Abstract
Trichomes, which are special structures on the surfaces of plants, play an important role in plant defense. Trichomes in tomatoes are composed of multiple cells that are divided into glandular and non-glandular trichomes. Glandular trichomes can secrete a diverse array of specialized metabolites and terpenes are the main types. Previous studies have shown that JA and TOR signaling are associated with trichomes, and there may be an interaction between these, but the mechanism remains unclear. In this study, JA signaling and its key transcription factor SlMYC1 were shown to regulate the transcript levels of terpene synthesis precursor-related genes (MEP/MVA pathways). SlTOR positively regulated the formation of type VI trichomes through GC-MS, qRT-PCR, and transient transgenic assays, and the synthesis and accumulation of monoterpenes and sesquiterpenes were positively regulated through the expression of key genes for terpene synthesis. Subsequently, SlTOR was the direct target of SlMYC1 by yeast one-hybrid, GUS, and transient expression assays. Collectively, this study revealed that tomato trichomes were regulated by SlTOR, and SlMYC1 mediated the growth and development of trichomes and the synthesis of terpenes by directly binding the promoter of SlTOR to activate its expression. This lays the foundation for the molecular regulatory mechanism of multicellular trichomes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Plant trichomes, which are widely dispersed throughout the aboveground portions of plants, are hair-like extensions that originate from epidermal cells and possess distinct structures (Liu et al. 2016; Chang et al. 2018). As a physical barrier, trichomes, which are divided into glandular and non-glandular (Gasparini et al. 2021), can provide a physical defense against various external abiotic and biotic stresses, and glands can also secrete a variety of secondary metabolites to provide chemical protection (Kariyat et al. 2017; Galdon-Armero et al. 2018; Ashfaq et al. 2019; Tang et al. 2020; Huebbers et al. 2022). In addition, certain metabolites can also be used as pharmaceutical and industrial raw materials with important economic value (Serna et al. 2006). Hence, the investigation into the molecular mechanisms underlying the growth and development of plant trichomes establishes the basis for the theory of cell differentiation and holds significant implications for the protective role of trichomes in agricultural production.
Tomato trichomes, an important model for studying the mechanism of multicellular trichomes, are typically multicellular structures and a rich species with different shapes (Song et al. 2017). Tomatoes yield a variety of trichomes, encompassing eight distinct types, classified as either glandular (type I, type IV, type VI, and type VII) or non-glandular trichomes (type II, type III, type V, type VIII) (Glas et al. 2012). Glandular trichomes, which serve as the primary site for production of various specialized secondary metabolites, can synthesize, secrete, or store diverse specialized metabolites (Pichersky and Raguso 2018; Zhou and Pichersky 2020). Among them, monoterpenes and sesquiterpenes, which are derived from the VI glandular trichomes, represent the most varied and plentiful groups of plant metabolites (Xu et al. 2018). Research has indicated that the process of terpene synthesis can be divided into three distinct segments. First, the terpene precursors are obtained from two distinct pathways, namely the plastidic methyl erythritol phosphate (MEP) pathway and the mevalonate (MEV) pathway. Subsequently, the fundamental carbon framework of the terpene core is established. Ultimately, a multitude of terpenes are produced as the carbon skeleton undergoes alterations catalyzed by terpene synthases (TPSs) (Pichersky and Raguso 2018; Yang et al. 2021). Therefore, the study of MEP and MVA pathways and TPSs for terpene synthesis and accumulation is particularly important.
This study shows differences in the regulation of tomato trichomes from unicellular trichomes (Serna and Martin 2006). The unicellular trichome represented by Arabidopsis is primarily governed by transcription factors, such as R3-MYB, Basic helix-loop-helix (bHLH), R2R3-MYB, C2H2 zinc finger protein (C2H2), and WD40 repeat (WD40) (Hung et al. 2020; Zheng et al. 2021; Wang et al. 2022). Although tomato trichomes are also regulated by a variety of genes, they mainly include transcription factors of the HD-Zip IV subfamily, such as C2H2, R2R3-MYB, bHLH, and other gene family transcription factors (Chalvin et al. 2020). Downregulation of SlMX1, which belongs to the R2R3-MYB subfamily, decreases the density of tomato trichomes, whereas overexpression increases their density (Ewas et al. 2017). Furthermore, the Woolly (Wo) transcription factor belonging to the HD-ZIP family can enhance the initiation of type I trichomes. There is an elevation in the density of type I trichomes in Wo mutant plants, while the density of type I trichomes experiences a substantial reduction in Wo-RNAi plants (Chang et al. 2018). The formation of multicellular trichomes can be regulated by hormones, and different hormones contribute to the growth of specific types of trichomes (Yu et al. 2018; Chen et al. 2020; Yuan et al. 2021). Jasmonic acid (JA) plays a crucial role in the regulation of the formation and growth of type VI glandular trichomes (Yan et al. 2017; Gong et al. 2021).
The application of exogenous JA resulted in a notable augmentation in the trichome density of Artemisia annua and Lycopersicum esculentum (Wang et al. 2021). The interaction between the Jasmonate ZIM domain (JAZ) proteins and bHLH transcription factors (GL3, EGL3, and TT8), as well as MYB transcription factors (MYB75 and GL1), plays a crucial role in the regulation of trichome initiation and development. Upon degradation by JA, these proteins release a complex consisting of WD repeat/bHLH/MYB and subsequently activate downstream factors involved in trichome formation (Qi et al. 2011; Wang et al. 2021). The inhibition of H and HL activity in tomatoes is accomplished by the physical interaction of SlJAZ2, a JA signaling inhibitor. This interaction then triggers the activation of THM1, which acts as a suppressor for trichome formation (Hua et al. 2022). SlMYC1, an essential helix-loop-helix transcription factor and a modulator of JA signaling, has a vital function in controlling the development of type VI glandular trichomes; furthermore, it has unique regulatory roles in the production of mono- and sesquiterpenes in the glandular cells. Downregulation of SlMYC1 led to lower densities of type VI glandular trichomes. Strikingly, the SlMYC1 protein, which governs the growth of type VI glandular trichomes, concurrently oversees the transcriptional activity of numerous terpene synthases (Xu et al. 2018). In addition, SlMYC1 plays a role in the biosynthesis of terpenes mediated by JA. The regulatory module of Wo/SlMYC1 is hindered by SlJAZ2 through a mechanism of competitive binding, leading to a precisely regulated JA response in the trichomes of tomato plants (Hua et al. 2021a). Although we know the importance of JA and SlMYC1 for trichomes, certain underlying mechanisms are still not fully resolved.
