Abstract
Due to the increasing demand for biofuel feedstock production, Styrax tonkinensis, as a woody oilseed tree species, has received much more attention. However, the effects of various plant growth regulators on its seed development haven’t been explored deeply. Here, we applied 24-epibrassinolide (EBL) and methyl jasmonate (MJ) on the whole trees, and the aim of our study was to explore their effects on seed development of S. tonkinensis as well as seed chemical compositions, especially seed lipid metabolism. The results showed that EBL and MJ promoted the seed development, which was reflected in the increase of fresh and dry weight, as well as transverse and longitudinal diameter. The concentration of crude fat (CF) exhibited an ‘increase–decrease-increase’ trend, and the peak appeared at 70 days after flowering. EBL (in concentration of 5 μM, EBL5) and MJ (in concentration of 200 μM, MJ200) had the most significant effect on the accumulation of CF and the CF content in single seed. In addition, the activities of enzymes related to fatty acid (FA) synthesis were also higher under the two treatments. 12 FAs were detected using gas chromatography-mass spectrometry, among which palmitic acid, oleic acid, and linoleic acid were the main components. EBL5, EBL10, and MJ200 favored the accumulation of soluble sugar, especially in the middle and late stages. The starch concentration in seeds was lower, but it significantly increased after the application of EBL5. Furthermore, our results demonstrated that EBL and MJ enhanced SOD and POD activity while decreased MDA content. We highlighted that EBL and MJ promoted seed development of S. tonkinensis, affected seed chemical compositions and contributed to the accumulation of CF and FA.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The consumption of biological oil has increased at an annual rate of 5% over the past 50 years due to a growing global population and rapid industrialization (Harwood et al. 2017). Biodiesel is a renewable, safe, and green energy, which is regarded as an alternative resource to alleviate the energy crisis (Xiong et al. 2018; Dai et al. 2018). Therefore, woody oilseed species have attracted researchers’ attention. The dynamic change of plant oil is a momentous feature of oilseed plants during seed development. Plant oils, stored in seeds of most plants, are mainly in the form of triacylglycerols (Pokotylo et al. 2014). Fatty acids (FAs) are the main components of lipids and their synthesis is mainly carried out in plastids. This process is controlled by the complicate transcriptional and enzymatic regulatory network. Acetyl-CoA carboxylase (ACCase) is one of the basilic enzymes in FA biosynthesis and affects the entire life process of plants. ACCase catalyzes the carboxylation of acetyl CoA to form malonyl CoA, which is a pivotal regulatory step for FA synthesis and lipid formation. The synthesis rate of FA and the accumulation of oil are closely related to the activity of ACCase (Baud et al. 2003; Wang et al. 2006). Studies have shown that the expression of diacylglycerol acyltransferase (DGAT) affects plant seed development, seed oil content, and FA composition (Taylor et al. 2009). Overexpression of AtDGAT1 makes the DGAT activity in transgenic Arabidopsis thaliana seeds 10% to 70% higher than that in the wild type, and the oil content is also higher in transgenic plant (Jako et al. 2001). Besides, great efforts were made to investigate the FA components in seeds. Zonuz et al. (2020) compared the differences of FA composition in seeds of three Achillea species. Results from this study revealed that oleic acid and linoleic acid were major components in these species, which were indispensable FAs for human health. Salicylic acid (SA) and jasmonic acid (JA) significantly increased the content of FA in Glycine max seeds, and the compositions of FA changed drastically. Ghassemi-Golezani and Farhangi-Abriz (2018) revealed that SA and JA reduced oleic acid content, but enhanced linoleic acid and linolenic acid contents, thus improved oil quality.
Styrax tonkinensis, as a fast-growing tree species with strong adaptability and high ornamental values (Chen et al. 2019a, b), is native to Laos and Vietnam and widely distributed in the southern provinces of China (Zhang et al. 2018a, b). The flowers of S. tonkinensis are in strings with light fragrance and can be used as medicine to relieve pain (Chen et al. 2019a, b). Its bark is the source of benzoin, which can be used as a flavoring agent and produce incense, perfume and medicine (Burger et al. 2016; Courel et al. 2019). Moreover, S. tonkinensis is a vital oil plant, and previous research has elaborated the changing trend of crude fat (CF) concentration in its seeds at different days after flowering (DAF). Specifically, S. tonkinensis seeds has abundant oil and excellent FA composition with a high percent of unsaturated FAs (UFAs) (Zhang et al. 2017). The CF content rose sharply from 50 to 80 DAF, then dropped continuously and rose again at 120 DAF. As the seeds mature, the main UFAs (oleic acid and linoleic acid) accounted for 40% of the oil content and then increased to 87%, while the major saturated FAs (SFAs) (palmitic acid and stearic acid) decreased from 50 to 12% (Zhang et al. 2017; Wu et al. 2020a, b). Although S. tonkinensis seeds have high oil content and good FA compositions, there is still room for improvement. Breeding and cultivation are the major means to increase plant yield, and plant growth regulator treatment is one of the common cultivation methods. Janeczko et al. (2010) considered that 24-epibrassinolide (EBL) altered grain chemical compositions, increased soluble sugar content, but decreased total fats and calcium in wheat. Additionally, it was well documented that EBL increased the content of β-carotene and tocopherols in oilseed rape (Janeczko et al. 2009). Methyl jasmonate (MJ) was verified to promote Triticum aestivum fruit setting (Javadipour et al. 2019). Extensive work has been carried out on the role of EBL and MJ in fruiting and alteration in chemical compositions, while whether they can improve the yield and quality of S. tonkinensis seeds, especially the increase of CF concentration and the optimization of FA compositions, remains unknown.
