Abstract
Purpose
While retroperitoneal lymph node dissection (RPLND) is traditionally reserved for nonseminomatous germ cell tumors, recent efforts to reduce long-term toxicities of radiation and chemotherapy have turned attention to its application for testicular seminomas. Currently, RPLND is reserved for the post-chemotherapy for stage II testicular seminomas; we aimed to describe current utilization of RPNLD for testicular seminomas by stage and implications for survival.
Methods
A national sample of men diagnosed with stage IA/IB/IS/IIA/IIB/IIC testicular seminoma (1988–2013) was evaluated from SEER Program registries. Stage-specific utilization of RPLND was determined. Cox proportional hazards models, adjusted for age, race, and radiotherapy, evaluated overall (OS) and cancer-specific survival (CSS) for the RPLND cohort. Adjusted models assessed predictors of RPLND.
Results
A total of 17,681 men (mean age 38.1 years) with testicular seminoma were included with low utilization of RPLND for stage I disease (1.3% overall) and higher rates for stage II disease (10.6% overall). There were no appreciable trends over time. Patients receiving RPLND did not appear to have worse OS or CSS on adjusted stage-by-stage analysis. Higher stage disease (IIA-IIC) was associated with greater need for RPLND while radiotherapy was associated with decreased use [OR 0.40 (0.32–0.51), p < 0.001].
Conclusions
Utilization of RPLND for testicular seminomas in the post-chemotherapy setting has remained stable over a 25-year period. Patients undergoing RPLND are a higher risk cohort but stage-by-stage survival outcomes appeared comparable to men not undergoing RPLND. Upcoming trials implementing RPLND as a first-line modality for testicular seminoma or isolated retroperitoneal relapse will help better quantify relative recurrence and survival.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Retroperitoneal lymph node dissection (RPLND) is traditionally reserved for nonseminomatous germ cell tumors due to the high cure rates of primary chemotherapy or radiotherapy for seminoma. An analysis of the Surveillance, Epidemiology, and End Results (SEER) Program through 2006 showed utilization of RPLND was about 20% in recent years for stage I disease but remained stable around 60% for stage II nonseminomatous germ cell tumors [1]. For primary testicular seminoma, the National Comprehensive Cancer Network (NCCN) uses a stage-stratified approach to recommend active surveillance, adjuvant radiotherapy, or adjuvant chemotherapy after orchiectomy without a role for RPLND in first-line management [2].
In recent years, radiotherapy and chemotherapy for testicular seminoma have been associated with long-term toxicities including the risk of secondary malignant neoplasms [3,4,5]. Consequently, use of radiotherapy has decreased from > 80 to < 20% for stage I disease but remains about 50% for stage IIA [4]. Therefore, attention has turned to the potential application of RPLND for the primary treatment of early-stage testicular seminoma in an effort to reduce long-term toxicity with a small, but acceptable, risk of short-term surgery-related side effects. A recent case series of 4 patients undergoing RPLND for lymph node positive seminoma experienced no recurrence at a mean of 25 months and two subsequent multicenter trials assessing efficacy of RPLND have been initiated [6, 7].
Currently, RPLND is reserved for the post-chemotherapy setting for stage II testicular seminomas and a potential option for the rare stage I patient who recurs and progresses despite other adjuvant therapy. Requiring a RPLND for testicular seminoma, therefore, may be thought to be a poor prognostic marker in the modern era, but survival outcomes have not been adequately studied in the past. Given the potential expansion of indications for RPLND in the future and recognizing that SEER registries lack information regarding prior chemotherapy, we aimed to describe current utilization of RPNLD for testicular seminoma by quantifying utilization of RPLND by stage, impact on survival compared to patients not undergoing RPLND, and predictors of undergoing a RPLND.
Methods
Patient population, variables, and outcomes
With Institutional Review Board approval, we identified men with stage I and stage II testicular seminoma from 1988 to 2013 from cancer registries captured by the SEER Program. Extragonadal germ cell tumors and patients with distant metastases were excluded. Demographic data including age, race, and year of diagnosis were obtained along with post-orchiectomy testicular seminoma staging data (IA, IB, IS, IIA, IIB, and IIC; AJCC, 7th Edition). Given known staging limitations of SEER data, patients prior to 2004 were assigned the most appropriate stage based on extent of disease, lymph node, and tumor marker data rather than predefined SEER AJCC categories. Patients after 2004 also had AJCC staging assignments adjusted based on T and N stage assignments. Sufficient data was not available to validly identify patients with stage III disease. The independent variable of interest was performance of RPLND. Outcomes included progression to performance of RPLND by stage, overall survival (OS), cancer-specific survival (CSS; deaths due to testicular cancer), and predictors associated with receiving a RPLND.
