Abstract
Purpose
Although the role of second transurethral resection of bladder tumor (TURB) is well established in high-risk non-muscle-invasive bladder cancer, to the best of our knowledge, there is no study regarding the role of a second transurethral resection (TUR) after a complete first TURB in multimodal therapy (MMT). The aim of this study was to evaluate the role of a second TUR on disease-specific survival (DSS) and overall survival (OS) rates in muscle-invasive bladder cancer (MIBC) patients who were treated with MMT.
Methods
We assessed the data of 90 patients (stage T2-4, N0-1, M0 urothelial cancer) who were treated with MMT at our clinic between January 2000 and June 2014. Patients with incomplete initial TURB were excluded. A total of 43 patients had a second TUR before starting radiochemotherapy of MMT (group 1), and 47 patients (group 2) were treated with MMT without having a second TUR. The impact of second TUR on DSS and OS rates was the primary outcome measure of the study.
Results
Mean (SD, range) age and mean follow-up of the patients were 65.1 (7.1, 52–81) years and 60.3 (38.3, 6–159) months, respectively. The two groups were similar with regard to sex, age, presence of hydronephrosis, lymph node involvement and stage. The 5-year DSS rate was better in group 1 compared to group 2 (68 vs. 41 %) (p = 0.046). The 5-year OS rates of the patients were 63.7 and 40.1 % in groups 1 and 2, respectively (p = 0.054). Multivariate analysis revealed that second TUR, lymph node involvement, presence of hydronephrosis and tumor stage were independent prognostic factors for DSS.
Conclusions
Second TUR should be performed in patients with MIBC who are going to be treated with bladder-preserving MMT protocols.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Radical cystectomy (RC) is the standard treatment modality for muscle-invasive bladder cancer (MIBC) patients. Although it provides long-term disease-specific survival (DSS) in a significant percentage of MIBC patients, RC is associated with a considerable morbidity and mortality rates [1, 2]. Recent interest in patients’ quality of life has promoted the trend toward bladder preservation with multimodal therapy (MMT) which combines transurethral resection of bladder tumor (TURB), chemotherapy and radiation [3–5].
In well-selected patients, MMT leads to acceptable outcomes with 5-year DSS rates of ≥50 % [5, 6]. In this treatment modality, many studies support the positive prognostic role of maximal TURB at the beginning of the treatment [3, 4]. In high-risk non-muscle-invasive bladder cancer (NMIBC), today we know that second TUR (re-TUR) has a positive impact on the long-term outcome of patients with respect to recurrence, progression and DSS [7, 8]. However, to our knowledge, there are no data evaluating the prognostic role of a second TUR in patients who will undergo MMT for MIBC.
In this study, we aimed to evaluate the role of a second TUR after a complete first TURB on DSS and overall survival (OS) rates in MIBC patients who were treated with MMT.
Materials and methods
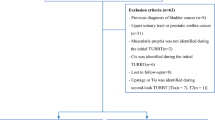
This single-institution, retrospective study consists of 90 patients with urothelial MIBC who were treated with MMT between January 2000 and June 2014. A total of 19 patients had an initial NMIBC diagnosis and progressed after BCG therapy. The eligibility criteria included patients with MIBC, stage T2-4, N0-1, M0 urothelial cancers, according to the American Joint Committee on cancer staging system. All patients were evaluated by history, physical examination, complete blood cell count, blood chemistry, and chest radiography and computed tomography (CT) of the abdomen and pelvis. Patients were provided with informed consent. Patients who received either radiotherapy (RT) or chemotherapy in a neoadjuvant setting for planned RC and who had incomplete initial TURB and concomitant malignancies were excluded.
In all patients, macroscopically complete resection was performed including the edges of the resection area. The underlying bladder wall with the detrusor muscle was also resected. Biopsies were taken from abnormal-looking urothelium. After this stage, a second TUR within 2–6 weeks after initial resection was performed if the patient approved. This second TUR included resection of the primary tumor site and any other tumor in the bladder which was not detected in the first TURB. A total of 43 patients had a second TUR before starting chemoradiotherapy of MMT (group 1), and 47 patients (group 2) were treated with MMT without having a second TUR.
