Abstract
Purpose
Metastatic prostate cancer (mPCa) rarely occurs under the age of 60, and we aim to evaluate the clinical outcomes and prognosis of mPCa patients ≤ 60-year-old.
Methods
Two thousand and eighty-three patients were treated with mPCa between April 2003 and May 2020. Clinicopathological characteristics between groups, biochemical recurrence (BCR)-free survival, and overall survival (OS) were assessed. Subgroup analysis was performed on patients ≤ 60 years. Multivariable cox regression was used for survival analysis.
Results
Three hundred and seventy-five patients (> 60 years: older) and 115 patients (≤ 60 years: young) were identified. 5-year BCR-free survival rates were 38.8% in young and 74.1% in older group (p < 0.001). 5-year OS were 88.1% in young and 96.5% in older group (p = 0.006). The significant factor associated with BCR was age > 60 (hazard ratio [HR] = 0.67, 95% confidence [CI]: 0.36–0.94, p = 0.017). The significant predictors of OS were age > 60 (HR 0.40, CI 0.18–0.91, p = 0.028) and local definitive treatment (HR 0.29, CI 0.13–0.64, p = 0.002). For the subgroup analysis, median BCR-free survival was significantly shorter in younger (≤ 56) group (14 mo vs. 27 mo, p = 0.026), and the median OS was significantly different (p = 0.048).
Conclusions
In mPCa patients ≤ 60-year-old, BCR occurs earlier and OS is significantly reduced than older patients. Therefore, special caution is mandatory when treating these mPCa patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The median age of prostate cancer (PCa) diagnosis is 66 years, and the majority of prostate cancer survivors (64%) are over 70-year-old and it is rare to receive a PCa diagnosis < 50-year-old (< 1%) [1]. Only 28% of distantly metastatic prostate cancer (mPCa) shows 5-year survival in the US [2]. The prognosis of mPCa depends on different parameters of the clinicopathological factors, such as the prostate-specific antigen (PSA) level, the Gleason score (GS), the lymph node (LN) status, and recurrence [3]. There are few studies to date that have focused on the role of age in the survival of patients with mPCa.
Biological behavior of PCa is difficult to interpret in young patients because most of these cases are either reported as case reports or are included in the case series of older men. Because of its rarity, there is no standard guideline for management of mPCa in young patients and thus it is treated in the same way as in older patients. Some studies have reported that age is an independent prognostic factor for mPCa and young age is associated with poor outcome [4,5,6,7,8]. Nevertheless, most of their results were concluded from relatively small cohorts in the era when chemotherapy and new hormonal agents, like enzalutamide or abiraterone, were not commonly used. We aimed to compare the clinical features and survival of mPCa according to age at diagnosis.
Patients and methods
Data collection
After institutional review board (IRB) approval of study (IRB number: B-2007-622-102), among the 2083 patients treated with mPCa in a tertiary referral institution from May 2003 to May 2020, 375 patients > 60 and 115 patients ≤ 60 were included in the study.
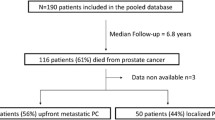
Inclusion criteria of the study are as follows; a histologically confirmed diagnosis of PCa (neuroendocrine tumors excluded), metastasis at initial presentation or occurred over follow-up after initial treatment (including metastatic castration resistance developed over time). We recorded their pre-androgen deprivation therapy (ADT) history of PCa (GS and the presence/absence of metastasis at the time of diagnosis, the interval between the PCa diagnosis and the development of metastatic or non-metastatic castration-resistant prostate cancer [CRPC], and the ADT duration before developing CRPC), their characteristics at the time of starting chemotherapy or androgen receptor signaling axis-targeting agents, their chemotherapy treatment history and outcomes, and their post-chemotherapy history. Exclusion criteria are as follows; patients with incomplete medical records unable to evaluate the patient's treatment method, recurrence, or survival status, and patients diagnosed with malignant tumors in other organs at the time of PCa diagnosis or metastasized from other organs to prostate (Fig. 1).
All data were collected from our prospectively maintained database, and pathological specimens were reviewed in detail by our uropathologists according to standard pathological procedures using the modified definition of the 2005 International Society of Urological Pathology (ISUP) Consensus conference [9]. Tumor stage and grading were evaluated according to the 2002 American Joint Committee on Cancer (AJCC) TNM classification. Patients were followed up and screened every 3 months through serum PSA and, if possible, imaging studies (computed tomography, bone scan, or magnetic resonance imaging). Biochemical recurrence (BCR) is defined as a rise in PSA to 0.2 ng/mL and a confirmatory value of 0.2 ng/mL or greater following radical prostatectomy, or a rise of 2 ng/mL or more above the nadir PSA after radiation therapy. BCR in the metastatic setting is defined as PSA progression, adapted from the study of Prostate Cancer Clinical Trials Working Group 3, an increase in PSA greater than 25% and > 2 ng/ml above nadir, confirmed by progression at 2 time points at least 3 weeks apart. [10].
