Abstract
Purpose
We aimed to determine incidence, pathologic findings, prognostic factors and clinical outcomes for patients with clinically localized papillary RCC.
Methods
Demographic, clinical and pathologic findings were collected on all patients with PRCC undergoing surgery at four academic medical centers. The primary endpoint was cancer-specific survival (CSS). Relapse-free survival (RFS) and overall survival (OS) were secondary endpoints. Kaplan–Meier estimates were obtained, and Cox proportional hazard regression models were used to assess predictors of mortality and relapse.
Results
We identified 626 PRCC, of which 373 (60 %) were type 1 and 253 (40 %) were type 2, with three-quarters of all tumors being pT1. Compared to patients with type 1, those with type 2 were older (mean age: 63 vs 61; p = 0.02), presented more commonly with symptoms (13 vs 7 %; p = 0.02) and had larger mean tumor size (5.2 vs 4.3 cm; p = 0.001). With a median follow-up of 41 months (IQR: 16–68), 92 patients had died of PRCC (15 %), 48 (8 %) experienced relapse, and 101 died from all causes (16 %). The estimated 5-year CSS, RFS and OS were 83, 91 and 82 %, respectively. In multivariable analysis, older age, T stage and nodal status were predictors of CSS and OS. However, PRCC subtype was not a predictor of CSS, RFS or OS.
Conclusion
While patients with type 2 PRCC appear to present with more advanced disease than patients with type 1, PRCC subtype does not appear to be an independent predictor of CSS, RFS or OS for treated localized disease.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Papillary renal cell carcinoma (PRCC) is the second most frequent histologic type among all renal cortical neoplasms, accounting for up to 15 % [1]. Based on Delahunt and Eble’s description, PRCC is morphologically subclassified into two groups, type 1 and type 2, depending on nuclear features and growth pattern characteristics [2, 3].
Since this subclassification was delineated, the aggressive potential of PRCC type 2 has been observed [4, 5], as patients with type 2 PRCC are diagnosed at a higher stage and harbor a higher nuclear grade compared to type 1 [6, 7]. Additionally, type 2 appears to be predominant in cases of PRCC with inferior vena cava thrombus [8] and has been reported to have lower cancer-specific survival compared to type 1 [5, 9].
Based on these findings, subclassification of PRCC has been suggested. However, there have been equivocal findings when the prognostic utility of PRCC subclassification has been assessed [5, 9–11].
Our aim was to evaluate a cohort of patients with clinically localized PRCC, to examine clinicopathologic factors, assess clinical outcomes and determine prognostic factors for recurrence and mortality.
Materials and methods
After obtaining institutional review board approval were retrospectively queried four institutional databases to identify patients with clinically localized PRCC (cT1-2 N0 M0) who underwent radical or partial nephrectomy between January 2002 and December 2012. The clinical information extracted included patient age, gender, symptoms at presentation, tumor size, operative data, pathologic findings and clinical follow-up.
All surgical procedures were performed by different surgeons using open or minimally invasive approaches. Lymph node dissection was performed when deemed necessary by preoperative imaging. RCC stage was assigned according to the American Joint Committee on Cancer 2010 TNM classification. A dedicated genitourinary pathologist re-reviewed each case and assigned histologic type following the recommendations of the Vancouver classification [12]. Patients with a history of synchronous or metachronous clear cell RCC were excluded from analysis. In addition, cases with mixed type 1 and type 2 or history of metachronous type 2 after type 1 PRCC were also excluded.
The patients were followed after surgery at regular intervals during visits to the outpatient clinic with history and physical examination. Radiographic surveillance imaging of the chest, abdomen and pelvis was obtained during different intervals according to each institution surveillance protocol. The length of the follow-up was considered the last information on file until the date of death.
The primary endpoint (PE) of the study was cancer-specific survival (CSS). This was defined as time from surgery to date of death from PRCC or last follow-up. The secondary endpoints were relapse-free survival (RFS) and overall survival (OS). Survival was determined from the respective institutional database, hospital records and the US Social Security Death Index. The evaluation of PE and SE was conducted using Kaplan–Meier estimates and the log-rank test. The impact of clinicopathological factors on mortality and recurrence was analyzed using Cox proportional hazard regression models, obtaining the HR and 95 % CI. Statistical analysis was conducted using Stata 12 (StataCorp, College Station, TX, USA).
Results
We identified 626 patients from the four institutions that underwent radical or partial nephrectomy between January 2002 and December 2012 and had PRCC at final pathology. After contemporary pathologic re-review, there were 373 (60 %) cases PRCC type 1 and 253 (40 %) type 2. There were 17 cases with mixed histology and therefore excluded from final analysis.
