Abstract
In endotherms, such as mammals and birds, internal organs can specialise to function within a narrow thermal range. Consequently, these organs should become more sensitive to changes in body temperature. Yet, organs at the periphery of the body still experience considerable fluctuations in temperature, which could select for lower thermal sensitivity. We hypothesised that the performance of soleus muscle taken from the leg would depend less on temperature than would the performance of diaphragm muscle taken from the body core. Soleus and diaphragm muscles were isolated from mice and subjected to isometric and work-loop studies to analyse mechanical performance at temperatures between 15 and 40 °C. Across this thermal range, soleus muscle took longer to generate isometric force and longer to relax, and tended to produce greater normalised maximal force (stress) than did diaphragm muscle. The time required to produce half of maximal force during isometric tetanus and the time required to relax half of maximal force were both more sensitive to temperature in soleus than they were in diaphragm. However, thermal sensitivities of maximal force during isometric tetani were similar for both muscles. Consistent with our hypothesis, power output (the product of speed and force) was greater in magnitude and more thermally sensitive in diaphragm than it was in soleus. Our findings, when combined with previous observations of muscles from regionally endothermic fish, suggest that endothermy influences the thermal sensitivities of power output in core and peripheral muscles.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The strategies animals adopt for coping with variation in temperature can be mapped against two continuous dimensions, namely thermal sensitivity (specialist to generalist) and thermoregulation (thermoconforming to thermoregulating) (Angilletta 2009). A thermal specialist has higher thermal sensitivity but higher peak performance than a thermal generalist, enabling it to perform relatively well over a relatively narrow range of temperatures. Endotherms thermoregulate, normally maintaining relatively high and constant body temperature compared to ectotherms. Theory predicts that a key benefit of such precise thermoregulation in endotherms is that physiological processes will be enhanced via high thermal specialisation, leading to high thermal sensitivity (Angilletta et al. 2010). Many endotherms are homeothermic, tightly regulating their core temperature within a range of less than 3 °C (Refinetti 1999; Wooden and Walsberg 2004). However, peripheral muscles of endotherms can still endure much variation in temperature; for example, some peripheral muscles of humans undergo changes of more than 15 °C as their environment warms or cools (Ducharme et al. 1991; Ranatunga 1998). Even large peripheral muscles undergo appreciable temperature changes. During exercise in controlled environments, peripheral muscles of humans warm by 3–4 °C (Saltin et al. 1968; Kenny et al. 2003; Castle et al. 2006; Yaicharoen et al. 2012). Importantly, changes in air temperature could exacerbate changes caused by physiological states such as exercise.
The temperature of a muscle has profound effects on its contractile performance. As a muscle warms, it produces force more rapidly, shortens and relaxes more quickly, and achieves a greater peak force (Bennett 1984; Rall and Woledge 1990; Marsh 1994; Syme 2006; James 2013). These changes in the intrinsic properties of muscle lead to greater power output at higher temperatures, as long as temperature does not get too high (Rome and Swank 1992; Swoap et al. 1993; Herrel et al. 2007; James et al. 2012). Recent findings also indicate that warmer muscles use less energy to produce power, possibly due to a reduction in passive stiffness (Seebacher et al. 2014). Endothermic thermoregulation enables mammals and birds to maintain warm bodies and enhance performance even when the environment cools. Indeed, muscular and locomotor performances are usually maximised at the set-point temperature (James 2013). All endotherms thermoregulate regionally since they can either defend core temperature or surface temperature, but not both (Lovegrove et al. 1991). For instance, tuna and lamnid sharks have an endothermic core that keeps interior muscles at a higher temperature than superficial ones, yielding more power and faster swimming. Skeletal muscle from the warm core produces high power over a narrow range of temperatures, which exceed sea temperatures. In fact, slow fibres from the endothermic core produce greater peak power but are more sensitive to temperature than either superficial muscles from the same species or core muscles from ectothermic species (Altringham and Block 1997; Bernal et al. 2005; Donley et al. 2007, 2012). These findings indicate a specialist-generalist trade-off that constrains the evolution of skeletal muscle.
