Abstract
The impact of seven clonal rootstocks (Colt, MaxMa 14, Krymsk 6, Adara, Cigančica, Gisela 5, and Gisela 6) and one local plum (Myrobalan seedlings) on yield, fruit weight, leaf mineral content at 120 days after full bloom (DAFB), and deviation from optimum percentage (DOP) for macro- and microelements of the ‘Šumadinka’ sour cherry cultivar was evaluated in orchard conditions for two consecutive years. Results showed that yield was higher on Adara, Gisela 5 and MaxMa 14, intermediate on Cigančica and Krymsk 6, and lower on Colt, Gisela 6, and Myrobalan rootstocks. The average fruit weight (FW) was highest on Gisela 6 and lowest on Myrobalan seedlings, and FW was higher in the second year of the trial. Significant effect of rootstock was found on the leaf mineral analysis properties evaluated. Thus, Adara showed the best capacity to uptake and translocate to the scion leaves for most macro- and microelements, whereas the poorest nutritional status was, in general, obtained for Myrobalan, probably due to the incidence of graft incompatibility disturbances with the ‘Šumadinka’ cultivar. MaxMa 14 showed the best balanced nutritional values (ΣDOP) whereas the wider imbalance among elements was induced by Myrobalan seedlings. ‘Krymsk 6’ had, in general, lower values for most leaf mineral elements but higher ΣDOP macro and ΣDOP micro-indexes, showing more unbalanced nutritional index than the rest of rootstocks, with the exception of Myrobolan, and both of them followed by Colt. This work demonstrates that the rootstock strongly influences some important sour cherry attributes such as yield, fruit size, leaf macro- and microelements. The significant positive correlations between yield and mineral elements as Mn and Ca could indicate the interest of rootstocks having higher absorption and uptake for these elements in the present growing conditions. Considering their overall performance and tolerance to heavy and acidic soils, and according to the PCA results, Adara, MaxMa 14, and Gisela 6 appear as new promising rootstocks and can be recommended for sour cherries growing under similar soil conditions. We believe that sufficient information on the influence of different rootstocks on yield, fruit size, and leaf mineral composition of sour cherry would enable less ambiguous comparisons within and among them and ensure the best choice for growing a specific cultivar in similar environmental conditions.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The vegetative growth and yield of fruit trees are influenced by many factors such as climate, soil, cultivar, rootstock, training, pruning, plant protection, weed control, irrigation, and fertilization (Milošević and Milošević 2019). While some of them can be controlled by orchard management, others are genotype dependent. Plant fertilization, alongside with irrigation, is a paramount cultural measure for successful fruit trees culture, and it is among the easier controllable factors (Herrera 2001). These two measures have contributed to a strong increase in fruit production in the last few decades around the world. Optimal mineral nutrition is essential to reach high productivity, and correction of nutritional problems has resulted in enhanced profit (Righetti and Wilder 1987). Fertilization, also known as mineral nutrition of crops, is an important tool used by growers to boost crop yield and quality. However, excessive fertilization has been verified, especially in the horticultural enterprises, where the fertilizer costs represented less than 10% of the variable crop costs as mentioned by Milošević and Milošević (2019). These authors also reported that, except for an economic point of view, excessive fertilization has been connected to soil, groundwater, and stream water contamination, and causing an increment of pest and diseases occurrence. The new doctrine of fruit nutrition, also called “smart fertilization,” is based on several key pillars: application of necessary fertilizers, i.e., elements in optimal amounts at the right time, prevention of environmental pollution (soil, water), and fruit contamination by harmful chemical compounds and heavy metals, and decrease fertilizers costs. According to the presence in plants, the necessary elements are divided into macro- (N, P, K, Ca, Mg) and microelements (Fe, Mn, Cu, Zn, B). In addition, several heavy metals may be present in soils and plants, in a lower extent.
Soil analysis plays an important role in fertilization management of fruit trees. Nevertheless, it is also informed by plant analyses since the amount of nutrients in plants, determined in general by leaf analyses, reflects actual plant nutrient uptake (Uçgun and Gezgin 2017). As known, soil analysis is the initial basis for planning a fertilization program, but it is not in itself sufficient for the quality application of this measure in the field (Milošević and Milošević 2019). The most commonly used plant prognosis method is the leaf mineral analysis (Leece 1975). In this sense, both soil and leaf analyses are synergistic and complement each other by providing the most reliable data on the condition and quantity of elements, especially from sufficiency, deficiency, and/or excess aspects. Some studies recommend chemical mineral analysis of flowers because it gives the earliest data in the current season on the nutritional status of fruit trees (Jiménez et al. 2004; Font i Forcada et al. 2020). There are numerous sources in the literature that refer the best time to perform leaf analysis, depending on fruit species and harvest time of scion cultivars. Consequently, leaf analysis could be performed at 60 days after full bloom (DAFB) (Betrán et al. 1997), at 90 DAFB (Jiménez et al. 2004), or in mid-summer, i.e., approximately at 120 DAFB (Leece 1975; Neilsen and Kappel 1996; Jiménez et al. 2007). However, the routine sampling time for leaf nutrient diagnosis for perennial fruit tree species is assessed at mid-summer (Leece 1975). It could be adequately described as “late foliar analysis” or “postmortem” since it may give accurate information on nutritional disorders that can only be corrected adequately in the following growing season (Abadía 1992). However, some authors recommended leaf chemical analysis at mid-summer as a valuable tool for adequate sampling time and determination of leaf composition standards (Leece 1975). Nutritional concentrations substantially lower or higher than reference values are associated with decreases in crop growth, yield, or fruit quality in different fruit tree species (Righetti 1987; Reig et al. 2018; Font i Forcada et al. 2020; Chaleshtori et al. 2020).
Sour cherry is a very important fruit tree species for Serbian agriculture and economy. Most of the production is exported to EU countries, especially to Germany as raw material for processing, mostly in the frozen state. It is grown on an average area of 23,030 ha, and production per year ranged between 66,224 t and 128,023 t in the last decade (FAOSTAT 2022). The predominant sour cherry cultivar is ‘Oblačinska’ (~ 55%) which is propagated by rooted suckers (without grafting) (Milošević et al. 2020). It has a dwarfing tree and small fruits rich in soluble dry matter, and suitable for processing, especially into juices. Nevertheless, other sour cherry cultivars with larger fruits as ‘Šumadinka’ can also be found in Serbian orchards in smaller extent, and they are usually grafted on seedlings of Prunus avium L. (Mazzard) and/or P. mahaleb L. rootstocks. However, the experiences of Serbian growers with new international and local clonal rootstocks for cherries are very modest. In particular, and to the best of our knowledge, none study has reported their requirements for the environmental conditions and growing technology in Serbia, using the ‘Šumadinka’ sour cherry cultivar budded on Prunus spp. rootstocks with different genetic background.
