Abstract
Purpose
Vesicovaginal fistulae (VVF) have a significant negative impact on quality of life, with failed surgical repair resulting in ongoing morbidity. Our aim was to characterize the rate of VVF repair and repair failures over time, and to identify predictors of repair failure.
Methods
We completed a population-based, retrospective cohort study of all women who underwent VVF repair in Ontario, Canada, aged 18 and older between 2005 and 2018. Risk factors for repair failure were identified using multivariable cox proportional hazard analysis; interrupted time series analysis was used to determine change in VVF repair rate over time.
Results
814 patients underwent VVF repair. Of these, 117 required a second repair (14%). Mean age at surgery was 52 years (SD 15). Most patients had undergone prior gynecological surgery (68%), and 76% were due to iatrogenic injury. Most repairs were performed by urologists (60%). Predictors of VVF re-repair included iatrogenic injury etiology (HR 2.1, 95% CI 1.3–3.45, p = 0.009), and endoscopic repair (HR 6.1, 95% CI 3.1–11.1, p < 0.05,); protective factors included combined intra-abdominal/trans-vaginal repair (HR 0.51, 95% CI 0.3–0.8, p = 0.009), and surgeon years in practice (21 + years—HR 0.5, 95% CI 0.3–0.9, p = 0.005). Age adjusted annual rate of VVF repair (ranging from 0.8 to 1.58 per 100,000 women) and re-repair did not change over time.
Conclusions
VVF repair and re-repair rates remained constant between 2005 and 2018. Iatrogenic injury and endoscopic repair predicted repair failure; combined intra-abdominal/trans-vaginal repair, and surgeon years in practice were protective. This suggests surgeon experience may protect against VVF repair failure.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Vesicovaginal fistula (VVF) is an abnormal tract connecting the bladder to the vagina and is the most commonly acquired urogenital fistula, often presenting as continuous incontinence of urine per vagina [1]. Iatrogenic injury from surgery is the most common cause for VVF in North America (> 75%), with hysterectomy as the most common surgical cause [2]. Other etiologies include obstructed labour, radiation, malignancy, trauma, and inflammatory conditions [3].
VVF, and urinary incontinence in general, is associated with a significant decrease in quality of life (QoL). It has been associated with discomfort, social isolation, changes in sexual activity, depression and anxiety [4]. Urinary incontinence has been reported as one of the most common causes of loss of health-related QoL at the population level, on par with depression and back problems [5].
Considerable research has been done on obstetrical VVF in the developing world, but there is a lack of high-level evidence on fistula repair in North America. A 2012 study by Brown et al. reviewed trends in lower reproductive tract fistula repair and estimated 33,221 patients required VVF surgical repair in the United States from 1979 to 2006 [6]. The success rate of VVF surgical repair is estimated at 77.5% [7]. Re-repair rates of genitourinary (GU) fistula are highly variable in the literature, ranging from 9 to 42% [8,9,10].
This study will identify rates of fistula repair and need for secondary repair in a large Canadian cohort, examine practice patterns of repair, and identify predictors for fistula repair failure.
Methods
Data sources and setting
REB approval was obtained prior to study initiation at our institution (REB# 290–2019). We conducted a retrospective, population-based cohort study of all patients ≥ 18 years of age in Ontario, Canada, using linked health administrative databases. All analyses were performed between 2019 and 2021.
In Ontario, all necessary healthcare services, physician services and prescription medication information are recorded and held at ICES (Institute for Clinical Evaluative Sciences) (http://www.ices.on.ca). ICES is an independent, non-profit research institute whose legal status under Ontario’s health information privacy law allows it to collect and analyze health care and demographic data, without individual patient consent, for health system evaluation and improvement. We linked the following validated data sets: the Canadian Institute for Health Information Discharge Abstract Database (CIHI-DAD), which contains records for all hospitalizations; the Canadian Institute for Health Information National Ambulatory Care Reporting System (NACRS), which contains records for emergency department visits; and the Ontario Health Insurance Plan database (OHIP), which tracks claims paid for physician billings, laboratories, and out-of-province providers (physicians, allied health, and hospitals). These datasets were linked using unique, encoded identifiers, and were analyzed at ICES. Individual informed consent was waived owing to the anonymous, aggregated nature of the data.