Study endpoints
Our primary endpoints are BCR-free survival and overall survival (OS). Secondary endpoint is subgroup analysis of patients ≤ 60 years. Clinicopathological characteristics between groups were investigated. Subgroup analysis was performed on patients ≤ 60 years classified into younger (≤ 56 years) group (n = 58) and young (57–60 years) group (n = 57) based on median age of 56 in the subgroup. We also analyzed CRPC-free survival between groups. CRPC-free survival is the time from ADT initiation to development of CRPC.
Statistical analysis
The relationships between the background variables and the age groups were assessed using the chi-squared test for categorical variables and the independent t test or Mann–Whitney U test for continuous variables. OS and BCR-free survival were assessed by the Kaplan–Meier method. If multiple events (BCR, CRPC, or death) occurred in the same patient, the time of appearance of the first of these events was deemed to be the time of appearance of the event in this patient. Univariate and multivariate Cox proportional hazard regression analyses were performed to evaluate the significant variables associated with the survival outcomes. The univariate results were used to determine the candidate variables for the final multivariate model in a backward model selection process. In all variables remaining in the final multivariate analysis, the p value was set to 0.05. All data were analyzed with SPSS version 22, and all tests were two-sided with a p value of 0.05 considered statistically significant (IBM SPSS Statistics, IBM Corp., Armonk, NY, USA).
Results
Demographic and clinical characteristics
A total of 490 eligible cases were identified, with a median follow-up of 16.0 months (mo) (interquartile range [IQR]: 9.0–35.0). Patients’ base characteristics are detailed in Table 1. 32 patients (6.5%) died of PCa and 115 patients (23.5%) had occurred BCR. 5-year BCR-free survival rates were 38.8% in young and 74.1% in older group (median survival: 25 mo vs not reached, p < 0.001) (Fig. 2A). 5-year OS was 88.1% in young and 96.5% in older group (median survival: not reached for both groups, p = 0.006) (Fig. 2B). Young group had significantly shorter median CRPC-free survival than older group (11 mo vs 18 mo, p = 0.002) (Fig. 2C). The significant factor associated with BCR was age > 60 (hazard ratio [HR] = 0.67, 95% confidence [CI]: 0.36–0.94, p = 0.017) (Table 2). The significant predictors of OS were age > 60 (HR 0.40, CI 0.18–0.91, p = 0.028) and local definitive treatment (HR 0.29, CI 0.13–0.64, p = 0.002) (Table 2).
Subgroup analysis—young (57–60) versus younger (≤ 56) group
The mean age in the subgroup was 52.2 years (younger group) and 58.5 years (young group), respectively (p < 0.001), and the median follow-up period was 13.0 mo. The rates of metastasis at the time of diagnosis were 74.1% and 66.7% (p = 0.900), respectively, and 41.4% and 43.9% (p = 0.480) of each group received local definitive treatment (radical prostatectomy or radiation therapy). Differences in clinical and pathological characteristics between groups were not significant except for hypertension (p = 0.005). Median BCR-free survival was significantly shorter in younger group (14 mo vs. 27 mo, p = 0.026), and the median OS was significantly different (87 mo for younger group vs not reached for young group, p = 0.048). In multivariate analysis, factor related to BCR-free survival and OS was local definitive treatment (supplementary tables 2 and 3).
Discussion
A study based on Surveillance Epidemiology and End Results (SEER) showed that the general 10-year, cancer-specific survival (CSS) of younger men with low-grade PCa is similar to that of their older counterparts, although the youngest men with high-grade and advanced PCa at initial diagnosis had poor prognosis: in particular, men aged 35–44 years with stage IV PCa had an approximately 1.5-fold greater risk of cancer-specific mortality when compared with men aged 65–74 years [11]. However, most of the patients were treated in the predocetaxel era, which makes it difficult to apply the results to the contemporary clinical scenario in which a number of agents like docetaxel, cabazitaxel, enzalutamide, or abiraterone have led to a significant survival gain in patients with mCRPC [12,13,14,15,16,17]. One study [18] reported 333 mCRPC patients but it stratified the patients based on their age at PCa diagnosis and does not provide any information concerning the age when they developed mCRPC. They concluded that the age at PCa diagnosis affects the outcome of the patients who subsequently develop mCRPC, with the shortest survival being observed in the oldest (> 75-year-old) and youngest (< 55-year-old) groups.