It is a well-known fact that the availability of suitable and compatible rootstocks is the key to economically justified and sustainable fruit industry. The role of rootstock in the horticultural plants is of paramount importance as it drives nutrient and growth-regulating substances from source (root) to sink (leaves) tissues. The vigor and performances of trees can be manipulated by the selection of proper rootstock. Namely, rootstocks directly control the absorption of water and uptake of nutrients from the soil to the scion (Milošević and Milošević 2019). There are many horticultural traits of fruit trees that are affected or controlled by rootstocks including nutrient uptake, vegetative growth, flowering intensity, yield capacity, and fruit quality (Moreno et al. 2001; Milošević et al. 2020). The mechanism of the influence of the rootstock on the scion cultivar and vice versa is very complex and in many cases insufficiently known, nor is there one main hypothesis. Some authors reported that relationship, i.e., interaction between the rootstock and scion is bidirectional through the xylem and phloem and includes water, nutrients, hormones, metabolites, peptides, small organic molecules, and nucleic acids (Albacete et al. 2015). In general, the genetic and physiological mechanisms of the influence of the rootstock on scion tree vigor and crop load have been better studied and more understood in relation to the impact of the rootstock on fruit quality (Anthony and Musacchi 2021).
The main goal of this study was evaluation of seven new clonal and one generative rootstocks through their effect on yield, fruit size, and leaf mineral composition of the ‘Šumadinka’ sour cherry cultivar grown under western Serbian conditions. Results will provide valuable information for growers to choose the best rootstock for intensive cultivation of sour cherries and, in particular, for this interesting cultivar exhibiting large sized fruits.
Material and Methods
Plant Material, Experimental Layout, and Environmental Conditions
A trial was established on a commercial sour cherry orchard in the village of Prislonica (43°33′N; 16°21′E) near the Čačak town (western Serbia), at 300 m above the sea level. The ‘Šumadinka’ sour cherry cv. was budded on eight rootstocks. Trees were planted in 2016, spaced at 4.0 m × 2.0 m, in a randomized complete-block design with five trees for each rootstock-scion combination in four blocks (n = 20). Rootstocks included in this trial were Colt, MaxMa 14 (syn.: Brokforest, Maxma Delbard® 14), Krymsk 6 (syn.: LC-52), Adara, Cigančica (syn.: Cigány Meggy), Gisela 5, Gisela 6, and Myrobalan seedlings from open pollination (Table 1). Trees were pruned and trained as a modified Brunner-spindle bush. The experiment was performed in 2019 and 2020. Standard cultural practices were applied without irrigation. The regular nutrition program of the orchard consisted of soil application of 150 kg ha–1 of calcium ammonium nitrate—CAN (contained 27% total N) and 200 kg ha–1 of compound NPK (15–15–15) mineral fertilizers. CAN was added to the soil before onset of vegetative cycle in 2019 and 2020, whereas the compound NPK was applied in late fall, both in 2018 and 2019. Foliar pesticides and insecticides were used for plant protection.
The soil in the orchard had a clay-loam texture with 1.62% organic matter and low soil pH (4.86) in the 0–30 cm soil depth. Contents of total N, available P2O5 and K2O, CaO and MgO were 0.16%, 178 μg g–1, 220 μg g–1, 0.39%, and 6.2 μg g–1 on dry matter basis, respectively. In this region (moderate temperate climate), the average annual temperature (from 2016 to 2020) was 12.9 °C, and the annual rainfall was 810.9 mm.
Yield and Fruit Size Measurements
Yield per tree (kg) was determined at the harvest period using an ACS System Electronic Scale (Zhejiang, China). Fruit weight (g) was measured using a digital balance FCB 6 K (Kern & Sohn GmbH, Balingen, Germany). Twenty five fruits per each individual tree of each rootstock–scion graft combination and four replications (n = 100) were randomly picked at commercial harvest to determine FW (g).
Analysis of Leaf Elements Content and Deviation from Optimum Percentage
Leaf mineral analyses were carried out at the fourth and fifth year after planting. Leaf sampling (about 100 leaves in average per tree) was carried out at 120 DAFB (approximately two weeks after harvest). Leaf samples were collected from the middle part of one-year-old non-bearing shoots of the current year’s growth (approximately 30–50 cm long) of each graft combination. The leaf concentrations of essential macro- (N, P, K, Ca, Mg) and microelements (Fe, Mn, Cu, Zn, B) were assessed from the dried leaf samples. The mineral elements were determined using the methods and equipment described in our previous studies (Milosevic and Milosevic 2011; Milošević et al. 2013, 2014). Briefly, N total was measured by the modified Kjeldahl method using the Tecator, Kjeltec-system 1003 Distilling Unit (Rochester, New York, USA); P was analyzed by the phosphor-vanadate colorimetric method using the UV–Vis spectrophotometer Thermo Fisher Scientific Genesys 180 (Waltham, MA, USA); K and Mg were determined using a flame photometer Flapho 4 (Carl Zeiss, Jena, Germany); and leaf B was quantified colorimetrically using kinalizarin on a colorimeter MK 6/6 (Carl Zeiss, Jena, Germany). The remaining elements were determined using the flame atomic absorption spectrometer Perkin-Elmer PinAAcle 500 (Waltham, MA, USA). Data were expressed as % and mg kg–1 on dry weight (dw) basis depending of the nutrient evaluated. All nutrients were analyzed by triplicate per each rootstock-cultivar combination in 2019 and 2020. Final values are means ± SE for the two years, due to the absence of statistical significant differences between years.
The deviation from the optimum percentage (DOP) is an index that indicates the relative tendency of nutrient deficiency (DOP < 0), optimum (DOP = 0), or excess (DOP > 0) in plants. The DOP index is calculated from the leaf analysis by using the following mathematical expression (Montañés et al. 1993):
where C is the nutrient concentration in the sample to be studied and Cref is the nutrient concentration considered as optimum, both values given on a dry matter basis. The Cref has been taken from optimum values proposed by Heckman (2004). The ΣDOP is obtained by adding the values of DOP indices irrespective of sign. The lower the ΣDOP, the greater is balances among nutrients and vice versa.
Statistical Analysis
Data were evaluated by analysis of variance (ANOVA) with the Microsoft Office Excel software (Microsoft Corporation, Redmond, WA, USA). When the F test was significant, means were separated by LSD test (P ≤ 0.05). Pearson correlation analyses were performed to study correlations among traits evaluated using the R corrplot package (Wei and Simko 2017). Correlation values were different from 0 with a significance level α = 0.05 and α = 0.01. A principal component analysis (PCA) was performed to simultaneously analyze all the yield and mineral elements traits of ‘Šumadinka’ sour cherry cultivar and to understand how these traits contribute to variability, using SPSS Statistics 23.0 software (IBM Corp., Armonk, NY).