Target of rapamycin (TOR), the serine/threonine protein kinase, is widely conserved among eukaryotes and plays a crucial role in regulating the growth and development of plants (Boutouja et al. 2019). Our laboratory study found that after SlTOR was inhibited, tomato trichomes significantly changed, but the relationship between SlTOR and trichomes was not involved. TOR signaling pathway significantly interacts with JA signaling (Zheng et al. 2019; Awasthi et al. 2020; Wang et al. 2020). In cotton, JA biosynthesis and signaling mutants (jar1, coi1-2, and myc2-2) displayed a TOR inhibitor-resistant phenotype, whereas COI1-overexpressing transgenic lines and jaz10 exhibited sensitivity to AZD8055 (Song et al. 2017). However, the relationship between JA and TOR in the regulation of trichomes and related molecular mechanisms remains unclear.
Currently, there is limited information available regarding the control of JA and SlMYC1 in the growth, development, and terpene production of tomato trichomes. However, further research is required to uncover the underlying mechanisms involved in these processes (Xu et al. 2018; Hua et al. 2021a).
The study conducted on the model plant tomato identified the role of JA and SlMYC1 in the MEP/MEV pathway. Additionally, it was found that SlTOR plays a crucial role in trichome formation and the synthesis and accumulation of terpenoids. The study further demonstrated that SlMYC1 regulates the growth and development of trichomes and the synthesis of terpenes by targeting the SlTOR promoter and activating its expression. These findings provide a basis for understanding the molecular regulatory mechanism of multicellular trichomes.
Materials and Methods
Plant Material and Growing Environment
Tomato (Solanum lycopersicum L. cultivars Jinguan No. 5) and tobacco (Nicotiana tabacum L.) plants used in this study, and they were grown in a glasshouse with 23/18 °C day/night temperatures and a 16/8 h light/dark photoperiod cycle with a light intensity of 20,000 Lux.
RNA Isolation and qRT-PCR Analysis
The stems and the fourth leaf were gathered and then wrapped in aluminum foil before being stored at -80 °C. Prior to RNA extraction, the samples were rapidly frozen in liquid nitrogen. The RNA extraction process involved the use of Trizol reagent (CWBIO, Jiangsu, China) to isolate total RNA. Subsequently, cDNA synthesis was carried out using the FastKing cDNA First-Strand Synthesis Kit (TIANGEN, Beijing, China). The resulting cDNA was then subjected to qRT-PCR using the SYBR Green PCR Master Mix kit. The primer sequences used for qRT-PCR are seen from Table S1. To determine the expression levels, the transcript level of Actin was used as a reference for normalization.
Plant Treatments
For in planta experiments, 50 μM MeJA and 5 μM AZD8055 (TOR inhibitor) were applied to 4-week-old tomato seedlings for 24 h, respectively. Stem pieces and leaflets were collected for RNA isolation and volatile terpene measurements 24 h later. To prevent any potential cross-contamination from hormone treatment, the plants that underwent MeJA treatment were maintained separately from the control group.
Analysis of Trichomes Density and Morphology
Scanning electron microscope Regulus 8100 (Hitachi, Japan) was used to analyze the trichomes morphology, and stereo microscope SMZ171 (Motic, China) were used to analyze the trichomes density of tomato stems. 2-week-old tomato seedings were treated with 50 μM MeJA and 5 μM AZD8055 for 15 d, respectively, and treatment once in 2 d for trichomes density and morphology analyzing. To measure trichome density and morphology, stem pieces from the first internodes of 4-week-old plants were used.
Analysis of Volatile Terpenes
To measure volatile terpenes, the 4-week-old tomato was sprayed with 50 μM MeJA and 5 μM AZD8055 for 24 h, respectively, stem pieces from the first internodes of plants were collected and rinsed with dichloromethane, filtered it into an EP tube with two layers of filter paper, and then diluted the volume to 20 mL with dichloromethane, with 3 seedlings for each treatment as a repeat. The samples were concentrated by rotary evaporation with a rotary evaporation temperature of 39.9 °C and a rotation speed of 133 rpm. Finally, 1 mL of acetone was used to dissolve the organic phase extract, and 700 μL of the 0.22 μM filter was filtered into the liquid phase vial and analyzed by Gas chromatography-mass spectrometry (GC-MS) that performed as described in Xu et al. (2018).
Yeast One Hybrid
A yeast one-hybrid system was used to assay the relationship between the SlMYC1 protein and SlTOR promoters. The SlTOR promoter, which had a length of 1856 bp, was partitioned into three overlapping fragments, and each fragment had an overlap of approximately 50 bp to prevent disruption of the binding sites. The baits (pAbAi-PSlTOR1/2/3) were constructed by inserting the SlTOR promoters, which had a fragment size ranging from 400 to 900 bp, into the pAbAi vector. The BpiI-cut baits were transformed into the Y1HGold and then spread on SD/-Ura and cultured upside down at 30 °C for 2-4 d. Then, the identified positive plaques were screened for AbA resistance, that is, the minimum concentration of AbA without plaque growth, for subsequent yeast one-hybrid experiments. The prey vector (AD-SlMYC1) was generated by cloning the open reading frame of SlMYC1 into the GAL4 AD in the pGADT7 vector, serving as the effector construct. Subsequently, the Y1HGold yeast strain containing the linearized pAbAi vector was utilized to introduce the prey vector. The screened positive co-transformed cells were spread on SD/-Leu medium supplemented with AbA to observe the growth of plaques to detect the binding between SlMYC1 and SlTOR promoters. The primer sequences are seen from Table S2.
