Abstract
Introduction
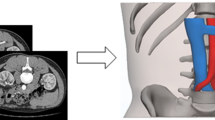
Kidney Stone Calculator (KSC) is a free, three-dimensional (3D) planning software for flexible ureteroscopy(fURS) with Holmium:YAG(Ho:YAG) endocorporeal lithotripsy (EL). KSC provides the stone volume (SV) and expected duration of lithotripsy (ExDL) estimations based on non-enhanced-CT scan (NECT) DICOM series. We aimed to provide a preclinical and clinical evaluation of KSC.
Patients and methods
A preclinical evaluation measured the SV by three operators (resident, endourology expert and research engineer) among 17 NECT cases. Between January and March 2020, a multicentric, prospective, observational double-blind clinical evaluation was conducted in patients presenting with renal stones treated with Ho:YAG-EL during fURS and preoperative NECT. Demographic and surgical data were collected. The primary endpoint was a significant median difference between ExDL and EffectiveDL (EfDL). Second, efficiency (J/mm3) and efficacy (mm3/min) ratios were calculated.
Results
The preclinical evaluation showed no significant difference in the SV measurements among operators (p > 0.05). Pearson and Kendall coefficients of 0.99 and 0.98, respectively, were found. Twenty-six patients were included in the clinical evaluation, with a median age of 55 years. In 66% of cases, there was a single stone located in the lower pole, with a density > 1000 Hounsfield Unit observed in 42% and 85% of cases. A 14% [Q1–Q3 (5.4–24.8); p = 0.36] median difference between ExDL and EfDL was noted, which was greater in the case of lower pole stones with no possible relocation (p = 0.008). Median values of 17.6 J/mm3 and 0.4 (0.32–0.56) mm3/s EL were also noted.
Conclusions
Kidney Stone Calculator is a reproducible and accurate software that allows for an estimation of the stone burden and provides an ExDL for URSf. Defining the influencing factors of EL will improve its ExDL.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
International guidelines for the surgical management of urolithiasis are based on the location and size of the stone, which are used to decide on the surgical modality [1]. The stone burden is mostly evaluated by the measurement of the maximum diameter (MD) [2, 3]. However, the real stone burden, i.e., stone volume (SV), could be different from that estimated by the MD due to various geometrical and non-standard shapes [4, 5]. For example, a cubic stone of 8 mm, a paving stone of 20 × 5 × 5 mm and a spherical stone of 10 mm present nearly similar SVs (500–523 mm3) but different MDs. These MDs could lead to different surgical options according to the international guidelines [1, 6]. Several formulas, such as Ackerman’s (0.6 × π × radius2), spherical (4/3 × π × radius3), half-cubic, surface area and cumulative diameter formulas, have been evaluated to estimate the stone burden, but none of them can assess the exact SV [4, 7].
However, several imaging techniques are now available to estimate the exact stone burden. As one of them, “Kidney Stone Calculator” (KSC), has been developed as a free three-dimensional (3D) software for the surgical planning of kidney stone endoscopic procedures [8]. It is an extension of 3DSlicer, a free software platform for medical image computing and 3D visualization [9]. KSC uses segmentation on a DICOM series of nonenhanced computed tomography (NECT) images. Consequently, KSC determines the stone burden using a volume assessment to help plan the procedure, giving an estimation of the lithotripsy duration based on the following parameters: laser fiber diameter, laser source [Holmium:Yttrium–Aluminium–Garnet (Ho:YAG) or Thulium Fiber Laser (Tm-Fiber)], pulse energy (J) and pulse rate (Hz), multiple stone estimation, and 3D-view of the stone. These parameters are based on in vitro data obtained by estimating the ablation volumes of both low power Ho:YAG and TmFiber and implemented in KSC for lithotripsy duration (DL) estimation [10]. After the development of KSC, we first aimed to evaluate its accuracy in estimating the stone burden based on stone volume measurements by segmentation. Then, a clinical evaluation was conducted to endorse its ability to predict DL based on a comparison with an estimated DL.
Patients and methods
Kidney stone calculator
KSC is a python-scripted free open-source add-on extension of 3DSlicer [11]. It measures the SV independently of its shape and the number of stones by segmentation of the NECT-DICOM series and provides an expected duration of lithotripsy (ExDL). A dedicated tutorial is available to realize SV measurement: https://www.youtube.com/watch?v=pZLXHdfJtP0&t=5s.
