Abstract
Objective
To describe our institution’s initial experience with robot-assisted radical prostatectomy (RARP) using the Senhance® robotic system.
Patients and methods
A prospective analysis of 127 robot-assisted radical prostatectomies was performed. Patient demographics, preoperative and intraoperative parameters, histopathological examination results, intraoperative and early postoperative complications were obtained and analyzed.
Results
The median patient age was 61.0 ± 6.36 (from 37 to 73) years, with a mean body mass index of 26.2 ± 3.79 kg/m2. Of 127 patients, 16.5% (n = 21) underwent a pelvic lymph node dissection, 29.1% (n = 37) underwent one sided or bilateral nerve sparing. Post-operative extracapsular invasion (≥ pT3) was found in 15% (n = 19) of the cases and a Gleason score ≥ 7 in 74.8% of all patients. Our median operative time was 180 ± 41.98 min [interquartile range (IQR) 150–215], and median blood loss was 250 ± 236 (IQR 175–430) ml. Of 127 patients, 33.9% (n = 43) had positive margins, of them 28.7% in pT2 and 57.9% in pT3. Fifteen patients (11.8%) experienced complications, of them only three had Clavien–Dindo ≥ 3. Operation time decreased by about 60 min and estimated blood loss decreased by about 200 ml from the initial experience of each surgeon.
Conclusions
Robotic prostatectomy using a Senhance® robotic system is feasible, and warrants further study to determine whether it can improve patient outcomes.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Prostate cancer (PCa) is the second most common type of cancer in men worldwide and the incidence rate of this cancer is currently increasing [1]. PCa is the sixth main cause of cancer related mortality in males [1, 2]. Radical prostatectomy is a treatment of choice for localized disease [3].
Robot-assisted radical prostatectomy (RARP) has been rapidly diffused for patients undergoing radical prostatectomy and increasing from less than 10% to over 80% of all radical prostatectomies in the USA during last 10 years [4]. RARP offers several advantages compared to standard laparoscopy, including the use of a high-resolution (HD) camera with three-dimensional (3D) visualization, working with robotic arms results in more accurate dissection leading to better preservation of functional structures, reduced positive surgical margins (PSM) rates and better perioperative results [5]. Da Vinci robotic system (Intuitive Surgical, Inc., Sunnyvale, CA, USA) was the only available robotic surgical system for RARP for a long time, but now several companies already have such a system or are in the process of establishing it [6, 7].
The Senhance® Surgical System (TransEnterix, Morrisville, NC, USA) is a novel robotic system used in several centers across the world [8]. The Senhance® system reveals some important benefits over laparoscopy and other robotic platforms. It has advanced technology such as 3D vision with eye tracking for better visualization, very important tactile sensitivity feedback, and a comfortable, special ergonomic chair, that decreases fatigue characteristic and precision [6].
Experience in performing different abdominal procedures with Transenterix has recently been published [7, 8].
The aim of this article was to describe our institution's initial experience with RARP using the Senhance® robotic system.
Materials and methods
The study was approved by Klaipeda University Hospital review board.
Senhance® robotic system was started in November 2018. Since November 2018 until October 2020 127 robot-assisted radical prostatectomies were performed in Klaipeda University Hospital, Lithuania. All operations were performed by two surgeons who are expert urologists, with at least 100 laparoscopic prostatectomies with lymphonodectomies performed each, without any expertise in Da Vinci robotic system. The data was collected during the learning curve for both surgeons.
Patient inclusion criteria were:
-
American Association of Anesthesiologists (ASA) score < 3.
-
Mean body mass index < 40 kg/m2.
-
Patient’s age < 75 years.
-
Patient’s written consent.
Surgical technique was selected by assessing the initial prostate-specific antigen (PSA) level, the magnetic resonance imaging (MRI) data, histopathological examination following systemic or adaptive biopsy. Standard pelvic lymph node dissection (obturator, internal and external iliac) was performed in all patients with a lymph node metastasis risk of 5% or higher according Memorial Sloan Kettering Cancer Center (MSKCC) nomograms [9].