GUS Staining and Enzyme Activity Assay
The CDS of SlMYC1 and the 1856 bp promoter region of SlTOR were cloned. Subsequently, the SlMYC1 was inserted into the pRI101-GFP vector, while the SlTOR promoter was inserted into the pRI101-GUS vector. The transactivation of the effectors was verified using the β-glucuronidase (GUS) gene as a reporter, which was controlled by the SlTOR promoter. The positive and negative controls for this experiment were the 35S::SlMYC1 and 35S::GFP effector constructs, respectively. The vectors were transformed into A. tumefaciens. A. tumefaciens cultures were grown at 28 °C, 200 rpm shaking until an OD600 between 0.8 and 1.0 was reached. Taking 20 mL of different bacterial solutions centrifuged at 5000 rpm for 10 min, resuspended and washed the bacteria in MM Buffer (pH 5.6, 10 mM MES, 10 mM MgCl2·6H2O), centrifuged at 5000 rpm for 10 min, and discarded the supernatant. Then, bacterial pellets were resuspended in MMA Buffer (pH 5.6, 10 mM MES, 10 mM MgCl2·6H2O, and 200 μM acetosyringone, Sigma-Aldrich) to OD600 ≈ 0.5, 28 °C in the dark for 3 h. Subsequently, the reporter and effector components were combined in equal volumes, with a ratio of 1:1, and used for the transformation of 5-week-old tobacco plants. GUS staining and enzyme activity assay were performed as described previously (Zhang et al. 2022). Primer sequence lists are seen from Table S3.
Data Processing
All the experiments in this study were carried out at least in triplicate and analyzing the data using Microsoft Excel 2010 and GraphPad Prism 9. The values obtained were analyzed using one-way ANOVA. The data presented in the graphs were expressed as mean ± SD. The errors represented the standard deviations observed among three biological replicates. Student’s t test was used to determine the significance of differences between means, with *P < 0.05 and **P < 0.01 indicating statistical significance. Non-significant differences were denoted as “ns.”
Results
The Critical Role of JA Signaling in the Regulation of Trichome Formation and Terpene Accumulation in Tomatoes
As shown in Fig. 1a, four main types of trichomes, namely, type I, type III, type VI, and type VII were observed in cultivated tomatoes. JA signaling plays a critical role in trichome formation and terpene synthesis (Gong et al. 2021). To explore the role of JA signaling in the growth and development of tomato trichomes, the changes of trichomes and terpene content of tomato treated with MeJA were detected. MeJA treatment significantly induced the formation of trichomes and the accumulation of terpenes compared with the control (Figs. 1b, c, 2a, b).
Effects of activated JA signaling on tomato trichomes formation. a Types of trichomes of tomato observed by scanning electron microscopy. b Stereoscopic microscopy was used to analyze the changes in the number of trichomes on stems treated with MeJA (scale bars, 500 μm). Control (Con.), MeJA treated (MeJA). c Statistical analysis of the trends of the number of trichomes of tomato treated with MeJA
In addition, the synthesis of terpenes was related to TPSs and the precursor of terpene synthesis. Compared with the control, MeJA treatment significantly changed the transcription of TPSs (Fig. S1). Besides, the expression levels of SlCMK, SlIDI1, SlHDS, and SlMDS on the MEP pathway were significantly upregulated (Fig. 2c), and the expression levels of SlHMGR4, SlHMGS2, SlAACT2, SlFPPS1, SlAACT3, SlMDC, and SlMK on the MVA pathway were significantly upregulated (Fig. 2d). These results showed that JA signaling not only changes the expression levels of SlTPSs, but the related genes of terpene synthesis precursor MEP/MVA pathway also have altered to regulate the accumulation of terpenes.
The Key Transcription Factor SlMYC1 in the JA Signaling Pathway Affects the Expression of Terpene Synthesis Precursor-Related Genes (MEP/MVA Pathways)
Compared with the control, the expression level of SlMYC1 was significantly increased after MeJA treatment (Fig. S2a) and studies have shown that SlMYC1 is essential for type VI glandular trichome development and modulates the expression of SlTPSs to affect terpene biosynthesis in tomato (Xu et al. 2018). However, the function of SlMYC1 in the MEP/MVA pathway of the precursor of terpene synthesis is still unclear. To deeply elucidate the function of SlMYC1 in terpene synthesis, transient silenced lines (SlMYC1-TRV) were constructed, and qRT-PCR results showed that SlMYC1 expression levels of the lines (SlMYC1-TRV) had significantly reduced compared with the control (TRV), which proved that the SlMYC1 gene was successfully silenced in transgenic tomato plants (Fig. S2b). Additionally, the expression levels of MEP pathway-related genes SlCMK, SlIDI1, SlMDS, SlDXR, SlHDS, and MVA pathway-related genes SlHMGR4, SlHMGS2, SlHMGS3, SlAACT2, SlAACT3, SlMDC2, and SlMK were reduced. By contrast, the expression levels of MEP pathway-related gene SlDXS2 is increased compared with the control (TRV) (Fig. 3a, b). These results indicate that SlMYC1 can regulate terpene synthesis through terpene synthesis precursor-related pathways.
SlMYC1 was involved in the transcript levels of MEP/MVA pathway-related genes. a Transcript levels of MEP pathway-related genes were analyzed in SlMYC1 transiently silenced lines. b Transcript levels of MVA pathway-related genes were analyzed in SlMYC1 transiently silenced lines. TRV, only infiltrated with empty vectors. SlMYC1-TRV, SlMYC1 transiently silenced lines
TOR Signaling is Involved in Trichome Formation and the Accumulation of Terpenes
To further explore the function of SlTOR in the growth and development of tomato trichomes, the TOR-specific inhibitor AZD8055 was used to treat tomato plants. Compared with the control, both glandular and non-glandular trichomes significantly increased the AZD8055-treated tomato (Fig. 4a). Further analyzing found that AZD8055 treatment increased the number of type I and type III trichomes, while the number of type VI trichomes was significantly reduced (Fig. 4b). The findings of this study demonstrated the involvement of SlTOR in the control of tomato trichomes. It was observed that SlTOR played a negative role in the growth of type I and type III tomato trichomes, while exerting a positive influence on the growth of type VI trichomes.
