Abstract
Purpose
The aim of the present study is to investigate the impact of the near-infrared (NIRF) technology with indocyanine green (ICG) in robotic urologic surgery by performing a systematic literature review and to provide evidence-based expert recommendations on best practices in this field.
Methods
All English language publications on NIRF/ICG-guided robotic urologic procedures were evaluated. We followed the PRISMA (Preferred Reporting Items for Systematic Review and Meta-Analyses) statement to evaluate PubMed®, Scopus® and Web of Science™ databases (up to April 2019). Experts in the field provided detailed pictures and intraoperative video-clips of different NIRF/ICG-guided robotic surgeries with recommendations for each procedure. A unique QRcode was generated and linked to each underlying video-clip. This new exclusive feature makes the present the first “dynamic paper” that merges text and figure description with their own video providing readers an innovative, immersive, high-quality and user-friendly experience.
Results
Our electronic search identified a total of 576 papers. Of these, 36 studies included in the present systematic review reporting the use of NIRF/ICG in robotic partial nephrectomy (n = 13), robotic radical prostatectomy and lymphadenectomy (n = 7), robotic ureteral re-implantation and reconstruction (n = 5), robotic adrenalectomy (n = 4), robotic radical cystectomy (n = 3), penectomy and robotic inguinal lymphadenectomy (n = 2), robotic simple prostatectomy (n = 1), robotic kidney transplantation (n = 1) and robotic sacrocolpopexy (n = 1).
Conclusion
NIRF/ICG technology has now emerged as a safe, feasible and useful tool that may facilitate urologic robotic surgery. It has been shown to improve the identification of key anatomical landmarks and pathological structures for oncological and non-oncological procedures. Level of evidence is predominantly low. Larger series with longer follow-up are needed, especially in assessing the quality of the nodal dissection and the feasibility of the identification of sentinel nodes and the impact of these novel technologies on long-term oncological and functional outcomes.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Indocyanine green (ICG) is a water-soluble molecule which binds albumin and immediately allows visualization of both the vasculature and contours of anatomic structures. It has been approved for intravenous administration by FDA since 1959 for a broad range of surgical indications including hepatic function and cardiac output assessment [1].
The use of near-infrared fluorescence (NIRF) technology with ICG has been explored in several surgical specialties [2]. It is able to provide an enhanced anatomical view of the surgical field with potentially improved perioperative surgical outcomes without compromising the oncological adequacy [3,4,5,6,7].
In complex surgical cases, the proper identification of key anatomic structures is mandatory to achieve successful outcomes. Since 2011 the potential uses of NIRF with ICG for robotic urologic surgery have been investigated [8, 9] and the fusion between 3D visualization with the integrated Firefly® technology in the Da Vinci Surgical platform (Intuitive Surgical®, Sunnyvale, CA, USA) was found to be a valuable instrument that delivers improved surgical guidance for oncological and non-oncological procedures [10].
The aim of the present study is to systematically investigate the impact of the NIRF technology with ICG in robotic urologic surgery. As a complement to the present systematic review an expert consensus of world leaders in the use of ICG/NIRF in robotic urological surgeries was carried out to provide guidelines on best practices in this field.
Evidence acquisition
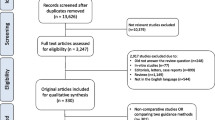
We performed a systematic review limited to articles in the English language, published until February 2019. A full update was performed on April 1st, 2019 (Fig. 1). A specific search on MEDLINE, Scopus, and Web of Science databases included “((indocyanine green fluorescence) OR ICG) OR NIRF OR Near Infrared Fluoroscopy) AND (((Robotic surgery) OR Urology))”.
All studies reporting data of interest were collected. Animal studies, editorials, commentaries, meeting abstracts, reviews, book chapters, studies reporting experimental studies on animals or cadavers were not included in the review. References were manually reviewed to identify additional studies of interest. Two authors (A.S. and A.T.) independently reviewed the literature using inclusion and exclusion criteria. All disagreements about eligibility were resolved by a discussion with a third reviewer (G.E.C) until a consensus was reached. This study was performed using guidelines set out by PRISMA (Preferred Reporting Items for Systematic Reviews and meta-analysis) statement [11]. All papers were distinguished according to the 2011 Oxford Centre for Evidence-based Medicine level of evidence for therapy studies [12]. All data retrieved from the systematically reviewed studies were recorded in an electronic database.
The composition of the consensus panel is made by expert researchers and clinicians who have published their experience in the use of NIRF/ICG in robotic urological procedures. The statements in Table 1 represent a “consensus on standard procedure” within the specific research groups on how NIRF/ICG can be used to enhance a given surgery.
In the present study, experts in the field provided detailed pictures and intraoperative video-clips of the NIRF/ICG-guided surgeries with recommendations for each procedure (Table 1). A unique QRcode was generated and linked to the underlying video-clip. This new exclusive feature makes the present the first “dynamic paper” that merges text and figure descriptions with their corresponding video, thus providing readers with an innovative, immersive, high-quality and user-friendly experience.
Evidence synthesis
Our electronic search identified a total of 576 papers in PubMed, Scopus, and Embase (Fig. 1). Of these, 243 publications were identified for detailed review, which yielded 36 studies included in the present systematic review reporting the use of NIRF and ICG in urological robotic procedures including (Supplementary Table): 13 robotic partial nephrectomy (RPN) series [8, 13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28], 7 robotic radical prostatectomy (RRP) [29] and lymphadenectomy (LND) series [30,31,32,33,34,35], 5 robotic ureteral re-implantation and reconstruction series [27, 36,37,38,39], 4 robotic adrenalectomy case series [40,41,42,43], 3 robotic radical cystectomy (RRC) series [44,45,46], 2 penectomy and robotic inguinal lymphadenectomy (RILND) series [47, 48], 1 robotic simple prostatectomy (RSP) series [49], 1 robotic kidney transplant (RAKT) series [50], and 1 localization of the ureter during robotic sacrocolpopexy series [37].
