Abstract
Purpose
This study evaluated the baseline patient characteristics associated with the time to biochemical progression and overall survival in patients who participated in a phase II trial on zoledronic acid combined with the initial androgen-deprivation therapy for treatment-naïve bone-metastatic prostate cancer.
Methods
Patients received zoledronic acid 4 mg intravenously every 4 weeks for up to 24 months, concomitantly started with bicalutamide 80 mg orally every day and goserelin acetate 10.8 mg subcutaneously every 12 weeks.
Results
A total of 53 Japanese patients were enrolled between July 2008 and April 2010, and 52 patients were evaluable. Median follow-up period was 41.6 months. Updated median time to biochemical progression was 25.9 months (95 % confidence interval 14.5–49.9). Higher serum bone-specific alkaline phosphatase was an independent risk factor for time to biochemical progression based on multivariate analysis (hazard ratio 6.51; 95 % confidence interval 2.71–15.62; P < 0.001). Median time to biochemical progression for patients with serum bone-specific alkaline phosphatase level higher than 26 μg/L was 12.7 months. Multivariate analysis indicated that higher serum C-terminal telopeptide of type I collagen independently increased the risk of death (hazard ratio 9.62; 95 % confidence interval 2.11–43.89; P = 0.003). Median overall survival for patients with serum C-terminal telopeptide of type I collagen level higher than 8.0 ng/ml was 31.1 months.
Conclusions
Baseline bone markers can be useful as predictors for disease progression and survival time in patients with bone metastasis from treatment-naïve prostate cancer treated with upfront zoledronic acid concomitantly started with androgen-deprivation therapy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Prostate cancer (PCa) is one of the primary diseases that cause bone metastasis most frequently [1]. Bone metastasis may evoke skeletal-related events (SRE) which lead to not only impaired quality of life, but also poor prognosis [2–4]. Therefore, prevention of SRE plays a very important role in treatment of patients with bony-metastatic PCa.
Zoledronic acid (ZA), a potent bone-modifying agent (BMA) that suppresses osteoclasts, proved its efficacy to prevent SRE compared with placebo in patients with castration-resistant prostate cancer (CRPC) and with bone metastasis based on a phase III trial [5]. However, there can be some patients who benefit from the administration of BMA in the earlier stages before CRPC, or other patients may progress earlier than expected despite adding BMA to the first-line androgen-deprivation therapy (ADT), for whom other treatment modalities may be beneficial, for example, more recent hormone therapy such as abiraterone and enzalutamide or other treatment with distinct mechanism of action like docetaxel or radium-223. It would be beneficial for the future development of novel treatment strategies if biomarkers that predict poor responsiveness to the existing therapy at diagnosis were established.
We previously performed and reported the prospective phase II study on ZA combined with the initial ADT for treatment-naïve bone-metastatic PCa [6]. The SRE-free survival rate at the first 24 months, the primary endpoint, was 84.4 % in the study. Here, we updated the time to prostate-specific antigen (PSA) progression (TPP) and overall survival (OS) and evaluated the baseline patient characteristics associated with early PSA progression or poor survival in patients who participated in the trial.
Materials and methods
Patients
This study was performed based on data from a single-arm, open-label, phase II clinical trial of ZA, in which patients with bone metastases from treatment-naïve PCa were enrolled [6]. Key inclusion criteria were as follows: radiologic evidence of bone metastasis; histologically confirmed adenocarcinoma of the prostate; no prior systemic or local therapy for PCa; Eastern Cooperative Oncology Group (ECOG) performance status (PS) of 0 or 1; no history of bisphosphonate therapy; and no history of another malignancy within the preceding 5 years [6]. ECOG PS of 2 due to the bone disease was permitted. The study was conducted according to the declaration of Helsinki with approval from ethics committees at each institution. Written informed consent was obtained from all patients consistent with local requirements.