Results and Discussion
Yield and Fruit Size
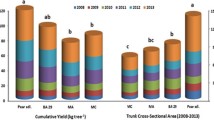
Differences in yield per tree were significantly found among rootstocks (Table 2). Adara, Gisela 5, and MaxMa 14 induced the highest yield with no significant differences among them. In contrast, Myrobalan, Colt, and Gisela 6 rootstocks promoted the lowest and statistically similar yield per tree. Colt and Gisela 6 were the most vigorous rootstocks (data not shown), whereas trees on Myrobalan were extremely dwarf with very pronounced thickening (swelling) at the grafting union. Differences of yield achieved on different rootstocks have been also previously reported for sour (Wociór 2008) and sweet cherry cultivars (Cantín et al. 2010; Font i Forcada et al. 2017). In the case of Myrobalan, it could be associated with scion–rootstock graft incompatibility anomalies, as mentioned by Reig et al. (2018) in different plum graft combinations. Precocity of cherries on invigorating rootstocks as Colt and Gisela 6 could be delayed (Milošević et al. 2014). On the other hand, the physiological scion-rootstock graft incompatibility can decrease vigor, yield, and fruit size (Moreno et al. 2001; Grzyb et al. 2005; Reig et al. 2018). In addition, sweet and sour cherry cultivars grafted on rootstocks of different genetic background may lead to incompatibility problems (Webster and Schmidt 1996; Stehr 1998; Moreno et al. 2001). Graft incompatibility is generally referred to as inability of the stock and scion to bind together to form a successful graft union and can be categorized as translocated and localized (Zarrouk et al. 2006). In our study, early, i.e., “translocated” incompatibility between Myrobalan rootstock and ‘Šumadinka’ cultivar was evident. Namely, scion and root growth tend to terminate at a very early stage, reduced carbohydrate translocation at the union, shriveling of leaves, leaf chlorosis leading to leaf reddening, and early leaf drop are commonly observed symptoms (Zarrouk et al. 2006; Rasool et al. 2020). Also, lack of compatibility has been connected with a pronounced accumulation of polyphenols above the graft union (Feucht et al. 1992), which are known to affect auxin transport (Errea et al. 1994; Errea 1998). Hence, hormonal regulations have been proposed as a mechanism through which rootstocks affect scion compatibility and vigor by modulating root–shoot chemical signaling (Pérez-Alfocea et al. 2010).
Fruit size is considered as the main benchmark in commercial cherry grading, being a large factor in consumer preference, and it is a huge determinant of both farm gate and market price (Pereira et al. 2020). Previous studies reported a high variability among rootstocks regarding this parameter, both in sweet cherry (Moreno et al. 2001; Cantín et al. 2010; Font i Forcada et al. 2017) and sour cherry cultivars (Kopytowski and Markuszewski 2010). The average FW of ‘Šumadinka’ trees on different rootstocks showed significant differences between years and rootstocks (Table 2). Higher FWs were found for all rootstocks in the second harvest season (2020) for 1.4 times compared to the first one. Considering the average of the two years of study, the greatest FW was recorded on Gisela 6 followed by Gisela 5, and both Adara and Krymsk 6 rootstocks. The highest FW induced by these rootstocks is a desirable marketable trait since thinning is not usually practiced in cherry production (Moreno et al. 2001). In contrast, the lowest FW was found on Myrobalan, followed by MaxMa 14 and Cigančica rootstocks. Due to cumulative effect of rootstocks on scion through years, our results will be confirmed in future, since in the case of MaxMa 14, Cantín et al. (2010) reported that this rootstock induced higher or intermediate FW values for two sweet cherry cultivars and four harvesting seasons. In previous data from the literature, FW of the ‘Šumadinka’ sour cherry ranged between 7.40 and 7.57 g (Nenadović-Mratinić et al. 2006; Blagojević et al. 2006), closer to our values for the second harvest season. In general, FW of ‘Šumadinka’ was higher than those obtained by Borowy et al. (2018) for several international large fruited sour cherry cultivars. Sour cherry breeding programs aim to obtain new cultivars with a FW ranging between 6 and 8 g (Iezzoni 1996). The interaction between the scion–rootstock has been reported to influence the quality and functioning in cherries. Namely, the rootstock has been found to influence the movement of water and the process of photosynthesis in sweet cherry trees whereas the scion chiefly exerts its influence on other physical and chemical quality traits in cherry (Gonçalves et al. 2006).
Leaf Nutrients Composition at 120 DAFB
The mean amounts of essential macro- and microelements from mature leaves for each scion-rootstock are shown in Tables 3 and 4. Significant influence of rootstock was found for all mineral elements evaluated, as previously reported in other sour cherry cultivars (Prodanov and Vitanova 1977; Ugorik and Holubowicz 1990; Anderson et al. 1996; Dencker and Toldam-Andersen 2005). On this point of view, Jadczuk (1993) reported that rootstock was the primary factor affecting leaf nutrient content. This author also stated that intensity of rootstock effect depended on cultivar and harvest season. In the present study, the lowest levels of N, P, Ca, Mg, Zn, and B at 120 DAFB found on Myrobalan, were probably due to graft incompatibility disorders exhibited with the grafted scion cultivar. The lowest leaf K, Mg, Fe, and Cu values were found on Krymsk 6, whereas the lowest leaf Mn value was found on Colt, probably due to its poorest mineral uptake efficiency (Anderson et al. 1996; Moreno et al. 1996). Dencker and Toldam-Andersen (2005) reported that trees of ‘Stevnsbaer’ sour cherry on Colt had very low-leaf Mn content which supported our results. Similar findings were reported for sweet cherry cultivars budded on Colt, compared to other Prunus rootstocks (Moreno et al. 1996, 2001).
In contrast, the highest leaf N content was shown on Colt, although it did not differ significantly from MaxMa 14. In different soil growing conditions (calcareous and clay-loam soil), Jiménez et al. (2004) reported that MaxMa 14 induced lower leaf N content and Colt intermediate values on the ‘Sunburst’ sweet cherry cultivar. MaxMa 14 alongside with Cigančica, Gisela or Adara induced, in general, higher leaf P, Mg, and B levels. Adara promoted the highest leaf K, Ca, Fe, Mn, Cu, and B values, although it did no differ significantly from Gisela 5 for Ca and from MaxMa 14 for B. It is interesting to note that although the Adara rootstock has been reported very convenient for heavy, clay-loamy, calcareous, and waterlogged soils (Moreno et al. 1995, 1996), its higher capacity to uptake different mineral nutrients in the acidic soil growing conditions of the present work was as uprising and interesting result. This rootstock appears to have a strong adaptability to different soil types as well as to a wide valence of soil pH values. Probably, the good buffering ability of its roots and good graft compatibility with ‘Šumadinka’ sour cherry could explain these results, in good agreement with Moreno et al. (1995) who stating that Adara is graft compatible with some sour cherry cultivars.