NIRF/ICG-guided selective perfusion assessment and renal mass differential fluorescence during robotic partial nephrectomy (Fig. 2a–d)
A total of 13 papers reported the use of NIRF/ICG during RPN [8, 13,14,15,16,17,18,19,20,21,22,23,24,25,26]. Two main applications have been reported: perfusion assessment [13, 16, 18, 19, 22, 24,25,26,27] and differential ICG uptake during renal mass resection [8, 13,14,15, 17, 18, 28].
NIRF/ICG-guided robotic urological surgeries. Left side: white light; right side: NIRF/ICG. a, b NIRF/ICG-guided vascular assessment during selective clamping robotic partial nephrectomy; c, d NIRF/ICG-guided tumor resection during robotic partial nephrectomy; e, f NIRF/ICG-guided vascular assessment of ileal segment during robotic radical cystectomy; g, h NIRF/ICG-guided ureteral isolation and vascular assessment during robotic radical cystectomy; i, j NIRF/ICG-guided ureteral vascularization during robotic ureteral re-implantation for benign stricture. (A linked video-clip is available scanning the QRcode on the right. For apple users: open the Camera app from your devices. Hold your device so that the QR code appears in the Camera app’s viewfinder. Your device recognizes the QR code and shows a notification. Tap the notification to open the link associated with the QR code. For Android users: download a QRcode scanner app and follow the above instructions)
In 2011 Tobis et al. first investigated the utility NIRF/ICG during 11 RPNs to identify the renal vasculature and to delineate cortical renal tumors from surrounding parenchyma. The median number of ICG injections was 3 (range 0.75–7.5 mg ICG per injection). No positive margins were reported. At the end of each case the surgeon rated the ICG-guided RPN; scoring the ability to differentiate the tumor from surrounding renal parenchyma (8/11) and the ability to characterize the renal vasculature (11/11) [8]. The same group confirmed their preliminary findings showing that the NIRF/ICG-guided minimally invasive PN may be useful to differentiate tumors from normal parenchyma [14]. Moreover, the author speculated that NIRF/ICG could discriminate between benign (isofluorescent or hyperfluorescent) and malignant (hypofluorescent) lesions.
Opposing findings were reported in this regard by Manny et al. A total of 100 RPN were reviewed. Their protocol consisted in a single ICG dose of 5–7.5 mg before vascular clamping. In determining malignant vs benign lesions, hypofluorescence had a positive predictive value of 87%, negative predictive value of 52%, sensitivity of 84%, and specificity of 57%. The authors concluded that ICG cannot predict malignancy in RPN [17].
Krane et al. compared white light RPN vs NIRF/ICG RPN in 94 patients with renal masses. The ICG dose used was 5–7.5 mg. The authors did not find a significant difference in term of positive surgical margins (8.5% vs 6%, respectively). The mean ischemia time was shorter in the NIRF/ICG group (17 min vs 15 min, p = 0.03); however, no differences in postoperative functional outcomes were noted [13].
Angell et al. investigated the optimal ICG dosing in assessing the utility of NIRF/ICG for differential fluorescence. In their study 79 patients underwent RPN with NIRF/ICG. Their protocol consisted of a minimum of two ICG doses, including an initial test dosage and a re-dosing just before vessel clamping for renal mass resection. With a median initial dose of 1.25 mg (0.62–2.5 mg) and a median re-dose of 1.875 mg (0.625–5.0 mg) the differential fluorescence was effectively achieved in 65 of 79 tumors (82%) [15].
Borofsky et al. underlined the utility of NIRF/ICG to facilitate super-selective arterial clamping in 34 patients undergoing zero-ischemia RPN. A matched pair analysis was performed by matching 27 patients undergoing zero-ischemia RPN with super-selective arterial clamping to 27 patients undergoing conventional RPN by the same surgeon. The author found comparable outcomes between cohorts, except for longer operating time (256 vs 212 min, p = 0.02) and less eGFR% loss (− 1.8% vs − 14.9%, p = 0.03) in the zero-ischemia cohort [16].
Confirmatory results were found by Harke et al. who compared the on clamp-RPN with selective clamping with the use of NIRF/ICG. After intravenous injection of 2.5 mg/ml (with a maximum dosage of 2 mg/kg), NIRF/ICG-selective clamping RPN was carried out in 15 patients. Comparing short-term renal function outcomes, there was significantly less eGFR% loss in the NIRF/ICG-selective clamping group with an absolute loss of 5.1 vs 16.1 ml/min in the on clamp-RPN cohort (p = 0.045).
McClintock et al. looked at the differences in renal functional outcomes between RPN and selective arterial clamping with the use of NIRF/ICG by performing a matched-pair comparison of patients that received intravenous injection of 5–7.5 mg ICG. From this, they found the use of NIRF imaging to be beneficial in improving short-term eGFR at the time of patient discharge (78.2 vs 68.5 ml/min/1.73 m2; p = 0.04), absolute reduction of eGFR (− 2.5 vs − 14.0 ml/min/1.73 m; p < 0.01), and percent change in eGFR (− 1.9% vs − 16.8%; p < 0.01). However, there were no differences at 3 months of follow-up. Of the four minor complications recorded in the selective arterial clamping group, none were ICG-related [19].
Herz et al. attempted to analyze the utility of NIRF/ICG-guided selective arterial clamping in children undergoing robotic heminephrectomy (RHN). Additionally, they assessed whether real-time ICG-guided selective arterial clamping would in fact lead to improved surgical outcomes. Using a descriptive series of six children that underwent successful RHN, they found that NIRF/ICG-guided selective arterial clamping is safe without toxicity or vascular complications and feasible to perform in conjunction with RHN [22].