Because endotherms regulate their core temperatures more tightly than their peripheral temperatures, muscle from the core should be more specialised. Thus, we predict that core muscles will have greater peak performance but will be more sensitive to temperature than peripheral muscles. Whilst thermal sensitivities of muscular and locomotor performances of ectotherms have been studied extensively, we know very little about thermal sensitivities of these performances in endotherms. Moreover, to our knowledge, no one has published a comparison of the thermal sensitivities of mammalian muscle from the core with mammalian muscle from the periphery (Angilletta et al. 2010; James 2013). Thus, we aimed to compare thermal sensitivities of performances by diaphragm muscle (core) and soleus muscle (periphery) in mice. For both types of muscle, we measured isometric (constant length) and work-loop performance (power production during length change cycles). Based on our hypothesis, we expected two patterns to emerge from our comparisons of these muscles within individuals. First, we expected diaphragm muscle to produce greater power at the core temperature than would soleus muscle. Second, we expected the performance of diaphragm muscle to depend more on temperature than would the performance of soleus muscle.
Materials and methods
Tissue samples
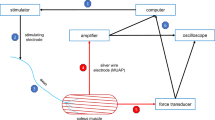
Mice (Mus musculus, Linnaeus 1758; strain CD1, Charles River, UK) were bred and maintained at Coventry University at 19–22 °C. Adult mice (n = 8; body mass = 35.2 ± 0.9 g mean ± s.e.m.) were euthanased by dislocation of the neck in accordance with British Home Office Animals (Scientific Procedures) Act 1986, Schedule 1. Body mass was determined to the nearest 0.1 g using an electronic balance. One hind-limb was removed and soleus muscle was rapidly isolated in chilled (4–6 °C), oxygenated (95 % O2; 5 % CO2) Krebs–Henseleit solution (composition, values in mM: NaCl 118; KCl 4.75; MgSO4 1.18; NaHCO3 24.8; KH2PO4 1.18; glucose 10; CaCl2 2.54; pH 7.55 at room temperature prior to oxygenation). A piece of bone was left at the end of both the proximal and distal tendons of soleus, and aluminium foil clips were placed around the tendons. Meanwhile a ventral section of the costal diaphragm was removed, whilst kept in Krebs as described above. A rib and part of the central tendon were left attached to the diaphragm preparation. An aluminium foil T-clip was placed around the central tendon of the diaphragm. The methods used for isometric and work-loop studies are based on those used in previous studies (Seebacher and James 2008; James et al. 2012).
Isometric studies
Isometric studies were used to determine the twitch and tetanus kinetics of isolated muscle. The bone or foil clip at one end of the muscle preparation was clamped via a crocodile clip to a strain gauge (UF1, Pioden Controls Ltd, Canterbury, Kent, UK), whereas the bone or foil clip at the other end was clamped via a crocodile clip to a motor arm (V201, Ling Dynamics Systems, Royston, Herts, UK) attached to an LVDT (Linear Variable Displacement Transformer, DFG 5.0, Solartron Metrology, Bognor Regis, Sussex, UK). The LVDT was used to monitor the length changes delivered to the muscle preparation. The whole of the muscle, tendon and bone preparation was then allowed to equilibrate within the organ bath at the first test temperature for 10–15 min in circulating, oxygenated (95 % O2; 5 % CO2) Kreb’s solution. The preparation was then held at constant length and stimulated via parallel platinum electrodes to deliver square wave stimuli of 2 ms pulse width that generated a series of twitches. Stimulus amplitude and muscle length were adjusted to determine maximal isometric twitch force. An isometric tetanic force response was elicited by subjecting the diaphragm muscle to a 220 ms train of stimulation and soleus to a 350 ms train of stimulation. Stimulation frequency was altered (140–160 Hz for diaphragm muscle; 120–140 Hz for soleus muscle) to determine maximal tetanic force. Time to half of maximal force during tetanus and time from last stimulus to half tetanus relaxation were measured. A rest period of 5 min was allowed between each tetanic response.
