Abstract
Objectives
To investigate the impact of three-dimensional (3D) printing on the surgical planning, potential of training and patients’ comprehension of minimally invasive surgery for renal tumors.
Methods
Patients of a T1N0M0 single renal tumor and indicated for laparoscopic partial nephrectomy were selected. CT data were sent for post-processing and output to the 3D printer to create kidney models with tumor. By presenting to experienced laparoscopic urologists and patients, respectively, the models’ realism, effectiveness for surgical planning and training, and patients’ comprehension of disease and procedure were evaluated with plotted questionnaires (10-point rating scales, 1—not at all useful/not at all realistic/poor, 10—very useful/very realistic/excellent). The size of resected tumors was compared with that on the models.
Results
Ten kidney models of such patients were fabricated successfully. The overall effectiveness in surgical planning and training (7.8 ± 0.7–8.0 ± 1.1), and realism (6.0 ± 0.6–7.8 ± 1.0) were reached by four invited urologists. Intraoperative correlation was advocated by the two performing urologists. Patients were fascinated with the demonstration of a tactile “diseased organ” (average ≥9.0). The size deviation was 3.4 ± 1.3 mm.
Conclusions
Generating kidney models of T1N0M0 tumors with 3D printing are feasible with refinements to be performed. Face and content validity was obtained when those models were presented to experienced urologists for making practical planning and training. Understandings of the disease and procedure from patients were well appreciated with this novel technology.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Three-dimensional (3D) printing is a technology that is developed rapidly in recent years. With the incorporation of computer aided designing, tactile 3D objects can be generated through special printing devices. The principle is almost identical to that in a classic spray printer, and multiple printed sheets are sintered together with stereolithography [1]. Theoretically, objects of any shape can be formed. Early application was in industrial manufacturing to produce specialized modules. The medical usage appeared later together with the revolution of imaging and bio-materials. The potential advantage, when compared with traditional two-dimensional (2D) films, is clear that it may help both physicians and patients comprehend the disease more. With a tangible copy of an organ or system, physicians may be able to make better determinations and plans, and/or design proper prostheses in replacement of dysfunctional tissues or organs. Encouraging results have been recently reported in craniofacial surgery, stomatology, and orthopedics [2–4], but little in urology.
In the current study, our effort using 3D printing in minimally invasive treatment of urology is presented. From June 2013 to January 2014, kidney models from patients with T1N0M0 renal tumors were successfully “printed.” The usefulness on surgical planning, potential of surgical training, dimensional satisfaction and patients’ comprehension of the disease and management was then investigated.
Materials and methods
-
1.
Patient selection
Patients diagnosed to have a single renal tumor of stage T1N0M0 and clinically indicated for laparoscopic partial nephrectomy were included.
-
2.
Generation of 3D kidney models
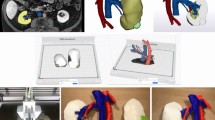
Prior to the surgery, the patients received standard contrast enhanced CT scan for the overall urinary tract, slice of 5 mm at 120 kV and 320 mA, and injection of 80–100 mL non-ionic contrast materials at 2–2.5 mL/s. Images were reconstructed at 1.25 mm interval slices. Data in DICOM format were sent for processing with Medical Imaging ToolKit (MITK) and 3DMed [5] by Institute of Automation, Chinese Academy of Sciences. Major vasculature (renal arteries and veins), collecting system (major calyces, pelvis, and ureter), and tumor were preserved, but perirenal tissue removed. Inter-relationships between the tumor, surrounding parenchyma and other key structures were attempted in various virtual sections. The final output was in standard STL format after denoise, smoothing, and meticulous filling. LaserCore5300® 3D printer (Longyuan Rapid Prototyping Ltd, Beijing, China) was used to produce the kidney models. The printing material was thermoplastic plastics, and the sheet thickness 0.2 mm. Manual coloring of the parenchyma (rose-red), vasculature (arteries in red and veins in blue), collecting system (yellow), and tumor (pink) was finally performed. The model creation took three-to-four working days and the price for each model was about $150, including post-processing, printing, and material. The 3D printer was an industrial type, worthy of $110,000, whose manager is dedicated to medical extensions.
