Abstract
We compared the influence of conspecifics and clutter on echolocation and flight speed in the bat Myotis daubentonii. In a large room, actual pairs of bats exhibited greater disparity in peak frequency (PF), minimum frequency (F MIN) and call period compared to virtual pairs of bats, each flying alone. Greater inter-individual disparity in PF and F MIN may reduce acoustic interference and/or increase signal self-recognition in the presence of conspecifics. Bats flying alone in a smaller flight room, to simulate a more cluttered habitat as compared to the large flight room, produced calls of shorter duration and call period, lower intensity, and flew at lower speeds. In cluttered space, shorter call duration should reduce masking, while shorter call period equals more updates to the bat’s auditory scene. Lower intensity likely reflects reduced range detection requirements, reduced speed the demands of flying in clutter. Our results show that some changes (e.g. PF separation) are associated with conspecifics, others with closed habitat (e.g. reduced call intensity). However, we demonstrate that call duration, period, and flight speed appear similarly influenced by conspecifics and clutter. We suggest that some changes reduce conspecific interference and/or improve self-recognition, while others demonstrate that bats experience each other like clutter.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Echolocating animals operating in proximity to conspecifics must distinguish or otherwise filter the biosonar signals of other individuals from their own if they are to effectively orient themselves and track objects in their surroundings (Surlykke and Moss 2000). In addition, when operating in closed habitat (i.e. clutter), echolocators are faced with the possibility that the echoes of objects being tracked will overlap in time with those of background vegetation (Schnitzler and Kalko 2001). Based on field data from several vespertilionid bat species, Obrist (1995) proposed that echolocating bats might contend with these two ostensibly different problems in similar ways, at least with respect to changes in echolocation behaviour. The Vespertilionidae is the most species-rich family of bats (>400 of ca. 1,200 species) (Simmons 2005) and the success of this family has been attributed to the flexibility they exhibit in echolocation call design and emission (Jakobsen and Surlykke 2010). In turn, Myotis (Vespertilionidae) is the most species rich of bat genera (>100 species; Simmons 2005), a success attributed to foraging flexibility (Ratcliffe and Dawson 2003).
Daubenton’s bat, Myotis daubentonii, is common and widespread across Europe (Dietz et al. 2009), and a well-studied species, especially with respect to echolocation and flight behaviour (Bogdanowicz 1994; Schnitzler and Kalko 2001; Surlykke et al. 2009). M. daubentonii weighs between 6 and 10 g (Dietz et al. 2009) and hunts insects by trawling prey near and from the surface of water, occasionally capturing prey in midair (Kalko and Schnitzler 1989). It changes the duration, emission rate, and frequency content of its calls according to habitat (Kalko and Schnitzler 1989), emphasizes different frequencies and modulates intensity to optimize task-specific performance (Surlykke et al. 2009).
Recent studies demonstrate that M. daubentonii and other vespertilionid species can dynamically change their acoustic field of view (Surlykke et al. 2009; Jakobsen and Surlykke 2010; Jakobsen et al. 2013). In open space, M. daubentonii uses a long, narrow sonar beam to maximize target detection distance while minimizing extraneous, off-axis echoes (Surlykke et al. 2009). In cluttered space it uses a shorter, broader beam, presumably to improve obstacle avoidance (Jakobsen et al. 2013). It uses a still broader, shallower beam during the final phase of attack on an airborne insect to improve target tracking (Jakobsen and Surlykke 2010). Many other vespertilionids are likely to behave similarly (Jakobsen et al. 2013; Ratcliffe et al. 2013). Here, we used M. daubentonii to compare vespertilionid echolocation and flight behaviour across two levels of acoustic clutter (medium and high; that is, the large and small rooms, respectively) to those changes made in echolocation and flight behaviour due to the presence of conspecifics.
Myotis daubentonii lives in groups (up to 1,000 individuals; Bogdanowicz 1994) and often hunts and commutes between sites in proximity to other bats, often conspecifics (Kalko and Schnitzler 1989; Surlykke et al. 2009). The “cocktail party effect” (Cherry 1953), our ability to attend to a single speaker in a loud and crowded room (reviewed in Bregman 1990), has long fascinated psychologists. Similarly, the influence of conspecifics on a bat’s echolocation behaviour has intrigued bioacousticians (Ulanovsky and Moss 2008). Specifically, how echolocating bats are able to differentiate their own call’s echoes from those of other bats and deal with the potentially interfering effects of conspecific signals (Ratcliffe et al. 2004; Gillam 2007). Although there have been a number of correlational studies on bat echolocation behaviour alone and in groups in the wild (e.g. Obrist 1995; Surlykke and Moss 2000; Ratcliffe et al. 2004), few are experimental (see Gillam 2007 for an exception). Fewer still have flown pairs of bats together in the lab and recorded their echolocation calls (for exceptions see Chiu et al. 2008, 2009).
