Abstract
Melatonin (N-acetyl-5-methoxy-tryptamine), derivative of tryptophan, manifested as a conserved domain, which is ubiquitously apportioned from bacteria to higher organisms extending to fungi and algae as well. Melatonin is entailed in umpteen developmental processes of plants, including stress responses. The pleiotropic impact of melatonin in regulating transcripts of manifold genes validate its imperative contribution as multi-regulatory substance. Albeit, the progressive research regarding plants is yet prelusive in contrast to orthodox melatonin physiology in animals. This reinforces the exigency for comprehensive reassessment pertaining to its potential in biochemical and physiological processes, anti-stress response against abiotic stimulators (temperatures, salinity, drought, toxins, etc.), detoxification mechanism, and its other salubrious effect. Stressors are known to create RNS and ROS, which induces oxidative damage in plants. Cellular deterioration and mortality are a result of negligence toward oxidative damage. Tremendous quantum leap has been made in comprehending, how melatonin safeguards plants against abiotic stress. Here, focus will be on mechanistic basis of melatonin-mediated protection to abate abiotic stress. Abiotic stress induces melatonin synthesis and this redeeming upsurge in melatonin succors plant to thrive under stress conditions. Melatonin is considered an excellent antioxidant because it effectively scavenges a wide range of RNS and ROS. Melatonin maintains ROS levels in peculiar ways: (a) chemical interaction between melatonin and ROS, causing their inactivation and (b) melatonin-induced activation of SOD, POD, APX, CAT, and GPX leads to ROS detoxification. The contemporary study gives a comprehensive review on abiotic stress response of melatonin, particularly, its mitigating impact when applied exogenously in plants under environmental stress conditions. The commentary will allow the researchers to comprehend the prevailing plant stress conditions and further contemplate the tendency of phytomelatonin in crop research.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Melatonin (N-acetyl-5-methoxytryptamine), a tryptophan derivative, discerned in bovine pineal gland during late 1950s was a radical discovery (Lerner et al., 1958). This is a pleiotropic molecule which is evolutionarily conserved and exists ubiquitously in fauna and flora (Hardeland et al., 2011). Melatonin is both amphiphilic and amphipathic molecules and can pass effortlessly through cell membrane to cytosol, mitochondria, and nucleus (Galano and Reiter, 2018). Melatonin occurs in numerous plant parts and is accountable for invigorating multifarious physiological process. Among plants, another major indication for diverse function of melatonin can be inferred from various species and tissue-specific differences in concentrations, ranging from very low (unnoticeable) to above 20 or 30 µg/g (Arnao & Hernández 2020). Umpteen investigations reveal unambiguous role of melatonin in plants (Fig. 1), acting as growth regulator (Sun et al., 2021), determining growth of the explants, roots, and shoot (Mao et al., 2020), stimulate rhizogenesis (Riaz et al., 2018), and defer leaf senescence (Zhao et al., 2021). The antioxidant potential of phytomelatonin may elucidate its few physiological functions viz, to reinforce plants under abiotic stress, such as heat, drought, pesticides, UV radiations, cold, and salinity, rendering melatonin as remarkable candidate to countermeasure field crops (Bose and Howlander, 2020). Melatonin is a powerful free radical scavenger that protects against dangerous reactive oxygen and nitrogen species. Membrane receptors and nuclear receptors regulate melatonin’s pleiotropic activity in living organisms (Back, 2021). Melatonin receptors also function independently, and their bioactive substance enables the replacement of reactive oxygen and nitrogen species (ROS and RNS) with melatonin (Arnao and Hernandez-Ruiz, 2019). Melatonin also plays a role in non-receptor mediated effects, such as ROS scavenging, antioxidant potential enhancement, and tissue protection from oxidative stress (Lee and Back, 2020). Eventually, the production and accumulation of ROS and RNS are rudimentary processes related to cellular and physiological pathogenesis.
Abiotic stress imparts detrimental effect on plants, limiting agricultural productivity and growth, thereby becoming a global issue. However, the proportion of agricultural lands experiencing multiple abiotic stresses is expected only to rise under changing global climate fueled by anthropogenic activities. These stresses retard plant growth and development by lowering metabolism, leading to nutrient deficiency, generating reactive oxygen species, and osmotic and ionic stress causing oxidative damage to essential components viz, proteins and lipids, and reduce root growth, enzymatic activity, water absorption, and mineral uptake efficiency (Kumar et al., 2019). Data suggest that crop productivity has severely decreased by ~ 50–82% depending upon type of stress, duration, and crop variety (Gao et al., 2007). Plants undergo numerous physiological and metabolic changes to safeguard in response to abiotic stress. Also plants are bestowed with inbuilt capacity to respond via signal transduction pathways for adjusting their metabolism (Jan et al., 2020). In order to promote sustainable agricultural practices various amendments practices have been implemented to achieve the goal (Sabkia et al., 2021).
Selection of some exogenous chemical priming can regulate plant metabolism and improve plant resistance. Integrating melatonin to agronomical practices reportedly provides numerous benefits. Mentioning particularly about melatonin priming, it has been observed to enhance photosynthesis of Solanum lycopersicum under low-temperature stress (Yang et al., 2018) and salt stress (Zhao et al., 2016) and it could be due to action of melatonin on redox regulation of photosynthetic electron transport and synthesis of D1 protein (Zhou et al., 2016). Seed priming with melatonin has shown improved seed germination and resilience under adverse conditions. Seed priming with melatonin is one such method used to regulate plants metabolic processes during early stages of germination under stress conditions. Seed priming is a promising method that confers uniform germination by reducing imbibitions time, activating germination inducers, repairing breaks in DNA, and regulating hydration levels (Marthandan et al., 2020). It has been documented that melatonin pre-treatment neutralized the detrimental aging effect in aged oats seeds. Primed seeds were found to have restored membrane integrity and up-regulated energy generation pathways (Yan and Mao, 2021). Melatonin-primed seeds possess higher content of osmoprotectants, up-regulation of energy-producing pathways (Jan et al., 2022), and enhanced protein content, which may lead to improve abiotic stress response in plants (Rajora et al., 2022). Various modes of melatonin priming are seed priming, leaf spray, and root application and seed priming and leaf spray are found to be work better in alleviating stress. Using melatonin to improve crop resistance and mitigate photo-inhibition is an important agricultural management measure.
Ergo, it is concluded that fundamental role of melatonin in life forms is to boost antioxidant defense system and serve as a primary respondent to stress conditions (Arnao and Hernandez-Ruiz, 2019). Promulgating the advances of melatonin in previous years, its mode of action in plants is explored extensively. The contemporary study gives a comprehensive review on abiotic stress response of melatonin, particularly, its mitigating impact when applied exogenously in plants under environmental stress conditions. The commentary will allow the researchers to comprehend the prevailing plant stress conditions and further contemplate the tendency of phytomelatonin in crop research.
Melatonin Modulates Plant’s Various Attributes
Multifarious role of melatonin among higher plants have been studied thoroughly yet data for all cases is scarce (Table 1). Among various experiments executed to identify exact role of phytomelatonin, its role as promising growth regulator has been extensively reviewed. The circadian oscillator in plants is capable of regulating various biological processes, viz metabolic modulation, gene regulation and protein stability, thereby increasing growth rate and photosynthesis and further may influence seed production and flowering Arnao and Hernandez-Ruiz, 2019). The subject discussed below outlines the physiological function of phytomelatonin and protecting plant’s integrity under stress conditions.
Plant Growth and Development
The tryptophan derivatives, melatonin, and IAA have alike chemical conformation and their structural homology allows them to regulate synonymous activity in plants. IAA and melatonin contain indole structure, yet differ in bonded functional groups (Fig. 2). Structurally, auxin and melatonin exhibit numerous similarities, for example, planar aromatic-ring, hydrophobic transition region, and carboxylic acid-binding site. While, melatonin improves different growth parameters in plants, but in majority of cases the mechanisms are partly defined (Tan and Reiter, 2020). Thereon, melatonin exhibit IAA-like activities. To give an instance, in Arabidopsis thaliana transcriptome data were analyzed where 16 auxin-related genes were identified and due to melatonin treatment, the expression pattern was metamorphosed. Melatonin works in correlation with auxin in order to foster secondary root growth of Arabidopsis thaliana (Ren et al., 2019). In woody plants, it was inferred that melatonin facilitates adventitious root development. Melatonin’s involvement in IAA signaling pathway was also ascertained by RT-qPCR which suggests that melatonin induces expression of IAA biosynthesis and signal transduction-associated genes (Mao et al., 2020). Melatonin-dosed soybean cultivars produce more pods and seeds relatively to untreated one (Bawa et al., 2020). Melatonin regulates root formation in concentration-dependent manner in tomato (Solanum lycopersicum) (Siddiqui et al., 2020), mustard (Brassica) (Dai et al., 2020), and various monocot plants (Danilova et al., 2020). Succinctly, various studies suggests that phytomelatonin invigorates seedling growth (Mir et al., 2020), promotes or prevents primary root growth (Yang et al., 2021), facilitates adventitious and lateral root progression (Gokbayrak et al., 2020), impedes delay-induced leaf senescence by improving photosynthesis, carbon dioxide uptake, and biomass accretion (Zhao et al., 2021), and stimulates caulogenesis and rhizogenesis in organotypic culture (Golovatskaya et al., 2020).
