Abstract
Introduction
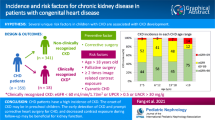
Crystalluria is a frequent finding in normal individuals and in patients suffering from urolithiasis. As nephrolithiasis has been associated with cardiovascular risk factors and most congenital heart disease (CHD) patients reach adulthood, the objective of this study is to determine the presence of crystalluria and if it influences their cardiovascular outcome.
Methods
Case–control and observational prospective study design of patients with CHD older than 14 years with a stable CHD verified with imaging tests and a control population.
Results
214 patients with CHD [median age 21 (17–35) years and 41 (19%) males] and 345 controls were studied and followed up. None of them had symptoms of renal calculi. Nine (4%) patients with CHD and 24 (7%) patients in the control group showed crystalluria (p = 0.180), all of them composed of calcium oxalate. No significant differences were seen in age, sex, body mass index, CHD complexity, cardiovascular risk factors, NYHA functional class, cyanosis, and medical treatment between CHD patients with and without crystalluria. In relation to survival, 18 patients with CHD had a major acute cardiovascular event (MACE) (3 strokes, 2 myocardial infarction, 9 cardiovascular death and 4 non cardiovascular mortality) during the follow up time [7.3 (4.4–8.5) years] without significant differences in the Kaplan–Meier analysis (p = 0.358) between patients with and without crystalluria.
Conclusion
No significant differences were found between CHD and control patients in relation to crystalluria and it had no impact on the occurrence of cardiovascular events in the medium term follow up of patients with CHD.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Crystalluria may be seen in clinically healthy people causing limited symptoms unless large enough stones develop. In most instances the precipitation of crystals of calcium oxalate, uric acid triple phosphate, calcium phosphate and amorphous phosphates or urates is caused by transient supersaturation of the urine, ingestion of foods, or by changes of urine temperature and/or pH. In a minority of cases, however, crystalluria is associated with pathological conditions [1]. Moreover, it has been suggested that nephrolithiasis is associated with subclinical atherosclerosis, arterial hypertension, diabetes mellitus, metabolic syndrome, and adverse cardiovascular events such as myocardial infraction or stroke when compared to the general population [2,3,4].
As the number of patients with congenital heart disease (CHD) reaching adulthood continues to increase, it is becoming clear that pathophysiological derangement occurs not only in the heart but in other organs [5, 6]. In fact, renal impairment has been recognized as a common complication in patients with CHD [7] particularly in those with cyanosis [8] as long-standing hypoxemia may affect the glomerular and tubular function leading to acidosis, crystallization, and stones formation [9]. However, there is a paucity of data on crystalluria among patients with CHD.
The purpose of this study is (a) to determine the presence of crystalluria among patients with CHD and a control group, (b) which variables are associated with crystalluria in patients with CHD and (c) the cardiovascular outcome of patients with CHD depending on whether crystalluria is present or not.
Methods
A case–control study was carried out in first place to compare patients with CHD (cases) and a control population (controls) and thereafter, a prospective observational study was undertaken only in patients with CHD to watch for outcomes. Patients were recruited consecutively from a single adolescent and adult CHD outpatient unit between January 2010 and December 2015. Inclusion criteria were being older than 14 years and having a stable CHD verified with imaging tests. Controls were drawn from patients older than 14 years attending the primary health care centers, of a same geographical area, between July 2017 and December 2018 due to preventive activities or minor illnesses such as anxiety, palpitations, or muscle aches. Controls were matched for age and sex to patients with CHD. Patients excluded from the study were those with hospital admission in the previous 6 months, pregnancy, clinical symptoms of urinary tract infection at the time of the urine collection, a life expectancy under 1 year or who did not give written informed consent to participate in the study. All patients or their parents/tutors gave the informed consent for participation in the study. Approval of the research protocol was carried out by our Institutional Reviewer Board.