Close clutter and nearby conspecifics both present perceptual and mechanical constraints (Fenton 1990; Obrist 1995; Ratcliffe et al. 2004). In the study, we report here, we flew individual M. daubentonii in large and small indoor flight rooms. We then flew the same bats in pairs in the large room. Using multi-microphone arrays we recorded their echolocation calls and used time-of-arrival differences at the microphones to position the bats in 3D space and calculate flight speed (Jakobsen and Surlykke 2010; Ratcliffe et al. 2011). For on-axis recordings with good signal-to-noise ratios, we measured call duration, intensity, peak frequency (PF), minimum frequency (F MIN), maximum frequency (F MAX), BW (BW), and call period (time from beginning of one call to beginning of the next). We tested the hypothesis suggested by Obrist (1995) that some changes observed in bats flying with conspecifics are similar to those observed in single bats flying in closed (Fenton 1990) or cluttered habitat (Schnitzler and Kalko 2001). To our knowledge, ours is the first study to empirically compare the influence of conspecifics versus clutter on echolocation call design in bats.
Specifically, we tested the prediction that the echolocation behaviour of M. daubentonii would differ between cluttered space (large room) and even more cluttered space (small room). In the more cluttered situation, we predicted shorter call duration and period (Fenton 1990; Obrist 1995; Schnitzler and Kalko 2001), lower call intensity (Surlykke et al. 2009; Brinkløv et al. 2011; Jakobsen et al. 2013), and slower flight speed (Norberg and Rayner 1987; Jakobsen et al. 2013). For bats flying in the large flight room, we also predicted that the echolocation behaviour would differ between bats flying alone versus bats flying in pairs. Those predicted included PF and F MIN changes to maximize the absolute difference between paired individuals (Obrist 1995; Ratcliffe et al. 2004; Ulanovsky et al. 2004; Chiu et al. 2009), absolute differences in call period (Obrist 1995), perhaps to aid in self-recognition, and shorter call durations to reduce the incidence signal masking (Schnitzler and Kalko 2001) between individuals (Obrist 1995). Assuming bats track other bats, we predicted that paired bats would call more often than bats flying alone. Assuming bats experience one another, to some extent, as clutter, we also predicted paired bats would use calls of shorter duration and lower intensity.
Materials and methods
Bats
We used 16 adult (8 male, 8 female) Daubenton’s bats, M. daubentonii caught from the wild at Skovsøen in Odense, Denmark. Bats were housed at the University of Southern Denmark and given access to mealworms (Tenebrio molitor) and water in their cages. Bats were not fed during trials. After experiments, we released the bats at the site of capture.
Flights: solo (small room), solo (large room), and pairs (large room)
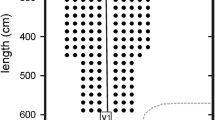
We made our recordings in two flight rooms at the University of Southern Denmark. One, our small flight room, was w 3 m × d 3.5 m × h 2.4 m, the other, our large flight room, was w 7 m × d 4.8 m × h 2.4 m (Fig. 1). Both flight rooms were constructed from aluminium poles covered in cotton batting and foam and had walls of thin mesh nylon. The difference in volume between these two rooms was more than threefold (25.4 versus 80.7 m3), and we believe that the smaller rooms would have been perceived by the bats as being more closed, or cluttered.
We released and recorded single bats as they flew in our small flight room. We released and recorded single bats and pairs of bats flying in our large flight room. We recorded four pair combinations for each bat and randomized flight order between pairs. For pairs, we only recorded sound files when both bats were in full flight (see below).
Sound recording
We used three multi-microphone arrays to record the bats’ echolocation calls. Array 1 (Fig. 1a), in our small flight room, was a cross-shaped array with seven ¼” 40 BF microphones (grids off) (G.R.A.S. Sound and Vibration A/S, Holte, Denmark). Microphones were set 50 cm apart on the horizontal axis and 35 cm apart on the vertical axis.
Arrays 2 and 3, in our large flight room, each consisted of twelve ¼” 40 BF G.R.A.S. microphones (grids off) arranged in two flat plane arrays, one at each end of the flight room (Fig. 1b). Microphones were set 70 cm apart on the horizontal axis and 100 cm apart on the vertical axis.