Photosynthesis and Pigmentation
Plants perform photosynthesis by converting light energy to chemical energy and subsequently utilize converted energy for the proliferation. Plants being sessile are incessantly exposed to array of environmental stresses, causing extensive yield loss. Abiotic stresses, for example, cold, salinity, drought, heavy metal, and pesticide, result in electron transport chain (ETC) distortion, inducing photo-oxidation and thereby decreasing photosynthetic efficacy (Jan et al., 2020). Stress causes ultrastructural changes in leaf chloroplasts and mitochondria, resulting in uncontrolled generation of reactive oxygen species (ROS) (Debnath et al., 2021). The photosynthetic efficiency is determined by total chlorophyll content, and an increase in ROS produces a significant fall in chlorophyll content inside plants (Siddiqui et al., 2020). Plants with a strong root system and high photosynthetic capability benefit from melatonin pre-treatment. Melatonin supports plant development, accelerates photosynthetic carbon absorption, and aids in the protection of cellular proteins (Meng et al., 2021). Many studies were conducted to validate the active participation of melatonin in enhancing photochemical efficiency of photosystem II in plants. Melatonin treatment remarkably improved Fv/Fm and PLABS in zinc oxide stressed plants, indicating stimulatory role of melatonin on quantum yield of photosystem II and light assimilation efficiency (Zuo et al., 2017). Likewise, experimental evaluation validates the active participation of phytomelatonin in enhancing photosynthetic efficiency. In Solanum lycopersicum melatonin treatment (100 µM) elevated endogenous melatonin level, photosynthetic pigment content, and up-regulated gene expression of photosynthetic pigments under stress conditions. Melatonin amplified CO2 assimilation, that is, Vc,max (maximum rate of ribulose-1,5-bisphosphate carboxylase, Rubisco) and Jmax (electron transport of Rubisco formation) along with increased Rubisco activity and FBPase activity. Under stress conditions melatonin increased electron transport rate (ETR), PSII efficacy (ФPSII), and photochemical quenching co-efficient (qP). Concisely, melatonin treatment enhanced chlorophyll a fluorescence which in turn maximizes electron transport efficiency (Jahan et al., 2021). Stomata in leaves are the primary path for gas exchange and have a major role in photosynthesis and respiration. Implementation of melatonin improves stomatal conductance in Vitis vinifira and Malus domestica (Yang et al., 2020; He et al., 2020). Also melatonin seed treatment increases stomatal size and regulates its opening in plants during stress conditions thereby boosting stomatal conductance and capacity. In Cucumis sativus plants, melatonin treatment improved the photosynthetic rate, antioxidant capacity, and lowered chlorophyll degradation ultimately mitigating abiotic stress (Ali Shah et al., 2020). Findings also reveal that melatonin delays dark-induced leaf senescence and decreases ROS generation in Vitis vinifera. It enables scavenging of antioxidant enzymes, such as CAT, POD, and SOD. In cessation, the results also manifest that melatonin subdues translation of leaf senescence associated genes (SAGs) (Shi et al., 2019). In drought-stressed Glycine max seedling the transcriptome analysis unveiled the expression of photosynthetic genes up-regulated by melatonin. Distinctly, some interesting genes up-regulated by melatonin are PsaK and PsaG (subunits of photosystem I), PsbO and PsbP (proteins of photosystem II), PetF (ferredoxin gene) and VTC4 (involved in ascorbate biosynthesis) (Imran et al., 2021). In microalga Ulva sp. melatonin showed few additive effects on chlorophyll content (Hardeland 2019). Similar evidences were reported where melatonin imparted protective role on photosynthetic pigments in cold-stressed Triticum (Zhang et al., 2021), salt-stressed Cucumis sativus (Zhang et al., 2020), drought-stressed citrus (Jafari and Shahsavar 2020), and salt-stressed tomato (Altaf et al., 2021).
Anti-Senescence
From very long melatonin has been recognized as anti-aging component in mammals (Reiter et al., 2018). Being a potent antioxidant in living species its salubrious effects have been discussed in detail (Bose and Howlader 2020). Melatonin behaves as a signal to activate antioxidant enzymes or bolster other antioxidants in particular GSH, AsA, and polyphenols (Jahan et al., 2021). For instance, CAND2/PMTR1 maintains ROS equilibrium via modulating gene expression and activity of ROS scavenging enzymes such as CAT, POD, and SOD in Arabidopsis (Li et al., 2020a, b). Albeit, the impact of melatonin on plant sustenance remained unexamined comprehensively, yet its involvement in leaf senescence has received limelight (Zhao et al., 2021). It was promulgated that melatonin decelerated dark-induced senescence and this serendipitous effect was verified in Actinidia deliciosa (Liang et al., 2018). The impact of melatonin on anti-senescence contributes to its exceptional antioxidant properties. Through various examinations it is evident that melatonin regulates redox parameters viz, lowering ROS levels and rising AsA and GSH levels along with antioxidant enzymes which corresponds to its anti-senescence effect. Howbeit, studies on melatonin-induced leaf senescence fail to present a reconcilable picture. Multifarious phytohormones viz, salicylic acid, ethylene, and jasmonic acid involved in inducing or stimulating senescence were up-regulated by prodigious set of genes and this phenomenon was mediated by melatonin as conferred from whole-genome expression analysis of melatonin-treated Arabidopsis plant (Zia et al., 2019). Irrespective of influence of melatonin on senescence, synergy between melatonin and aforementioned phytohormones should be envisaged. To prevent leaf senescence, it is essential to preserve chlorophyll activity, ergo, role of melatonin in regulating photosynthetic activity demands preferential attention. In trailblazing study, exogenous melatonin safeguards chlorophyll from deterioration in Chinese flowering cabbage (Tan et al., 2019). Impact of melatonin in protecting chlorophyll was also studied in other cultivars, including Solanum lycopersicum (Yin et al., 2019) and Oryza sativa (Choi and Back, 2019). In transcriptome analysis, treatment of melatonin up-regulated expression of PsaK and PsaG (two subunits of photosystem I), PsbO and PsbP (two sub units of photosystem II), and ferredoxin PetF, while it down-regulated expression of chlorophyllase and PaO (Cao et al., 2019). Melatonin prevents leaf senescence by preserving chlorophyll, as evidenced by several studies. The proteome transformation that occurs during leaf senescence was studied in a groundbreaking study. Such discoveries provide a plethora of data and reveal many of the functions of melatonin in plant physiological systems. Natural senescence affected 622 proteins, but melatonin therapy altered just 309 proteins, according to climactic proteomic data. Melatonin affected many proteins involved in the senescence process during cessation, further down-regulating those proteins that are normally up-regulated during natural senescence. Furthermore, during a natural senescence event, proteins involved in photosynthesis were shown to be up-regulated (Zhao et al., 2021).
Increase in Melatonin level and their corollary for plant protection and stress tolerance is presented in pictorial form. (1) Pathogen attack, (2) senescence, (3) Chemical stressors, (4) Drought, (5) Heat, (6) Salinity, (7) Developmental stage—Fruit ripening, (8) Developmental stage—Flower bud, (9) Cold, and (10) Intense UV radiation. 1 – 9 correspond to the increased melatonin content. HSPs; Heat shock proteins
Melatonin for Abiotic stress Forbearance
Tremendous quantum leap has been made in comprehending, how melatonin safeguards plants from abiotic stress. Here, focus will be on mechanistic basis of melatonin-mediated protection to abate abiotic stress. Abiotic stress induces melatonin synthesis and this redeeming upsurge in melatonin succors plant to thrive during stress conditions.