Therefore, the aim of this study was to evaluate the effect of EBL and MJ on seed development as well as seed chemical composition of S. tonkinensis. Additionally, we also analyzed antioxidant enzymes activity and membrane peroxidation level in seeds. Fatty acid synthesis-related enzymes activity was measured to reveal the mechanism of impact of plant growth regulators on lipid metabolism. Data we obtained could help lay a foundation for boosting the industrialization of S. tonkinensis oil and provide a theoretical support for improving the seed quality and yield of S. tonkinensis.
Materials and Methods
Plant Material and Experiment Design
The experiment was conducted in planting base of Jiangsu Guoxing Biotechnology Co. Ltd., located in Luhe district, Nanjing (32°54' N, 118°50' E). The average temperature, hours of sunshine, and rainfall per year were 15.3 °C, 2200 h, and 970 mm, respectively. The experimental site is hilly land, and the soil fertility is suitable for plant growth.
Ten-year-old S. tonkinensis plants were chosen as experimental objects. These trees grew under natural conditions and no fertilizer was applied. S. tonkinensis starts to have flowers in late May and quickly enters the blooming stage.
In 30th June, 2020 (40 DAF), 42 trees with similar height, growth and good condition were selected and tagged. We designed seven treatments, including control (CK), EBL (in concentration of 1 μM, 5 μM, and 10 μM; marked as EBL1, EBL5, and EBL10) and MJ (in concentration of 50 μM, 200 μM, and 500 μM; marked as MJ50, MJ200, and MJ500), so each treatment contained six trees.
EBL (24-epibrassinolide, purchased from Shanghai Yuanye Biological Technology Co., Ltd., China) was dissolved in a small volume of alcohol and then diluted with distilled water into different concentrations (Zhang et al. 2018a, b). MJ (methyl jasmonate, purchased from Aladdin, USA) was diluted with distilled water to make different concentrations. Approximately 1800 mL of EBL or MJ was sprayed on each sampled tree (spray on the whole tree). CK plants were treated with equal amount of distilled water. Exogenous treatments were applied every ten days from 45 to 135 DAF and always performed between 6:00 to 8:00 am.
Sample Collection of S. tonkinensis After EBL and MJ Application
According to our previous research on seed development of S. tonkinensis (Zhang et al. 2017; Wu et al. 2019, 2020a, b), we selected samples from four representative time points in order to explore the effects of EBL and MJ on seed development and CF concentration:
-
(1)
50 DAF, the previous stage before seed dry matter rapid increase
-
(2)
70 DAF, during the aforementioned steep rise in nutrient concentration
-
(3)
100 DAF, the stage with decreasing oil concentration and increasing starch concentration
-
(4)
130 DAF, the final maturation stage
About fifty fresh fruits were randomly sampled from each individual tree at each sampling time, so one treatment had three hundred fruits. Then, these samples were placed on an insulated box with dry ice for transport to the laboratory. In each treatment, thirty fruits were peeled to measure the size of the seeds, fifty fruits were peeled to determine the weight of the seeds, some of the remaining fruits were used to estimate the nutrient concentration in seeds, and some were frozen in liquid nitrogen and stored at − 80 ℃ for assessing the enzyme activities.
Morphological Characteristics of Seeds Collected from Experimental Trees
Thirty seeds per treatment at four time points were selected, and the transverse and longitudinal diameter was measured using a vernier caliper. Fifty seeds per treatment at four time points were weighed with an electronic balance (FW) and then dried at 65 ℃ for 72 h (DW).
Crude Fat Concentration and Fatty Acid Composition in Seeds Collected from Experimental Trees
Forty-five seeds per treatment were randomly selected (divided into three replicates) and dried at 65 °C for 72 h. Then seeds were crushed and transferred into filter paper, which were dried and weighed (M0). The seeds and filter paper were weighed (M1) after drying for 30 min. Petroleum ether (30–60 ℃) was used as the extraction solution and the Soxhlet apparatus was adopted to extract CF. The extraction process lasted for 24 h and the water temperature was controlled at 65 ℃. After extraction, the filter paper covering the seeds was dried at 65 ℃ for 30 min (M2). The CF content in a single seed was calculated by multiplying the average CF concentration in seeds of sample trees under different treatments by the corresponding dry seed weight. The CF concentration was calculated according to the formula below:
In the EBL and MJ-treatments, we selected the treatment that had the great impact on the CF concentration for FA composition examination using gas chromatography-mass spectrometry (GC–MS). 0.2 mL of seed oil that extracted above was first dissolved in 2 mL of petroleum ether/benzene (1:1, v/v) and then fully mixed with 2 mL of 400 mM KOH-CH3OH to prepare FA methyl esters. Shaking was required during the 15 min conversion at room temperature. The product was diluted to a final volume of 10 mL with deionized water, and the upper phase was used for analysis (Zhang et al. 2018a, b). The GC–MS analysis was performed on a TRACE DSQ GC–MS (Thermo Fisher Scientific Inc., USA) coupled with an Agilent DB-5 ms capillary column (30 m, 0.33 mm, 0.25 μm). Refer to our previous studies (Chen et al. 2021a, b; Ji 2020; Wu et al. 2020a, b), we made minor modifications for measurement process.
The GC oven temperature was initially 60 °C for 2 min and then gradually increased at a rate of 10 °C/min to 280 °C, which was held for 5 min. Electron ionization was carried out with 70 eV of ionization energy. 1 μL of the sample was injected in non-split mode, and the injector temperature was set at 260 °C. The mass spectra were obtained using the full scan mode with a mass scan range of m/z 33–450. To identify the oil constituents, the spectra and retention times of peaks were compared using X-caliber with reference to the NIST database. An ion current peak area normalization method was used to calculate the relative amount of each component (Ji 2020; Yang et al. 2016):
M, the peak area of the individual compounds; N, the total peak area.
Moreover, in order to clarify the proportion relationship between unsaturated fatty acid (UFA) and saturated fatty acid (SFA) in seeds under different treatments at each sampling period, we calculated the ratio of UFA to SFA.