Work-loop analysis
The work-loop technique was used to determine the power output of muscles during cyclical length changes (Josephson 1985). Unlike fixed-length isometric studies and fixed-load isotonic studies of muscle performance, the work-loop technique allows measurement of muscle power output under length and activation changes that are generally more indicative of in vivo contractile performance (James et al. 1996; Caiozzo 2002). Each muscle preparation was subjected to a set of four sinusoidal length changes symmetrical around the length found to generate maximal twitch force. The muscle was stimulated using the stimulation amplitude and stimulation frequency found to yield maximal isometric force. Electrical stimulation and length changes were controlled via a data acquisition board (KUSB3116, Keithley Instruments, Cleveland, OH, USA) and a custom-designed program developed with TestPoint software (CEC Testpoint version 7, Measurement Computing, Norton, MA, USA). Muscle force was plotted against muscle length for each cycle to generate a work-loop, the area of which equated to the net work produced by the muscle during the cycle of length change (Josephson 1985). Instantaneous power output was calculated for every data point in each work-loop (1,000 data points per work-loop) by multiplying instantaneous velocity by instantaneous force. Instantaneous power output values were averaged to generate an average power output for each length change cycle. The cycle frequency of length change was altered to determine the cycle frequency for maximal power output. Muscle strain was kept at 0.10 (10 % peak to peak) of muscle fibre length for soleus (James et al. 1995), 0.13 of muscle fibre length for diaphragm (Altringham and Young 1991) at each cycle frequency as these strains have previously been found to yield maximal power output and fixing strain, but varying cycle frequency simplified the procedure used. Every 5 min the muscle was subjected to a further set of four work-loop cycles with cycle frequency, stimulation duration and stimulation phase parameters being altered in between each set until maximum power output was determined.
After maximal power output was determined the temperature of the Kreb’s solution bathing the muscle was altered to a new temperature over 10–20 min, allowing at least a further 10 min for the muscle to equilibrate to the new temperature. The isometric and work-loop studies were then repeated at the new temperature. Each muscle was subjected to four different temperatures and then the first temperature was repeated twice as a control for time (i.e. 1, 2, 3, 1, 4, 1). To maximize our power to compare thermal sensitivities, we randomly selected test temperatures from a continuous distribution between 15 and 40 °C (Steury and Murray 2005). A set of control parameters for sinusoidal length change and stimulation were imposed on the muscle every three to five sets of work-loops, whenever the muscle was at temperature 1, to monitor variation in the muscles ability to produce power over the time-course of the experiment. There were significant changes in absolute muscle power output over the time-course of the experiments (P = 0.0074; Table 3). Determination of the effects of power output on muscle performance typically took 4.5 h per muscle, during which time diaphragm and soleus muscle power output typically decreased by about 6 %, with no significant difference in the effect of time between muscles. Any variation in power was found to be due to a matching change in ability to produce force. Therefore, the power produced by each preparation was corrected to the control run at temperature 1 that yielded the highest power output, assuming that alterations in power generating ability were linear over time between the control runs delivered at temperature 1.
At the end of the isometric and work-loop experiments, the bones, foil clips and tendons were removed and each muscle was blotted on absorbent paper to remove excess Kreb’s solution. Wet muscle mass was determined to the nearest 0.0001 g using an electronic balance (Mettler-Toledo B204-S, Greifensee, Switzerland). Mean muscle cross-sectional area was calculated from muscle length and mass assuming a density of 1,060 kg m−3 (Mendez and Keys 1960). Maximum isometric muscle stress at each test temperature was then calculated as maximal tetanic force divided by mean cross-sectional area (kN m−2). Normalised muscle power output at each test temperature was calculated as power output divided by wet muscle mass (W kg−1).