Melatonin levels and epigenetic changes in plants during stress conditions
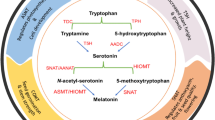
Melatonin perpetuates in plants under normal condition. While, melatonin concentration is relentlessly distressed by pernicious conditions, including heat, cold, UV intensity, drought, salinity, and metal stress (Sajjad et al., 2020). Melatonin concentration within cell is responsive to extraneous condition. Briefly enumerating, in Arabidopsis thaliana significant increase in melatonin levels under UV-B stress condition was observed which in turn provided resistance against UV-B stress (Yao et al., 2021). In melatonin synthesis pathway serotonin N-acetyletransferase (SNAT) and N-acetylserotonin methyltransferase (ASMT) are vital enzymes. The endogenous melatonin level increases by 2–threefold in Cucumis sativus amid salt stress conditions (Qi et al., 2020). The enzymes involved in melatonin biosynthesis are over-expressed by stress stimuli. In Oryza sativa, melatonin biosynthesis inflates under heat and dark conditions because of increased regulation of SNAT and ASMT (Choi and Back 2019). Hydroxyindole-O-methyltransferase (HIOMT; also known as ASMT) shows higher activity in Zea mays cotyledons under extreme salt conditions, culminating with increased melatonin levels (Ahmad et al., 2020). Various reports on mechanisms rudimentary to regulation of melatonin synthesis during stress stimuli emphasize on up-regulated transcription factors of aforementioned enzymes and isoenzymes. Over-expressing HIOMT/AANAT increases melatonin accumulation in switchgrass under salinity stress and simultaneously provides tolerance under such conditions (Huang et al., 2017). Up-regulation of MdTDC1, MdASMT1, MdT5H4, and MdAANAT2 (genes involved in melatonin biosynthesis) regulates water balance in Malus hupehensis under drought conditions (Ahn et al., 2021). Melatonin endows drought resistance by improving photosynthetic efficiency and promotes accretion of proline and total soluble sugars in grafted Carya cathayensis. Also, metabolomic study uncovers that melatonin modulates metabolic pathways viz, carbon fixation, sugar metabolism, chlorophyll, carotenoids, and phenylpropanoid synthesis (Sharma et al., 2020). Forbye, selenium induces transcription of TDC, SNAT, ASMT, and T5H genes associated with melatonin biosynthesis in Solanum lycopersicum, leading to increased melatonin levels and assists in cadmium detoxification (Mathur and Pramanik 2020). External factors certainly affect the melatonin level in plant tissues. Plants raised indoor under controlled conditions possess less melatonin content comparatively to field grown plants under diverse condition. Plants cultivated in daylight have 2.5–3 times increased melatonin within roots and leaves, respectively, in comparison to plant grown under in vitro conditions (Yu et al., 2018). Similarly, in Solanum lycopersicum the monochromatic red and blue light change fruit quality and increase endogenous melatonin levels during fruit ripening (Li et al., 2021). The above segment divulges that melatonin partakes in stress tolerance.
Melatonin-Mediated Abiotic Stress Tolerance
The cloned gene of phytomelatonin synthesizing enzyme corroborate those plants possess indispensible enzymatic machinery for melatonin synthesis. Apart from biosynthesis, plants can imbibe exogenous melatonin to accumulate within tissues (Zhang et al., 2021). The impact of exogenous melatonin varies from being restorative to ineffective or even toxic. The concentration is an actual basis of this marginal distinction. Melatonin plays heterogeneous roles in modulating plant’s development and growth under varying concentrations within plant species (Table 2) (See Fig. 3).
Drought Stress
The repercussion of drought stress is extensive crop loss, thus posing jeopardy to sustainable agriculture. Numerous studies are carried out to examine the relation between melatonin and drought stress (Sharma et al., 2020). Limited watering triggers melatonin synthesis, and melatonin application mitigates drought-induced encumbrance on root elongation and seed germination (Dai et al., 2020). It has been proven that during drought stress, melatonin enhances antioxidant enzyme activity and increases content of antioxidants viz, GSH, AsA, and phenolic compounds, consequently decreases ROS accretion and palliates oxidative damage (Xia et al., 2020). Water deficiency curbs plant growth and development attributable to decreased photosynthetic rate. Besides, protecting chlorophyll, melatonin also boosts stomatal function in association with abscisic acid, causing closure of stomata which maximizes tolerance against drought-induced stress (Wang et al. 2021a, b). It is revealed that melatonin down-regulated abscisic acid synthesizing gene, namely 9-cis-epoxycarotenoid dioxygenase 3 gene MdNCED3, and up-regulated its catabolic genes MdCYP707A1 and MdCYP707A2, thus lowering abscisic acid contents and retains stomatal function during drought conditions (Ahn et al., 2021). The enhanced stomatal function acts synergistically with antioxidant network to skirmish drought stress.
Cold and Heat Stress
Ambient temperature is predominant aspect for determining momentum of plant growth. Both heat and cold stress alters redox homeostasis, leading ROS accretion and membrane impairment. Exposure to cold temperatures stimulates physiological and biochemical changes which provoke restricted growth and loss of vigor in plants. Primary site of cold-induced injuries are cell membranes. Cold stress causes alteration in cell structure and cell wall components. The defensive role of melatonin during cold stress has been studied in Capsicum annuum. The seeds treated with melatonin activated the antioxidant defense system and minimum disorganized cell ultra-structure was observed. In order to combat oxidative damage, melatonin up-regulated CaAPX, CaSOD, CaCAT, and CaPOD genes (Kong et al., 2020). In Solanum lycopersicum, melatonin regulates osmo-protective and nitro-oxidative homeostasis to reinforce tolerance against heat stress. Melatonin attenuates heat-induced stress in Solanum lycopersicum by maintaining redox status and regulating nitric oxide and polyamine synthesis (Jahan et al., 2019). Heat stress leads to four types of mutilation: oxidative stress sourced from ROS; loss of membrane integrity; dicarbonyl stress elicited by methylglyoxal that reacts abruptly with lipids, nucleic acids, and proteins; and cell dehydration due to water deficiency caused by osmotic stress (Pardo-Hernandez et al., 2020). Heat stress elevates endogenous melatonin levels, thus enhancing thermo-tolerance due to strong antioxidant capacity of phytomelatonin (Ahammed et al., 2019). Melatonin action against heat stress is principally regulated by heat shock proteins and factors. It is revealed that HSP90 and HSP101 (heat shock proteins) and HSFA2 and HSA32 (heat shock factors) modulate melatonin-induced thermo-tolerance in Solanum lycopersicum (Jahan et al., 2019).
Salt Stress
Salinity stress is another major menace for agriculture, causing huge economic loss globally. Crescent data indicate that melatonin enhances salt tolerance in many plant species including Zea mays, Solanum lycopersicum, Oryza sativa, Triticum, and cucumis sativus (Li et al., 2019a, b). Melatonin networking with nitric oxide regulates glutathione reductase activity and increases glutathione levels in cotyledons of Brassica napus during salt stress (Zhao et al., 2018). For plants under saline conditions, maintaining Na+ and K+ ion homeostasis turns out more difficult. Melatonin under salt-stressed conditions up-regulates AKT1 and NHX1 genes which are responsible for controlling ionic balance in plants (Ren et al., 2020). Exogenous melatonin rescued amassing of Cl− and Na+ in leaves and roots of Oryza sativa plants and also it increased the transcription of OsCLC1, OsCLC2, and OsSOS1 in leaves and roots (Moustafa-Farag et al., 2020). Exogenous melatonin also stimulates expression of salt-inhibited genes, consequently assuaging inhibitory impact of salinity on gene expression. Genes associated with carbohydrate metabolism, ascorbate metabolism, cell division, photosynthesis, and fatty acid biosynthesis are activated by melatonin treatment in Phaseolus vulgaris (Elsayed et al., 2021). Melatonin treatment helps maintain Na+ and K+ homeostasis by triggering fatty acid β-oxidation and triacylglycerol breakdown in order to control H+ ATPase activity of plasma membrane, thereby maintaining Na+ and K+ ion homeostasis in Ipomoea batatas (Yu et al., 2018). The signaling molecules were recently identified that acts downstream of melatonin in salt-sensitive Oryza sativa. These signaling molecules cause uptake of K+ in cell during saline-stressed conditions (Yan et al 2021).
Heavy Metal Stress
Mounting level of heavy metals has constantly affected our ecosystem, arousing toxicity and stress in flora. Melatonin is well used for safeguarding plants against heavy metal stress. Seed priming with melatonin protects Cucumis melo from toxic copper ions. Protective role of melatonin on lateral roots has been demonstrated in copper-stressed Cucumis melo. Melatonin pre-treatment enhances root vigor and antioxidant activities and also decline in malondialdehyde and proline content was observed. Melatonin propelled glutathione levels, which in turn chelates excess copper ions (Hu et al., 2020). Exogenous melatonin assuages chromium stress in Zea mays via improving diameter, surface area, tips, and volume of roots, respectively. Melatonin assuages chromium-induced inhibition of carotenoids and chlorophyll, enhances water retention, and confers better plant growth (Malik et al., 2021). Melatonin significantly diminishes cadmium toxicity, as it reduces amassing of nitric oxide in Chinese cabbage. Also melatonin augments photosynthetic parameters and biomass under cadmium stress (Wang et al., 2021a). Improved plant performance, eventuated after melatonin treatment, along with thrust expression of melatonin-synthesizing genes, imply that manipulating phytomelatonin activity can direct toward developing highly resistant crop varieties against heavy metal toxicity (Bose and Howlader 2020). A paragon is an evaluation by Sun et al. in 2020, where exogenous melatonin induces antioxidant enzyme activity, elevates antioxidant levels, and obstructs ROS generation in Zea mays subjected to aluminum stress. Melatonin helps plant to re-establish redox homeostasis during heavy metal stress, by nitric oxide signaling. Melatonin offers resistance to lead and cadmium-induced oxidative damage by mediating nitric oxide cell signaling (Kaya et al., 2019). Melatonin lowers cadmium amassing by stimulating heavy metal transporter genes, such as PCR2 and ABC transporter genes in Arabidopsis (He et al., 2020). In summation, all studies propose that melatonin plays a prominent role in plant acclimatization toward heavy metal stress, by serving dynamically.