Lanchon et al. focused on the potential positive impact that NIRF/ICG-guided super-selective arterial clamping may have on functional renal outcomes. By conducting a prospective study examining patients undergoing successful RPN for treatment of a single tumor, they concluded that the addition of super-selective clamping leads to improved outcomes of renal function after RPN. Improved eGFR was noted at the time of patient discharge through 6 months of follow-up [24].
Mattevi et al. compared the functional and operative outcomes of 62 RPN with NIRF/ICG-guided selective arterial clamping vs a cohort of patients who underwent standard RPN (S-RPN) without selective arterial clamping. Selective clamping was achieved in 15 patients, due to inadequate ischemic appearance of the tumor during the NIRF/ICG-guided selective clamping. No major complications were reported in the NIRF/ICG-guided RPN group, while three acute hemorrhages were recorded in the S-RPN group. The analysis of renal scan data showed a greater loss of eGFR after S-RPN compared to NIRF/ICG-guided RPN (21.5% vs 5.5%; p = 0.046), as well as a greater total eGFR loss (8% vs 0%; p = 0.007) [25].
Simone et al. investigated the utility of the ICG in ten consecutive patients with totally endophytic renal masses and performed off-clamp RPN following super-selective transarterial delivery of ICG–lipiodol mixture (1.5 ml ICG + 0.75 ml lipiodol). Preoperatively, using a femoral approach, they marked the tumor with an ICG–lipiodol solution by performing super-selective angiographic catheterization of tertiary and quaternary renal arterial branches supplying the tumors to avoid rapid ICG washout. Intraoperatively, after the Gerota’s capsule incision, surgical margins were scored under NIRF/ICG. Once resection was completed, the lesion bed was examined, and ICG-marked areas were further excised. Surgical margins were negative in all cases and within 1-year follow-up, a median eGFR% decrease of 12.2% was noted [26].
NIRF/ICG-guided robotic radical prostatectomy and lymphadenectomy (Fig. 3e–f)
A total of seven studies evaluated the application of intraoperative NIRF/ICG during RRP [29,30,31,32,33,34,35, 51] six of which evaluated the role of indocyanine in lymph node evaluation during RRP with ePLND [30,31,32,33,34,35, 51].
NIRF/ICG-guided robotic urological surgeries. Right side: white light; left side: NIRF/ICG. a, b NIRF/ICG-guided robotic partial adrenalectomy; c, d NIRF/ICG-guided robotic groin lymphadenectomy for penile cancer; e, f NIRF/ICG-guided robotic lymphadenectomy during robotic radical prostatectomy; g–j NIRF/ICG-guided urethral vascular assessment during robotic simple prostatectomy; i–l NIRF/ICG-guided graft vascular assessment during robotic renal transplantation. (A linked video-clip is available scanning the QRcode on the right. For Apple users: open the Camera app from your devices. Hold your device so that the QR code appears in the Camera app’s viewfinder. Your device recognizes the QR code and shows a notification. Tap the notification to open the link associated with the QR code. For Android users: download a QRcode scanner app and follow the above instructions)
Mangano et al. investigated the use of NIRF/ICG to visualize the peri-prostatic neurovascular bundle [29]. After bladder neck incision and seminal vesicle dissection, a single dose of 1.25 ml of ICG was administrated allowing the visualization of the arteries within the neurovascular bundle. They concluded that the application of NIRF/ICG during RRP may be helpful to identify and preserve the neurovascular bundle [29].
During initial studies with the da Vinci S system Van der Poel et al., preoperatively injected ICG in the prostate of 11 patients before RRP. Here a formulation was used wherein ICG was non-covalently bound to the SN-specific nanocarrier molecule 99mTc-nanocolloid (standard sentinel node radiotracer in Europe), yielding the hybrid tracer ICG-99mTcNanoColloid (radioactive and fluorescent). Use of ICG-99mTc-nanocolloid (0.4 ml; 0.05 mg ICG and 0.1 mg nanocolloid) allowed for preoperative lymphoscintigraphy and single-SPECT/CT imaging of the tracer uptake, while intraoperative ICG fluorescence guidance could accurately identify pelvic nodes. The ex vivo evaluation of the lymph nodes (LNs) confirmed a correlation between the radioactive and fluorescent uptake. The authors concluded that ICG-99mTc-nanocolloid, in combination with laparoscopic fluorescence (Karl Stroz technology applied through the assistant port), facilitates and optimizes dissection of nodes during RRP [30]. KleinJan et al. refined the tracer administration and fluorescence laparoscope procedure by changing the conditions in two complementary patient groups. In group 2 (n = 13), the amount of ICG-99mTc-nanocolloid injected was increased to 2 ml; 0.25 mg ICG and 0.5 mg 99mTc-nanocolloid and the laparoscopic fluorescence imaging (LFI) system was updated to HD. In the group 3 (n = 16) the tracer formulation was identical to that used in group 2, but the filter settings of the LFI system were modified to allow fluorescence imaging in anatomical (white light) context. The value of these tracer and technical optimizations was reflected by the improvement in intraoperative fluorescence-based sentinel node identification, going from 63.7 to 85.2% and finally to 93.5% for groups 1, 2 and 3, respectively.
Following an upgrade to the da Vinci Si system the same group evaluated the same study setup (optimized ICG-99mTc-nanocolloid amount) in a similar patient group, but this time in using the Firefly laparoscope that is integrated into the da Vinci Si platform (standard fluorescence settings in 50 patients and with custom settings in 5 patients). With the Firefly camera intraoperative fluorescence imaging using standard fluorescence settings visualized 80.4% of sentinel LNs vs 85.7% with customized fluorescence settings. In the custom settings the intensity of the white light background in the fluorescence image could be reduced, which improved the detection accuracy: 78.6% vs 78.6% vs 85.7% for 30%, 15% and 0% white light background intensity, respectively [32]. Combined with the initial study this suggests that nodal identification improves when a camera allows for identification of fluorescence uptake in anatomical context, but that the sensitivity of detection increases when the fraction of white light background is reduced.