Krymsk 6 [P. cerasus × (P. cerasus × P. maackii)] has been selected as a promising rootstock for cherries (Eremin 2005; Maas et al. 2014). In the present work, it showed the lowest uptake capacity for K, Mg, Fe, and Cu, although ‘Šumadinka’ showed positive cropping results on this rootstock, satisfying health and good tree growth until now (data not shown). Probably, external factors such as leaching, nutrient buffer capacity, temperature, and soil moisture or oxygen content may affect the ability of Krymsk 6 roots to take up nutrients from the soil and disturb the content of leaf mineral elements, as reported in other works (Kangueehi et al. 2011). Similarly, Archibald and Cline (1962) earlier found that sour cherry trees grown on soils maintained under sod contained more K in their leaves than those from clean cultivated soil. These authors reported that leaves of the ‘Montmorency’ sour cherry cultivar contained less N, P, K, and Mg and more Ca in dry and warm years than in wet years which was not case in our trial. It may be that the Krymsk 6 is still adapting to the acidic soil conditions.
Regarding to the influence of rootstocks on mineral concentration in scion leaf and fruits, plant growth, yield potential, and quality traits of some fruit trees, Amiri et al. (2014) found that rootstocks exert their influence on scion yield, quality, and vigor by influencing the amount of minerals reaching the scion. It can be said that various rootstocks had different uptake ability of macro- and micronutrients, i.e., inherent capacity of different rootstocks to major nutrient absorption and translocation is different (Milošević et al. 2013, 2014; Shahkoomahally et al. 2020). Namely, different types of rootstocks can restrict nutrient translocation because of variation in xylem dimensions (Tombesi et al. 2011), and morphological and physiological aspects of root morphology, which can directly contribute to ion absorption and translocation, and redistribution (Hell and Stephan 2003) and, consequently, to the final nutrient amount for plant growth (Nawaz et al. 2016).
DOP and ΣDOP Indexes
DOP index of mineral elements was determined to evaluate the optimum nutrition content according to different studies (Montañés et al. 1993; Jiménez et al. 2004; Font i Forcada et al. 2020). According to this index, deficiency of leaf N content was found in all rootstocks in the present study (Table 4), in good agreement with other sweet cherry studies (Moreno et al. 2001). Negative DOPN (individual DOP index for nitrogen) is related to problems in soil availability and nutrient uptake (Leece 1975). The low soil N total content, even when it was supplied with 150 kg ha−1 N fertilizer and 200 kg ha−1 compound NPK, could be insufficient to reach optimal leaf nutrient content. Nevertheless, Colt and MaxMa 14 appear to induce lower leaf N deficiency in ‘Šumadinka’ sour cherry in comparison with the other rootstocks, according to Heckman (2004) values.
Negative DOPP values were also found for all rootstocks, although MaxMa 14 and Cigančica were closer to the optimum and the contrary for Myrobalan showing the higher P deficiency. In the case of DOPK values, it is interesting to note that Adara and MaxMa 14 showed normal values for this macronutrient. In contrast, Myrobolan induced the highest leaf K deficiency, followed by Krymks 6. Behaviors of the previously mentioned elements in sour cherry leaves may be explained by the antagonistic interaction of Cu with P. The K deficiency in sweet cherry orchards with different rootstocks was also reported and associated with heavier cropping seasons for cherry (Bould 1966; Neilsen and Kappel 1996) and different performance of rootstock genotypes (Moreno et al. 1996; Jiménez et al. 2004). However, in our study, soil was a good source of available P2O5 and K2O, and yield of trees was smaller on Myrobalan and intermediate on Krymsk 6 rootstocks (Table 1), implying that crop load was not a primary factor which determined leaf K level. This paradox can be connected with results of Tombesi et al. (2011) who revealed that different genotypes of rootstocks can restrict nutrient translocation because of variation in xylem dimension. The antagonistic effect of Ca, Mn, Ni, and Cd may also play an important role in K reduction in plants (Kalavrouziotis and Koukoulakis 2010). In the current study, the optimal K values induced by Adara and MaxMa 14, followed by Gisela 5 and Gisela 6, could indicate their best adaptation to heavy and acidic soils. It seems that Adara, as a rootstock suitable for heavy and calcareous soils with high pH (Moreno et al. 1995, 1996), has also a good adaptation to low pH in other type of soils, showing a resilient aptitude.
As seen in Table 5, MaxMa 14, Cigančica, Gisela 6, and Colt appear to have optimal leaf Ca level of the ‘Šumadinka’ sour cherry. In contrast, Myrobalan and Krymsk 6 induced leaf Ca deficiency, according to reference values for sour cherry described by Heckman (2004). Gisela 5 and Adara showed leaf Ca values slightly higher than optimum. MaxMa 14, Gisela 5, and Colt rootstocks promoted leaf Mg contents in the optimal range, whereas Cigančica, Gisela 6, and Adara showed values higher than the normal level. Neilsen and Kappel (1996) reported that Colt was much less susceptible to show Mg deficiency than other cherry rootstocks which confirmed our data. The negative DOPMg of trees on Myrobalan and Krymsk 6 could indicate their tendency to Mg deficiency. Some authors (Milošević et al. 2013) reported that deficiency of leaf Mg can be induced by antagonism with other cations such as Zn, Fe, and K. Similarly, a negative correlation between K and Mg in peach cultivars grafted on different rootstocks was also observed (Shahkoomahally et al. 2020). Otherwise, the Ca and Mg insufficiencies are extremely rare in cherry orchards (Jiménez et al. 2004), although some cases of both elements deficiency has been previously reported (Milošević et al. 2014). Leaf Ca deficiency can be explained also by its lower content in the soil conditions (Milošević and Milošević 2019), very low mobility in the plant (von Bennewitz et al. 2011) or small Ca uptake efficiency of some rootstocks (Hrotkó et al. 2014).
Significant differences for leaf macronutrients were observed across rootstocks for the ΣDOP index (Table 5), as previously reported (Jiménez et al. 2004; Milošević et al. 2014). The best balanced nutritional values (the lowest ΣDOP index) were found on MaxMa 14, followed by Colt, Gisela 5, Gisela 6, and Adara. In contrast, Myrobalan showed the most unbalanced leaf macronutrient status, followed by Krymsk 6. Concerning Krymsk 6, it could induce a higher imbalance across macronutrients probably due to the weak capacity of adaptation to the growing conditions. In an earlier study with different cherry rootstocks, Colt showed more balanced nutritional values for macronutrients than Mazzard, probably due to its better uptake capacity for them (Milošević et al. 2014).