Before and after each recording session, microphones were calibrated using a 1 kHz pure tone at 94 dB SPL (type 4231 calibrator, Brüel and Kjær Sound and Vibration Measurement A/S, Nærum, Denmark). Microphones were amplified using AA G.R.A.S. amplifiers (20 dB amplification, high-pass filtered at 13.5 kHz). Signals were sampled by an Avisoft USGH 1216 A/D converter (Avisoft Bioacoustics, Berlin, Germany) at 500 kHz sample rate (16-bit). We recorded multi-channel .wav files of 3 s in duration (2 s pre-trigger, 1 s hold time). Files were recorded to a ThinkPad X201 laptop computer (Lenovo, Morrisville, USA) using Avisoft Recorder USGH software.
Flight paths
We used a custom Matlab script (written by L. Jakobsen, Lund University) to position the bats using time-of-arrival differences at the microphones, by cross-correlating the same signals on the multiple channels and then triangulating each bat’s position at the time of each call emission. This provided us with x, y, z coordinates, which were then processed using a second custom Matlab script (written by B. Charlton, University of Southampton) to produce a visual 3D reconstruction of flight paths.
In paired trials, usable flight paths consisted of those where both bats were clearly flying (and echolocating) within the same 1-s time window and where call assignment to each bat was unambiguous. In solo trials, usable flight paths consisted of those where the bat was in full flight (not in the act of landing or taking off). In all situations we selected only files containing flight paths with >4 consecutive calls per bat. To avoid pseudo-replication, we used only one file/bat/condition (i.e. one for each bat in each of the three conditions: bat flying alone in small room, bat flying alone in the large room and bat flying with conspecific in large flight room) (Hurlbert 1984; Ratcliffe and Dawson 2003; Biscardi et al. 2004).
Sound analysis
From recordings selected based on flight path screening, we split the multi-channel files into single channel files and simultaneously screened them in Adobe Audition (Adobe Systems Inc., San Jose, CA, USA). We chose only sequences with good signal-to-noise ratios (signal >2 times greater than background noise) and analysed them using BatSound (version 4, Pettersson Elektronik AB, Uppsala, Sweden). Where possible we analysed recordings from the central microphones of the cluster of microphones used for positioning.
We measured call duration and call period (start time of the focal call to the start time of the next call) from the oscillograms. From the power spectrum [automatic fast Fourier transform function (FFT), size 1,024, Hann window, frequency resolution 244 Hz] of each call, we measured PF and BW at −10 and −20 dB from the PF. To estimate the intensity of the calls, we filtered positioned calls using a third octave band-pass filter at 45 kHz. The filtered calls were then compensated for spherical spreading loss (−6 dB per doubling of distance), atmospheric attenuation (ANSI 1978) and microphone directionality. The resulting call intensities refer to the RMS pressure at 10 cm from the bat’s mouth. Only on-axis calls were used for intensity estimation (i.e. calls for which maximum source level estimates came from the centre microphones). We used JMP v. 10 (SAS Institute, Cary, NC, USA) for all statistical analyses.
Echolocation and flight behaviour across three conditions: sampling and sub-sampling
We conducted all experiments in the dark and decided not to mark the bats (e.g. arm bands, LED lights) since that might influence their behaviour. Thus, we could not keep track of individual bats, when flying bats in pairs, and we therefore included enough bats (16) in the study, to achieve statistical valid comparisons between flying alone and in pairs.
We first compared parameters (as described above) from single flight paths from each of 16 bats flying alone in the small and large flight rooms, respectively, using paired t tests (Table 1). Similarly, we measured the same parameters for these same bats when flown in pairs in the large room. To roughly determine these for bats when flying in pairs we selected 16 of these paired bat recordings such that each bat was sampled twice. We calculated the mean values for each parameter for these 16 pairs and presented these means and standard deviation values (Table 1, bold column). These paired data were not used in any quantitative statistical treatment (e.g. t tests or ANOVA) but give the results (and not just differences) based on all bats flown, when they were flying in pairs.
To investigate potential quantitative differences in echolocation behaviour and flight speed between paired bats versus solo bats in the large and the small room, we also sub-sampled our dataset such that we made three independent groups (i.e. no bat was in more than one group). We randomly sampled without replacement. That is, we randomly selected five bats and used their solo recordings from the small room. Out of the remaining bats, we randomly selected six bats (representing 3 actual pairs) and used their recordings from the large room when flying in those pairs. For the remaining five bats, we used their solo recordings from the large room. We then compared the echolocation and flight speed data from each of these three groups. By creating these three independent groups, we ensured that no bat was sampled twice and thus met this assumption of ANOVA (samples are independent). We replicated this random sampling procedure and re-ran these analyses an additional four times.