Statistical analysis
Baseline characteristic, including age, race, year of diagnosis, laterality, and stage, were tabulated. Utilization of RPLND over time was stratified by stage. Kaplan–Meier curves assessed OS and CSS based on receipt of RPLND. Univariable and multivariable Cox proportional hazards regression models were constructed to compare the RPLND cohort to patients not receiving a RPLND with adjustment for age, race, and radiotherapy. The effect of positive lymph node status was also assessed. Logistic regression models assessed predictors of receiving a RPLND. Multivariable logistic regression models were adjusted for statistically significant variables on univariable analysis. Statistical analyses were performed using STATA v.12.0 (STATA Corp, College Station, TX, 2011) with two-sided alpha set to 0.05.
Results
Cohort and utilization
A total of 17,681 patients diagnosed with primary stage I or stage II testicular seminoma were identified, of which 349 (2.0%) ultimately required a RPLND (Supplemental Table 1). A median of 14 nodes (mean 19.8; interquartile range 6–28.5) were evaluated per patient. About 1.3% of men with stage I disease ultimately underwent a RPLND compared to 10.6% with stage II disease (Supplemental Table 2). The proportion of men receiving a RPLND by stage was stable over time with no appreciable trends (Fig. 1).
Survival analysis
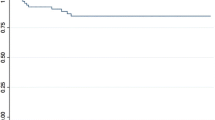
At a mean follow-up of 99.1 months (median 88 (interquartile range 40–144)), 791 (4.5%) of patients died from any cause including 114 (0.64%) from testis cancer. For the overall cohort, patients who required a RPLND appeared to have worse OS [HR 1.55 (1.03–2.32), p = 0.034] and CSS [HR 2.51 (1.09–5.78), p = 0.030] after adjusting for age, race, and radiotherapy (Table 1). Figure 2 shows Kaplan–Meier survival curves for the overall cohort. However, given that RPLND was more often performed for higher stage disease, a stage-by-stage analysis was performed and revealed no statistically significant differences in survival comparing those receiving a RPLND to those who did not. Patients found to have node positive disease experienced worse CSS [HR 3.60 (1.06–10.84), p = 0.039] although the difference in OS did not reach statistical significance [HR 1.79 (0.89–3.60), p = 0.100] when adjusted for age, race, and radiotherapy.
Predictors of RPLND
Logistic regression models did not show age to be a predictor of receiving a RPLND (Table 2). Adjuvant radiotherapy with the first course of therapy was associated with decreased progression to RPLND [HR 0.40 (0.32–0.51), p < 0.001]. Stage of disease was found to be the strongest variable associated with RPLND with odds ratios ranging between 8 and 10 for stage IIA, IIB, and IIC compared to stage IA.
Discussion
A very small proportion of patients with stage I testicular seminoma progress to requiring a RPLND while about 10.6% with stage II disease will eventually undergo RPLND. Patients in the post-chemotherapy setting requiring RPLND are a higher risk cohort, but stage-by-stage survival outcomes are notably similar with intermediate follow-up to patients who did not undergo RPLND. Initial stage of disease was the factor most strongly associated with receiving a RPLND as may be expected due to an increased risk of disease progression with higher stage. The findings suggest outcomes for patients with testicular seminoma who are able to undergo a post-chemotherapy RPLND are favorable and lend support to the notion that RPLND in the primary setting may be a viable alternative to explore due to known long-term risks associated with adjuvant chemotherapy and radiotherapy.
The management of stage I testicular seminoma has experienced a paradigm shift toward active surveillance due to data supporting low recurrence rates, excellent oncologic survival, and minimal need for salvage therapy [8]. For men with more advanced disease, chemotherapy is most often preferred modality due to excellent cure rates as a single modality treatment, although radiotherapy may be used 50% of the time for stage IIA seminoma [2, 4]. The rare patient who relapses or has residual mass(es) may be considered for RPLND or pursue second-line or salvage chemotherapy options. Long-term cardiovascular toxicity with increased risk of myocardial infarction has led to a risk-adapted approach for chemotherapy regimens for testicular seminoma while the increased incidence of secondary malignant neoplasms has tapered enthusiasm for radiotherapy [2,3,4]. RPLND is certainly not devoid of risks with historical complication rates around 20%, largely due to transfusion requirements and surgical site infections [9]. Post-chemotherapy RPLND, in particular, can be a technically challenging procedure with the potential to require performing en bloc resections and adjuvant procedures [10, 11]. However, contemporary series of primary RPLND at expert centers demonstrate much lower complications rates at about 7% [12, 13].