Stone volume measurement
To evaluate the accuracy of KSC in measuring the SV, we performed a preclinical evaluation consisting of the SV measurement of a panel of 17 NECT in the bone window. This test cohort included ex vivo human stones of various compositions [calcium-oxalate monohydrate (COM), cystine and uric acid (UA)], 1 cm3 cubes of artificial hard and soft begostones (Bego©, Germany) and two clinical cases. This panel presents various shapes, known and unknown volumes, in vitro and in vivo cases, and human and artificial stones.
After a ten-minute formation of KSC, three participants, i.e., a urology resident, a senior endourologist and a research engineer without prior experience in medical image computing or urology, were asked to assess the volumes of each sample of the panel. The evaluation was conducted in full-blind mode.
Lithotripsy duration estimation
To evaluate the accuracy of the KSC in estimating DL, a prospective observational study was conducted in three centres from January 2020 to March 2020. Approval from the Ethics Committee of the French Association of Urology (CERU-AFU) was obtained (reference: CERU_2020/003). An official declaration to the National Commission of Informatics and Freedoms (CNIL) was also made (reference: 2216615V0-MR-004).
The included patients were aged above 18 years, with single or multiple renal calculi, observed on preoperative NECT and planned for flexible ureterorenoscopy (fURS) with endocorporeal lithotripsy (EL) using a low-power Ho:YAG laser generator and 272 µm laser fibers. For DL estimation, a stone maximum density (SMD) over 1000UH was retained as “hard” and under 1000UH as “soft” stones. The exclusion criteria were patients with ureteric stones, the presence of an incrusted double-J stent, standard or mini-percutaneous nephrolithotomy (PCNL), monobloc extraction, the absence of preoperative NECT, and a known history of struvite stones.
Written informed consent was obtained from all patients after explaining the study protocol and providing an information sheet.
fURS with EL was conducted following an observational design. The collected data included demographic characteristics, personal urolithiasis history and actual characteristics of the stones [side, localization, number, MD (mm), SV (mm3), and SMD (UH)]. Peri-operative data were also collected: surgeon’s expertise [junior/senior (< 100 fURS per year) or expert (> 100 fURS per year)], endourology devices used, laser settings (dusting: 0.5–0.8 J/15–30 Hz; fragmentation: 1–1.5 J/10–15 Hz), operative duration, effective DL (EfDL), delivered total energy (J), volumetric energy (J/mm3), ablation rate (mm3/s), and presence of residual fragments. The EfDL was defined as the duration between the initiation of lasering and its termination. In the case of separate lasering times (basketing between two lithotripsy periods), several durations were recorded and added to obtain the overall EfDL. The postoperative data concerned the stone-free rate level defined by the absence of residual fragments or residual fragments lower than 3 mm MD on postoperative NECT or the absence of endoscopically residual stones at the end of the procedure. Complications were consigned according to the Clavien–Dindo classification. The SV was calculated postoperatively based on the preoperative NECT using KSC. Consecutively, the ExDL was defined according to the chosen laser settings. The investigator was not aware of any data other than the laser settings at the moment of SV and ExDL measurement. Clinical and imaging data were anonymized at inclusion.
The primary endpoint of this study was the difference between ExDL and EfDL. The secondary endpoints were five a priori influencing factors of the DL: presence or absence of relocation of a lower-pole stone, absence of possible relocation of a lower-pole stone, 10–12CH or 12–14CH ureteral access sheath diameters, lithotripsy mode(Dusting/Fragmentation), and surgeon’s expertise(> 100/ < 100 fURS per year).
Statistical analysis
The measured volumes were compared using a two-tailed paired Student’s t-test. The correlation strength was studied using Pearson correlation and Kendall concordance coefficients. In the clinical study, qualitative and quantitative data are presented as percentages and medians with interquartile ranges (Q1–Q3), respectively. Primary and secondary endpoints were compared using a two-tailed paired Student’s t test or chi-square test and Bonferroni’s correction. All statistics used Rstudio and GraphPad Prism. p values less than 0.05 were regarded as statistically significant.