Chennamsetty et al. aimed to assess the ideal dosing and the value of fluorescent sentinel LN detection with ICG in detecting LN metastases in intermediate/high-risk prostate cancer patients who underwent RRP and PNLD after intraprostatic trans-perineal ultrasound-guided ICG injection. Patients were cycled through 5 doses (1.25, 2.5, 3.75, 5, and 7.5 mg). The median number of fluorescent LNs packets was 4.0, 6.0, and 4.5 for the respective doses of 3.75, 5.0, and 7.5 mg. Compared to the higher ICG doses, the 1.25 and 2.5 mg doses had fewer fluorescent LN packets and were abandoned. The NIRF/ICG showed 62% sensitivity, 50% specificity, 8% positive predictive value, and 95% negative predictive value in detecting LNs metastases. The authors concluded that lymph node dissection with ICG cannot be used as an alternative to ePLND [33].
During robotic pelvic lymphadenectomy and RRP in patients with intermediate/low-risk prostate cancer Van den Berg et al. evaluated the potential of multispectral fluorescence imaging with the above-mentioned hybrid tracer ICG-99mTc-nanocolloid and the free-dye fluorescein (visible fluorescence), using a Karl Storz NIRF laparoscope. They were able to identify 85.3% of the sentinel LNs using NIRF/ICG-99mTc-nanocolloid in 10/10 patients and visible fluorescence imaging with Fluorescein visualized 44.1% of lymphatic ducts in 8/10 patients. This study underlined the increase in nodal specificity that the 99mTc-nanocolloid carrier provides. They also concluded that multispectral fluorescence image-guided surgery is clinically feasible providing additional information during sentinel LN dissection [34]. A preclinical study from the same group demonstrates that similar multispectral imaging concepts are also feasible when using a Firefly NIRF laparoscope [52].
In a prospective randomized trial, Harke et al. evaluated 120 intermediate/high-risk prostate cancer patients divided into two groups to demonstrate the benefits of ICG/NIRF-guided ePLND (using a total of 2.5 mg of ICG injected transrectally into two basal, two apical and one central prostatic lobes before docking) compared to regular ePLND in RRP. There was a total yield of 2609 LNs with significantly more LNs after ICG-supported ePLND. Nodal metastases were detected in 6 patients in the control group (25 cancerous LN) vs 9 patients in intervention group (62 positive LN, p = 0.40). In seven of nine patients, ICG-ePLND identified at least one cancer-positive LN with a sensitivity of 78%. The authors concluded that the sensitivity was not sufficient to recommend stand-alone ICG-ePLND, nevertheless ICG-ePLND may help the surgeon in a better evaluation of lymphatic drainage as well as a more meticulous diagnostic approach [35].
NIRF/ICG-guided robotic adrenalectomy for benign and malignant adrenal masses (Fig. 3a, b)
Four studies assessed the role of intraoperative NIRF/ICG during robot-assisted adrenalectomy (RAA) for symptomatic benign adrenal lesions and malignant lesions [40,41,42,43].
Manny et al. examined if NIRF/ICG improved intraoperative tumor identification and excision during robotic partial adrenalectomy (RPA). All the cases were hypofluorescent during intraoperative ICG/NIRF evaluation. No cases showed positive surgical margins. The authors concluded that RPA with intraoperative NIRF/ICG is safe and feasible and may allow more precise resection of adrenal masses and encourage the use of adrenal-sparing surgery. However, they included only small lesions without cancerous features [40].
Colvin et al. evaluated the impact of NIRF/ICG in delineating adrenal tumor borders compared to conventional robotic white light in a cohort of 40 patients undergoing RAA. The ICG dose used was a 2.5 mg/ml ICG solution. NIRF/ICG was found to be superior in 46.5% of patients, equivalent in 25.6% and inferior in 27.9% of cases. Additionally, adrenocortical tumors were displayed more accurately on ICG imaging. The ICG dosage was sufficient to provide enough contrast between adrenal parenchyma and surrounding retroperitoneal tissues irrespective of the patient body mass index. They concluded that ICG is useful to guide the dissection and removal of adrenal tumors during RAA [41].
Sound et al. studied ten patients with benign and malignant lesions who underwent RAA. The total dose per patient ranged between 7.5 and 18.8 mg. NIRF/ICG imaging was found to be helpful in real-time delineation of the adrenal gland in eight out of ten procedures. In one patient who underwent a right retroperitoneal-RAA, there was significant background fluorescence from the liver and therefore, contrast distinction between the adrenal and retroperitoneal tissues was not possible. In the second patient a 6.5-cm adrenocortical neoplasm that did not show ICG fluorescence [42].
In a large, prospective series, Kahramangil et al. evaluated the NIRF/ICG use characteristics of different adrenal pathologic conditions and attempted to define the best clinical indications for each. Globally 100 patients were evaluated of which 96 patients underwent RAA and 4 patients underwent adrenal-sparing surgery. A single dose of 2 ml of ICG solution was used. A total of 74% of the tumors were hyperfluorescent. The contrast distinction between the tumor and the retroperitoneum was found to be better (41%), similar (27%), or inferior (32%) on ICG fluorescence compared with the non-fluoresced view. On multivariate logistic regression, the origin of adrenocortical tissue was the only predictor of hyper-fluorescence. The authors concluded that adrenal tumors have different fluorescence patterns according to histologic origin and the best utility of ICG was found in adrenocortical tumors and during cortical-sparing adrenalectomy [43].