The effects of SlTOR on the number of trichomes and the relative contents of terpenes in type VI trichomes in tomato stem. a Stereoscopic microscopy was used to analyze the changes in the number of trichomes on stem treated with 5 μM of AZD8055 (scale bars, 500 μm). b Statistical analysis of the trends of the number of trichomes. c, d Levels of monoterpenes and sesquiterpenes after AZD8055 treatment. Control (Con.), AZD8055 treated (AZD8055). AZD8055, TOR inhibitor
In cultivated tomatoes, mono- and sesquiterpenes are mainly derived from glands at the tips of VI glandular trichomes (Gong et al. 2021). GC-MS analysis of the volatiles of tomato stems treated with AZD8055. It can be seen from Fig. 4c and d that four monoterpenes and one sesquiterpene were detected, namely, (+)-4-carene, α-phellandrene, sabinene, and D-limonene. Among them, the relative contents of monoterpenes and sesquiterpene significantly reduced, and the percentage of each monoterpene reduction was higher than 20% of the AZD8055-treated tomato compared with the control, which demonstrated that SlTOR positively regulates the accumulation of terpenes in tomato trichomes.
TOR Signaling Regulation the Expression of SlTPSs and MEP/MVA Pathway-Related Genes
Results of qRT-PCR analysis of the expression levels of SlTPSs showed that SlTPS5, SlTPS9, SlTPS12, SlTPS20, and SlTPS39 were significantly decreased after AZD8055 treatment compared with the control (Fig. 5a), which suggests that SlTOR might affect the accumulation of terpenes by regulating the transcript levels of SlTPSs. In addition, the precursors were needed to increase the accumulation of volatile terpenes. As shown in Fig. 5b and c, AZD8055 treatment could significantly inhibit the expression levels of SlCMK, SlIDI1, SlMDS, and SlDXR, but the SlDXS2 was activated compared with that in the control. However, SlHDS did not change significantly after treatments with AZD8055. Additionally, the expression levels of SlHMGR4 and SlHMGS2 in the MVA pathway were significantly decreased, while SlAACT2, SlAACT3, SlFPPS1, SlMDC2, and SlMK were significantly increased. These results indicate that SlTOR might affect the biosynthesis of monoterpenes and sesquiterpenes by regulating expression levels of SlTPSs and synthetic precursor MVA/MEP pathway-related genes in tomato trichomes.
The expressions by SlTOR signaling regulation of SlTPSs and genes in terpene synthesis precursor MEP/MVA pathway. Tomatoes were treated with AZD8055 and relative transcript levels of SlTPSs a and genes in MEP/MVA pathway-related b, c were analyzed. Control (Con.), AZD8055 treated (AZD8055). AZD8055, TOR inhibitor
SlMYC1 Activated SlTOR Expression Through Direct Binding to the SlTOR Promoter
Both JA and TOR signaling regulate the growth and development of trichomes. To explore their connections, the promoter of SlTOR gene was analyzed by Plantcare and found that there was one MYC-binding site (5′-CACGTT-3′) and four G-Box motifs (5′-CACGTG-3′) capable of binding to MYC1 (Fig. 6a), indicating that SlMYC1 might regulate the expression of SlTOR.
SlMYC1 directly bound to the promoter of SlTOR and activated its transcript levels. a Schematic diagram of segments of SlTOR promoter in yeast one hybrid. PSlTOR1, PSlTOR2, and PSlTOR3 are segments of the SlTOR promoter. The green was the MYC-binding site (5′-CACGTT-3′), and yellow was the G-box motif (5′-CACGTG-3′) of MYC1-binding site. b Yeast one hybrid. AD-SlMYC1 as the prey, pAbAi-PSlTOR as the bait, and AD and pAbAi-PSlTOR as the negative controls. c Schematic diagram of the reporter gene and effector vector in the GUS experiment. d The enzymatic activity of GUS was determined. Tobacco leaves co-transformed with the reporter and the empty effector vector and transformed with the reporter used as negative control. e The expression of SlTOR in SlMYC1-silenced and overexpression lines. TRV, only infiltrated with empty vectors. SlMYC1-TRV, SlMYC1-silenced lines. GFP, only infiltrated with empty vectors. SlMYC1-GFP, SlMYC1 overexpression lines
To investigate how SlMYC1 regulates SlTOR, the yeast one-hybrid assay was carried out to detect the binding of SlMYC1 to the SlTOR promoter. Due to the inherent self-activating nature of the SlTOR promoter, it was necessary to partition the promoter into three distinct fragments that overlapped with each other, encompassing the MYC1-binding motifs (Fig. 6a). Next, the pAbAi vector was utilized to create the bait (pAbAi-PSlTOR1/2/3) by inserting fragments from the SlTOR promoter. It was observed that both pAbAi-PSlTOR1 and pAbAi-PSlTOR2 exhibited self-activation (Fig. S3). Subsequently, the prey vector was introduced into the Y1HGold yeast strain, which contained the linearized pAbAi vector, and the mixture was spread on SD/-Leu medium. Results provided evidence that SlMYC1 has ability to directly bind to the SlTOR promoter (Fig. 6b).
Besides, GUS staining experiments were performed to examine the effect of SlMYC1 on the transcript activity of the SlTOR promoter. The coding region of SlMYC1 was cloned into the pRI101-GFP (GFP) vector driven by the 35S promoter to obtain the SlMYC1 overexpression vector (Fig. 6c). The empty GUS vector was used as a positive control, and the empty GFP vector served as a negative control. Results showed that tobacco leaves in the positive control group and experimental group had obvious blue plaques, but the negative control group had no blue plaques (Fig. 6d). The experimental group exhibited a significantly higher level of GUS enzyme activity compared to the negative control group (Fig. 6d). Furthermore, the levels of SlTOR expression were notably reduced in SlMYC1-silenced lines, whereas they were significantly elevated in SlMYC1 overexpression lines (Fig. 6e). These findings indicate that SlMYC1 plays a crucial role in positively controlling the transcription of SlTOR.