Study subjects
We identified all residents of Ontario, Canada, who were ≥ 18 years of age between January 1, 2005, and December 31, 2018 who had VVF repair, using OHIP surgical billing codes (index surgery date defined by operation code, available in Appendix 1). The last date of follow-up was March 31, 2019. A 3-year look-back window was completed to ensure index case surgery date and a life-long look-back window was used to identify patient baseline characteristics.
Baseline characteristics
We collected patient, physician, and health-care utilization variables, listed in Table 1. Surgeon covariates, listed in Table 2, were extracted from the ICES physician database (IPDB). The total sample (n = 814) was divided into those patients who had VVF repair success (n = 697) and those who underwent VVF re-repair (n = 117). Each group was summarized using descriptive statistics and expressed as mean and standard deviations (SD) and median and interquartile range (IQR) for continuous variables, and frequency and percentage for categorical variables. Baseline patient characteristics, physician factors, and health care utilization were compared. A two-sample Student’s t-test and Wilcoxon rank-sum test were used to compare the difference in means and medians, respectively. Chi-squared tests were used to compare categorical variables of non-paired data. When expected cell counts were < 5, the Fisher’s exact test was used.
Primary objective
To determine the rate of VVF surgical repair failure, defined as OHIP surgical billing code for VVF surgical re-repair. Multi-variable cox proportional hazard model was conducted to determine predictors for VVF re-repair. The model used a stepwise selection of variables based on the smallest AIC and best subsets, with age as a pre-selected variable.
Secondary objective
To characterize the change in rate of VVF repair and failures over time. Primary exposure was calendar year of VVF surgical repair. The dependent variable was number of cases of VVF repairs done annually, operationalized as raw number of cases per year, number of cases per 100,000 population, and number of cases per 100,000 population age adjusted. To assess the rate of VVF repair over time we used interrupted time series analysis. Model diagnostics to determine the best fit models are outlined in Appendix 2.
Adjusted annual rate of re-repair of VVF was determined using interrupted time series analysis, adjusting for surgeon type, repair type, fistula etiology, hospital location and prior pelvic radiation in logistic regression, where adjusted rate is estimated from logistic regression results for each year, outlined in Appendix 2.
All statistics were performed using SAS® software and statistical significance was defined as p < 0.05.
Results
Patient baseline characteristics
We identified 814 patients who had surgical repair of VVF between January 1, 2005, and December 31, 2018, in Ontario, Canada. Of these patients, 117 had a second VVF repair within the study period and were defined as VVF repair failures.
Baseline patient characteristics are presented in Table 1. Mean age at time of surgery was 52.5 years. 6.4% of patients had a BMI > 40. The patient cohort was spread evenly across income quartiles. 84% were from an urban setting. The majority had a Charlson Comorbidity Score of 0 (81%). 8% had been diagnosed with endometriosis, 7% diagnosed with cervical/vaginal malignancy, 7% diagnosed with bladder malignancy, and 4% had undergone pelvic radiation. The majority had pelvic surgery, with 68% having undergone a gynecological surgical procedure. 12% had pelvic mesh placement. 25% had had vaginal deliveries, and 15% had undergone caesarean sections. Iatrogenic injury was the primary cause of VVF (76%).
Of the 814 VVF repair patients, 117 were deemed repair failures, requiring a second surgical repair. Surgical failures were more likely to occur in women who were younger at time of initial repair (age 50 vs 53, p = 0.046), who were from rural settings (23% vs 15%, p = 0.026), who had been diagnosed with a cervical/vaginal malignancy (13% vs 6%, p = 0.01), and who had undergone a prior gynecological surgery (83% vs 66%, p < 0.001). Those undergoing a secondary repair were more likely to have had the cause of their VVF attributed to iatrogenic injury (88% vs 74%, p < 0.001) (Table 1).
