Abstract
The foliar application of plant growth regulators to sugarcane can increase crop growth and yield per cultivated area, improve crop productivity and quality, and mitigate possible abiotic stresses by optimizing photosynthesis. The productive potential of sugarcane has not been fully tapped, and plant growth regulator technology via foliar application could greatly benefit the production of food and renewable energy from the sugarcane production chain. In this study, we conducted 15 sugarcane field trials to evaluate the effects of plant growth regulators (17 ppm GA3 activity, 817 ppm IAA activity and 43 ppm zeatin) via foliar application at the vegetative stage (V) or vegetative and maturation stages (VM) on the photosynthetic and antioxidant enzyme activities, carbohydrate production and yield production of three harvest periods (early, mid-late and late harvest sugarcane). In general, foliar application increased the enzymatic, agronomic, quality and energy parameters of sugarcane. The application of plant growth regulators in V and VM increased the activities of the photosynthetic enzymes phosphoenolpyruvate carboxylase and ribulose-1,5-bisphosphate carboxylase-oxygenase, decreased the contents of malondialdehyde and hydrogen peroxide, and increased superoxide dismutase, catalase, ascorbate peroxidase, and proline content. The average stalk yield over the three harvest times increased by 5.4 and 8.0% in V and VM, respectively, compared to the control (101 Mg ha−1). In addition, V and VM increased the sucrose concentration, theoretical recoverable sugars (TRS), and sugar productivity by averages of 2.9%, 2.6% and 9.3%, respectively compared to the control (13.9% of sucrose, 139 kg sugar stalk−1 of TRS, and 13.9 Mg ha−1 of stalk yield), across all harvest seasons. The best results for straw, bagasse and energy production were observed in VM, with average increases of 8.0%, 7.7% and 8.0% compared with the control (14.1 Mg ha−1, 6.1 Mg ha−1 and 69.8 kWh, respectively). Thus, plant growth regulator application can increase sugarcane metabolism, growth and development. Although single plant growth regulator application in the vegetative stage improved all sugarcane parameters, the double application of plant growth regulators in the vegetative and maturation stages ensured improvements in yield and product quality.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The growing population will inevitably increase the global demand for food and renewable energy (FAO 2021), which is driving the search for improvements in productivity per unit area and the quality of crops, especially sugarcane (Saccharum spp.) (Cardozo et al. 2014, 2020). Brazil remains the world’s largest producer of sugarcane, responsible for approximately 40.5% of global production and has the potential to continue in this position, as a producer of sugar and energy in the sector (Hughes et al. 2020; FAO 2021). Hence, exploring technologies, such as the use of plant growth regulators, is crucial for enhancing sugarcane productivity and addressing abiotic stresses that restrict the genetic potential of crops and threaten global food security (Mahalingam 2015).
In the central-south region of Brazil, sugarcane is harvested from March to November (Leite et al. 2008). A combination of varieties with different genetic characteristics are used to ensure a continuous supply of raw material for the industry throughout the harvest period. Thus, sugarcane varieties can be classified as early, middle-late or late harvest (Cardozo and Sentelhas 2013; Tischler et al. 2021). This study evaluates the nutritional, physiological and climatic demands of sugarcane in each harvest period. The plant’s nutritional and physiological characteristics at the time of harvest have important implications for producing sugarcane of industrial quality.
Plant primary and specialized metabolism are coordinated by signaling compounds called hormones, which are produced at specific locations and act as chemical signals to neighboring or distal cells via the xylem and phloem to induce the synthesis of specialized proteins that stimulate photosynthesis under appropriate environmental conditions (Raza et al. 2019; Weng et al. 2021). Plants under abiotic stress coordinate hormone production with biochemical, physiological, and metabolic adjustments, including the production of specific enzymatic and non-enzymatic antioxidants for plant protection, to increase productivity and plant quality (Davies 2010; Akhtar et al. 2020; Jogawat et al. 2021). Hormones influence photosynthesis directly and indirectly (Müller and Munné-Bosch 2021). In photoautotrophic organisms, the interaction of light and hormones directly regulates chloroplast development by directly influencing pigment accumulation, organelle size and division, the organization of the thylakoid membrane and the number of copies of the chloroplast genome (Stern et al. 2004).
Hormone such as auxins (AX), gibberellins (GAs) and cytokinins (CKs) modulate the photosynthetic rate under ideal conditions, whereas hormones such as abscisic acid (ABA), jasmonates (Jas), salicylic acid (SA) and ethylene regulate photosynthesis under non-ideal conditions, that is, under stress (Müller and Munné-Bosch 2021). Under stress conditions, the CO2 assimilation rate drops considerably due to stomatal, mesophilic and biochemical limitations imposed by the reduction in electron transport in the photosynthetic apparatus, photoinhibition and photooxidative stress caused by excess incident light associated with abiotic stresses (Takahashi and Badger 2011; Muñoz and Munné-Bosch 2018). Plant physiology research has focused on the modulation of photosynthesis by redox and hormonal signaling, particularly the action of hormones in the regulation of the production and elimination of reactive oxygen species (ROS) derived from photosynthesis and in photoprotection (Foyer 2018; Mandal et al. 2022). Exogenous plant growth regulators have been used to stimulate plant growth and development and to mitigate the deleterious effects of abiotic and biotic stresses (Egamberdieva et al. 2017). The main plant growth regulators are AXs (IAA), CKs (zeatin) and Gas (GA3), which have promising effects on yields when applied to the leaves of sugarcane (Silva et al. 2010). However, gaps remain in the understanding of the effects of these plant growth regulators in the last growth stages of sugarcane. We conjecture that applying IAA, zeatin and GA3 at the end of the vegetative and maturation stages could increase sugarcane productivity and quality by increasing the photosynthetic rate and stimulating the synthesis of sugars, given the effects of these plant growth regulators on the photosynthetic apparatus and the production of antioxidant enzymes.
Endogenous AXs play important roles in cellular metabolism, including indole-3-butyric acid (IBA), phenoxyacetic acid (PAA) and indole-3-acetic acid (IAA), which is synthesized from l-Tryptophan, synthesized from chorismate, which is the end product of the shikimate pathway (Ljung 2013). These AXs stimulate growth and development through changes in plant gene expression patterns (Asgher et al. 2015). High AX concentrations are present in organs with active cell division, that is, the apical meristems of root cells, where it is assumed that a large number of genes are involved in IAA biosynthesis (Ljung 2013). AXs have positive effects on the control of stomatal opening and closing, as high atmospheric CO2 concentrations stimulate the photosynthetic rate and, consequently, the production of photosynthates (Pemadasa 1982a, b; Snaith and Mansfield 1984).