Melatonin-Mediated Detoxification and Attenuation of Oxidative Stress
Melatonin production is stimulated in plants under abiotic stress. Stressors are known to create RNS and ROS, which induce oxidative damage in plants ‘crosstalk with phytohormones.’
Plants have built-in systems to resist oxidative damage in order to flourish in harsh environments. These mechanisms include an antioxidative defense system and increased antioxidant synthesis. Melatonin is considered an excellent antioxidant because it effectively scavenges a wide range of RNS and ROS (Debnath et al., 2019) (Fig. 4). Melatonin maintains ROS levels in peculiar ways: (a) chemical interaction between melatonin and ROS, causing their inactivation (Arnao and Hernandez-Ruiz, 2019) and (b) melatonin-induced activation of SOD, POD, APX, CAT, and GPX leads to ROS detoxification (Khan et al., 2020). During stress conditions plants naturally enhance their melatonin level to protect from damage. Since melatonin is a ROS scavenger and assuages oxidative damage induced by stressors in plants, concurrently, melatonin improves antioxidant capacity of organelles and regulates expression of stress-responsive genes viz, C-repeat-binding factors (CBFs) to increase plant resilience during stressed conditions (Huang et al., 2020). Mechanistic studies divulge that aforementioned response occurs at gene expression level. Melatonin controls H2O2 burst in plants, probably by quenching superfluous ROS and improves antioxidative enzyme activity and capacity of AsA-GSH cycle (Liang et al., 2018). Through cascade reaction, one molecule of melatonin scavenges ten ROS species, which is in contrast with typical antioxidants, as they normally detoxify one free radical per molecule (Balaji and Varadarajan, 2021). Ergo, it is inferred that rudimentary role of melatonin as potent antioxidant scavenge free radical species, including ROS and RNS (Back 2021). Exogenous melatonin reduces accretion of oxidized proteins, remits photo-oxidation damage, and increases oxidative stress-induced autophagy in Arabidopsis (Zhao et al., 2019). Seed treatment with melatonin assuages lipid peroxidation and electrolyte leakage caused by malondialdehyde content during plant stress response. Abiotic stresses viz, heat, cold, drought and salinity elevate the levels of ROS and MDA, which directly affects the cell membrane, causing increased electrolyte leakage in plants (Xie et al., 2020). Lately, melatonin-mediated reduction of oxidative stress was evaluated in context to phyto-toxic agents. In cassava, melatonin up-regulated ascorbate peroxidase activity and enhanced the antioxidant capacity. Howbeit, it was revealed that two enzymes (MeTDC2 and MeASMT2) involved in melatonin biosynthesis pathway show direct interaction with ascorbate peroxidase (MeAPX2). In lieu, melatonin facilitated stress tolerance and regulated redox homeostasis in cassava, which is elucidated as a survival stratagem to eradicate oxidative stress-damaged cell organelles (Bai et al., 2020). Credible evidence showed that melatonin interacts with wide array of signaling molecules including nitric oxide (NO) which generates S-nitrosylation and NO2- which is regarded as prominent antioxidant-related proteins, thereby maintaining the antioxidant capacity of Asa/GSH cycle under nitro-oxidative conditions. This is a vital action to balance antioxidant potential of AsA/GSH cycle during nitro-oxidative conditions (Pardo-Hernandez et al., 2020). Also, a dynamic synchronization was discerned between melatonin and ROS, where it was seen that ROS has an ability to up-regulate melatonin biosynthesis pathway gene which leads to upsurge of endogenous melatonin content (Zhan et al., 2019). Hydrogen peroxide detoxification in plants is mediated by melatonin using glutathione which depends upon time, concentration, and plant under contemplation.
Crosstalk with Other Phytohormones
The process of plant development and growth starts at germination and pursues till senescence. Roots are requisite for carbohydrate storage, nutrient and water uptake, mechanical support, and anchoring to plants (Shen et al., 2021). Thus, root architecture holds eloquent role for plant development and growth. Auxin regulates root architecture and its significance in shaping root has been assessed. Structurally, auxin and melatonin show homology, such as hydrophobic transition region, planar aromatic rings, and carboxylic acid-binding site (Arnao & Hernández-Ruiz 2018). Besides, they share common precursor, namely tryptophan for their synthesis. On the basis of various corroborations, melatonin and auxin probably co-participate in multifarious processes related to plant development and growth, since structural similarity may hold similar roles. Also, both have powerful antioxidant potential too (Sharif et al., 2018). Melatonin works as a growth enhancer in etiolated lupin (Lupinus albus), behaving alike as auxin (IAA), and stimulates growth of hypocotyls at very less concentrations while imparting inhibitory effect at higher concentrations. Growth-promoting effect induced by melatonin is 63% comparative to auxin, regarded as auxinic effect (Hernandez-Ruiz and Arnao, 2008). Under the apprehension of various studies, the auxin-like activity of melatonin emerges more enigmatic than before. Melatonin down-regulates 1AA17, auxin-resistant 3, AXR3, acting as transcriptional suppressor of many auxin-inducible genes. Thus melatonin may obliquely cause up-regulation of auxin-dependent genes (Jahan et al., 2021). The findings stipulate that phytomelatonin is involved in stress responses at transcriptional as well as post-transcriptional levels. It was discerned that upsurge in intrinsic melatonin levels considerably stimulates root growth in Hypericum perforatum in vitro conditions, while accretion of serotonin, a melatonin precursor directs shoot formation. Likewise, melatonin actively participates in shaping root architecture indicating auxin-like roles (Arnao & Hernández-Ruiz 2018). Contemporary studies unfold that auxin and melatonin share dose-dependent relation to accelerate their activities, simultaneously modulating root development in plants. Moreover, auxin synthesizing gene transcripts viz, YUC1, YUC2, YUC5, YUC6, and TAR2 are depleted upon melatonin treatment at a dose of 600 µM and further decreased auxin levels in melatonin-treated roots (Ren et al., 2019).
Cytokinins participate in cell cycle modulation and promote numerous developmental mechanisms in plants (Li et al., 2020a, b). Intrinsic cytokinin levels impede cytokinin signaling pathways in order to expedite senescence under diverse stress conditions. ARR genes in cytokinin signaling pathway is a downstream transcriptional controller. These genes modulate the communication among cytokinin and abscisic acid (Huo et al., 2020). It is contemplated that exogenous melatonin up-regulates transcripts of cytokinin synthesizing genes along with its transcriptional factors. Furthermore, melatonin and cytokinin treatment collectively alleviates dark-induced chlorophyll reduction in Brassica rapa leaves (Tan et al., 2019). Leaf senescence intensifies during heat stress, thereby thwarting photosynthesis and chlorophyll synthesis in leaves (Rossi et al., 2020), since cytokinin is involved in stress induced or inherent senescence. So, in order to pioneer cytokinin and melatonin interaction, specifically under heat stress an experiment was conducted on perennial grass during winter season. It was revealed that melatonin lowered leaf senescence via integrating with cytokinin and abscisic acid and also controlling their metabolic pathways (Zhang et al., 2017). Apparently, an experiment was carried out to scrutinize melatonin and abscisic acid interaction in stressed plants. Abscisic acid is altered by melatonin adjunct also exogenous melatonin begets up-regulation of abscisic acid catabolism genes that is two CYP707 monooxygenase and down-regulation of 9-cis-epoxycarotenoid dioxygenase. Melatonin-amended plants manifested higher production of abscisic acid, thereby alleviating oxidative damage, reducing ROS and malondialdehyde level, and up-regulating antioxidants (ascorbate and glutathione) and enzymes activity (SOD, CAT, GR, and APX) (Arnao and Hernandez-Ruiz, 2021).
Salicylic acid and melatonin synthesis in plants have a common terminal precursor, namely chorismic acid, produced by shikimic acid – a condensation product of erythrose 4-phosphate from pentose phosphate pathway and phosphoenolpyruvic acid from glycolysis. Increased melatonin level secondarily transcribes iso-chorismate Synthase-1 gene, which mediates salicylic acid biosynthesis (Hernandez-Ruiz and Arnao, 2018). Neoteric work uncovered crosstalk between melatonin and salicylic acid. An evaluation ascertains the impact of melatonin and salicylic acid on antioxidants in M. arvensis var piperascens and M. × piperita under stress condition. The combinatorial application of salicylic acid and melatonin increased antioxidant activity and ROS quenching in stressed plants (Haydari et al., 2019). Analogous results were retrieved from a study where cadmium-exposed safflower plants showed noticeable decline in activity of lipoxygenase (LOX) when supplemented with melatonin and salicylic acid. Along with this H2O2 and MDA (malondialdehyde) contents were reduced which indicate an important role of these phytohormones in skirmishing oxidative stress. Also, melatonin and salicylic acid increase the production of ascorbate and glutathione to alleviate the metal-induced toxicity by regulating cellular redox potential. It is accorded well that glutathione and ascorbate activate the enzymes which are present in ascorbate–glutathione cycle and succor in H2O2 degradation. Further melatonin and salicylic acid elevated ascorbate levels and ascorbate/dehydroascorbate ratio in metal-stressed safflower plants and promote biosynthesis of non-enzymatic antioxidants to eliminate ROS produced by metals (Amjadi et al., 2021).