Study design
Patients received ZA (Zometa; Novartis Pharma, Tokyo, Japan) 4 mg intravenously every 4 weeks for up to 24 months, concomitantly started with ADT of maximum androgen blockade consisting of bicalutamide (Casodex; AstraZeneca, Osaka, Japan) 80 mg orally every day, and goserelin acetate (Zoradex; AstraZeneca, Osaka, Japan) 10.8 mg subcutaneously every 12 weeks [6]. PSA progression and survival were also monitored and updated for the current analyses. Serum PSA levels were assessed every 4 weeks. PSA progression was defined as the date that a 25 % or greater increase and an absolute increase of 2 ng/mL or more from the nadir was documented, which was confirmed by a second value obtained three or more weeks later, or as the date that a 25 % increase from the baseline value along with an increase in absolute value of 2 ng/mL or more after 12 weeks of treatment where no decline from baseline was documented [7]. Baseline patient characteristics associated with TPP or OS were analyzed in the current study.
Bone markers evaluation
Serum bone-specific alkaline phosphatase (BAP) was determined by the chemiluminescent enzyme immunoassay kit (Access OSTASE; Beckman Coulter, Fullerton, CA, USA). Urinary N-terminal cross-linked telopeptide of type I collagen (uNTx) was determined by the enzyme-linked immunosorbent assay kit (Osteomark NTx urine ELIZA; Alere, Waltham, MA, USA). Level of uNTx was adjusted for urinary creatinine. Serum C-terminal telopeptide of type I collagen (ICTP) was determined by the radioimmunoassay kit (UniQ ICTP RIA; Orion Diagnostica, Espoo, Finland).
Statistical analyses
TPP and OS were estimated using the Kaplan–Meier method. The significance of difference in the Kaplan–Meier curves was determined using the log-rank test. Univariate and multivariate analyses were performed using the Cox proportional-hazard model with step-up procedure for evaluation of baseline patient clinical factors associated with TPP and OS. A level of P < 0.05 was accepted as the statistical significance. S-Plus Ver. 6.1 (NTT DATA Mathematical Systems Inc., Tokyo, Japan) was used for the Kaplan–Meier analysis. Data were analyzed with IBM SPSS Statistics ver. 22.0 (IBM, Armonk, NY, USA).
Results
Patient demographics
A total of 53 Japanese men with bone metastases from treatment-naïve PCa were enrolled between July 2008 and April 2010, and 52 patients were evaluable. Patient characteristics were described in detail in the previous report [6]. Median age was 72 years (range 55–86). Most patients (85 %) had an ECOG PS of 0. Majority of patients had Gleason score (GS) of 8 or higher (63 %) and local stage of T3b or greater (56 %). Table 1 shows baseline values of PSA and bone markers.
Time to prostate-specific antigen progression
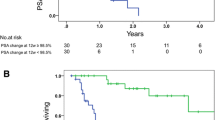
At the time of analysis, median follow-up period was 41.6 months (range 5.9–60.0). Updated median TPP was 25.9 months (95 % confidence interval [CI] 14.5–49.9). Univariate analysis revealed that baseline GS, extent of bone disease (EOD), BAP, ICTP, and uNTx had significant impact on TPP (Table 2). Higher BAP was an independent risk factor for TPP based on multivariate analysis (hazard ratio [HR] 6.51; 95 % CI 2.71–15.62; P < 0.001). Figure 1 shows that median TPP for patients with BAP level higher than 26 μg/L was 12.7 months (95 % CI 8.6–15.4).
Overall survival
Median OS was not yet reached at the time of analysis. Univariate analysis revealed that baseline EOD, ALP, BAP, ICTP, and uNTx were significantly associated with OS (Table 3). Multivariate analysis indicated that higher ICTP independently increased the risk of death (HR 9.62; 95 % CI 2.11–43.89; P = 0.003). Figure 2 shows that median OS for patients with ICTP levels higher than 8.0 ng/ml was 31.1 months (95 % CI 19.0 to not available).
Discussion
The current study presents the possible usefulness of baseline bone markers as predictors for biochemical progression and overall survival in patients with bone metastasis from treatment-naïve PCa when they commence treatment with ADT and ZA. The balance between bone resorption and formation is disrupted, and bone metabolism is disorderly upregulated in metastatic bone lesions [8, 9]. The potential prognostic and predictive values of bone markers have been reported in metastatic PCa patients. BAP is indicative of bone formation and has been reported as a possible risk factor for SRE and death in bone-metastatic PCa [10–12]. ICTP, a bone resorption marker, has indicated a correlation with the extent of disease in bone and bone pain in PCa [13]. However, these reports have thus far conveyed their findings based on CRPC. The current study is the first report that addresses hormone-naïve PCa patients.