Although multiple guidelines with congruent recommendations exist, treatment variation continues to be pervasive for patients with testicular germ cell tumors [2, 14,15,16]. Part of the problem may be due to limited availability of some treatment options and uncertainty in weighing the pros and cons of different options. RPLND utilization has been shown to vary greatly across different community and academic cancer centers for nonseminomatous germ cell tumors, but practice for seminoma appears to be more consistent across centers [16]. While some argue that performance of RPLND should be restricted to high-volume centers, over half of RPLNDs appear to be performed by urologists logging only 1–2 cases in a year [17]. Practice variation may increase as potential indications for RPLND expand.
The present study is among the first to quantify the population-based rate of post-chemotherapy RPLND for patients with testicular seminomas and suggest largely favorable outcomes among all-comers requiring RPLND. One potential explanation is that RPLND exerts a therapeutic effect and salvage option in the post-chemotherapy setting improving survival sufficiently that a statistically significant detriment to survival could not be detected in the current sample size. However, some patients may be encountered who have additional poor prognostic markers; one of these may be viable seminoma at post-chemotherapy RPLND where the largest reported cohort of 36 patients was noted to have a 5-year CSS of 54% despite some patients undergoing additional resections and chemotherapy courses [18]. Additionally, there is minimal experience with the application of RPLND for primary treatment of testicular seminomas. One case series, including only 4 patients, reported favorable results and has led to two multi-institutional phase II trials evaluating recurrence-free survival [6]. Because perioperative outcomes for primary RPLND are more favorable than post-chemotherapy RPLND, demonstration of a durable recurrence-free survival may expand the indications for RPLND in testicular seminoma.
Limitations of the current study include its retrospective nature, lack of chemotherapy data in SEER, and inability to evaluate RPLND in the primary setting. However, it is well established that RPLND is currently performed exclusively in the post-chemotherapy setting for testicular seminoma with a prior study from the National Cancer Database using the same definition [16]. Data for staging was limited in earlier years for SEER leading to the decision to use 1988 as a cutoff, exclusion of earlier years, and adjustment of staging for included patients as previously mentioned. Lastly, SEER does not capture perioperative complications and morbidity that may be related to performance of RPLND. Some of the morbidity is related to surgical approach, but SEER does not capture this data; however, the appropriateness and selection criteria for a laparoscopic approach in the post-chemotherapy continues to be debated [19].
Despite the limitations, the analysis shows about 10.6% of men undergo post-chemotherapy RPLND for stage II testicular seminoma with favorable stage-by-stage survival outcomes compared to men not requiring RPLND. While there has traditionally been no experience with the use of primary RPLND for testicular seminoma, indications may be expanding with emerging data.
Conclusions
The utilization of RPLND for testicular seminomas in the post-chemotherapy setting has remained stable over a 25-year period. Patients receiving RPLND are a higher risk cohort, largely due to use for stage II disease, but stage-by-stage survival outcomes appeared comparable to men not requiring RPLND. Upcoming trials implementing RPLND as a first-line modality for testicular seminoma or for isolated retroperitoneal relapse will help better quantify relative recurrence and survival.
