Abstract
Purpose
To assess the performance of EAU risk classification in PCa patients according to the biopsy pathway (standard versus MRI guided) and to develop a new, more accurate, targeted biopsy (TB)-based classification.
Materials and methods
We included 1345 patients consecutively operated by radical prostatectomy (RP) since 2014, when MRI and TB were introduced in the diagnostic pathway. Patients underwent systematic biopsy (SB) only (n = 819) or SB and TB (n = 526) prior to RP during the same time period. Pathological and biochemical outcomes were compared between PCa men undergoing SB (SB cohort) and a combination of TB and SB (TB cohort). Kaplan–Meier and Cox regression models were used to assess biochemical recurrence-free survival (RFS).
Results
Both cohorts were comparable regarding final pathology and RFS (p = 0.538). The EAU risk classification accurately predicted outcomes in SB cohort, but did not significantly separate low from intermediate risk in TB cohort (p = 0.791). In TB cohort, the new proposed three-group risk classification significantly improved the recurrence risk prediction compared with the EAU risk classification: HR 4 (versus HR 1.2, p = 0.009) for intermediate, and HR 15 (versus HR 6.5, p < 0.001) in high-risk groups, respectively. A fourth group defining very high-risk cases (≥ T2c clinical stage or grade group 5) was also proposed.
Conclusions
The new classification integrating TB findings we propose meaningfully improves the recurrence prediction after surgery in patients undergoing a TB-based diagnostic pathway, compared with standard EAU risk classification which is still relevant for patients undergoing only SB. External validation is needed.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Treatment decision-making in prostate cancer (PCa) is mainly based on accurate, clinically meaningful, reproducible risk classifications including prognostic parameters such as PSA, clinical stage, and grade group on biopsies [1,2,3]. The EAU risk classification that classified newly diagnosed PCa within three well-established categories (low, intermediate, and high risk) aims at predicting the risk of recurrence after curative treatment and adapt PCa management according to that risk [1,2,3]. However, the EAU risk classification has been validated in cohorts of patients undergoing systematic, not imaging-targeted biopsies (SB). Recent literature has demonstrated the superiority of targeted biopsies (TB) over SB for the detection of clinically significant PCa [4,5,6,7]. This new diagnostic pathway modifies grade group distribution with more high-grade disease detected [8]. This could lead to important changes in the pre-treatment prognostic models with less marked survival differences between EAU risk groups.
We acknowledge that the widely used pre-treatment risk classification may no longer apply to men undergoing MRI-guided biopsy. Therefore, given the increasing utilization of MRI-guided biopsy in contemporary practice, we think it is critical to revise prognostic tools to account for MRI-targeted biopsy [9]. Few studies have assessed the improvement of prediction of adverse pathology prediction at the time of radical prostatectomy by MRI and TB [10]. However, to our knowledge, no series of patient undergoing TB has included survival outcomes after surgery and has validated recurrence risk prediction models.
Therefore, we aimed to evaluate the performance of the EAU risk classification in a cohort of patients undergoing SB and fusion TB and to compare it with its accuracy in a contemporary cohort of patients who were diagnosed by a standard, not imaging-guided biopsy pathway.
Materials and methods
Study population
Between January 2014 and May 2019, 526 patients underwent an RP for pathologically biopsy-proven prostate cancer after a pre-biopsy positive (PI-RADS ≥ 3) multiparametric (mp) MRI (“targeted biopsy” or TB cohort). During the same period, 819 patients underwent RP after a diagnosis based only on systematic biopsy (“standard biopsy” or SB cohort), because of pre-biopsy negative MRI or absence of imaging prior to biopsy. For these patients, MRI could have been performed after the diagnosis for staging purposes and did not influence the biopsy procedure.
In the TB cohort, the imaging protocol consisted of multiplanar T2-weighted images, diffusion-weighted imaging, dynamic contrast-enhanced MRI, and T1-weighted images with fat suppression according to the European Society of Urogenital Radiology guidelines [11]. Both institutions used a 1.5-T MR unit and a 16-channel coil. No endorectal coil was used. The maximal b value used for diffusion-weighted imaging was b 2000. The mpMRI images were scored and reported according to Prostate Imaging-Reporting and Data System v.2 (PI-RADS) [12]. MRI lesions with a PI-RADS ≥ 3 on mpMRI were submitted to targeted biopsy using real-time transrectal ultrasound (TRUS) guidance via a software registration system with elastic fusion (Koelis® system). A minimum of two targeted cores were taken for each suspicious lesion on mpMRI. All patients also underwent concomitant systematic biopsy at the time of the targeted biopsy, with at least ten random cores taken.