NIRF/ICG-guided robotic ureteral re-implantation/reconstruction (Fig. 2i, j)
Four studies assessed the role of intraoperative NIRF/ICG during robotic ureteral re-implantation/reconstruction [27, 36, 38, 39].
In 2013, Lee et al. initially reported the use of NIRF/ICG in localizing ureteral strictures by instilling 25 mg of ICG in 10 ml of distilled water in either an anterograde and/or retrograde fashion into the ureter and subsequently performing robotic ureteroureterostomy in seven patients. NIRF/ICG was utilized when identification of the ureter was difficult due to inflammation or obliteration of dissection planes, or both. In addition, ICG fluorescence was used in the evaluation of the vascularity of the ureteral margins. There were no complications attributable to ICG use. During a short-term follow-up period, no patients had any clinical or radiographic evidence of stricture recurrence [36].
The same group confirmed their results with the use of NIRF/ICG and expanded their cohort by evaluating 25 patients who underwent robot-assisted ureteral reconstruction procedures including ureterolysis, pyeloplasty, ureteroureterostomy and ureteroneocystostomy. At a mean follow-up of 11.6 months no clinical or radiographic evidence of recurrent disease was found. They found ICG to be safe, easy to perform and reproducible when used during robot-assisted ureteral reconstruction by allowing real-time delineation of the ureter and discerning healthy ureter from diseased tissue [38].
Bjurlin et al. studied the use of ICG in robotic upper tract reconstruction. A dose of 5–10 mg of ICG was injected intravenously and NIRF was used to evaluate tissue perfusion before performing the anastomosis, re-implantation, or omental wrap during pyeloplasty, ureteral re-implantation, ureterolysis or ureteroureterostomy. No complications attributable to ICG were reported. They reported an overall success rate of 95.2%. Two of the patients who underwent ureterolysis failed to improve and required further surgical management [27].
Lee et al. studied the use of ICG and NIRF during robotic ureteroenteric re-implantation for the treatment of ureteroenteric strictures. Their cohort consisted of eight patients who underwent ten robotic ureteroenteric re-implantations. A dose of 25 mg of ICG dissolved in 10 ml of water was injected antegrade and/or retrograde into the lumen of the ureter and urinary diversion and assessed intraoperatively under NIRF. Seven patients had an ileal conduit and one had a Studer neobladder. Median stricture length was 2 cm. There were no complications related to ICG use. All the ureteroentertic re-implantations were clinically and radiological successful at a median follow-up of 29 months [39].
NIRF/ICG-guided robotic radical cystectomy for bladder cancer (Fig. 2e–h)
Three studies reporting the use of ICG and NIRF during cystectomy were reviewed [44,45,46].
In 2014 Manny and Hemal initially reported on the feasibility of identification of sentinel node drainage, and tumor marking, after submucosal intravesical ICG injection in ten patients, and mesenteric arcade identification after intravenous ICG injection in eight patients. They injected ICG in the bladder submucosa and detrusor circumferentially around the tumor before robotic docking and then recorded parameters describing the time course of tissue fluorescence and pelvic lymphangiography. Bladder tumor marking and identification of sentinel nodes were achieved in 9 out of 10 patients and at a median of 15 and 30 min, respectively, while mesenteric angiography was successful in 8 of 8 patients at a median of < 1 min after intravenous injection [44].
Chopra et al. underlined the utility of intraoperative ICG and NIRF in assessing the vascularity of bowel and avoiding mesenteric arcades when preparing to isolate a segment of bowel during intracorporeal ileal neobladder reconstruction after RRC [45].
Ahmadi et al. investigated the impact of the use of NIRF/ICG on decreasing complications after robotic radical cystectomy and intracorporeal urinary diversion. Intraoperative ICG was used to assess the vascular integrity of the distal ureter before the ureteroenteric anastomosis was performed. Specifically, they compared the incidence of ureteroenteric strictures between 132 patients in the non-ICG arm and 47 patients in the ICG arm. Patients in the non-ICG arm were found to have a lower stricture rate than patients in the ICG arm (10.6 vs 0%, p = 0.020) and had a greater length of ureter excised (2.7 vs 2.2 cm, p = 0.001) [46].
NIRF/ICG-guided robotic inguinal lymphadenectomy for penile cancer (Fig. 3c, d)
Two studies reported the feasibility and oncological adequacy of the use of NIRF with ICG-guided robotic inguinal lymphadenectomy (RILND) for penile cancer [47, 48].
Bjurlin et al. first described a novel technique of RILND in patients with penile cancer using NIRF with ICG to identify both superficial and deep inguinal nodes. According to the authors’ protocol, intradermal ICG was injected at the base of the penis (0.5 ml of 2 mg/kg). Fifteen minutes after the injection the lymphatic channels and nodes were visualized using the Firefly® technology integrated with the Da Vinci System. The novel technique was tested on ten groin LNDs in five patients. With a mean follow-up of 10 months, no postoperative infections, lymphatic leak, necrosis or wound breakdown was recorded [47].
Savio et al. reported their novel technique of combined partial penectomy with bilateral RILND using NIRF and ICG. 5 ml of ICG was injected subcutaneously below the tumor and bilateral robotic modified inguinal lymphadenectomy was performed using NIRF and ICG guidance via the DaVinci Firefly ® technology. The procedure was carried out without complications and a total of 24 nodes were retrieved [48].
NIRF/ICG-guided graft vascular assessment during robotic kidney transplant (Fig. 3i, j)
One study explored the utility of NIRF/ICG to assess ureteral and graft reperfusion in six patients undergoing RAKT [50]. After the completion of the vascular anastomoses and positioning of the graft within the extraperitoneal pouch, 0.3 mg/kg of ICG in 5% of glucose was injected. A few seconds later it was possible to examine the fluorescence at the level of renal parenchyma, ureter and vascular anastomosis. The NIRF/ICG fluorescence was successfully performed in all patients with mean duration of 15 min. At 12 month follow-up the mean eGFR was 64.2 ml/min/1.73 m2 [50].