Clinical data
Cardiovascular imaging, mainly through echocardiography, established the diagnosis of CHD and patients were classified into diagnostic groups: simple, moderate, or great complexity according to the anatomical defect [10]. The type of cardiac intervention (none, surgical or percutaneous) and the moment in which it was carried out (infancy or adulthood) were also determined. Cardiovascular risk factors were categorized as previously reported [11], the body mass index (BMI) was calculated with the formula BMI = kilograms/metres2 and the Modification of Diet in Renal Disease (MDRD) equation was used to estimate glomerular filtration rate [12]. Medication taken by patients with CHD was determined and patients were identified as cyanotic if their hemoglobin oxygen saturation, measured using a digital oximeter (Model 512 Handheld Pulse Oximeter; Novametrix Medical Systems Inc., Wallingford, CT, USA), was 90% or less. Information on personal history of renal colic and family history of kidney stone formation was also obtained.
Blood test, 24-h urinalysis and urine test strips
Following a 10-h overnight fast, baseline blood samples were collected. An Olympus AU 2700 (Olympus Diagnostic, Hamburg, Germany) and a Siemens Stratus CS Acute Care Diagnostic System (Siemens Healthcare Diagnostics, Inc, Newark, DE, USA) analyzers were used for blood test. Urine test strip analyzer (AtionMax AX-430, Menarini Diagnostics, Italy) was used to measure pH, urine specific gravity, glucose, leukocytes, blood, proteins, bilirubin, urobilinogen, and ketones. Meanwhile, the urine sediment analyzer (SediMax2, Menarini Diagnostic, Italy) identified the sample, shaked it, performed centrifugation and took the cuvette to the microscope to determine cells and crystals. An increased amount of glucose, leukocytes, blood, proteins, bilirubin, urobilinogen and ketones, above normal lab reference values, were considered as a “positive” chemical test result.
Follow up
Major adverse cardiac event (MACE) included cardiovascular and non-cardiovascular mortality and arterial thrombotic events (myocardial infarction or stroke) [13]. Follow-up was carried out in all patients with CHD until December 2022 and patients were censored when the first event of interest occurred. Data were obtained through the clinical terms coded with the International Classification of Diseases (ICD) system, the clinical history or telephone calls.
Statistical analysis
Continuous variables are presented as mean ± standard deviation or median (interquartile range), depending on the normality of distribution. Comparisons between two groups were performed using Student’s t test for continuous variables or the Mann–Whitney U test for continuous non-parametric variables. Categorical values were compared using the χ2 test. Survival analysis was estimated by the Kaplan–Meier method and the log rank test. A p value < 0.05 was considered statistically significant. Statistical Package for the Social Sciences (SPSS 24, Chicago, IL, USA) was used for data analysis.
Results
Study population
Two hundred fourteen out of 225 (95%) Caucasian CHD patients (cases) and 345 controls were included in the study. Eleven patients in the case group were excluded: 10 did not collect the urine sample despite having given informed consent and one due to previous hospitalization. No patient was excluded due to a reduced life expectancy or pregnancy. Table 1 shows the type of CHD and the classification according to the cardiac complexity [120 (56%) patients had simple defects, 65 (30%) patients showed moderate defects and 29 (14%) patients presented great defects], the type of intervention and the life stage in which it was done.
Demographic and blood and urine test data in patients with CHD and the control population
Table 2 shows a comparison between patients with CHD and a control population. As can be seen from the table, patients with CHD had statistically significant higher levels of serum urea, creatinine, calcium, uric acid and haemoglobin than patients in the control group. Similarly, the urine pH was significantly higher in CHD patients than controls. Meanwhile, no significant differences were seen related to the squamous cells and the presence of crystalluria in the urinary sediment of both groups.