CKs act on several plant tissues simultaneously and thus regulate many plant developmental processes, including growth, leaf senescence and acclimatization to environmental stresses (Li et al. 2016). Hormonal regulation is an important factor in the flowering process in sugarcane by transcriptional analysis, expressing genes involved in the biosynthesis and signaling of hormones, such as CKs, GAs and abscisic acid, and other plant regulators, such as ethylene and jasmonic acid. All these regulators influence aspects related to plant response to abiotic stress, photoprotection, photosynthesis, light harvesting and pigment biosynthesis (Manechini et al. 2021). CKs directly participate in chloroplast development and function and in the biosynthesis of chlorophyll and carotenoids. Carotenoids protect plastids against photooxidative damage (Alabadí et al. 2008; Cheminant et al. 2011; Cortleven and Schmülling 2015). Carotenoids are also important for PSII chlorophyll a/b binding proteins and ribulose-1,5-bisphosphate carboxylase/oxygenase (Rubisco) activity (Yuan and Xu 2001; Iqbal et al. 2011; Brenner and Schmülling 2012) and thus have direct and indirect effects on photosynthesis. According to Yang et al., (2018), the exogenous application of 6-benzylaminopurine (BA) increases the electron transport rate (ETR), reduces the relative variable fluorescence intensity and increases the quantum yield in wheat, leading to a higher photosynthetic rate. In addition, endogenous zeatin (Zt) stimulates the activity of antioxidant enzymes and reduces malondialdehyde (MDA) content.
GAs stimulate the growth of most organs through cell elongation and help stimulate cell division (Colebrook et al. 2014). Environmental signaling by light actively participates in the formation of functional chloroplasts and their survival. In the dark, chlorophyll precursors can have harmful effects when illuminated; thus, plants produce plastids as a form of adaptive mechanism for developmental control in the transition to light. In addition to stimulating photosynthesis, exogenous GA3 alters differential gene expression in sugarcane, enabling greater tolerance to drought (Tripathi et al. 2019).
GA3 is an important hormonal regulator, coordinating the growth and elongation of sugarcane internodes, promoting a greater concentration of sucrose in the stalks (Chen et al. 2021). Studies on the exogenous application of growth regulators at the final stages of the sugarcane development or in the maturation phenological phases are scarce, with little understanding of the effects of plant growth regulators, specifically AXS (IAA), CKS (Zeatin) and GAs (GA3).
The aim of this study was to evaluate the effects of the foliar application of exogenous plant growth regulators on sugarcane, including photosynthesis, sugar and stalk yields, and the regulation of antioxidant enzymes. The central hypothesis of this study is that applying plant growth regulators such as AXs (IAA), CKs (zeatin) and GAs (GA3) to sugarcane will increase the photosynthetic rate by stimulating the enzymes phosphoenolpyruvate carboxylase (PEPcase; EC:4.1.1.31) and Rubisco and antioxidant enzymes involved in ROS scavenging, leading to improvements in sugarcane yield, raw material quality, biometric parameters, and biomass production.
Materials and Methods
Site Description
The study was carried out in 15 commercial sugarcane areas in Brazil in 2017, 2019 and 2020 in the early (May–June), mid-late (August) and late (November–December) harvest seasons. The field experiments were located in Pontal-SP (1), Santa Maria da Serra-SP (2), Luís Antônio-SP (3), Paulo Faria-SP (4), Mirassol-SP (5), Olímpia-SP (6), Uberaba- MG (7), Agissê-SP (8), Cruzeiro do Oeste-PR (9), Sertãozinho-SP (10 and 13), Igaraçu do Tietê-SP (11 and 14) and Pradópolis-SP (12 and 15). The details of the harvest times, geographic coordinates, cultivars, crop management, row spacing, and applications are shown in Table 1. All cultivars were managed according to recommendations for the specific environmental conditions (Daros et al. 2010; UDOP 2018).
The predominant climates in the experiments were Aw, with hot, rainy summers; Cfa, temperate without a dry season and with hot summers; and Cwa, temperate with hot, humid summers according to the Köppen-Geiger climate classification. The average temperatures in the early, mid-late and late seasons were 22.4 °C, 21.6 °C and 21.7 °C, respectively. Precipitation data were obtained from meteorological stations located near each site (Table 2).
Soil classification was performed using the international system (Soil Survey Staff 2014). The soil characteristics (0.00–0.25 and 0.25–0.50 m depths) were determined prior to the installation of the experiments according to van Raij et al. (1997). The soil data are shown in Table 3.
Experimental Design and Treatment Applications
The plots consisted of eight rows with a length of 10 m; the inter-row spacing at each site is provided in Table 1. The experimental design consisted of three treatments of foliar plant growth regulator application in completely randomized blocks with 8 replications in the early, mid-late, and late harvest seasons. The treatments were as follows: (i) control with no application of plant growth regulator (control), (ii) foliar application of plant growth regulator at the vegetative stage of sugarcane (V), and (iii) foliar application of plant growth regulator at the vegetative and maturation stages of sugarcane (VM). Plant growth regulator application at the vegetative stage was performed 120, 160 and 60 days before harvest (DBH) in the early, mid-late and late harvest seasons, respectively; plant growth regulator application at the maturation stage was applied 35 DBH in all sugarcane harvest seasons (Fig. 1).
Foliar plant growth regulator application was performed in each plot by spraying with pressurized backpack equipment (CO2) coupled to a 2.6-m-long rod with a single tip, brass jet type 1/4KLC-9 with an average flow of 100 L ha−1 and a pressure of 4 kgf cm2 or 58.0 PSI. The plant growth regulator doses were 17 ppm GA3 activity, 817 ppm IAA activity and 43 ppm zeatin.
The preparation of the plant regulator was carried out by combining two commercial products, 2 kg ha−1 of Raizal composed by 400 ppm kg−1 of IAA, and 0.5 l ha−1 of Biozyme composed by 33 ppm L−1 of GA3 activity, 33 ppm L−1 of IAA activity and 85 ppm L−1 of zeatin activity (UPL Brazil).
Photosynthetic and Antioxidant Enzymes
Fully expanded (+1) or top visible dewlap (TVD) leaves were collected from early-harvest canes, stored in 50-mL Falcon tubes and frozen in liquid nitrogen immediately after collection for the analysis of photosynthetic enzymes and antioxidants. The leaves were collected between 8:00 and 10:00 a.m. in all plots of site 5 (Mirassol-SP). After collection, the samples were taken to the laboratory and stored in a freezer at − 80 °C. Enzymatic analyses were performed only at site 5 due to the high cost, distance and difficulty of storing samples.
Leaf samples were crushed in a mortar with liquid N to obtain a fine powder, and 1 part crude extract was homogenized with 2 parts extraction buffer on ice for 20 s in an Ultra-Stirred (BIOMT: 0.5 to 250 mL, 5 mm stainless steel rod; 50–60 HZ).