Repair, surgeon, and cost characteristics
These characteristics are presented in Table 2. Most patients had a trans-vaginal repair (66%). The surgeon undertaking the VVF repair was more likely to be a urologist (59%), with a mean of 16 years in practice. 17% of VVF repairs were done by an OBGYN. Mean number of fistula repairs for the surgeon was 1.3 in the year prior to patient repair. Healthcare utilization 1 year following surgery included a mean of 6.8 visits to family physician, 3.2 visits to a urologist, 1.6 visits to an OBGYN, and 1.2 visits to an emergency room.
Patients who underwent re-repair were less likely to have had an intra-abdominal VVF repair (15% vs 32%, p < 0.001). 66% were done by urology, 20% by obstetrics and gynecology (OBGYN) and 15% by “other,” (p = 0.036). Those undergoing re-repair had a surgeon with a lower number of years in practice (mean 13 years, median 14 years, vs mean 16 years, median 17 years, p = 0.003, 0.004). They had more emergency department visits, visits to a family physician, a urologist, and to an OBGYN (Table 2).
Primary analysis
Multi-variable cox proportional hazards model was conducted to examine factors associated with VVF re-repair and represented in Table 3. Predictors of VVF re-repair were iatrogenic injury as etiology of VVF (HR 2.13, 95% confidence interval, CI 1.28–3.50, p = 0.0094) compared to those without iatrogenic injury, or endoscopic treatment as initial repair (HR 6.05, 95% CI 3.07–11.1), p < 0.05). Protective factors included having a combined intra-abdominal/trans-vaginal repair surgical approach to VVF repair (HR 0.51, 95% CI 0.30–0.83, p = 0.009). Total number of physician years in practice was protective, where having 21 + years’ experience predicted the patient was half as likely to require a re-repair (HR 0.53, 95% CI 0.31–0.89, p = 0.0162), compared to those with 1–5 years of experience.
Secondary analysis
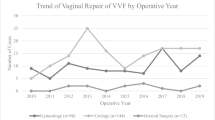
The rate of VVF repair over time was analyzed using interrupted time series analysis. No significant decrease in VVF repairs over time were found (Fig. 1a). When analysing annual VVF repair rate per 100,000 Ontario women, the annual number of VVF repair significantly decreased by 0.14 per 100,00 Ontario women each year from 2005 to 2009, and insignificantly decreased from 2009 to 2018 (Fig. 1b). However, when age-standardized to the 2001 Ontario female population, no significant decrease was found (Fig. 1c). In the adjusted annual rate of re-repair of VVF two regression lines are seen, though neither show significant decrease over time (Fig. 1d).
a Interrupted Time Series model: VVF annual repair rate over time. b VVF annual repair rate over time per 100,000 Ontario women. c VVF annual repair rate over time per 100,000 Ontario women age standardized to the 2001 Ontario female population. d Adjusted VVF annual re-repair rate over time among baseline VVF patients
Discussion
There is a lack of large-scale studies examining VVF re-operation rates and its success. Our large population-based study found a 14% re-repair rate over 13 years, with no significant decrease in re-repair rates over time. Flynn et al. reported that 42% of their patients failed first time fistula repair, and 12% failed two or more repairs. However, this study was undertaken at a tertiary referral centre, where a high rate of prior repair failure is expected [8]. A recent meta-analysis identified 23 VVF series that reported surgical outcomes, with an overall surgical failure rate of 13% (of 2–32%)—reviewing a total of 4737 patients [11]. This is in keeping with our findings.