Results
Stone volume measurement
Table 1 presents the 3D views of each sample obtained with KSC and the volumes measured by the three practitioners. Various shapes, sizes and compositions were segmented, showing the ability of KSC to provide a 3D reconstruction of renal calculi, independent of the NECT protocol. No significant difference among the operators was noted (Op1–Op2: p = 0.35, Op1–Op3: p = 0.69, Op2–Op3: p = 0.29, Table 2). In the subgroup analysis, there was no difference in the volume assessment for human stones, artificial stones and clinical cases. A mean difference of 0.28% to 1%, including all cases, was noticed. When measuring the known 1 cm3 cubes, a 1% maximum variation occurred. The maximum difference among operators was observed with a clinical case (15%). The Pearson’s correlation coefficient was r = 0.99 among the three operators, and Kendall’s concordance coefficient value was 0.98. Furthermore, there was no systematic over- or underestimation from any operator.
Clinical evaluation
From January 2020 to March 2020, 26 patients were included in the clinical evaluation of KSC. Table 3 presents the demographic and renal calculi characteristics. Our cohort had a median age and BMI of 55.5 (38–69.5) years, 28.7 (24.4–32.1) kg/m2, respectively. Sixty-six percent of patients presented a single renal stone and were localized in the lower pole calyx in 42% of cases. Eighty-five percent of patients presented a MSD over 1000UH, with a median MD and SV of 10 (8–12.5) mm and 479 (268–4517) mm3, respectively. A total of 42%, 31% and 27% of procedures were conducted by an expert in endourology (> 100 fURS/year) and senior or junior urologists, respectively (Tables 4, 5, 6, 7). The median difference between ExDL and EfDL was 14% (5.4–24.8); p = 0.36. A median volumetric energy of 17.6 (13.6–24.7) J/mm3 and ablation rate of 0.4 (0.324–0.56) mm3/s were noted. The stone-free rate was 81%, considering both endoscopic and postoperative NECT methods. We reported two postoperative complications: one Grade II event of urosepsis that was medically treated and one Grade IV event of cardiac arrest at postoperative day 1 related to rhythmic dysfunction.
Among the five supposed influencing factors, only the absence of relocation for a lower-pole stone significantly increased the difference between ExDL and EfDL (p = 0.008) after Bonferroni’s correction (Table 6).
Discussion
Volume measurement for kidney stone surgical procedures
Kidney Stone Calculator provides a 3D measurement of the SV by a segmentation process [8]. Although volume is instinctively better than the diameter for the stone burden estimation, previous studies have failed to show that the SV can be used as the new standard [12]. Other devices have been developed for this goal, but none of them aimed to predict the lithotripsy duration [2, 13, 14]. Thus, the main interest of 3D evaluation pertains to its surgical application. The surgeon would be able to manage his laser settings and equipment and estimate the procedure’s duration and the possible iterative sessions of fURS. He could also pre- and postoperatively evaluate himself based on the ExDL and the EfDL. Moreover, as an education programme, KSC could be used to evaluate junior urologists who are working to master the learning curve.
The segmentation process inherent to KSC does not calculate a volume based on two-dimensional measurements and a mathematical formula [4, 5, 15]; rather, it refers to the sum of voxels collected by a manual density scale in a region of interest (ROI) [8, 9]. Segmentation has been shown to be effective in quantifying vascular calcifications or muscular mass as predictive factors in carcinologic or noncarcinologic diseases [16,17,18,19]. Consequently, this concept was also used for in vitro evaluation of ablation rates (mm3/s) for low power Ho:YAG and Tm-Fiber devices, which are secondarily implemented in KSC for ExDL calculation [10]. To date, only volumetric data have been generated, satisfying new concepts of lithotripsy efficiency, namely, ablation rates and volumetric energy [20].