The activity of PEPcase (EC:4.1.1.31) was determined by monitoring the oxidation of NADH in a spectrophotometer at 340 nm for 120 s and expressed in μmol CO2 min−1 mg−1 protein using a molar absorptivity of 6.22 M−1 cm−1 (Degl’Innocenti et al. 2003).
Rubisco (EC4.1.1.39) activity was determined by grinding leaves in liquid N and extraction in ice-cold Eppendorf tubes containing 0.2 M KPi (pH 7.8), 5 mM MgCl2, 1 mM DTT, 1% (w/v) polyvinylpyrrolidone PM 40,000 (PVP-40), and 5 mM acid ascorbic acid (Sigma-Aldrich). The tubes were centrifuged at 14,000×g and 4 °C for 30 min, and the supernatant was immediately used to measure Rubisco activity at 25 °C (ASHTON et al. 1990). Rubisco was activated by incubation (35 μL of crude extract) with 450 μL of buffer containing 100 mM Bicine (pH 8.0), 25 mM KHO3, 20 mM MgCl2, 3.5 mM ATP, 5 mM phosphocreatine, 0.25 mM NADH, 80 nkat G-3-P dehydrogenase (EC1.2.1.12), 80 nkat 3-phosphoglycerate phosphokinase (EC 2.7.2.3), 80 nkat creatine phosphokinase (EC 2.7.3.2) and 0.25 mM NADH for 15 min. The oxidation of NADH was initiated by the addition of 0.5 mM RuBP (Sigma-Aldrich). The difference in absorbance at 0 and 3 min was used to calculate the activity expressed in μmol CO2 min−1 mg−1 protein using a molar absorptivity of NADH of 6.22 M−1 cm−1. Rubisco (EC4.1.1.39) activity was determined by measuring the rate of NADH oxidation at 340 nm on lambda 3B spectrophotometer (Raij et al. 1997).
Superoxide dismutase (SOD; EC:1.15.1.1) and catalase (CAT; EC:1.11.1.6) activity were extracted according to the methodology proposed by Silva et al. (2020). Extraction was performed in buffer [200 mM KPi (pH 7.8) containing 10 mM EDTA, 20 mM ascorbic acid, 1% PVP-40 (Sigma-Aldrich) and 1 mM 1,4-dithiothreitol (DTT, Sigma-Aldrich)]. Crude extracts for SOD and CAT activity were obtained from the supernatant after centrifugation at 12,000×g for 30 min at 4 °C. The units of the photochemical activity of SOD were expressed in mg of protein and obtained in an assay system consisting of 13 mM methionine (Sigma-Aldrich), 100 nM EDTA, 2 µM riboflavin (Sigma-Aldrich) and 75 µM NBT (Sigma-Aldrich) in 50 mM KPi buffer. The initial rate of the reaction was determined as increase of absorbance at 560 nm to superoxide dismutase (SOD; EC:1.15.1.1) (Giannopolitis and Ries 1977). Catalase activity was determined by measuring the rate of decrease in absorbance at 240 nm of a solution of 12.5 mM H202 in 50 mm K-phosphate (pH 7.0) at 30 °C. CAT activity was assayed according to Havir and Mchale (1987) by monitoring the consumption of 250 μM hydrogen peroxide and expressed in μmol min−1 mg−1 protein using a molar extinction coefficient of 39.4 mM−1 cm−1.
Ascorbate peroxidase (APX; EC:1.11.1.11) activity was measured by extraction in 10 mM EDTA, 1% PVP-40, 1 mM DTT, and 200 mM KPi (pH 6.0). The extract obtained after homogenization was centrifuged for 30 min at 12,000×g and 4 °C. The reaction was initiated by adding 1 mM hydrogen peroxide and 80 μM ascorbic acid to the crude extract. The decrease in absorbance at 290 nm was monitored for 120 s, and the results were expressed in μmol min−1 mg−1 protein (Nakano and Asada 1981; Koshiba 1993).
Free peroxidase activity (POD; EC:1.11.1.7) was analyzed in buffered solution containing 10 mM EDTA, 1% PVP-40, and 200 mM KPi (pH 6.7) using an extract collected by centrifugation at 8,000 ×g for 20 min at 4 °C. The rate of hange in absorbance of the mixture was measured at 500 nm at 37 °C (Allain et al. 1974).
MDA levels were determined by reacting leaf tissue extracts with thiobarbituric acid (TBA). The resulting TBA-MDA adduct was measured spectrophotometrically at 532 nm. The MDA concentration was calculated from a standard curve of 1,1,3,3-tetramethoxypropane (TPE) and expressed in nanomoles of MDA per g of fresh weight (Little and Gladen 1999). The concentration of hydrogen peroxide (H2O2) was determined according to the method of Alexieva et al. (2001) using a standard curve prepared with known concentrations of H2O2. Proline content was measured based on the methodology proposed by Bates et al. (1973).
Sugarcane Measurements
The biometric parameters consisted of plant height of 10 sugarcane plants per replicate, which was measured from the base of the stalk to the base of the TVD (+3) (Dillewijn 1960); stalk diameter, which was measured at the third internode in the same 10 sugarcane plants that were measured at height; and stalk yield (StY), which was determined by harvesting the sugarcane plants in 4 linear m, with two mirrored in central two rows of each plot and extrapolating to tons of sugarcane per hectare.
Sucrose, juice purity (PUR), fiber (FIB), reducing sugars (RS) and theoretical recoverable sugars (TRS) were determined by collecting 10 sugarcane plants per replicate (Supplementary Material). Laboratory analyses were performed according to the methodology of Fernandes (Fernandes 2011). Sugar per hectare, i.e., sugar yield (SY), was calculated by multiplying StY by TRS and dividing by 1000.
Energy production was analyzed using the FIB and StY results at 50% humidity to determine bagasse productivity. Straw yield was calculated by multiplying StY by 140, and energy production was calculated assuming that 1 Mg of straw produces 4.96 MWh of primary energy (Waldheim et al. 2001).
Data Analysis
The homogeneity of variances and data normality were evaluated with the F-Bartlett (Snedecor and Cochran 1983) and Shapiro–Wilk tests (Shapiro and Wilk 1965), respectively. The values were submitted to analysis of variance (ANOVA) to compare means between the treatments in each sugarcane harvest (early, mid-late and late) by the LSD test (p < 0.10) in SISVAR (Ferreira 2014).
Results
Photosynthetic Enzyme Activities, Oxidative Stress and Antioxidant Enzyme Activities
In early-harvest sugarcane, foliar plant growth regulator application increased PEPcase activity in VM (56.1 µmol CO2 min−1 mg−1 prot) and V (52.3 µmol CO2 min−1 mg−1 prot) compared with the control (34.3 µmol CO2 min−1 mg−1 prot) (Fig. 2A). Sugarcane Rubisco activity in V and VM was 0.68 and 0.77 µmol CO2 min−1 mg−1 prot higher than in the control (1.55 µmol CO2 min−1 mg−1 prot) (Fig. 2B).