All patients had pathologically confirmed prostate cancer in both localized and locally advanced stages. With prior written informed consent, clinical data were recorded during the hospital stay, including perioperative parameters, as well as histopathological results. All patient’s data were collected prospectively. Preoperative parameters included prostate-specific antigen (PSA), Gleason score after biopsy, positive biopsy rate and disease location, clinical disease stage as assessed by digital rectal examination, endorectal ultrasound, and pelvic computed tomography (CT) scan or prostate multiparametric magnetic resonance imaging (MRI). Intraoperative parameters including console time (min) (duration spent by the surgeon using the joystick), type of nerve sparing (bilateral/one sided/none), lymphadenectomy status as well as blood loss (we used only visual estimation of blood loss. This includes the estimation of blood volumes in sponges, suction containers and the recording of external blood losses; the amount of water used with water irrigation is deducted from the suction containers) were assessed. Histopathological examination results were recorded in the database with emphasis on the pTNM staging, histological type, Gleason score, positive surgical margin, tumor tissue volume and prostate volume, lymphovascular invasion, perineural invasion, number of excised lymph nodes and metastatic lymph nodes. Intraoperative and early postoperative complications were defined according to Clavien–Dindo classification of surgical complications [10].
The clinical database was created using Microsoft Excel and the statistical analysis was performed using SPSS Statistics for Windows, Version 26.0 (Armonk, NY: IBM Corp.). Descriptive statistics were used for the overall results (mean and standard deviation or median with 95% confidence intervals, depending on data distribution) and chi-squared test or paired-samples t test. p value < 0.05 was considered statistically significant.
Surgical technique
Patients were hospitalized one day prior the surgery. Operative time was calculated from the beginning of the incision to the wound closure (skin to skin). For radical prostatectomy we used three robotic arms: two on the left side of the patient and one on the right. Four robotic arms were abandoned due to lack of space in the operating room and using only four trocars for surgery. The position of trocars depends on operation: in radical prostatectomy without lymphonodectomy, we used 10 mm trocar infraumbilical for camera. In right and left lower quadrants—two 5 mm trocars (for bipolar, scissors, forceps, ultrasonic knife). The position of the lateral trocars can be changed depending on the circumference of the abdomen and the position of the pelvis. On the left between the 5 mm trocar and 10 mm infraumbilical trocar, inserting 10 mm trocar for the assistant (Fig. 1).
During lymphonodectomy, a 10 mm trocar is inserted over 10 mm infraumbilical trocar into abdominal cavity, sometimes an additional 5 mm trocar is inserted to the right of the assistant. Lymphonodectomy was performed laparoscopically, not robotically, for convenience and speed, but sometimes robotically.
The operation is started with infraumbilical incision, blunt dissection with the finger to access the retropubic space, insertion of the 10 mm camera trocar retroperitoneally. 10 mm and 5 mm trocars are placed on each side under visual control. The assistant surgeon for inserting a laparoscopic suction device, grasper, clip applicator, and/or advanced bipolar instrument uses 10 mm trocar. The operation protocol is the same as for laparoscopic prostatectomy. For prostate lifting, we use an instrument inserted directly through the skin—a small scar remains and there is no need to use a fourth robot arm. For nerve sparing, vessels are ligated using XL Click ligating clips or small titan clips (Grena Ltd Think Medical, Brentford-London, UK). Venous vascular fibers were not sutured—bipolar coagulation or ultrasonic knif is enoughe. The continuous connection of the bladder and urethra is made with Stratafix (Ethicon Inc., New Jersey, USA) 16 cm thread and two 17 mm needles and 5 mm not wristed (most often) or 10 mm wristed instruments. Drain is left for 2–3 days after the surgery. The Foley catheter is removed after 10 days.
Results
The median patient age at the time of RARP was 61.0 ± 6.36 (from 37 to 73) years, with a mean body mass index (BMI) of 26.2 ± 3.79 kg/m2. Median PSA value at the time of diagnosis was 5.5 ± 3.86 ng/ml (from 2 to 26.8) [95% confidence interval (CI) 6.09–7.48]. Of the 127 patients, 21 (16.5%) underwent a pelvic lymph node dissection, 37 (29.1%) underwent one sided or bilateral nerve sparing. Post-operative extracapsular invasion (≥ pT3) was found 15% of the cases and a Gleason score ≥ 7 in 74.8% of all patients. Clinical data, histopathological results are presented in Table 1.
