Abstract
Purpose
The mid-urethral synthetic sling (MUS) procedure has become the standard of care for treatment of female stress urinary incontinence. However, a small number of patients will have complications following MUS including failure, obstructive voiding, sling erosion, or chronic pain. This paper discusses the role of 2D and 3D ultrasound imaging in the evaluation of the female patient with complications following placement of a synthetic mid-urethral sling.
Results
The MUS is easily visualized as an echogenic structure on ultrasound and can be imaged by transperineal, transvaginal and introital approaches. Ultrasound allows dynamic assessment of the sling and can assist in the diagnosis of sling failure, obstruction, erosion and mesh related pain.
Conclusions
Pelvic floor ultrasound has an emerging role in the assessment of complications following MUS surgery. 3D ultrasound can assist the clinician in assessment of the complex patient with multiple slings or meshes in situ.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The mid-urethral synthetic sling (MUS) procedure has become the standard of care for treatment of female stress urinary incontinence with durable medium to long-term outcomes. Multiple studies have demonstrated the efficacy of the MUS with a low morbidity [1]. However, a small number of patients will have complications following MUS including failure, obstructive voiding, sling erosion, or chronic pain [2, 3]. These issues are especially topical with the current controversy regarding the safety of synthetic mesh use in vaginal surgery for treatment of pelvic organ prolapse.
Apart from a thorough history and careful physical examination, the evaluation of a patient with potential sling complications includes functional assessment (such as urinary flow study, post-void residual urine measurement, and urodynamics), cystourethroscopy, and imaging. For complex patients, multiple investigative modalities may be necessary to fully evaluate the situation and formulate a management plan.
Whilst there is ample gynaecologic literature on application of pelvic floor ultrasound including 3D/4D ultrasound in assessment of pelvic organ prolapse over the past 15 years [4], the urological audience may not be as aware of the potential value of ultrasound imaging in assessment of the suburethral sling and mesh complications. Ultrasound equipment is widely available, relatively low cost; and there is increasing interest in clinician-performed ultrasound. The examination is non-invasive, quick, and easily performed at the bedside or clinic setting.
This paper discusses the role of ultrasound imaging in the evaluation of the female patient with complications following placement of a synthetic mid-urethral sling.
Principles of ultrasound imaging of suburethral slings
The female pelvic floor can be imaged by transabdominal, transperineal, and transvaginal/introital ultrasound approaches (Fig. 1). The transperineal (sometimes referred to as ‘translabial’) approach is ideal as the examination is performed entirely externally and hence readily acceptable to most female patients and does not distort the pelvic organ anatomy/relationships compared to transvaginal approach (Fig. 2).
a, b Transducer placement for transperineal (translabial) and introital ultrasound (Fig. 1c)
The MUS is very echogenic and easily visualized on ultrasound compared to other imaging modalities such as CT scan and MRI where the mesh is generally difficult to visualize. The development of 3D and dynamic 3D (so-called ‘4D’) ultrasound technologies allows the assessment of sling and pelvic structures in multiple imaging planes, similar to what can be achieved with MRI (Fig. 3).
Furthermore, ultrasound allows real-time dynamic imaging of the sling and other pelvic structures with a better temporal resolution than single-plane functional MRI and can contribute to functional as well as anatomic assessment of sling problems.
Applications of pelvic floor ultrasound in sling complications
Sling failure
There is imaging evidence to suggest that the synthetic suburethral sling provides posterior support to the urethra, and in particular, there is compression of the urethra by the sling during cough/Valsalva demonstrated on real-time imaging, which suggest that this is a mechanism of action of these types of sling (video 1) [5,6,7]. Ultrasound has a role in the evaluation of the patient who has ongoing stress incontinence following placement of a suburethral sling. The various parameters reported in the literature include distance of sling to urethra, sling to pubic symphysis, sling angle, and location of the sling relative to mid-urethra or bladder neck [4, 8].
Whilst the reported studies correlating these measurement parameters to clinical outcomes/success have been inconclusive [6, 9, 10], it is important to realize that there can be multiple reasons for sling failure rather than just inadequate compression or poor positioning. Imaging findings such as an open bladder neck or proximal urethra may suggest the presence of intrinsic urethral sphincter deficiency (Fig. 4a, b).
a Failed mid-urethral sling. Transperineal ultrasound in a patient with persisting urinary incontinence following mid-urethral sling surgery. Note open bladder neck/proximal urethra (arrow) due to intrinsic urethral sphincter deficiency. b Post-injection of bulking agent. Transperineal ultrasound in the same patient (as Fig. 4a) following injection of bulking agent (Bulkamid®, hypoechoic area—arrow) demonstrating coaptation of the proximal urethra above the level of the sling (s)
The lack of dynamic compression on imaging may suggest either loosening of the sling which may have occured if the patient return to vigorous activity early postoperatively (video 2) or, indeed, technical failure with poor positioning of the sling at the time of the procedure. Longitudinal ultrasound studies of sling location have shown that there is no significant change in sling position over time [11].