Activities of phosphoenolpyruvate carboxylase (PEPcase) (A) and ribulose-1,5-bisphosphate carboxylase-oxygenase (Rubisco), (B) in early harvest sugarcane in 2020 as a function of foliar plant growth regulator application. The treatments were as follows: control, control with no foliar application of plant growth regulator (white); V, foliar application of plant growth regulator at the vegetative stage (blue); VM, foliar application of plant growth regulator at the vegetative and maturation stages (green). Means followed by the same letter do not differ by the t test (LSD) at 10% probability (Color figure online)
In general, foliar plant growth regulator application decreased MDA and H2O2 contents (Fig. 3A and B). H2O2 content decreased by 36.7% in VM compared with the control (3.85 µmol CO2 min−1 mg−1 prot). MDA content decreased by 14.2% and 36.2% in V and VM, respectively, compared with the control.
Malondialdehyde (MDA) content (A), hydrogen peroxide (H2O2) content (B), superoxide dismutase (SOD) activity (C), peroxidase (POD) activity (D), catalase activity (CAT) (E), ascorbate peroxidase (APX) activity (F) and proline content (G) in early harvest sugarcane in 2020 as a function of foliar plant growth regulator application. The treatments were as follows: control, control with no foliar application of plant growth regulator (white); V, foliar application of plant growth regulators at the vegetative stage (blue); VM, foliar application of plant growth regulators at the vegetative and maturation stages (green). Means followed by the same letter do not differ by the t test (LSD) at 10% probability (Color figure online)
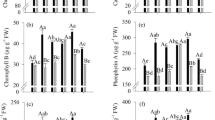
Foliar plant growth regulator application did not affect SOD activity but did increase POD, CAT, and APX activities and proline content (Fig. 3C–G). POD activity increased by 3.23-fold in VM compared with the control (0.13 µmol CO2 min−1 mg−1 prot) (Fig. 3D). CAT activity was 9.87 and 23.8% higher in V and VM than in the control (Fig. 3E). APX activity increased by 18.4% and 23.5% in V and VM, reaching 0.64 µmol CO2 min−1 mg−1 prot (Fig. 3F). Finally, proline content increased by 12.5 and 16.9% in V and VM, respectively, compared with the control (0.31 µmol g−1 FW) (Fig. 3G).
Agronomic Parameters
Foliar plant growth regulator application did not affect plant height or stalk diameter (Fig. 4A–F) but significantly (p < 0.10) increased sugarcane StY in all harvest seasons (Fig. 4G, H and I). In early, mid-late, and late harvest sugarcane, the average StY was 127, 101, and 91 Mg ha−1 in V, 130, 104, and 93 Mg ha−1 in VM, and 118, 97, 87 Mg ha−1 in the control (Fig. 4G, H and I). These values correspond to average increases of 7.1%, 4.8% and 4.4% in V and 9.7%, 7.7% and 6.7% in VM.
Plant height (A–C), stalk diameter (D–F) and stalk yield (G–I) of early, mid-late and late harvest sugarcane as a function of foliar plant growth regulator application. The treatments were as follows: control, control with no foliar application of plant growth regulator (white); V, foliar application of plant growth regulators at the vegetative stage (blue); VM, foliar application of plant growth regulators at the vegetative and maturation stages (green). Means followed by the same letter do not differ by the t test (LSD) at 10% probability (Color figure online)
Quality Parameters
In general, foliar plant growth regulator application increased sucrose content and TRS in early-harvest sugarcane and sugar production in all harvest seasons (p < 0.10) (Fig. 5A). The highest sucrose contents were 10.9% in VM in 2019 and 12.4% in both V and VM in 2020. The largest increases in TRS occurred in early-harvest sugarcane and were 6.1% (111 kg of sugar ha−1) in VM in 2019 and 5.7 and 5.8% (124 kg of sugar ha−1) in V and VM, respectively, in 2020; TRS was lowest in the control in 2019 and 2020, with values of 104 and 117 kg of sugar ha−1, respectively (Fig. 5D). Foliar application also increased sugar yield, which averaged 15.2 Mg ha−1 in V and VM (Fig. 5G, H and I). Compared with the control (14.4, 15.4 and 11.9 Mg ha−1), sugar yield increased by 11.0%, 7.1% and 5.1% in V and 14.6%, 10.3% and 7.7% in VM in early, mid-late and late harvest sugarcane, respectively. In early and mid-late harvest sugarcane, sugar yield was similar in V and VM, whereas in late harvest sugarcane, sugar yield was higher in VM than in V (2017).
Sucrose (A–C), theoretical recoverable sugar (TRS) (D–F) and sugar yields (G–I) in early, mid-late and late harvest sugarcane as a function of foliar plant growth regulator application. The treatments were as follows: control, control with no foliar application of plant growth regulator (white); V, foliar application of plant growth regulators at the vegetative stage (blue); VM, foliar application of plant growth regulators at the vegetative and maturation stages (green). Means followed by the same letter do not differ by the t test (LSD) at 10% probability (Color figure online)
Energy Parameters
Foliar plant growth regulator application increased straw yield significantly in early harvest sugarcane (Fig. 6A) but not mid-late and late harvest sugarcane (Fig. 6B and C), with average increases of 9.7% in VM compared with the control (16.6 Mg ha−1) in early harvest sugarcane (Fig. 6A). V and VM also increased bagasse production in early harvest sugarcane (Fig. 6D) but not mid-late and late harvest sugarcane (Fig. 6E and F). Compared with the control, V and VM significantly increased bagasse by averages of 6.9% and 10.2% compared with the control (6.8 Mg ha−1) (Fig. 6D). Finally, energy production in early harvest sugarcane was 7.1% higher in V and 9.7% higher in VM than in the control (82.1 kWh) (Fig. 6G); foliar plant growth regulator application did not affect energy production in mid-late and late harvest sugarcane (Fig. 6H and I).
Straw (A–C), bagasse (D–F) and energy production (G–I) in early, mid-late and late harvest sugarcane as a function of foliar plant growth regulator application. The treatments were as follows: control, control with no foliar application of plant growth regulators (white); V, foliar application of plant growth regulators at the vegetative stage (blue); VM, foliar application of plant growth regulators at the vegetative and maturation stages (green). Means followed by the same letter do not differ by the t test (LSD) at 10% probability (Color figure online)
Discussion
Plant growth regulators are a diverse class of biomolecules that promote plant acclimatization to environmental conditions by regulating development, growth, nutrient acquisition and allocation, and molecular and physiological crop responses (Sabagh et al. 2021; Hirayama and Mochida 2022).