Measurement accuracy
The preclinical evaluation revealed that KSC was reproducible, independent of the shape, size or composition of the stone and showed little difference between operators, with a Pearson’s correlation coefficient of 0.99. Moreover, the Kendall concordance coefficient (0.98) confirmed the excellent reproducibility of KSC. We found no difference between operators or cases in the subgroup analysis. A previous study showed similar results (0.97 interobserver-correlation) with experienced radiologists [19]. In our protocol, rapid training was necessary to provide familiarity with the basics of 3DSlicer, but no prior personal experience seems to be required. However, we have to acknowledge some limits. First, the maximum interobserver variation occurred in a clinical case (15%), which was not the complex staghorn case. A further evaluation of high-burden cases would be necessary to confirm our findings. Moreover, we present a comparison of measured volumes, without reference volumes in most cases. An alternative experiment comparing referenced stone volumes to segmented ones could support the validation of KSC, as performed by Wilhelm et al. [15]. Finally, special attention must be paid to the NECT protocol, which has been shown to influence outcomes. Insufficient slice thickness (3–5 mm) and low-dose irradiation protocols may alter the detection or precision of the segmented volume, especially in obese patients, as well as the calibration window [21, 22]. In the present work, the window was manually adjusted, and the NECT panel included various settings, but changes in these settings did not alter the correlation between operators. Further improvements in KSC would include automatic detection using deep learning, as used for kidney segmentation [22].
Clinical evaluation
We reported a 14% median difference between ExDL and EfDL, which increased in the absence of relocation of a lower-pole stone (Tables 4, 5, 6). This study first aimed to estimate the EL duration with a small number of participants. Considering this specific aspect, we interrupted patient enrolment because of the COVID-19 pandemic crisis, which led to the postponement of kidney stone surgical procedures in our country for 3 months [24]. Among the 26 included patients, we found similar demographic characteristics to those in the available data [25, 26]. We also present a complication rate similar to that reported in the available literature [27]. Whereas two-thirds of our population presented with a single stone, multiple calculi were found in 9 patients, possibly increasing the difference in ExDL-EfDL for two reasons: First, with “stop-and-start” laser emission, multiple stone cases and irrigation flow have increased. Second, due to the increasing probability of producing significant residual fragments, basketing is required. These arguments could consequently explain the relatively low volumetric energy (17 J/mm3) and ablation rate (0.4 mm3/s) compared to the available in vitro or clinical data [10, 20, 28]. Ventimiglia et al. reported 19 J/mm3 and 0.7(0.4–0.9) mm3/s ablation outcomes in their cohort, which included 30 patients with a high stone burden [1599 (630–3502) mm3] treated in a urolithiasis expert centre [20]. Consequently, their dusting ability could have been superior to ours in the present study. Regarding the in vitro data, we acknowledge the ideal conditions of lasering: minimizing the burnback effect and optimizing the ablation rate with a single shot or robotic displacement of the laser fiber, with a new fiber tip used for each procedure. The present study concerned in vivo conditions with irrigation flow, stone retropulsion, deterioration of the fiber tip, and various surgeons’ dusting ability. These lasering parameters could not be explored without possible logistic regression analysis because of the small number of patients. We a priori defined potential influencing factors and analysed the p values with Bonferroni’s correction. We acknowledge that other factors could have been selected. The 81% stone-free rate, defined endoscopically and by the absence of 3 mm fragments, also limits our findings. “Dust” has been recently defined as fragments floating under irrigation [29]. Whereas Keller et al. demonstrated in vitro that fragments smaller than 250 µm, except for struvite (125 µm), met this definition; the SFR level is still under discussion [30, 31]. Consequently, we left the SFR assessment to the surgeon’s preference. Moreover, we studied only the Ho:YAG EL during the clinical evaluation using a low-power(LP) laser generator. High-power (HP) Ho:YAG has been suggested to reduce the lasering time, but the data herein concerned only LP-Ho:YAG and Tm-Fiber. Consequently, we cannot draw conclusions on the ability of KSC to predict DL for HP-Ho:YAG devices or Moses’ technology. The present work consisted of a pilot study, and more patients are needed to investigate the viability of KSC application, including Tm-Fiber EL.
Conclusion
Kidney stone calculator is a free, reproducible, safe and accurate software for stone burden and lithotripsy duration estimations. Further studies with a larger population and endocorporeal laser lithotripsy procedures using Tm-Fiber are needed to confirm our preliminary results.
