and Carlsberg foundation (JO). The stay of K·S. in Aarhus was supported by the project Postdok_BIOGLOBE (CZ.1.07/2.3.00/30.0032) co-financed by the European Social Fund and the state budget of the Czech Republic.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by I.D. Hume.
Rights and permissions
About this article
Cite this article
Fisker, K.V., Bouvrais, H., Overgaard, J. et al. Membrane properties of Enchytraeus albidus originating from contrasting environments: a comparative analysis. J Comp Physiol B 185, 389–400 (2015). https://doi.org/10.1007/s00360-015-0895-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00360-015-0895-7