The clinical and pathological features according to PRCC subtype are given in Table 1. Mean age at surgery was 61 (SD = 12) for type 1 and 63 (SD = 12) for type 2 (p = 0.02). Patients with type 2 tumors presented with a larger tumor size [5.2 cm (SD = 4)] than patients with type 1 PRCC [4.3 cm (SD = 3), p = 0.001]. There were more males with type 1 PRCC (82 vs 72 %; p = 0.004). Patients with type 2 were more commonly symptomatic at presentation (13 vs 7 %; p = 0.02) and had higher incidence of vein thrombus (4 vs 0 %; p = 0.001). There were no differences with regard to surgical approach (p = 0.5).
Overall, 74 % of the tumors were pT1 and 84 % had organ-confined disease (pT2b or lower). There were no observable differences in the distribution according to T stage (p = 0.8). A lymph node dissection was undertaken in 98 patients (16 %), and in 21 % of the cases, a positive lymph node was detected (21/98). Type 2 patients were deemed for lymphadenectomy more commonly than type 1 (32 vs 11 %; p < 0.001) and were more likely to harbor lymph node metastasis (42 vs 9 %; p = 0.01).
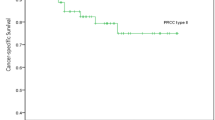
At a median follow-up of 41 months (IQR: 16–68), 92 patients had died of PRCC (15 %), 48 with type 1 and 44 with type 2. A total of 48 (8 %) experienced relapse, and 101 died from all causes (16 %). The median time to recurrence was 45 and 32 months for type 1 and type 2, respectively. The estimated 5-year CSS was 83 % (95 % CI = 79–87). The estimated 5-year RFS was 91 % (95 % CI = 88–94), and the 5-year overall survival (OS) was 82 % (95 % CI = 78–86). There were 21 relapses in the type 1 group, the majority in the lungs (11/21). In type 2 patients, there were 27 relapses; most detected in the lung (16) or lymph nodes (10).
To evaluate the role of PRCC subtype in the prediction of recurrence and survival after surgical treatment, we compared the 5-year Kaplan–Meier CSS, RFS and OS (Fig. 1a–c) estimates by histologic subtype.
On univariable analysis, type 2 PRCC (HR = 1.7; 95 % CI = 1.1–2.5, p = 0.02), older age (HR = 1.07; 95 % CI = 1.04–1.09, p < 0.001), T stage (HR = 3.4; 95 % CI = 2–5, p < 0.001) and nodal status (HR = 7.6; 95 % CI = 4–14, p < 0.001) were predictors of CSS. With regard to relapse, type 2 PRCC (HR = 2.3; 95 % CI = 1.3–4, p = 0.005), T stage (HR = 3.5; 95 % CI = 2–6, p < 0.001) and nodal status (HR = 26; 95 % CI = 14–51, p < 0.001) were variables associated. Older age (HR = 1.07; 95 % CI = 1.05–1.09, p = 0.001), T stage (HR = 3; 95 % CI = 2.1–4.5, p = 0.001) and lymph node involvement (HR = 6.8; 95 % CI = 3.6–12, p = 0.001) were predictors of overall mortality (Table 2).
On multivariable analysis, T stage higher than pT1 (HR = 2.1; 95 % CI = 1.1–4, p = 0.02) and positive nodes (HR = 15; 95 % CI = 6.8–35, p = 0.001) were predictors of RFS. Age (HR = 1.05; 95 % CI = 1–1.07, p < 0.001), T stage (HR = 2.5; 95 % CI = 1.6–3.9, p < 0.001) and nodal involvement (HR = 2.8; 95 % CI = 1.4–6, p = 0.005) were independently associated with cancer-specific and overall mortality. PRCC subtype was not a predictor for CSS, RFS or OS in multivariable analyses (Table 2).
Discussion
We present a contemporary experience of surgically managed, clinically localized PRCC with the goal of better understanding differences in clinical outcomes as stratified by histologic subtype. Over the last few decades, there has been a rising incidence of renal cell carcinoma in the USA [13] with papillary being the second most common histologic subtype, accounting for approximately 13 % of all renal neoplasms [14].
PRCC generally has a favorable prognosis after surgical treatment [1, 14]; however, this entity represents a heterogeneous subset of patients with unique clinical characteristics [15]. Compared to patients with clear cell RCC, those with papillary histology are less likely to present with advanced stage and have a lower risk of cancer-specific and overall mortality [1, 14, 16].