Risk factors for VVF repair failure are not widely reported outside of the obstetrical trauma literature. Yang et al. reported a case–control study of 60 patients, with 15 unsuccessful VVF repairs. They found fistula size ≥ 1 cm and repeat trans-vaginal repair predicted VVF repair failure [12]. Zhou et al. reviewed 139 VVF repairs and found fistula number, size, and peri-fistula fibrosis predicted repair failure [13]. Zaghbib et al. reviewed 132 patients who underwent VVF repair, finding vaginal fibrosis, fistula at the trigone, > 1 cm diameter, and “complex and complicated fistula” predicted repair failure [14].
In this study, which did not examine specific surgical findings, risk factors for secondary repair were iatrogenic injury as VVF etiology, and endoscopic treatment (fulguration or fibrin glue) as repair. It is not surprising that endoscopic repair was a risk factor for repair failure. This approach is often used for elderly patients attempting to avoid invasive surgery, or as a first resort with the knowledge that definitive repair may become necessary. Review of the literature revealed minimal data on endoscopic treatment for VVF, with failure rates of 1/3, 1/5 and 1/1 [15,16,17]. Iatrogenic injury as VVF etiology was twice as likely to result in the need for VVF re-repair, as compared to malignancy, obstetrical trauma, endometriosis, radiation, and other. This finding is difficult to explain, as one might expect the aforementioned etiologies to result in poor tissue healing following initial repair. We theorize that this finding may be due to unknown factors not accounted for in this study – perhaps anatomic location or size of VVF, or time from diagnosis to repair.
Protective factors found included combined intra-abdominal/trans-vaginal repair and physician years in practice. Specifically, an intra-abdominal approach decreased the risk of a re-repair by 50%. We suspect this is due to the nature of the fistula itself, and less so the surgical approach. Location of fistula is often the determining factor in surgical approach, which we did not capture in our study.
Physician years in practice was associated with a decreased risk of VVF re-repair. Index repairs by surgeons with 21 + years of practice compared to those with 1–5 years of practice were half as likely to result in re-repair. In the literature, whether surgeon experience improves outcomes and complication rates has been mixed. Papageorge et al. completed a SEER meta-analysis on 2,450 patients undergoing pancreaticoduodenectomy and found that while hospital volume affected outcomes, surgeon volume did not [18]. A large patient database study of 27,714 patients undergoing laparoscopic Roux-en-Y gastric bypass found no association between surgeon experience and patient outcomes [19]. However, Patel et al. examined 642 patients undergoing cervical fusion, and found increased surgical volume was associated with decreased operative complications [20].
We examined changes to repair rates over time and found a significant decrease in repair rates of annual VVF repair per 100,000 women, by 0.14 each year from 2005 to 2009. From 2009 onward this rate continued to decrease, but not significantly. When age-standardized this decrease was not found. Over our 13-year study period the rate of VVF repairs and re-repairs did not change year to year. This may be due to lack of surgical advances in VVF repair, specifically robot-assisted repair, which have not become common place in Canada. Robotic-assisted VVF repair has become popular in other countries and may represent an opportunity for improved repair rates. A prospective study of 73 patients undergoing robot-assisted VVF repair were analyzed, with only two patients requiring re-repair (3%) [21]. Other recent studies have shown similar failure rates, with three studies reporting a 0% fistula recurrence, and one study report a 7% recurrence rate [22,23,24,25].
Limitations to our study include the use of re-repair as a surrogate for overall surgical failure. We did not capture the overall rate of fistula repair failure, only those patients who went on to have a second surgery. It is possible that some patients had recurrence but elected not to proceed with repeat repair or had repeat repair outside of Ontario or outside of the study period. Other limitations include the data we were able to collect; as this was a large population-based database study we did not collect data on individual surgical findings, such as size of fistula, number of fistulae, location of fistula, or operative complications, which are likely to play a role in the risk of fistula recurrence. For all studies using administrative databases, there is a risk of misclassification. In this study, the use of OHIP billing to identify fistula repair has not been validated, and billing practices are up to the discretion of the individual surgeon.