In the present study, foliar application of plant growth regulators stimulated the photosynthetic process by increasing Rubisco and PEPcase activities, inducing high yield and quality of sugarcane when performed once (vegetative growth stage) or twice (vegetative and maturation growth stages).
Plant growth regulators can directly affect photosynthesis by regulating the expression of genes involved in the photosynthetic process, or can indirectly regulate physiological processes that affect photosynthesis, such as the opening and closing mechanism of stomata and consequent CO2 entry (Poonam et al. 2015). CKs promote cell division and plant growth, but also stimulate photosynthetic rate in plant leaves. AXs, GAs and strigolactones also influence photosynthesis and play an important role in reducing the production of reactive oxygen species (ROS) that can damage the photosynthetic machinery (Müller et al. 2021). In summary, plant grow regulators play an important role in regulating photosynthesis in plants, both under optimal conditions and under stress conditions.
Both timings of plant growth regulator application (V and VM) helped decrease ROS levels, as H2O2 and MDA contents were reduced. These decreases were the result of higher antioxidant enzyme activities (SOD, CAT, POD and APX) in plant growth regulators treated sugarcane (Wu et al. 2018). SOD, CAT, POD and APX form a complex enzymatic antioxidant system that protects the photosynthetic process from oxidative stress and prevents ROS production (Gill and Tuteja 2010; Farooq et al. 2019). These four key cellular detoxification enzymes convert H2O2 to H2O during the plant cycle (Gupta et al. 2018). Thus, foliar plant growth regulator application can promote antioxidant activity in sugarcane and mitigate unfavorable environmental conditions.
Several studies have reported that hormones, e.g., ethylene, AXs, GAs, and CKs, are important metabolic engineering targets for stimulating crop development and production and improving abiotic stress tolerance in plants (Fahad et al. 2015; Raza et al. 2019). Foliar plant growth regulator application is a recent practice in agriculture around the world (Jiang and Asami 2018; Jalil and Ansari 2019; Khan et al. 2023), and there are few studies of its effectiveness in sugarcane in different harvest seasons in tropical regions.
The application of plant growth regulators (IAA, zeatin and GA3) during sugarcane production has positive effects on crop development, as evidenced by gains in StY. Plant growth regulators help plants overcome various environmental deficits, such as abiotic stresses and low availability of soil nutrients (Fraire-Velázquez et al. 2011) which may have positively influenced the increase in profitability and sugarcane quality.
The first application performed at the vegetative stage of sugarcane promoted greater stalk productivity, consequently greater sugar production through the high concentration of IAA, which stimulated cell elongation and regulation of gene expression (Cohen and Gray 2006; Tuan et al. 2019). Other functions of IAA include promoting adequate nutrition and controlling plant growth under stress (Mano and Nemoto 2012; Raza et al. 2019). Although the essential role of IAA in plants is well known (Javid et al. 2011; Kazan 2013), IAA-related metabolism and pathways and the interactions of IAA with nutrients and other plant growth regulators in specific crops, such as sugarcane, are still unclear, particularly under different growth conditions.
GAs act in germination, stem elongation, leaf expansion, trichome initiation and plant development (Yamaguchi 2008). GAs positively influence the photosynthetic rate, light interception, nutrient use, and the regulation of several processes throughout the plant life cycle (Khan et al. 2007). Additionally, GAs promoted a mechanism of low stomatal processes related to plant stress resistance, an increase of water use efficiency and consequent high crop yields (Maggio et al. 2010). GA3 frequently interact with other plant growth regulators and promote crop development and pathway activation (Wang and Irving 2011; Gupta and Chakrabarty 2013).
The plant growth regulators are extremely important in different stages of crop growth and development, which can explain the differences in the effects of foliar plant growth regulator application once at the vegetative stage and twice at the vegetative and maturation stages of sugarcane. Single foliar plant growth regulator application at the sugarcane vegetative stage stimulated growth, i.e., more parenchymatous cells to store sucrose in the next stages.
At vegetative stage of sugarcane, IAA activates cell division and plant development by stimulating the growth of roots, stalks and leaves (McSteen 2010; Phillips et al. 2011), and GA3 regulate cell division and elongation, promote hypocotyl and stem growth, and increase root and leaf meristem size (Hedden and Thomas 2016). Our results suggest that plant growth regulator application at the vegetative stage can increase sugarcane development and yields by enhancing plant photosynthesis overall.
In the treatments with two foliar applications of plant growth regulators (vegetative and maturation stages), the first plant growth regulator application stimulated vegetative development and the formation of parenchymatous cells to store sucrose, and the second plant growth regulator application increased photoassimilate and sucrose production. The benefits of foliar application of plant growth regulators at two stages of sugarcane growth included increases in biometric parameters and sucrose, TRS and sugar yields. Plant growth regulators can promote shoot growth, increase xylem and decrease root growth (Guo et al. 2015; Wang et al. 2015). Chen et al. (2021) showed that applying plant growth regulators at the maturation stage increases sucrose phosphate synthase activity in leaves and stalks while decreasing soluble acid invertase activity in stalks, thereby increasing sucrose production and accumulation.
Sucrose content and sugar production are important indicators of sugarcane quality, and sugarcane industry efforts are focused on improving sucrose content and accumulation (Rossetto et al. 2003; Cunha et al. 2020). Products that can be applied at the maturation stage to increase sucrose accumulation are desirable, especially plant growth regulators that enhance sugarcane yield and mitigate environmental stresses in plants. In this study, the increases in stalk productivity and sucrose content were accompanied by gains in bagasse, straw and energy production, suggesting potential economic gains.
In most countries, the economy is dependent on agriculture, which relies on suitable climate conditions and fertile soil. An extensive body of research has examined the molecular mechanisms that regulate hormone synthesis, signaling, and actions; plant growth regulators have many functions in plant development and responses to abiotic stresses. Our results indicate that appropriate supplementation of sugarcane with plant growth regulators can enhance yields and quality by improving plant metabolism, regardless of harvest season.
Conclusion
This study found that a single application of plant growth regulators was sufficient to enhance sugarcane production when applied at 100, 75, and 60 days before harvest in early, mid-late and late harvest seasons. Both a single application at the vegetative stage and two applications at the vegetative and maturation stages (applied at 30 days before harvest in all sugarcane harvest seasons) increased sugarcane growth and productivity. We found that foliar plant growth regulator application at the vegetative and maturation stages stimulated sugarcane development and enhanced photosynthetic and antioxidant metabolism (SOD, CAT, POD and APX). Application at both stages resulted in metabolic improvements that increased sucrose accumulation, stalk and sugar yields, and crop development and productivity. Relevant questions about which hormone are the main drivers of plant metabolism processes in this specific application timing and its synergetic or antagonistic impact deserve further investigations.