Gibberellic acid plays pivotal role in plants particularly during seed germination (Tuan et al., 2018). Since, melatonin augments plant growth under various physiological processes and attempts have been made to assess its probable crosstalk with gibberellic acid, an uninterrupted link was discerned between melatonin and gibberellic acid in regard to mitigate oxidative stress induced by NaCl in Solanum lycopersicum. Researchers proclaim that both phytohormones were effective in lowering ROS and malondialdehyde. Melatonin behaves as an antioxidant and plunges ROS generation, whereas gibberellic acid boots the activity of antioxidant system. Besides, melatonin + gibberellic acid treatment up-regulated activity of Gly I and Gly II involved in methylglyoxal detoxification system in order to detoxify methylglyoxal (a potent cytotoxin) under NaCl stress. It is observed that methylglyoxal overproduction leads to glutathione oxidation and formation of oxidized glutathione (Siddiqui et al., 2020) Overall, phytohormone synthesis, catabolism, and crosstalk between melatonin and other phytohormones are propitious for comprehending melatonin-induced mechanisms to counteract abiotic stress-mediated oxidative damage.
Conclusion and Future Aspect
Melatonin is a nascent molecule possessing pleiotropic effects with multifarious functions in plants. It is a growth stimulator and stress alleviator in plants. The cardinal role of phytomelatonin is to provide first line of defense against oxidative stress that befalls during unfavorable conditions. Within plant cells, melatonin, ROS, and NO form an intriguing triad which maintains redox homeostasis, perhaps melatonin acting as a vital component in redox network. Ergo, impact of melatonin in redox network can be exploited as bio-stimulator to produce healthy cultivars exhibiting enhanced photosynthesis and anti-senescence genes. An interesting and contentious discovery reveals that phytomelatonin works as indispensable regulatory element for many other phytohormones. Contemporary evidences proposes that melatonin stimulates expression of numerous compounds such as receptors, enzymes and genes associated with other hormones, namely auxin, abscisic acid, salicylic acid, gibberellic acid, and ethylene and also lately identified other signaling molecules strigolactones, nitric oxide, and brassinosteroids. Melatonin interacts with other phytohormones to regulate gene expression, stabilize proteins, and induce miRNA-mediated epigenetic modifications. Furthermore, stress-response pathways are activated, including antioxidant defense systems, ROS scavenging, and osmoprotectants, as well as the recovery of leaf ultra-structure, which helps to prevent membrane oxidation and delays senescence. Endogenous melatonin, on the other hand, is incapable of shielding plants from acute harm. In light of this, exogenous melatonin has proven to be an excellent coping mechanism in extreme situations. In conclusion, further research is needed to fully understand the effects of melatonin across the plant kingdom, as well as creative approaches to improve crop production and the agricultural economy.
References
Ahammed GJ, Xu W, Liu A, Chen S (2019) Endogenous melatonin deficiency aggravates high temperature-induced oxidative stress in Solanum lycopersicum L. Environ Exp Bot 161:303–311
Ahmad S, Kamran M, Ding R, Meng X, Wang H, Ahmad I, Han Q (2019) Exogenous melatonin confers drought stress by promoting plant growth, photosynthetic capacity and antioxidant defense system of maize seedlings. PeerJ 7:e7793
Ahmad S, Cui W, Kamran M, Ahmad I, Meng X, Wu X, Su W, Javed T, El-Serehy HA, Jia Z, Han Q (2020) Exogenous application of melatonin induces tolerance to salt stress by improving the photosynthetic efficiency and antioxidant defense system of maize seedling. J Plant Growth Regul 40(3):1270–1283
Ahn HR, Kim YJ, Lim YJ, Duan S, Eom SH, Jung KH (2021) Key genes in the melatonin biosynthesis pathway with circadian rhythm are associated with various abiotic stresses. Plants 10(1):129
Alam MN, Zhang L, Yang L, Islam MR, Liu Y, Luo H, Chan Z (2018) Transcriptomic profiling of tall fescue in response to heat stress and improved thermotolerance by melatonin and 24-epibrassinolide. BMC Genomics 19(1):1–14
Ali Shah A, Ahmed S, Yasin NA (2020) Cadmium stress consolation in melatonin supplemented Cucumis sativus through modulation of antioxidative defense system. Iranian J Plant Physiol 10(2):3135–3154
Altaf MA, Shahid R, Ren MX, Altaf MM, Khan LU, Shahid S, Jahan MS (2021) Melatonin alleviates salt damage in tomato seedling: A root architecture system, photosynthetic capacity, ion homeostasis, and antioxidant enzymes analysis. Sci Hortic 285:110145
Amjadi Z, Namdjoyan S, Soorki AA (2021) Exogenous melatonin and salicylic acid alleviates cadmium toxicity in safflower (Carthamus tinctorius L.) seedlings. Ecotoxicology 30(3):387–401
Arnao MB, Hernández-Ruiz J (2018) Melatonin and its relationship to plant hormones. Ann Bot 121(2):195–207
Arnao MB, Hernández-Ruiz J (2019) Melatonin: a new plant hormone and/or a plant master regulator? Trends Plant Sci 24(1):38–48
Arnao MB, Hernández-Ruiz J (2020) Melatonin in flowering, fruit set and fruit ripening. Plant Reproduction 33(2):77–87
Arnao MB, Hernández-Ruiz J (2021) Melatonin as a regulatory hub of plant hormone levels and action in stress situations. Plant Biol 23:7–19
Back K (2021) Melatonin metabolism, signaling and possible roles in plants. Plant J 105(2):376–391
Bai Y, Guo J, Reiter RJ, Wei Y, Shi H (2020) Melatonin synthesis enzymes interact with ascorbate peroxidase to protect against oxidative stress in cassava. J Exp Bot 71(18):5645–5655
Bajwa VS, Shukla MR, Sherif SM, Murch SJ, Saxena PK (2014) Role of melatonin in alleviating cold stress in Arabidopsis thaliana. J Pineal Res 56(3):238–245
Balaji TMM, Varadarajan S, Bandyopadhyay D, Jagannathan R, Patil S, Raj T (2021) A potential protection of melatonin on pathogenesis of oral sub-mucous fibrosis (OSMF): a current update. Melatonin Res 4(1):84–98
Bawa G, Feng L, Shi J, Chen G, Cheng Y, Luo J, Wu W, Ngoke B, Cheng P, Tang Z, Pu T (2020) Evidence that melatonin promotes soybean seedlings growth from low-temperature stress by mediating plant mineral elements and genes involved in the antioxidant pathway. Funct Plant Biol 47(9):815–824
Bose SK, Howlader P (2020) Melatonin plays multifunctional role in horticultural crops against environmental stresses: A review. Environ Exp Bot 176:104063
Cao L, Jin XJ, Zhang YX (2019) Melatonin confers drought stress tolerance in soybean (Glycine max L.) by modulating photosynthesis, osmolytes, and reactive oxygen metabolism. Photosynthetica 57(3):812–819
Cen H, Wang T, Liu H, Tian D, Zhang Y (2020) Melatonin application improves salt tolerance of alfalfa (Medicago sativa L.) by enhancing antioxidant capacity. Plants 9(2):220
Chen L, Liu L, Lu B, Ma T, Jiang D, Li J, Zhang K, Sun H, Zhang Y, Bai Z, Li C (2020) Exogenous melatonin promotes seed germination and osmotic regulation under salt stress in cotton (Gossypium hirsutum L.). PLoS ONE 15(1):e0228241
Choi GH, Back K (2019) Suppression of melatonin 2-hydroxylase increases melatonin production leading to the enhanced abiotic stress tolerance against cadmium, senescence, salt, and tunicamycin in rice plants. Biomolecules 9(10):589
Dai L, Li J, Harmens H, Zheng X, Zhang C (2020) Melatonin enhances drought resistance by regulating leaf stomatal behaviour, root growth and catalase activity in two contrasting rapeseed (Brassica napus L.) genotypes. Plant Physiol Biochem 149:86–95