Conclusions
VVF repair and re-repair rates have remained constant between 2005 and 2018 in Ontario, Canada, with a 14% re-repair rate. Iatrogenic injury and VVF repair done endoscopically were predictors of repair failure, while protective factors included combined intra-abdominal/trans-vaginal repair surgical approach to VVF repair, and surgeon years in practice. This suggests surgeon experience may protect against the need for a second VVF surgery.
References
Gerber GS, Schoenberg HW (1993) Female urinary tract fistulas. J Urol 149(2):229–236
Armenakas NA, Pareek G, Fracchia JA (2004) Iatrogenic bladder perforations: longterm followup of 65 patients. J Am Coll Surg 198(1):78–82
Stamatakos M, Sargedi C, Stasinou T et al (2014) Vesicovaginal fistula: diagnosis and management. Indian J Surg 76(2):131–136
Melville JL, Fan MY, Rau H et al (2009) Major depression and urinary incontinence in women: temporal associations in an epidemiologic sample. Am J Obstet Gynecol 201(5):490.e1–7
Saarni SI, Harkanen T, Sintonen H et al (2006) The impact of 29 chronic conditions on health-related quality of life: a general population survey in Finland using 15D and EQ-5D. Qual Life Res 15(8):1403–1414
Brown HW, Wang L, Bunker CH et al (2012) Lower reproductive tract fistula repairs in inpatient US women, 1979–2006. Int Urogynecol J 23(4):403–410
Oakley SH, Brown HW, Greer JA et al (2014) Management of vesicovaginal fistulae: a multicenter analysis from the Fellows’ Pelvic research network. Female Pelvic Med Reconstr Surg 20(1):7–13
Flynn MK, Peterson AC, Amundsen CL et al (2004) Functional outcomes of primary and secondary repairs of vesicovaginal fistulae via vaginal cuff scar excision. Int Urogynecol J Pelvic Floor Dysfunct 15(6):394–398 (discussion 8)
Gupta NP, Mishra S, Mishra A et al (2012) Outcome of repeat supratrigonal obstetric vesicovaginal fistula repair after previous failed repair. Urol Int 88(3):259–262
Javed A, Abdullah A, Faruqui N et al (2015) Doctor! Will I be dry? Factors determining recurrence after vesicovaginal fistula repair. J Pak Med Assoc 65(9):954–959
Shrestha DB, Budhathoki P, Karki P et al (2022) Vesico-vaginal fistula in females in 2010–2020: a systemic review and meta-analysis. Reprod Sci. https://doi.org/10.1007/s43032-021-00832-8
Yang Y, Chen YK, Che XY et al (2021) Prognostic factors for failure of transvaginal repair of vesicovaginal fistula: a nested case-control study. Beijing Da Xue Xue Bao Yi Xue Ban 53(4):675–679
Zhou L, Yang TX, Luo DY et al (2017) Factors influencing repair outcomes of vesicovaginal fistula: a retrospective review of 139 procedures. Urol Int 99(1):22–28
Zaghbib S, Chakroun M, Saadi A et al (2021) Vesico-vaginal fistula in Tunisia: epidemiology and risk factors of treatment failure. Prog Urol 31(17):1175–1181
Zhang WY, Hu H, Zhang XP et al (2017) Comparison and discussion of different surgical methods used to treat vesicovaginal fistulas. Beijing Da Xue Xue Bao Yi Xue Ban 49(5):889–892
Shah SJ (2010) Role of day care vesicovaginal fistula fulguration in small vesicovaginal fistula. J Endourol 24(10):1659–1660
Mubeen RM, Naheed F, Anwar K (2007) Management of vesicovaginal fistulae in urological context. J Coll Physicians Surg Pak 17(1):28–31
Papageorge MV, de Geus SWL, Woods AP et al (2022) The effect of hospital versus surgeon volume on short-term patient outcomes after pancreaticoduodenectomy: a seer-medicare analysis. Ann Surg Oncol. https://doi.org/10.1016/j.hpb.2022.05.743
Chao GF, Yang J, Thumma J et al (2021) Volume-outcome relationships for Roux-en-Y gastric bypass patients in the sleeve gastrectomy era. Surg Endosc. https://doi.org/10.1007/s00464-021-08705-6
Patel MR, Jacob KC, Shah VP et al (2021) Impact of surgeon experience on outcomes of anterior cervical discectomy and fusion. J Am Acad Orthop Surg. https://doi.org/10.5435/JAAOS-D-21-01080
