Abstract
We examined the circadian rhythms of locomotor activity in three spider species in the Family Theridiidae under light–dark cycles and constant darkness. Contrary to previous findings in other organisms, we found exceptionally high variability in endogenous circadian period both within and among species. Many individuals exhibited circadian periods much lower (19–22 h) or much higher (26–30 h) than the archetypal circadian period. These results suggest relaxed selection on circadian period as well as an ability to succeed in nature despite a lack of circadian resonance with the 24-h daily cycle. Although displaying similar entrainment waveforms under light–dark cycles, there were remarkable differences among the three species with respect to levels of apparent masking and dispersion of activity under constant dark conditions. These behavioral differences suggest an aspect of chronotype adapted to the particular ecologies of the different species.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Circadian rhythms are near 24-h cyclic outputs of biological clock systems and are nearly ubiquitous among eukaryotes. The biological clocks governing circadian rhythms enable the organism to anticipate important events in the environment and underlie the daily scheduling of many critical biological processes, including the sleep–wake cycle, changes in basal metabolic rate, and release of hormones (reviewed in Halberg et al. 2003). In the absence of external time cues (‘zeitgebers’) such as light or temperature cycles, circadian rhythms persist (free run) with an endogenous period that is close to 24 h. In nature, these endogenous circadian rhythms are not independent of the external world but are regularly adjusted (‘entrained’) by daily zeitgebers to remain in synchrony with the Earth’s rotation (reviewed in Dunlap 1996).
From both physiological and ecological perspectives, the interaction between the organism’s internal circadian clock-driven state and the external environment is crucial for survival. Malfunctions in this internal–external interaction can lead to significant fitness costs. For example, eastern chipmunks with surgically ablated suprachiasmatic nuclei (SCN), the anatomical location of the mammalian master circadian oscillator, exhibited significantly higher mortality rates when released into their natural habitat compared to sham-operated and intact controls (DeCoursey et al. 2000). Such SCN ablations cause arrhythmicity and it is presumed that, in this case, the SCN-ablated chipmunks were active rather than quiescent in their burrows at night and alerting predators to their location. In Drosophila, loss-of-function mutations of the canonical circadian clock genes period, timeless, cycle, or clock resulted in both reduced sperm quality in males and reduced production of oocytes in females, which lowered fertility, a metric for fitness (Beaver et al. 2002, 2003). Thus, the absence of rhythmicity may have serious consequences for the organism.
While the loss of a circadian clock apparently presents a significant cost to fitness, even a functioning circadian system must operate within certain temporal constraints. More precisely, the degree of resonance between the organism’s endogenous circadian free-running period (FRP) and the period of the external LD cycle is critical. For example, strains of the cyanobacterium Synechococcus whose FRP closely matched the period of the LD cycle were found to outcompete strains whose FRPs were not concordant (Ouyang et al. 1998; Woelfle et al. 2004). When placed in competition under LD 11:11 h (i.e., 11 h of light alternating with 11 h of darkness, comprising a period of 22 h), Synechococcus strains with endogenous periods of 23 h outcompeted 28-h strains. However, under LD 15:15 h, the 28-h strain exhibited a competitive advantage (Ouyang et al. 1998). Similarly, in pitcher-plant mosquitos, Wyeomyia smithii, individuals raised in a non-resonant LD cycle had significantly lower fecundity (Emerson et al. 2009). Similar results have been obtained with hamsters (Martino et al. 2008), mice (Spoelstra et al. 2016), Drosophila melanogaster (Pittendrigh and Minis 1972), and ants (Lone et al. 2010). These results strongly suggest that a close match (“resonance”) between the endogenous circadian period and exogenous environmental cycles promotes individual fitness.
In most organisms surveyed thus far, circadian clocks have endogenous periods, under constant dark (DD) and constant temperature conditions, within about two hours of 24 h (Johnson et al. 2004; DeCoursey 2004; Refinetti 2012). For example, the range is 23.9 to 24.5 h in humans (Czeisler et al. 1999). Because the distribution of endogenous periods is very narrow in humans and a wide variety of other species, it has been proposed that the endogenous period of the circadian oscillator is under tight genetic control (Czeisler et al., 1999). Extrapolating to the natural world, these findings suggest that an organism’s fitness may be dependent upon the possession of a functioning circadian clock with a period that resonates with the Earth’s 24-h solar day.
Despite the selective pressure favoring endogenous circadian clocks with a circa 24-h period, the trashline orb-weaving spider Cyclosa turbinata (Family: Araneidae), collected directly from the field, has a mean FRP in DD of about 18.7 h (Moore et al. 2016). This represents the fastest (i.e., shortest period) naturally-occurring circadian clock described thus far, on par with lab-generated short-period mutants, including the tau hamster with an FRP of about 20 h (Ralph and Menaker 1988) and the perS mutant in Drosophila (FRP = 19 h; Konopka and Benzer 1971). Based on resonance studies, such fast-running clocks should not exist without significant fitness costs, yet these spiders are widely distributed across southern North America (Bradley 2013). Whether C. turbinata’s short period represents an outlier among spiders, however, is unknown. Currently, knowledge of spider circadian rhythms is rather limited. To date, circadian rhythmicity in locomotor activity has been examined only in seven other species from six families (Seyfarth 1980; Suter 1993; Ortega-Escobar 2002; Soriano-Morales et al. 2013; Jones et al. 2018). All of these species have more or less ‘typical’ FRPs within the range of 24 ± 2 h.
Much of our current knowledge concerning the mechanistic properties of circadian rhythms derive from studies conducted under laboratory conditions using a relatively small number of model organisms (Ralph et al. 1990; Allada et al. 2001; Lowrey and Takahashi 2011; Dubowy and Sehgal 2017). Complementing these studies are those concerned with systems that are weakly circadian or exhibit various degrees of circadian plasticity. For example, newly emerged adult worker honey bees are arrhythmic and perform around-the-clock behaviors such as nursing the brood (Moore et al. 1998). However, by the time the worker bee reaches foraging age (typically about 3 weeks) (Moore et al. 1998), or if a nurse bee is removed from the colony (Shemesh et al. 2010), her behavior becomes circadian. Moreover, rhythmic foragers can revert to arrhythmic nursing behavior if the colony experiences a dearth of nurse bees (Bloch and Robinson 2001; Bloch et al. 2001). Another example of circadian plasticity is the diversity of daily activity rhythms exhibited by breeding birds under constant light conditions during the arctic summer solstice: these rhythms were 24-h entrained, arrhythmic, or free-running, depending on the species, sex, and breeding stage (Steiger et al. 2013). Other examples include migratory birds in which the decoupling of two different oscillators, one responsible for daytime activity and the other for nighttime (Zugunruhe) activity, occurs during migration (Bartell and Gwinner 2005) and fishes that show seasonal changes in FRP (Kavaliers 1978, 1980, 1981). Recently, it has been proposed that to better understand biological rhythms, an integrative approach that combines the mechanistic perspectives of chronobiology and the functional adaptation perspectives of ecology are necessary (Helm et al. 2017). The present study is one attempt to combine the two disciplines.
The purpose of the present study is to examine circadian rhythms of locomotor activity in three species in the family Theridiidae: the subsocial spider Anelosimus studiosus; the common house spider Parasteatoda tepidariorum; and the southern black widow Latrodectus mactans. More specifically, the pattern and phasing of activity with respect to entraining LD cycles, the presence or absence of masking by light during the photophase, and the period and pattern of the free-running rhythms are assessed. In addition to describing both the entrainment waveforms and free-running behavior of these three species, we draw links between their life-history, specifically foraging behavior, and any circadian patterns we find. Furthermore, because our three study-species are all from the same family, we also can determine how circadian rhythms vary within families and use this information to develop an ecologically-relevant model for understanding the adaptive benefits of circadian rhythms.
Materials and methods
Study species
All spiders in this study were adult females collected from their webs at night. Anelosimus studiosus females were taken from pine trees (Pinus virginiana) along the banks of Boone Lake in Washington and Sullivan Counties, Tennessee, USA, in June 2012 and August 2017. These spiders were exposed to no nighttime urban lighting or only at long distances (at least 500 m) from the opposite shore of the lake. Latrodectus mactans females were collected from rock-lined drainage ditches at Willow Springs Park in Washington County, Tennessee in August and September 2017. The park is surrounded mostly by farmland but bordered on one edge by a residential area. Because of the topography, none of the drainage ditches were directly exposed to light at night: one ditch was approximately 80 m from indirect light emanating from incandescent bulbs and a high-pressure sodium lamp and four other ditches were at least 190 m from diffuse incandescent light from frosted-glass post lamps. Parasteatoda tepidariorum females were captured in June 2017 in Washington County, Tennessee from a diversity of urban structures and exposures to nighttime light. Collections were haphazard but included doorways, eaves and soffits, window sills, barns, and parking garages. Care of the animals followed Association for the Study of Animal Behavior/Animal Behavior Society (ASAB/ABS) guidelines, and the spiders were released near the site of collection following experiments.