Obstructive sling
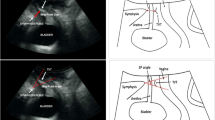
Patients with an obstructive suburethral sling may present with voiding dysfunction, elevated post-void residual, worsening of overactive bladder symptoms, or pain. A high index of suspicion is warranted in such patients. Whilst urodynamics remains the gold standard evaluation in male bladder outlet obstruction, there is currently no consensus regarding the urodynamic parameters for diagnosis of female bladder outlet obstruction. As a result, the diagnosis of sling obstruction is often a clinical decision based on de-novo symptomatology post-sling and the presence of elevated post-void residual urine. Ultrasound imaging adds to the evaluation armamentarium for the patient with an obstructive sling and can provide information on sling location, bladder/urethral axis, and dynamics of sling motion during rest and on Valsalva. This can be demonstrated easily with the echogenicity of the synthetic sling but can also be seen in patients with pubovaginal fascial slings (video 3). This is important especially as some patients are Valsalva voiders. A common finding in patients with an obstructive sling is angulation of the urethral axis (Fig. 5), especially if this is observed in the resting state [12]. Other sonographic findings reported in obstructive slings include a short distance between the sling and the urethra/pubic symphysis and abnormalities of sling configuration around the urethra [10, 12].
Sling erosion
Whilst sling exposure or extrusion into the vagina is easily assessed on clinical examination and erosion of the sling into the urethra is generally diagnosed on cystourethroscopy, these findings can also be demonstrated on ultrasound (Fig. 6) [13, 14]. Slings which have eroded into the urinary tract for a long period of time may become calcified and easily visible on ultrasound. Furthermore, information about the configuration of the arms of the sling outside the urethra obtained on imaging especially using 3D ultrasound volume reconstruction may assist the surgeon in operative management.
The patient with multiple slings
Both 2D and 3D ultrasound can be useful in assessment of the complex patient who has undergone several sling procedures or has persisting problems despite surgery for sling-related complications. In the patient who has persisting voiding dysfunction following sling division for obstruction, 3D ultrasound with multi-planar reconstruction can assess whether sufficient sling division has been achieved (Fig. 7a, b).
a and b Transperineal 3D ultrasound images in patient with persisting voiding dysfunction post-sling division demonstrating insufficient division of the sling with a segment of sling continuity (Fig. 7b)
3D TPUS is of particular value in the assessment of complex patients with multiple slings or other meshes in the anterior or apical compartment of the pelvis. 3D imaging can demonstrate the location of sling/s, whilst 2D imaging can demonstrate the dynamics of the slings with activity and can be useful if division or excision of one or more slings is planned (Fig. 8).
Assessment of pain
Pain following mesh procedures is often multi-factorial and best managed in the context of multi-disciplinary team and multi-modal approach. Imaging is of value in assessing the extent of mesh and the surrounding pelvic structures. 2D dynamic ultrasound can demonstrate the position of the mesh with movement, different postures, and activity. 3D ultrasound is of use in demonstrating mesh material and the relationship with pelvic organs. However, the retropubic or trans-obturator parts of the MUS may be difficult to visualize on ultrasound due to shadow artifact from adjacent bony structures. In the patient with suspected infected mesh material as the cause of their pain, an MRI scan is of value in detecting inflammatory changes around mesh (increased signal intensity on T2-weighted sequence). Transvaginal ultrasound can be used to correlate pain on examination with the presence or absence of mesh material at the site of pain (video 4). This information may be valuable to assist the surgeon in planning the optimal surgical approach and providing informed consent as a number of such patients will eventually undergo some excision of the mesh [13].
Conclusions
2D and 3D pelvic floor ultrasound has an emerging role in the evaluation of patients with complications following synthetic suburethral sling surgery. Imaging findings should be interpreted in the context of clinical presentation, physical examination, and urodynamic findings. 3D pelvic floor ultrasound can assist the clinician in the management of complex patients with sling complications, especially those who have multiple MUS or transvaginal prolapse meshes in situ.