After TURB with or without second TUR, patients received 64–66 Gy (fraction dose, 200 cGy/day) radiation to the small pelvis over 4-week time with two cycles of concurrent cisplatin (20 mg/day for 5 days) during the first and fourth weeks of RT. Carboplatin was given to patients who were not fit for cisplatin treatment. Four weeks after the completion of MMT, the response was evaluated by cystoscopy, urine cytology, TUR and CT scan. Side effects were also recorded. Clinical complete response was defined as no tumor palpable on bimanual examination under anesthesia (BEUA), no tumor visible on cystoscopy, negative tumor site biopsy and negative urine cytology. Patients with conserved bladders were followed up with cystoscopy, tumor site biopsy, BEUA, urine cytology and CT. Cystoscopic surveillance was performed every 3 months in the first year, every 3–4 months in the second year, every 6 months for 3 years and then annually. Patients with NMIBC local failures were treated with intravesical therapy, and those with a muscle-invasive local failure underwent salvage RC. The impact of second TUR on DSS and OS rates was the primary outcome measure of the study.
Statistical analyses
All statistical analyses were performed with SPSS version 16.0 (Chicago, IL). Normal distribution of the data was investigated by the Kolmogorov–Simirnov test, and data were expressed as the mean ± SD. Survival curves were constructed by the Kaplan–Meier method. Univariate and multivariate logistic regression analyses were carried out to determine associations between the variables. For statistical significance, p values of <0.05 were accepted.
Results
Among the 90 patients, second TUR was performed in 43 (47.8 %) patients (group 1) and was not performed in 47 (52.2 %) patients (group 2). Patient characteristics and comparison of the two groups are shown in Table 1. There were no differences between the groups in age, sex, T stage, lymph node involvement, presence of hydronephrosis and mean follow-up period (Table 1). In 6 patients (6.7 %), cystectomy was required ultimately (two patients in group 1 and four patients in group 2).
The 5-year DSS rates of the patients were 68.8 and 41.5 % in groups 1 and 2, respectively (p = 0.046, Fig. 1). In group 1, 29 (67.4 %) patients had residual tumor at their second TUR specimen. This 29 patients exhibited worse 5-year DSS rates compared to 14 patients who had no residual tumor at their second TUR specimen, but the difference was not statistically different (5-year DSS rates 51 and 75 %, respectively, p = 0.242). The 5-year OS rates of the patients were 63.7 and 40.1 % in groups 1 and 2, respectively (p = 0.054, Fig. 2).
On univariate analysis, tumor stage (p = 0.006, OR 2.3, 95 % CI 0.659–9.348), presence of hydronephrosis (p = 0.001, OR 3.4, 95 % CI 1.135–9.631), lymph node involvement (p = 0.001, OR 2.9, 95 % CI 0.914–8.743) and not performing a second TUR (p = 0.001, OR 2.5, 95 % CI 0.548–6.647) were associated with worse DSS. Tumor stage (p = 0.001, OR 3.1, 95 % CI 0.246–8.048), presence of hydronephrosis (p = 0.0001, OR 4.1, 95 % CI 1.562–7.634) and lymph node involvement (p = 0.001, OR 2.1, 95 % CI 0.862–9.512) were identified as predictors of worse OS on univariate analysis.
On multivariate analysis, lymph node involvement, presence of hydronephrosis, not performing a second TUR and higher stage were detected as predictors of worse DSS (Table 2). We also identified lymph node involvement, presence of hydronephrosis and higher stage as predictors of OS on multivariate analysis. However, performing second TUR was not detected as a predictor of OS on multivariate analysis (Table 3).