In the case of the assessed microelements (Table 6), rootstocks induced optimal, insufficient, and excessive leaf values, according to standard values proposed by Heckman (2004). Myrobalan, MaxMa 14, Gisela 5, and Gisela 6 induced optimal levels of leaf Fe, whereas MaxMa 14 induced optimal leaf Mn, followed by Gisela 5 and Gisela 6 with close to normal levels for this element. In Cigančica, Colt and Krymsk 6, leaf Fe levels were deficient but acute symptoms of leaf Fe-chlorosis were not found. Hence, Fe insufficiency was not lime induced in this trial because soil has both low pH and Ca values. Probably, some rootstocks have a stronger or weaker Fe uptake capacity or more flexible to the antagonism of Fe:P, Fe:Cu, Fe:Mn or Fe:(Cu + Mn) which contributes to the Fe deficiency or excess (Tisdale and Nelson 1966). In this trial, scion leaves on Adara showed the tendency to induce higher leaf Fe and Mnvalues in good agreement with Jiménez et al. (2004). According to Leece (1975), those values could be classified as adequate for Mn but slightly higher than adequate in the case of Fe. The higher leaf Mn values reported on Adara have been also associated with higher yields and best adaptation to heavy calcareous soils (Moreno et al. 1996; Jiménez et al. 2007). Myrobalan and Krymsk 6 induced higher Cu and B deficiencies whereas other rootstocks induced normal Cu concentrations and close to optimal B levels in leaves of ‘Šumadinka’ sour cherry. All rootstocks showed high Zn deficiency, with exception of Krymsk 6 and MaxMa14 who also promoted negative Zn amounts, but they were closer to the optimum. The chronic low-leaf Zn and adequate Cu contents have already been described by other authors in different type of soils (Neilsen and Kappel 1996; Moreno et al. 2001; Milošević et al. 2014). In the present case, soil pH was acidic which indicates that alkalinity was not hindering Zn uptake (Tisdale and Nelson 1966). Otherwise, Prunus rootstocks had varying ability to absorb and translocate Zn, due to different root system architecture and susceptibility to mycorrhizal infection (Havlin et al. 2005; Milošević and Milošević 2019).
As known, the B element is very important in fruit production because it plays a major role in the reproductive development and fruit set. It is usually deficient in compact soils because it is adsorbed to clay minerals, hydrous metal oxides, and organic matter in soils (Milošević and Milošević 2019). However, with exception of Myrobalan and Krymsk 6, all rootstocks could have adequate B uptake capacity and translocation. Although the leaves mineral composition significantly depends on the rootstock, other factors as cultivar and season, geographical latitude, and accompanying light intensity, temperature, and moisture conditions can affect optimal leaf element contents (Proebsting and Kenworthy 1954; Jadcuzk 1993).
Data in Table 6 showed that ΣDOP index of leaf microelements significantly varied among rootstocks. The highest unbalanced values were observed on Myrobalan, followed by Krymsk 6 and Colt rootstocks. The wider imbalanced value on Myrobolan could be probably due to scion-rootstock graft incompatibility, as previously mentioned. In contrast, the lowest ΣDOP index value was found on MaxMa 14, therefore, exhibiting the best balance among micronutrients for the ‘Šumadinka’ cultivar. Different results on ΣDOP index were observed for other cherry (Jiménez et al. 2004; Milošević et al. 2014) and Prunus rootstocks studies (Shahkoomahally et al. 2020; Font i Forcada et al. 2020). It can be said that the ‘Šumadinka’ sour cherry grafted on MaxMa 14, followed by Gisela 5, showed the best nutritional balance in the growing conditions as compared with the other rootstocks.
Pearson Correlations and Principal Component Analysis
Data in Fig. 1 show the correlations found between yield per tree, FW, and leaf mineral content. Most of the significant correlations found had moderate to high coefficients. Among them, it is noteworthy the high correlations found among K and Ca (r = 0.869, P ≤ 0.01), K and Mn (r = 0.803, P ≤ 0.05), K and Cu (r = 0.871, P ≤ 0.01), and K and B (r = 0.878, P ≤ 0.01). Other significant correlations were found between B and P (r = 0.839, P ≤ 0.01), Ca (r = 0.794, P ≤ 0.05), Mn (r = 0.715, P ≤ 0.05), and Cu (r = 0.783, P ≤ 0.05). Fe and Mn were also positively correlated (r = 0.826, P ≤ 0.05). It is interesting to note that deficiencies for Fe and Mn are very commonly found in different cherry growing conditions of the Mediterranean area (Moreno et al. 1996; Jiménez et al. 2004). Both ΣDOP macro- and ΣDOP micronutrients were also positively correlated (r = 0.819, P ≤ 0.05). The significant positive correlations between yield per tree and Ca (r = 0.780, P ≤ 0.05) and with Mn (r = 0.791, P ≤ 0.05) could indicate the interest of rootstocks having higher absorption and uptake for these elements in the present growing conditions (Supplementary files 1 and 2). Also, the significant and positive correlations were observed between ΣDOPmacro and ΣDOPmicro values (r = 0.819, P ≤ 0.05) (Supplementary files 3).
Pearson’s correlation coefficients for the traits studied for the eight Prunus rootstocks assessed and the two years of study. The size of the circle for each correlation and the color depicts the significance and the magnitude of the correlation coefficient, respectively. FW fruit weight, Y yield per tree (Color figure online)
A principal component analysis (PCA) was performed to understand how mineral elements and yield traits contribute to variability among the different rootstocks budded with the ‘Šumadinka’ sour cherry cultivar (Fig. 2). The first two components of the PCA performed on the different traits explained 73.33% of the variation in the dataset, with 57.35% being captured by the first (PC1) and 15.98% by the second principal component (PC2) (Fig. 2). The PC1 mainly contributes to B, K, Ca, P, Cu, and Mg, FW and yield per tree, in the positive side. In contrast, the ΣDOP macro and ΣDOP micro-indexes were situated in the negative side of PC1. Therefore, these results showed that rootstocks on the positive side of PC1 corresponding to Adara, Gisela 5, and MaxMa 14 induced, in general, higher yield per tree, FW, and lower ΣDOP macro and ΣDOP micro-indexes, and consequently, they were among the most balanced rootstocks. In contrast, trees on the negative side of PC1, corresponding to Myrobalan and Krymsk 6 had, in general, lower values for most leaf mineral elements but higher ΣDOP macro and ΣDOP micro-indexes, showing the more unbalanced nutritional index, especially when compared to MaxMa 14. On the other hand, the positive side of the PC2 mostly explained the Fe and Mn mineral elements, and therefore, demonstrating the aptitude of Adara rootstock to induce higher contents for these two elements.