For all patients from both cohorts, RP was performed by high-volume surgeons in one of the two study centres. Delay between biopsy and RP was < 6 months (median 3.2 months, from 0.8 to 5.7). All imaging and biopsy procedures have been performed in two institutions by radiology and urology seniors experienced in prostate cancer diagnosis. Biopsy and RP specimens were evaluated by senior dedicated uropathologists from the two institutions. PSA recurrence was defined by any PSA above 0.2 ng/ml. Biochemical follow-up was standardized with a PSA test at 6 weeks, 3 months and then every 6 months after surgery. No patient received any adjuvant treatment. Data from clinical evaluation, biopsy and RP specimens, and follow-up were recorded in a prospective database.
Analyses
The clinical, biological, and pathological findings were assessed in the overall population, and compared between the two cohorts (SB versus TB cohorts).
The primary endpoint was the prognostic performance of three-group EAU risk classification for predicting biochemical recurrence-free survival (RFS) in both cohorts. A multivariable Cox regression analysis was performed to develop a new risk classification which could outperform the EAU classification in the context of TB diagnostic pathway.
Statistics
The qualitative data were tested using a Chi-square test, or Fisher’s exact test as appropriate and the continuous data were tested using Student’s t test. The Mann–Whitney’s test was used in case of no normal distribution. Survival curves were assessed using Kaplan–Meier method and multivariable analyses were run using Cox regression models. ROCs were also built to assess AUC values. The limit of statistical significance was defined as p < 0.05. The SPSS 22.0 (Chicago, Illinois) software was used for analysis.
Results
Overall population characteristics
Main patient characteristics are listed in Table 1. We found significant variability in the number of patients within each cancer risk category due to TB when compared to SB (p < 0.001). Overall, 61% of low-risk PCa cases defined by SB alone were reclassified as intermediate-risk cases by adding MRI-TB. PCa cases classified as intermediate risk using only SB were reclassified as high risk in 7% of cases when adding MRI-TB.
Biopsy features’ comparison between SB and TB cohorts is also shown in Table 1. The mean number of biopsy cores taken was superior in the SB cohort (16.2 versus 14.7, p < 0.001) compared with the TB cohort, but the mean number of positive cores was lower (5.4 versus 5.9, p = 0.016). Grade group 2 and 3 was reported in 52.1% and 22.6% of cases, respectively, in the TB cohort, compared with 39.1% and 13.9%, respectively, in the SB cohort (p < 0.001).
Pathological outcomes
Pathological features in RP specimens are reported in Table 2, as well as the comparisons between both cohorts. Main pathological features were comparable in the two cohorts. Grade group on final pathology did not differ significantly between the SB and TB cohorts (p = 0.751), as well as pT (p = 0.501) and pN stage (p = 0.101). Patients in the TB cohort had a lower risk of upgrading (31.0% versus 48.2%, p < 0.001) with an improved concordance rate (53.2% versus 43.5%) compared with those included in the SB cohort. The proportion of high-grade disease within each risk group significantly differed according to the biopsy pathway. The rate of grade group ≥ 3 or ≥ 4 disease was significantly lower in the TB cohort. In the SB cohort, 22.2% of grade group ≥ 3 disease was reported in the low-risk group, as compared with only 8.9% in the TB cohort low-risk group (p < 0.001).