Our median operative time was 180 [interquartile range (IQR) 150–215] min, and median blood loss was 250 (IQR 175–430) ml. Of the 127 patients with pathologic information available, 43 (33.9%) had positive margins, out of which 28.7% in pT2 and 57.9% in pT3. Fifteen patients experienced a complication (11.8%). Complete operative characteristics can be seen in Table 2.
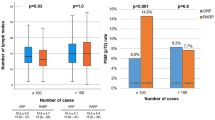
The skin to skin operation time decreased by about 60 min from the initial experience of each surgeon. Estimated blood loss (EBL) decreased by about 200 ml from the initial experience of each surgeon (see in Figs. 2 and 3).
Discussion
In our study, we found the median operative time of 180 ± 41.98 min with median blood loss 250 ± 236 ml. Of 127 patients, 33.9% (n = 43) had positive margins, of them 28.7% in pT2 and 57.9% in pT3. Fifteen patients (11.8%) experienced complications, of them only three had Clavien–Dindo ≥ 3.
The Senhance® operating system can be used for abdominal, gynecological and urological surgical procedures [6, 11,12,13]. The majority of the literature available about Senhance® focuses on gynecologic surgery and abdominal surgery confirming the feasibility of these procedures using this robotic platform [11,12,13]. Unfortunately, there is a great lack of literature on the use of Senhance® in urology.
Stephan et al. reported the results of The TransEnterix European Patient Registry for Robotic-Assisted Laparoscopic Procedures in Urology, Abdominal, Thoracic, and Gynecologic Surgery (“TRUST”), where procedures were performed in five European centers between February 2017 and July 2020 by experienced laparoscopic surgeons. They included 168 robotic assisted prostatectomies [14].
Our mean operation time was similar compared to RARP Da Vinci (179 min) but shorter than LRP (236 min) as shown by De Carlo et al. [15]. Our data showed that increasing number of operations resulted in decrease of operative time by about 60 min from the initial experience of each surgeon. Our estimated blood loss was less compared to LRP (442 ml) and similar to RARP Da Vinci -191 ml [15]. Increasing number of surgeries leads to declining tendency in blood loss. Eight patients (6.3%) required postoperative blood transfusions, which is comparable to LRP (6.3%) and RARP Da Vinci (4.66%) [15].
The overall incidence of complications was 11.8%, which is lower than for LRP (13.4%) and RARP Da Vinci (18.5%) reported by De Carlo et al. [15]. Three patients (2.4%) had Clavien III severity complications: one had urethral stricture and required dilation without general anesthesia, one had lymphocele requiring aspiration under local anesthesia and one had vesico-abdominal fistula requiring reoperation under general anesthesia.
Of 127 patients, 43 (33.9%) had positive margins, of them 28.7% in pT2 and 57.9% in pT3. In the literature, positive surgical margin (PSM) rates following the RARP range from 6.5‒32% [16, 17]. De Carlo et al. reported PSM in LRP group of 22%, and 21.1% in the RARP Da Vinci [15]. PSM in our pT2 group was 28.7%, compared LRP—17.4% and 10.5% in RARP Da Vinci reported by same authors. PSM in pT3 group was 57.9%, compared LRP—49.6%, and RARP Da Vinci—53.3% [15].We hope that with increasing experience we will reduce our positive margin rates. It is difficult to explain the higher frequency of PSM, and biochemical relapse and overall survival data should be analyzed. We encountered changes between the first cases and the latest cases. We see a second peak of PSM when nerve-sparing surgery techniques were initiated and we think that both aspects are related with the learning curve. Moreover, we think that some might be because of false positive report reporting all the iatrogenic damages during manipulation of the prostate.
Disadvantages of this platform are space in operation room, time to docking arms and limited instruments movement. The pros of the Da Vinci system are the construction, where all manipulative hands are in one stand and less space is needed in the operating room. One of the Senhance® cons are additional time required for docking the robot arms. Moreover, the Senhance® system has a relatively large arms, which reduces the working space for the assistant surgeon. In addition, Da Vinci manipulators have a greater freedom of movement, and the Senhance® system has limited movements and is essentially the same as laparoscopic surgery.