Locomotor activity
Following collection, individuals were exposed to an LD 12:12 h cycle (12 h of light and 12 h of the dark with lights-on occurring at 08:00 h) in the laboratory for three days in clear plastic containers (6 cm diameter × 3.6 cm height), during which time the spiders were fed once. Individuals from L. mactans, A. studiosus, and P. tepidariorum were placed in clear glass or plastic tubes (25 mm diameter × 15 cm length) and inserted into a locomotor activity monitor (model LAM25, Trikinetics Inc., Waltham, MA, USA). The monitor then was placed into a temperature-controlled (24 ± 0.5 °C) environmental chamber. In the monitors, individuals were first exposed to LD 12:12 h for five (A. studiosus) or eight (L. mactans and P. tepidariorum) complete days, followed by at least 10 complete days of total darkness (DD). Light during photophase was provided by four vertically mounted, 32 W fluorescent tubes. Brightness at the level of the monitors was measured at 1400–1600 lx, somewhat brighter than an average overcast day (approx. 1000 lx) but about an order of magnitude dimmer than full daylight. All transitions between light and dark were sharp, not ramped.
Activity for each individual was measured by recording the number of interruptions of three infrared beams transmitting across the middle of the clear tube. Each interruption was registered as an event, and events were counted in 1-min bins with the exception of the 2012 dataset of A. studiosus, for which the bin size was 5 min. The resulting activity patterns were analyzed using ClockLab Analysis 6 Software (Actimetrics, Wilmette, IL, USA) and graphed as double-plotted actograms, using 5-min bins, to facilitate visual inspection. Significant periods in free-running activity patterns under DD were determined using the Lomb-Scargle periodogram. This method is noted for its detection efficiency with incomplete, noisy, or unequally spaced data (Van Dongen et al. 1999; Ruf 1999). For confirmation in period detection, we also used the chi-square periodogram (Sokolove and Bushell 1978). For A. studiosus and P. tepidariorum, periods were accepted for an individual only if both the chi-square and Lomb-Scargle periodograms indicated significance at P < 0.001. Both periodogram analyses were performed using ClockLab Analysis 6 Software. The L. mactans datasets were more problematic. The locomotor activity patterns were exceptionally noisy, typically leading to the detection of indistinguishable, multiple significant periods by the chi-square periodogram method. However, the Lomb-Scargle periodograms drew the major significant periods out of the data. Only those periods indicated by the Lomb-Scargle periodogram at P < 0.001 were accepted. In the case of the September 2017 L. mactans dataset, the recordings were much longer (most were 26 days in DD) than the “short-duration” August 2017 dataset (most were 12 days in DD) and often showed a period change during that 26-day span. To capture these changing periods in the longer dataset, but still maintain objectivity, we divided the 26 days for each individual into three consecutive segments of 8, 9, and 9 days and found the FRP for each segment. The reported period for each individual was simply the average of the three values. For each species, the FRPs are reported as means ± SEM. As a measure of relative variability of FRPs within each species, we used percent coefficient of variation (% CV), the ratio of standard deviation to the mean, expressed as a percentage.
Entrainment waveforms were constructed for each species by finding the average number of activity counts for each time-stamped 1-min bin throughout the day. This value was calculated for all of the complete days of LD 12:12 for each individual spider. Then, to complete the waveform, the mean number of counts for the individual values was determined for each 1-min bin and graphed over the 24-h day. For the A. studiosus waveform, only the individuals from the 2017 dataset were used because of the lower temporal resolution in the 2012 data set. For each individual in all three species (using the same individuals used to determine the entrainment waveforms), the phase angles of entrainment were determined for activity onset, acrophase, and activity offset using the ClockLab Analysis 6 Software. The results were recorded as time (h) elapsed after the light-off transition, expressed as means ± SD for each species. Phase angles could not be determined for one P. tepidariorum individual due to activity occurring only during two days of LD 12:12. Comparisons among species for each phase angle measure were accomplished using the Kruskal–Wallis test in VassarStats, followed if indicated, by Dunn’s post hoc test with Benjamini–Hochberg FDR adjustment using The Kruskal–Wallis Rank Sum Test Calculator (https://astatsa.com/KruskalWallisTest/). For each of the three species, comparisons of activity levels between LD and DD were made by determining the activity counts for the last five days of the LD cycle and the first five days of DD for each individual. Correlations between LD and DD activity levels were then made via linear regression.
For P. tepidariorum, to confirm or reject the existence of masking during LD entrainment, we followed the procedure of Garmany et al. (2019), using measures of activity onset times in LD and the onsets on the first and second days of DD. To determine the extent of masking for A. studiosus, the above procedure was not used because the locomotor activity under DD conditions was often sparse during the first or second day of DD or showed inconsistencies from day-to-day. Instead, we extrapolated back from the onsets of the free-running rhythms in DD to the phase position on the last day of LD indicated by the free-run. The time difference between the mean activity onset phase during LD and the mean extrapolated phase were compared using a t-test for correlated samples. Quantitative determination of the extent of masking was not possible for L. mactans because the presence of activity throughout DD precluded the determination of activity onsets.
Spearman rank-order correlations were used to test for the potential relationship between FRP and phase angles of activity onset (corrected for apparent masking effects) and offset in A. studiosus and P. tepidariorum. Because activity onsets in DD were not possible to decipher in L. mactans, a correlation between FRP and phase angle of activity onset was not performed on this species. However, a correlation was done on the phase angle of offsets and FRP in L. mactans. Because of the variation in FRP over time in the long-duration data set, we correlated the first FRP detected during DD with the phase of activity offset. For the short-duration data set (about 12 days of DD), we simply used the FRP detected during that span. No correlations between FRP and acrophase were implemented in any of the three species because of the putative inhibition of activity in late photophase due to masking and the resultant biasing of acrophase from activity in early scotophase.
The power of the locomotor activity rhythms under both LD and DD were determined for each individual using the highest peak indicated by Lomb-Scargle periodograms. The periodograms were calculated using the Astropy package (https://docs.astropy.org/en/stable/timeseries/lombscargle.html) implemented in custom Python script and the periodogram scale was normalized with respect to variance according to Press and Rybicki (1989). All peak values were independently confirmed with Lomb-Scargle periodograms using ClockLab 6 Analysis software. The power measures are presented as means ± SEM. A t test for correlated samples was used to compare the difference in power levels between DD and LL for each species.
Results
Entrainment
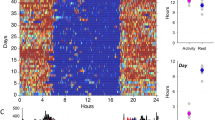
As illustrated in the entrainment waveforms (Fig. 1), locomotor activity in all three species was concentrated largely during the scotophase of the LD 12:12 h cycle. The DiNoc ratio (mean daytime activity—mean nighttime activity/mean total activity), a metric for quantifying the degree of diurnality vs. nocturnality (Suter and Benson 2014), indicated that locomotor behavior was predominately nocturnal (DiNoc ratio < − 0.7, Table 1) for all three species. Also common among the three species were minimal activity levels immediately after the lights-off transition, a sharp activity peak (corresponding to the highest activity level of the diel rhythm) occurring approximately 1 h after lights-off, and a general decline in activity throughout most of the scotophase. Comparing the three species (Fig. 1), A. studiosus exhibited markedly lower overall levels of activity relative to the other two species. Also, A. studiosus showed relatively low-level, sporadic peaks interspersed among intervals with little or no activity throughout photophase. In contrast, both P. tepidariorum and L. mactans displayed low levels of consistent activity throughout photophase. P. tepidariorum displayed a prominent peak of activity shortly after the lights-on transition; this peak is not apparent in the other two species. Further comparisons among the three species were accomplished by determining the phase angles of entrainment (Fig. 2) for three behavioral indices: activity onset, acrophase, and activity offset. There were no significant differences among the three species with respect to the mean phase (time of occurrence) of acrophase (Kruskal–Wallis test, H = 3.26, df = 2, P = 0.196) nor for the mean phase of activity offset (Kruskal–Wallis test, H = 0.4, df = 2, P = 0.819). However, there were significant differences among the values for activity onset (Kruskal–Wallis test, H = 9.63, df = 2, P = 0.008) despite the fact that the means were all within a span of 0.26 h. Post-hoc analysis (Dunn’s test with Benjamini–Hochberg FDR adjustment) revealed that the mean phases of activity onset for both A. studiosus (P = 0.028) and P. tepidariorum (P = 0.032) were significantly later than that of L. mactans but not significantly different from each other (P = 0.480).