Chandna A, Mavuduru RS, Bora GS et al (2020) Robot-assisted repair of complex vesicovaginal fistulae: feasibility and outcomes. Urology 144:92–98
Kumar S, Modi P, Mishra A et al (2021) Robot-assisted laparoscopic repair of injuries to bladder and ureter following gynecological surgery and obstetric injury: a single-center experience. Urol Ann 13(4):405–411
Antonelli A, Veccia A, Morena T et al (2021) Robot-assisted vesico-vaginal fistula repair: technical nuances. Int Braz J Urol 47(2):684–685
Bora GS, Singh S, Mavuduru RS et al (2017) Robot-assisted vesicovaginal fistula repair: a safe and feasible technique. Int Urogynecol J 28(6):957–962
Jairath A, Sudharsan BS, Mishra S et al (2016) Robotic repair of vesicovaginal fistula - initial experience. Int Braz J Urol 42(1):168–169
Acknowledgements
This study was supported by ICES, which is funded by an annual grant from the Ontario Ministry of Health (MOH) and the Ministry of Long-Term Care (MLTC). This study also received funding from: The University of Toronto Functional Urology Research Program supported by an unrestricted grant from Astellas Pharma Canada. Parts of this material are based on data and information compiled and provided by: MOH and Canadian Institute for Health Information (CIHI). The analyses, conclusions, opinions and statements expressed herein are solely those of the authors and do not reflect those of the funding or data sources; no endorsement is intended or should be inferred. Parts of this material are based on data and information provided by Cancer Care Ontario (CCO). The opinions, results, view, and conclusions reported in this paper are those of the authors and do not necessarily reflect those of CCO. No endorsement by CCO is intended or should be inferred.
Author information
Authors and Affiliations
Contributions
SN: project development, data analysis, manuscript writing/editing. JAL: project development, manuscript writing/editing. BZ: data collection, data analysis, manuscript editing. RS: data collection, data analysis, manuscript editing. SH: project development, manuscript editing.
Corresponding author
Ethics declarations
Conflict of interest
S Neu, JA Locke, B Zhang, R Saskin and S Herschorn declares that they have no conflict of interest.
Research involving human subjects and informed consent
This study was completed using ICES data. In Ontario, all necessary healthcare services, physician services and prescription medication information are recorded and held at ICES (Institute for Clinical Evaluative Sciences) (http://www.ices.on.ca). ICES is an independent, non-profit research institute whose legal status under Ontario’s health information privacy law allows it to collect and analyze health care and demographic data, without individual patient consent, for health system evaluation and improvement. We linked the following validated data sets: the Canadian Institute for Health Information Discharge Abstract Database (CIHI-DAD), which contains records for all hospitalizations; the Canadian Institute for Health Information National Ambulatory Care Reporting System (NACRS), which contains records for emergency department visits; and the Ontario Health Insurance Plan database (OHIP), which tracks claims paid for physician billings, laboratories, and out-of-province providers (physicians, allied health, and hospitals). These datasets were linked using unique, encoded identifiers, and were analyzed at ICES. Individual informed consent was waived owing to the anonymous, aggregated nature of the data.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Neu, S., Locke, J.A., Zhang, B. et al. Prevalence and repair patterns of vesicovaginal fistula: a large retrospective population-based cohort analysis. World J Urol 42, 149 (2024). https://doi.org/10.1007/s00345-024-04812-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00345-024-04812-w