Our study obviously has some limitations. First, this is a single center single arm analysis of the data. However, so far this is a larger study with learning curve assessment. Moreover, we assessed only in-hospital rate of complications. No analysis of long-term results and functional outcome was performed. Obviously, future multicenter randomized studies comparing with laparoscopic procedures and assessing the long-term oncologic and functional results are needed.
Conclusions
According to our data, it is possible to perform robot-assisted radical prostatectomy using the Senhance® operating system. It is user-friendly operating system. However, further studies comparing whit laparoscopy and other robotic systems are needed.
References
Ferlay J, Soerjomataram I, Dikshit R et al (2015) Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 136(5):E359-386
Siegel RL, Miller KD, Jemal A (2017) Cancer statistics, 2017. CA Cancer J Clin 67(1):7–30
Ilic D, Evans SM, Allan CA et al (2017) Laparoscopic and robotic-assisted versus open radical prostatectomy for the treatment of localised prostate cancer. Cochrane Database Syst Rev 9(9):CD009625
Oberlin DT, Flum AS, Lai JD et al (2016) The effect of minimally invasive prostatectomy on practice patterns of American urologists. Urol Oncol 34(6):255.e1–5
Gandaglia G, Montorsi F, Karakiewicz PI et al (2015) Robot-assisted radical prostatectomy in prostate cancer. Future Oncol 11(20):2767–2773
Kastelan Z, Hudolin T, Kulis T, Knezevic N, Penezic L, Maric M, Zekulic T (2021) Upper urinary tract surgery and radical prostatectomy with Senhance® robotic system: single center experience—first 100 cases. Int J Med Robot. https://doi.org/10.1002/rcs.2269
Rao PP (2018) Robotic surgery: new robots and finally some real competition! World J Urol 36(4):537–541
Stephan D, Salzer H, Willeke F (2018) First experiences with the new Senhance1 telerobotic system in visceral surgery. Visc Med 34:31–36
Godoy G, Chong KT, Cronin A et al (2011) Extent of pelvic lymph node dissection and the impact of standard template dissection on nomogram prediction of lymph node involvement. Eur Urol 60(2):195–201
Dindo DA, Demartines N, Clavien PA (2004) Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 240(2):205–213
Alletti SG, Rossitto C, Cianci S et al (2018) The Senhance™ surgical robotic system (“Senhance”) for total hysterectomy in obese patients: a pilot study. J Robot Surg 12(2):229–234
Samalavicius NE, Janusonis V, Siaulys R et al (2020) Robotic surgery using Senhance® robotic platform: single center experience with first 100 cases. J Robot Surg 14:371–376
Rumolo V, Rosati A, Tropea A et al (2019) Senhance robotic platform for gynecologic surgery: a review of literature. Updates Surg 71(3):419–427
Stephan D, Darwich I, Willeke F (2021) The TransEnterix European patient registry for robotic-assisted laparoscopic procedures in urology, abdominal, thoracic, and gynecologic surgery (“TRUST”). Surg Technol Int. 38:sti38/1394
De Carlo F, Celestino F, Verri C et al (2014) Retropubic, laparoscopic, and robot-assisted radical prostatectomy: surgical, oncological, and functional outcomes: a systematic review. Urol Int 93(4):373–383
Yossepowitch O, Briganti A, Eastham JA et al (2014) Positive surgical margins after radical prostatectomy: a systematic review and contemporary update. Eur Urol 65(2):303–313
Adili AF, Di Giovanni J, Kolesar E et al (2017) Positive surgical margin rates during the robot-assisted laparoscopic radical prostatectomy learning curve of an experienced laparoscopic surgeon. Can Urol Assoc J 11(11):E409–E413
Acknowledgements
RV, TT, MV, VJ and AD performed the research; AD, NES designed the research study; RV, MJ and TT performed the surgeries; TT, MV, VJ analysed the data; TT, MV, RV, NES and AD wrote the paper.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no competing interests.
Patient confidentiality and consent to publication
Informed, written consent has been obtained, that studies have been performed according to the Declaration of Helsinki, and that the procedures have been approved by a local ethics committee.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Venckus, R., Jasenas, M., Telksnys, T. et al. Robotic-assisted radical prostatectomy with the Senhance® robotic platform: single center experience. World J Urol 39, 4305–4310 (2021). https://doi.org/10.1007/s00345-021-03792-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00345-021-03792-5