Conclusions
In the studied growing conditions, with heavy and acidic soil and moderate climate, yield per tree was higher on Adara, Gisela 6, and MaxMa 14 rootstocks, whereas the largest fruits were induced by Gisela 6. Although Adara was selected for heavy and calcareous soils, it showed a high degree of adaptability to acidic soils and appears as a new promising rootstock for these conditions. Rootstocks significantly affected leaf macro- and microelements content and nutritional balance, probably due to their different uptake and potential for translocation to the leaves. Leaves of ‘Šumadinka’ sour cherry on all rootstocks showed the tendency to show leaf deficiency for N, P, and Zn, whereas in most cases, MaxMa 14 and Gisela 6 induced optimal nutrients levels. This phenomenon confirmed the good adaptation of these rootstocks to acidic soils. In contrast, Krymsk 6 did not show important positive features. Considering their overall performance and tolerance to heavy and acidic soil, Adara, MaxMa 14, and Gisela 6 appears as new promising rootstocks and can be recommended for sour cherries growing under similar soil conditions. On the contrary, the studied Myrobalan seedlings should not be used as rootstock for sour cherry due to its anomalous performance with the ‘Šumadinka’ cultivar. The DOP index may be accurate tool of estimating sour cherry yield and fruit quality as affected by the amount of different macro- and micronutrients which is of very high economic and environmental importance, worldwide. Nevertheless, further study should be done to confirm and relate growth and fruit quality parameters with leaf mineral composition and productivity. Also, this study should be conducted before alterations of current recommendations concerning clonal sour cherry rootstocks can be made and the continued widespread practice of using seedlings of Mazzard or Mahaleb rootstocks is altered. Finally, we assume that it is possible to select most efficient sour cherry rootstocks in capturing and translocation mineral elements in the soil with more effective combinations with fertilizers.
References
Abadía J (1992) Leaf responses to Fe deficiency: a review. J Plant Nutr 15(10):1699–1713. https://doi.org/10.1080/01904169209364432
Albacete A, Martínez-Andújar C, Martínez-Pérez A, Thompson AJ, Dodd IC, Pérez-Alfocea F (2015) Unravelling rootstock × scion interactions to improve food security. J Exp Bot 66(8):2211–2226. https://doi.org/10.1093/jxb/erv027
Amiri ME, Fallahi E, Safi-Songhorabad M (2014) Influence of rootstock on mineral uptake and scion growth of ‘golden delicious’ and ‘royal gala’ apples. J Plant Nutr 37(1):16–29. https://doi.org/10.1080/01904167.2013.792838
Anderson JL, Lindstrom TE, del Real-Laborde JI (1996) Rootstock effects on growth and productivity of ‘montmorency’ sour cherry. Acta Hortic 410:511–518. https://doi.org/10.17660/ActaHortic.1996.410.84
Anthony B, Musacchi S (2021) Dwarfing mechanisms and rootstock-scion relationships in apple. Italus Hortus 28(2):22–36. https://doi.org/10.26353/j.itahort/2021.2.2236
Archibald JA, Cline RA (1962) Factors affecting leaf nutrient composition of ‘Montmorency’ cherry. Annual Report of Experiment Station of Production Laboratory, Vineland, pp 39–41
Betrán JA, Val J, Millán LM, Monge E, Montañés L, Moreno MA (1997) Influence of rootstock on the mineral concentrations of flowers and leaves from sweet cherry. Acta Hortic 448:163–167. https://doi.org/10.17660/ActaHortic.1997.448.24
Blagojević M, Miletić R, Rakićević M, Mitrović M, Glišić I, Karaklajić-Stajić Ž (2006) Initial cropping of sour cherry under the dense planting system. Agroznanje 7(4):5–10 (in Serbian with English abstract)
Borowy A, Chrzanowska E, Kapłan M (2018) Comparison of three sour cherry cultivars grown in Central-Eastern Poland. Acta Sci Pol Hortoru 17(1):63–73. https://doi.org/10.24326/asphc.2018.1.6
Bould C (1966) Leaf analysis of deciduous fruits. In: Childers NF (ed) Nutrition of fruit crops Horticultural Publications. Rutgers University, New Brunsvick, NJ, pp 651–684
Cantín CM, Pinochet J, Gogorcena Y, Moreno MA (2010) Fruit quality and yield of ‘Van’ and ‘Stark Hardy Giant’ sweet cherry cultivars as influenced by grafting on different rootstocks. Sci Hortic 123:329–335. https://doi.org/10.1016/j.scienta.2009.09.016
Chaleshtori AA, Panahpour E, Iranipour R, Moezzi A (2020) Diagnosing the nutritional balance of almond (Prunus sp.) Orchards Using DRIS and DOP Methods. J Plant Growth Regul 40(8):1640–1651. https://doi.org/10.1007/s00344-020-10214-0
Dencker I, Toldam-Andersen TB (2005) Effects of rootstock, winter temperature and potassium fertilization on yield components of young sour cherries. Acta Hortic 667:409–414. https://doi.org/10.17660/ActaHortic.2005.667.59
Errea P (1998) Implications of phenolic compounds in graft incompatibility in fruit tree species. Sci Hortic 74(3):195–205. https://doi.org/10.1016/S0304-4238(98)00087-9
Errea P, Felipe A, Herrero M (1994) Graft establishment between compatible and incompatible Prunus spp. J Exp Bot 45(3):393–401. https://doi.org/10.1093/jxb/45.3.393
Eremin G (2005) Prunus plant named ‘LC-52’. United States Plant Patent US PP16, 114 P3
Eremin GV, Podorozhniy VN, Eremina OV (2017) Use of genetic diversity of the genus Prunus L. in selection of clonal rootstocks for stone fruit crops and features of their reproduction. Proc Latv Acad Sci B 71(3):173–177. https://doi.org/10.1515/prolas-2017-0029
FAOSTAT (2022) http://www.fao.org/faostat/en/#data/QC. Accessed 26 April 2022
Feucht W, Treutter D, Christ E (1992) Localization and quantitative determination of catechins and proanthocyanidins in the phloem of elm and cherry. Tree Physiol 10(2):169–177. https://doi.org/10.1093/treephys/10.2.169
Font i Forcada C, Pinochet J, Gogorcena Y, Moreno MÁ (2017) Effect of eight different rootstocks on agronomic and fruit quality parameters of two sweet cherry cultivars in Mediterranean conditions. Acta Hortic 1161:315–320. https://doi.org/10.17660/ActaHortic.2017.1161.51