Entrainment waveforms under LD 12:12 h, constant temperature (24 °C) conditions for the three theridiid species examined in the present study: A. studiosus (n = 16), P. tepidariorum (n = 30), and L. mactans (n = 33). Data presented are mean locomotor activity counts compiled in 1-min bins. Note that the ordinate scale is different for each species. Abscissa shows zeitgeber time: light-on transition occurs at 0 h and light-off transition at 12 h. Scotophase is indicated by gray background
Phase angles of entrainment for three different behavioral indices (locomotor activity onset, acrophase, and activity offset) for each spider species. Data are presented as means ± SD, plotted as time elapsed after the light-off transition under LD 12:12 h, constant temperature conditions. Gray background represents scotophase
Circadian free-running behavior
Anelosimus studiosus
Of the 50 A. studiosus placed in the monitors, 44 survived at least 10 days in DD and 23 yielded significant circadian periodicities according to our criteria (see Methods). The mean FRP was 23.14 ± 0.51 h, as reported by Lomb-Scargle periodograms. Figure 3a–c illustrates actograms (left column) from three representative individuals, showing significant circadian periods in both chi-square (middle column) and Lomb-Scargle (right column) periodograms. Filled triangles in the chi-square periodograms indicate a peak that most closely matches the major peak reported in the Lomb-Scargle periodograms. The first representative individual (Fig. 3a) exhibited almost no activity during the photophase of the LD cycle and its activity (mostly short bursts lasting 20 min or less) in scotophase began shortly after the lights-off transition and ended before the lights-on transition. In DD, the activity continued to be expressed in bursts and the onsets of free-running activity visibly extrapolated back to the onset of activity during the LD cycle. Locomotor activity in DD, though expressed in short bursts with large gaps between bursts, clearly exhibited a circadian rhythm (period = 20.83 h per the Lomb-Scargle periodogram). Similar to the first example, the second (Fig. 3b) and third (Fig. 3c) spiders showed activity occurring in short bursts in both LD and DD as well as a similar nocturnal entrainment pattern during LD. For most spiders of this species, despite the scattered nature of activity in DD, the free-running activity onsets extrapolated back to late photophase rather than to the entrained onsets of activity in early photophase (e.g., Figs. 3b and c but not a), suggesting that locomotor activity was masked by light during late photophase. To test for the existence of masking, extrapolations of activity onsets in DD back to the last day of LD were performed on 18 individuals possessing both significant FRPs and at least four consecutive days of activity in LD. The results revealed that the extrapolated mean onset phase occurred significantly earlier (by 1.49 h) than the mean onset phase observed during LD (t-test for correlated samples, t = 2.17, df = 17, P = 0.044).
Examples of entrained and free-running activity patterns for three representatives (a, b, c) A. studiosus females. Left panels Actograms show locomotor activity, complied in 5-min bins, under LD (5 days) and thereafter under DD conditions. Middle panels Chi-square periodograms show significant circadian periodicities (peaks above diagonal gray line indicated by filled triangles; P < 0.001) under DD conditions. Right panels Lomb-Scargle periodograms indicate significant circadian periodicities (peaks above horizontal gray line; P < 0.001) under DD conditions
The second individual (Fig. 3b) showed a clear free-run, with a period of 22.83 h. The free-running locomotor behavior in the third individual (Fig. 3c) was more complex than that shown in the other two examples. During the first four days in DD, the activity appeared to free-run with a period shorter than 24 h but, thereafter, the period was much longer than 24 h (the Lomb-Scargle periodogram indicated a significant period of 28.58 h). Such apparent changes in FRP were observed in 3 of the 23 confirmed circadian individuals.
There was no significant correlation between individual FRPs and the extrapolated phases during entrainment of activity onset (Spearman rank-order correlation: rs = − 0.18, t = − 0.73, df = 16, P = 0.476) nor between individual FRPs and phases of activity offset during entrainment (rs = 0.05, t = 0.19, df = 16, P = 0.852).
Parasteatoda tepidariorum
Of 32 P. tepidariorum sampled, 17 demonstrated a significant circadian rhythm according to our criteria with an overall mean FRP in DD of 21.70 ± 0.27 h (Lomb-Scargle). The spiders that failed to show significant circadian periodicity all survived through the entirety of the experiment but exhibited extremely sparse activity during DD. All three individuals illustrated in Fig. 4a–c exhibited significant circadian FRPs in both the chi-square and Lomb-Scargle periodograms. Each individual showed nocturnal entrainment patterns during LD with consistent bouts of activity in early to mid-scotophase with an obvious absence of activity during the last 2–3 h before lights-on. In agreement with the conspicuous, early photophase activity in the entrainment waveform (Fig. 1), the vast majority of spiders exhibited a short burst of activity occurring within about one hour after lights-on; this burst is quite prominent in the first two examples (Fig. 4a, b) but minimal in the third (Fig. 4c).
Examples of entrained and free-running activity patterns for three representatives (a, b, c) P. tepidariorum females. Left panels Actograms show locomotor activity, compiled in 5-min bins, under LD (8 days) and thereafter under DD conditions. Dotted lines on right side of each double-plotted actogram indicate that the free-running activity under DD extrapolates back to mid- or late-photophase rather than to the onset of activity in early scotophase. Middle panels Chi-square periodograms show significant circadian periodicities (peaks above diagonal gray line; P < 0.001) under DD conditions. Right panels Lomb-Scargle periodograms indicate significant circadian periodicities (peaks above horizontal gray line; P < 0.001) under DD conditions
Interestingly, in 15 of the 17 confirmed circadian individuals, the onset of the free-run extrapolated back to mid- or late-photophase (indicated by dotted lines in the actograms, Fig. 4a–c), rather than to the activity onsets in LD, suggesting a masking effect of light on activity during entrainment to LD cycles. To estimate the extent of this apparent masking effect, we compared the time difference between the phase angle of entrainment during LD (potentially delayed because of masking) and the activity onset during the first day of DD (no masking by light) with the time difference between the activity onsets on the first and second days of DD (neither of which would be subject to masking). The time difference in the second measure reflected the phase change due solely to the endogenous FRP of the circadian clock. Because the first measure, − 6.81 ± 0.33 h (mean ± SEM), was significantly greater (t-test for correlated samples, t = − 7.22, df = 16, P < 0.0001) than the second measure (-3.32 ± 0.50 h), we conclude that there is, on average, 3.49 h of masking during LD entrainment.
There was no significant correlation between individual FRPs and the phases during entrainment, corrected for apparent masking, of activity onset (Spearman rank-order correlation: rs = − 0.10, t = 0.38, df = 14, P = 0.710) nor between individual FRPs and phases of activity offset (rs = − 0.49, t = 2.13, df = 14, P = 0.051).
Latrodectus mactans
Three representative actograms (Fig. 5) illustrate the rather typical nocturnal entrainment pattern exhibited by black widows under LD conditions. In all three examples, locomotor activity begins shortly after lights-off, is particularly intense during early scotophase, and anticipates lights-on by terminating activity 2–3 h before the lights-on transition. Also common to all three examples is the near absence of activity during photophase except for a small number of short bursts, usually occurring in mid-photophase. The mid-photophase burst occurred in 25 of the 33 individuals sampled. In contrast to the archetypal nocturnal entrainment patterns, the behavior elicited during DD conditions is highly unusual in that there are no visually apparent circadian free-running rhythms. Instead, locomotor activity is extremely fragmented and dispersed throughout the entire subjective day and night, with no coalescence into coherent bands. The presence of activity throughout the free-running circadian cycle, in contrast with the absence of activity during the photophase of the LD cycles, suggests that masking of activity by light may occur throughout the photophase. Relatively coarse to relatively fine levels of granularity (i.e., fragmentation) are depicted, from left to right, in Fig. 5a–c.
Examples of entrained and free-running activity patterns for three L. mactans females. Double-plotted actograms depict locomotor activity, compiled in 5-min bins, under LD (8 days) followed by DD conditions. Individuals were chosen to illustrate different levels of granularity (occurrences of short activity bouts) under DD conditions, from relatively coarse (a), to medium (b), to relatively fine (c)
Of the 33 black widows (L. mactans) sampled, 26 survived long enough (8 days in LD and at least 10 days in DD) to assess the presence or absence of a circadian rhythm in locomotor activity. In agreement with the apparent arrhythmicity in DD shown in Fig. 5, chi-square periodograms failed to indicate any predominant circadian periodicity in any individual. However, further analyses using Lomb-Scargle periodograms were able to discern significant circadian rhythmicity in 22 individuals as well as a great deal of complexity in the records. For example, Fig. 6a shows the actogram for one individual with a typical nocturnal entrainment pattern under LD conditions but fragmented activity under DD. The chi-square periodogram for this individual (Fig. 6b) showed a multitude of significant periods but no predominant one. On the other hand, the Lomb-Scargle periodogram (Fig. 6c) revealed a prominent peak at 24.58 h, another peak at approximately half the period of the first (at 12.00 h), and a cluster of significant periods in the ultradian range between 2 and 6 h. Common to all of the black widows living at least 10 days in DD were significant periods in the ultradian range (between about 2 and 10 h). When the activity under DD was examined in 3 consecutive segments (approximate one-thirds) of eight (Fig. 6d), nine (Fig. 6e), and nine days (Fig. 6f), the Lomb-Scargle periodogram indicated that the significant circadian period, the secondary period, and the ultradian periods all were maintained throughout the 26 days under DD. This particular analysis was performed only on the long-duration dataset, for which the spiders were kept under DD conditions for 26 days. Such continuity of circadian period throughout DD occurred in only 4 of the 17 spiders subjected to this analysis. The more representative pattern for L. mactans under DD contained at least one change, by at least 2 h, in FRP. For instance, Fig. 7a shows the actogram for one such individual. As was typical, the entrainment pattern under LD was nocturnal and the activity in DD was fragmented, though not as severely as in the previous examples (Figs. 5 and 6). While the chi-square periodogram failed to indicate a predominant circadian period (Fig. 7b), the Lomb-Scargle periodogram indicated three significant periods within the circadian range (Fig. 7c): 20.92, 21.92, and 23.75 h, from shortest to longest. The shortest of these periods occurred with precisely the same period as the predominant peak in the first third of DD (Fig. 7d), the middle period approximated the period (21.17 h) of the prominent peak in the second third (Fig. 7e), and the longest period closely approximated the peak period (24.08 h) in the last third of DD (Fig. 7f). In some spiders, the change in the period was even more drastic: from 27.58 to 19.42 h in one individual and from 34.5 to 20.92 h in another.