The findings of this study provide further evidence that SlMYC1 exerts control over transcription by directly interacting with the promoter region of SlTOR. This interaction leads to a positive regulatory effect on SlTOR transcription, ultimately influencing the growth and development of trichomes in tomatoes.
Discussion
This study clarifies the mechanism which SlMYC1 interact with SlTOR in JA signals to regulate tomato trichomes growth and development and terpene synthesis and accumulation, which lays a foundation for revealing the mechanism of multicellular trichomes generation and development.
JA Signaling Regulates the Expression of Multiple Genes in TPSs, MEP, and MVA Pathways Through MYC1 to Affect the Accumulation of Terpenes
JA play a crucial role in governing numerous physiological processes during the growth and development of plants (Hong et al. 2020; Han et al. 2020; Hua et al. 2021b). This research has validated that the external administration of MeJA has effectively augmented the abundance of type VI glandular trichomes in young tomato plants. These findings align with the outcomes reported by Hua et al. (Hua et al. 2021a). Moreover, it is worth noting that trichomes serve as the primary locations for the secretion of volatile components, type VI trichomes is closely related to the accumulation of terpenes (Xu et al. 2018; Gao et al. 2022). TPSs mainly modify terpene precursors to generate different kinds of terpenes, and terpene precursors are primarily synthesized by the MEP and MVA pathways (Pu et al. 2021). The transcript levels of related genes in the MEP and MVA pathways are significantly increased, and corresponding terpene contents are significantly increased in SlSCL3 overexpression lines of tomatoes (Yang et al. 2021). Similarly, after AtDXS in Arabidopsis is transferred into Lavandula angustifolia, the content of monoterpene essential oil produced by Lavandula is significantly increased. JA signaling activates biosynthetic genes, thereby inducing the biosynthesis of secondary compounds (Wasternack and Strnad 2019). JA plays a specific role in stimulating the activation of the ‘late’ anthocyanin biosynthetic genes, namely DFR, LDOX, and UF3GT, resulting in the enhanced production of anthocyanins in Arabidopsis (Shan et al. 2009). Following the examination of gene expression through qRT-PCR, it was observed that the levels of SlTPSs transcripts, as well as several genes involved in the MVA and MEP pathways, underwent significant alterations upon treatment with MeJA. In particular, the transcript levels of SlHMGR4 increased ten-fold compared to control treated with MeJA. In addition, MeJA treatment significantly increased terpene accumulation compared to the control group, supporting previous research and confirming the reliability of our results (Hong et al. 2012).
SlMYC1, an essential element in the JA signaling pathway, also assumes a critical function in governing the progression of trichome growth and development as well as the biosynthesis of terpenes (Spyropoulou et al. 2014). The transcript levels of SlTPSs were significantly altered due to the downregulation of SlMYC1, and the absence of type VI glandular trichomes was observed upon the knockout of this gene (Xu et al. 2018). This study showed that SlMYC1 functions as a positive regulator of terpenes biosynthesis. The downregulation of SlMYC1 suppressed the expression of SlTPSs, and the expression of genes related to the MEP/MEA pathway also significantly changed. However, the trends in terpene synthesis-related genes were different from the MeJA treatment, which indicates that although MYC1 is a key transcription factor in the JA signaling pathway, there were differences in its downstream regulation and the existence of posttranscriptional regulation or multiple factors in the JA signaling pathway.
The Regulation of Trichomes Quantity and Terpene Accumulation by JA Signaling is Related to TOR Kinase
JA signaling regulates the biosynthesis of terpenes in plant tissues through transcription factors. It was found that the number and type of trichomes changed, and terpene content decreased significantly in tomato with TOR signaling inhibited. What’s more, JA signaling and TOR signaling were functionally consistent in regulating trichomes number, species, and terpene accumulation. JA signaling activates stem cells and regulates cell development and tissue regeneration (Zhou et al. 2019). Whole-genome gene expression profiling of Arabidopsis indicates that MeJA altered the cellular metabolism and progression of the cell cycle, while inhibiting the activation of genes associated with the M-phase, thus, it caused a halt in the cell cycle at the G2 phase (Pauwels et al. 2008). Besides, the JA signaling pathway assumes a crucial function in the biosynthesis of secondary metabolites in plants, whereby the activation of biosynthetic genes is facilitated through JA signaling (Wasternack et al. 2019). The administration of exogenous MeJA induces the activation of molecular signal transduction, thereby regulating the expression of genes and ultimately resulting in the accumulation of secondary metabolites (Ho et al. 2020). TOR can regulate cell cycle and cell differentiation and integrate hormone signals to balance energy metabolism (Rodriguez et al. 2019; Meng et al. 2022). TOR tightly controls various downstream processes through direct or indirect TOR effectors, involving in the cell cycle, primary and secondary metabolism, transcription, translation, or more (Li et al. 2021a, b). Recent research has brought to light that TOR effectively integrates intricate nutrient and hormonal signaling pathways to coordinate a multitude of subsequent processes (Liu et al. 2022). Here, MYC1 and TOR are functionally consistent, and MYC1 activates the transcription of TOR. In summary, MYC1 regulation of TOR transcription is part of the integration of TOR to plant hormone signaling.