Our study is the first in describing a contemporary series of patients ≤ 60-year-old when they were diagnosed as mPCa and received ADT and/or radiotherapy and subsequent first-line chemotherapy or further newer agents or progressed to mPCa from localized PCa with local definitive treatment. As there was control group of older patients (> 60-year-old), the possible comparison is with the clinical outcomes observed in single-center retrospective data of mPCa patients. Patients who received enzalutamide or abiraterone, though they have relatively shorter follow-up, were also included in the analyses. Although the survival of younger patients after the appearance of castration resistance seems to be comparable with that obtained in the pivotal trials, they may develop castration resistance more quickly. Our data support this finding, as the median time between the initial diagnosis and the development of mCRPC was 13.0 months [Interquartile range = 8.0–23.0].
Younger PCa men are more likely to receive radical definitive treatment, as they tend to reveal more favorable disease affordable to cure and fewer comorbidities [11]. Longer life expectancy in these younger patients also means longer time to deal with possible side effects of treatment. Urinary incontinence and erectile dysfunction often have a greater impact on quality of life in the younger cohort [19]. Yuh et al. [20] showed results of the first planned Phase 1 study on cytoreductive prostatectomy (CRP). With more than 6 months of follow-up, 67.9% had PSA nadir ≤ 0.2 ng/mL. The 90-day overall complication rate was 31.2%, of which 6.25% were considered major complications. Though limited to phase I trial, careful interpretation can be deducted that CRP might be effective for patients with mPCa under 60 of operation-tolerable general condition. Moreover, Culp et al. [21] identified 8185 men from the SEER database with mPCa between 2004 and 2010 and found that CRP was performed in 245 of these men. They found that the 5-year OS was significantly higher in patients who had CRP compared to those who had no local therapy (67.4% vs. 22.5%, p < 0.001). Interestingly, this discrepancy in survival was most pronounced in patients with visceral metastases who underwent CRP, suggesting that even patients with the poorest prognoses may benefit from surgery. Nevertheless, there is no Level 1 evidence supporting the role of surgery in mPCa [22]. Recent data indicated the feasibility of salvage prostatectomy as one of modality for local definitive treatment. Martinez et al. [23] advocated estimated BCR-free survival rate in the open approach group and robot-assisted group was 67% (95% CI 53.7–80.3) and 60.9% (95% CI 40.5–81.3), respectively (log-rank test p: 0.873). Robotic salvage RP reported significantly less complications. This suggests that, as evidence to support our results, it is helpful for patients to implement surgery (robotic approach) for metastatic prostate cancer, if feasible.
Findings from the STAMPEDE trial [24] support the treatment of primary tumor by radiotherapy in patients with oligometastatic mPCa and can be applied to our results. OS was improved significantly in patients with a low metastatic burden who underwent radiotherapy (HR: 0.68, 95% CI 0.52–0.90; p = 0.007). In addition, failure-free survival was also improved in men with low metastatic burden (HR: 0.59, 95% CI 0.49–0.72; p < 0.0001). Though no improvement in unselected patients, radiotherapy could improve survival in men with a low metastatic burden.
Contrary to our results, similar findings have been observed by a study [25] that older men are more likely to have high risk PCa and low OS because older patients are more likely to be treated with primary ADT rather than potentially curative local therapy. A study [26] of 151 patients < 50-year-old has shown that young patients have similar morphology and outcome as older patients. Patients received different treatment modality as surgery, radiotherapy, and hormonal therapy depending upon the disease stage and treatment availability at that time. It was suggested that young PCa patients present with similar symptomatology, histological grade, stage, and prognosis as the older population. Patients in these findings are those who were treated before the emergence of new agents, and careful clinical application should be taken when applying those results in the current clinical practice.
Nevertheless, several groups have reported poor prognoses in young PCa patients like ours. One report [27] reported PCa in a young boy of 11-year-old and summarized the clinicopathological characteristics of PCa in infants and adolescents. It was thought that aggressive behavior of young PCa can be originated from undifferentiated histology. In another study [28], 41 mPCa patients < 50-year-old and bone metastasis were identified from 1952 to 2005. Patients received different kind of palliative treatment like bilateral orchiectomy and adrenalectomy in 1970s and oral or parenteral ADT in recent years. Additionally, patients received palliative radiotherapy and other symptomatic treatment. Median survival was 16.1 months and all patients died of progressive disease. A recent study has also reported poorer outcomes in 3006 young mPCa patients receiving primary ADT [29]. In this study, age was an independent prognostic factor for mPCa patients and 5-year OS was poorer in young patients, at just 22% in patients < 50-year-old.
