Abstract
Purpose
Selective serotonin reuptake inhibitors are associated with high rates of nonadherence and sexual dysfunction, yet the correlation between these findings in young adult men is poorly characterized. We aimed to evaluate if young adult men are less willing to adhere to antidepressant treatment due to intolerable side effects, such as sexual dysfunction.
Methods
Deidentified, compensated survey that assessed baseline demographics, PHQ-8 and GAD-7 scores, attitudes towards antidepressant medication side effects, and perceptions of antidepressant medications including selective serotonin reuptake inhibitors, bupropion, and mirtazapine.
Results
From 665 delivered surveys, 505 respondents completed their survey (response rate: 76%), of which 486 were included for final analysis. After seeing common side effect profiles, our sample’s willingness to take sexual function-sparing agents, such as bupropion or mirtazapine, was significantly greater than selective serotonin reuptake inhibitors (p < 0.001), with no significant difference between bupropion and mirtazapine (p = 0.263). The negative influence of erectile dysfunction and anorgasmia scored significantly higher than other common antidepressant side effects like weight gain, nausea, and dry mouth (range: p < 0.001, p = 0.043). With the exception of insomnia, participants indicated that experiencing sexual dysfunction while taking an antidepressant medication would lead to nonadherence at a significantly higher frequency than any other side effect assessed (range: p < 0.001, p = 0.005).
Conclusion
The risk of experiencing sexual side effects when taking antidepressants could lead young adult men to become nonadherent to these medications. Strategies to augment the effectiveness of antidepressants, such as shared decision-making and the use of sexual function-sparing agents, are critical.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The prevalence of Major Depressive Disorder (MDD), Generalized Anxiety Disorder (GAD), and/or suicidal ideations amongst young adults has substantially increased over the past 10 years, along with a rise in the provision of mental health care [1]. The prevalence of GAD amongst young adults in the United States is 19.1%; MDD is seen in about one in five young adults [2]. The use of first-line antidepressant therapies for these mental illnesses, such as selective serotonin reuptake inhibitors (SSRIs), has also risen to match these trends [3]. Nonetheless, it is estimated that less than half of primary care patients diagnosed with depression and prescribed antidepressants take their medication longer than 6–9 months [4,5,6].
Concurrently, a growing body of evidence cites sexual dysfunction as a prominent adverse effect of antidepressants, particularly SSRIs [7, 8]. While illnesses such as MDD and GAD may cause de novo sexual dysfunction, many SSRI-class drugs garner a substantial independent risk for these symptoms emerging with treatment [9, 10]. It is believed that 40–65% of all adult taking SSRIs are afflicted by some sexual dysfunction, including erectile dysfunction, anorgasmia, and/or decreased libido [11].
In the past two decades, atypical antidepressants, such as bupropion and mirtazapine, have emerged as potential alternative therapies to SSRIs and carry significantly reduced risks of sexual dysfunction [10, 12]. Bupropion acts as a norepinephrine and dopamine reuptake inhibitor used to treat depression, smoking cessation, and attention-deficit/hyperactivity disorder; mirtazapine enhances the release of both norepinephrine and serotonin by antagonizing alpha-2 adrenergic auto- and heteroreceptors and is used to treat depression, anxiety, and insomnia [13].
Unfortunately, young adult men experiencing psychological distress are the least likely of all demographics across the lifespan to seek help [14]. The suicide rate among men in 2021 was approximately four times higher than the rate among women [15]. Further, compared with women, men are significantly less likely to pursue any lifetime depression care or to take antidepressants [16].
There is a paucity of research assessing the potential correlation between antidepressant associated sexual dysfunction and nonadherence to these medications. Hence, the current project aims to assess the attitudes and perceptions of young men toward antidepressants and how these beliefs correlate to medication adherence. We evaluated the hypotheses that young men are hesitant to pursue antidepressant treatment because of fear and/or experience of sexual dysfunction, and that alternative therapies with less risk of sexual dysfunction, such as bupropion or mirtazapine, are more amenable to the average patient of this demographic.