Danilova ED, Efimova MV, Kolomeichuk LV, Kuznetsov VV (2020) Melatonin supports photochemical activity of assimilation apparatus and delays senescence of leaves of monocotyledonous plants. Doklady Biochem Biophys 495(1):271–275
Dawood MG, El-Awadi ME (2015) Alleviation of salinity stress on Vicia faba L. plants via seed priming with melatonin. Acta Biológica Colombiana. 20(2):223–235
Debnath B, Islam W, Li M, Sun Y, Lu X, Mitra S, Hussain M, Liu S, Qiu D (2019) Melatonin mediates enhancement of stress tolerance in plants. Int J Mol Sci 20(5):1040
Debnath B, Sikdar A, Islam S, Hasan K, Li M, Qiu D (2021) Physiological and molecular responses to acid rain stress in plants and the impact of melatonin, glutathione and silicon in the amendment of plant acid rain stress. Molecules 26(4):862
ElSayed AI, Rafudeen MS, Gomaa AM, Hasanuzzaman M (2021) Exogenous melatonin enhances the reactive oxygen species metabolism, antioxidant defense-related gene expression, and photosynthetic capacity of Phaseolus vulgaris L. to confer salt stress tolerance. Physiol Plant 173(4):1369–1381
Fu J, Wu Y, Miao Y, Xu Y, Zhao E, Wang J, Hu T (2017) Improved cold tolerance in Elymus nutans by exogenous application of melatonin may involve ABA-dependent and ABA-independent pathways. Sci Rep 7(1):1–11
Galano A, Reiter RJ (2018) Melatonin and its metabolites vs oxidative stress: From individual actions to collective protection. J Pineal Res 65(1):e12514
Gao JP, Chao DY, Lin HX (2007) Understanding abiotic stress tolerance mechanisms: recent studies on stress response in rice. Journal of Integrative Plant Biology 49(6):742–750
Gao W, Zhang Y, Feng Z, Bai Q, He J, Wang Y (2018) Effects of melatonin on antioxidant capacity in naked oat seedlings under drought stress. Molecules 23(7):1580
Gökbayrak Z, Engin H, Kiraz H (2020) Effects of Melatonin and IAA on Adventitious Root Formation in Rootstock 5BB and cv. Cabernet Sauvignon (Vitis vinifera L.). Kahramanmaraş Sütçü İmam Üniversitesi Tarım Ve Doğa Dergisi 23(4):835–841
Golovatskaya IF, Boyko EV, Reznichenko AE, Plyusnin IN (2020) Melatonin and Selenium Regulate Growth and Oxidative Status of Saussurea orgaadayi In Vitro Cell Cultures Derived from Different Explants. Russ J Plant Physiol 67(6):1036–1045
Gu Q, Chen Z, Yu X, Cui W, Pan J, Zhao G, Shen W (2017) Melatonin confers plant tolerance against cadmium stress via the decrease of cadmium accumulation and reestablishment of microRNA-mediated redox homeostasis. Plant Sci 261:28–37
Hardeland R (2019) Melatonin in the evolution of plants and other phototrophs. Melatonin Res 2(3):10–36
Hardeland R, Cardinali DP, Srinivasan V, Spence DW, Brown GM, Pandi-Perumal SR (2011) Melatonin—A pleiotropic, orchestrating regulator molecule. Progress Neurobiol 93(3):350–384
Hasan M, Ahammed GJ, Yin L, Shi K, Xia X, Zhou Y, Zhou J (2015) Melatonin mitigates cadmium phytotoxicity through modulation of phytochelatins biosynthesis, vacuolar sequestration, and antioxidant potential in Solanum lycopersicum L. Front Plant Sci 6:601
Haydari M, Maresca V, Rigano D, Taleei A, Shahnejat-Bushehri AA, Hadian J, Basile A (2019) Salicylic acid and melatonin alleviate the effects of heat stress on essential oil composition and antioxidant enzyme activity in Mentha× piperita and Mentha arvensis L. Antioxidants 8(11):547
He J, Zhuang X, Zhou J, Sun L, Wan H, Li H, Lyu D (2020) Exogenous melatonin alleviates cadmium uptake and toxicity in apple rootstocks. Tree Physiol 40(6):746–761
Hernández-Ruiz J, Arnao MB (2008) Melatonin stimulates the expansion of etiolated lupin cotyledons. Plant Growth Regul 55(1):29–34
Hernández-Ruiz J, Arnao MB (2018) Relationship of melatonin and salicylic acid in biotic/abiotic plant stress responses. Agronomy 8(4):33
Hu Z, Fu Q, Zheng J, Zhang A, Wang H (2020) Transcriptomic and metabolomic analyses reveal that melatonin promotes melon root development under copper stress by inhibiting jasmonic acid biosynthesis. Horticulture Research 7(1):1–15
Huang YH, Liu SJ, Yuan S, Guan C, Tian DY, Cui X, Zhang YW, Yang FY (2017) Overexpression of ovine AANAT and HIOMT genes in switchgrass leads to improved growth performance and salt-tolerance. Sci Rep 7(1):1–13
Huang B, Chen YE, Zhao YQ, Ding CB, Liao JQ, Hu C, Yuan M (2019) Exogenous melatonin alleviates oxidative damages and protects photosystem II in maize seedlings under drought stress. Front Plant Sci 10:677
Huang Z, Xie W, Wang M, Liu X, Ashraf U, Qin D, Zhuang M, Li W, Li Y, Wang S, Tian H (2020) Response of rice genotypes with differential nitrate reductase-dependent NO synthesis to melatonin under ZnO nanoparticles’(NPs) stress. Chemosphere 250:126337
Huo R, Liu Z, Yu X, Li Z (2020) The interaction network and signaling specificity of two-component system in Arabidopsis. Int J Mol Sci 21(14):4898
Imran M, Latif Khan A, Shahzad R, Aaqil Khan M, Bilal S, Khan A, Lee IJ (2021) Exogenous melatonin induces drought stress tolerance by promoting plant growth and antioxidant defence system of soybean plants. AoB Plants. https://doi.org/10.1093/aobpla/plab026
Jafari M, Shahsavar A (2020) The application of artificial neural networks in modeling and predicting the effects of melatonin on morphological responses of citrus to drought stress. PLoS ONE 15(10):e0240427
Jahan MS, Shu S, Wang Y, Chen Z, He M, Tao M, Guo S (2019) Melatonin alleviates heat-induced damage of tomato seedlings by balancing redox homeostasis and modulating polyamine and nitric oxide biosynthesis. BMC Plant Biol 19(1):1–16
Jahan MS, Shu S, Wang Y, Hasan M, El-Yazied AA, Alabdallah NM, Hajjar D, Altaf MA, Sun J, Guo S (2021) Melatonin pretreatment confers heat tolerance and repression of heat-induced senescence in tomato through the modulation of ABA-and GA-mediated pathways. Front Plant Sci 12:381
Jan S, Singh R, Bhardwaj R, Ahmad P, Kapoor D (2020) Plant growth regulators: a sustainable approach to combat pesticide toxicity. 3 Biotech. https://doi.org/10.1007/s13205-020-02454-4
Kaya C, Okant M, Ugurlar F, Alyemeni MN, Ashraf M, Ahmad P (2019) Melatonin-mediated nitric oxide improves tolerance to cadmium toxicity by reducing oxidative stress in wheat plants. Chemosphere 225:627–638
Khan A, Numan M, Khan AL, Lee IJ, Imran M, Asaf S, Al-Harrasi A (2020) Melatonin: Awakening the defense mechanisms during plant oxidative stress. Plants 9(4):407
Kobylińska A, Posmyk MM (2016) Melatonin restricts Pb-induced PCD by enhancing BI-1 expression in tobacco suspension cells. Biometals 29(6):1059–1074
Kong XM, Ge WY, Wei BD, Zhou Q, Zhou X, Zhao YB, Ji SJ (2020) Melatonin ameliorates chilling injury in green bell peppers during storage by regulating membrane lipid metabolism and antioxidant capacity. Postharvest Biol Technol 170:111315
Kumar A, Patel JS, Meena VS, Ramteke PW (2019) Plant growth-promoting rhizobacteria: strategies to improve abiotic stresses under sustainable agriculture. J Plant Nutr 42(11–12):1402–1415
Lee HY, Back K (2020) The phytomelatonin receptor (PMRT1) Arabidopsis Cand2 is not a bona fide G protein–coupled melatonin receptor. Melatonin Res 3(2):177–186
Lerner AB, Case JD, Takahashi Y, Lee TH, Mori W (1958) Isolation of melatonin, the pineal gland factor that lightens melanocyteS1. J Am Chem Soc 80(10):2587–2587
Li C, Tan DX, Liang D, Chang C, Jia D, Ma F (2015) Melatonin mediates the regulation of ABA metabolism, free-radical scavenging, and stomatal behaviour in two Malus species under drought stress. J Exp Bot 66(3):669–680
Li C, Liang B, Chang C, Wei Z, Zhou S, Ma F (2016a) Exogenous melatonin improved potassium content in Malus under different stress conditions. J Pineal Res 61(2):218–229