The heterogeneity observed in outcomes of patients with PRCC is also seen at the histologic level, and two morphologically different groups have been postulated [2]. Type 1 is constituted by small cells with basophilic cytoplasm, and type 2 has larger cells with eosinophilic cytoplasm. This morphologic observation has been correlated with cytogenetic differences [3]. Jiang et al. [17] described that 7p and 17p gains are more commonly seen in type 1 than type 2. Moreover, this higher frequency of trisomy 17 present in type 1 has been associated with lower T stage, lower nodal involvement, less metastatic spread and longer survival [7]. Interestingly, unique features also have been delineated in type 2 PRCC, with a greater number of chromosomal aberrations than type 1 [17] (e.g., loss of 1p, loss of 3p and gain of 5q) [7].
The differing histologic and genetic features provide support to divide papillary RCC into two different entities, raising the question of variable recurrence and survival of PRCC based on histologic subtype.
Although patients with type 2 PRCC presented with larger tumors, were more likely to exhibit nodal disease and were more likely to harbor venous tumor thrombus, we did not find differences in CSS, OS and RFS probabilities between type 1 and type 2 PRCC on multivariate analyses. Previously, investigators have observed papillary type 2 as an independent predictor of cancer-specific mortality [5, 9]. However, this has not been reproduced by more contemporary series, suggesting subclassification of PRCC does not provide independent prognostic information at a given tumor stage [7, 10, 11].
The variability noticed across the PRCC literature may be due to the molecular biology of PRCC subtypes. Yang et al. [18] were able to identify subtle nuances by analyzing the differential gene expression profiles of 35 cases of PRCC. Using this methodology along with immunohistochemistry, they identified 2 different classes of PRCC. In class 1, three different histologic subtypes were aggregated: type 1, low-grade type 2 and mixed type 1/low-grade type 2. These patients had excellent survival, and their tumors had high expression of cytokeratin 7. The class 2 tumors were highly aggressive and characterized by having high-grade type 2 histology and expressing elevated levels of topoisomerase IIα. Clinically, they presented with higher rates of metastasis at surgery and poorer survival.
Aside from the underlying differences detected at the genetic level across the two different subtypes of PRCC, other factors play a role in predicting relapse and mortality. We identified stage and lymph node involvement as strong predictors of relapse in our multivariable model. This finding is in agreement with previous reports assessing factors related to recurrence and survival in PRCC. Margulis et al. [19] identified T stage, M status, presence of venous tumor thrombus and Fuhrman grade as independent predictors of disease-specific mortality by assessing 245 surgically treated PRCC. In a large multi-institutional experience summarized by Zucchi et al. [20], lymph node involvement, presence of metastases and Fuhrman grade were predictors of RFS and CSS. In order to provide further accuracy to the assessment of prognosis, a comprehensive nomogram has been proposed by Klatte et al. [21]. Incidental detection, TNM stage, vascular invasion and tumor necrosis were independent prognostic factors of DSS with an externally validated accuracy of 94 %. Consequently, the vast majority of the literature emphasizes the predominant role of TNM classification in determining the prognosis of a patient with PRCC.
The nuclear grading assessment of PRCC deserves special attention. This grading system was established before understanding that various types of RCC have distinctive clinical, genetic and histological features. Despite its widespread usage, there is debate regarding the reproducibility of its criteria and scant evidence to indicate that Fuhrman grading has prognostic utility for tumor types other than clear cell RCC [22]. Furthermore, it is unclear whether nucleolar grade alone or Fuhrman grade should be used in papillary RCC [23, 24]. A new grading system was recently proposed by the International Society of Urological Pathology, although further validation by confirmatory studies is warranted [25]. Based on the aforementioned reasons, we do not routinely quantify the Fuhrman grade in cases of PRCC at our institutions.
It has been previously suggested that PRCC has higher predilection for lymph node involvement [26, 27]. Compared to clear cell tumors, PRCC is more likely to have synchronous nodal metastases at the time of presentation [26], having a higher tendency to regional lymph node spread, especially in tumors measuring more than 8 cm [27]. In our cohort, we observed that 10 of 27 patients with papillary type 2 RCC who have a relapse exhibited metastatic disease in the lymph nodes, whereas most patients with type 1 PRCC metastasized to the lungs (11/21). This is consistent with data reported by Paparel et al. [28], who noted papillary type 2 accounts for up to 20 % of the surgically resected local recurrences. As such, it may be reasonable to obtain retroperitoneal imaging more frequently among patients with type 2 PRCC, in order to survey for nodal recurrences.