Locomotor activity under LD (8 days) and DD for one L. mactans female. a Double-plotted actogram, b chi-square periodogram shows no predominant circadian periodicity, c Lomb-Scargle periodogram for the entire DD free-run reveals three significant (P < 0.001) circadian periodicities (values indicated in graph) plus numerous significant ultradian periods between 2 and 12 h. Lomb-Scargle periodograms for the first 8 days of activity in DD (d), days 9–17 in DD (e), days 18–26 in DD (f) indicate significant circadian periods (values indicated within each graph) as well as numerous ultradian periods between 2 and 12 h. Note rough agreement between circadian periodicities in c and those in d, e, and f
Locomotor activity under LD (8 days) and DD for one L. mactans female. a Double-plotted actogram, b chi-square periodogram shows no predominant circadian periodicity, c Lomb-Scargle periodogram for the entire DD free-run reveals one significant (P < 0.001) circadian periodicity as well as a second component at approximate half the circadian period (values indicated within graph), plus numerous significant ultradian periods between 2 and 8 h. Lomb-Scargle periodograms for (d) the first 8 days of activity in DD, (e) days 9–17 in DD, and (f) days 18–26 in DD indicate significant circadian periods (values indicated within each graph), a second component at approximately half the circadian period, and numerous ultradian periods between 2 and 8 h. Note close agreement between the three circadian periods indicated in c and those in d, e, and f
The period changes detected in the long-duration dataset posed a problem for determining the endogenous FRP for this species. To provide at least an estimate of the FRP, and to enable comparisons with the other two species, the separate values in the approximate one-third sections were simply averaged. The FRPs for the short duration dataset were those reported for the entire span of time in DD. Using the values from the combined datasets, the mean FRP in DD for L. mactans (n = 22) was 24.83 ± 0.64 h.
The presence of activity throughout DD (thus preventing determination of activity onsets and, in turn, the degree of masking of activity during LD) rendered a correlation between activity onset phase and FRP impossible. However, a comparison of individual FRPs and phases of activity offset revealed no significant correlation (Spearman rank-order correlation: rs = 0.01, t = 0.06, df = 20, P = 0.952).
Comparisons within and among the three species
Figure 8 depicts individuals’ FRPs observed under DD for each of the three theridiid species examined in this study. The species with the smallest range of FRPs was P. tepidariorum (4.34 h). The ranges were much larger in A. studiosus (9.08 h) and L. mactans (10.17 h). Another metric for describing the distribution of FRPs, % CV (Czeisler et al. 1999), yielded values of 10.54, 5.14, and 12.13 for A. studiosus, P. tepidariorum, and L. mactans, respectively. It is instructive to note that, within these distributions, the proportions of FRPs that differed by more than 1 h from 24 h (the period of the solar day) were 74%, 94%, and 91% for A. studiosus, P. tepidariorum, and L. mactans, respectively. The proportions of FRPs that differed by more than 2 h from 24 h were 52%, 53%, and 45%, respectively.
For each species, comparisons of activity levels within individuals between LD and DD showed a significant correlation: A. studiosus (R2 = 0.803, F(1,14) = 57.14, P < 0.0001); P. tepidariorum (R2 = 0.610, F(1,27) = 42.31, P < 0.0001); L. mactans (R2 = 0.756, F(1,27) = 83.78, P < 0.0001). The ratio of LD:DD activity was about 2:1 for all three species: A. studiosus (LD:DD = 1.98 ± 0.34); P. tepidariorum (LD:DD = 1.75 ± 0.25); L. mactans (LD:DD = 2.37 ± 0.77).
Although there were no within-species correlations between FRP and phase angles of activity onset (corrected for masking) nor FRP and phase angles of activity offset, the unmasked phase angle of activity onset was significantly earlier in P. tepidariorum relative to that in A. studiosus (t test: t = 3.05, df = 32, P = 0.0046). As might be predicted based on the earlier activity onset in P. tepidariorum, the FRP was significantly shorter in P. tepidariorum than in A. studiosus (t = 2.61, df = 32, P = 0.0137). L. mactans was not included in this comparison because the inability to decipher activity onsets in DD precluded the ability to determine masked activity onsets in LD.
The mean amplitude of the circadian locomotor rhythms for all three spider species, determined by the power measure of Lomb-Scargle periodograms, was stronger in LD compared to DD. However, the difference in power between LD and DD was statistically significant (t-test for correlated samples) in P. tepidariorum (t = 2.34, df = 32, P = 0.013) and L. mactans (t = 3.58, df = 42, P < 0.001) but not in A. studiosus (t = 0.55, df = 34, P = 0.293). The increases in mean power from DD to LD were 22% for A. studiosus (from 196.3 ± 53 to 239.3 ± 58), 132% for P. tepidariorum (from 185.5 ± 29 to 431.7 ± 101.3) and 556% for L. mactans (from 59.5 ± 6.7 to 390.4 ± 92.1).
Discussion
Inter-individual and inter-species variation in free-running period
The free-running locomotor behavior under DD varied extensively, both within and among the species examined in this study. All three species exhibited extraordinarily wide distributions of FRPs (Fig. 8), ranging from about 19.1 to 23.4 h in P. tepidariorum, 19.9 to 29.0 h in A. studiosus, and 20.1 to 30.2 h in L. mactans. Using another metric for variation, % CV, the three species studied here exhibited values larger, and often about an order of magnitude larger, than those of most animals examined thus far (see Table 2 for examples). The high levels of variance in spider FRPs within each species are in sharp contrast to the low levels of variance in other organisms where it is suggested that the endogenous period of the circadian clock is under tight genetic control (Ebihara et al. 1978; Ralph and Menaker 1988; Czeisler et al. 1999; Shimizu and Masaki 1997; Helm and Visser 2010). However, the range of FRPs among the three spider species is similar to the 22.3 to 35.0 h range exhibited by cave-dwelling millipedes (Glyphiulus cavernicolus) that inhabit an environment completely devoid of zeitgebers (Koilraj et al. 2000). Presumably released from selection for FRP, this cave-dwelling species has a % CV of 12.8, approximately the same level of FRP variance as A. studiosus (10.54) and L. mactans (12.13). In comparison, a surface-dwelling species of millipede (Syngalobolus sp.) has a % CV of 4.2 (Koilraj et al. 1999).
Also contrary to findings from other organisms, the vast majority of the spiders observed in the present study exhibited endogenous circadian periods that were out of resonance with the Earth’s 24-h solar day: most deviated more than 1 h from 24 h, and many by more than 2 h (Fig. 8). Several studies have shown that organisms suffer fitness costs (Ouyang et al. 1998; Beaver et al. 2002, 2003; Emerson et al. 2009) and reduced lifespans (Pittendrigh and Minis 1972; von Saint Paul and Aschoff 1978; Dodd et al. 2005) when subjected to environmental cycles with periods that differ from their FRPs. While endogenous circadian period is a selectable trait (Shimizu and Masaki 1997; Helm and Visser 2010) and likely contributes to microevolutionary adjustments (Kyriacou et al. 2008; Allebrandt and Roenneberg 2008), it also appears to operate under a severe constraint—the daily need to re-entrain to 24-h environmental zeitgebers. Precise re-entrainment is critical for the organism to maintain appropriate temporal phase relationships with critical environmental factors such as food, predators, and parasitism (Kronfeld-Schor et al. 2017). However, the daily re-entrainment of the circadian clock may come with a cost (Daan and Beersma 2002; Turek 2008; Wyse et al. 2010). For instance, heterozygous tau mutant (22 h FRP) hamsters developed a variety of disease states when housed under 24-h LD cycles. Homozygous individuals (20-h clocks), on the other hand, showed no such disease profile: they failed to entrain to the LD cycle and, instead, free-ran through it according to their endogenous circadian periods (Martino et al. 2008).