Statistical modelling
We modelled the thermal sensitivity of four muscle performances: time to half of maximal force during tetanus; time to half tetanus relaxation; maximal absolute force; maximal absolute power. In each model, temperature and cross-sectional area of the muscle (or muscle mass) were treated as continuous independent variables, and muscle type (diaphragm versus soleus) was considered a fixed factor. Cross-sectional area was used as an independent variable for force measurements, whereas mass was used as the independent morphometric variable for power measurements as force production is highly dependent on muscle cross-sectional area and power production is highly dependent on muscle mass. Since each preparation of muscle was tested repeatedly at different temperatures, we included time as a fixed factor to account for possible effects of fatigue or other form of time-dependent deterioration of muscle. Multiple model types were tested for each muscle performance measure. The Akaike Information Criterion (AIC) was used to determine the best, most likely, model for each muscle performance. We started with the maximal model and then eliminated terms from the model, starting with the highest order term, until we arrived at the model with the lowest AIC (Crawley 2007). All models were fit using the R Statistical Package (R Development Core Team 2011).
A Cox proportional hazards model was fit to data for the time to half of maximal force during tetanus and another such model was fit to data for time to half tetanus relaxation. Often used to fit data on survival, a proportional hazards model relates the time until some event occurs to a set of independent variables. Unlike parametric survival models, the nonparametric proportional hazards model makes few assumptions about the distribution of residuals. Because the responses of each muscle preparation throughout the experiment were likely correlated, we also included a robust sandwich estimator of the variance attributable to this random factor. Parameters were estimated using R’s survival library (Therneau and Lumley 2009). Both the model for time to half of maximal force during tetanus and the model for time to half tetanus relaxation that had the lowest AIC included the terms muscle type (i.e. diaphragm versus soleus) and temperature.
A General Additive Model was fit to data for absolute force production. An additive model enabled us to estimate a nonlinear response to temperature, without knowing the form of the nonlinear function in advance (Zuur et al. 2009). Consequently, we preferred this approach to those that assume an exponential, an asymptotic, or a piecewise function (e.g. Arrhenius breakpoints). To avoid pseudoreplication, the identity of each muscle preparation was included as a random factor. Parameters were estimated using the mgcv (Wood 2004) and nlme (Pinheiro et al. 2011) libraries. The model for absolute force production that had the lowest AIC included the terms muscle cross-sectional area, muscle type (i.e. diaphragm versus soleus) and temperature.
A General Linear Model was fit to data for absolute power production. As in our statistical analysis of absolute force production, the identity of each muscle preparation was included as a random factor. Parameters were estimated using the nlme library (Pinheiro et al. 2011). The model for power production that had the lowest AIC included the terms muscle mass, muscle type (i.e. diaphragm versus soleus), temperature and time.
Results
The best model for time to half of maximal force during tetanus and time to half tetanus relaxation described the vast majority of the variations in these traits (95 and 90 %, respectively; Table 1) and included muscle type (diaphragm vs soleus), temperature and the interaction between muscle type and temperature. Both of these tetanus times decreased with increasing temperature in diaphragm and soleus muscles (Figs. 1, 2; P < 0.001 in each case), but the thermal effects were greater in soleus muscle as indicated by significant interactions between muscle type and test temperature (P < 0.007 in each case; Table 1). Soleus muscle force took longer to relax than did diaphragm muscle (P = 0.007; Table 1; Fig. 2), but there was no significant difference in time to half of maximal tetanus force between muscles (P = 0.52).