References
Akhtar SS, Mekureyaw MF, Pandey C, Roitsch T (2020) Role of cytokinins for interactions of plants with microbial pathogens and pest insects. Front Plant Sci. https://doi.org/10.3389/fpls.2019.01777
Alabadí D, Gallego-Bartolomé J, Orlando L et al (2008) Gibberellins modulate light signaling pathways to prevent Arabidopsis seedling de-etiolation in darkness. Plant J 53:324–335. https://doi.org/10.1111/j.1365-313X.2007.03346.x
Alexieva V, Sergiev I, Mapelli S, Karanov E (2001) The effect of drought and ultraviolet radiation on growth and stress markers in pea and wheat. Plant Cell Environ 24:1337–1344. https://doi.org/10.1046/j.1365-3040.2001.00778.x
Allain CC, Poon LS, Chan CSG et al (1974) Enzymatic determination of total serum cholesterol. Clin Chem 20:470–475. https://doi.org/10.1093/clinchem/20.4.470
Asgher M, Khan MIR, Anjum NA, Khan NA (2015) Minimising toxicity of cadmium in plants—role of plant growth regulators. Protoplasma 252:399–413. https://doi.org/10.1007/s00709-014-0710-4
Ashton AR, Burnell JN, Furbank RT et al (1990) 3—Enzymes of C4 photosynthesis. In: Lea PJ (ed) Methods in plant biochemistry. Academic Press, London, pp 39–72
Bates LS, Waldren RP, Teare ID (1973) Rapid determination of free proline for water-stress studies. Plant Soil 39:205–207. https://doi.org/10.1007/BF00018060
Brenner WG, Schmülling T (2012) Transcript profiling of cytokinin action in Arabidopsis roots and shoots discovers largely similar but also organ-specific responses. BMC Plant Biol 12:112. https://doi.org/10.1186/1471-2229-12-112
Cardozo NP, Sentelhas PC (2013) Climatic effects on sugarcane ripening under the influence of cultivars and crop age. Sci Agric 70:449–456. https://doi.org/10.1590/S0103-90162013000600011
Cardozo NP, Sentelhas PC, Panosso AR, Ferraudo AS (2014) Multivariate analysis of the temporal variability of sugarcane ripening in south-eastern Brazil. Crop Pasture Sci 65:300–310. https://doi.org/10.1071/CP13160
Cardozo NP, de Bordonal RO, Panosso AR, Crusciol CAC (2020) A multivariate approach to determine the economic profitability of sugarcane production under diverse climatic conditions in Brazil. Sugar Tech 22:954–966. https://doi.org/10.1007/s12355-020-00854-7
Cheminant S, Wild M, Bouvier F et al (2011) DELLAs regulate chlorophyll and carotenoid biosynthesis to prevent photooxidative damage during seedling deetiolation in Arabidopsis. Plant Cell 23:1849–1860. https://doi.org/10.1105/tpc.111.085233
Chen D, Zhou W, Yang J et al (2021) Effects of seaweed extracts on the growth, physiological activity, cane yield and sucrose content of sugarcane in China. Front Plant Sci 12. https://doi.org/10.3389/fpls.2021.659130
Cohen JD, Gray WM (2006) Auxin metabolism and signaling. Plant hormone signaling. John Wiley & Sons, Ltd, Hoboken, pp 37–66
Colebrook EH, Thomas SG, Phillips AL, Hedden P (2014) The role of gibberellin signalling in plant responses to abiotic stress. J Exp Biol 217:67–75. https://doi.org/10.1242/jeb.089938
Cortleven A, Schmülling T (2015) Regulation of chloroplast development and function by cytokinin. J Exp Bot 66:4999–5013. https://doi.org/10.1093/jxb/erv132
Cunha FN, Teixeira MB, Soares FAL et al (2020) Industrial quality of sugarcane under fertigation with nitrogen and zinc. Sugar Tech 22:232–240. https://doi.org/10.1007/s12355-019-00762-5
Daros E, Oliveira RA, Zambon JLC, Bespalhok Filho J (2010) Catálogo nacional de variedades “RB” de cana-de-açúcar
Davies PJ (2010) The plant hormones: their nature, occurrence, and functions. In: Davies PJ (ed) Plant hormones: biosynthesis, signal transduction, action! Springer, Netherlands, pp 1–15
de Silva MA, Cato SC, Costa AGF (2010) Produtividade e qualidade tecnológica da soqueira de cana-de-açúcar submetida à aplicação de biorregulador e fertilizantes líquidos. Cienc Rural 40:774–780. https://doi.org/10.1590/S0103-84782010005000057
Degl’Innocenti E, Vaccà C, Guidi L, Soldatini GF (2003) CO2 photoassimilation and chlorophyll fluorescence in two clover species showing different response to O3. Plant Physiol Biochem 41:485–493. https://doi.org/10.1016/S0981-9428(03)00057-3
Dillewijn CV (1960) Botany of sugar cane. Botany of sugar cane
Egamberdieva D, Wirth SJ, Alqarawi AA et al (2017) Phytohormones and beneficial microbes: essential components for plants to balance stress and fitness. Front Microbiol. https://doi.org/10.3389/fmicb.2017.02104
Fahad S, Hussain S, Matloob A et al (2015) Phytohormones and plant responses to salinity stress: a review. Plant Growth Regul 75:391–404. https://doi.org/10.1007/s10725-014-0013-y
FAO (2021) Food and agriculture organization, commission on population and development. Population, food security, nutrition and sustainable development—Report of the secreary-general
Farooq MA, Niazi AK, Akhtar J et al (2019) Acquiring control: the evolution of ROS-Induced oxidative stress and redox signaling pathways in plant stress responses. Plant Physiol Biochem 141:353–369. https://doi.org/10.1016/j.plaphy.2019.04.039
Fernandes AC (2011) Cálculos na Agroindústria da Cana-de-Açúcar, 3rd edn. STAB, Piracicaba
Ferreira DF (2014) Sisvar: a guide for its bootstrap procedures in multiple comparisons. Ciência e Agrotecnol 38:109–112. https://doi.org/10.1590/S1413-70542014000200001
Foyer CH (2018) Reactive oxygen species, oxidative signaling and the regulation of photosynthesis. Environ Exp Bot 154:134–142. https://doi.org/10.1016/j.envexpbot.2018.05.003
Fraire-Velázquez S, Rodríguez-Guerra R, Sánchez-Calderón L (2011) Abiotic and biotic stress response crosstalk in plants. IntechOpen
Giannopolitis CN, Ries SK (1977) Superoxide dismutases: II. Purification and quantitative relationship with water-soluble protein in seedlings 1 2. Plant Physiol 59:315–318. https://doi.org/10.1104/pp.59.2.315
Gill SS, Tuteja N (2010) Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol Biochem 48:909–930. https://doi.org/10.1016/j.plaphy.2010.08.016
Guo H, Wang Y, Liu H et al (2015) Exogenous GA3 application enhances xylem development and induces the expression of secondary wall biosynthesis related genes in Betula platyphylla. Int J Mol Sci 16:22960–22975. https://doi.org/10.3390/ijms160922960
Gupta R, Chakrabarty SK (2013) Gibberellic acid in plant: still a mystery unresolved. Plant Signal Behav. https://doi.org/10.4161/psb.25504
Gupta DKJ, Palma JM, Corpas FJ (2018) Antioxidants and antioxidant enzymes in higher plants. Springer, Cham
Havir EA, McHale NA (1987) Biochemical and developmental characterization of multiple forms of catalase in tobacco leaves. Plant Physiol 84:450–455. https://doi.org/10.1104/pp.84.2.450
Hedden P, Thomas SG (2016) Annual plant reviews. John Wiley & Sons