Rhythm phase (i.e., phase angles of entrainment) may be at least partly dependent on FRP (Hall 1995; Hamblen et al. 1998) and, therefore, may influence behavioral chronotypes (Johnson et al. 2003). In humans, for example, differences in FRP are associated with “lark” and “owl” chronotypes: faster endogenous periods are associated with earlier circadian phase and slower periods with later phase (Duffy et al. 2001; Brown et al. 2008). Among-species differences in FRP could be adaptive if those differences result in different phase angles of activity (i.e. chronotypes) which, in turn, temporally partition the day, thereby easing resource competition. Fleury et al. (2000) found such evidence among three sympatric species of parasitoid wasps that oviposition on Drosophila larvae. Such deviations in FRP driven by selection acting on phase angle are not indicated in the present study. Comparisons between the two theridiid species for which circadian phase of activity onset could be determined showed that the species with the significantly faster clock (P. tepidariorum) possessed an earlier circadian phase of activity onset than the species with the slower clock (A. studiosus). However, at the within-species level, we found no correlations between FRP and phase angles of activity onset or offset for any of our three species. If deviations in FRP are driven by selection acting on phase angle, there should be a correlation between FRP and phase angle within the species. These results are not unexpected because, even though there could be overlap in prey types, any advantage of temporal niche partitioning (both within and among the three species) would be negated because the spiders forage territorially in their individual webs and, thus, do not interfere with each other’s foraging success (reviewed in Wise 1993).
Based upon the entrainment waveforms, the consistent phase angles of entrainment, and the existence of free-running rhythms that extrapolate back to the activity patterns in LD (at least in A. studiosus and P. tepidariorum), it is apparent that the spiders in all three species successfully entrained to the 24-h LD cycles. In light of the extraordinary variability in endogenous circadian period as well as the existence of many individuals with circadian periods deviating by more than 2 h from the 24-h LD cycle (Fig. 8), a plausible explanation is that selection for near 24-h clock period has been relaxed. Furthermore, because all of the spiders were collected in the wild, non-resonance with the 24-h day apparently was not a critical factor for their existence. We hypothesize that these theridiid spiders evolved an exceptionally robust response to light, thus enabling the large daily phase advances or phase delays necessary to maintain stable entrainment. Such robust responses to light presumably could reflect a circadian system operating according to a high amplitude-phase response curve (Johnson 1992). This idea of relaxed selection may be extended to the exceptionally short-period clock (mean FRP of about 18.7 h) of the trashline-orb weaving spider, Cyclosa turbinata (Moore et al. 2016), which belongs to a related family, Araneidae. As with the three theridiid species examined in the present study, C. turbinata shows a consistent entrainment waveform but the average C. turbinata must accomplish a 5.3 h phase delay each day to maintain entrainment with the Earth’s 24-h solar cycle.
Another, not mutually exclusive, explanation for the apparently robust responses to light in our three theridiid species is a modification in how the output signals from the master oscillator in the brain are processed by peripheral clocks throughout the body. For example, Yamaguchi et al. (2013) generated mutants in mice that lacked vasopressin receptors, thus accelerating re-entrainment to large LD phase-shifts. Similarly, accelerated resynchronization to LD phase-shifts occurred after knockdown of pdf mRNA and PDF (pigment-dispersing factor) peptide levels in crickets (Hassaneen et al. 2011). Whatever the mechanism underlying the vigorous response to light in spiders, its potential for alleviating at least some of the cost of daily re-entrainment may have permitted the relaxation of selection for near 24-h clock periods.
Another possible explanation for the broad range of FRPs in all three species examined in this study might be the amplitude of the circadian oscillators. It has been proposed that a weak (low amplitude) oscillator offers a flexibility of rhythm adjustment for an organism that relies heavily on environmental conditions (Pittendrigh et al. 1991; Vitaterna et al. 2006; Brown et al. 2008; Vaze and Helfrich-Förster 2016). One property of a weak oscillator is that the oscillator amplitude is higher under entrainment than under free-running conditions (Abraham et al. 2010; Kurosawa and Goldbeter 2006; Vaze and Helfrich-Förster 2016). Two of our species (P. tepidariorum and L. mactans) demonstrated significantly higher rhythm power in LD relative to DD (by factors of about 2.3 and 6.6, respectively). In A. studiosus, the rhythm power also was higher in LD relative to DD (by a factor of about 1.2) but the difference was not statistically significant. Although we are measuring rhythmic outputs (locomotor activity) downstream from the circadian oscillator rather than oscillations of components within the clock itself, these differences in amplitude may provide evidence for weak circadian oscillators. Further support for the presence of weak circadian oscillators in our species will require measurements that show a wide entrainment range over different zeitgeber strengths, rapid re-entrainment in response to LD phase shifts, and a Type 0 phase response curve (Vitaterna et al. 2006).
Yet another potential contributor to the broad range of FRPs in the species examined in this study is differential exposures to artificial light at night prior to capture and placement in our activity monitors. After-effects, sometimes long-lasting, to a number of different 24-h and non-24-h LD cycles have been described in a variety of species (Pittendrigh and Daan 1976; Aschoff 1979; Barrett and Page 1989). For example, Tomioka et al. (1997) showed that wild-type Drosophila melanogaster FRPs measured under DD lengthened after pre-exposure to 24-h LD cycles with short light periods (e.g. LD 4:20 and LD 8:16) and shortened after pre-exposure to LD cycles with longer light periods (e.g. LL, LD 20:4, and LD 16:8).
In rats, the presence of dim light at night has been shown to shift the time of food intake (Fonken et al. 2010) and also to attenuate rhythms of PER1 and PER2 clock gene expression in both the SCN and liver (Fonken et al. 2013). It is suspected that nighttime dim light may affect circadian clock function in free-living European blackbirds: individuals from an urban environment were found to have significantly shorter FRPs (by 50 min) of locomotor activity under DD than conspecifics from a forest environment (Dominoni et al. 2013). Individuals from one of our study species, P. tepidariorum, were collected from a variety of urban structures and likely experienced a variety of different light intensities during nighttime hours. It is presumed that a wide variety of light level exposures at night would yield a wide variety of after-effects if the species were susceptible. It is interesting to note that, although P. tepidariorum individuals showed the shortest mean FRP among the three species, they also exhibited the smallest range of FRPs (4.3 h) and the smallest % CV (5.14). In contrast, the species with the least possible exposure to artificial light at night, A. studiosus, showed a 9.1 h range of FRPs and a % CV of 10.54. Similarly, L. mactans, collected at considerable distances from artificial lights, possessed the largest range of FRPs (10.2 h) and the highest % CV (12.13). Important next steps in our effort to understand the broad distributions of FRPs in our three species will be determining the influence of LD cycles with different levels of dim light substituting for darkness on subsequent FRPs under DD conditions and comparing the effects of gradual vs. instantaneous transitions between photophase and scotophase.
Chronotypes
The assignment of chronotype typically is based on the phase angle of entrainment to environmental (e.g., LD) cycles (Refinetti et al. 2016; Zakharenko et al. 2018). Our approach is to extend the definition of chronotype by examining the phasing of locomotor activity under DD conditions relative to the phasing during LD entrainment, focusing on the extent of apparent masking of activity under LD.
Among the three species surveyed in this study, the patterns by which the locomotor activity was expressed during LD cycles (Figs. 1 and 2) exhibit a great deal of similarity, thus suggesting similar chronotypes. Spiders in all three species are strongly nocturnal (Table 1) and display a short delay in activity onset after the light-off transition and a peak in activity in early scotophase, followed by a gradual reduction in activity culminating in activity offset shortly before the light-on transition. All three species are about two times more active during LD cycles than under DD conditions, indicating that LD cycles enable the expression of locomotor activity beyond the circadian clock-governed activity expressed during DD.
Despite the correspondence among the three theridiid species in activity patterns under LD cycles, there are remarkable differences in the expression of locomotor behavior (i.e., chronotypes) under DD. We hypothesize that these differences relate to their respective ecologies. For example, the three species differ dramatically in the degree of apparent masking of activity during LD entrainment. Such masking typically is revealed by the occurrence of free-running activity during the first day of exposure to DD at an earlier or later time of day than the phasing of activity predicted by the FRP. In all three species, the onset of activity begins shortly after the light-off transition under LD cycles. However, in P. tepidariorum, activity onsets during DD extrapolate back to mid- or late-photophase (Fig. 4) rather than the early scotophase portion of the LD cycle, indicating that circadian clock-driven locomotor activity is inhibited (masked) by light during the last half of the photophase. Specifically, our measures indicate an average of about 3.5 h of masking in female P. tepidariorum. In nature, P. tepidariorum tend to build their webs under rock outcrops and likely experience higher variation in the onset of local darkness relative to more exposed species (due to cloudiness as well as depth and angle of cover). They forage on both crawling and flying insects, capturing them at a fairly even rate throughout the day (Riechert and Cady 1983). Because the phase angle of entrainment for locomotor activity onset is in mid-to-late photophase (but apparently hidden by masking), individuals may be ‘neurologically primed’ during mid-to-late photophase (in addition to their nocturnal foraging), thus allowing them to capture prey opportunistically during these daylight hours should local environmental conditions (i.e., low light levels) permit. Such opportunistic behavior shares some similarity with nocturnal mammals that awake from an extended daytime sleep before dusk so that they can “light sample” to ensure that their emergence from the den is safely under the cover of darkness (DeCoursey 1990).