Actual versus virtual pairs: comparison of absolute value differences for echolocation and flight behaviour
Following Obrist (1995) and Ratcliffe et al. (2004), we compared our echolocation data from actual pairs of two known bats flying together in the large flight room to virtual pairs, in our case data derived from these same two bats when flying alone in the same large flight room (described in detail below). In the study we report here, we made our recordings under the more controlled conditions of the laboratory (versus the field) and did so using known individuals (see Obrist 1995; Ratcliffe et al. 2004 for comparison).
For each of our 16 bats (hereafter the ‘focal bat’), we considered all pair-wise, two bat combinations that it had flown in. Each focal bat had flown with 3–4 other bats (i.e. we had 3–4 analysed actual pair trials for each bat). We then built a complimentary set of 3–4 virtual pairs for each bat (i.e. we matched call data from the focal bat flying alone in the large flight room with solo data from the same bats it had flown with in actual pairs).
For each focal animal, we then calculated the 3–4 absolute differences between each of the call parameters and flight speeds for it and each of the conspecifics it had been paired with (actual pairs) and for the virtual pairs (focal bat alone and ‘virtual’ paired bat alone, both taken from the large room recordings). For both actual and virtual pairs, we took these 3–4 absolute value differences for the focal bat versus each of the bats it was paired with. We then calculated the average of these absolute differences for each parameter for each bat.
For example, bat 1 was flown in pairs with bats 2, 3 and 4. Actual pair absolute value differences for call duration were: bat 1 versus bat 2 = 0.3 ms, bat 1 versus bat 3 = 0.4 ms, bat 1 versus bat 4 = 0.2 ms. Thus, bat 1, when considered as focal animal, had an average absolute difference for actual pairs of 0.3 ms for call duration.
We took the absolute value difference of each averaged call parameter from known pairs of bats, meaning that the 3D flight paths could clearly be assigned to one bat or the other. However, while the bats were in flight, we could not idenitfy which bat was which for a given pair of known individuals. By using absolute differences for both actual and virtual pairs, knowledge of which bat was which was not necessary for our analysis.
Focal bat data for virtual pairs were derived in the same way using the parameters measured from recordings of bats flying in pairs in the large flight room. To illustrate, let us say bat 1 flew in actual pairs with bats 2, 3, and 4. Bat 1 produced calls, on average, that were 3 ms in duration when flying alone in the large flight room. Let us also say that bats 2, 3 and 4 had average call durations of 2.7, 3.1, and 3.2 ms, respectively, when each was flying alone in the large flight room. Therefore, the absolute differences for these virtual pairs (bat 1/bat 2, bat 1/bat 3, bat 1/bat 4) are 0.3, 0.1 and 0.2 ms, respectively. Thus, bat 1, when considered as focal animal, had an average absolute difference for virtual pairs of 0.2 ms for call duration.
Each of our 16 bats was considered in turn as the focal animal, resulting in 16 data points for each parameter for both actual and virtual pairs. Focal absolute differences for virtual and actual pairs were analysed using paired t tests for each parameter (Table 1). Please note that echolocation and flight data were replaced after each focal animal analysis (i.e. all pair-wise combinations flown were included when calculating average absolute values for each bat when treated as focal animal in both actual and virtual pairs).
Results
Bats, flight paths, and echolocation calls used
We analysed the calls of 16 bats. For each bat, we analysed a single solo flight path in each of the two rooms, and three paired flight paths (see “Materials and methods”). In total, we analysed 741 echolocation calls, for which we were able to derive intensity data for 663 calls. These data breakdown as follows. We analysed 175 calls for solo bats in the small flight room (range 7–16 calls/bat). Of these 175 calls, we were able to estimate call intensity for 145 calls (range 3–16 calls/bat). We analysed 171 calls for solo bats in the large flight room (range 9–16 calls/bat). Of these 171 calls, we were able to estimate call intensity for 151 calls (range 3–13 calls/bat). We analysed 495 calls for bats flying in pairs in the large flight room (range 5–14 calls/bat). Of these 495 calls, we were able to estimate call intensity for 367 calls (range 3–11 calls/bat).