-
3.
Model evaluation from urologists and patients, and tumor size comparison (resected vs. printed)
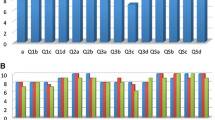
Two questionnaires with open ended questions of ordinal 10-point rating scales (1—not at all useful/not at all realistic/poor, 10—very useful/very realistic/excellent) were plotted [6, 7]. In the questionnaire for experienced laparoscopic urologists (laparoscopic partial nephrectomy ≥30 cases), five questions of overall usefulness (1 item), realism (3 items) and whether a useful tool in surgical planning and training (1 item) were asked to test face and content validity. The other questionnaire was for patients, in which four questions on whether the model a tool for surgeon–patient communications were designed—overall satisfaction of conversation (1 item), model assistance in understanding the disease and procedure (2 items), and preference of using 3D models by urologists (1 item). After the surgery, the size of the tumors resected was compared with those measured on the models.
Results
From June 2013 to January 2014, 10 patients with a unilateral single renal tumor of stage T1N0M0 and clinical indication of laparoscopic partial nephrectomy were selected. Demographics and surgical variables are shown in the Table 1.
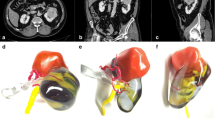
Tumor-specific kidney models for all patients were successfully generated. Overall kidney contour, tumor, vasculature, and collecting system were clearly presented with regards to the virtual reconstructions (Fig. 1a, b). Through different sectioning, the infiltration of the tumor into the parenchyma and its relationship with other key structures could be further investigated (Fig. 1c, d). Preoperatively, four experienced laparoscopic urologists, including two operating urologists, were surveyed for the face and content validation. The overall usefulness, realism and usefulness in surgical planning and training were rated as 7.8 ± 0.7 (Q1), 7.3 ± 0.5 (Q2, graphics), 6.0 ± 0.6 (Q2a, details of vasculature and collecting system), 7.8 ± 1.0 (Q2b, tumor size and inter-relationship), and 8.0 ± 1.1 (Q3), respectively (Fig. 2). All participating urologists agreed and advocated that it be a beneficial advancement for surgical planning and potential training of demanding procedures, when combined with 2D data. The rating from the patients was nine or over in all four questions (Fig. 3).
Laparoscopic partial nephrectomy for tumor resection was performed for all those patients. Apart from one case of perirenal hematoma formation controlled by blood transfusion, most cases were uneventful. The size deviation between model and specimen was 3.4 ± 1.3 mm (Table 1). Pathology correlated well with the preoperative diagnosis and no positive margin was encountered. The two operating urologists claimed that intraoperative navigation with those models was helpful in presenting tumor details, its relationship of surroundings, resection range, and avoidance of injury to key structures, especially for those in the hilum.
Discussions
Surgery has shifted into the era of minimal invasiveness. However, surgical planning at present still depends on transferring the solid lesion into multiple 2D images. With brain-work or computerized softwares, a virtual reconstruction is created and then validated by actual manipulations on individual patients. However, this process might be reformed by 3D printing which brings this virtual reconstruction into a tangible stage. The emerging application for preoperative planning has been successful in some surgical specialties mentioned. Attempts were also reported in some challenging clinical scenarios and surgical training. Rohner et al. [4] successfully used 3D printing for planning and navigation in a complicated case of facial reconstruction. 3D printed templates of Charcot foot were individually fabricated by Giovinco et al. [8] for surgical simulation. The practice on patient-specific templates effectively affected the final correction. Hurson et al. [9] studied 12 cases of acetabular fracture with 3D printing. Apart from preoperative planning and surgical refinement, the used models were further presented to younger surgeons, medical students, nurses, and operating staff for further training.