References
Fusco F, Abdel-Fattah M, Chapple CR, Creta M, La Falce S, Waltregny D et al (2017) Updated systematic review and meta-analysis of the comparative data on colposuspensions, pubovaginal slings, and midurethral tapes in the surgical treatment of female stress urinary incontinence. Eur Urol 72(4):567–591
Blaivas JG, Purohit RS, Benedon MS, Mekel G, Stern M, Billah M et al (2015) Safety considerations for synthetic sling surgery. Nat Rev Urol. 12(9):481–509
Brubaker L, Norton PA, Albo ME, Chai TC, Dandreo KJ, Lloyd KL et al (2011) Adverse events over two years after retropubic or transobturator midurethral sling surgery: findings from the Trial of Midurethral Slings (TOMUS) study. Am J Obstet Gynecol 205(5):498e1–498e6
Shek KL, Dietz HP (2014) Imaging of slings and meshes. Australas J Ultrasound Med. 17(2):61–71
Shek KL, Chantarasorn V, Dietz HP (2010) The urethral motion profile before and after suburethral sling placement. J Urol 183(4):1450–1454
Hegde A, Nogueiras M, Aguilar VC, Davila GW (2017) Dynamic assessment of sling function on transperineal ultrasound: does it correlate with outcomes 1 year following surgery? Int Urogynecol J 28(6):857–864
Dietz HP, Wilson PD (2004) The ‘iris effect’: how two-dimensional and three-dimensional ultrasound can help us understand anti-incontinence procedures. Ultrasound Obstet Gynecol 23(3):267–271
Dietz HP (2011) Pelvic floor ultrasound in incontinence: what’s in it for the surgeon? Int Urogynecol J 22(9):1085–1097
Kociszewski J, Fabian G, Grothey S, Kuszka A, Zwierzchowska A, Majkusiak W et al (2017) Are complications of stress urinary incontinence surgery procedures associated with the position of the sling? Int J Urol 24(2):145–150
Takacs P, Larson K, Scott L, Cunningham TD, DeShields SC, Abuhamad A (2017) Transperineal sonography and urodynamic findings in women with lower urinary tract symptoms after sling placement. J Ultrasound Med 36(2):295–300
Majkusiak W, Pomian A, Tomasik P, Horosz E, Zwierzchowska A, Kociszewski J et al (2017) Does the suburethral sling change its location? Int J Urol 24:848–853
Larson K, Scott L, Cunningham TD, Zhao Y, Abuhamad A, Takacs P (2017) Two-dimensional and three-dimensional transperineal ultrasound findings in women with high-pressure voiding after midurethral sling placement. Female Pelvic Med Reconstr Surg. 23(2):141–145
Manonai J, Rostaminia G, Denson L, Shobeiri SA (2016) Clinical and ultrasonographic study of patients presenting with transvaginal mesh complications. Neurourol Urodyn 35(3):407–411
Staack A, Vitale J, Ragavendra N, Rodriguez LV (2014) Translabial ultrasonography for evaluation of synthetic mesh in the vagina. Urology. 83(1):68–74
Author information
Authors and Affiliations
Contributions
LC: project development, data collection, and manuscript writing. VT: data collection and manuscript editing.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no potential conflicts of interest to disclose.
Human participants or animals rights
This article does not contain any studies with human participants or animals performed by any of the authors.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary material 1 (AVI 7937 kb) Video 1 Transperineal 2D ultrasound of MUS imaged in para-sagittal plane showing dynamic compression of the mid-urethra by sling during coughing
Supplementary material 2 (AVI 23449 kb) Video 2 Transperineal ultrasound of patient with MUS who reported recurrence of urinary incontinence following heavy physical exercise at 3-weeks post surgery. Note lack of dynamic compression of the urethra suggesting that the sling may have become loose after exercise
Supplementary material 3 (AVI 21818 kb) Video 3 Transperineal ultrasound (imaged in sitting position) of patient with voiding dysfunction following pubovaginal fascial sling demonstrating marked angulation of proximal urethra/ bladder neck by sling during Valsalva
Supplementary material 4 (AVI 78750 kb) Video 4 Transvaginal ultrasound in patient with pelvic pain, previous MUS, and mesh repair of rectocele. Pain was reproduced by gentle pressure of transducer on MUS but not on posterior mesh—this patient subsequently underwent excision of sling with resolution of pain
Rights and permissions
About this article
Cite this article
Chan, L., Tse, V. Pelvic floor ultrasound in the diagnosis of sling complications. World J Urol 36, 753–759 (2018). https://doi.org/10.1007/s00345-018-2253-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00345-018-2253-3