Echolocation and flight behaviour in large and small room: sampling and sub-sampling
When we compared the call parameters of bats flying solo in the large room to the same bats flying solo in the small room, we found call duration and period were significantly shorter in the small room (Table 1). Call intensity was significantly lower, and both PF and F MIN were significantly lower, in the small room (Table 1). Flight speed was significantly slower in the small room than in the large room (Table 1). We found no significant difference in the ranges of PF and F MIN used by individual bats flying solo in the small room versus these same bats flying solo in the large room (paired t test, P = 0.47), nor did visual inspection of the data reveal that bats were alternating between two distinct peak frequencies (and by extension two distinct minimum frequencies) when operating in the small room.
Our ANOVA results from our first run of the three independent groups (N = 5–6 bats/group) indicate that call duration, intensity, F MIN, and flight speed were significantly lower for solo bats in the small room than for solo bats in the large room (Table 2). Paired bat call parameters and flight speed in the large room mostly fell in between values from large room alone and the small room alone, but did not differ significantly from either large or small room solo bats, with the exception of F MIN, which was significantly lower in solo bats in the small room (Table 2). Additional runs yielded similar results with respect to mean ± SD for each parameter (i.e. groups differed from one another qualitatively in the same ways). However, in a single additional run, call period was significantly greater (P < 0.05) for bats flying solo in the large room compared to bats flying solo in the small and bats flying in pairs in the large room.
Actual versus virtual pairs: comparison of absolute value differences for echolocation and flight behaviour
We found significantly greater absolute value differences in PF, F MIN and call period in actual pairs versus virtual pairs flying in the large room (Table 1; Fig. 2). We found no significant differences in absolute values in our paired samples of actual and virtual pairs for call duration, intensity, PF, BW and flight speed (Table 1; Fig. 2).
a Spectrogram to show calls emitted by M. daubentonii when flying together in an actual pair in the large flight room. b FFTs of one call from each bat within the pair. c Spectrogram and d FFT of one call from one of the two bats from the actual pair while flying alone in the large room. e Spectrogram and f FFT of one of the calls from the other bat in the actual pair when flying alone. The absolute difference between the PF of the actual pair of these bats was 3.5 kHz, the PF of the virtual pair was 1.2 kHz
Discussion
We compared the echolocation and flight speed of the vespertilionid bat M. daubentonii when flying in two levels of clutter (our large and small flight rooms, mimicking medium and high levels of clutter, respectively) and when flying in conspecific pairs in the large flight room, a situation where the echolocation calls of each individual might interfere with the other bat’s echolocation. Actual pairs of bats flying in the large room exhibited greater disparity in PF, F MIN and call period compared to these same bats flying alone but matched as virtual pairs (sensu Obrist 1995; Ratcliffe et al. 2004; Table 1). Bats flying solo in the small flight room produced calls of shorter duration and call period, lower intensity and flew at slower speed than when flying solo in the large room (Tables 1, 2).
Some of the changes observed between actual and virtual pairs (e.g. greater PF disparity between actual versus virtual pairs) suggest a JAR to the echolocation signals of other bats (Habersetzer 1981; Obrist 1995; Ratcliffe et al. 2004; Ulanovsky et al. 2004; Gillam 2007). Others, like reduced call intensity in more cluttered space, are probably more a reaction to a highly cluttered environment (Brinkløv et al. 2011; Surlykke et al. 2009; Nørum et al. 2012; Jakobsen et al. 2013). As hypothesized by Obrist (1995), we found that other changes (e.g. reduced call duration, period, flight speed) may be influenced by conspecifics in a way that partly reflect the changes made in response to clutter. For these parameters, conspecific influence in the large room falls somewhere between solo flight in the small room and solo flight in the large room (Table 1).
Conspecifics influence echolocation call design and emission
Greater inter-individual disparity in PF and F MIN may help M. daubentonii, and other vespertilionids (Miller and Degn 1981; Obrist 1995; Ibáñez et al. 2004; Ratcliffe et al. 2004; Ulanovsky et al. 2004; Bartonicka et al. 2007), reduce acoustic interference and/or increase self-recognition of their own echoes from the calls and echoes of conspecifics (Obrist 1995). Playbacks of bat calls alone are sometimes sufficient to elicit changes in echolocation behaviour, even in the absence of a ‘physical’ conspecific (Bates et al. 2008; Gillam 2007). However, for M. daubentonii and most other low-duty cycle species when flying in edge or cluttered habitat (e.g. the big brown bat, Eptesicus fuscus; see Surlykke and Moss 2000; Bates et al. 2008; Chiu et al. 2008, 2009), PF and F MIN differences between two bats in proximity of <5 kHz would mean that the BWs of the two bat’s echolocation calls would still largely overlap (Tables 1, 2). Thus PF separation should only reduce, not eliminate, acoustic interference, but in doing so might also improve self-recognition (Obrist 1995; Ratcliffe et al. 2004; Ulanovsky et al. 2004).