Materials and methods
The Young Adult Men Antidepressant Nonadherence (YAMAN) study was a cross-sectional, deidentified, compensated questionnaire distributed to young adult men via social media through a virtual flyer. Funding was provided through the 2022 Scholars in Sexuality Research Grant sponsored by the Sexual Medicine Society of North America.
Study population and distribution
The inclusion criteria for this study were biological males between 18 and 35 who voluntarily agreed to participate in our questionnaire. Submission of the survey served as participants’ consent to participate in the research. Subjects who could not complete the survey in its entirety, or did not consent to research upon accessing the study, were excluded.
We designed our sign-up and study surveys using the HIPAA-compliant Qualtrics XM (Provo, UT, USA). Each survey was deidentified from its corresponding submitter using this software. Digital Amazon gift cards (10 USD) were obtained from Amazon Incentives Services (Seattle, WA, USA). These digital gift cards were securely delivered to the unique email addresses of each study respondent that had fully completed the survey.
Survey development
We created our survey by selecting previously validated, well-established questionnaires (i.e., PHQ-8 and GAD-7 severity scales for depression and anxiety). Additionally, we self-constructed questions to evaluate participants’ attitudes toward common antidepressants medications through collaboration with our multidisciplinary team. The survey evaluated the following domains (see Supplementary Document 1 for the exact survey questions):
-
1.
Consent to Research and Confirmation of Inclusion Criteria.
-
2.
Demographic Information: participants reported on their age, ethnicity, gender identity, sexual orientation, relationship status, and occupation.
-
3.
Medication Side Effects: respondents read a brief vignette about being prescribed an unidentified “medication.” Following the vignette, participants rated twelve potential side effects of the “medication” on how much each would impact their lifestyle, on a scale of one to ten, if they were to occur were to occur monthly, weekly, or daily.
-
4.
Healthcare Utilization and Sexual Health: respondents were asked how often they visit the doctor for routine check-ups, if they were sexually active in the past year, and how many sexual partners they had in their lifetime.
-
5.
Most Important and Most Difficult-to-discuss Areas of Health: respondents were prompted to select the top three of seven options that they felt were (1) most important to them and (2) most difficult to talk about with a doctor. The areas of health that were assessed in this survey item were chosen based on the clinical experience of our multidisciplinary research team. These ranked topics were subsequently transformed to calculate a “reported score” for analysis (3 points = ranked 1st; 2 points = ranked 2nd; 1 point = ranked 3rd; 0 points = unranked).
-
6.
Past Psychiatric History: respondents responded to questions asking if they had ever been diagnosed by a healthcare provider with GAD, MDD, or Seasonal Affective Disorder (SAD).
-
7.
Comparison of Antidepressant Side Effect Profiles: Respondents read a vignette describing that they were recently diagnosed with a mental illness and that their doctor recommended they take an unidentified medication, providing only a list of its four most common side effects. Using evidence from the literature, in subsequent questions we displayed the top 4 most common side effects for escitalopram, an SSRI-class drug (Medicine “A”) [11, 17, 18], bupropion (Medicine “B”) [19,20,21], and mirtazapine (Medicine “C”) [22, 23]. Respondents were then asked how willing they would be to take each of these medicines, and which of these three medications they would prefer to take if given the choice.
-
8.
Beliefs about Antidepressant Medications and Communicating Sexual Problems: respondents completed a 5-point Likert scale matrix pertaining to their beliefs regarding various statements.
-
9.
Depression symptoms were evaluated with the well-established PHQ-8 [24].
-
10.
Anxiety symptoms were evaluated with the well-established GAD-7 [25].
-
11.
Past Antidepressant Use: Patients were asked about their history of past or present antidepressant use.
Distribution strategy
Digital flyers were delivered to our target demographic through email listservs and social media posts. Through this flyer, potential participants submitted a phone number and email address. Then, through Qualtrics XM automations, a study survey was automatically delivered to the associated email address of each respondent.