Clinical, blood and urine test data in patients with CHD
Nine (4%) patients showed crystalluria, all of them composed of calcium oxalate, and 20 (9%) patients presented squamous cells in the urine samples. From a clinical point of view, no significant differences were seen in age, sex, BMI, CHD complexity, NYHA functional class, cardiovascular risk factors, medical treatment, and cyanosis between CHD patients with and without crystalluria (Table 3). On the contrary, patients with crystalluria showed lower NT-pro-BNP levels and higher 24-h calciuria than those without it. Similarly, patients with crystalluria showed in the urine test strips higher nitrites, bilirubin, and urobilinogen concentrations than patients without crystalluria.
In relation to urine pH, patients with urinary acidosis (pH ≤ 5) did not show statistically more crystalluria than patients with pH levels above 5 [2 out of 67 patients with acidosis vs. 7 out of 147 patients with a pH > 5, p = 0.548]. Similarly, no significant differences were found between CHD patients who had renal failure (GFR ≤ 60 ml/min/1.73 m2) and those patients who did not (GFR > 60 ml/min/1.73 m2) according to crystalluria [0 out of 13 patients with renal failure vs. 9 out of 201 patients with a GFR > 60 ml/min/1.73 m2, p = 0.436]. No patient with CHD had a history of renal colic before or during the study enrolment and similarly no patient reported having a family history of kidney stone formation.
Cardiovascular outcome
In relation to the cardiovascular outcome, 18 patients had a MACE (3 patients had a stroke, 2 suffered a myocardial infarction, 9 presented cardiovascular death and 4 non cardiovascular mortality) during a follow up time of 7.3 (4.4–8.5) years. As can be seen from Table 3, all the events occurred in patients without crystalluria although without reaching statistical significance (p = 0.368). Similarly, the Kaplan–Meier analysis showed no significant differences, in relation to the occurrence of cardiovascular events during the follow-up time, in patients with CHD regardless of the existence or not of crystalluria (p = 0.358).
Discussion
The pathophysiology in CHD can lead to changes in the structure and function of the kidney. Although there are a significant number of gaps in the knowledge related to renal function in patients with CHD current evidence demonstrates that chronic renal disease is increasing far beyond that of the general population [14], predicting a worse prognosis. However, little is known about crystalluria among patients with CHD despite it may induce kidney problems such as nephrolithiasis, nephrocalcinosis, kidney stone formation and renal failure [15].
Crystalluria is not rare in the general population where some series [16] have reported prevalence rates of 8% which is similar to ours. Likewise, the calcium oxalate, the only urine crystal seen in our patients, is the most common type of kidney stone to be generated by endogenous overproduction, increased ingestion, or excessive intestinal absorption. In fact, when a supersaturation status is reached, oxalates combine with calcium crystallize to form 80% of the urinary stones.
As a history of kidney stones has been associated with an increased risk of coronary heart disease [17], systemic arterial hypertension [18] and diabetes mellitus [19] it may be hypothesized that crystalluria, which is an inexpensive and valuable tool for the detection and the monitoring of inherited and acquired diseases associated with urinary stone formation [20], may also be. However, we found no association between crystalluria and classic cardiovascular risk factors or MACE presumably due to the young age of our patients. Moreover, we found no significant differences in creatinine levels between CHD patients with and without crystalluria despite literature has linked crystalline microparticles with mechanical obstructions, local intrarenal inflammation, and tissue renal injury [21]. Moreover, no significant differences were seen in crystalluria levels between patients with CHD and the control population what implies that crystalluria must not be a key factor in the impairment of the renal function among young adult patients with CHD. Similarly, the CHD complexity, the NYHA functional class and being under loop diuretic treatment did not reach statistical significance among CHD patients with and without crystalluria probably because in both groups the NT-pro BNP level, a cardiac natriuretic peptide that is raised in heart failure, was within normal limits [22].
On the other hand, urine specific gravity, which correlates well with urine osmolality and gives important insight into hydration status and the concentrating ability of the kidneys obtained statistically significant differences between CHD patients with and without crystalluria showing patients with crystalluria higher levels. Similarly, we found a positive urine reagent strip reaction between nitrite, bilirubin, and urobilinogen and crystalluria. However, small quantities of urobilinogen may also be found in normal urine, where it contributes to the typical yellow colour of the specimen.