Font i Forcada C, Reig G, Mestre L, Mignard P, Betrán JA, Moreno MA (2020) Long-term Prunus rootstocks performance under root asphyxia conditions, heavy-calcareous soil and hot climate. Agronomy 10(8):e1159. https://doi.org/10.3390/agronomy10081159
Gonçalves B, Moutinho-Pereira J, Santos A, Silva AP, Bacelar E, Correia C, Rosa E (2006) Scion–rootstock interaction affects the physiology and fruit quality of sweet cherry. Tree Physiol 26(1):93–104. https://doi.org/10.1093/treephys/26.1.93
Gruppe W (1985) An overview of the cherry breeding program at Giessen 1965–1984. Acta Hortic 169:189–198. https://doi.org/10.17660/ActaHortic.1985.169.27
Grzyb Z-S, Sitarek M, Guzowska-Batko B (2005) Results of a sweet cherry rootstock trial in northern Poland. Acta Hortic 667:207–210. https://doi.org/10.17660/ActaHortic.2005.667.30
Havlin JL, Tisdale SL, Nelson W, Beaton JD (2005) Soil fertility and fertilizers: An introduction to nutrient management. Pearson Prentice Hall, Upper Saddle River, NJ
Heckman J (2004) Leaf analysis for fruit trees. Rutgers Cooperative Research & Extension NJAES, Rutgers the State University of New Jersey, New Brunswick, NJ, pp 1–2
Hell R, Stephan UW (2003) Iron uptake, trafficking and homeostasis in plants. Planta 216(4):541–551. https://doi.org/10.1007/s00425-002-0920-4
Herrera EA (2001) Fertilization programs for apple orchards. Guide H-319. Extension Horticulturist College of Agriculture and Home Economics, New Mexico State University. http://aces.nmsu.edu
Hrotkó K, Magyar L, Borsos G, Gyeviki M (2014) Rootstock effect on nutrient concentration of sweet cherry leaves. J Plant Nutr 37(9):1395–1409. https://doi.org/10.1080/01904167.2014.911317
Iezzoni AF (1996) Sour cherry cultivars: objectives and methods of fruit breeding and characteristics of principal commercial cultivars. In: Webster AD, Looney NE (eds) Cherries: crop physiology, production and uses. CAB International, Wallingford, pp 113–123
Jadcuzk E (1993) Some factors affecting potassium nutrition of sour cherry trees. In: Fragoso MAC, van Beusichem ML (eds) Optimization of plant nutrition. Development in plant and soil sciences. Springer, Dordrecht, pp 127–132. https://doi.org/10.1007/978-94-017-2496-8_21
Jiménez S, Garín A, Gogorcena Y, Betrán JA, Moreno MA (2004) Flower and foliar analysis for prognosis of sweet cherry nutrition: influence of different rootstocks. J Plant Nutr 27(4):701–712. https://doi.org/10.1081/PLN-120030376
Jiménez S, Pinochet J, Gogorcena Y, Betrán JA, Moreno MA (2007) Influence of different vigour cherry rootstocks on leaves and shoots mineral composition. Sci Hortic 112(1):73–79. https://doi.org/10.1016/j.scienta.2006.12.010
Kalavrouziotis IK, Koukoulakis P (2010) Elemental antagonism in vegetables under treated municipal wastewater. J Plant Interact 5(2):101–109. https://doi.org/10.1080/17429140903438092
Kangueehi GN, Stassen PJC, Theron KI, Wooldridge J (2011) Macro and microelement requirements of young and bearing apple trees under drip fertigation. S Afr J Plant Soil 28(2):136–141. https://doi.org/10.1080/02571862.2011.10640025
Kopytowski J, Markuszewski B (2010) The effect of the rootstock on growth, yielding and fruit quality of three cultivars of sour cherry cultivated in the Warmia region. J Fruit Ornam Plant Res 18(2):177–184
Leece D (1975) Diagnostic leaf analysis for stone fruits. 5. Sweet cherry. Aust J Exp Agric 15(72):118–122. https://doi.org/10.1071/EA9750118
Maas FM, Balkhoven-Baart J, van der Steeg PAH (2014) Evaluation of Krymsk®5 (VSL-2) and Krymsk®6 (LC-52) as rootstocks for sweet cherry ‘Kordia.’ Acta Hortic 1058:531–536. https://doi.org/10.17660/ActaHortic.2014.1058.66
Milosevic T, Milosevic N (2011) Seasonal changes in micronutrients concentrations in leaves of apricot trees influenced by different interstocks. Agrochimica 55(1):1–14
Milošević T, Milošević N (2019) Soil fertility: plant nutrition vis-à-vis fruit yield and quality of stone fruits. In: Sirvastava AK, Chengxiao H (eds) Fruit crops: diagnosis and management of nutrient constraints. Elsevier Inc., pp 583–605. https://doi.org/10.1016/B978-0-12-818732-6.00041-1
Milošević T, Milošević N, Glišić I, Bošković-Rakočević L, Milivojević J (2013) Fertilization effect on trees and fruits characteristics and leaf nutrient status of apricots which are grown at Cacak region (Serbia). Sci Hortic 164:112–123. https://doi.org/10.1016/j.scienta.2013.09.028
Milošević T, Milošević N, Milivojević J, Glišić I, Nikolić R (2014) Experiences with Mazzard and Colt sweet cherry rootstocks in Serbia which are used for high density planting system under heavy and acidic soil conditions. Sci Hortic 176(12):261–272. https://doi.org/10.1016/j.scienta.2014.07.020
Milošević T, Milošević N, Mladenović J (2020) Combining fruit quality and main antioxidant attributes in the sour cherry: the role of new clonal rootstock. Sci Hortic 265:e109236. https://doi.org/10.1016/j.scienta.2020.109236
Montañés L, Heras L, Abadía J, Sanz M (1993) Plant analysis interpretation based on a new index: deviation from optimum percentage (DOP). J Plant Nutr 16(7):1289–1308. https://doi.org/10.1080/01904169309364613
Moreno MA, Tabuenca CM, Cambra R (1995) Adara, a plum rootstock for cherries and other stone fruit species. HortScience 30(6):1316–1317. https://doi.org/10.21273/HORTSCI.30.6.1316
Moreno MA, Montañés L, Tabuenca MC, Cambra R (1996) The performance of Adara as a cherry rootstock. Sci Hortic 65(1):58–91. https://doi.org/10.1016/0304-4238(95)00862-4
Moreno MA, Adrada R, Aparicio J, Betrán JA (2001) Performance of ‘Sunburst’ sweet cherry grafted on different rootstocks. J Hortic Sci Biotechnol 76(2):167–173. https://doi.org/10.1080/14620316.2001.11511345