References
Türk C, Petřík A, Sarica K, Seitz C, Skolarikos A, Straub M et al (2016) EAU guidelines on interventional treatment for urolithiasis. EurUrol mars 69(3):475–482
Patel SR, Nakada SY (2011) Quantification of preoperative stone burden for ureteroscopy and shock wave lithotripsy: current state and future recommendations. Urology 78(2):282–285
Hyams ES, Bruhn A, Lipkin M, Shah O (2010) Heterogeneity in the reporting of disease characteristics and treatment outcomes in studies evaluating treatments for nephrolithiasis. J Endourol 24(9):1411–1414
Merigot de Treigny O, Bou Nasr E, Almont T, Tack I, Rischmann P, Soulié M et al (2015) The cumulated stone diameter: a limited tool for stone burden estimation. Urology 86(3):477–481
De Coninck V, Traxer O (2018) The time has come to report stone burden in terms of volume instead of largest diameter. J Endourol 32(3):265–266
Assimos D, Krambeck A, Miller NL, Monga M, Murad MH, Nelson CP et al (2016) Surgical management of stones: American urological association/endourological society guideline, guideline, part I. J Urol. 196(4):1153–1160
Ackermann D, Griffith DP, Dunthorn M, Newman RC, Finlayson B (1989) Calculation of stone volume and urinary stone staging with computer assistance. J Endourol. 3(4):355–360
Panthier F, Doizi S, Illoul L, Berthe L, Traxer O (2020) Developing free three-dimensional software for surgical planning for kidney stones: volume is better than diameter. Eur Urol Focus [Internet]. 24 juin 2020 [cité 24 juin 2020]; Disponible sur: http://www.sciencedirect.com/science/article/pii/S2405456920301619
Fedorov A, Beichel R, Kalpathy-Cramer J, Finet J, Fillion-Robin J-C, Pujol S et al (2012) 3D Slicer as an image computing platform for the Quantitative Imaging Network. MagnReson Imaging 30(9):1323–1341
Panthier F, Doizi S, Lapouge P, Chaussain C, Kogane N, Berthe L et al (2020) Comparison of the ablation rates, fissures and fragments produced with 150 µm and 272 µm laser fibers with superpulsed thulium fiber laser: an in vitro study. World J Urol 6:1–9
Panthier F (2020) fredericpanthier/SlicerKidneyStoneCalculator [Internet]. 2019 [cité 11 févr 2020]. Disponible sur: https://github.com/fredericpanthier/SlicerKidneyStoneCalculator. Accessed 23 Nov 2019
Diamand R, Idrissi-Kaitouni M, Coppens E, Roumeguère T, Legrand F (2018) Evaluation of stone size before flexible ureteroscopy: which measurement is best? Progres En Urol J AssocFrancaiseUrolSocFrancaise Urol. 28(1):62–70
Ziemba JB, Li P, Gurnani R, Kawamoto S, Fishman E, Fung G et al (2018) A user-friendly application to automate CT renal stone measurement. J Endourol 32(8):685–691
Patel SR, Stanton P, Zelinski N, Borman EJ, Pozniak MA, Nakada SY et al (2011) Automated renal stone volume measurement by noncontrast computerized tomography is more reproducible than manual linear size measurement. J Uroldéc 186(6):2275–2279
Wilhelm K, Miernik A, Hein S, Schlager D, Adams F, Benndorf M et al (2018) Validating automated kidney stone volumetry in CT and mathematical correlation with estimated stone volume based on diameter. J Endourol 32(7):659–664
Goo JM, Tongdee T, Tongdee R, Yeo K, Hildebolt CF, Bae KT (2005) Volumetric measurement of synthetic lung nodules with multi-detector row CT: effect of various image reconstruction parameters and segmentation thresholds on measurement accuracy. Radiology 235(3):850–856
Ko JP, Rusinek H, Jacobs EL, Babb JS, Betke M, McGuinness G et al (2003) Small pulmonary nodules: volume measurement at chest CT–phantom study. Radiology 228(3):864–870
Fukushima H, Takemura K, Suzuki H, Koga F (2018) Impact of sarcopenia as a prognostic biomarker of bladder cancer. Int J Mol Sci 19(10):2999
Demehri S, Kalra MK, Rybicki FJ, Steigner ML, Lang MJ, Houseman EA et al (2011) Quantification of urinary stone volume: attenuation threshold-based CT method—a technical note. Radiology 258(3):915–922