PF and F MIN differences, could potentially act as ‘markers’ for bats to better recognize the echoes of their own calls as compared to the calls and echoes of a nearby conspecific (Obrist 1995; Ratcliffe et al. 2004; Ulanovsky et al. 2004). Similarly, the observed absolute differences in call period between actual versus virtual pairs of bats indicate that when flying together, two bats also use more divergent call emission rates than when flying alone in the same room. These differences might equate to recognizable patterns of echoes and, like frequency shifting, aid the bat in distinguishing its own echoes from the signals of other bats.
In general, the broadband signals used by M. daubentonii, and other myotids, may contain more detailed spectral information (e.g. be more easily discriminated) than the narrow band signals used by the molossids, Tadarida brasiliensis and T. tenitous, both of which have been proposed to increase PF disparity in response to conspecifics primarily as a JAR (Ulanovsky et al. 2004; Gillam 2007). For these molossids and other narrowband species (e.g. open-space foraging vespertilionids; Obrist 1995), an equivalent disparity in PF in pairs will result in less overlap than in broadband species (Ulanovsky et al. 2004), but may also be more important for narrowband species for self-recognition.
Increasing clutter influences echolocation call design and emission
Call duration, consistent with a response to increasing levels of clutter (Obrist 1995), was shorter in the small room, than in the large room (Tables 1, 2). Shorter call durations reduce forward masking (i.e. call–echo overlap). In both rooms bats, used shorter call durations and call periods than reported from the wild (Kalko and Schnitzler 1989; Surlykke et al. 2009). Shorter call periods equate to greater call emission rates and thus a more often updated auditory scene (Surlykke and Moss 2000; Schnitzler and Kalko 2001). In open habitat, M. daubentonii characteristically uses calls with a PF of 45 kHz. In all conditions tested here M. daubentonii used calls with a PF of >50 kHz, again consistent with a clutter reaction resulting in better resolution of small objects (Schnitzler and Kalko 2001; Ratcliffe and Dawson 2003). Lower call intensity in the small room likely reflects reduced range detection requirements (Brinkløv et al. 2011; Jakobsen et al. 2013) and reduces echo intensity at the bat’s ears (Kick and Simmons 1984; Hartley 1992; Ratcliffe et al. 2011; Nørum et al. 2012). Lower flight speed reflects the greater sensorimotor demands of flying in cluttered space (Norberg and Rayner 1987; Fenton 1990; Jakobsen et al. 2013).
Neither in the small room nor in actual pairs, was BW broader than that used by solo bats in the large room (Tables 1, 2). However, in all conditions, BW was greater than that observed in more open habitat in the wild (Obrist 1995; Kalko and Schnitzler 1989; Siemers and Schnitzler 2004; Surlykke et al. 2009). Broader BW equates to more accurate range determination (Moss and Schnitzler 1995) and improves target resolution in clutter (Siemers and Schnitzler 2004). M. daubentonii is an aerial or trawling insectivore whose preferred habitat is background cluttered space, placing it in between aerial insectivores, preferring uncluttered space and species that prefer highly cluttered space (Schnitzler and Kalko 2001). We therefore suggest that M. daubentonii had already reached the upper limit of BW in the large room, corroborating the results of Siemers and Schnitzler (2004) which showed that M. daubentonii had a lower maximum BW than M. nattereri, a species which specializes on taking airborne and suspended prey in highly cluttered habitat (Siemers et al. 2001).
Last, we were surprised by the significant drop in PF and F MIN (of approximately 3 kHz for both parameters) in bats flying solo in the small room as compared to bats flying alone or in pairs in the large room (Tables 1, 2). This is the opposite of what has typically been reported in vespertilionid and molossid bats. We investigated this difference further by visually inspecting the data for all bats in the three conditions and statistically comparing the range of PF and F MIN values used by individual bats flying solo in the large and small rooms. We found no indication that bats were alternating between call frequencies (e.g. PF at 55 kHz, followed by an PF of 50 kHz), which might have explained the lower averages in the small room and been corroborated by a recent study by Hiryu et al. (2010) which demonstrated E. fuscus alternates between two peak frequencies when flying in highly cluttered laboratory conditions. We have no obvious explanation why PF (and F MIN) is decreased in the small room. A 3 kHz PF decrease from 55 kHz will have no noteworthy effect on the beam width.