Data analysis and survey validation
Descriptive statistical analysis was performed using Stata 17 (College Station, TX, USA) and Microsoft Excel (Redmond, WA, USA) with biostatistical support from the Biostatistics, Epidemiology And Data Management (BEAD) Core at Johns Hopkins University. Hypothesis testing was conducted using two-sided parametric tests, such as Chi-Square tests and Student’s t test. One-way repeated measures analysis of variances (ANOVA) with post-hoc comparisons using the Tukey method were used to compare differences in the detrimental influence of side effects on respondents’ lifestyles.
We ensured the validation of each submitted questionnaire through two methods. First, the emails and phone numbers submitted to our team were verified to be unique and non-duplicated from other respondents. Second, we evaluated the amount of time (in s) each participant spent completing the survey. After collecting all responses, the study team determined that forms completed in under 180 s could not have reasonably been completed with accuracy. These forms were excluded from statistical analysis.
Results
Overview
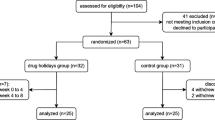
Six hundred and sixty-five respondents completed the sign-up survey, of which 505 fully participated in the study (response rate: 76%). We included 486 responses for analysis and excluded 19 responses completed in under 180 s. The median time to complete the valid study surveys was 11 min and 48 s (IQR: 6 min 40 s, 17 min 43 s).
As illustrated in Table 1, age was normally distributed with a mean of 27.4 (SD 3.6). Most of our sample identified as White (59.8%). Regarding sexual orientation, about 21% of our sample identified as LGBTQ+. Most respondents were employed (59.9%), and roughly one-third were undergraduate, graduate, or professional students. About half (53.5%) of participants reported current or previous antidepressant use. Mean PHQ-8 and GAD-7 severity scores for all respondents were 8.7 (SD 4.5) and 7.3 (SD 4), respectively. When taken together, 83.5% of respondents displayed at least mild symptoms of depression or anxiety by these validated instruments.
Preference for atypical antidepressants over selective serotonin reuptake inhibitors
We displayed the top 4 most common side effects for escitalopram, an SSRI-class drug, bupropion, and mirtazapine (Fig. 1). When the atypical antidepressants were compared to escitalopram, respondents were significantly more willing to take mirtazapine (p < 0.001) or bupropion (p < 0.001) after being prompted with their side effect profiles. Also, the willingness of respondents to take either mirtazapine or bupropion was not significantly different when compared to each other (p = 0.263). These data suggest the willingness of our sample to take the atypical antidepressants (bupropion and mirtazapine) was significantly higher than that of the SSRI when comparing the most common side effect profiles.
Histogram displaying the willingness of respondents to take deidentified antidepressants after being prompted with only their common side effect profiles. Hypothesis testing via student’s t test independently comparing SSRI-class drug to bupropion and mirtazapine. * p < 0.05; ** p < 0.01; *** p < 0.001; **** p < 0.0001
A proceeding survey item requested respondents to pick which previously described, deidentified drug they would prefer to take as a treatment. Accordingly, only 26.7% (129/483) of respondents chose escitalopram when also given the option of either atypical antidepressant (p < 0.001). Individually, bupropion (200/483, 41.4%) was preferred significantly more than escitalopram (p < 0.001) and mirtazapine (p = 0.002). However, mirtazapine (154/483, 31.9%) was not significantly preferred over escitalopram (p = 0.078). Taken together, roughly 3 of 4 respondents, after being prompted only with the relevant side effect profiles of these medications, stated a preference for the atypical antidepressants over SSRIs if given a choice.