Due to the sharp contrast to the peripheral tissue in those bony structured specialties, imaging data can be precisely extracted and translated through 3D printing into the anatomy to be operated. Image processing is relatively easy and materials ready for printing. For the supple viscera full of intra-organic intubations like the liver and kidney, however, the fabrication is far more complicated. Studies in this regard are limited, focusing mainly on the dimensional accuracy. Materials for printing are usually nonflexible in nature, including thermoplastic plastics and light solid resin. In his review, Ikegami [10] sorted out publications on 3D printing in liver transplantation. Preoperative evaluation was feasible and helpful in delineating the volume data of the allograft, especially in pediatric patients. In a recent comparative study by Zein et al. [11], flexible materials of TangoPlus® series were utilized to simulate the donated liver, including the parenchyma, portal vein, intra-hepatic biliary tree, and arteries. The average dimensional deviation was lower than 4 mm and that of tubular structures lower than 1.3 mm. The authors claimed that this methodology may provide transplant guidance and upgrade the donor-recipient compatibility, thus improve the success rate of living donor liver transplantation. In particular, the TangoPlus FullCure®, a flexible material close to the texture of latex, was used in fabricating human vessels elsewhere [12]. 3D printing of other non-bony organs for surgical planning and training were also reported, including neurosurgery of brain tumors and cardiovascular surgery of primary cardiac tumor or valve replacement [13–15].
Our study of 3D printing was focused on a minimally invasive treatment in urology. The target organ was the kidney with single T1 tumor clinically indicated for the management of laparoscopic partial nephrectomy. Currently, the 3D printing recreation was feasible with tolerable length of time and price. Models fabricated were tested for the acceptance from urologists and patients, together with the dimensional matching. From the urologists invited, satisfaction on the overall usefulness (mean 7.8), help in surgical planning and training (mean 8.0) and realism (mean 6.0–7.8) was recorded, which proved the face and content validity. In their view, this tumor-specific simulation was impressive because the lesion could be seen and touched before the surgery. The tumor infiltration and its relationship with other important structures could be further examined from different artificial sections. The two operating urologists claimed that, besides preoperative planning, some models were reviewed intraoperatively for individual navigation. In the aspect from patients, high score was documented in every question of the survey (≥9). A tactile “diseased organ” helped them understand the anatomy, lesion, and surgical risk better. Conversation over the consent was efficient because the explanation was straightforward. The patients surveyed were fascinated about this novel way of communication. The actual size of tumors was measured immediately after the specimen was taken out of the patient’s body. The deviation was 3.4 ± 1.3 mm with the resected tumors universally bigger than that of the models. Possible explanation was that the tumor confined in the surrounding parenchyma might stretch a bit after resection. However, this was not appreciated by the urologists from the survey (Q2b), showing the printed models as an acceptable fabrication of the kidneys with single T1 tumor.
The perspective of our preliminary study using 3D printing in urology is profound. Traditional surgical planning may be revolutionized and transformed from the format of reality–virtuality (lesion–films) into that of reality–virtuality–reality. The actual lesion (reality) data are acquired with modernized imaging system (virtuality), processed and output to a sophisticated 3D printer. The diseased organ model (reality) is then visualized, touched, and sectioned for surgeons to make practical plans and communications with colleagues and patients. With the demand of patient safety and strict practice, current surgical skill training has incorporated tools of simulation with various trainers and virtual reality. The novel 3D printing technology may become an added-on training modality with the potential for surgeons to practice lesion-specific skills before the actual procedure. Multiple models of diseased organs may be fabricated and presented to junior surgeons, residents, medical students, and nurses for the comprehension and practice of “normal variations”. Surgeon–patient communications may be optimized by obviating misunderstandings from the explanation of complicated terminologies and 2D films.
The current study is not without Limitations. Although the overall realism was accepted, the vasculature and collecting system details were moderate only. The size deviation of 3.4 ± 1.3 mm may become significant enough in misinterpretation. And not only is the tumor size of critical importance, but also vascular structures and tumor vessels should be presented in more detail to shorten, for example, the ischemia time. Highlighting of anatomical structures and tumor was still performed manually. Thus, imaging data processing should be refined to delineate with better quality, and coloring done at the same time during processing and output. Our printing material was hard thermoplastic plastics, which did not allow hands-on practice. The latex textured material TangoPlus FullCure®, though flexible, was still unfit for surgical manipulations. Proper close-to-tissue materials are to be sought for or invented so that skill trainings and lesion-specific rehearsals can be finally accomplished. Research in this regard should be on-going, and validated by well designed, randomized trials of larger patient volume.