Recurrence-free survival outcomes
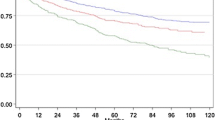
Mean follow-up after RP was 26.6 months, and comparable between the two cohorts (25.8 versus 27.0 months, p = 0.125). RFS curves did not differ significantly between the SB and the TB cohorts (Fig. 1a, log rank test; p = 0.538). The 2-year RFS rates were 84.9% and 88.1% in the SB and TB cohorts, respectively.
a Biochemical recurrence-free survival curves (bRFS) stratified by the biopsy pathway: standard (SB) versus targeted biopsy (TB) cohort (p = 0.538). b Biochemical recurrence-free survival curves (bRFS) stratified by the risk group classification and by the cohort. LR low risk, IR intermediate risk, HR high risk
Figure 1b shows the RFS curves stratified by two factors, the EAU risk classification and the cohort. In the SB cohort, the EAU risk classification was also significantly predictive for PSA outcomes in the overall cohort (p < 0.001). The risk of biochemical recurrence was increased by fourfold in intermediate (95% CI 1.6–10) and by 15.7-fold (95% CI 6.2–39.8) in high-risk groups, compared with low-risk group.
In the TB cohort, the EAU risk classification was also significantly predictive for PSA outcomes in the overall cohort (p < 0.001). However, only the high-risk group curve was significantly different with a HR 6.5 (95% CI 1.5–27.5, p = 0.011). Indeed, the risk of biochemical recurrence did not differ significantly between the low- and the intermediate-risk group (p = 0.791; HR 1.2; 95% CI 0.28–5.2) in the TB cohort.
PSA, clinical stage and grade group on biopsy were included into a multivariable Cox regression model in the TB cohort (Table 3). The aim was to develop a new risk classification to improve the accuracy of the prognostic model within the TB cohort. The new risk groups were defined as follows: PSA < 10 and grade group 1–2 and T1–T2a clinical stage (low risk); PSA 10–20 or grade group 3 or T2b clinical stage (intermediate risk); PSA ≥ 20 or ≥ T2c clinical stage or grade group 4–5 (high risk).
The new risk classification outperformed the EAU risk classification in the TB cohort for predicting biochemical recurrence (Fig. 2a). The risk of biochemical recurrence was increased by fourfold in intermediate (p = 0.009) and by 15-fold (p < 0.001) in high-risk groups, compared with low-risk group (Table 3).
a Biochemical recurrence-free survival curves (bRFS) in the TB cohort, stratified by the new classification. LR low risk: PSA < 10 and grade group 1–2 and T1–T2a clinical stage; IR intermediate risk PSA 10–20 or grade group 3 or T2b clinical stage; HR high risk: PSA ≥ 20 or T3 clinical stage or grade group 4–5. b Biochemical recurrence-free survival curves (bRFS) in the TB cohort, stratified by the new 4-group classification. LR low risk: PSA < 10 and grade group 1–2 and T1–T2a clinical stage; IR intermediate risk: PSA 10–20 or grade group 3 or T2b clinical stage; HR high risk: PSA ≥ 20 or grade group 4; VHR very high risk: ≥ T2c clinical stage or or grade group 5
Then, we developed a new four-group classification to better define patients at very high risk of recurrence (Fig. 2b, log rank test: p < 0.001). The four risk groups were defined as follows: PSA < 10 and grade group 1–2 and T1–T2a clinical stage (low risk); PSA 10–20 or grade group 3 or T2b clinical stage (intermediate risk); PSA ≥ 20 or grade group 4 (high risk); ≥ T2c clinical stage or grade group 5 (very high risk). The risk of biochemical recurrence was significantly increased by fourfold in intermediate, by ninefold in high-risk groups, and by 29-fold in very high-risk groups, compared with low-risk group (p < 0.001, Table 3). The overall 2-year recurrence rates significantly differed between new classification groups: 1.9%, 8.7%, 16.9%, and 42.9% in the low-, intermediate-, high-, and very high-risk groups, respectively. AUC for predicting recurrence was calculated for each classification. The AUC of the EAU risk classification was 0.686 compared with 0.734 for the AUC of the new risk classification. The improvement of AUC obtained by the new risk classification compared with the EAU classification was strictly comparable in both centres: + 0.048 in centers 1 and 2.