NIRF/ICG-guided robotic urethra-sparing simple prostatectomy (Fig. 3g, h)
Simone et al. evaluated the selective dissection of prostatic lobes during urethra-sparing RSP. After dissecting the bladder neck and exposing the proximal prostatic urethra, 50 ml of ICG was instilled through an 18 Fr urethral catheter into the intraprostatic urethra. Prostatic adenoma dissection was performed using NIRF/ICG in order to spare the intraprostatic urethra. Continuous bladder irrigation was avoided in 83.4% of patients with a median hospital stay of 3 days and promising functional outcomes, including ejaculation preservation after 1 year follow-up [49].
NIRF/ICG during robotic sacrocolpopexy
One study was reviewed reporting the use of ICG during robotic sacrocolpopexy. Siddighi et al. examined the used of ICG for identification of the ureter during robotic sacrocolpopexy in > 10 patients. 25 mg of ICG dissolved in 10 ml of sterile water was injected retrograde into the ureter before commencing the robotic procedure. During the procedure, NIRF was use intermittently to allow definitive identification of the ureter throughout the case. In all patients they were able to visualize bilateral ureters; however, variations in brightness of fluorescence were noted depending on the depth of the ureter from the peritoneal surface. No complications due to ICG were noted [37].
Conclusion
NIRF/ICG technology has now emerged as a safe and feasible tool for an enhanced surgical experience. It has been shown to improve the identification of key anatomical landmarks and pathological structures for oncological and non-oncological procedures. The applications of NIRF/CG are versatile, where it can be injected intravenously to assess the vascularity of specific tissues or “in situ” directly into various organs to identify diseased parenchyma or assess the lymphatic pathways. This has made a significant impact on facilitating challenging reconstructive and oncologic robotic procedures. NIRF/ICG has been found to be useful during RPN in guiding selective/super-selective clamping of arteries, while differential fluorescence may play a role in discerning between pathological and normal renal tissue resulting in minimal renal parenchymal loss. The use of this technology during robotic surgery for different types of adrenal pathologies (pheochromocytoma, metastatic RCC, lymphangioma, adrenocortical adenoma, adrenal hemorrhagic cyst, adrenal simple cyst, cystic lymphangioma) is compelling in the identification of the mass, excision, and promoting the use of adrenal-sparing surgery. The utility of NIRF/ICG during ePLND at the time of RRP has been found to better-assist in understanding lymphatic drainage and in achieving a more scrupulous diagnostic approach. However, it cannot be used as an alternative to ePLND. Sentinel LN biopsy could decrease morbidity caused by ePLND and improve staging by targeted removal of cancerous LNs outside the standard ePLND template. Initial data show that multimodal NIRF/ICG-99mTc-NanoColloid, can be used to facilitate and optimize dissection of sentinel nodes during RRP procedures. Encouraging studies have shown that NIRF/ICG-guided robotic lymphadenectomy of the superficial and deep inguinal nodes for penile cancer may result in reduced morbidity. However, larger series with longer follow-up are needed to obtain more robust results assessing the quality of the nodal dissection and the feasibility of the identification of sentinel LNs and the impact of these novel techniques on long-term oncological and functional outcomes. While this technology assists the surgeon, the actual clinical benefit for the patient and in surgical training remains to be determined. Although the applications of ICG/NIRF in urology are promising, the level of evidence is low. Further investigations are needed to improve the understanding on the impact of NIRF/ICG. The integration of fluorescence imaging within upcoming robotic systems entering the market soon should also be contemplated.
References
Nair R, Aggarwal R, Khanna D (2011) Methods of formal consensus in classification/diagnostic criteria and guideline development. Semin Arthritis Rheum 41(2):95–105
Reinhart MB, Huntington CR, Blair LJ, Heniford BT, Augenstein VA (2016) Indocyanine green: historical context, current applications, and future considerations. Surg Innov 23(2):166–175
Veccia A, Antonelli A, Hampton LJ, Greco F, Perdona S, Lima E et al (2019) Near-infrared fluorescence imaging with indocyanine green in robot-assisted partial nephrectomy: pooled analysis of comparative studies. Eur Urol Focus. https://doi.org/10.1016/j.euf.2019.03.005
Huang SW, Ou JJ, Wong HP (2018) The use of indocyanine green imaging technique in patient with hepatocellular carcinoma. Transl Gastroenterol Hepatol 3:95
Newton AD, Predina JD (2018) Intraoperative fluorescence imaging in thoracic surgery. J Surg Oncol 118(2):344–355
Nie S, Low PS, Singhal S, Mangano A, Masrur MA, Bustos R et al (2018) Near-infrared indocyanine green-enhanced fluorescence and minimally invasive colorectal surgery: review of the literature. J Surg Oncol 33:77–83
Spinoglio G, Bertani E, Borin S, Piccioli A, Petz W (2018) Green indocyanine fluorescence in robotic abdominal surgery. Updates Surg 70(3):375–379
Tobis S, Knopf J, Silvers C, Yao J, Rashid H, Wu G et al (2011) Near infrared fluorescence imaging with robotic assisted laparoscopic partial nephrectomy: initial clinical experience for renal cortical tumors. J Urol 186(1):47–52