It is interesting to note that P. tepidariorum males express an entrainment waveform under LD 12:12 that is remarkably similar, though at a smaller amplitude, to that in females (Fig. 2) including a peak shortly after the lights-off transition followed by a steady decline in activity through scotophase and a small activity burst in the photophase shortly after the lights-on transition (Garmany et al. 2019). Also, the mean FRP under DD conditions in males (about 21.2 h) is not significantly different from the mean FRP of about 21.7 h in females (Garmany et al. 2019). Furthermore, the variance in FRPs, represented as % CV, is similar in males (7.48) and females (5.14). However, males show a more extremely nocturnal DiNoc ratio (− 0.88 compared to − 0.72) and a smaller degree of masking (about 2.2 h compared to about 3.5 h) relative to females. As proposed in Garmany et al. (2019), the higher level of nocturnality in males (as well as the shorter duration of masking by light during late photophase) relative to females possibly may be related to gender-specific behavioral differences: mature males leave their webs in search of mates (thereby increasing their vulnerability to predation) whereas females do not (Ewing 1918).
A more extreme form of apparent masking is observed in L. mactans. Although its entrainment waveform is very similar to both of the other theridiids, activity under DD consists of short bouts of activity that are distributed throughout the entire circadian cycle (Figs. 5–7). In accord with these widely distributed activity bouts in DD, it is interesting to note that periodogram analyses (Figs. 6c–f and 7c–f) reveal consistent ultradian cycles (2–10 h periodicity) that are not present in the other two species. The cycle-wide presence of ultradian activity bouts under DD suggests that masking occurs throughout the photophase under LD cycles. Latrodectus species are well-known for seeking particularly secluded and dark places to build webs (Salomon et al. 2010; Pruitt et al. 2011) and are specialized in capturing crawling prey (Hódar and Sánchez‐Piñero 2002; Salomon 2011). Their most abundant prey type (Family: Carabidae) contains both diurnal and nocturnal species (Lövei and Sunderland 1996). Thus, L. mactans appears to be even more chronobiologically primed than P. tepidariorum to respond to prey at any time of day. An informative comparison to L. mactans is the presence of both circadian and ultradian (1–4 h periodicity) control of general locomotor behavior (all movements including running wheel activity) in the common vole, Microtus arvalis (Gerkema et al. 1990). Under LD conditions, if the running wheel is blocked, the nocturnal wheel running pattern (previously confirmed as circadian) disappears and the result is purely ultradian activity, including ultradian bouts that were masked by the circadian wheel-running activity. Furthermore, some individuals under DD show a spontaneous loss of circadian wheel-running behavior. The persistence of ultradian rhythms under constant conditions (Gerkema and van der Leest 1991) and the discovery of ultradian gene expression in vitro (van der Veen and Gerkema 2017) strongly suggest that ultradian rhythms are intrinsically driven. In L. mactans, periodogram analyses (Figs. 6 and 7) indicate simultaneous contributions of both circadian and ultradian components to locomotor activity under DD conditions. The widespread occurrence of circadian period changes under DD conditions in L. mactans (e.g., Fig. 7) and occasionally in A. studiosus (Fig. 3c) suggests control from multiple circadian oscillators (Daan and Berde 1978; Christensen and Lewis 1982).
A. studiosus showed a smaller amount of masking (according to our measurements, an average of about 1.5 h) during the late photophase under LD conditions relative to that observed in P. tepidariorum and L. mactans. A. studiosus builds exposed webs near branch tips of trees and shrubs (Brach 1977), capturing flying prey exclusively (Jones and Parker 2002). These prey species show a strong daily cycle in abundance, being lowest around dawn and peaking around dusk (Watts et al. 2015). Of the three species in this study, A. studiosus appears to be the most chronobiologically specialized (including possession of the most extreme DiNoc ratio, Table 1), suggesting that this species is specifically ‘primed’ to capture prey at night. We currently are exploring if the apparent masking of activity in photophase leads to differences among species in their ability to quickly ‘ramp up’ activity at different times during photophase.
Comparisons of chronotypes among spider species in this study suggest a novel chrono-ecological hypothesis. Just as selection has led to specialist and generalist species (Futuyma and Moreno 1988) in traditional aspects of ecology (e.g., habitat, prey types, etc.), we propose that species can evolve to be circadian specialists or generalists. More specifically, perhaps some species use their circadian clocks to tightly focus their neurological arousal at specific times of the diel cycle, while others may distribute neurological arousal more evenly around the clock by disengaging it from their circadian clock. Intrinsic to this idea is the assumption that neurological arousal is energetically limited (Laughlin et al. 1998; Ames 2000) and that individuals cannot maintain peak arousal throughout the entire diel cycle. With particular respect to spiders, circadian-specialist species might be especially effective predators within a narrow time window, but less able to exploit prey opportunities at other times. The tradeoffs would be reversed for circadian-generalist species: they might be alert enough to exploit opportunities with even effectiveness at any time of day, but not especially effective at any time. Considering foraging effort, a prediction of this hypothesis would be that species in which foraging opportunities (factoring in their own predation risks; Lima and Dill 1990) are concentrated during a limited part of the diel cycle should be circadian-specialists, while species which encounter prey throughout the diel cycle should be circadian generalists. As stated earlier, there is a growing call to integrate classic chronobiology and ecology to more deeply understand biological clocks from an evolutionary perspective (Helm et al. 2017; Schwartz et al. 2017). We believe that spiders may comprise an excellent model system to further this goal.
References
Abraham U, Granada AE, Westermark PO, Heine M, Kramer A, Herzel H (2010) Coupling governs entrainment range of circadian clocks. Mole Syst Biol 6:438. https://doi.org/10.1038/msb.2010.92
Allada R, Emery P, Takahashi JS, Rosbash M (2001) Stopping time: the genetics of fly and mouse circadian clocks. Ann Rev Neurosci 24:1091–1119
Allebrandt KV, Roenneberg T (2008) The search for circadian clock components in humans: new perspectives for association studies. Braz J Med Res 41:716–721
Ames A (2000) CNS energy metabolism as related to function. Brain Res Re 34:42–68
Aschoff J (1979) Circadian rhythms: influences of internal and external factors on the period measured in constant conditions. Z Tierpsychol 49:225–249
Barrett RK, Page TL (1989) effects of light on circadian pacemaker development. I. The freerunning period. J Comp Physiol 165:41–49
Bartell PA, Gwinner E (2005) A separate circadian oscillator controls nocturnal migratory restlessness in the songbird Sylvia borin. J Biol Rhythms 20:538–549
Beaver LM, Gvakharia BO, Vollintine TS, Hege DM, Stanewsky R, Giebultowicz JM (2002) Loss of circadian clock function decreases reproductive fitness in males of Drosophila melanogaster. Proc Nat Acad Sci USA 99:2134–2139
Beaver LM, Rush BL, Gvakharia BO, Giebultowicz JM (2003) Noncircadian regulation and function of clock genes period and timeless in oogenesis of Drosophila melanogaster. J Biol Rhythms 18:463–472
Bertossa RC, van Dijk J, Diao W, Saunders D, Beukeboom LW, Beersma DGM (2013) Circadian rhythms differ between sexes and closely related species of Nasonia wasps. PLoS ONE 8(3):e60167. https://doi.org/10.1371/journal.pone.0060167
Bloch G, Robinson GE (2001) Reversal of honeybee behavioural rhythms. Nature 410:1048
Bloch G, Toma DP, Robinson GE (2001) Behavioral rhythmicity, age, division of labor and period expression in the honeybee brain. J Biol Rhythms 16:444–456