Correlation between sexual dysfunction and medication adherence in young adult men
We next asked respondents to rank how detrimental potential medication side effects would be to their current lifestyles if they were to experience these effects every week (Fig. 2). One-way repeated measures ANOVA illustrated a significant difference in the perceived influence of the various side effects listed (p < 0.001). Post-hoc comparisons revealed that weekly occurrences of erectile dysfunction or orgasmic dysfunction were viewed as the most detrimental among the sexual complaints and received scores similar to weekly occurrences of seizures (p = 1.000 and p = 0.957, respectively) or heart problems (p = 0.982 and p = 0.261, respectively). Erectile dysfunction or orgasmic dysfunction were also viewed as significantly more detrimental than weight gain, nausea, diarrhea, or dry mouth (range: p < 0.001, p = 0.043) while low libido was only viewed as significantly more detrimental than dry mouth (p < 0.001).
Respondents were then asked to answer if experiencing any of these potential medication side effects every week would prompt them to stop taking the responsible drug. Erectile dysfunction (341/481, 70.9%), low libido (333/479, 69.5%), and orgasmic dysfunction (343/481, 71.3%) all received higher scores than insomnia (333/481, 69.2%), weight gain (290/477, 60.8%), headaches (290/479, 60.5%), nausea (284/480, 59.2%), diarrhea (280/478, 58.6%), dry mouth (271/478, 56.7%), or fatigue (258/480, 53.8%). Chi-Square tests illustrated that the three sexual side effects assessed would reportedly lead to nonadherence at a significantly higher frequency than the other common side effect assessed (range: p < 0.001, p = 0.005) except for insomnia.
Discussion
Key findings
To our knowledge, our survey is the first primary study to date investigating the perceptions and attitudes of young adult men toward antidepressant medications. Nearly three-quarters of respondents favored atypical, sexual-function-sparing antidepressants (bupropion and mirtazapine) to SSRIs when prompted with their deidentified side effect profiles. About 70% stated they would become nonadherent to medications they believed were responsible for weekly sexual side effects. Low libido, erectile dysfunction, and orgasmic dysfunction were viewed as disproportionally detrimental to the lifestyles of our respondents compared to most other common side effects of antidepressants, including weight gain, nausea, and dry mouth.
Shared decision-making and challenges to the SSRI paradigm
The endemic nonadherence to antidepressants, and the findings of our study, both challenge the current antidepressant dogma. Present treatment guidelines to address SSRI-associated sexual dysfunction contain prolonged titration periods, sometimes months in duration, to properly taper or augment regimens. Subsequently, the successful adjustment in dosing of SSRIs is often contingent on high engagement and motivation from patients [26]. Given our results, the impetus to engage patients in direct, open dialogues regarding the side effect profiles of SSRIs, bupropion, and mirtazapine must be encouraged. Our findings support informing the patient upfront of the alternative options for treatment that are known to better spare sexual function.
Limitations and future directions
Ultimately, our final sample size was determined my optimizing the cost–benefit of our study, balancing maximum participation with appropriate remuneration. We believe this conservative recruitment strategy enabled our response rate of 76%. Moreover, we recruited our subjects through convenience sampling and voluntary participation, which may have introduced a response bias. Additionally, we cannot individually follow up with respondents for future work as we deidentified our participants’ responses to assure confidentiality. While our study did not include women, this does not reflect a lack of clinical relevance of the sexual health concerns discussed for this population. Accordingly, we plan to conduct an analogous future study assessing our hypotheses among women [27]. Moreover, granulation of our findings amongst LGBTQ + individuals in our sample and determining the extent of the influence of education and age on the findings we’ve reported are critical.
Conclusions
Our findings support a correlation between reported nonadherence to antidepressants and their risk of sexual side effects in young adult men. Moreover, the strong preference displayed by our respondents for atypical antidepressants adds to evidence challenging the current treatment dogma of depression and anxiety. As mental health illness and suicide continue to rise at a high rate among young adult men, measures to augment the effectiveness of antidepressants through shared decision-making and improved adherence are critical.
Data availability
The data that support the findings of this study are available on request from the corresponding author.