Our study has limitations that should be incorporated into the interpretation of our findings. We report a multi-institutional retrospective experience in which four prospectively attained databases were queried to identify patients with PRCC. The inclusion and exclusion criteria were uniformly applied, and the output data were analyzed. Since not all databases were created with the exact same variables, we do not have the specific details regarding adjuvant treatment utilized in patients with T3 or N + disease. There may be patients with this high-risk feature who received some type of adjuvant treatment on a clinical trial. Nevertheless, the most common practice in the USA in these cases is to follow a close surveillance protocol since there are not drugs approved by the FDA to be utilized in the adjuvant setting. In addition, our study was not conceived to test different templates utilized when a retroperitoneal lymph node dissection was performed; therefore, we cannot provide more accurate data on template utilization in this setting. An additional drawback may be possible variations on surveillance imaging protocols utilized at the different institutions participating that could implicate differences in follow-up. Moreover, the patterns of care for postoperative relapse may have varied over the timeframe of our study, which may have influenced our study endpoints. It is also possible that longer follow-up would lead to different findings. However, our 1-, 3- and 5-year survivals are consistent with previous reports [1, 20].
In spite of its limitations, this study has the strength of being a multi-institutional experience of four academic centers representing a contemporary high-volume practice. In addition, we were able to gather what to our knowledge is the largest cohort of PRCC reported in the literature.
Conclusion
Patients with surgically treated type 2 PRCC present with larger tumor size, are more likely to exhibit synchronous nodal metastases and are more likely to harbor venous tumor thrombus than patients with type 1 PRCC. Nevertheless, when controlling for clinicopathological variables, PRCC subtype is not a predictor of CSS, RFS or OS. Type 2 PRCC more commonly recurs in regional lymph nodes.
Abbreviations
- PRCC:
-
Papillary renal cell carcinoma
- CSS:
-
Cancer-specific survival
- RFS:
-
Relapse-free survival
- OS:
-
Overall survival
- HR:
-
Hazard ratio
- SD:
-
Standard deviation
- CI:
-
Confidence interval
- IQR:
-
Interquartile range
References
Steffens S, Janssen M, Roos FC (2012) Incidence and long-term prognosis of papillary compared to clear cell renal cell carcinoma—a multicentre study. Eur J Cancer 48:2347–2352
Delahunt B, Eble JN (1997) Papillary renal cell carcinoma: a clinicopathologic and immunohistochemical study of 105 tumors. Mod Pathol 10:537–544
Shuch B, Amin A, Armstrong AJ, Eble JN, Ficarra V, Lopez-Beltran A, Martignoni G, Rini BI, Kutikov A (2015) Understanding pathologic variants of renal cell carcinoma: distilling therapeutic opportunities from biologic complexity. Eur Urol 67:85–97
Delahunt B, Eble JN, McCredie MR (2001) Morphologic typing of papillary renal cell carcinoma: comparison of growth kinetics and patient survival in 66 cases. Hum Pathol 32:590–595
Allory Y, Ouazana D, Boucher E (2003) Papillary renal cell carcinoma. Prognostic value of morphological subtypes in a clinicopathologic study of 43 cases. Virchows Arch 442:336–342
Mejean A, Hopirtean V, Bazin JP (2003) Prognostic factors for the survival of patients with papillary renal cell carcinoma: meaning of histological typing and multifocality. J Urol 170:764–767
Klatte T, Pantuck AJ, Said JW (2009) Cytogenetic and molecular tumor profiling for type 1 and type 2 papillary renal cell carcinoma. Clin Cancer Res 15:1162–1169
Kim KH, You D, Jeong IG (2012) Type II papillary histology predicts poor outcome in patients with renal cell carcinoma and vena cava thrombus. BJU Int 110:673–678
Pignot G, Elie C, Conquy S (2007) Survival analysis of 130 patients with papillary renal cell carcinoma: prognostic utility of type 1 and type 2 subclassification. Urology 69:230–235
Ku JH, Moon KC, Kwak C (2009) Is there a role of the histologic subtypes of papillary renal cell carcinoma as a prognostic factor? Jpn J Clin Oncol 39:664–670
Sukov WR, Lohse CM, Leibovich BC (2012) Clinical and pathological features associated with prognosis in patients with papillary renal cell carcinoma. J Urol 187:54–59
Srigley JR, Delahunt B, Eble JN (2013) The International Society of Urological Pathology (ISUP) Vancouver classification of renal neoplasia. Am J Surg Pathol 37:1469–1489