Discussion
MRI followed by TB directed to suspicious lesions has also been shown to optimize PCa prognostic assessment and risk stratification compared with SB alone [8, 13,14,15,16,17,18,19]. However, despite the growing body of knowledge highlighting the benefits of performing MRI and MRI-TB to SB, understanding is still lacking on the effects of their implementation into patient care and prognostic assessment of newly diagnosed disease. Thus, the pathway including MRI followed by TB improves risk stratification of patients suspected of having PCa and has the potential to alter clinical decision-making for PCa patients. However, to date, treatment decision-making is mainly based on risk classifications that have been defined and validated in cohorts of patients undergoing systematic, not imaging-targeted biopsies [1,2,3]. Current tools predicting recurrence after curative treatment and guiding PCa management are driven by the results of systematic, not imaging-guided biopsies. We acknowledge the fact that these models do not apply for targeted biopsies which improve the grade group assessment and thereby, modify risk stratification obtained by SB only. Thus, the accuracy of well-established risk classification should be re-assessed in cohorts of patients undergoing both SB and TB, or at least TB.
Our study corroborated current body of the literature in showing that the use of TB decreased the risk of upgrading, and improved the grading concordance between biopsy and RP specimens [20]. Patients in the TB cohort had a lower risk of upgrading (31.0% versus 48.2%) with an improved concordance rate (53.2% versus 43.5%) compared with those included in the SB cohort. This supported the improvement of initial assessment of suspected PCa in terms of diagnosis, staging, and appropriate PCa management. This also led to a significant variability in the number of patients within each cancer risk category due to TB when compared to SB (p < 0.001). Our findings were in line with previous published reports [21, 22].
Interestingly, although the SB and the TB were strictly comparable in terms of final pathology and PSA outcomes, prognosis of each risk category was statistically different according to the biopsy pathway. As expected, the standard risk classification accurately predicted recurrence outcomes in the SB groups, with three well-separated groups in terms of survival curves. However, such a stratification was not relevant in the TB cohort, with statistical similarity between low- and intermediate-risk groups (p = 0.791). This could be explained by a not negligible proportion of grade group 1 on SB that were reclassified as grade group 2 disease by MRI-targeted biopsies (61%).
Patients in the TB cohort had a lower risk of upgrading (31.0% versus 48.2%, p < 0.001) with an improved concordance rate (53.2% versus 43.5%) compared with those included in the SB cohort.
Widely used pre-treatment risk classification may no longer apply to men undergoing MRI-guided biopsy. And given the increasing utilization of MRI-guided biopsy in contemporary practice, we thought it was critical to revise prognostic tools to account for MRI-targeted biopsy.
Given this contamination between low- and intermediate-risk groups in the TB cohorts leading to modified prognosis as compared with that calculated in the SB cohort, we built new risk classification by pooling grade groups 1 and 2 cases into the same risk group. This led to three new well-separated survival estimates. We also developed a four-group classification to better identify patients at very high risk of early recurrence who could benefit from more aggressive adjuvant strategies.
Improved prognostic accuracy by TP may help for a better counseling of PCa patients during treatment decision-making and for anticipating the potential need for multimodal strategies in very high-risk disease. First, the added value of TB for risk stratification may also play a role on the decision to perform lymphadenectomy and on the guidance of its extent. Gandaglia et al. have recently updated the Briganti’s nomogram which defined the risk of lymph node involvement and the need for lymph node dissection [23]. The integration of MRI and TB into a new nomogram significantly improved its accuracy, in addition to other standard parameters. All patients in the present study had a positive MRI and underwent targeted biopsies. Therefore, we could not guarantee that this new risk classification was also accurate for patients having a negative MRI. It would be interesting to assess the performance of standard/new risk classifications in the subset of patients with negative MRI. Unfortunately, our dataset only included MRI-positive patients which represent about 80% of contemporary patients receiving biopsies according to recent prospective trials [4, 7].
Second, the improvement of risk stratification may modify treatment planning before radiotherapy decision-making. Reed et al. reported that TB led to intensified strategies in 16% of patients (Reed). Dix et al. demonstrated that TB resulted in 13% more androgen deprivation therapy regimens and in a 26% increase of more aggressive radiotherapy treatments compared to SB pathology results [22]. Nevertheless, no final pathology based on RP specimens was available in such series.
The main limitation was the lack of strong clinical endpoints, such as metastasis-free, cancer-specific or overall survival. However, the recommendations favouring the MRI pathway have been recently updated and we cannot get enough follow-up to assess such endpoints. Moreover, EAU guidelines support the use of such recurrence risk prediction tools for treatment decision-making [1]. The two-center design of our study may be a limitation. Nevertheless, all radiologists and biopsy operators involved in the study were highly experienced and beyond their learning curves since the beginning of the study period. The same fusion computer-assisted software was used in the two institutions which reduced interpretation biases [24]. Pathology was not formally re-reviewed, but was interpreted by senior, fellowship trained, uropathologists in both centers. Analyses were not altered after stratification by center status.