Carbohydrate Concentration in Seeds Collected from Experimental Trees
Seeds (0.5 g) were ground and diluted to 10 mL. After two times of 30-min extraction with boiling water, dilute them to 25 mL. Add 0.2 mL of extracting solution, 1.8 mL of distilled water, 0.5 mL of anthrone ethyl acetate and 5 mL of 98% concentrated sulfuric acid respectively. Calculate soluble sugar concentration according to the method described by Li (2006). Transfer the residues from extracting soluble sugar into test tubes and dilute them to 10 mL. Place the tubes in boiling water for fully extraction for 15 min and add 2 mL of 9.2 mol/L perchloric acid, then extract it for another 15 min. Then, the measurement of starch was the same as above.
Antioxidant Enzyme Activity in Seeds Collected from Experimental Trees
Seeds (0.5 g) were weighed and ground with 5 mL of 0.1 mol/L phosphate buffer. The homogenate was transferred to centrifuge tube and centrifuged at 6000 rpm for 20 min. 2.9 mL of the reaction solution (manufacturing method see Chen et al. (2021a, b)) and 0.1 mL of the supernatant were mixed to measure the peroxidase (POD) activity with spectrophotometer. The calculation of POD activity was referred to Li (2006).
Seeds (0.5 g) were weighed and ground with 5 mL of 0.05 mol/L phosphate buffer. The homogenate was transferred to centrifuge tube and centrifuged at 10,000 rpm for 20 min. According to the method of Li (2006) and Chen et al. (2021a, b), we made the reaction system and determined the superoxide dismutase (SOD) activity with spectrophotometer at 560 nm.
Malondialdehyde (MDA) Content in Seeds Collected from Experimental Trees
Seeds (0.5 g) were weighed and ground with 5 mL of 5% trichloroacetic acid. The homogenate was transferred to centrifuge tube and centrifuged at 3000 rpm for 20 min. 2 mL of supernatant and 2 mL of 0.6% thiobarbituric acid were added to the tube and placed in boiling water for 20 min. MDA content was measured according to the method of Li (2006).
Fatty Acid Synthesis-Related Enzymes Activity in Seeds Collected from Experimental Trees
Seeds (0.5 g) were weighed to measured fatty acid synthase (FAS) activity with an assay kit (Suzhou Comin Biotechnology Co., Ltd., China). As described by Brusselmans et al. (2005), samples were homogenized in buffer, and reactions (manufacturing method see Zhang et al. (2017)) were performed in a total volume of 1000 μL. Decreases in NADPH absorbance at 340 nm were detected with a spectrophotometer (Bays et al. 2009).
ELISA (enzyme-linked immunosorbent assay) kit (Suzhou Comin Biotechnology Co., Ltd., China) was used for analyzing the activity of acetyl-CoA carboxylase (ACCase) and diacylglycerol acyltransferase (DGAT). The microtiter plates have been pre-coated with an antibody specific to plant ACCase (or DGAT). After antibody-antigen interactions, signals were amplified by the biotin-avidin system and the antigen targets in samples were qualified through HRP (horseradish peroxidase)-TMB (3,3′,5,5′-tetramethylbenzidine) colorimetric detection system. The color change was measured spectrophotometrically at a wavelength of 450 nm. ACCase (or DGAT) concentration in samples was then determined by comparing OD value to the standard curve.
Statistics Analysis
Values were expressed as mean ± SD for three replicates. Excel (Office 2019 Pro Plus, Microsoft Corporation, USA) was used to process data and figures were drawn by Prism 9 (GraphPad, USA). One-way analysis of variance (ANOVA) was performed using SPSS 26.0 (IBM, USA) followed by Duncan’s multiple range test. Associations between various physiological and biochemical indexes and CF concentration were established using Pearson’s correlation analysis. P-values less than 0.05 were considered to indicate significance within groups.
Results
Effects of EBL and MJ on Seed Development of S. tonkinensis
The fresh and dry weight of single seed increased continuously with the seed development (Table 1). From 50 to 70 DAF, the fresh weight increased by 27% in CK, then the increase slowed, and the dry weight reached 0.14 g per seed at 130 DAF. When the fruits were treated with EBL5, the seed fresh and dry weight were significantly higher than those in CK. Among three MJ-treatments, MJ500 had the better effect on the increase of seed fresh and dry weight.
The similar trend was observed in seed transverse and longitudinal diameter (Table 2). At 50 DAF, when trees were treated with EBL5, seed transverse and longitudinal diameter were significantly higher than those in other treatments with the values of 5.98 mm and 8.55 mm, respectively. Thereafter, seed transverse and longitudinal diameter continued to ascend and reached maximum of 6.96 mm and 9.87 mm at 130 DAF. Overall, EBL and MJ apparently enhanced the seed size.
Effects of EBL and MJ on Seed Nutrients Concentration of S. tonkinensis
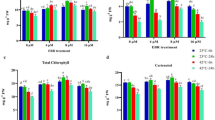
The patterns of CF concentration under different treatments at 50, 70, 100, and 130 DAF were demonstrated in Fig. 1a. Generally, the concentration of CF was lower at 50 DAF and the highest concentration was noted under EBL5 (129.44 mg/g DW), which had no significant difference with MJ50, but was significantly higher than those of other treatments. After 20 days, the concentration went up rapidly, and the highest and lowest values were 478.97 mg/g DW (EBL5) and 366.11 mg/g DW (MJ50), respectively, with significant difference. Then the concentration began to descend, but it was much higher than that at 50 DAF. At 130 DAF, the CF concentration rose again and the top three for concentration were EBL10, EBL5, and MJ200. As for the change in CF content per seed, we found it increased continuously, and EBL and MJ greatly increased CF content (Fig. 1b). From 50 to 70 DAF, the increase rate was large, then the rate became smaller. At 130 DAF, CF content per seed could reach up to 48.13 mg (EBL5), followed by 42.11 mg (MJ200). To sum up, EBL5 had the greatest impact on the concentration of CF in seeds, whereas MJ200 was optimal of three MJ-treatments, consequently we performed GC–MS to determine FA compositions of seeds in these two treatments.