Ventimiglia E, Pauchard F, Gorgen ARH, Panthier F, Doizi S, Traxer O (2021) How do we assess the efficacy of Ho:YAG low-power laser lithotripsy for the treatment of upper tract urinary stones? Introducing the Joules/mm3 and laser activity concepts. World J Urol. 39(3):891–896. https://doi.org/10.1007/s00345-020-03241-9
Brisbane W, Bailey MR, Sorensen MD (2016) An overview of kidney stone imaging techniques. Nat Rev Urol 13(11):654–662
Danilovic A, Rocha BA, Marchini GS, Traxer O, Batagello C, Vicentini FC et al (2019) Computed tomography window affects kidney stones measurements. Int Braz J Urol Off J BrazSocUrol 45(5):948–955
Sharma K, Rupprecht C, Caroli A, Aparicio MC, Remuzzi A, Baust M et al (2017) Automatic segmentation of kidneys using deep learning for total kidney volume quantification in autosomal dominant polycystic kidney disease. Sci Rep. 7(1):1–10
Pinar U, Anract J, Duquesne I, Dariane C, Chartier-Kastler E, Cussenot O et al (2020) Impact of the COVID-19 pandemic on surgical activity within academic urological departments in Paris. Progres En Urol J AssocFrancaiseUrolSocFrancaiseUrol 30:439–447
Scales CD, Smith AC, Hanley JM, Saigal CS (2012) Urologic diseases in America Project. Prevalence of kidney stones in the United States. EurUrol 62(1):160–165
Roger C, Abid N, Dubourg L, Auvergnon C, Lemoine S, Machon C (2020) Composition of urinary calculi: lessons from a French epidemiologic retrospective study. Progres En Urol J AssocFrancaiseUrolSocFrancaise Urol. 30(6):339–345
Lildal SK, Andreassen KH, Baard J, Brehmer M, Bultitude M, Eriksson Y et al (2020) Consultation on kidney stones, Copenhagen 2019: aspects of intracorporeal lithotripsy in flexible ureterorenoscopy. World J Urol. https://doi.org/10.1007/s00345-020-03481-9
Panthier F, Ventimiglia E, Berthe L, Chaussain C, Daudon M, Doizi S et al (2020) How much energy do we need to ablate 1 mm3 of stone during Ho:YAG laser lithotripsy? An in vitro study. World J Urol 38:2945–2953
Doizi S, Keller EX, De Coninck V, Traxer O (2018) Dusting technique for lithotripsy: what does it mean? Nat Rev Urol 15(11):653–654
Keller EX, De Coninck V, Doizi S, Daudon M, Traxer O (2021) What is the exact definition of stone dust? An in vitro evaluation. World J Urol. 39(1):187–194. https://doi.org/10.1007/s00345-020-03178-z
Somani BK, Desai M, Traxer O, Lahme S (2014) Stone-free rate (SFR): a new proposal for defining levels of SFR. Urolithiasis 42(2):95
Funding
Frédéric Panthier received a French Association of Urology research grant in 2018.
Author information
Authors and Affiliations
Contributions
Protocol/project development: FP, OT, SD, FA, AM, LB, LI. Data collection or management: FPOT, SD, FA, LY. Data analysis: FP, SD, FA. Manuscript writing/editing: FP, OT, FA, TL, MOT, AM.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest to disclose other than the following: Olivier Traxer is a consultant for Boston Scientific Corporation, Coloplast, Wolf, B-Braun, IPG photonics, Olympus and Rocamed. Steeve Doizi is a consultant for Boston Scientific Corporation and Coloplast.
Ethics approval
Approval from the Ethics Committee of the French Association of Urology (CERU-AFU) was obtained (reference: CERU_2020/003). An official declaration to the National Commission of Informatics and Freedoms (CNIL) was also made (reference: 2216615V0-MR-004).
Research involving human participants
The present study included human participants who provided informed written consent. Data anonymization was assured by the principal investigator.
Informed consent
Written informed consent was obtained from all patients after explaining the study protocol and providing an information sheet.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Panthier, F., Traxer, O., Yonneau, L. et al. Evaluation of a free 3D software for kidney stones’ surgical planning: “kidney stone calculator” a pilot study. World J Urol 39, 3607–3614 (2021). https://doi.org/10.1007/s00345-021-03671-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00345-021-03671-z