Brach V (1977) Anelosimus studiosus (Araneae: Theridiidae) and the evolution of quasisociality in theridiid spiders. Evolution 31:154–161
Bradley RA (2013) Common spiders of North America. University of California Press, Los Angeles
Brown SA, Kunz D, Dumas A, Westermark PO, Vanselow K, Tilmann-Wahnschaffe A, Herzel H, Kramer A (2008) Molecular insights into human daily behavior. Proc Nat Acad Sci USA 105:1602–1607
Christensen ND, Lewis R (1982) the circadian locomotor rhythms of Hemideina thoracica (Orthoptera, Stenopelmatidae): the circadian clock as a population of interacting oscillators. Physiol Entomol 7:1–13
Czeisler CA, Duffy JF, Shanahan TL, Brown EN, Mitchell JF, Rimmer DW, Ronda JM, Silva EJ, Allan JS, Emens JS, Dijk DJ, Kronauer RE (1999) Stability, precision, and near-24-hour period of the human circadian pacemaker. Science 284:2177–2181
Daan S, Berde C (1978) Two coupled oscillators: simulations of the circadian pacemaker in mammalian activity rhythms. J Theoret Biol 70:297–313
Daan S, Beersma DGM (2002) Circadian frequency and its variability. In: Kumar V (ed) Biological rhythms. Springer, Berlin, pp 24–37
DeCoursey PJ (1990) Circadian photoentrainment in nocturnal mammals: ecological overtones. Biol Behav 15:213–238
DeCoursey PJ (2004) The behavioral ecology and evolution of biological timing systems. In: Dunlap JC, Loros JJ, DeCoursey PJ (eds) Chronobiology: biological timekeeping. Sunderland, MA, Sinauer, pp 27–65
DeCoursey PJ, Walker JK, Smith SA (2000) A circadian pacemaker in free-living chipmunks: essential for survival? J Comp Physiol A 186:169–180
Dodd AN, Salathia N, Hall A, Keyei E, Toth R, Nagy F, Hibberd JM, Millar AJ, Webb AA (2005) Plant circadian clocks increase photosynthesis, growth, survival, and competitive advantage. Science 309:630–633
Dominoni DM, Helm B, Lehmann M, Dowse HB, Partecke J (2013) Clocks for the city: circadian differences between forest and city songbirds. Proc Roy Soc B 280:20130593
Dubowy C, Sehgal A (2017) Circadian rhythms and sleep in Drosophila melanogaster. Genetics 205:1373–1397
Duffy JF, Rimmer DW, Czeisler CA (2001) Association of intrinsic circadian period with morningness-eveningness, usual wake time, and circadian phase. Behav Neurosci 115:895–899
Dunlap JC (1996) Genetic and molecular analysis of circadian rhythms. Annu Rev Genet 30:579–601
Ebihara S, Tsuji K, Kondo K (1978) Strain differences of the mouse’s free-running circadian rhythm in continuous darkness. Physiol Behav 20:795–799
Emerson KJ, Bradshaw WE, Holzapfel CM (2009) Concordance of the circadian clock with the environment is necessary to maximize fitness in natural populations. Evolution 62–4:979–983
Ewing HE (1918) The life and behavior of the house spider. Proc Iowa Acad Sci 25:177–204
Fleury F, Allemand R, Vavre F, Fouillet P, Bouletreau M (2000) Adaptive significance of a circadian clock: temporal segregation of activities reduces intrinsic competitive inferiority in Drosophila parasitoids. Proc R Soc Lond B 267:1005–1010
Fonken LK, Workman JL, Walton JC, Weil ZM, Morris JS, Haim A, Nelson RJ (2010) Light at night increases body mass by shifting the time of food intake. Proc Nat Acad Sci USA 107:18664–18669
Fonken LK, Aubrecht TG, Melendez-Fernandez H, Weil ZM, Nelson RJ (2013) Dim light at night disrupts molecular circadian rhythms and affects metabolism. J Biol Rhythms 28:262–271
Futuyma DJ, Moreno G (1988) The evolution of ecological specialization. Annu Rev Ecol Syst 19:207–233
Garmany M, Moore D, Jones TC (2019) Diel and circadian rhythms of locomotor activity in male Parasteatoda tepidariorum (Araneae: Theridiidae). J Arachnol 47:310–316
Gerkema MP, Groos GA, Daan S (1990) Differential elimination of circadian and ultradian rhythmicity by hypothalamic lesions in the common vole Microtus arvalis. J Biol Rhythms 5:81–95
Gerkema MP, van der Leest F (1991) Ongoing ultradian activity rhythms in the common vole, Microtus arvalis, during deprivations of food, water and rest. J Comp Physiol A 168:591–597
Halberg F, Cornélissen G, Katinas G, Syutkina EV, Sothern RB, Zaslavskaya R, Halberg F, Watanabe Y, Schwartzkopff O, Otsuka K, Tarquini R, Frederico P, Siggelova J (1950s) Transdisciplinary unifying implications of circadian findings in the 1950s. J Circadian Rhythms 1:2
Hall JC (1995) Tripping along the trail to the molecular mechanisms of biological clocks. Trends Neurosci 18:230–240
Hamblen MJ, White NE, Emery PTJ, Kaiser K, Hall JC (1998) Molecular and behavioral analysis of four period mutants in Drosophila melanogaster encompassing extreme short, novel long, and unorthodox arrhythmic types. Genetics 149:165–178
Hassaneen E, Sallam AE, Abo-Ghalia A, Moriyama Y, Karpova SG, Abdelsalam S, Matushima A, Shimohigashi Y, Tomioka K (2011) Pigment-dispersing factor affects nocturnal activity rhythms, photic entrainment, and the free-running period of the circadian clock in the cricket Gryllus bimaculatus. J Biol Rhythms 26:3–13
Helm B, Visser ME (2010) Heritable circadian period length in a wild bird population. Proc R Soc B 277:3335–3342
Helm B, Visser ME, Schwartz W, Kronfeld-Schor N, Gerkema M, Piersma T, Bloch G (2017) Two sides of a coin: ecological and chronobiological perspectives of timing in the wild. Phil Trans R Soc B 372:20160246. https://doi.org/10.1098/rstb.2016.0246
Hódar JA, Sánchez-Piñero F (2002) Feeding habits of the black widow spider Latrodectus lilianae (Araneae: Theridiidae) in an arid zone of south-east Spain. J Zool 257:101–109
Johnson CH (1992) Phase response curves: What can they tell us about circadian clocks? In: Hiroshige T, Honma K (eds) Circadian clocks from cell to human. Hokkaido University Press, Sapporo, pp 209–246
Johnson CH, Elliott JA, Foster R (2003) Entrainment of circadian programs. Chronobiol Int 20:741–774
Johnson CH, Elliott J, Foster R, Honma K-I, Kronauer R (2004) Fundamental properties of circadian rhythms. In: Dunlap JC, Loros JJ, DeCoursey PJ (eds) Chronobiology: biological timekeeping. Sunderland, MA, Sinauer, pp 67–105
Jones TC, Parker PG (2002) Delayed dispersal benefits both mother and offspring in the cooperative spider Anelosimus studiosus (Araneae: Theridiidae). Behav Ecol 13:142–148
Jones TC, Wilson R, Moore D (2018) Circadian rhythms of locomotor activity in Metazygia wittfeldae (Araneae: Araneidae). J Arachnology 46:26–30
Kavaliers M (1978) Seasonal changes in the circadian period of the lake chub, Couesius plumbeus. Can J Zool 56:2591–2596
Kavaliers M (1980) Circadian locomotor activity rhythms of the burbot, Lota lota: seasonal differences in period length and the effect of pinealectomy. J Comp Physiol 136:215–218
Kavaliers M (1981) Seasonal effects on the freerunning rhythm of circadian activity of longnose dace (Rhinichthys cataractae). Environ Biol Fish 6:203–206
Koilraj AJ, Sharma VK, Marimuthu G, Chandrashekaran MK (2000) Presence of circadian rhythms in the locomotor activity of a cave-dwelling millipede Glyphiulus cavernicolus Sulu (Cambalidae, Spirostreptida). Chronobiol Int 17:757–765
Koilraj AJ, Marimuthu G, Sharma VK (1999) Circadian rhythms in the locomotor activity of a surface-dwelling millipede Syngalobolus sp. Biol Rhythm Res 30:529–533
Konopka RJ, Benzer S (1971) Clock mutants of Drosophila melanogaster. Proc Natl Acad Sci USA 68:2112–2116
Kronfeld-Schor N, Visser ME, van Gils JA (2017) Chronobiology of interspecific interactions in a changing world. Phil Trans R Soc B 372:20160248. https://doi.org/10.1098/rstb.2016.0248
Kurosawa G, Goldbeter A (2006) Amplitude of circadian oscillations entrained by 24-h light-dark cycles. J Theoret Biol 242:478–488
Kyriacou CP, Peixoto AA, Sandrelli F, Costa R, Tauber E (2008) Clines in clock genes: fine-tuning circadian rhythms to the environment. Trends Genet 24:124–132
Laughlin SB, de Ruyter van Steveninck RR, Anderson JC (1998) The metabolic cost of neural information. Nat Neurosci 1:36–41
Lima S, Dill L (1990) Behavioral decisions made under the risk of predation: a review and prospectus. Can J Zool 68:619–640
Lohmann M (1967) Ranges of circadian period length. Experientia 23:788–790
Lone SR, Ilangovan V, Murugan M, Sharma VK (2010) Circadian resonance in the development of two sympatric species of Campanotus ants. J Insect Physiol 56:1611–1616
Lövei GL, Sunderland KD (1996) Ecology and behavior of ground beetles (Coleoptera: Carabidae). Ann Rev Entomol 41:231–256
Lowrey PL, Takahashi JS (2011) Genetics and circadian rhythms in mammalian model organisms. Adv Genet 74:175–230