A notable finding from this study was that GS, local stage, lymph-node metastases, and PSA level were less important than bone markers for predicting disease progression and survival. This result suggests that the extent of deregulated bone metabolism, from the baseline, due to metastatic disease has a greater significance on the responsiveness to treatment and prognosis than the malignant potential and local progression level of the primary lesion even in hormone-sensitive PCa. Thus, it further emphasizes the necessity for early treatment intervention against metastatic bone lesions.
Metastatic bone diseases can provide a favorable environment for the epithelial–mesenchymal transition (EMT) of cancer cells. EMT is a phenomenon in which cancer cells essentially having epithelial characteristics acquire mesenchymal features leading to ability to metastasis, resistance against therapy, or avoidance from apoptosis [14]. Bone marrow contains elevated levels of transforming growth factor-β which promotes EMT of cancer cells; thus, EMT may be highly promoted in hyper-metabolic bone lesions [15]. EMT might also contribute to resistance against ADT in PCa. It was reported that ADT induced EMT in PCa in vivo [16]. Moreover, ZA was suggested to reverse EMT of breast cancer cell lines. Therefore, adding ZA to ADT may result in the inhibition of EMT and sustained sensitivity of PCa cells to ADT.
Based on the result from the current study, however, treatment modalities other than ZA might be more beneficial to patients with higher levels of baseline bone markers. Denosumab, a fully human monoclonal antibody against RANKL, has a distinct mechanism of action relative to ZA and demonstrated better performance than ZA for prevention of SRE in CRPC [17]. Radium-223, an alpha emitter, selectively targets bone metastases with alpha particles and improved OS compared with placebo in metastatic CRPC [18]. Cabozantinib, a tyrosine kinase inhibitor with activity against MET and vascular endothelial growth factor receptor 2, indicated improvement on bone scan in 68 % of patients with CRPC, including complete resolution in 12 % based on a phase II trial [19]. The efficacy of these new treatments against metastatic bone diseases should be theoretically promising not only for CRPC, but also for hormone-naïve PCa.
There are some limitations to the current study, namely being that findings from the study are based on the data from a non-randomized single-arm phase II trial with a small number of subjects. In addition, the markers used are well known. Although no direct clinical impact can be derived because of the absence of a control arm, this study provides proof of principle that baseline bone markers may serve as predictors for biochemical progression and overall survival. We did not evaluate the association between the transition of bone markers during treatment and clinical outcomes. In CRPC, the association between bone marker levels and clinical outcomes were stronger for on-study bone marker levels compared with baseline levels although the statistical heterogeneity in the strength of these correlations has not been reported [20]. We did not investigate the effect of treatments after disease progression with the initial ADT on OS. Docetaxel, abiraterone, enzalutamide, radium-223, and cabazitaxel can influence OS. The landscape for the treatment of hormone-naïve PCa has changed since the conception of the present study; docetaxel with ADT can improve overall survival compared with ADT alone [21], and abiraterone or enzalutamide may prove their value in this setting in the future (NCT01715285, NCT01957436) [22].
In conclusion, baseline bone markers may be useful as predictors for disease progression and survival in patients with bone metastases from treatment-naïve PCa who start treatment with ZA concomitant with ADT. Development of novel treatment strategies against metastatic bone diseases may improve the prognosis even in patients with hormone-sensitive PCa.