Effects of EBL and MJ, applied at various concentrations on S. tonkinensis trees, on crude fat concentration and carbohydrate concentrations in developing seeds at 50, 70, 100 and 130 days after flowering. a Crude fat concentration; b Crude fat content per seed; c Soluble sugar concentration; d Starch concentration. Data points show mean ± SD, the same below
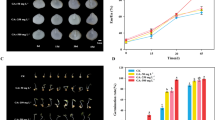
The results showed that 12 FAs were detected, including 8 SFAs (tetradecanoic acid, C14:0; pentadecanoic acid, C15:0; palmitic acid, C16:0; heptadecanoic acid, C17:0; stearic acid, C18:0; nonadecanoic acid, C19:0; eicosanoic acid, C20:0; docosanoic acid, C22:0) and 4 UFAs (palmitoleic acid, C16:1; oleic acid, C18:1; linoleic acid, C18:2; linolenic acid, C18:3). There was no obvious change in their composition under two treatments during the whole observation period (Table 3). The major and common compositions under EBL5 and MJ200 were C16:0, C18:1 and C18:2. The total concentration of three compositions was over 77.5% in all FAs. Among SFAs, C16:0 concentration kept at a high level, followed by C18:0, nevertheless C18:1 and C18:2 were the two main UFAs. As shown in Fig. 2, C16:0 concentration moved downwards with the development of seeds, which was opposite to that of C18:1 and C18:2 concentrations. EBL5 greatly boosted the accumulation of C16:0 at 50 DAF, nonetheless C18:1 concentration at 50 DAF was steeply increased by MJ200. EBL5 and MJ200 did not significantly affect the concentration of major compositions. In general, the concentration of C18:2 was higher than that of C18:1 and C16:0. UFA/SFA among all the treatments increased continuously during seed development. Except the value in CK at 50 DAF, the rest values were all more than 1, indicating that UFA concentration was higher than that of SFA (Fig. 3).
Relative concentration of palmitic acid (C16:0), oleic acid (C18:1) and linoleic acid (C18:2) in S. tonkinensis seeds exposed to EBL5 and MJ200 compared to CK. Color scale represented the variation in concentration of these three compositions, from low (green) to high (red) at 50, 70, 100 and 130 DAF, respectively (from top to bottom in each treatment)
Soluble sugar concentration showed upwards trend in CK at four sampling periods (Fig. 1c) and the concentration increased from 32.51 mg/g FW (50 DAF) to 112.71 mg/g FW (130 DAF). Although EBL and MJ had effects on soluble sugar concentration at 50 DAF, these effects were slight when compared with the other three periods. At 70 DAF and 100 DAF, EBL10 significantly increased soluble sugar concentration, while MJ200 dominated in enhancing soluble sugar concentration at 130 DAF.
There were significant differences for starch concentration at different sampling points. Initially, starch concentration was relatively high in CK, EBL1, and EBL10. Afterwards, the concentration increased rapidly under EBL5 and touched the maximum level at 130 DAF (Fig. 1d). Among three MJ-treatments, starch concentration in seeds under MJ200 was significantly higher than that of MJ50 and MJ500 at 130 DAF, while in other periods, the difference among the three was not significant.
Effects of EBL and MJ on Antioxidant Enzymes Activity and MDA Content of S. tonkinensis
The activity of SOD exhibited ‘A’ trend and peaked at 100 DAF (Fig. 4a). The highest and lowest SOD activity at 50 DAF was 169.90 U/min/g FW (EBL1) and 111.30 U/min/g FW (CK), respectively, and there was significant difference between them. From 70 to 100 DAF, SOD activity increased slightly, and then decreased sharply. At 130 DAF, the activity in seeds of EBL1-treated trees was the highest (176.39 U/min/g FW). It could be seen that different concentrations of EBL and MJ enhanced the SOD activity in seeds.
Although the changing trend of POD activity under different treatments was diverse, they all reached the maximum at 130 DAF (Fig. 4b). At 50 DAF, the activities of POD in seeds of different treatments were all lower than 9 U/min/g FW, thereafter it began to rise. At 70 DAF and 100 DAF, EBL1 and MJ500 caused the highest POD activity in seeds, respectively. POD activity in seeds of S. tonkinensis treated with MJ200 was significantly higher than that of other treatments at 130 DAF.
MDA contents under various treatments at different days after flowering were displayed in Fig. 4c. In any period, MDA content in seeds from treated trees was generally lower than that of seeds from untreated trees. It could be concluded that the exogenous treatments reduced the MDA content in seeds. At 50 DAF and 70 DAF, the diversity in MDA content under different treatments was small and there was no significant difference between them. At 100 DAF and 130 DAF, the MDA contents in seeds of untreated trees were the highest, which were 10.87 nmol/g FW and 11.91 nmol/g FW, respectively.
Effects of EBL and MJ on FA Synthesis-Related Enzymes Activity of S. tonkinensis
The trend of FAS activity rose first and then declined, and the plateau presented at 100 DAF (Fig. 5a). FAS activity in seeds under MJ50 and EBL5 was higher at 50 DAF, 5.07- and 3.72-fold of CK, respectively, and the difference between them was significant. At the second time point, the activity of FAS in seeds under EBL5 elevated sharply to 148.47 nmol/min/mg protein, which was significantly higher than that of other treatments. MJ50, MJ200, and MJ500 caused a reduction in FAS activity at 100 DAF, which were all lower than that of CK. On the contrary, EBL5 and EBL10 significantly increased the FAS activity by 77.9% and 76.7% when compared to CK. Then, there was a downward trend in FAS activity, and seeds of CK and EBL5 had the lowest activity (67.54 nmol/min/mg protein) and the highest activity (232.62 nmol/min/mg protein), respectively.