References
Wiens K, Bhattarai A, Pedram P et al (2020) A growing need for youth mental health services in Canada: examining trends in youth mental health from 2011 to 2018. Epidemiol Psychiatr Sci 29:e115. https://doi.org/10.1017/S2045796020000281
Barker MM, Beresford B, Bland M, Fraser LK (2019) Prevalence and incidence of anxiety and depression among children, adolescents, and young adults with life-limiting conditions: a systematic review and meta-analysis. JAMA Pediatr 173(9):835–844. https://doi.org/10.1001/jamapediatrics.2019.1712
Pirraglia PA, Stafford RS, Singer DE (2003) Trends in prescribing of selective serotonin reuptake inhibitors and other newer antidepressant agents in adult primary care. Prim Care Companion J Clin Psychiatry 5(4):153–157. https://doi.org/10.4088/pcc.v05n0402
Demyttenaere K, Enzlin P, Dewé W et al (2001) Compliance with antidepressants in a primary care setting, 1: beyond lack of efficacy and adverse events. J Clin Psychiatry 62(Suppl 22):30–33
Cantrell CR, Eaddy MT, Shah MB, Regan TS, Sokol MC (2006) Methods for evaluating patient adherence to antidepressant therapy: a real-world comparison of adherence and economic outcomes. Med Care 44(4):300–303. https://doi.org/10.1097/01.mlr.0000204287.82701.9b
Olfson M, Marcus SC, Tedeschi M, Wan GJ (2006) Continuity of antidepressant treatment for adults with depression in the United States. Am J Psychiatry 163(1):101–108. https://doi.org/10.1176/appi.ajp.163.1.101
Sansone RA, Sansone LA (2012) Antidepressant adherence. Innov Clin Neurosci 9(5–6):41–46
Ramic E, Prasko S, Gavran L, Spahic E (2020) Assessment of the antidepressant side effects occurrence in patients treated in primary care. Mater Sociomed 32(2):131–134. https://doi.org/10.5455/msm.2020.32.131-134
Huang X, Lu Y, Luo S, Wang F, Xie Z, Wang X (2009) Efficacy and safety of selective serotonin re-uptake inhibitors in the treatment of premature ejaculation: a systematic evaluation. Zhonghua Nan Ke Xue 15(3):248–255
Serretti A, Chiesa A (2009) Treatment-emergent sexual dysfunction related to antidepressants: a meta-analysis. J Clin Psychopharmacol 29(3):259–266. https://doi.org/10.1097/JCP.0b013e3181a5233f
Jing E, Straw-Wilson K (2016) Sexual dysfunction in selective serotonin reuptake inhibitors (SSRIs) and potential solutions: a narrative literature review. Ment Health Clin 6(4):191–196. https://doi.org/10.9740/mhc.2016.07.191
Zimmerman M, Posternak MA, Attiullah N et al (2005) Why isn’t bupropion the most frequently prescribed antidepressant? J Clin Psychiatry 66(5):603–610. https://doi.org/10.4088/jcp.v66n0510
Stahl SM (2021) Stahl’s essential psychopharmacology: neuroscientific basis and practical applications, 5th edn. Cambridge University Press, Cambridge
Lynch L, Long M, Moorhead A (2018) Young men, help-seeking, and mental health services: exploring barriers and solutions. Am J Mens Health 12(1):138–149. https://doi.org/10.1177/1557988315619469
Suicide Data and Statistics | Suicide | CDC. Published 06 April 2023. https://www.cdc.gov/suicide/suicide-data-statistics.html. Accessed 01 May 2023
Seidler ZE, Dawes AJ, Rice SM, Oliffe JL, Dhillon HM (2016) The role of masculinity in men’s help-seeking for depression: a systematic review. Clin Psychol Rev 49:106–118. https://doi.org/10.1016/j.cpr.2016.09.002
Sussman N, Ginsberg DL, Bikoff J (2001) Effects of nefazodone on body weight: a pooled analysis of selective serotonin reuptake inhibitor- and imipramine-controlled trials. J Clin Psychiatry 62(4):256–260
Cascade E, Kalali AH, Kennedy SH (2009) Real-world data on SSRI antidepressant side effects. Psychiatry (Edgmont) 6(2):16–18