Time to half of maximal force during tetanus decreased with increased test temperature in diaphragm and soleus muscles from mice (P < 0.001). Each symbol represents a measurement made on one individual. n = 8 muscles were used for both soleus and diaphragm. Each muscle was subjected to four different test temperatures
Time to half tetanus relaxation decreased with increased test temperature in diaphragm and soleus muscles from mice (P < 0.001). Each symbol represents a measurement made on one individual. n = 8 muscles were used for both soleus and diaphragm. Each muscle was subjected to four different test temperatures
The best model of maximal absolute isometric tetanic force described 86 % of the variation, including effects of temperature, cross-sectional area, muscle type, and the interaction between cross-sectional area and muscle type (Table 2). At all temperatures, soleus muscle tended to produce greater isometric tetanic force (P = 0.072) and tetanic stress (Fig. 3) than did diaphragm muscle. Since the best model excluded an interaction between muscle type and test temperature, thermal effects on isometric force are probably similar for soleus and diaphragm muscles. Maximal isometric force increased with a rise in temperature (P < 0.0001).
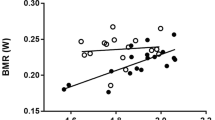
Maximal isometric tetanic stress (force normalised to muscle cross-sectional area) increased with increased test temperature in diaphragm and soleus muscles from mice. Each symbol represents a measurement made on one individual. n = 8 muscles were used for both soleus and diaphragm. Each muscle was subjected to four different test temperatures
When we adjusted for muscle mass, the maximal net power generated during a work- loop increased with increasing temperature in diaphragm and soleus muscles (Fig. 4). At higher temperature, power output was optimised at higher length change cycle frequency, yet still produced larger work-loops (Fig. 5). Diaphragm muscle produced greater net work-loop power than did soleus muscle on an absolute scale (P = 0.006) and relative to muscle mass (Fig. 4). Absolute muscle power output was significantly more thermally sensitive in diaphragm than it was in soleus (Table 3; Muscle type × Temperature P < 0.0001). The thermal sensitivity of absolute muscle power output significantly increased with increased muscle mass in soleus and diaphragm muscles (Table 3; P < 0.0001).
Maximal work-loop net power output, normalised to muscle mass, increased with increased test temperature in diaphragm and soleus muscles from mice. Each symbol represents a measurement made on one individual. n = 8 muscles were used for both soleus and diaphragm. Each muscle was subjected to four different test temperatures
a Diaphragm work-loop shapes that generated maximal power output at 17 °C (broken line) and 37.7 °C (solid line) in the same muscle preparation. Maximal power output was produced at a length change cycle frequency of 2 Hz at 17 °C and 7 Hz at 37.7 °C; b Soleus work-loop shapes that generated maximal power output at 15.3 °C (broken line) and 37.4 °C (solid line) in the same muscle preparation. Maximal power output was produced at a length change cycle frequency of 1 Hz at 15.3 °C and 5 Hz at 37.4 °C
Discussion
We hypothesised that a muscle from the core of a mammalian body would be more sensitive to temperature than would a muscle from the periphery. This hypothesis follows from the premise that no organism can regulate its core and surface temperatures at the same time (Lovegrove et al. 1991). Since endotherms regulate core temperatures, peripheral temperatures will still fluctuate according to environmental conditions. A muscle in the extremities, such as the soleus, will experience even greater fluctuations in temperature than will a muscle at the periphery of the torso. In a given environment, the disparity between the thermal variances of core and peripheral muscles will increase as thermoregulation within the core becomes more precise.
To test our hypothesis, we compared the thermal sensitivities of diaphragm (core) and soleus (peripheral) muscles of mice. Both types of muscles were affected by temperature in ways that resembled thermal sensitivities previously reported for skeletal muscles of other species, including endotherms and ectotherms (Ranatunga 1982; Bennett 1984; Rall and Woledge 1990; Rome and Swank 1992; Swoap et al. 1993; Altringham and Block 1997; De Ruiter et al. 1999; Herrel et al. 2007; Donley et al. 2012; James et al. 2012; James 2013). Muscles produced force more quickly, relaxed more quickly, and generated more power at higher temperatures. Presumably, the thermal optima for power output exceed 40 °C in the muscles that we tested. Maximal activity of enzymes has also been found to occur at temperatures that exceed body temperatures (Bernal et al. 2003). These findings suggest that natural selection optimizes rather than maximises performance at body temperature. A few studies have considered how variables affecting power output, such as length and stimulation parameters, are differentially optimised for power output or efficiency (Curtin and Woledge 1993a, b), but this work has not been extended to consider the effects of temperature. Such studies would help us to better understand the relationship between a muscle’s temperature and its performance.