This study has several limitations. First, its retrospective nature makes it methodologically difficult to evaluate treatment outcomes because activity measures such as the OS can be greatly affected by differences in imaging frequency (which may shorten or lengthen OS or fail to detect a radiological response or progression) or the interpretation bias because objective responses are determined by individual physicians. Another limitation is that the differences in the number of previously administered hormonal therapy regimens among urologists in a single institution reflected differences in treatment responses. A selection bias from securing study population and a confounding bias concerning the impacts of novel antiandrogens’ introduction time on OS, BCR-free survival period or treatment outcomes are also limitations. Finally, the question is the age at which a patient with mPCa can be considered “young,” which has still not been clearly and universally defined. We selected a cut-off age of 60 years, though considered purely arbitrary, because mPCa is mainly diagnosed in patients older than 60. Furthermore, our hypothesis that some of the patients who are “young” at the time of mPCa diagnosis may have aggressive disease and rapidly develop castration resistance warrants further investigation in larger prospective studies.
Conclusions
In mPCa patients ≤ 60-year-old, BCR occurs earlier and OS is significantly reduced than in older patients. Nevertheless, patients who received local definitive treatment (RP or RT) during disease course tend to have less BCR and longer OS. Therefore, special caution is mandatory when treating these mPCa patients.
References
Miller KD, Nogueira L, Mariotto AB, Rowland JH, Yabroff KR, Alfano CM et al (2019) Cancer treatment and survivorship statistics, 2019. CA Cancer J Clin 69(5):363–385
Gillessen S, Attard G, Beer TM, Beltran H, Bossi A, Bristow R et al (2018) Management of patients with advanced prostate cancer: the report of the advanced prostate cancer consensus conference APCCC 2017. Eur Urol 73(2):178–211
Guo Y, Mao S, Zhang A, Wang R, Zhang Z, Zhang J et al (2019) Prognostic significance of young age and non-bone metastasis at diagnosis in patients with metastatic prostate cancer: a SEER population-based data analysis. J Cancer 10(3):556–567
Wilson JM, Kemp IW, Stein GJ (1984) Cancer of the prostate. Do younger men have a poorer survival rate. Br J Urol 56(4):391–396
Tjaden HB, Culp DA, Flocks RH (1965) Clinical adenocarcinoma of the prostate in patients under 50 years of age. J Urol 93:618–621
Weitzner S, Sarikaya H, Furness TD (1980) Adenocarcinoma of prostate in a twenty-seven-year-old man. Urology 16(3):286–288
Madan R, Singh L, Haresh KP, Rath GK (2015) Metastatic adenocarcinoma of prostate in a 28-year-old male: the outcome is poor in young patients. Indian J Palliat Care 21(2):242–244
Caffo O, Ortega C, Di Lorenzo G, Sava T, De Giorgi U, Cavaliere C et al (2015) Clinical outcomes in a contemporary series of “young” patients with castration-resistant prostate cancer who were 60 years and younger. Urol Oncol 33(6):265.e15–21
Epstein JI, Allsbrook WC, Amin MB, Egevad LL (2005) The 2005 International Society of Urological Pathology (ISUP) consensus conference on Gleason grading of prostatic carcinoma. Am J Surg Pathol 29(9):1228–1242
Scher HI, Morris MJ, Stadler WM, Higano C, Basch E, Fizazi K, Antonarakis ES, Beer TM, Carducci MA, Chi KN, Corn PG, de Bono JS, Dreicer R, George DJ, Heath EI, Hussain M, Kelly WK, Liu G, Logothetis C, Nanus D, Stein MN, Rathkopf DE, Slovin SF, Ryan CJ, Sartor O, Small EJ, Smith MR, Sternberg CN, Taplin ME, Wilding G, Nelson PS, Schwartz LH, Halabi S, Kantoff PW, Armstrong AJ, Prostate Cancer Clinical Trials Working Group 3 (2016) Trial design and objectives for castration-resistant prostate cancer: updated recommendations from the Prostate Cancer Clinical Trials Working Group 3. J Clin Oncol 34(12):1402–1418
Lin DW, Porter M, Montgomery B (2009) Treatment and survival outcomes in young men diagnosed with prostate cancer: a Population-based Cohort Study. Cancer 115(13):2863–2871
Tannock IF, de Wit R, Berry WR, Horti J, Pluzanska A, Chi KN et al (2004) Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med 351(15):1502–1512