Conclusion
In sum, our results suggest that a subset of the observed changes in echolocation behaviour (e.g. greater inter-individual PF separation in actual versus virtual pairs of bats) is associated with conspecifics effects (Habersetzer 1981; Surlykke and Moss 2000; Ratcliffe et al. 2004; Ulanovsky et al. 2004). Other changes reflect responses to closed habitats (e.g. significantly reduced call intensity) (Brinkløv et al. 2011; Surlykke et al. 2009; Jakobsen et al. 2013). However, as hypothesized by Obrist (1995), shorter call duration, call period, and flight speed may be influenced by conspecifics and clutter in similar ways (Tables 1, 2) (see also Ulanovsky et al. 2004). This as suggested by the lack of significant differences between paired bats versus either solo bats in the large or small rooms, despite a number of significant differences between these two solo groups (Table 2). Reductions in each of these parameters should decrease the probability of collision with nearby vegetation and other bats. We therefore suggest that some changes observed during conspecific interactions represent strategies to reduce echolocation interference and/or improve signal recognition, while others reflect clutter-like reactions to the bodies of bats in proximity.
Abbreviations
- BW:
-
Call bandwidth (F MAX −F MIN)
- FM:
-
Frequency-modulated
- F MAX :
-
Maximum call frequency (−10 dB from PF)
- F MIN :
-
Minimum call frequency (−10 dB from PF)
- JAR:
-
Jamming avoidance response
- PF:
-
Peak frequency
References
Bartonička T, Řehák Z, Gaisler J (2007) Can pipistrelles, Pipistrellus pipistrellus (Schreber, 1774) and Pipistrellus pygmaeus (Leach, 1825), foraging in a group, change parameters of their signals? J Zool 272:194–201
Bates ME, Stamper SA, Simmons JA (2008) Jamming avoidance response of big brown bats in target detection. J Exp Biol 211:106–113
Biscardi S, Orprecio J, Fenton MB, Tsoar A, Ratcliffe JM (2004) Data, sample sizes and statistics affect the recognition of species of bats by their echolocation calls. Acta Chiropterol 6:347–363
Bogdanowicz W (1994) Myotis daubentonii. Mamm Species 475:1–9
Bregman AS (1990) Auditory scene analysis: the perceptual organization of sound. University of Chicago, Chicago
Brinkløv S, Jakobsen L, Ratcliffe JM, Kalko EKV, Surlykke A (2011) Echolocation call intensity and directionality in flying short-tailed fruit bats, Carollia perspicillata (Phyllostomidae). J Acoust Soc Am 129:427–435
Cherry EC (1953) Some experiments on the recognition of speech, with one and with two ears. J Acoust Soc Am 25:975–979
Chiu C, Xian W, Moss CF (2008) Flying in silence: echolocating bats cease vocalizing to avoid sonar jamming. Proc Natl Acad Sci USA 105:13116–13121
Chiu C, Xian W, Moss CF (2009) Adaptive echolocation behaviour in bats for the analysis of auditory scenes. J Exp Biol 212:1392–1404
Dietz C, von Helversen O, Nill D (2009) Bats of Britain, Europe and northwest Africa. A and C Black, London
Fenton MB (1990) The foraging behaviour and ecology of animal-eating bats. Can J Zool 68:411–422
Gillam EH (2007) Eavesdropping by bats on the feeding buzzes of conspecifics. Can J Zool 85:795–801
Habersetzer J (1981) Adaptive echolocation sounds in the bat Rhinopoma hardwickei: a field study. J Comp Physiol A 144:559–566
Hartley DJ (1992) Stabilization of perceived echo amplitudes in echolocating bats. II. The acoustic behavior of the big brown bat, Eptesicus fuscus, when tracking moving prey. J Acoust Soc Am 91:1132–1149
Hiryu S, Bates ME, Simmons JA, Riquimaroux H (2010) FM echolocating bats shift frequencies to avoid broadcast–echo ambiguity in clutter. Proc Natl Acad Sci USA 107:7048–7053
Hurlbert SH (1984) Pseudoreplication and the design of ecological field experiments. Ecol Monogr 54:187–211
Ibáñez C, Juste J, Lopez-Wilchis R, Nunez-Garduno A (2004) Habitat variation and jamming avoidance in echolocation calls of the sac-winged bat (Balantiopteryx plicata). J Mamm 85:38–42