Chow WH, Devesa SS, Warren JL (1999) Rising incidence of renal cell cancer in the United States. JAMA 281:1628–1631
Keegan KA, Schupp CW, Chamie K (2012) Histopathology of surgically treated renal cell carcinoma: survival differences by subtype and stage. J Urol 188:391–397
Ronnen EA, Kondagunta GV, Ishill N (2006) Treatment outcome for metastatic papillary renal cell carcinoma patients. Cancer 107:2617–2621
Pal SK, Nelson RA, Vogelzang N (2013) Disease-specific survival in de novo metastatic renal cell carcinoma in the cytokine and targeted therapy era. PLoS ONE 8:e63341
Jiang F, Richter J, Schraml P (1998) Chromosomal imbalances in papillary renal cell carcinoma: genetic differences between histological subtypes. Am J Pathol 153:1467–1473
Yang XJ, Tan MH (2005) Kim HL.A molecular classification of papillary renal cell carcinoma. Cancer Res 65:5628–5637
Margulis V, Tamboli P, Matin SF (2008) Analysis of clinicopathologic predictors of oncologic outcome provides insight into the natural history of surgically managed papillary renal cell carcinoma. Cancer 112:1480–1488
Zucchi A, Novara G, Costantini E (2012) Prognostic factors in a large multi-institutional series of papillary renal cell carcinoma. BJU Int 109:1140–1146
Klatte T, Remzi M, Zigeuner RE (2010) Development and external validation of a nomogram predicting disease specific survival after nephrectomy for papillary renal cell carcinoma. J Urol 184:53–58
Delahunt B, Bethwaite PB, Nacey JN (2007) Outcome prediction for renal cell carcinoma: evaluation of prognostic factors for tumors divided according to histological subtype. Pathology 39:459–465
Sika-Paotonu D, Bethwaite PB, McCredie MR (2006) Nuclear grade but no Fuhrman grade is applicable to papillary renal cell carcinoma. Am J Surg Pathol 30:1091–1096
Klatte T, Anterasian C, Said JW (2010) Fuhrman grade provides higher prognostic accuracy than nuclear grade for papillary renal cell carcinoma. J Urol 183:2143–2147
Delahunt B, Cheville JC, Martignoni G (2013) The International Society of Urological Pathology (ISUP) grading system for renal cell carcinoma and other prognostic parameters. Am J Surg Pathol 37:1490–1504
Renshaw AA, Richie JP (1999) Subtypes of renal cell carcinoma. Different onset and sites of metastatic Disease. Am J Clin Pathol 111:539–543
Mai KT, Landry DC, Robertson SJ (2001) A comparative study of metastatic renal cell carcinoma with correlation to subtype and primary tumor. Pathol Res Pract 197:671–675
Paparel P, Bigot P, Matillon X (2014) Local recurrence after radical nephrectomy for kidney cancer: management and prediction of outcomes. A multi-institutional study. J Surg Oncol 109:126–131
Authors’ contribution
Ledezma involved in protocol and project development, data collection, data management and data analysis, wrote and edited the manuscript. Negron involved in data collection or management. Paner involved in project development and data collection. Rjepaj involved in data collection or management. Lascano involved in data collection or management. Haseebuddin involved in data collection or management. Dangle involved in data collection or management. Shalhav involved in protocol/project development, wrote and edited the manuscript. Crist involved in data collection or management. Raman involved in protocol/project development, wrote and edited the manuscript. DeCastro involved in protocol/project development, wrote and edited the manuscript. Harik involved in data collection or management. Paroder involved in data collection or management. Uzzo wrote and edited the manuscript. Kutikov wrote and edited the manuscript. Eggener involved in protocol/project development and data analysis, wrote and edited the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical standards
The manuscript entitled “Clinically Localized Type 1 and 2 Papillary Renal Cell Carcinomas Have Similar Survival Outcomes Following Surgery” was an investigation approved by the Internal Board Review Committee of the University of Chicago (IRB number # 13-0461). Therefore, the manuscript has been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments.
Informed consent
In addition, all patients gave their informed consent prior to their inclusion in the study.
Rights and permissions
About this article
Cite this article
Ledezma, R.A., Negron, E., Paner, G.P. et al. Clinically localized type 1 and 2 papillary renal cell carcinomas have similar survival outcomes following surgery. World J Urol 34, 687–693 (2016). https://doi.org/10.1007/s00345-015-1692-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00345-015-1692-3