Petrylak DP, Tangen CM, Hussain MH, Lara PN, Jones JA, Taplin ME et al (2004) Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N Engl J Med 351(15):1513–1520
de Bono JS, Logothetis CJ, Molina A, Fizazi K, North S, Chu L et al (2011) Abiraterone and increased survival in metastatic prostate cancer. N Engl J Med 364(21):1995–2005
Scher HI, Fizazi K, Saad F, Taplin ME, Sternberg CN, Miller K et al (2012) Increased survival with enzalutamide in prostate cancer after chemotherapy. N Engl J Med 367(13):1187–1197
Ryan CJ, Smith MR, de Bono JS, Molina A, Logothetis CJ, de Souza P et al (2013) Abiraterone in metastatic prostate cancer without previous chemotherapy. N Engl J Med 368(2):138–148
de Bono JS, Oudard S, Ozguroglu M, Hansen S, Machiels JP, Kocak I et al (2010) Prednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: a randomised open-label trial. Lancet 376(9747):1147–1154
Humphreys MR, Fernandes KA, Sridhar SS (2013) Impact of age at diagnosis on outcomes in men with castrate-resistant prostate cancer (CRPC). J Cancer 4(4):304–314
Tan L, Wang LL, Ranasinghe W, Persad R, Bolton D, Lawrentschuk N et al (2018) Survival outcomes of younger men (< 55 years) undergoing radical prostatectomy. Prostate Int 6(1):31–35
Yuh BE, Kwon YS, Shinder BM, Singer EA, Jang TL, Kim S et al (2019) Results of Phase 1 study on cytoreductive radical prostatectomy in men with newly diagnosed metastatic prostate cancer. Prostate Int 7(3):102–107
Culp SH, Schellhammer PF, Williams MB (2014) Might men diagnosed with metastatic prostate cancer benefit from definitive treatment of the primary tumor? A SEER-based study Eur Urol 65(6):1058–1066
Jenjitranant P, Touijer KA (2019) Role of surgery in oligometastatic prostate cancer. Prostate Int 7(4):125–130
Martinez PF, Romeo A, Tobia I, Isola M, Giudice CR, Villamil WA (2021) Comparing open and robotic salvage radical prostatectomy after radiotherapy: predictors and outcomes. Prostate Int 9(1):42–47
Parker CC, James ND, Brawley CD, Clarke NW, Hoyle AP, Ali A et al (2018) Radiotherapy to the primary tumour for newly diagnosed, metastatic prostate cancer (STAMPEDE): a randomised controlled phase 3 trial. Lancet 392(10162):2353–2366
Bechis SK, Carroll PR, Cooperberg MR (2011) Impact of age at diagnosis on prostate cancer treatment and survival. J Clin Oncol 29(2):235–241
Aprikian AG, Zhang ZF, Fair WR (1994) Prostate adenocarcinoma in men younger than 50 years. A retrospective review of 151 patients. Cancer 74(6):1768–1777
Shimada H, Misugi K, Sasaki Y, Iizuka A, Nishihira H (1980) Carcinoma of the prostate in childhood and adolescence: report of a case and review of the literature. Cancer 46(11):2534–2542
Astigueta JC, Abad MA, Morante C, Pow-Sang MR, Destefano V, Montes J (2010) Characteristics of metastatic prostate cancer occurring in patients under 50 years of age. Actas Urol Esp 34(4):327–332
Kimura T, Onozawa M, Miyazaki J, Matsuoka T, Joraku A, Kawai K et al (2014) Prognostic impact of young age on stage IV prostate cancer treated with primary androgen deprivation therapy. Int J Urol 21(6):578–583
Funding
No funding was received specifically for this project.
Author information
Authors and Affiliations
Contributions
HK and SKH contributed to protocol/project development; HK and SKH were involved in data collection or management; HK, SL, SSB, and SKH analyzed the data; HK and SKH contributed to manuscript writing/editing; HK, SL, SSB, and SKH were involved in critical review; SKH supervised the study.
Corresponding author
Ethics declarations
Conflict of interest
All authors have no conflict of interest to declare.
Ethical statements
All study protocols were in accordance with the principles of the Helsinki Declaration. We removed personal identifiers and anonymized all data, which exempted the study from the need to obtain informed consent from patients. Institutional review board (IRB) approval of study from Seoul National University Bundang Hospital was obtained (IRB number: B-2007-622-102).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kim, H., Lee, S., Byun, SS. et al. Clinical outcomes and prognosis of metastatic prostate cancer patients ≤ 60-year-old. World J Urol 39, 4319–4325 (2021). https://doi.org/10.1007/s00345-021-03785-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00345-021-03785-4