Conclusions
MRI-TB mainly leads to reclassification to a higher risk group, which is likely to be accompanied by important changes in risk group distribution and pre-treatment prognostic assessment compared with a diagnostic pathway only based on SB. Thus, the EAU risk group classification which is historically based on tumor grade obtained by SB does not accurately dichotomize low- and intermediate-risk PCa in terms of biochemical recurrence, when MRI and TB are integrated into the diagnostic pathway. The new easy-to-use three-group classification we propose includes grade group 2 cancer within the low-risk group and improves the risk prediction of biochemical recurrence for patients undergoing a TB-based diagnostic pathway. We have also built a four-group classification that better defines very high-risk patients (grade group 5 or clinical ≥ T2c disease) who could benefit from more aggressive adjuvant therapies. External studies and longer follow-up are needed to confirm the superiority of this new classification for recurrence prediction in the opening era of MRI and TB-driven biopsy pathway.
References
Mottet N, Bellmunt J, Bolla M et al (2017) EAU-ESTRO-SIOG guidelines on prostate cancer. Part 1: screening, diagnosis, and local treatment with curative intent. Eur Urol 71:618–629
Spratt DE, Zhang J, Santiago-Jiménez M, Dess RT, Davis JW, Den RB, Dicker AP, Kane CJ, Pollack A, Stoyanova R, Abdollah F, Ross AE, Cole A, Uchio E, Randall JM, Nguyen H, Zhao SG, Mehra R, Glass AG, Lam LLC, Chelliserry J, du Plessis M, Choeurng V, Aranes M, Kolisnik T, Margrave J, Alter J, Jordan J, Buerki C, Yousefi K, Haddad Z, Davicioni E, Trabulsi EJ, Loeb S, Tewari A, Carroll PR, Weinmann S, Schaeffer EM, Klein EA, Karnes RJ, Feng FY, Nguyen PL (2018) Development and validation of a novel integrated clinical-genomic risk group classification for localized prostate cancer. J Clin Oncol 36(6):581–590
D'Amico AV, Whittington R, Malkowicz SB et al (1998) Biochemical outcome after radical prostatectomy, external beam radiation therapy, or interstitial radiation therapy for clinically localized prostate cancer. JAMA 280:969–974
Kasivisvanathan V, Rannikko AS, Borghi M et al (2018) MRI-targeted or standard biopsy for prostate-cancer diagnosis. N Engl J Med 378:1767–1777
Siddiqui MM, George AK, Rubin R, Rais-Bahrami S, Parnes HL, Merino MJ, Simon RM, Turkbey B, Choyke PL, Wood BJ, Pinto PA (2016) Efficiency of prostate cancer diagnosis by MR/ultrasound fusion-guided biopsy vs standard extended-sextant biopsy for MR-visible lesions. J Natl Cancer Inst. https://doi.org/10.1093/jnci/djw039
van der Leest M, Cornel E, Israël B et al (2019) Head-to-head comparison of transrectal ultrasound-guided prostate biopsy versus multiparametric prostate resonance imaging with subsequent magnetic resonance-guided biopsy in biopsy-naïve men with elevated prostate-specific antigen: a large prospective multicenter clinical study. Eur Urol 75:570–578
Rouvière O, Puech P, Renard-Penna R et al (2019) Use of prostate systematic and targeted biopsy on the basis of multiparametric MRI in biopsy-naive patients (MRI-FIRST): a prospective, multicentre, paired diagnostic study. Lancet Oncol 20:100–109
Siddiqui MM, Rais-Bahrami S, Truong H et al (2013) Magnetic resonance imaging/ultrasound-fusion biopsy significantly upgrades prostate cancer versus systematic 12-core transrectal ultrasound biopsy. Eur Urol 64:713–719
Dell'Oglio P, Stabile A, Dias BH et al (2019) Impact of multiparametric MRI and MRI-targeted biopsy on pre-therapeutic risk assessment in prostate cancer patients candidate for radical prostatectomy. World J Urol 37:221–234
Rayn KN, Bloom JB, Gold SA et al (2018) Added value of multiparametric magnetic resonance imaging to clinical nomograms for predicting adverse pathology in prostate cancer. J Urol 200:1041–1047
Barentsz JO, Richenberg J, Clements R et al (2012) ESUR prostate MR guidelines 2012. Eur Radiol 22:746–757