Li H, Dong Y, Chang J, He J, Chen H, Liu Q, Zhang X (2016b) High-throughput microRNA and mRNA sequencing reveals that microRNAs may be involved in melatonin-mediated cold tolerance in Citrullus lanatus L. Front Plant Sci 7:1231
Li X, Wei JP, Scott ER, Liu JW, Guo S, Li Y, Han WY (2018) Exogenous melatonin alleviates cold stress by promoting antioxidant defense and redox homeostasis in Camellia sinensis L. Molecules 23(1):165
Li J, Liu J, Zhu T, Zhao C, Li L, Chen M (2019a) The role of melatonin in salt stress responses. Int J Mol Sci 20(7):1735
Li ZG, Xu Y, Bai LK, Zhang SY, Wang Y (2019b) Melatonin enhances thermotolerance of maize seedlings (Zea mays L.) by modulating antioxidant defense, methylglyoxal detoxification, and osmoregulation systems. Protoplasma 256(2):471–490
Li D, Wei J, Peng Z, Ma W, Yang Q, Song Z, Sun W, Yang W, Yuan L, Xu X, Chang W (2020a) Daily rhythms of phytomelatonin signaling modulate diurnal stomatal closure via regulating reactive oxygen species dynamics in Arabidopsis. J Pineal Res 68(3):e12640
Li SM, Zheng HX, Zhang XS, Sui N (2020b) Cytokinins as central regulators during plant growth and stress response. Plant Cell Rep 40(2):271–282
Li Y, Liu C, Shi Q, Yang F, Wei M (2021) Mixed red and blue light promotes ripening and improves quality of tomato fruit by influencing melatonin content. Environ Exp Bot 185:104407
Liang D, Shen Y, Ni Z, Wang Q, Lei Z, Xu N, Deng Q, Lin L, Wang J, Lv X, Xia H (2018) Exogenous melatonin application delays senescence of kiwifruit leaves by regulating the antioxidant capacity and biosynthesis of flavonoids. Front Plant Sci 9:426
Liu G, Zhang Y, Yun Z, Hu M, Liu J, Jiang Y, Zhang Z (2020) Melatonin enhances cold tolerance by regulating energy and proline metabolism in litchi fruit. Foods 9(4):454
Malik Z, Afzal S, Dawood M, Abbasi GH, Khan MI, Kamran M, Zhran M, Hayat MT, Aslam MN, Rafay M (2021) Exogenous melatonin mitigates chromium toxicity in maize seedlings by modulating antioxidant system and suppresses chromium uptake and oxidative stress. Environ Geochem Health 44(5):1451–1469
Mao J, Niu C, Li K, Chen S, Tahir MM, Han M, Zhang D (2020) Melatonin promotes adventitious root formation in apple by promoting the function of MdWOX11. BMC Plant Biol 20(1):1–11
Marta B, Szafrańska K, Posmyk MM (2016) Exogenous melatonin improves antioxidant defense in cucumber seeds (Cucumis sativus L.) germinated under chilling stress. Fron Plant Sci 7:575
Marthandan V, Geetha R, Kumutha K, Renganathan VG, Karthikeyan A, Ramalingam J (2020) Seed priming: a feasible strategy to enhance drought tolerance in crop plants. Int J Mol Sci 21(21):8258
Mathur P, Pramanik S (2020) Prospective Role of Melatonin in Signaling and Alleviation of Stress in Plants. In: Baluška F, Mukherjee S, Ramakrishna A (eds) Neurotransmitters in Plant Signaling and Communication. Springer, Cham, pp 213–240
Meng JF, Xu TF, Wang ZZ, Fang YL, Xi ZM, Zhang ZW (2014) The ameliorative effects of exogenous melatonin on grape cuttings under water-deficient stress: antioxidant metabolites, leaf anatomy, and chloroplast morphology. J Pineal Res 57(2):200–212
Meng S, Wang X, Bian Z, Li Z, Yang F, Wang S, Yoder JI, Lu J (2021) Melatonin enhances nitrogen metabolism and haustorium development in hemiparasite Santalum album Linn. Environ Exp Bot 186:104460
Mir AR, Siddiqui H, Alam P, Hayat S (2020) Melatonin modulates photosynthesis, redox status, and elemental composition to promote growth of Brassica juncea—a dose-dependent effect. Protoplasma 257(6):1685–1700
Moustafa-Farag M, Elkelish A, Dafea M, Khan M, Arnao MB, Abdelhamid MT, Ai S (2020) Role of melatonin in plant tolerance to soil stressors: Salinity, pH and heavy metals. Molecules 25(22):5359
Mukherjee S, David A, Yadav S, Baluška F, Bhatla SC (2014) Salt stress-induced seedling growth inhibition coincides with differential distribution of serotonin and melatonin in sunflower seedling roots and cotyledons. Physiol Plant 152(4):714–728
Nabaei M, Amooaghaie R (2019) Interactive effect of melatonin and sodium nitroprusside on seed germination and seedling growth of Catharanthus roseus under cadmium stress. Russ J Plant Physiol 66(1):128–139
Nawaz MA, Jiao Y, Chen C, Shireen F, Zheng Z, Imtiaz M, Huang Y (2018) Melatonin pretreatment improves vanadium stress tolerance of watermelon seedlings by reducing vanadium concentration in the leaves and regulating melatonin biosynthesis and antioxidant-related gene expression. J Plant Physiol 220:115–127
Pardo-Hernández M, López-Delacalle M, Rivero RM (2020) ROS and NO regulation by melatonin under abiotic stress in plants. Antioxidants 9(11):1078
Posmyk MM, Kuran H, Marciniak K, Janas KM (2008) Presowing seed treatment with melatonin protects red cabbage seedlings against toxic copper ion concentrations. J Pineal Res 45(1):24–31
Qi C, Zhang H, Liu Y, Wang X, Dong D, Yuan X, Li X, Zhang X, Li X, Zhang N, Guo YD (2020) CsSNAT positively regulates salt tolerance and growth of cucumber by promoting melatonin biosynthesis. Environ Exp Bot 175:104036
Rajora N, Vats S, Raturi G, Thakral V, Kaur S, Rachappanavar V, Deshmukh R (2022) Seed priming with melatonin: a promising approach to combat abiotic stress in plants. Plant Stress 4:100071
Reiter RJ, Tan DX, Rosales-Corral S, Galano A, Zhou XJ, Xu B (2018) Mitochondria: central organelles for melatonin′ s antioxidant and anti-aging actions. Molecules 23(2):509
Ren S, Rutto L, Katuuramu D (2019) Melatonin acts synergistically with auxin to promote lateral root development through fine tuning auxin transport in Arabidopsis thaliana. PLoS ONE 14(8):e0221687
Ren J, Ye J, Yin L, Li G, Deng X, Wang S (2020) Exogenous melatonin improves salt tolerance by mitigating osmotic, ion, and oxidative stresses in maize seedlings. Agronomy 10(5):663
Riaz HR, Hashmi SS, Khan T, Hano C, Giglioli-Guivarc’h N, Abbasi BH (2018) Melatonin-stimulated biosynthesis of anti-microbial ZnONPs by enhancing bio-reductive prospective in callus cultures of Catharanthus roseus var. Alba. Artif Cells, Nanomed Biotechnol 46(sup2):936–950
Rossi S, Chapman C, Huang B (2020) Suppression of heat-induced leaf senescence by γ-aminobutyric acid, proline, and ammonium nitrate through regulation of chlorophyll degradation in creeping bentgrass. Environ Exp Bot 177:104116
Sabkia, M. H., Ongb, P. Y., Ibrahimc, N., Leea, C. T., Klemešd, J. J., Lie, C., & Gaoe, Y. (2021). A review on abiotic stress tolerance and plant growth metabolite framework by plant growth-promoting bacteria for sustainable agriculture. CHEMICAL ENGINEERING, 83.
Sajjad, N., Bhat, E.A., Hassan, S., Ali, R. and Shah, D., 2020. Role of Melatonin in Amelioration of Abiotic Stress‐induced Damages. Protective Chemical Agents in the Amelioration of Plant Abiotic Stress: Biochemical and Molecular Perspectives, pp.306–317.
Sharif R, Xie C, Zhang H, Arnao MB, Ali M, Ali Q, Muhammad I, Shalmani A, Nawaz MA, Chen P, Li Y (2018) Melatonin and its effects on plant systems. Molecules 23(9):2352
Sharma A, Wang J, Xu D, Tao S, Chong S, Yan D, Li Z, Yuan H, Zheng B (2020) Melatonin regulates the functional components of photosynthesis, antioxidant system, gene expression, and metabolic pathways to induce drought resistance in grafted Carya cathayensis plants. Sci Total Environ 713:136675
Shen, J., Wang, L., Wang, X., Jin, K. and Xiong, C., 2021. Interplay Between Root Structure and Function in Enhancing Efficiency of Nitrogen and Phosphorus Acquisition. The Root Systems in Sustainable Agricultural Intensification, pp.121–157.