Nawaz MA, Imtiaz M, Kong Q, Cheng F, Ahmed W, Huang Y, Bie Z (2016) Grafting: a technique to modify ion accumulation in horticultural crops. Front Plant Sci 7:1457. https://doi.org/10.3389/fpls.2016.01457
Neilsen G, Kappel F (1996) ‘Bing’ sweet cherry leaf nutrition is affected by rootstock. HortScience 31(7):1169–1172. https://doi.org/10.21273/HORTSCI.31.7.1169
Nenadović-Mratinić E, Milatović D, Đurović D (2006) Biological characteristics of sour cherry cultivars in the Danube region of Belgrade. Zbor Nauč Rad 12(3):24–29 (in Serbian with English abstract)
Nyéki J, Soltész M, Popovics L, Szabó T, Thurzó S, Holb IJ, Fári MG, Veres Z, Harsányi G, Szabó Z (2005) Strategy of the sour cherry verticum in the Northern Great Plain Region Hungary (Analytic study). Int J Hortic Sci 11(4):7–31. https://doi.org/10.31421/IJHS/11/4/601
Pereira S, Silva V, Bacelar E, Guedes F, Silva AP, Ribeiro C, Gonçalves B (2020) Cracking in sweet cherry cultivars Early Bigi and Lapins: correlation with quality attributes. Plants 9:e1557. https://doi.org/10.3390/plants9111557
Pérez-Alfocea F, Albacete A, Ghanem ME, Dodd IC (2010) Hormonal regulation of source-sink relations to maintain crop productivity under salinity: a case study of root-to-shoot signaling in tomato. Funct Plant Biol 37(7):592–603. https://doi.org/10.1071/FP10012
Perry RL (1987) Cherry rootstocks. In: Rom RC, Carlson RF (eds) Rootstocks for fruit crops. Wiley, New York, pp 217–264
Prodanov G, Vitanowa I (1977) Influence of the rootstock on the contents of nitrogen and some other elements in sour cherry leaves. Ovoscharstvo 56:36–37 (in Bulgarian)
Proebsting EL, Kenworthy AL (1954) Growth and leaf analysis of Montmorency cherry trees as influenced by solar radiation and intensity of nutrition. Proc Am Soc Hortic Sci 63:41–48
Rasool A, Mansoor S, Bhat KM, Hassan GI, Baba TR, Alyemeni MN, Alsahli AA, El-Serehy HA, Paray BA, Ahmad P (2020) Mechanisms underlying graft union formation and rootstock scion interaction in horticultural plants. Front Plant Sci 11:590847. https://doi.org/10.3389/fpls.2020.590847
Reig G, Font i Forcada C, Mestre L, Jiménez S, Betrán JA, Moreno MA (2018) Horticultural, leaf mineral and fruit quality traits of two “Greengage” plum cultivars budded on plum based rootstocks in Mediterranean conditions. Sci Hortic 232:84–91. https://doi.org/10.1016/j.scienta.2017.12.052
Righetti TL, Wilder KL (1987) Interpreting cherry leaf analyses. In: Willet M (ed) Conference: Sweet Cherry Production in the Pacific Northwest. Proceedings of the 1987 Pacific Northwest Tree Fruit Shortcourse. Washington State University, pp 120‒145
Shahkoomahally S, Chaparro JX, Beckman TG, Sarkhosh A (2020) Influence of rootstocks on leaf mineral content in the subtropical peach cv. UF Sun Hortsci 55(4):496–502. https://doi.org/10.21273/HORTSCI14626-19
Stehr R (1998) First results with dwarfing rootstocks in northern Germany as part of a National German Rootstock Trial. Acta Hortic 468:297–306. https://doi.org/10.17660/ActaHortic.1998.468.35
Tisdale SL, Nelson WLL (1966) Soil fertility and fertilisers, 2nd edn. The Macmillan Company, New York
Tombesi S, Almehdi A, DeJong TM (2011) Phenotyping vigour control capacity of new peach rootstocks by xylem vessel analysis. Sci Hortic 127(3):353–357. https://doi.org/10.1016/j.scienta.2010.11.007
Uçgun K, Gezgin S (2017) Interpretation of leaf analysis performed in early vegetation in apple orchards. Commun Soil Sci Plant Anal 48(14):1719–1725. https://doi.org/10.1080/00103624.2017.1383415
Ugorik M, Holubowicz T (1990) The influence of rootstock and cultivar on the leaf content of nutrient elements growth and yield of three sour cherry cultivars. Acta Hortic 274:491–500. https://doi.org/10.17660/ActaHortic.1990.274.63
von Bennewitz E, Cooper T, Benavides C, Losak T, Hlusek J (2011) Response of ‘Jonagold’ apple trees to Ca, K and Mg fertilization in an andisol in southern Chile. J Soil Sci Plant Nutr 11(3):71–81. https://doi.org/10.4067/S0718-95162011000300006
Webster AD (1981) Dwarfing rootstocks for plums and cherries. Acta Hortic 114:201–207. https://doi.org/10.17660/ActaHortic.1981.114.29
Webster AD, Schmidt H (1996) Rootstocks for sweet and sour cherries. Cherries: crop physiology, production and uses. CAB International, Cambridge, pp 127–167
Wei T, Simko V (2017) R package "corrplot": Visualization of a Correlation Matrix (Version 0.84). https://github.com/taiyun/corrplot
Wociór S (2008) The effect of rootstocks on the growth and yielding of sour cherry cv. ‘Łutowka.’ Acta Agrobot 61(1):123–127. https://doi.org/10.5586/aa.2008.01
Zarrouk O, Gogorcena Y, Moreno MA, Pinochet J (2006) Graft compatibility between peach cultivars and Prunus rootstocks. HortScience 41(6):1389–1394. https://doi.org/10.21273/hortsci.41.6.1389
Acknowledgements
This research did not receive any specific grants from funding agencies in the public, commercial, or not-for-profit sectors. However, the authors acknowledge Dr. B. Krška, Dr. T. Jemrić, Dr. K. Dugalić, and Dr. G. Fruk which assisted in providing rootstocks for research as well as “Agromillora Group,” Barcelona, Spain.
Author information
Authors and Affiliations
Contributions
TM: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Software, Validation, Writing—original draft, Writing—review & editing. MÁM: Investigation, Writing—original draft, Writing—review & editing. NM: Data curation, Formal and statistical analysis, Investigation, Validation. MM: Chemical and soil analysis, Investigation, Methodology.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Handling Editor: Jose M. Miguel.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Milošević, T., Moreno, M.Á., Milošević, N. et al. Regulation of Yield, Fruit Size, and Leaf Mineral Nutrients of the ‘Šumadinka’ Sour Cherry Cultivar with Help of Rootstocks. J Plant Growth Regul 42, 5587–5599 (2023). https://doi.org/10.1007/s00344-023-10939-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00344-023-10939-8