Barentsz JO, Weinreb JC, Verma S et al (2016) Synopsis of the PI-RADS v2 guidelines for multiparametric prostate magnetic resonance imaging and recommendations for use. Eur Urol 69:41–49
Porpiglia F, Manfredi M, Mele F et al (2017) Diagnostic pathway with multiparametric magnetic resonance imaging versus standard pathway: results from a randomized prospective study in biopsy-naïve patients with suspected prostate cancer. Eur Urol 72:282–288
Gordetsky JB, Thomas JV, Nix JW et al (2017) Higher prostate cancer grade groups are detected in patients undergoing multiparametric MRI-targeted biopsy compared with standard biopsy. Am J Surg Pathol 41:101–105
Meng X, Rosenkrantz AB, Mendhiratta N et al (2016) Relationship between prebiopsy multiparametric magnetic resonance imaging (MRI), biopsy indication, and MRI-ultrasound fusion-targeted prostate biopsy outcomes. Eur Urol 69:512–517
Ploussard G, Beauval JB, Lesourd M et al (2019) Added value of concomitant systematic biopsies for grade group prediction based on radical prostatectomy final pathology in MRI-positive patients undergoing fusion targeted biopsies. J Urol. https://doi.org/10.1097/ju.418
Ouzzane A, Renard-Penna R, Marliere F et al (2015) Magnetic resonance imaging targeted biopsy improves selection of patients considered for active surveillance for clinically low risk prostate cancer based on systematic biopsies. J Urol 194:350–356
Ploussard G, Beauval JB, Lesourd M et al (2019) Performance of systematic, MRI-targeted biopsies alone or in combination for prediction of unfavourable disease in MRI-positive low-risk prostate cancer patients eligible for active surveillance. World J Urol. https://doi.org/10.1007/s00345-019-02848
Hu JC, Chang E, Natarajan S et al (2014) Targeted prostate biopsy in select men for active surveillance: do the Epstein criteria still apply? J Urol 192:385–390
Turkbey B, Mani H, Shah V et al (2011) Multiparametric 3T prostate magnetic resonance imaging to detect cancer: histopathological correlation using prostatectomy specimens processed in customized magnetic resonance imaging based models. J Urol 186:1818–1824
Reed A, Valle LF, Shankavaram U et al (2017) Effect of prostate magnetic resonance imaging/ultrasound fusion-guided biopsy on radiation treatment recommendations. Int J Radiat Oncol Biol Phys 97:947–951
Dix DB, McDonald AM, Gordetsky JB et al (2018) How would MRI-targeted prostate biopsy alter radiation therapy approaches in treating prostate cancer? Urology 122:139–146
Gandaglia G, Ploussard G, Valerio M et al (2019) A novel nomogram to identify candidates for extended pelvic lymph node dissection among patients with clinically localized prostate cancer diagnosed with magnetic resonance imaging-targeted and systematic biopsies. Eur Urol 75:506–514
Cornud F, Roumiguié M, Barry de Longchamps N et al (2018) Precision matters in MR imaging-targeted prostate biopsies: evidence from a prospective study of cognitive and elastic fusion registration transrectal biopsies. Radiology 287:534–542
Author information
Authors and Affiliations
Contributions
Protocol/project development: GP, BM, MR. Data collection or management: GP, CM, JB, ML, CA, JG, GL, AS, MS, CT, BM, MR; data analysis: GP, MR; manuscript writing/editing: GP, MR.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical standards
All procedures performed in studies involving human participants were in accordance with the ethical standards.
Informed consent
Informed consent was obtained.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ploussard, G., Manceau, C., Beauval, JB. et al. Decreased accuracy of the prostate cancer EAU risk group classification in the era of imaging-guided diagnostic pathway: proposal for a new classification based on MRI-targeted biopsies and early oncologic outcomes after surgery. World J Urol 38, 2493–2500 (2020). https://doi.org/10.1007/s00345-019-03053-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00345-019-03053-6