Hirayama T, Mochida K (2022) Plant hormonomics: a key tool for deep physiological phenotyping to improve crop productivity. Plant Cell Physiol. https://doi.org/10.1093/pcp/pcac067
Hughes N, Mutran VM, Tomei J et al (2020) Strength in diversity? Past dynamics and future drivers affecting demand for sugar, ethanol, biogas and bioelectricity from Brazil’s sugarcane sector. Biomass Bioenerg 141:105676. https://doi.org/10.1016/j.biombioe.2020.105676
Iqbal N, Nazar R, Khan MIR et al (2011) Role of gibberellins in regulation of source–sink relations under optimal and limiting environmental conditions. Curr Sci 100:998–1007
Jalil SU, Ansari MI (2019) Role of phytohormones in recuperating salt stress. In: Akhtar MS (ed) Salt stress, microbes, and plant interactions: mechanisms and molecular approaches. Springer, Singapore, pp 91–104
Javid MG, Sorooshzadeh A, Moradi F, Allahdadi I (2011) The role of phytohormones in alleviating salt stress in crop plants. Aust J Crop Sci 5:726–734
Jiang K, Asami T (2018) Chemical regulators of plant hormones and their applications in basic research and agriculture*. Biosci Biotechnol Biochem 82:1265–1300. https://doi.org/10.1080/09168451.2018.1462693
Jogawat A, Yadav B et al (2021) Crosstalk between phytohormones and secondary metabolites in the drought stress tolerance of crop plants: a review. Physiol Plant 172:1106–1132. https://doi.org/10.1111/ppl.13328
Kazan K (2013) Auxin and the integration of environmental signals into plant root development. Ann Bot 112:1655–1665. https://doi.org/10.1093/aob/mct229
Khan NA, Singh S, Nazar R, Lone PM (2007) The source-sink relationship in mustard. Asian Aust J Plant Sci Biotechnol 9:10
Khan MIR, Singh A, Poor P (2023) Plant hormones in crop improvement, 1st edn. Academic Press
Koshiba T (1993) Cytosolic ascorbate peroxidase in seedlings and leaves of maize (Zea mays). Plant Cell Physiol 34:713–721. https://doi.org/10.1093/oxfordjournals.pcp.a078474
Leite GHP, Crusciol CAC, de Silva MA, Venturini Filho WG (2008) Reguladores vegetais e qualidade tecnológica da cana-de-açúcar em meio de safra. Ciênc e Agrotecnol 32:1843–1850. https://doi.org/10.1590/S1413-70542008000600024
Li W, Herrera-Estrella L, Tran L-SP (2016) The Yin–Yang of cytokinin homeostasis and drought acclimation/adaptation. Trends Plant Sci 21:548–550. https://doi.org/10.1016/j.tplants.2016.05.006
Little RE, Gladen BC (1999) Levels of lipid peroxides in uncomplicated pregnancy: a review of the literature. Reprod Toxicol 13:347–352. https://doi.org/10.1016/S0890-6238(99)00033-7
Ljung K (2013) Auxin metabolism and homeostasis during plant development. Development 140:943–950. https://doi.org/10.1242/dev.086363
Maggio A, Barbieri G, Raimondi G, De Pascale S (2010) Contrasting effects of GA3 treatments on tomato plants exposed to increasing salinity. J Plant Growth Regul 29:63–72. https://doi.org/10.1007/s00344-009-9114-7
Mahalingam R (2015) Consideration of combined stress: a crucial paradigm for improving multiple stress tolerance in plants. In: Mahalingam R (ed) Combined stresses in plants: physiological, molecular, and biochemical aspects. Springer, Cham, pp 1–25
Mandal M, Sarkar M, Khan A et al (2022) Reactive oxygen species (ROS) and reactive nitrogen species (RNS) in plants—maintenance of structural individuality and functional blend. Adv Redox Res 5:100039. https://doi.org/10.1016/j.arres.2022.100039
Manechini JRV, da Santos PHS, Romanel E et al (2021) Transcriptomic analysis of changes in gene expression during flowering induction in sugarcane under controlled photoperiodic conditions. Front Plant Sci. https://doi.org/10.3389/fpls.2021.635784
Mano Y, Nemoto K (2012) The pathway of auxin biosynthesis in plants. J Exp Bot 63:2853–2872. https://doi.org/10.1093/jxb/ers091
McSteen P (2010) Auxin and monocot development. Cold Spring Harb Perspect Biol 2:a001479. https://doi.org/10.1101/cshperspect.a001479
Müller M, Munné-Bosch S (2021) Hormonal impact on photosynthesis and photoprotection in plants. Plant Physiol 185:1500–1522. https://doi.org/10.1093/plphys/kiaa119
Muñoz P, Munné-Bosch S (2018) Photo-oxidative stress during leaf, flower and fruit development. Plant Physiol 176:1004–1014. https://doi.org/10.1104/pp.17.01127
Nakano Y, Asada K (1981) Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol 22:867–880. https://doi.org/10.1093/oxfordjournals.pcp.a076232
Pemadasa MA (1982a) differential abaxial and adaxial stomatal responses to indole-3-acetic acid in Commelina communis L. New Phytol 90:209–219. https://doi.org/10.1111/j.1469-8137.1982.tb03253.x
Pemadasa MA (1982b) Effects of phenylacetic acid on abaxial and adaxial stomatal movements and its interaction with abscisic acid. New Phytol 92:21–30. https://doi.org/10.1111/j.1469-8137.1982.tb03359.x
Phillips KA, Skirpan AL, Liu X et al (2011) vanishing tassel2 encodes a grass-specific tryptophan aminotransferase required for vegetative and reproductive development in maize. Plant Cell 23:550–566. https://doi.org/10.1105/tpc.110.075267
Poonam, Khatkar SP, Kumar R et al (2015) Synthesis, characterization, enhanced photoluminescence and biological activity of Eu(III) complexes with organic ligands. J Mater Sci: Mater Electron 26:7086–7095. https://doi.org/10.1007/s10854-015-3330-7
Raza A, Mehmood SS, Tabassum J, Batool R (2019) Targeting plant hormones to develop abiotic stress resistance in wheat. In: Hasanuzzaman M, Nahar K, Hossain MDA (eds) Wheat production in changing environments: responses, adaptation and tolerance. Springer, Singapore, pp 557–577
Rossetto MRM, Purgatto E, do Nascimento JRO et al (2003) Effects of gibberellic acid on sucrose accumulation and sucrose biosynthesizing enzymes activity during banana ripening. Plant Growth Regul 41:207–214. https://doi.org/10.1023/B:GROW.0000007508.91064.8c
Sabagh AE, Mbarki S, Hossain A et al (2021) Potential role of plant growth regulators in administering crucial processes against abiotic stresses. Front Agron. https://doi.org/10.3389/fagro.2021.648694
Shapiro SS, Wilk MB (1965) An analysis of variance test for normality (complete samples). Biometrika 52:591–611. https://doi.org/10.1093/biomet/52.3-4.591
Silva VM, Tavanti RFR, Gratão PL et al (2020) Selenate and selenite affect photosynthetic pigments and ROS scavenging through distinct mechanisms in cowpea (Vigna unguiculata (L.) walp) plants. Ecotoxicol Environ Saf 201:110777. https://doi.org/10.1016/j.ecoenv.2020.110777
Snaith PJ, Mansfield TA (1984) Studies of the inhibition of stomatal opening by naphth-1-ylacetic acid and abscisic acid. J Exp Bot 35:1410–1418. https://doi.org/10.1093/jxb/35.10.1410
Snedecor GW, Cochran WG (1983) Statistical Methods. 6th Edition, Oxford and IBH, New Delhi
Soil Survey Staff (2014) Keys to Soil Taxonomy. 12th Edition, USDA-Natural Resources Conservation Service, Washington DC.