DailyMed—WELLBUTRIN XL- bupropion hydrochloride tablet, extended release. https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=a435da9d-f6e8-4ddc-897d-8cd2bf777b21. Accessed 15 September 2021
Fava M, Rush AJ, Thase ME et al (2005) 15 Years of clinical experience with bupropion HCl: from bupropion to bupropion SR to bupropion XL. Prim Care Companion J Clin Psychiatry 7(3):106–113
Settle EC, Stahl SM, Batey SR, Johnston JA, Ascher JA (1999) Safety profile of sustained-release bupropion in depression: results of three clinical trials. Clin Ther 21(3):454–463. https://doi.org/10.1016/s0149-2918(00)88301-0
Hartmann PM (1999) Mirtazapine: a newer antidepressant. AFP 59(1):159
Jilani TN, Gibbons JR, Faizy RM, Saadabadi A (2022) Mirtazapine. In: StatPearls. StatPearls Publishing, Treasure Island. http://www.ncbi.nlm.nih.gov/books/NBK519059/. Accessed 24 January 2022
Kroenke K, Strine TW, Spitzer RL, Williams JBW, Berry JT, Mokdad AH (2009) The PHQ-8 as a measure of current depression in the general population. J Affect Disord 114(1):163–173. https://doi.org/10.1016/j.jad.2008.06.026
Sapra A, Bhandari P, Sharma S, Chanpura T, Lopp L (2020) Using generalized anxiety disorder-2 (GAD-2) and GAD-7 in a primary care setting. Cureus 12(5):e8224. https://doi.org/10.7759/cureus.8224
Lam RW, Kennedy SH, Grigoriadis S et al (2009) Canadian network for mood and anxiety treatments (CANMAT) clinical guidelines for the management of major depressive disorder in adults: III. Pharmacotherapy. J Affect Disord 117:S26–S43. https://doi.org/10.1016/j.jad.2009.06.041
Lorenz T, Rullo J, Faubion S (2016) Antidepressant-induced female sexual dysfunction. Mayo Clin Proc 91(9):1280–1286. https://doi.org/10.1016/j.mayocp.2016.04.033
Acknowledgements
We thank the Sexual Medicine Society of North America for their generous “Scholars in Sexuality Research Grant” award enabling the creation of this work. We would also like to recognize Dr. James Aluri and Dr. Vinay Parekh for their contribution to the conceptualization of this project from the Department of Psychiatry. We also recognize the Johns Hopkins University Biostatistics, Epidemiology and Data Management (BEAD) Core for their assistance. Finally, we thank Dr. Dolores Lamb and Dr. Arthur Burnett for their invaluable mentorship and guidance throughout the duration of this project.
Funding
This project was funded by the 2022 SMSNA Scholars in Sexuality Research Grant Program (PI: Matthew Rabinowitz; Grant Title: Anti-Depressant Non-adherence and the Prioritization of Sexual Function in Young Men) and generous philanthropic donations from patients of Dr. Amin Herati.
Author information
Authors and Affiliations
Contributions
M. J. Rabinowitz: Protocol/project development; Data Analysis; Manuscript writing/editing. O. Li: Data collection or management; Manuscript writing/editing. E. Pi: Data collection or management; Manuscript writing/editing. C. K. Eaton: Protocol/project development; Data collection or management; Data Analysis; Manuscript writing/editing. T. P. Kohn: Protocol/project development; Manuscript writing/editing. N. M. Haney: Manuscript writing/editing. A. S. Herati: Protocol/project development; Manuscript writing/editing.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Rabinowitz, M.J., Li, O., Pil, E.H. et al. Antidepressant nonadherence and sexual dysfunction among young adult males: the cross-sectional YAMAN study. World J Urol 42, 295 (2024). https://doi.org/10.1007/s00345-024-05003-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00345-024-05003-3