Basile G, Breda A, Gomez Rivas J, Cacciamani G, Okhunov Z, Dourado A et al (2019) Comparison between near-infrared fluorescence imaging with indocyanine green and InfraRed imaging: on-bench trial for kidney perfusion analysis. A project by ESUT-YAUWP group. Minerva Urol Nefrol 71(3):280–285. https://doi.org/10.23736/S0393-2249.19.03353-8
Autorino R, Zargar H, White WM, Novara G, Annino F, Perdona S et al (2014) Current applications of near-infrared fluorescence imaging in robotic urologic surgery: a systematic review and critical analysis of the literature. Urology 84(4):751–759
Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 6(7):e1000097
Howick J, Chalmers I, Glasziou P, Greenhalgh T, Heneghan C, Liberati A, Moschetti I, Phillips B, Thornton H (2016) Explanation of the 2011 Oxford Centre for Evidence-Based Medicine (OCEBM) Levels of Evidence (Background Document). Oxford Centre for Evidence-Based Medicine. http://www.cebm.net/index.aspx?o=5653. Accessed 01 Jan 2019
Krane LS, Manny TB, Hemal AK (2012) Is near infrared fluorescence imaging using indocyanine green dye useful in robotic partial nephrectomy: a prospective comparative study of 94 patients. Urology 80(1):110–116
Tobis S, Knopf JK, Silvers C, Messing E, Yao J, Rashid H et al (2012) Robot-assisted and laparoscopic partial nephrectomy with near infrared fluorescence imaging. J Endourol 26(7):797–802
Angell JE, Khemees TA, Abaza R (2013) Optimization of near infrared fluorescence tumor localization during robotic partial nephrectomy. J Urol 190(5):1668–1673
Borofsky MS, Gill IS, Hemal AK, Marien TP, Jayaratna I, Krane LS et al (2013) Near-infrared fluorescence imaging to facilitate super-selective arterial clamping during zero-ischaemia robotic partial nephrectomy. BJU Int 111(4):604–610
Manny TB, Krane LS, Hemal AK (2013) Indocyanine green cannot predict malignancy in partial nephrectomy: histopathologic correlation with fluorescence pattern in 100 patients. J Endourol 27(7):918–921
Harke N, Schoen G, Schiefelbein F, Heinrich E (2014) Selective clamping under the usage of near-infrared fluorescence imaging with indocyanine green in robot-assisted partial nephrectomy: a single-surgeon matched-pair study. World J Urol 32(5):1259–1265
McClintock TR, Bjurlin MA, Wysock JS, Borofsky MS, Marien TP, Okoro C et al (2014) Can selective arterial clamping with fluorescence imaging preserve kidney function during robotic partial nephrectomy? Urology 84(2):327–332
Bjurlin MA, McClintock TR, Stifelman MD (2015) Near-infrared fluorescence imaging with intraoperative administration of indocyanine green for robotic partial nephrectomy. Curr Urol Rep 16(4):20
Dominique I, Paparel P (2016) Indications and technique of intraveinous indocyanine green-based fluorescence during robotic-assisted partial nephrectomy. Prog Urol FMC 26(4):F76–F79
Herz D, DaJusta D, Ching C, McLeod D (2016) Segmental arterial mapping during pediatric robot-assisted laparoscopic heminephrectomy: a descriptive series. J Pediatr Urol 12(4):266.e1–266.e6
Arora S, Rogers C (2018) Partial nephrectomy in central renal tumors. J Endourol 32(S1):S63–S67
Lanchon C, Arnoux V, Fiard G, Descotes JL, Rambeaud JJ, Lefrancq JB et al (2018) Super-selective robot-assisted partial nephrectomy using near-infrared fluorescence versus early-unclamping of the renal artery: results of a prospective matched-pair analysis. Int Braz J Urol 44(1):53–62
Mattevi D, Luciani LG, Mantovani W, Cai T, Chiodini S, Vattovani V et al (2019) Fluorescence-guided selective arterial clamping during RAPN provides better early functional outcomes based on renal scan compared to standard clamping. J Robot Surg 13:391–396
Simone G, Tuderti G, Anceschi U, Ferriero M, Costantini M, Minisola F et al (2019) “Ride the green light”: indocyanine green-marked off-clamp robotic partial nephrectomy for totally endophytic renal masses. Eur Urol 75:1008–1014
Bjurlin MA, Gan M, McClintock TR, Volpe A, Borofsky MS, Mottrie A et al (2014) Near-infrared fluorescence imaging: emerging applications in robotic upper urinary tract surgery. Eur Urol 65(4):793–801
Mitsui Y, Shiina H, Arichi N, Hiraoka T, Inoue S, Sumura M et al (2012) Indocyanine green (ICG)-based fluorescence navigation system for discrimination of kidney cancer from normal parenchyma: application during partial nephrectomy. Int Urol Nephrol 44(3):753–759
Mangano MS, De Gobbi A, Beniamin F, Lamon C, Ciaccia M, Maccatrozzo L (2018) Robot-assisted nerve-sparing radical prostatectomy using near-infrared fluorescence technology and indocyanine green: initial experience. Urologia 85(1):29–31
van der Poel HG, Buckle T, Brouwer OR, Valdes Olmos RA, van Leeuwen FW (2011) Intraoperative laparoscopic fluorescence guidance to the sentinel lymph node in prostate cancer patients: clinical proof of concept of an integrated functional imaging approach using a multimodal tracer. Eur Urol 60(4):826–833
KleinJan GH, van den Berg NS, Brouwer OR, de Jong J, Acar C, Wit EM et al (2014) Optimisation of fluorescence guidance during robot-assisted laparoscopic sentinel node biopsy for prostate cancer. Eur Urol 66(6):991–998
KleinJan GH, van den Berg NS, de Jong J, Wit EM, Thygessen H, Vegt E et al (2016) Multimodal hybrid imaging agents for sentinel node mapping as a means to (re)connect nuclear medicine to advances made in robot-assisted surgery. Eur J Nucl Med Mol Imaging 43(7):1278–1287