Conclusion
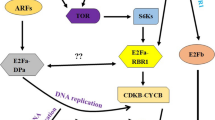
The present investigation aimed to explore the regulatory role of the interaction between JA and TOR signaling in the growth and development of tomato trichomes, as well as the biosynthesis of terpenes. To achieve this, a comprehensive approach involving GC-MS, qRT-PCR, yeast one hybrid, GUS, and transient transgenic assays was employed. JA signaling and the essential transcription factor, SlMYC1, play a vital role in the growth and development of tomato trichomes and the biosynthesis of terpenes. Here, the transcript levels of terpene synthesis-related genes were significantly altered in both MeJA-treated and SlMYC1-silenced tomato plants. What’s more, SlTOR positively regulated the formation of type VI trichomes and positively regulated the synthesis and accumulation of (+)-4-carene, α-phellandrene, sabinene, D-limonene of monoterpenes, and δ-elemene of sesquiterpene by regulating the transcript levels of key genes for terpene synthesis. In addition, we also found that there were binding motifs of SlMYC1 in the SlTOR promoter. Further confirmation of SlMYC1’s direct interaction with the SlTOR promoter was obtained through additional yeast one-hybrid investigations. The results of GUS staining, enzyme activity assays, and qRT-PCR showed that SlMYC1 activated the expression of SlTOR. In conclusion, the investigation found that SlMYC1 is crucial for connecting JA signaling and TOR signaling and regulating trichome growth and terpene production in tomatoes (Fig. 7).
Schematic diagram of the regulation pattern of SlMYC1-SlTOR module on trichomes. JA signaling promoted the expression of SlMYC1, and the activated SlMYC1 directly bounds to the SlTOR promoter and activates its expression, thereby promoting the growth and development of type VI trichomes, as well as the expressions of SlTPSs, the related genes in terpene synthesis precursor MEP/MVA pathways to promote the accumulations of terpenes
Data Availability
The datasets generated and analyzed during this study are available from the corresponding author upon reasonable request.
References
Ashfaq S, Ahmad M, Zafar MM, Sultana S, Bahadur S, Ullah F, Zaman W, Ahmed SN, Nazish M (2019) Foliar micromorphology of convolvulaceous species with special emphasis on trichome diversity from the arid zone of Pakistan. Flora 255:110–124. https://doi.org/10.1016/j.flora.2019.04.007
Awasthi A, Nain V, Srikanth CV, Puria R (1867) A regulatory circuit between lncRNA and TOR directs amino acid uptake in yeast. BBA-Mol Cell Res 6:118680. https://doi.org/10.1016/j.bbamcr.2020.118680
Boutouja F, Stiehm CM, Platta HW (2019) mTOR: a cellular regulator interface in health and disease. Cells 8(1):18. https://doi.org/10.3390/cells8010018
Chalvin C, Drevensek S, Dron M, Bendahmane A, Boualem A (2020) Genetic control of glandular trichome development. Trends Plant Sci 25(5):477–487. https://doi.org/10.1016/j.tplants.2019.12.025
Chang J, Yu T, Yang Q, Li C, Xiong C, Gao S, Xie Q, Zheng F, Li H, Tian Z, Yang C, Ye Z (2018) Hair, encoding a single C2H2 zinc-finger protein, regulates multicellular trichome formation in tomato. Plant J 96(1):90–102. https://doi.org/10.1111/tpj.14018
Chen Y, Su D, Li J, Ying S, Deng H, He X, Zhu Y, Li Y, Chen Y, Pirrello J, Bouzayen M, Liu Y, Liu M (2020) Overexpression of SlbHLH95, a basic helix-loop-helix transcription factor family member, impacts trichome formation via regulating gibberellin biosynthesis in tomato. J Exp Bot 71(12):3450–3462. https://doi.org/10.1093/jxb/eraa114
Ewas M, Gao Y, Ali F, Nishawy EM, Shahzad R, Subthain H, Amar M, Martin C, Luo J (2017) RNA-seq reveals mechanisms of SlMX1 for enhanced carotenoids and terpenoids accumulation along with stress resistance in tomato. Sci Bull 7:476–485. https://doi.org/10.1016/j.scib.2017.03.018
Galdon-Armero J, Fullana-Pericas M, Mulet PA, Conesa MA, Martin C, Galmes J (2018) The ratio of trichomes to stomata is associated with water use efficiency in Solanum lycopersicum (tomato). Plant J 96(3):607–619. https://doi.org/10.1111/tpj.14055
Gao W, Meng Q, Wang X, Chen F, Zhou Y, He M (2022) Overexpression of CiMYC2 transcription factor from Chrysanthemum indicum var. aromaticum resulted in modified trichome formation and terpenoid biosynthesis in transgenic tobacco. J Plant Growth Regul 42:4161–4175. https://doi.org/10.1007/s00344-022-10881-1
Gasparini K, da Silva MF, Costa LC, Martins SCV, Ribeiro DM, Peres LEP, Zsögön A (2021) The Lanata trichome mutation increases stomatal conductance and reduces leaf temperature in tomato. J Plant Physiol 260:153413. https://doi.org/10.1016/j.jplph.2021.153413
Glas JJ, Schimmel BC, Alba JM, Escobar-Bravo R, Schuurink RC, Kant MR (2012) Plant glandular trichomes as targets for breeding or engineering of resistance to herbivores. Int J Mol Sci 13(12):17077–17103. https://doi.org/10.3390/ijms131217077
Gong Z, Luo Y, Zhang W, Jian W, Zhang L, Gao X, Hu X, Yuan Y, Wu M, Xu X, Zheng X, Wu G, Li Z, Li Z, Deng W (2021) SlMYB75-centred transcriptional cascade regulates trichome formation and sesquiterpene accumulation on tomato. J Exp Bot 213(3):1145–1155. https://doi.org/10.1111/nph