Although warming generally enhanced muscle performance, thermal sensitivities of power output differed between soleus and diaphragm muscles. Consistent with our predictions, the power generated by diaphragm muscle was higher at core body temperature and changed more dramatically during warming than did the power generated by soleus muscle. The difference in thermal sensitivity between the soleus and diaphragm muscles of mice accords with differences between muscles of regionally endothermic fish. Power generated by core muscles of tuna was more sensitive to warming than was power generated by peripheral muscles (Altringham and Block 1997). Similar differences in thermal sensitivity have also been found when comparing ectothermic and endothermic species (Rall and Woledge 1990; Choi et al. 1998; James 2013). For instance, temperature affects the power generated by red muscle of regionally endothermic sharks more than it affects core muscles of ectothermic sharks (Donley et al. 2007, 2012). Collectively, these results support the idea that endothermy imposes divergent selective pressures on core and peripheral muscles.
The thermal sensitivity of power output depends on the thermal sensitivities of contractile properties such as passive stiffness, force generation, and velocities of shortening and lengthening (James 2013). In endothermic fishes, both power output and isometric force generation of core muscle were more sensitive to temperature than were the same properties of peripheral muscle (Bernal et al. 2005; Donley et al. 2007, 2012). We observed no statistically significant differences between the thermal sensitivities of maximal isometric force of diaphragm and soleus muscles. However, diaphragm muscles relaxed faster than did soleus muscles, probably enabling diaphragm to undergo more frequent cycles of shortening and lengthening to generate higher maximal power output. Greater diaphragm muscle power output at higher temperatures stemmed from faster changes in length and higher force during shortening (Fig. 5). Both of these properties infer an increase in the maximal shortening velocity of the muscle. Therefore, any difference in thermal sensitivities of maximal shortening velocity between soleus and diaphragm could help to explain the observed thermal sensitivities of power output. Contrary to our expectation, temperature had a greater effect on the time to half maximal tetanus force in soleus than in diaphragm; however, since soleus produces maximal power at lower cycle frequencies, variation in the time required to produce force should only weakly influence net power output in soleus (James et al. 1996).
In conclusion, we provide the first evidence that thermal sensitivities differ between muscles within a mammal. Consistent with our hypotheses, power output was greater in magnitude and more sensitive to temperature in diaphragm than it was in soleus. When combined with previous studies on regionally endothermic fish, our findings suggest that temperature affects the power production of an endotherm’s skeletal muscle more in the core of the body than in the periphery. This finding has important implications for thermal adaptation in endotherms, which might have to choose between a muscle specialised to perform at the mean body temperature or a muscle that performs well over a broader range of temperature (Angilletta et al. 2010). Given the initial support for this trade-off, researchers should compare thermal sensitivities of core and peripheral muscles in a wider range of mammals, including those that frequently undergo torpor. These studies should also examine a broader set of contractile properties and muscle types. Replicating these comparisons among species and among muscles within species will enable researchers to determine whether differences in the thermal physiology of muscle stem from thermal adaptation rather than potentially confounding factors, such as fibre type distribution.