Discussion
In this retrospective study with a mean follow-up of 60.3 months, performing a second TUR after an initial complete TURB revealed a significantly better 5-year DSS rate of 68.8 % compared with 41.5 % in those who did not have a second TUR (p = 0.046). On multivariate analysis, performing a second TUR was also found to be a significant predictor of DSS. Indeed, the effect of performing a second TUR on recurrence and progression rates in high-risk NMIBC patients is well known and accepted by the urology community [7, 8]. However, the effect of second TUR on the MMT outcome of MIBC patients who were treated was not studied previously. To our knowledge, although retrospective, this is the first study demonstrating the effect of second TUR in patients who had invasive bladder cancer and planned to be treated with MMT. Herr HW published a retrospective series assessing 151 MIBC patients after restaging TUR (second TUR) and a minimum follow-up >10 years [9]. T0 and T1 disease was found in 99 patients, and they managed with active surveillance and had a comparable outcome to those reported in the remaining 52 patients undergoing radical cystectomy. Similar to our study, they have demonstrated a residual tumor in second TUR as a negative prognostic factor. A total of 18 % of patients who were treated by TUR alone with T0 disease in second TUR have died because of bladder cancer. This rate was 42 % for patients who had T1 tumors during second TUR. Although that was a study that demonstrated the effect of second TUR in MIBC patients, that study differs from ours, as it did not evaluate the effect of second TUR in a MMT protocol.
In many studies, a safe TURB as complete as possible is the main requisite for MMT for optimal bladder preservation [3–6, 10, 11]. Mak et al. [6] reported that a visibly complete TURB was associated with a higher complete response rate to induction chemoradiation on both univariable and multivariable analyses, but this was not true for DSS and OS on multivariate analysis. In Krause et al’s [4] study, putative residual tumor after TURB was assessed histologically by biopsies from all the resection margins, with pR0 indicating microscopically complete TURB, pR1 a microscopically residual tumor and cR2 a macroscopically residual tumor. R0 status was found to be a highly significant parameter for long-term outcome with survival rates of 70, 46 and 35 % after 5, 10 and 15 years, respectively, compared to patients with R1 or R2. Additionally, R1 seemed to be more favorable, with better survival rates than R2 [4]. In the MGH series, patients with a complete TURB revealed statistically better complete response rates to induction therapy and required statistically less cystectomy rates when compared to patients with incomplete TURB [3]. These findings and the established value of second TUR in high-risk NMIBC patients led us to evaluate the value of second TUR after initial complete TURB in MIBC patients. In these patients, the role of second TUR would be to decrease residual tumor volume and to optimize radiation therapy. Our study revealed that patients who underwent a second TUR had a statistically better 5-year DSS rate compared to patients who had no second TUR. The 5-year OS rates of the patients who underwent a second TUR also had a better 5-year OS rate of 63.7 % compared with 40.1 % in those who had no second TUR, but this was not statistically significant (p = 0.054). This might be due to the relatively low number of patients. Efstathiou et al. [3] found statistically better 5- and 10-year DSS and OS rates in patients who had visibly complete TURB compared with not visibly complete TUR. Similarly, In Krause et al’s study, a tumor-free resection (R0) was found to be a significant parameter for 15-year OS [4]. In our study, 29 patients who had residual tumor at their second TUR specimen exhibited worse 5-year DSS rate compared to 14 patients who had no residual tumor at their second TUR specimen (5-year DSS rates 51 and 75 %, respectively, p = 0.242). We think that these results revealed that performing a second TUR in MIBC patients who are going to be treated with bladder-preserving MMT protocols might benefit from a second TUR, as a second TUR will probably decrease residual tumor volume, if any.
Many MMT studies revealed that the probability of saving the bladder is higher in the case of a small, unifocal, early-stage tumor (T2–T3), the absence of hydronephrosis, a visibly and microscopically complete TURB, no lymph node metastasis and no in situ tumors [3–6]. Our study also revealed that on multivariate analysis, lymph node involvement, presence of hydronephrosis, higher stage and not performing second TUR were predictors of worse DSS. A second TUR, probably by decreasing the residual tumor volume and increasing the microscopically tumor-free status, has a positive impact on DSS.