At 50 DAF, ACCase activity of each treatment was the lowest during the whole sampling periods (Fig. 5b). The activity of seeds in CK was 0.31 nmol/mg protein, which was significantly lower than that of other treatments. From 70 to 130 DAF, ACCase activity showed an overall downward trend. EBL and MJ enhanced ACCase activity, among which EBL5, EBL10 and MJ200 were more prominent.
The activity of DGAT increased first and then decreased, and there were significant differences in each period (Fig. 5c). Detailly, the highest activity appeared in EBL10 (4.31 nmol/mg protein) at 50 DAF, which was significantly higher than that of other treatments and was 2.51-fold of the lowest (MJ50, 1.72 nmol/mg protein). In the next three sampling periods, EBL5, EBL10 and MJ200 had greater impacts on DGAT activity in seeds. During the entire observation period, DGAT activity in seeds of MJ50-treated trees was always lower than that of untreated trees.
Discussion
EBL and MJ Promoted Seed Development and Boosted the Yield
EBL was confirmed as the sixth class of plant hormones, which was widely involved in regulating the plant growth and development. It was of great significance in improving plant yield and quality, photosynthesis, and resistance to stress (Li et al. 2021; Faizan et al. 2021). Also, MJ could not only enhance the stress tolerance of plants, but also has a strong influence on regulating secondary metabolism (Karami et al. 2013). In the present study, different concentrations of EBL and MJ were applied on S. tonkinensis, thereby investigating its morphological parameters. The results showed that EBL and MJ promoted the dry and fresh weight, transverse and longitudinal diameter of seeds at four periods, especially EBL5 and M500. Likewise, a few studies stated that EBL could increase the size, weight, and number of A. thaliana seeds (Jiang et al. 2013; Huang et al. 2013). We speculated that different concentrations of exogenous EBL and MJ could help to increase the size and weight of S. tonkinensis seeds, so as to achieve the purpose of increasing the yield. This could be attributed to the fact that EBL and MJ may enhance the photosynthetic assimilates in pericarp, thereby contributing to seed development (Zhang et al. 2018a, b).
EBL and MJ Improved Carbohydrates Accumulation and Stimulated Lipid Metabolism in S. tonkinensis Seeds
S. tonkinensis is a high oil value tree species, whose seeds are rich in UFAs, and the seed oil has perfect biodiesel properties, satisfying the standards of China, Germany, the USA and the European Union (Wu et al. 2020a, b). Based on this, it is more attractive to study the effect of exogenous plant growth regulators on the concentration of CF and composition of FA in S. tonkinensis seeds. In this study, we found that EBL5, EBL10, and MJ200 all promoted the accumulation of CF in each period, but the extent was varying. Also, these three treatments increased CF content per seed (Fig. 1b). Flores and Luisa (2016) explained that these treatments could promote the activity of enzymes related to FA synthesis, resulting in higher concentration of CF. In addition, EBL helped to accumulate FA in Carthamus tinctorius seeds (Zafari et al. 2020), which was coincident with our findings. Combined with the information we mentioned above, it could be concluded that EBL and MJ helped get higher yield of S. tonkinensis.
For edible oils, UFAs play an important role in human health. However, many UFAs cannot be synthesized by human themselves, which leads to a large demand. They can regulate blood lipids, enhance immunity, prevent cardiovascular diseases and so on (Bloedon and Szapary 2004). Thus, increasing the concentration of UFAs is a crucial goal when making edible oil. C18:1 and C18:2 were the main UFAs in S. tonkinensis seeds, and both reached the maximum at 130 DAF (Table 3). Hence, this period was the best time to extract UFAs, which would help augment the value of FAs in S. tonkinensis seeds. From 50 to 130 DAF, the trend of UFA/SFA was consistent with that of seed dry weight, which meant it continued to rise (Fig. 3, Table 1). It indicated that seed dry weight could be used to reflect the development of FA components and provide a theoretical basis for determining the optimal harvest period of fruits.
FAS, ACCase and DGAT, key enzymes in the synthesis of FA, correlated closely with the synthesis rate of FA (Baud et al. 2003). Turnham et al. (1982) expounded that the rate of FA synthesis was controlled by the level of ACCase activity, and he also believed that ACCase was a key limiting enzyme for oil accumulation in rape seeds. Moreover, increase in FA content of seeds was reported to be in accordance with increase in ACCase activity in soybean (Charles et al. 1986). Overall, FAS, ACCase and DGAT activities in CK were relatively low, whilst during the vigorous period of CF accumulation (70 DAF and 100 DAF), the activities of these enzymes in seeds of EBL-treated trees were significantly enhanced (Fig. 5), and positively correlated with CF (Table 4). It concluded that FAS, ACCase and DGAT were the key factors affecting CF concentration in S. tonkinensis seeds. When we further analyzed the CF concentration and FA synthesis-related enzyme activities in seeds after EBL treatments, we found that the CF concentration in S. tonkinensis seeds from EBL5- and EBL10-treated trees was always higher than that of CK during seed development, and the ACCase and DGAT activities in seeds were also significantly higher than those of CK. Therefore, a strong positive correlation could be established between CF and FA synthesis-related enzymes, which was proved in Table 4. This enlightened us that the appropriate concentration of EBL could enhance the activities of FAS, ACCase and DGAT in seed, and subsequently increase CF concentration. Our result was similar to previous research by Zhang et al. (2018a, b) that FAS activity in S. tonkinensis seeds under 10 μM EBL maintained at a higher level, and CF concentration was correspondingly higher. Whereas there was no significant association between ACCase activity and FA accumulation in rape seeds, the possible reason was that ACCase played different roles in different stages of lipid accumulation and FA synthesis (Kang et al. 1994).