References
Altringham JD, Block BA (1997) Why do tuna maintain elevated slow muscle temperatures? Power output of muscle isolated from endothermic and ectothermic fish. J Exp Biol 200:2617–2627
Altringham JD, Young IA (1991) Power output and the frequency of oscillatory work in mammalian diaphragm muscle: the effects of animal size. J Exp Biol 157:318–389
Angilletta MJ Jr (2009) Thermal adaptation. A theoretical and empirical synthesis. Oxford University Press, Oxford
Angilletta MJ Jr, Cooper BS, Schuler MS, Boyles JG (2010) The evolution of thermal physiology in endotherms. Front Biosci E2:861–881
Bennett AF (1984) Thermal dependence of muscle function. Am J Physiol 247:R217–R229
Bernal D, Smith D, Lopez G, Weitz D, Grimminger T, Dickson K, Graham JB (2003) Comparative studies of high performance swimming in sharks II. Metabolic biochemistry of locomotor and myocardial muscle in endothermic and ectothermic sharks. J Exp Biol 206:2845–2857
Bernal D, Donley JM, Shadwick RE, Syme DA (2005) Mammal-like muscles power swimming in a cold-water shark. Nature 437:1349–1352
Caiozzo VJ (2002) Plasticity of skeletal muscle phenotype: mechanical consequences. Muscle Nerve 26:740–768
Castle PC, Macdonald AL, Philp A, Webborn A, Watt PW, Maxwell NS (2006) Precooling leg muscle improves intermittent sprint exercise performance in hot, humid conditions. J Appl Physiol 100:1377–1384
Choi IH, Cho Y, Oh YK, Jung NP, Shin HC (1998) Behavior and muscle performance in heterothermic bats. Physiol Zool 71:257–266
Crawley MJ (2007) The R book. Wiley, New York
Curtin NA, Woledge RC (1993a) Efficiency of energy conversion during sinusoidal movement of white muscle fibres from the dogfish Scyliorhinus canicula. J Exp Biol 183:137–147
Curtin NA, Woledge RC (1993b) Efficiency of energy conversion during sinusoidal movement of red muscle fibres from the dogfish Scyliorhinus canicula. J Exp Biol 185:195–206
De Ruiter CJ, Jones DA, Sargeant AJ, De Haan A (1999) Temperature effect on the rates of isometric force development and relaxation in the fresh and fatigued human adductor pollicis muscle. Exp Physiol 84:1137–1150
Donley JM, Shadwick RE, Sepulveda CA, Syme DA (2007) Thermal dependence of contractile properties of the aerobic locomotor muscle in the leopard shark and shortfin mako shark. J Exp Biol 210:1194–1203
Donley JM, Sepulveda CA, Aalbers SA, McGillivray DG, Syme DA, Bernal D (2012) Effects of temperature on power output and contraction kinetics in the locomotor muscle of the regionally endothermic common thresher shark (Alopias vulpinus). Fish Physiol Biochem 38:1507–1519
Ducharme MB, VanHelder WP, Radomski MW (1991) Tissue temperature profile in the human forearm during thermal stress at thermal stability. J Appl Physiol 71:1973–1978
Herrel A, James RS, Van Damme R (2007) Fight versus flight: physiological basis for temperature dependent behavioral shifts in lizards. J Exp Biol 210:1762–1767
James RS (2013) A review of the thermal sensitivity of the mechanics of vertebrate skeletal muscle. J Comp Physiol 183:723–733
James RS, Altringham JD, Goldspink DF (1995) The mechanical properties of fast and slow skeletal muscles of the mouse in relation to their locomotory function. J Exp Biol 198:491–502
James RS, Young IS, Cox VM, Goldspink DF, Altringham JD (1996) Isometric and isotonic muscle properties as determinants of work loop power output. Pflug Arch Eur J Physiol 432:767–774
James RS, Tallis J, Herrel A, Bonneaud C (2012) Warmer is better: thermal sensitivity of both maximal and sustained power output in the iliotibialis muscle isolated from adult Xenopus tropicalis. J Exp Biol 215:552–558
Josephson RK (1985) Mechanical power output from striated muscle during cyclic contraction. J Exp Biol 114:493–512