Han X, Zhang M, Yang M, Hu Y (2020) Arabidopsis JAZ proteins interact with and suppress RHD6 transcription factor to regulate jasmonate-stimulated root hair development. Plant Cell 32(4):1049–1062. https://doi.org/10.1105/tpc.19.00617
Ho TT, Murthy HN, Park SY (2020) Methyl jasmonate induced oxidative stress and accumulation of secondary metabolites in plant cell and organ cultures. Int J Mol Sci 21(3):716. https://doi.org/10.3390/ijms21030716
Hong GJ, Xue XY, Mao YB, Wang LJ, Chen XY (2012) Arabidopsis MYC2 interacts with DELLA proteins in regulating sesquiterpene synthase gene expression. Plant Cell 24:2635–2648. https://doi.org/10.1105/tpc.112.098749
Hong SY, Sun B, Straub D, Blaakmeer A, Mineri L, Koch J, Brinch-Pedersen H, Holme IB, Burow M, Lyngs Jørgensen HJ, Albà MM, Wenkel S (2020) Heterologous micro protein expression identifies LITTLE NINJA, a dominant regulator of jasmonic acid signaling. Proc Natl Acad Sci USA 117(42):1–9. https://doi.org/10.1073/pnas.2005198117
Hua B, Chang J, Han X, Xu Z, Hu S, Li S, Wang R, Yang L, Yang M, Wu S, Shen J, Yu X, Wu S (2022) H and HL synergistically regulate jasmonate-triggered trichome formation in tomato. Hort Res. https://doi.org/10.1093/hr/uhab080
Hua B, Chang J, Wu M, Xu Z, Zhang F, Yang M, Xu H, Wang LJ, Chen XY, Wu S (2021a) Mediation of JA signaling in glandular trichomes by the woolly/SlMYC1 regulatory module improves pest resistance in tomato. Plant Biotechnol J 19(2):375–393. https://doi.org/10.1111/pbi.13473
Hua B, Chang J, Xu Z, Han X, Xu M, Yang M, Yang C, Ye Z, Wu S (2021b) Homeodomain PROTEIN8 mediates jasmonate-triggered trichome elongation in tomato. New Phytol 230(3):1063–1077. https://doi.org/10.1111/nph.17216
Huebbers JW, Büttgen K, Leissing F, Mantz M, Pauly M, Huesgen PF, Panstruga R (2022) An advanced method for the release, enrichment and purification of high-quality Arabidopsis thaliana rosette leaf trichomes enables profound insights into the trichome proteome. Plant Methods 18(1):12. https://doi.org/10.1186/s13007-021-00836-0
Hung FY, Chen JH, Feng YR, Lai YC, Yang S, Wu K (2020) Arabidopsis JMJ29 is involved in trichome development by regulating the core trichome initiation gene GLABRA3. Plant J 103(5):1735–1743. https://doi.org/10.1111/tpj.14858
Kariyat RR, Smith JD, Stephenson AG, De Moraes CM, Mescher MC (2017) Non-glandular trichomes of Solanum carolinense deter feeding by Manduca sexta caterpillars and cause damage to the gut peritrophic matrix. Proc Biol Sci 284(1849):20162323. https://doi.org/10.1098/rspb.2016.2323
Li L, Liu KH, Sheen J (2021a) Dynamic nutrient signaling networks in plants. Annu Rev Cell Dev Biol 37:341–367. https://doi.org/10.1146/annurev-cellbio-010521-015047
Li R, Chen P, Zhu L, Wu F, Chen Y, Zhu P, Ji K (2021b) Characterization and function of the 1-deoxy-D-xylose-5-phosphate synthase (DXS) gene related to terpenoid synthesis in Pinus massoniana. Int J Mol Sci 22(2):848. https://doi.org/10.3390/ijms22020848
Liu X, Bartholomew E, Cai Y, Ren H (2016) Trichome-related mutants provide a new perspective on multicellular trichome initiation and development in cucumber (Cucumis sativus L.). Front Plant Sci 7:1187–1187. https://doi.org/10.3389/fpls.2016.01187
Liu Y, Xiong Y (2022) Plant target of rapamycin signaling network: complexes, conservations, and specificities. J Integr Plant Biol 64(2):342–370. https://doi.org/10.1111/jipb.13212
Meng Y, Zhang N, Li J, Shen X, Sheen J, Xiong Y (2022) TOR kinase, a GPS in the complex nutrient and hormonal signaling networks to guide plant growth and development. J Exp Bot 73(20):7041–7054. https://doi.org/10.1093/jxb/erac282
Pauwels L, Morreel K, De Witte E, Lammertyn F, Van Montagu M, Boerjan W, Inzé D, Goossens A (2008) Mapping methyl jasmonate-mediated transcriptional reprogramming of metabolism and cell cycle progression in cultured Arabidopsis cells. Proc Natl Acad Sci USA 105(4):1380–1385. https://doi.org/10.1073/pnas.0711203105
Pichersky E, Raguso RA (2018) Why do plants produce so many terpenoid compounds? New Phytol 220(3):692–702. https://doi.org/10.1111/nph.14178
Pu X, Dong X, Li Q, Chen Z, Liu L (2021) An update on the function and regulation of methylerythritol phosphate and mevalonate pathways and their evolutionary dynamics. J Integr Plant Biol 63(7):1211–1226. https://doi.org/10.1111/jipb.13076
Qi T, Song S, Ren Q, Wu D, Huang H, Chen Y, Fan M, Peng W, Ren C, Xie D (2011) The jasmonate-ZIM-domain proteins interact with the WD-repeat/bHLH/MYB complexes to regulate jasmonate-mediated anthocyanin accumulation and trichome initiation in Arabidopsis thaliana. Plant Cell 23:1795–1814. https://doi.org/10.1105/tpc.111.083261
Rodriguez M, Parola R, Andreola S, Pereyra C, Martínez-Noël G (2019) TOR and SnRK1 signaling pathways in plant response to abiotic stresses: do they always act according to the “yin-yang” model? Plant Sci 288:110220. https://doi.org/10.1016/j.plantsci.2019.110220
Serna L, Martin C (2006) Trichomes: different regulatory networks lead to convergent structures. Trends Plant Sci 11(6):274–280. https://doi.org/10.1016/j.tplants.2006.04.008
Shan X, Zhang Y, Peng W, Wang Z, Xie D (2009) Molecular mechanism for jasmonate-induction of anthocyanin accumulation in Arabidopsis. J Exp Bot 60(13):3849–3860. https://doi.org/10.1093/jxb/erp223