Flores and Luisa (2016) investigated the effect of MJ on FA in black currant seeds and pointed out that MJ did not have obvious impact on FA content, which was different from our findings. They regarded that this phenomenon was probable that MJ affected variously individual FAs in such a way that they were balanced in the total content. Also, C18:2 was the primary UFA in black currant seeds, and MJ reduced its content. Furthermore, MJ significantly increased the content of FA in soybean, among which C18:3 increased the most (Mohamed and Latif 2017). The differences in FA concentration and composition could be due to species differences and/or distinctive cultivation managements (Maestri et al. 1998; Bellaloui et al. 2012).
Soluble sugar, as the main respiratory substrate, is an important source of nutrients for seed development, and closely related to FA synthesis in oil seeds (Hill 2003). It was reported that there was a distinct soluble sugar content and composition change during the development of oil seeds, which affected the accumulation of seed FAs in the later stage. Increasing photosynthetic metabolites (soluble sugar) could apparently promote the oil content of seeds (Borisjuk et al. 2004). In this study, the concentrations of soluble sugar and CF in seeds of CK soared simultaneously from 50 to 70 DAF in S. tonkinensis seeds, suggesting that high concentration of soluble sugar contributed to the accumulation of CF (Fig. 1a, 1c). After that the CF concentration dropped from 70 to 100 DAF. The possible reason was that soluble sugar was converted into starch, which led to the increase of starch concentration from 70 to 100 DAF (Fig. 1d). EBL changed the profile of soluble sugar accumulation. We noted that EBL5 and EBL10 had a greater promoting effect on soluble sugar concentration, especially from 70 to 130 DAF, at the same time, CF concentration also increased. The significant positive correlation between CF and soluble sugar was a solid evidence that high soluble sugar concentration was beneficial to CF accumulation (Table 4, r = 0.719, P < 0.05). The accumulation of CF in oil seeds could be promoted by modifying some genes related to FA synthesis by means of molecular improvement and genetic breeding. However, seed FA accumulation is a very complex metabolic process, and the activities of enzymes are usually regulated by the feedback of the whole metabolic network, which may not make seed FA accumulation achieve the expected goal (Bates et al. 2014). In contrast, the application of plant growth regulator is a simple way to increase FA content. Therefore, in the middle and late stage of S. tonkinensis seeds development, EBL5 and EBL10 would increase the concentration of soluble sugar, and consequently increase the concentration of CF in seeds. Our results were consistent with the results of Wang et al. (2019) that spraying appropriate concentration of EBL was beneficial to soluble sugar accumulation.
As for the exogenous application of MJ, Dong et al. (2020) compared the effects of two different MJ spraying methods (the whole tree and only fruit) on fruit quality of citrus ‘Huangguogan’, and they found that the whole tree spray of 10 μM MJ had the greatest impact on fruit quality, which led to the highest soluble sugar content. In the current study, we also sprayed different concentrations of MJ on the whole tree and came to the similar conclusion with MJ200. It was inferred that different tree species had different response to MJ concentration, whether there was a more suitable MJ concentration for the development of S. tonkinensis seeds was worth further exploration.
Although starch content in seeds was relatively low, it acted as an energy source for seed development and one of indispensable nutrients. According to Fig. 1c, soluble sugar concentration in seeds under EBL5 was low, which was perhaps caused by that a large amount of soluble sugar was converted into starch and stored in seeds, inducing an increase in starch concentration. With the development of seeds, plenty of soluble sugar was utilized, and starch was converted into soluble sugar for FA synthesis and lipid accumulation (Zhang and Liu 2016). A significant positive correlation between CF and starch was found in the present study (Table 4, r = 0.436, P < 0.05), indicating that CF concentration went up as starch concentration increased. We didn’t observe the significant change of seed starch concentration at 50 DAF under EBL, because this was the first sampling after EBL spraying, and the effect of EBL has not been performed. However, EBL5 drastically increased starch concentration at 70 DAF, 100 DAF, and 130 DAF. It could be summarized that the effect of EBL on starch concentration required a cumulative process. MJ didn’t influence starch concentration noticeably all the time.
EBL and MJ Enhanced Seed Antioxidant Enzymes Activity and Declined Membrane Peroxidation Level
Several researchers devoted to elucidating the effect and mechanism of EBL and MJ on seed stress resistance. EBL played a role in modulating plant metabolic reactions and regulating plasma membrane proteins, thus leading to alleviation of stress influence and increasement of plant productivity (Li et al. 2012; Pokotylo et al. 2014). In general, SOD and POD activities in seeds from EBL-treated trees were higher than those of CK during seed development, indicating that EBL could activate the defense system to improve the ability of plants to resist unfavorable environmental condition. The third and fourth sampling (100 DAF and 130 DAF, respectively) were in August and September, so S. tonkinensis might be subjected to high temperature stress, consequently the MDA content as well as the degree of membrane peroxidation increased. After spraying EBL, MDA content decreased significantly, implying that EBL alleviated the damage of the membrane system under high temperature stress. In conclusion, EBL enhanced the tolerance of S. tonkinensis seeds by regulating POD and SOD activities and MDA content. Accordingly, it was feasible to manipulate EBL to achieve this goal in the middle and late stage of seed development.
MJ could protect plants from damage by activating various mechanisms and increasing the activity of antioxidant enzymes, such as catalase (CAT), SOD and POD (Kolupaev and Yastreb 2021). MJ contributed to higher SOD and POD activities and lower MDA content during S. tonkinensis seed development, which revealed a similar pattern with that of EBL and agreed well with the conclusion obtained in Actinidia deliciosa and Oryza sativa (Pan et al. 2020; Dai et al. 2021). However, a reduction in POD activity in Averrhoa carambola was observed when fruits treated with MJ (Mustafa et al. 2016).