Chennamsetty A, Zhumkhawala A, Tobis SB, Ruel N, Lau CS, Yamzon J et al (2017) Lymph node fluorescence during robot-assisted radical prostatectomy with indocyanine green: prospective dosing analysis. Clin Genitourin Cancer 15(4):e529–e534
van den Berg NS, Buckle T, KleinJan GH, van der Poel HG, van Leeuwen FWB (2017) Multispectral fluorescence imaging during robot-assisted laparoscopic sentinel node biopsy: a first step towards a fluorescence-based anatomic roadmap. Eur Urol 72(1):110–117
Harke NN, Godes M, Wagner C, Addali M, Fangmeyer B, Urbanova K et al (2018) Fluorescence-supported lymphography and extended pelvic lymph node dissection in robot-assisted radical prostatectomy: a prospective, randomized trial. World J Urol 36(11):1817–1823
Lee Z, Simhan J, Parker DC, Reilly C, Llukani E, Lee DI et al (2013) Novel use of indocyanine green for intraoperative, real-time localization of ureteral stenosis during robot-assisted ureteroureterostomy. Urology 82(3):729–733
Siddighi S, Yune JJ, Hardesty J (2014) Indocyanine green for intraoperative localization of ureter. Am J Obstet Gynecol 211(4):436.e1–436.e2
Lee Z, Moore B, Giusto L, Eun DD (2015) Use of indocyanine green during robot-assisted ureteral reconstructions. Eur Urol 67(2):291–298
Lee Z, Sterling ME, Keehn AY, Lee M, Metro MJ, Eun DD (2019) The use of indocyanine green during robotic ureteroenteric reimplantation for the management of benign anastomotic strictures. World J Urol 37:1211–1216
Manny TB, Pompeo AS, Hemal AK (2013) Robotic partial adrenalectomy using indocyanine green dye with near-infrared imaging: the initial clinical experience. Urology 82(3):738–742
Colvin J, Zaidi N, Berber E (2016) The utility of indocyanine green fluorescence imaging during robotic adrenalectomy. J Surg Oncol 114(2):153–156
Sound S, Okoh AK, Bucak E, Yigitbas H, Dural C, Berber E (2016) Intraoperative tumor localization and tissue distinction during robotic adrenalectomy using indocyanine green fluorescence imaging: a feasibility study. Surg Endosc 30(2):657–662
Kahramangil B, Kose E, Berber E (2018) Characterization of fluorescence patterns exhibited by different adrenal tumors: determining the indications for indocyanine green use in adrenalectomy. Surgery 164(5):972–977
Manny TB, Hemal AK (2014) Fluorescence-enhanced robotic radical cystectomy using unconjugated indocyanine green for pelvic lymphangiography, tumor marking, and mesenteric angiography: the initial clinical experience. Urology 83(4):824–829
Chopra S, de Castro Abreu AL, Berger AK, Sehgal S, Gill I, Aron M et al (2017) Evolution of robot-assisted orthotopic ileal neobladder formation: a step-by-step update to the University of Southern California (USC) technique. BJU Int 119(1):185–191
Ahmadi N, Ashrafi AN (2019) Use of indocyanine green to minimise uretero-enteric strictures after robotic radical cystectomy. BJU Int. https://doi.org/10.1111/bju.14733
Bjurlin MA, Zhao LC, Kenigsberg AP, Mass AY, Taneja SS, Huang WC (2017) Novel use of fluorescence lymphangiography during robotic groin dissection for penile cancer. Urology 107:267
Savio LF, Panizzutti Barboza M, Alameddine M, Ahdoot M, Alonzo D, Ritch CR (2018) Combined partial penectomy with bilateral robotic inguinal lymphadenectomy using near-infrared fluorescence guidance. Urology 113:251
Simone G, Misuraca L, Anceschi U, Minisola F, Ferriero M, Guaglianone S et al (2019) Urethra and ejaculation preserving robot-assisted simple prostatectomy: near-infrared fluorescence imaging-guided Madigan technique. Eur Urol 75(3):492–497
Vignolini G, Sessa F, Greco I, Cito G, Vanacore D, Cocci A et al (2019) Intraoperative assessment of ureteral and graft reperfusion during robotic kidney transplantation with indocyanine green fluorescence videography. Minerv Urol Nefrol 71(1):79–84
Xia L, Zeh R, Mizelle J, Newton A, Predina J, Nie S et al (2017) Near-infrared intraoperative molecular imaging can identify metastatic lymph nodes in prostate cancer. Urology 106:133–138
Meershoek P, KleinJan GH, van Oosterom MN, Wit EMK, van Willigen DM, Bauwens KP et al (2018) Multispectral-fluorescence imaging as a tool to separate healthy from disease-related lymphatic anatomy during robot-assisted laparoscopy. J Nucl Med 59(11):1757–1760
Author information
Authors and Affiliations
Contributions
Protocol/project development: GEC, AS, AT. Data collection or management: GEC, AS, AT, KG, JH. Data analysis: GEC, AS, AT. Manuscript writing/editing: GEC, AS, AT, NA, PAH, MG, GS, RC, GV, WCH, JT, EB, FWBL, HGP, LPV, AKH, AB, RA, RS, MA, MMD, ALCA.
Corresponding author
Ethics declarations
Conflict of interest
Disclosures Dr. Mihir Desai is a consultant for Auris Robotics and PROCEPT BioRobotics. Dr. Monish Aron is a consultant for Intuitive Surgical.
Informed consent
The study complied with the Declaration of Helsinki. All patients provided written informed consent prior to their surgical procedure.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Cacciamani, G.E., Shakir, A., Tafuri, A. et al. Best practices in near-infrared fluorescence imaging with indocyanine green (NIRF/ICG)-guided robotic urologic surgery: a systematic review-based expert consensus. World J Urol 38, 883–896 (2020). https://doi.org/10.1007/s00345-019-02870-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00345-019-02870-z