References
Sun M, Abdollah F, Budaüs L et al (2011) Trends of retroperitoneal lymphadenectomy use in patients with nonseminomatous germ cell tumor of the testis: a population-based study. Ann Surg Oncol 18(10):2997–3004
Motzer RJ, Jonasch E, Agarwal N, Beard C, Bhayani S, Bolger GB et al (2015) Testicular cancer, version 2.2015. J Natl Compr Canc Netw 13(6):772–799
Meinardi MT, Gietema JA, van der Graaf WT et al (2000) Cardiovascular morbidity in long-term survivors of metastatic testicular cancer. J Clin Oncol 18(8):1725–1732
Patel HD, Srivastava A, Alam R et al (2017) Radiotherapy for stage I and II testicular seminomas: secondary malignancies and survival. Urol Oncol. doi:10.1016/j.urolonc.2017.06.051
Travis LB, Fosså SD, Schonfeld SJ, McMaster ML, Lynch CF, Storm H et al (2005) Second cancers among 40,576 testicular cancer patients: focus on long-term survivors. J Natl Cancer Inst 97(18):1354–1365
Hu B, Shah S, Shojaei S, Daneshmand S (2015) Retroperitoneal lymph node dissection as first-line treatment of node-positive seminoma. Clin Genitourin Cancer. 13(4):e265–e269
Lusch A, Gerbaulet L, Winter C, Albers P (2017) Primary retroperitoneal lymph node dissection (RPLND) in Stage II A/B seminoma patients without adjuvant treatment: a phase II trial (PRIMETEST). Eur Urol Suppl. 16(3):e1899
Kollmannsberger C, Tandstad T, Bedard PL et al (2015) Patterns of relapse in patients with clinical stage I testicular cancer managed with active surveillance. J Clin Oncol 33(1):51–57
Patel HD, Ball MW, Cohen JE, Kates M, Pierorazio PM, Allaf ME (2015) Morbidity of urologic surgical procedures: an analysis of rates, risk factors, and outcomes. Urology 85(3):552–559
Djaladat H, Nichols C, Daneshmand S (2012) Adjuvant surgery in testicular cancer patients undergoing postchemotherapy retroperitoneal lymph node dissection. Ann Surg Oncol 19(7):2388–2393
Johnston P, Beck SD, Cheng L et al (2013) Incidence, histology and management of intraluminal thrombus at post-chemotherapy retroperitoneal lymph node dissection. J Urol 190(3):874–877
Williams SB, McDermott DW, Winston D, Bahnson E, Berry AM, Steele GS, Richie JP (2010) Morbidity of open retroperitoneal lymph node dissection for testicular cancer: contemporary perioperative data. BJU Int 105(7):918–921
Baniel J, Sella A (1999) Complications of retroperitoneal lymph node dissection in testicular cancer: primary and post-chemotherapy. Semin Surg Oncol 17(4):263–267
Beyer J, Albers P, Altena R et al (2013) Maintaining success, reducing treatment burden, focusing on survivorship: highlights from the third European consensus conference on diagnosis and treatment of germ-cell cancer. Ann Oncol 24(4):878–888
Nichols CR, Roth B, Albers P et al (2013) Active surveillance is the preferred approach to clinical stage I testicular cancer. J Clin Oncol 31(28):3490–3493
Hugen CM, Hu B, Jeldres C et al (2016) Utilization of retroperitoneal lymph node dissection for testicular cancer in the United States: Results from the National Cancer Database (1998–2011). Urol Oncol 34(11):487.e7–487.e11
Flum AS, Bachrach L, Jovanovic BD, Helenowski IB, Flury SC, Meeks JJ (2014) Patterns of performance of retroperitoneal lymph node dissections by American urologists: most retroperitoneal lymph node dissections in the United States are performed by low-volume surgeons. Urology 84(6):1325–1328
Rice KR, Beck SD, Bihrle R, Cary KC, Einhorn LH, Foster RS (2014) Survival analysis of pure seminoma at post-chemotherapy retroperitoneal lymph node dissection. J Urol 192(5):1397–1402
Daneshmand S, Gill IS (2013) Minimally invasive postchemotherapy retroperitoneal lymph node dissection: caution and prudence. Eur Urol. 63(6):1018–1019 (discussion 1020)
Author information
Authors and Affiliations
Contributions
HDP project development, data management, data analysis, manuscript writing/editing. GAJ: project development, manuscript editing. ZRS: project development, manuscript editing. AS: project development, manuscript editing, other (supervision). RA: project development, data management, manuscript editing. AS: project development, data management, manuscript editing. MEA: project development, manuscript editing, other (supervision). PMP: project development, manuscript editing, other (supervision).
Corresponding author
Ethics declarations
Conflict of interest
There are no financial conflicts of interest.
Research involving human participants and/or animals
The study was approved by the institutional review board for retrospective, de-identified analysis. No animals were involved.
Informed consent
The institutional review board waived informed consent given the retrospective, de-identified nature of the study using SEER data.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Patel, H.D., Joice, G.A., Schwen, Z.R. et al. Retroperitoneal lymph node dissection for testicular seminomas: population-based practice and survival outcomes. World J Urol 36, 73–78 (2018). https://doi.org/10.1007/s00345-017-2099-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00345-017-2099-0