In relation to hypoxemia, patients with cyanotic CHD have physiological compensatory erythrocytosis associated with a shortened red blood cell lifespan. Increased red cell turnover may lead to high purine metabolism, uric acid production and stone formation as seen in the tumor lysis syndrome or in patients with leukemia. Despite of this, none of our cyanotic patients with CHD showed crystalluria and no uric acid crystals were observed in any patient of the population under study. However, relatively few patients with severe cyanotic CHD and polycythemia extreme enough to lead to hyperuricemia survive into adult life when the manifestations are most common [23].
Regarding survival, kidney stones have been associated with more morbidity than expected [3]. However, in our series no significant differences were seen in cardiovascular outcome irrespective of having or not crystalluria in the medium term follow up even though nephrolithiasis seems to be the result of crystal formation, aggregation, and retention in the kidney during crystalluria. An explanation to this lack of significance could be the young age of our patients with CHD and that crystalluria may just be an incipient state of stone formation.
There are, however, limitations in our study that may impact our findings. Firstly, the low number of patients with crystalluria and the low number of MACE observed during the follow up time, typical of young populations. Secondly, we did not determine vitamin D supplementation or medications such as amoxicillin or sulphonamides, both related to crystalluria, and used to treat some infections. Thirdly, including only Caucasian patients may have led to an under-representation of other minority ethnic groups. Also, we did not carry out a routine and periodic analysis of the urine to determine crystalluria as any of our patients with CHD showed findings compatible with urinary stone diseases. In such cases, the 24-h urine seems to be the most useful type of urine collection [24]. Finally, we did not use renal ultrasound, as an accurate imaging technique to diagnose stones, as none of our patients had acute renal colic or suspicion of urinary stone disease.
In conclusion, there was not significant difference in the presence of crystalluria between patients with CHD and the control group. Similarly, no significant differences were seen in cardiovascular risk factors, cyanosis, sex, age, NYHA functional class, CHD complexity or creatinine levels between patients with CHD with or without crystalluria. Likewise, crystalluria had no impact on the occurrence of cardiovascular events in the medium term follow up of patients with CHD.
Referencess
Fogazzi GB (1996) Crystalluria: a neglected aspect of urinary sediment analysis. Nephrol Dial Transplant 11(2):379–387
Ferraro PM, Taylor EN, Eisner BH, Gambaro G, Rimm EB, Mukamal KJ, Curhan GC (2013) History of kidney stones and the risk of coronary heart disease. JAMA 310(4):408–415
Domingos F, Serra A (2011) Nephrolithiasis is associated with an increased prevalence of cardiovascular disease. Nephrol Dial Transplant 26(3):864–868
Arafa A, Eshak ES, Iso H (2020) Oxalates, urinary stones and risk of cardiovascular diseases. Med Hypotheses 137:109570
Billett J, Cowie MR, Gatzoulis MA, Vonder Muhll IF, Majeed A (2008) Comorbidity, healthcare utilisation and process of care measures in patients with congenital heart disease in the UK: cross-sectional, population-based study with case-control analysis. Heart 94:1194–1199
Dimopoulos K, Diller GP, Koltsida E, Pijuan-Domenech A, Papadopoulou SA, Babu-Narayan SV, Salukhe TV, Piepoli MF, Poole-Wilson PA, Best N, Francis DP, Gatzoulis MA (2008) Prevalence, predictors, and prognostic value of renal dysfunction in adults with congenital heart disease. Circulation 117(18):2320–2328