The present study is limited by its retrospective nature. Second, the second TUR was performed not due to a certain strict criteria. However, like in NMIBC cases, to evaluate the effect of second TUR on MMT outcomes, we only included patients without any visible residual tumors after initial TURB. This selection criterion for this study limited the number of patients in our study. Third, this relatively small number of patients made subgroup analysis difficult. However, regarding DSS, we managed to demonstrate that patients who underwent a second TUR had a statistically better 5-year DSS rate compared to patients who had no second TUR.
Conclusion
In conclusion, second TUR should be performed in patients with MIBC who are going to be treated with bladder-preserving MMT protocols. In such cases, to our knowledge, this is the first study demonstrating the effect of second TUR on patient outcomes. A prospective randomized study is needed to validate these findings.
References
Hautmann RE, de Petriconi RC, Pfeiffer C et al (2012) Radical cystectomy for urothelial carcinoma of the bladder without neoadjuvant or adjuvant therapy: long-term results in 1100 patients. Eur Urol 61:1039–1047
Hautmann RE, de Petriconi RC, Volkmer BG (2010) Lessons learned from 1,000 neobladders: the 90-day complication rate. J Urol 184:990–994
Efstathiou JA, Spiegel DY, Shipley WU et al (2012) Long-term outcomes of selective bladder preservation by combined-modality therapy for invasive bladder cancer: the MGH experience. Eur Urol 61:705–711
Krause FS, Walter B, Ott OJ et al (2011) 15-year survival rates after transurethral resection and radiochemotherapy or radiation in bladder cancer treatment. Anticancer Res 31:985–990
Ploussard G, Daneshmand S, Efstathiou JA et al (2014) Critical analysis of bladder sparing with trimodal therapy in muscle-invasive bladder cancer: a systematic review. Eur Urol 66:120–137
Mak RH, Hunt D, Shipley WU et al (2014) Long-term outcomes in patients with muscle-invasive bladder cancer after selective bladder-preserving combined-modality therapy: a pooled analysis of Radiation Therapy Oncology Group protocols 8802, 8903, 9506, 9706, 9906, and 0233. J Clin Oncol 32:3801–3809
Divrik RT, Sahin AF, Yildirim U, Altok M, Zorlu F (2010) Impact of routine second transurethral resection on the long-term outcome of patients with newly diagnosed pT1 urothelial carcinoma with respect to recurrence, progression rate, and disease-specific survival: a prospective randomised clinical trial. Eur Urol 58:185–190
Babjuk M, Burger M, Zigeuner R et al (2013) EAU guidelines on non-muscle-invasive urothelial carcinoma of the bladder: update 2013. Eur Urol 64:639–653
Herr HW (2001) Transurethral resection of muscle-invasive bladder cancer: 10-year outcome. J Clin Oncol 19:89–93
Rödel C, Grabenbauer GG, Kühn R et al (2002) Combined-modality treatment and selective organ preservation in invasive bladder cancer: long-term results. J Clin Oncol 20:3061–3071
Caffo O, Veccia A, Fellin G et al (2013) Trimodality treatment in the conservative management of infiltrating bladder cancer: a critical review of the literature. Crit Rev Oncol Hematol 86:176–190
Authors’ contribution
S Baltaci and K Turkolmez developed the protocol/project; N Hamidi: collected or managed the data and wrote/edited the manuscript; O Gulpinar and Y Beduk analyzed the data; E Suer wrote/edited the manuscript and analyzed the data; MI Gokce collected or managed the data.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None.
Rights and permissions
About this article
Cite this article
Suer, E., Hamidi, N., Gokce, M.I. et al. Significance of second transurethral resection on patient outcomes in muscle-invasive bladder cancer patients treated with bladder-preserving multimodal therapy. World J Urol 34, 847–851 (2016). https://doi.org/10.1007/s00345-015-1710-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00345-015-1710-5