Kenny GP, Reardon FD, Zaleski W, Reardon ML, Haman F, Ducharme MB (2003) Muscle temperature transients before, during, and after exercise measured using an intramuscular multisensor probe. J Appl Physiol 94:2350–2357
Lovegrove BG, Heldmaier G, Ruf T (1991) Perspectives of endothermy revisited: the endothermic temperature range. J Therm Biol 16:185–197
Marsh RL (1994) Jumping ability of anurans. In: Jones JH (ed) Comparative vertebrate exercise physiology. Academic Press, San Diego, pp 51–111
Méndez J, Keys A (1960) Density and composition of mammalian muscle. Metabolism 9:184–188
Pinheiro J, Bates D, DebRoy S, Sarkar D, the R Development Core Team (2011) nlme: linear and nonlinear mixed effects models. R PackageVersion 3.1-102
Rall JA, Woledge RC (1990) Influence of temperature on mechanics and energetics of muscle contraction. Am J Physiol 259:R197–R203
Ranatunga KW (1982) Temperature-dependence of shortening velocity and rate of isometric tension development in rat skeletal muscle. J Physiol 329:465–483
Ranatunga KW (1998) Temperature dependence of mechanical power output in mammalian (rat) skeletal muscle. Exp Physiol 83:371–376
Refinetti R (1999) Amplitude of the daily rhythm of body temperature in eleven mammalian species. J Therm Biol 2:477–481
Rome LC, Swank D (1992) The influence of temperature on power output of scup red muscle during cyclical length changes. J Exp Biol 171:261–281
Saltin B, Gagge AP, Stolwijk JA (1968) Muscle temperature during submaximal exercise in man. J Appl Physiol 25:679–688
Seebacher F, James RS (2008) Plasticity of muscle function in a thermoregulating ectotherm (Crocodylus porosus): biomechanics and metabolism. Am J Physiol 294:R1024–R1032
Seebacher F, Tallis JA, James RS (2014) The cost of muscle power production: muscle oxygen consumption per unit work increases at low temperatures in Xenopus laevis Daudin. J Exp Biol 217:1940–1945
Steury TD, Murray DL (2005) Regression versus ANOVA. Front Ecol Environ 3:356–357
Swoap SJ, Johnson TP, Josephson RK, Bennett AF (1993) Temperature, muscle power output, and limitations on burst locomotor performance of the lizard Dipsosaurus dorsalis. J Exp Biol 174:185–197
Syme DA (2006) Functional properties of skeletal muscle. In: Shadwick RE, Lauder GV (eds) Randall DJ, Farrell AP (series eds) Fish physiology: fish biomechanics, vol 23. Academic Press, Waltham, pp 179–240
Therneau T, Lumley T (2009) Survival: survival analysis, including penalised likelihood. R package version 2.35-8. http://www.CRAN.R-project.org/package=survival
Wood SN (2004) Stable and efficient multiple smoothing parameter estimation for generalized additive models. J Am Stat Assoc 99:673–686
Wooden KM, Walsberg GE (2004) Body temperature and locomotor capacity in a heterothermic rodent. J Exp Biol 207:41–46
Yaicharoen P, Wallman K, Morton A, Bishop D (2012) The effect of warm-up on intermittent sprint performance and selected thermoregulatory parameters. J Sci Med Sport 15:451–456
Zuur AF, Leno EN, Walker N, Saveliev AA, Smith GM (2009) Mixed effects models and extensions in ecology with R. Springer, New York
Acknowledgments
Thanks to Mark Bodycote, Bethan Grist and Roy Petticrew for technical assistance. This research was supported by Coventry University, UK. Many thanks to the referees for their constructive comments.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by I. D. Hume.
Rights and permissions
About this article
Cite this article
James, R.S., Tallis, J. & Angilletta, M.J. Regional thermal specialisation in a mammal: temperature affects power output of core muscle more than that of peripheral muscle in adult mice (Mus musculus). J Comp Physiol B 185, 135–142 (2015). https://doi.org/10.1007/s00360-014-0872-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00360-014-0872-6