Martínez-Quintana E, Barreto-Martín A, Estupiñán-León H, Rojas-Brito AB, Déniz-Déniz L, Rodríguez-González F (2021) Proteinuria versus albuminuria in 24-hour urine collection: prevalence and clinical outcome in non-hypoxemic adult patients with congenital heart disease. Am J Cardiovasc Dis 11(1):46–52
Martínez-Quintana E, Rodríguez-González F (2016) Medium-term follow-up of renal function in hypoxaemic congenital heart disease patients. Cardiol Young 26(6):1137–1143
Tasic V, Lozanovski VJ, Gucev Z, Blau N, Cheong HI, Sayer JA (2011) Failure to thrive and nephrolithiasis in a boy with congenital cyanotic heart anomaly. Pediatr Nephrol 26(12):2153–2157
Care of the Adult with Congenital Heart Disease (2001) Presented at the 32nd Bethesda conference, Bethesda, Maryland, October 2–3, 2000. J Am Coll Cardiol 37:1161–1198
Martínez-Quintana E, Rodríguez-Hernández JL, Rodríguez-González F, Riaño-Ruiz M, Fraguela-Medina C, Girolimetti A, Jiménez-Rodríguez S (2019) Cardiovascular risk factors and arterial thrombotic events in congenital heart disease patients. Int J Clin Pract 73(9):1–8
Stevens LA, Coresh J, Greene T, Levey AS (2006) Assessing kidney function—measured and estimated glomerular filtration rate. N Engl J Med 354:2473–2483
Hicks KA, Mahaffey KW, Mehran R et al (2018) Standardized Data Collection for Cardiovascular Trials Initiative (SCTI) Author information 2017. Cardiovascular and stroke endpoint definitions for clinical trials. Circulation 137(9):961–972
Opotowsky AR, Carazo M, Singh MN et al (2019) Creatinine versus cystatin C to estimate glomerular filtration rate in adults with congenital heart disease: Results of the Boston Adult Congenital Heart Disease Biobank. Am Heart J 214:142–155
Daudon M (2015) Cristallurie [Crystalluria]. Nephrol Ther 11(3):174–190
Verdesca S, Fogazzi G, Garigali G, Messa P, Daudon M (2011) Crystalluria: prevalence, different types of crystals and the role of infrared spectroscopy. Clin Chem Lab Med 49(3):515–520
Peng JP, Zheng H (2017) Kidney stones may increase the risk of coronary heart disease and stroke: a PRISMA-compliant meta-analysis. Medicine (Baltimore) 96(34):e7898
Borghi L, Meschi T, Guerra A, Briganti A, Schianchi T, Allegri F, Novarini A (1999) Essential arterial hypertension and stone disease. Kidney Int 55(6):2397–2406
Assimos DG (2006) Diabetes mellitus and kidney stone formation. Rev Urol 8(1):44
Frochot V, Daudon M (2016) Clinical value of crystalluria and quantitative morphoconstitutional analysis of urinary calculi. Int J Surg 36(Pt D):624–632
Mulay SR, Shi C, Ma X, Anders HJ (2018) Novel insights into crystal-induced kidney injury. Kidney Dis (Basel) 4(2):49–57
Baggen VJM, Baart SJ, van den Bosch AE, Eindhoven JA, Witsenburg M, Cuypers JAAE, Roos-Hesselink JW, Boersma E (2018) Prognostic value of serial N-terminal pro-B-type natriuretic peptide measurements in adults with congenital heart disease. J Am Heart Assoc 7(7):e008349
Somerville J (1961) Gout in cyanotic congenital heart disease. Br Heart J 23(1):31–34
Williams JC Jr, Gambaro G, Rodgers A et al (2021) Urine and stone analysis for the investigation of the renal stone former: a consensus conference. Urolithiasis 49(1):1–16
Author information
Authors and Affiliations
Contributions
EM-Q contributed to project development, data collection, data analysis, manuscript writing; FR-G contributed to data analysis, manuscript writing.
Corresponding author
Ethics declarations
Conflict of interest
No author has conflict of interest.
Research involving human participants and/or animals
None.
Informed consent
Informed consent was obtained from all participants.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Martínez-Quintana, E., Rodríguez-González, F. Crystalluria in adolescent and adult patients with congenital heart disease. World J Urol 41, 2839–2845 (2023). https://doi.org/10.1007/s00345-023-04557-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00345-023-04557-y