Conclusion
The current study implied that EBL and MJ promoted S. tonkinensis seed development and altered concentrations of seed chemical components. Generally, EBL and MJ significantly elevated the CF concentration in seeds, especially at 70 DAF. With regard to FA compositions, UFAs concentration was much higher than that of SFAs. CF was positively or significantly positively correlated with FA synthesis-related enzymes, and EBL and MJ facilitated lipid accumulation by increasing the activity of these enzymes. Among these exogenous treatments, the most effective were hormones applied in concentration of 5 μM EBL (EBL5) and 200 μM MJ (MJ200), which remarkably increased the carbohydrate concentrations in seeds. Our study also illuminated that EBL and MJ enhanced the resilience of seeds by improving SOD and POD activity and dropping MDA content. Hence, exogenous application of EBL and MJ could be considered as a potential approach to boost the yield and quality of seed oil of S. tonkinensis. Not only our results provided evidence for EBL and MJ role in the regulation of seed development, but also they grant basis for further research required for steady development of biofuel production.
Abbreviations
- ACCase:
-
Acetyl-CoA carboxylase
- CF:
-
Crude fat
- DAF:
-
Days after flowering
- DGAT:
-
Diacylglycerol acyltransferase
- ELISA:
-
Enzyme-linked immunosorbent assay
- EBL:
-
24-Epibrassinolide
- FA:
-
Fatty acid
- FAS:
-
Fatty acid synthase
- GC–MS:
-
Gas chromatography-mass spectrometry
- JA:
-
Jasmonic acid
- MDA:
-
Malondialdehyde
- MJ:
-
Methyl jasmonate
- POD:
-
Peroxidase
- SA:
-
Salicylic acid
- SFA:
-
Saturated fatty acid
- SOD:
-
Superoxide dismutase
- UFA:
-
Unsaturated fatty acid
References
Bates PD, Johnson SR, Cao X, Jia L, Browse J (2014) Fatty acid synthesis is inhibited by inefficient utilization of unusual fatty acids for glycerolipid assembly. P Natl Acad Sci USA 111:1204–1209
Baud S, Guyon V, Kronenberger J, Wuilleme S, Rochat C (2003) Multifunctional acetyl-CoA carboxylase 1 is essential for very long chain fatty acid elongation and embryo development in Arabidopsis. Plant J 33:75–86
Bays N, Hill AD, Kariv I (2009) A simplified scintillation proximity assay for fatty acid synthase activity: development and comparison with other FAS activity assays. J Biomol Screen 14:636–642
Bellaloui N, Mengistu A, Fisher DK, Abel C (2012) Soybean seed composition as affected by drought and phomopsis in phomopsis susceptible and resistant genotypes. J Crop Improv 26:428–453
Bloedon LT, Szapary PO (2004) Flaxseed and cardiovascular risk. Nutr Rev 64:18–27
Borisjuk L, Rolletschek H, Radchuk R, Weschke W, Wobus U, Weber H (2004) Seed development and differentiation: a role for metabolic regulation. Plant Biol 6:375–386
Brusselmans K, Vrolix R, Verhoeven G, Swinnen JV (2005) Induction of cancer cell apoptosis by flavonoids is associated with their ability to inhibit fatty acid synthase activity. J Biol Chem 280:5636–5645
Burger P, Casale A, Kerdudo A (2016) New insights in the chemical composition of benzoin balsams. Food Chem 210:613–622
Charles DJ, Hasegawa PM, Cherry JH (1986) Characterization of acetyl-CoA carboxylase in the seed of two soybean genotypes. Phytochemistry 25:55–59
Chen C, Cao YY, Wang XJ, Wu QK, Yu FY (2019a) Do stored reserves and endogenous hormones in overwintering twigs determine flower bud differentiation of summer blooming plant-Styrax tonkinensis. Int J Agric Biol 22:815–820
Chen C, Wang XJ, Cao YY, Wu QK, Yu FY (2019b) Time course morphological and histological changes in differentiating floral buds of Styrax tonkinensis. Int J Agric Biol 22:1491–1496
Chen C, Cao YY, Chen H, Yu FY (2021a) Floral scent compounds and emission patterns of three Styrax species. Dendrobiology 85:30–38
Chen C, Chen H, Ni M, Yu FY (2021b) A study on petal morphological and physiological characteristics of Styrax japonicus during the flowering period. Agronomy 11:1498
Courel B, Adam P, Schaeffer P (2019) The potential of triterpenoids as chemotaxonomic tools to identify and differentiate genuine, adulterated and archaeological balsams. Microchem J 147:411–421
Dai GH, Yang J, Lu SR, Huang CH, Jin J, Jiang P, Yan PB (2018) The potential impact of invasive woody oil plants on protected areas in china under future climate conditions. Sci Rep 8:1041
Dai ZH, Yuan Y, Huang HL, Hossain MM, Xiong SL, Cao MH, Ma LQ, Tu SX (2021) Methyl jasmonate mitigates high selenium damage of rice via altering antioxidant capacity, selenium transportation and gene expression. Sci Total Environ 756:143848
Dong TT, Wang BZ, Zhang HY, Wang JQ, Yao Y, Xu WX, Xiong B, Wang ZH (2020) Effects of different concentrations of methyl jasmonate on fruit quality of citrus Huangguogan. IOP Conference Series: Earth Env Sci 474:032026
Faizan M, Bhat JA, Noureldeen A, Ahmad P, Yu FY (2021) Zinc oxide nanoparticles and 24-epibrassinolide alleviates Cu toxicity in tomato by regulating ROS scavenging, stomatal movement and photosynthesis. Ecotox Environ Safe 218:112293
Flores G, Luisa RDCM (2016) Enhancement of nutritionally significant constituents of black currant seeds by chemical elicitor application. Food Chem 194:1260–1265