3D printing application in medicine has aroused concerns increasingly. In the aspect of lesion-specific surgical planning and skill training, non-comparative advantage is shown over the conventional methodology. Although still in its starting phase, this novel technology may become a breakthrough and bring surgical planning and training from virtuality back to reality. Ultimately, the efficiency of surgery and patient’s safety will be improved.
Conclusions
Generating kidney models of T1 renal tumors with 3D printing technology is feasible with refinement in certain aspects to be accomplished. The models were accepted by urologists in assisting surgical planning and training of minimally invasive treatment preoperatively and intraoperatively. Face and content validity was obtained. Understandings of the disease and the surgical procedure from patients were well appreciated with this novel technology.
References
Lipson H, Kurman M (2012) Fabricated: the new world of 3D printing [M] Indiana. Wiley, New Jersey, pp 14–17
Levine JP, Patel A, Saadeh PB et al (2012) Computer-aided design and manufacturing in craniomaxillofacial surgery: the new state of the art. J Craniofac Surg 23(1):288–293
Bilhan H, Arat S, Mumcu E et al (2012) Precision of implant placement with stereolithographic templates: a pilot in vitro study. J Oral Implantol 38(5):569–574
Rohner D, Guijarro-Martinez R, Bucher P et al (2013) Importance of patient-specific intraoperative guides in complex maxillofacial reconstruction. J Craniomaxillofac Surg 41(5):382–390
He H, Tian J, Yang H et al (2001) 3D medical image diagnosis workstation—3dmed. Chin J Stereol Imaging Anal 6(2):73–77
Schout BM, Bemelmans BL, Martens EJ et al (2009) How useful and realistic is the uro trainer for training transurethral prostate and bladder tumor resection procedures? J Urol 181(3):1297–1303 (discussion 1303)
Zhang Y, Yu CF, Jin SH et al (2014) Validation of a novel non-biological bench model for the training of percutaneous renal access. Int Braz J Urol 40(1):87–92
Giovinco NA, Dunn SP, Dowling L et al (2012) A novel combination of printed 3-dimensional anatomic templates and computer-assisted surgical simulation for virtual preoperative planning in Charcot foot reconstruction. J Foot Ankle Surg 51(3):387–393
Hurson C, Tansey A, O’Donnchadha B et al (2007) Rapid prototyping in the assessment, classification and preoperative planning of acetabular fractures. Injury 38(10):1158–1162
Ikegami T, Maehara Y (2013) Transplantation: 3D printing of the liver in living donor liver transplantation. Nat Rev Gastroenterol Hepatol 10(12):697–698
Zein NN, Hanouneh IA, Bishop PD et al (2013) Three-dimensional print of a liver for preoperative planning in living donor liver transplantation. Liver Transplant 19(12):1304–1310
Biglino G, Verschueren P, Zegels R et al (2013) Rapid prototyping compliant arterial phantoms for in vitro studies and device testing. J Cardiovasc Magn Reson 15:2
Spottiswoode BS, van den Heever DJ, Chang Y et al (2013) Preoperative three-dimensional model creation of magnetic resonance brain images as a tool to assist neurosurgical planning. Stereotact Funct Neurosurg 91(3):162–169
Schmauss D, Gerber N, Sodian R (2013) Three-dimensional printing of models for surgical planning in patients with primary cardiac tumors. J Thorac Cardiovasc Surg 145(5):1407–1408
Schmauss D, Schmitz C, Bigdeli AK et al (2012) Three-dimensional printing of models for preoperative planning and simulation of transcatheter valve replacement. Ann Thorac Surg 93(2):e31–e33
Conflict of interest
There is no actual or potential conflict of interest in relation to this article.
Ethical standard
Ethical approval was obtained from the Research and Ethics Committee of the institute.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhang, Y., Ge, Hw., Li, Nc. et al. Evaluation of three-dimensional printing for laparoscopic partial nephrectomy of renal tumors: a preliminary report. World J Urol 34, 533–537 (2016). https://doi.org/10.1007/s00345-015-1530-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00345-015-1530-7