Shi H, Tan DX, Reiter RJ, Ye T, Yang F, Chan Z (2015) Melatonin induces class A1 heat-shock factors (HSFA 1s) and their possible involvement of thermotolerance in Arabidopsis. J Pineal Res 58(3):335–342
Shi X, Xu S, Mu D, Sadeghnezhad E, Li Q, Ma Z, Wang L (2019) Exogenous melatonin delays dark-induced grape leaf senescence by regulation of antioxidant system and senescence associated genes (SAGs). Plants 8(10):366
Siddiqui MH, Alamri S, Alsubaie QD, Ali HM (2020) Melatonin and gibberellic acid promote growth and chlorophyll biosynthesis by regulating antioxidant and methylglyoxal detoxification system in tomato seedlings under salinity. J Plant Growth Regul 39(4):1488–1502
Sun L, Song F, Guo J, Zhu X, Liu S, Liu F, Li X (2020) Nano-ZnO-induced drought tolerance is associated with melatonin synthesis and metabolism in maize. Int J Mol Sci 21(3):782
Sun C, Liu L, Wang L, Li B, Jin C, Lin X (2021) Melatonin: A master regulator of plant development and stress responses. J Integr Plant Biol 63(1):126–145
Tan DX, Reiter RJ (2020) An evolutionary view of melatonin synthesis and metabolism related to its biological functions in plants. J Exp Bot 71(16):4677–4689
Tan XL, Fan ZQ, Kuang JF, Lu WJ, Reiter RJ, Lakshmanan P, Su XG, Zhou J, Chen JY, Shan W (2019) Melatonin delays leaf senescence of Chinese flowering cabbage by suppressing ABFs-mediated abscisic acid biosynthesis and chlorophyll degradation. J Pineal Res 67(1):e12570
Tiryaki I, Keles H (2012) Reversal of the inhibitory effect of light and high temperature on germination of Phacelia tanacetifolia seeds by melatonin. J Pineal Res 52(3):332–339
Tuan PA, Kumar R, Rehal PK, Toora PK, Ayele BT (2018) Molecular mechanisms underlying abscisic acid/gibberellin balance in the control of seed dormancy and germination in cereals. Front Plant Sci 9:668
Uchendu EE, Shukla MR, Reed BM, Saxena PK (2013) Melatonin enhances the recovery of cryopreserved shoot tips of A merican elm (U lmus americana L.). J Pineal Res 55(4):435–442
Wang D, Chen Q, Chen W, Guo Q, Xia Y, Wang S, Jing D, Liang G (2021a) Physiological and transcription analyses reveal the regulatory mechanism of melatonin in inducing drought resistance in loquat (Eriobotrya japonica Lindl.) seedlings. Environ Exp Bot 181:104291
Wang T, Song J, Liu Z, Liu Z, Cui J (2021b) Melatonin alleviates cadmium toxicity by reducing nitric oxide accumulation and IRT1 expression in Chinese cabbage seedlings. Environ Sci Pollut Res 28(12):15394–15405
Wu M, Wu J, Gan Y (2020) The new insight of auxin functions: transition from seed dormancy to germination and floral opening in plants. Plant Growth Regul 91(2):169–174
Xia H, Ni Z, Hu R, Lin L, Deng H, Wang J, Tang Y, Sun G, Wang X, Li H, Liao M (2020) Melatonin alleviates drought stress by a non-enzymatic and enzymatic antioxidative system in kiwifruit seedlings. Int J Mol Sci 21(3):852
Xie Z, Wang J, Wang W, Wang Y, Xu J, Li Z, Zhao X, Fu B (2020) Integrated analysis of the transcriptome and metabolome revealed the molecular mechanisms underlying the enhanced salt tolerance of rice due to the application of exogenous melatonin. Front Plant Sci 11:618680
Xu T, Chen Y, Kang H (2019) Melatonin is a potential target for improving post-harvest preservation of fruits and vegetables. Front Plant Sci 10:1388
Yan H, Mao P (2021) Comparative time-course physiological responses and proteomic analysis of melatonin priming on promoting germination in aged oat (Avena sativa L.) seeds. Int J Mol Sci 22(2):811
Yan F, Wei H, Ding Y, Li W, Chen L, Ding C, Tang S, Jiang Y, Liu Z, Li G (2021) Melatonin enhances Na+/K+ homeostasis in rice seedlings under salt stress through increasing the root H+-pump activity and Na+/K+ transporters sensitivity to ROS/RNS. Environ Exp Bot 182:104328
Yang XL, Xu H, Li D, Gao X, Li TL, Wang R (2018) Effect of melatonin priming on photosynthetic capacity of tomato leaves under low-temperature stress. Photosynthetica 56(3):884–892
Yang M, Wang L, Belwal T, Zhang X, Lu H, Chen C, Li L (2020) Exogenous melatonin and abscisic acid expedite the flavonoids biosynthesis in grape berry of Vitis vinifera cv. Kyoho Molecules 25(1):12
Yang L, Sun Q, Wang Y, Chan Z (2021) Global transcriptomic network of melatonin regulated root growth in Arabidopsis. Gene 764:145082
Yao JW, Ma Z, Ma YQ, Zhu Y, Lei MQ, Hao CY, Chen LY, Xu ZQ, Huang X (2021) Role of melatonin in UV-B signaling pathway and UV-B stress resistance in Arabidopsis thaliana. Plant, Cell Environ 44(1):114–129
Yin Z, Lu J, Meng S, Liu Y, Mostafa I, Qi M, Li T (2019) Exogenous melatonin improves salt tolerance in tomato by regulating photosynthetic electron flux and the ascorbate–glutathione cycle. J Plant Interact 14(1):453–463
Yu Y, Wang A, Li X, Kou M, Wang W, Chen X, Xu T, Zhu M, Ma D, Li Z, Sun J (2018) Melatonin-stimulated triacylglycerol breakdown and energy turnover under salinity stress contributes to the maintenance of plasma membrane H+–ATPase activity and K+/Na+ homeostasis in sweet potato. Front Plant Sci 9:256
Zhan H, Nie X, Zhang T, Li S, Wang X, Du X, Song W (2019) Melatonin: A small molecule but important for salt stress tolerance in plants. Int J Mol Sci 20(3):709
Zhang N, Zhao B, Zhang HJ, Weeda S, Yang C, Yang ZC, Guo YD (2013) Melatonin promotes water-stress tolerance, lateral root formation, and seed germination in cucumber (Cucumis sativus L.). J Pineal Res 54(1):15–23
Zhang N, Sun Q, Li H, Li X, Cao Y, Zhang H, Guo YD (2016) Melatonin improved anthocyanin accumulation by regulating gene expressions and resulted in high reactive oxygen species scavenging capacity in cabbage. Front Plant Sci 7:197
Zhang YP, Xu S, Yang SJ, Chen YY (2017) Melatonin alleviates cold-induced oxidative damage by regulation of ascorbate–glutathione and proline metabolism in melon seedlings (Cucumis melo L). J Hortic Sci Biotechnol 92(3):313–324
Zhang M, He S, Zhan Y, Qin B, Jin X, Wang M, Wu Y (2019a) Exogenous melatonin reduces the inhibitory effect of osmotic stress on photosynthesis in soybean. PLoS ONE 14(12):e0226542
Zhang Z, Hu Q, Liu Y, Cheng P, Cheng H, Liu W, Xing X, Guan Z, Fang W, Chen S, Jiang J (2019b) Strigolactone represses the synthesis of melatonin, thereby inducing floral transition in Arabidopsis thaliana in an FLC-dependent manner. J Pineal Res 67(2):e12582
Zhang T, Shi Z, Zhang X, Zheng S, Wang J, Mo J (2020) Alleviating effects of exogenous melatonin on salt stress in cucumber. Sci Hortic 262:109070
Zhang H, Liu L, Wang Z, Feng G, Gao Q, Li X (2021) Induction of low temperature tolerance in wheat by pre-soaking and parental treatment with melatonin. Molecules 26(4):1192
Zhao Y, Qi LW, Wang WM, Saxena PK, Liu CZ (2011) Melatonin improves the survival of cryopreserved callus of Rhodiola crenulata. J Pineal Res 50(1):83–88
Zhao G, Zhao Y, Yu X, Kiprotich F, Han H, Guan R, Shen W (2018) Nitric oxide is required for melatonin-enhanced tolerance against salinity stress in rapeseed (Brassica napus L.) seedlings. Int J Mol Sci 19(7):1912
Zhao G, Yu X, Lou W, Wei S, Wang R, Wan Q, Shen W (2019) Transgenic Arabidopsis overexpressing MsSNAT enhances salt tolerance via the increase in autophagy, and the reestablishment of redox and ion homeostasis. Environ Exp Bot 164:20–28
Zhao YQ, Zhang ZW, Chen YE, Ding CB, Yuan S, Reiter RJ, Yuan M (2021) Melatonin: a potential agent in delaying leaf senescence. Crit Rev Plant Sci 40(1):1–22
Zhou X, Zhao H, Cao K, Hu L, Du T, Baluška F, Zou Z (2016) Beneficial roles of melatonin on redox regulation of photosynthetic electron transport and synthesis of D1 protein in tomato seedlings under salt stress. Front Plant Sci 7:1823
Zia SF, Berkowitz O, Bedon F, Whelan J, Franks AE, Plummer KM (2019) Direct comparison of Arabidopsis gene expression reveals different responses to melatonin versus auxin. BMC Plant Biol 19(1):1–18
Zuo Z, Sun L, Wang T, Miao P, Zhu X, Liu S, Li X (2017) Melatonin improves the photosynthetic carbon assimilation and antioxidant capacity in wheat exposed to nano-ZnO stress. Molecules 22(10):1727
Author information
Authors and Affiliations
Contributions
SJ and PA: designed and conceptualized the study. SJ and BS: prepared the manuscript. SJ and SM: edited the manuscript. SJ, RS and RB: reviewed the edited version of manuscript. SJ, BS and PA: completed the final draft of manuscript.
Corresponding author
Additional information
Handling Editor: Tariq Aftab.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Jan, S., Singh, B., Bhardwaj, R. et al. Recent Advances on the Pragmatic Roles of Phytomelatonin and Its Exogenous Application for Abiotic Stress Management in Plants. J Plant Growth Regul 42, 4885–4900 (2023). https://doi.org/10.1007/s00344-022-10766-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00344-022-10766-3