Jakobsen L, Surlykke A (2010) Vespertilionid bats control the width of their biosonar sound beam dynamically during prey pursuit. Proc Natl Acad Sci USA 107:13930–13935
Jakobsen L, Ratcliffe JM, Surlykke A (2013) Convergent acoustic field of view in echolocating bats. Nature 493:93–96
Kalko EKV, Schnitzler H-U (1989) The echolocation and hunting behavior of Daubenton’s bat, Myotis daubentonii. Behav Ecol Sociobiol 24:225–238
Kick SA, Simmons JA (1984) Automatic gain control in the bat’s sonar receiver and the neuroethology of echolocation. J Neurosci 4:2725–2737
Miller LA, Degn HJ (1981) The acoustic behaviour of four species of vespertilionid bats studied in the field. J Comp Physiol 142:67–74
Moss CF, Schnitzler H-U (1995) Behavioral studies of auditory information processing. In: Fay RR, Popper AN (eds) Hearing by bats. Springer, New York, pp 87–145
Norberg UM, Rayner JMV (1987) Ecological morphology and flight in bats (Mammalia; Chiroptera): wing adaptations, flight performance, foraging strategy and echolocation. Phil Trans R Soc Lond B 316:335–427
Nørum U, Brinkløv S, Surlykke A (2012) New model for gain control of signal intensity to object distance in echolocating bats. J Exp Biol 215:3045–3054
Obrist MK (1995) Flexible bat echolocation: the influence of individual, habitat and conspecifics on sonar signal design. Behav Ecol Sociobiol 36:207–219
Ratcliffe JM, Dawson JW (2003) Behavioural flexibility: the little brown bat, Myotis lucifugus, and the northern long-eared bat, M. septentrionalis, both glean and hawk prey. Anim Behav 66:847–856
Ratcliffe JM, ter Hofstede HM, Avila-Flores R, Fenton MB, McCracken G, Biscardi S, Blasko J, Gillam E, Orpecio J, Spanjer G (2004) Conspecifics influence call design in the Brazilian free-tailed bat, Tadarida brasiliensis. Can J Zool 82:966–971
Ratcliffe JM, Jakobsen L, Kalko EKV, Surlykke A (2011) Frequency alternation and an offbeat rhythm indicate foraging behavior in the echolocating bat, Saccopteryx bilineata. J Comp Physiol A 197:413–423
Ratcliffe JM, Elemans CPH, Jakobsen L, Surlykke A (2013) How the bat got its buzz. Biol Lett 9:20121031
Schnitzler H-U, Kalko EKV (2001) Echolocation by insect-eating bats. Bioscience 51:557–569
Siemers BM, Schnitzler H-U (2004) Echolocation signals reflect niche differentiation in five sympatric congeneric bat species. Nature 429:657–661
Siemers BM, Stilz P, Schnitzler H-U (2001) The acoustic advantage of hunting at low heights above water: behavioural experiments on the European ‘trawling’ bats Myotis capaccinii, M. dasycneme and M. daubentonii. J Exp Biol 204:3843–3854
Simmons NB (2005) Order Chiroptera. In: Wilson DE, Reeder DM (eds) Mammal species of the world: a taxonomic and geographic reference, vol 1, 3rd edn. Johns Hopkins University Press, Baltimore, pp 312–529
Surlykke A, Moss CF (2000) Echolocation behaviour of big brown bats, Eptesicus fuscus, in the field and the laboratory. J Acoust Soc Am 108:2419–2429
Surlykke A, Pedersen SB, Jakobsen L (2009) Echolocating bats emit a highly directional sonar sound beam in the field. Proc R Soc Lond B 276:853–860
Ulanovsky N, Moss CF (2008) What the bat’s voice tells the bat’s brain. Proc Natl Acad Sci USA 105:8491–8498
Ulanovsky N, Fenton MB, Tsoar A, Korine C (2004) Dynamics of jamming avoidance in echolocating bats. Proc R Soc Lond B 271:1467–1475
Acknowledgments
We thank L. Jakobsen and B. Charlton for Matlab scripts. We thank S. Brinkløv, J. Christensen-Dalsgaard, A. Denzinger, B. Fenton, A. Surlykke for comments on the manuscript. L. Faber and T. Bojesen assisted with experiments. This study was funded by Danish Natural Sciences Research Council (FNU) grants to JMR and adhered to the legal requirements of Denmark and all institutional guidelines.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fawcett, K., Ratcliffe, J.M. Clutter and conspecifics: a comparison of their influence on echolocation and flight behaviour in Daubenton’s bat, Myotis daubentonii . J Comp Physiol A 201, 295–304 (2015). https://doi.org/10.1007/s00359-014-0977-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00359-014-0977-0