References
Coleman RE (2004) Bisphosphonates: clinical experience. Oncologist 9:14–27
Oefelein MG, Ricchiuti V, Conrad W, Resnick MI (2002) Skeletal fractures negatively correlate with overall survival in men with prostate cancer. J Urol 168:1005–1007
Nørgaard M, Jensen AØ, Jacobsen JB, Cetin K, Fryzek JP, Sørensen HT (2010) Skeletal related events, bone metastasis and survival of prostate cancer: a population based cohort study in Denmark (1999 to 2007). J Urol 184:162–167
Sathiakumar N, Delzell E, Morrisey MA et al (2011) Mortality following bone metastasis and skeletal-related events among men with prostate cancer: a population-based analysis of US medicare beneficiaries, 1999–2006. Prostate Cancer Prostatic Dis 14:177–183
Saad F, Gleason DM, Murray R et al (2002) A randomized, placebo-controlled trial of zoledronic acid in patients with hormone-refractory metastatic prostate carcinoma. J Natl Cancer Inst 94:1458–1468
Nozawa M, Inagaki T, Nagao K et al (2014) Phase II trial of zoledronic acid combined with androgen-deprivation therapy for treatment-naïve prostate cancer with bone metastasis. Int J Clin Oncol 19:693–701
Scher HI, Halabi S, Tannock I et al (2008) Design and end points of clinical trials for patients with progressive prostate cancer and castrate levels of testosterone: recommendations of the prostate cancer clinical trials working group. J Clin Oncol 26:1148–1159
Maeda H, Koizumi M, Yoshimura K, Yamauchi T, Kawai T, Ogata E (1997) Correlation between bone metabolic markers and bone scan in prostatic cancer. J Urol 157:539–543
Aruga A, Koizumi M, Hotta R, Takahashi S, Ogata E (1997) Usefulness of bone metabolic markers in the diagnosis and follow-up of bone metastasis from lung cancer. Br J Cancer 76:760–764
Cook RJ, Coleman R, Brown J et al (2006) Markers of bone metabolism and survival in men with hormone-refractory metastatic prostate cancer. Clin Cancer Res 12:3361–3367
Smith MR, Cook RJ, Coleman R et al (2007) Predictors of skeletal complications in men with hormone-refractory metastatic prostate cancer. Urology 70:315–319
Lara PN Jr, Ely B, Quinn DI et al (2014) Serum biomarkers of bone metabolism in castration-resistant prostate cancer patients with skeletal metastases: results from SWOG 0421. J Natl Cancer Inst 106: dju013
Berruti A, Dogliotti L, Gorzegno G et al (1999) Differential patterns of bone turnover in relation to bone pain and disease extent in bone in cancer patients with skeletal metastases. Clin Chem 45:1240–1247
Hollier BG, Evans K, Mani SA (2009) The epithelial-to-mesenchymal transition and cancer stem cells: a coalition against cancer therapies. J Mammary Gland Biol Neoplasia 14:29–43
Lévesque JP, Helwani FM, Winkler IG (2010) The endosteal ‘osteoblastic’ niche and its role in hematopoietic stem cell homing and mobilization. Leukemia 24:1979–1992
Sun Y, Wang BE, Leong KG et al (2012) Androgen deprivation causes epithelial-mesenchymal transition in the prostate: implications for androgen-deprivation therapy. Cancer Res 72:527–536
Fizazi K, Carducci M, Smith M et al (2011) Denosumab versus zoledronic acid for treatment of bone metastases in men with castration-resistant prostate cancer: a randomised, double-blind study. Lancet 377:813–822
Parker C, Nilsson S, Heinrich D et al (2013) Alpha emitter radium-223 and survival in metastatic prostate cancer. N Engl J Med 369:213–223
Smith DC, Smith MR, Sweeney C et al (2013) Cabozantinib in patients with advanced prostate cancer: results of a phase II randomized discontinuation trial. J Clin Oncol 31:412–419
Brown JE, Cook RJ, Major P et al (2005) Bone turnover markers as predictors of skeletal complications in prostate cancer, lung cancer, and other solid tumors. J Natl Cancer Inst 97:59–69
Sweeney CJ (2006) ECOG: CHAARTED–ChemoHormonal therapy versus androgen ablation randomized trial for extensive disease in prostate cancer. Clin Adv Hematol Oncol 4:588–590
Tombal B, Borre M, Rathenborg P et al (2014) Enzalutamide monotherapy in hormone-naive prostate cancer: primary analysis of an open-label, single-arm, phase 2 study. Lancet Oncol 15:592–600
Acknowledgments
The authors thank all patients and their families for participating in this study, all investigators and site staff for their contribution, and Marco De Velasco for reviewing the language.
Conflict of interest
The authors have no conflict of interest.
Ethical standard
The study was conducted according to the Declaration of Helsinki with approval from ethics committees at each institution. Written informed consent was obtained from all patients consistent with local requirements.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nozawa, M., Hara, I., Matsuyama, H. et al. Significance of baseline bone markers on disease progression and survival in hormone-sensitive prostate cancer with bone metastasis. World J Urol 33, 1263–1268 (2015). https://doi.org/10.1007/s00345-014-1431-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00345-014-1431-1