Song Y, Zhao G, Zhang X, Li L, Xiong F, Zhuo F, Zhang C, Yang Z, Datla R, Ren M, Li F (2017) The crosstalk between target of rapamycin (TOR) and jasmonic a cid (JA) signaling existing in Arabidopsis and cotton. Sci Rep 7:45830. https://doi.org/10.1038/srep45830
Spyropoulou EA, Haring MA, Schuurink RC (2014) RNA sequencing on Solanum lycopersicum trichomes identifies transcription factors that activate terpene synthase promoters. BMC Genomics 15(1):402. https://doi.org/10.1186/1471-2164-15-402
Tang T, Li CH, Li DS, Jing SX, Hua J, Luo SH, Liu Y, Li SH (2020) Peltate glandular trichomes of Colquhounia vestita harbor diterpenoid acids that contribute to plant adaptation to UV radiation and cold stresses. Phytochemistry 172:112285. https://doi.org/10.1016/j.phytochem.2020.112285
Wang X, Shen C, Meng P, Tan G, Lv L (2021) Analysis and review of trichomes in plants. BMC Plant Biol 21(1):70. https://doi.org/10.1186/s12870-021-02840-x
Wang Y, Qin Y, Li B, Zhang Y, Wang L (2020) Attenuated TOR signaling lengthens circadian period in Arabidopsis. Plant Signal Behav 15(2):1710935. https://doi.org/10.1080/15592324.2019.1710935
Wang Y, Zhou Q, Meng Z, Abid MA, Wang Y, Wei Y, Guo S, Zhang R, Liang C (2022) Multi-dimensional molecular regulation of trichome development in Arabidopsis and cotton. Front Plant Sci 13:892381. https://doi.org/10.3389/fpls.2022.892381
Wasternack C, Strnad M (2019) Jasmonates are signals in the biosynthesis of secondary metabolites—pathways, transcription factors and applied aspects—a brief review. N Biotechnol 48:1–11. https://doi.org/10.1016/j.nbt.2017.09.007
Xu J, van Herwijnen ZO, Dräger DB, Sui C, Haring MA, Schuurink RC (2018) SlMYC1 regulates type VI glandular trichome formation and terpene biosynthesis in tomato glandular cells. Plant Cell 30(12):2988–3005. https://doi.org/10.1105/tpc.18.00571
Yan T, Chen M, Shen Q, Li L, Fu X, Pan Q, Tang Y, Shi P, Lv Z, Jiang W, Ma YN, Hao X, Sun X, Tang K (2017) HOMEODOMAIN PROTEIN 1 is required for jasmonate-mediated glandular trichome initiation in Artemisia annua. New Phytol 213(3):1145–1155. https://doi.org/10.1111/nph.14205
Yang C, Marillonnet S, Tissier A (2021) The scarecrow-like transcription factor SlSCL3 regulates volatile terpene biosynthesis and glandular trichome size in tomato (Solanum lycopersicum). Plant J 107(4):1102–1118. https://doi.org/10.1111/tpj.15371
Yu X, Chen G, Tang B, Zhang J, Zhou S, Hu Z (2018) The Jasmonate ZIM-domain protein gene SlJAZ2 regulates plant morphology and accelerates flower initiation in Solanum lycopersicum plant. Plant Sci 267:65–73. https://doi.org/10.1016/j.plantsci.2017.11.008
Yuan Y, Xu X, Luo Y, Gong Z, Hu X, Wu M, Liu Y, Yan F, Zhang X, Zhang W, Tang Y, Feng B, Li Z, Jiang CZ, Deng W (2021) R2R3 MYB-dependent auxin signaling regulates trichome formation, and increased trichome density confers spider mite tolerance on tomato. Plant Biotechnol J 19(1):138–152. https://doi.org/10.1111/pbi.13448
Zhang Y, Xing H, Wang H, Yu L, Yang Z, Meng X, Hu P, Fan H, Yu Y, Cui N (2022) SlMYC2 interacted with the SlTOR promoter and mediated JA signaling to regulate growth and fruit quality in tomato. Front Plant Sci. https://doi.org/10.3389/fpls.2022.1013445
Zheng H, Zhang Y, Chen Y, Guo P, Wang X, Yuan X, Ge W, Yang R, Yan Q, Yang X, Xi Y (2019) Prominin-like, a homolog of mammalian CD133, suppresses di lp6 and TOR signaling to maintain body size and weight in Drosophila. Faseb J 33(2):2646–2658. https://doi.org/10.1096/fj.201800123R
Zheng K, Wang X, Wang Y, Wang S (2021) Conserved and non-conserved functions of the rice homologs of the Arabidopsis trichome initiation-regulating MBW complex proteins. BMC Plant Biol 21(1):234. https://doi.org/10.1186/s12870-021-03035-0
Zhou F, Pichersky E (2020) More is better: the diversity of terpene metabolism in plants. Curr Opin Plant Biol 55:1–10. https://doi.org/10.1016/j.pbi.2020.01.005
Zhou W, Lozano-Torres JL, Blilou I, Zhang X, Zhai Q, Smant G, Li C, Scheres B (2019) A Jasmonate signaling network activates root stem cells and promotes regeneration. Cell 177(4):942-956.e14. https://doi.org/10.1016/j.cell.2019.03.006
Acknowledgements
This research was funded by the National Key Research and Development Program of China (2019YFD1000300) and the Key Project of Science and Technology Research of Liaoning Provincial Education Department (LJKZ0631).
Funding
This study was funded by National Key Research and Development Program of China, 2019YFD1000300, Na Cui, Key Project of Science and Technology Research of Liaoning Provincial Education Department, LJKZ0631, Na Cui.
Author information
Authors and Affiliations
Contributions
LY and NC designed the research; LY, YZ, and QD performed the experiments; LY and YZ analyzed the data and wrote the manuscript; XM, YY, NC, and HF revised the paper. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no competing and conflict of interest.
Additional information
Handling Editor: Ariel D. Arencibia.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yu, L., Zhang, Y., Ding, Q. et al. The SlMYC1-TOR Module Regulates Trichome Formation and Terpene Biosynthesis in Tomatoes (Solanum lycopersicum L.). J Plant Growth Regul 43, 3282–3294 (2024). https://doi.org/10.1007/s00344-024-11292-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00344-024-11292-0