Stern DB, Hanson MR, Barkan A (2004) Genetics and genomics of chloroplast biogenesis: maize as a model system. Trends Plant Sci 9:293–301. https://doi.org/10.1016/j.tplants.2004.04.001
Takahashi S, Badger MR (2011) Photoprotection in plants: a new light on photosystem II damage. Trends Plant Sci 16:53–60. https://doi.org/10.1016/j.tplants.2010.10.001
Tischler AL, Jeronimo EM, Lúcio AD et al (2021) Sugarcane harvest time for processing and technological quality of brown sugar. Pesq Agropec Bras 56:e02435. https://doi.org/10.1590/S1678-3921.pab2021.v56.02435
Tripathi P, Chandra A, Prakash J (2019) Physio-biochemical assessment and expression analysis of genes associated with drought tolerance in sugarcane (Saccharum spp. hybrids) exposed to GA3 at grand growth stage. Plant Biol 21:45–53. https://doi.org/10.1111/plb.12919
Tuan PA, Yamasaki Y, Kanno Y et al (2019) Transcriptomics of cytokinin and auxin metabolism and signaling genes during seed maturation in dormant and non-dormant wheat genotypes. Sci Rep 9:3983. https://doi.org/10.1038/s41598-019-40657-9
UDOP (2018) União Nacional de Bioenergia. Características Agronômicas das Variedades IAC
van Raij B, Cantarella H, Quaggio JA, Furlani AMC (1997) Recomedações de adubação e calagem para o Estado de São Paulo. Instituto Agronômico (IAC), Campinas
Waldheim L, Monis M, Verde Leal MR (2001) Biomass power generation: sugar cane bagasse and trash. Progress in thermochemical biomass conversion. John Wiley & Sons, Ltd, pp 509–523
Wang YH, Irving HR (2011) Developing a model of plant hormone interactions. Plant Signal Behav 6:494–500. https://doi.org/10.4161/psb.6.4.14558
Wang G-L, Que F, Xu Z-S et al (2015) Exogenous gibberellin altered morphology, anatomic and transcriptional regulatory networks of hormones in carrot root and shoot. BMC Plant Biol 15:290. https://doi.org/10.1186/s12870-015-0679-y
Weng J-K, Lynch JH, Matos JO, Dudareva N (2021) Adaptive mechanisms of plant specialized metabolism connecting chemistry to function. Nat Chem Biol 17:1037–1045. https://doi.org/10.1038/s41589-021-00822-6
Wu ZZ, Ying YQ, Zhang YB et al (2018) Alleviation of drought stress in Phyllostachys edulis by N and P application. Sci Rep 8:228. https://doi.org/10.1038/s41598-017-18609-y
Yamaguchi S (2008) Gibberellin metabolism and its regulation. Annu Rev Plant Biol 59:225–251. https://doi.org/10.1146/annurev.arplant.59.032607.092804
Yang DQ, Luo YL, Dong WH et al (2018) Response of photosystem II performance and antioxidant enzyme activities in stay-green wheat to cytokinin. Photosynthetica 56:567–577. https://doi.org/10.1007/s11099-017-0708-1
Yuan L, Xu DQ (2001) Stimulation effect of gibberellic acid short-term treatment on leaf photosynthesis related to the increase in Rubisco content in broad bean and soybean. Photosynth Res 68:39–47. https://doi.org/10.1023/A:1011894912421
Acknowledgements
The first author received a scholarship from the CAPES (Coordination for the Improvement of Higher Level Personnel—Finance Code 001). The authors thank the BP Bunge Bioenergia (Moema sugar mill), COCAL (Paraguaçu Paulista sugar mill), COFCO International Brasil S.A. (Potirendaba sugar mill), Delta Sucroenergia S.A. (Delta sugar mill), Raízen (Barra sugar mill and Santa Elisa sugar mill), Santa Terezinha (Umuarama sugar mill), São Martinho (São Martinho sugar mill), and Tereos (Cruz Alta sugar mill) groups for providing the experimental areas and analytical support. The fifth author would like to thank the National Council for Scientific and Technological Development (CNPq) for an award for excellence in research.
Author information
Authors and Affiliations
Contributions
Design the experiment: CACC. Obtain and process the data: CMH, APAPF and LMJ. Analyze the data: CMH and APAPF. Wrote the paper: CMH, APAPF and LM with contribution of all co-authors. All authors confirm being contributor of this work and has approved it for publication.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Additional information
Handling Editor: Boon Chin Tan.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
de Morais Hervatin, C., de Almeida Prado Filho, A.P., Momesso, L. et al. Effects of Plant Growth Regulators on Sugarcane Productivity and Quality of the Art Through the Increase in Photosynthetic and Antioxidant Activity. J Plant Growth Regul (2024). https://doi.org/10.1007/s00344-024-11354-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00344-024-11354-3