Martino TA, Oudit GY, Herzenberg AM, Tata N, Koletar MM, Kabir GM, Belsham DD, Backx PH, Ralph MR, Sole MJ (2008) Circadian rhythm disorganization produces profound cardiovascular and renal disease in hamsters. Am J Physiol Regul Integr Comp Physiol 294:R1675–R1683
Moore D, Rankin MA (1985) Circadian locomotor rhythms in individual honeybees. Physiol Entomol 10:191–197
Moore D, Angel JE, Cheeseman IM, Fahrbach SE, Robinson GE (1998) Timekeeping in the honey bee colony: integration of circadian rhythms and division of labor. Behav Ecol Sociobiol 43:147–160
Moore D, Watts JC, Herrig A, Jones TC (2016) Exceptionally short-period circadian clock in Cyclosa turbinata: regulation of locomotor and web-building behavior in an orb-weaving spider. J Arachnol 44:388–396
Ortega-Escobar J (2002) Circadian rhythms of locomotor activity in Lycosa tarentula (Araneae, Lycosidae) and the pathways of ocular entrainment. Biol Rhythm Res 33:561–576
Ouyang Y, Andersson CR, Kondo T, Golden SS, Johnson CH (1998) Resonating circadian clocks enhance fitness in cyanobacteria. Proc Natl Acad Sci 95:8660–8664
Pittendrigh CS, Daan S (1976) A functional analysis of circadian pacemakers in nocturnal rodents. I. The stability and lability of spontaneous frequency. J Comp Physiol 106:223–252
Pittendrigh CS, Minis DH (1972) Circadian systems: longevity as a function of circadian resonance in Drosophila melanogaster. Proc Nat Acad Sci USA 69:1537–1539
Pittendrigh CS, Kyner WT, Takamura T (1991) The amplitude of circadian oscillations: temperature dependence, latitudinal clines, and the photoperiodic time measurement. J Biol Rhythms 6:299–313
Press WH, Rybicki GB (1989) Fast algorithm for spectral analysis of unevenly sampled data. Astrophys J 338:277–280
Prohaska F, Joplin KH, Moore D (2018) Effects of gender, age, and nutrition on circadian locomotor activity rhythms in the flesh fly Sarcophaga crassipalpis. J Insect Physiol 107:265–275. https://doi.org/10.1016/j.jinsphys.2017.11.007
Pruitt JN, DiRenzo N, Kralj-Fišer S, Johnson JC (2011) Individual-and condition-dependent effects on habitat choice and choosiness. Behav Ecol Sociobiol 65:1987–1995
Ralph MR, Menaker M (1988) A mutation of the circadian system in golden hamsters. Science 241:1225–1227
Ralph MR, Foster RG, Davis FC, Menaker M (1990) Transplanted suprachiasmatic nucleus determines circadian period. Science 247:975–978
Refinetti R (2012) Integration of biological clocks and rhythms. Compr Physiol 2:1213–1239
Refinetti R, Wassmer T, Chrukalady R, Pandey VK, Singaravel M, Giannetto C, Piccione G (2016) Variability of behavioral chronotypes of 16 mammalian species under controlled conditions. Physiol Behav 161:53–59
Riechert SE, Cady A (1983) Patterns of resource use and tests for competitive release in a spider community. Ecology 64:899–913
Ruf T (1999) The Lomb-Scargle periodogram in biological rhythm research: analysis of incomplete and unequally spaced time-series. Biol Rhythm Res 30:178–201
Salomon M, Vilbert S, Bennet RG (2010) Habitat use by western black widow spiders (Latrodectus Hesperus) in coastal British Columbia: evidence of facultative group living. Can J Zool 88:334–346
Salomon M (2011) The natural diet of a polyphagous predator, Latrodectus hesperus (Araneae: Theridiidae), over one year. J Arachnology 39:154–160
Schwartz WJ, Helm B, Gerkema MP (2017) Wild clocks: preface and glossary. Phil Trans R Soc B 372:20170211. https://doi.org/10.1098/rstb.2017.0211
Seggio JA (2013) The free-running period of Drosophila melanogaster is not affected by the length of the tube in a Drosophila Activity Monitor (DAM). Dro Inf Ser 96:197–199
Seyfarth E-A (1980) Daily patterns of locomotor activity in a wandering spider. Physiol Entomol 5:199–206
Shemesh Y, Eban-Rothschild A, Cohen M, Bloch G (2010) Molecular dynamics and social regulation of context-dependent plasticity in the circadian clockwork of the honey bee. J Neurosci 30:12517–12525
Shimizu T, Masaki S (1997) Geographical and species variation in circadian rhythm parameters in nemobiine crickets. Physiol Entomol 22:83–93. https://doi.org/10.1111/j.1365-3032.1997.tb01144.x
Sokolove PG, Bushell WN (1978) The chi square periodogram: its utility for analysis of circadian rhythms. J Theo Biol 72:131–160
Soriano-Morales S, Caballero-Hernández O, Dávila-Montes M, Morales-Malacara JB, Miranda-Anaya M (2013) Circadian locomotor activity and entrainment by light cycles in cave spiders (Dipluridae and Ctenidae) at the cave Los Riscos. Qro México Bio Rhythm Res 44:949–955
Spoelstra K, Wikelski M, Daan S, Loudon ASI, Hau M (2016) Natural selection against a circadian clock gene mutation in mice. Proc Nat Acad Sci USA 113:686–691
Steiger SS, Valcu M, Spoelstra K, Helm B, Wikelski M, Kempenaers B (2013) When the sun never sets: diverse activity rhythms under continuous daylight in free-living arctic-breeding birds. Proc Roy Soc B 280:20131016. https://doi.org/10.1098/rspb.2013.1016
Suter RB (1993) Circadian rhythmicity and other patterns of spontaneous motor activity in Frontinella pyramitela (Linyphiidae) and Argyrodes trigonum (Theridiidae). J Arachnol 21:6–22
Suter RB, Benson K (2014) Nocturnal, diurnal, crepuscular: activity assessments of Pisauridae and Lycosidae. J Arachnology 42:178–191
Tomioka K, Uwozumi K, Matsumoto N (1997) Light cycles given during development affect freerunning period of circadian locomotor rhythm of period mutants in Drosophila melanogaster. J Insect Physiol 43:297–305
Turek FW (2008) Staying off the dance floor: when no rhythm is better than bad rhythm. Am J Physiol Regul Integr Comp Physiol 294:R1672–1674
Van der Veen DR, Gerkema MP (2017) Unmasking ultradian rhythms in gene expression. FASEB J 31:743–750
Van Dongen H, Olofsen E, VanHartevelt JH, Kruyt EW (1999) Searching for biological rhythms: peak detection in the periodogram of unequally spaced data. J Biol Rhythms 14:617–620
Vaze KM, Helfrich-Förster C (2016) Drosophila ezoana uses an hour-glass or highly damped circadian clock for measuring night length and inducing diapause. Physiol Entomol 41:378–389
Vitaterna MH, Ko CH, Chang A-M, Buhr ED, Freuchte EM, Schook A, Antoch MP, Turek FW, Takahashi JS (2006) The mouse Clock mutation reduces circadian pacemaker amplitude and enhances efficacy of resetting stimuli and phase-response curve amplitude. Proc Nat Acad Sci USA 103:9327–9332
von Saint PU, Aschoff J (1978) Longevity among blowflies Phormia terraenovae R.D. kept in non-24-hour light-dark cycles. J Comp Physiol 127:191–195
Watts JC, Ross C, Jones TC (2015) Diel and life-history characteristics of personality: consistency versus flexibility in relation to ecological change. Anim Behav 101:43–49
Wise DH (1993) Spiders in ecological webs. Cambridge University Press, Cambridge
Woelfle MA, Ouyang Y, Phanvijhitsiri K, Johnson KH (2004) The adaptive value of circadian clocks: an experimental assessment in cyanobacteria. Curr Biol 14:1481–1486
Wyse C, Coogan A, Selman C, Hazlerigg D, Speakman J (2010) Association between mammalian lifespan and circadian free-running period: the circadian resonance hypothesis revisited. Biol Lett 6:696–698
Yamaguchi Y, Suzuki T, Mizoro Y, Kori H, Okada K, Chen Y, Fustin J-M, Yamazaki F, Mizuguchi N, Zhang J, Dong X, Tsujimoto G, Okuno Y, Doi M, Okamura H (2013) Mice genetically deficient in vasopressin V1a and V1b receptors are resistant to jet lag. Science 342:85–90
Zakharenko LP, Pterovskii DV, Putilov AA (2018) Larks, owls, swifts, and woodcocks among fruit flies: differential responses of four heritable chronotypes to long and hot summer days. Nat Sci Sleep 10:181–191
Acknowledgements
We gratefully acknowledge support from the National Science Foundation (IOS Grant No. 1257133). We additionally acknowledge support from the Washington and Lee University Summer Scholar’s Program. We thank the ETSU Department of Biological Sciences for logistical support and M. Miller, R. Wilson, and M. Gauck for assistance in collecting spiders. Finally, we thank F. Barth, B. Helm, and an anonymous reviewer for their exceptionally helpful comments on this manuscript. All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mah, A., Ayoub, N., Toporikova, N. et al. Locomotor activity patterns in three spider species suggest relaxed selection on endogenous circadian period and novel features of chronotype. J Comp Physiol A 206, 499–515 (2020). https://doi.org/10.1007/s00359-020-01412-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00359-020-01412-y