Ghassemi-Golezani K, Farhangi-Abriz S (2018) Changes in oil accumulation and fatty acid composition of soybean seeds under salt stress in response to salicylic acid and jasmonic acid. Russ J Plant Physiol 65:229–236
Harwood JL, Woodfield HK, Chen G, Weselake RJ (2017) Modification of oil crops to produce fatty acids for industrial applications. In Moghis U Ahmad (ed) Fatty acids-chemistry, synthesis, and applications. Academic Press, Elsevier Inc, pp 187–236
Hill LM (2003) Metabolism of sugars in the endosperm of developing seeds of oil seed rape. Plant Physiol 131:228–236
Huang HY, Jiang WB, Hu YW, Wu P, Zhu JY, Liang WQ, Wang ZY, Lin WH (2013) BR signal influences Arabidopsis ovule and seed number through regulating related genes expression by BZR1. Mol Plant 6:456–469
Jako C, Kumar A, Wei YD, Zou JT, Barton DL, Giblin EM, Covello PS, Taylor DC (2001) Seed-specific overexpression of an Arabidopsis cDNA encoding a diacylglycerel acyltransferase enhances seed oil content and seed weight. Plant Physiol 126:861–874
Janeczko A, Biesaga-Kościelniak J, Dziurka M (2009) 24-Epibrassinolide modifies seed composition in soybean, oilseed rape and wheat. Seed Sci & Technol 37:625–639
Janeczko A, Biesaga-Kościelniak J, Okleštková J, Filek M, Dziurka M, Szarek-Lukaszewska G, Kościelniak J (2010) Role of 24-epibrassinolide in wheat production: Physiological effects and uptake. J Agron Crop Sci 196:311–321
Javadipour Z, Balouchi H, Movahhedi D, Yadavi A (2019) Roles of methyl jasmonate in improving growth and yield of two varieties of bread wheat (Triticum aestivum) under different irrigation regimes. Agr Water Manage 222:336–345
Ji XY (2020) Comparative analysis of volatile organic compounds and bioactive compounds in typical coniferous and broad-leaved tree species. J Essent Oil Bear Pl 23:1105–1117
Jiang WB, Huang HY, Hu YW, Zhu SW, Wang ZY, Lin WH (2013) Brassinosteroid regulates seed size and shape in Arabidopsis. Plant Physiol 162:1965–1977
Kang F, Ridout CJ, Morgan CL, Rawsthorne S (1994) The activity of acetyl-CoA carboxylase is not correlated with the rate of lipid synthesis during development of oilseed rape (Brassica napus L.) embryos. Planta 193:320–325
Karami A, Shahbazi M, Niknam V, Shobbar ZS, Tafreshi RS, Abedini R, Mabood HM (2013) Expression analysis of dehydrin multigene family across tolerant and susceptible barley (Hordeum vulgare L.) genotypes in response to terminal drought stress. Acta Physiol Plant 35:2289–2297
Kolupaev TE, Yastreb TO (2021) Jasmonate signaling and plant adaptation to abiotic stressors. Appl Biochem Micro 57:1–19
Li HS (2006) The Principles and Technologies for Plant Physiology and Biochemistry Experiments. High Education Press, Beijing, China
Li BQ, Zhang CF, Cao BH, Qin GZ, Wang WH, Tian SP (2012) Brassinolide enhances cold stress tolerance of fruit by regulating plasma membrane proteins and lipids. Amino Acids 43:2469–2480
Li SM, Zheng HX, Lin L, Wang F, Sui N (2021) Roles of brassinosteroids in plant growth and abiotic stress response. Plant Growth Regul 93:1–10
Maestri DM, Labuckas DO, Guzman CA (1998) Correlation between seed size, protein and oil contents and fatty acid composition in soybean genotypes. Grasas Aceites 49:450–453
Mohamed HI, Latif HH (2017) Improvement of drought tolerance of soybean plants by using methyl jasmonate. Physiol Mol Biol Pla 23:545–556
Mustafa MA, Ali A, Seymour G, Tucker G (2016) Enhancing the antioxidant content of carambola (Averrhoa carambola) during cold storage and methyl jasmonate treatments. Postharvest Biol Tec 118:79–86
Pan LY, Zhao XY, Chen M, Fu YQ, Xiang ML, Chen JY (2020) Effect of exogenous methyl jasmonate treatment on disease resistance of postharvest kiwifruit. Food Chem 305:125483
Pokotylo IV, Kretynin SV, Khripach VA, Ruelland E, Blume Y, Kravets S (2014) Influence of 24-epibrassinolide on lipid signalling and metabolism in Brassica napus. Plant Growth Regul 73:9–17
Taylor DC, Zhang Y, Kumar A, Francis T, Giblin EM, Barton DL, Ferrie JR, Laroche A, Shah S, Zhu WM, Crystal S, Linda H, Gerhar R, John H, Randall W (2009) Molecular modification of triacylglycerol accumulation by over-expression of DGAT1 to produce canola with increased seed oil content under field conditions. Botany 87:533–543
Wang FL, Wu GT, Lang CX, Chen JQ (2006) Acetyl-CoA carboxylase in plant. Plant Physiol J 42:10–14
Wang Y, Fu XK, He W, Chen Q, Wang XR (2019) Effect of spraying brassinolide on fruit quality of Citrus grandis cv. ‘Huangjinmiyou’ and ‘Hongroumiyou.’ IOP Conference Series: Earth Env Sci 358:022029
Wu QK, Zhang ZH, Peng H, Wu YL, Yu FY (2019) The nutrient distribution in the continuum of the pericarp, seed coat, and kernel during Styrax tonkinensis fruit development. Peer J 7:e7996
Wu QK, Cao YY, Chen C, Gao ZZ, Yu FY, Guy RD (2020a) Transcriptome analysis of metabolic pathways associated with oil accumulation in developing seed kernels of Styrax tonkinensis, a woody biodiesel species. BMC Plant Biol 20:121
Wu QK, Zhao X, Chen C, Zhang ZH, Yu FY (2020b) Metabolite profiling and classification of developing Styrax tonkinensis kernels. Metabolites 10:21
Xiong B, Zhang Z, Dong S (2018) Biodiesel from Lindera glauca oil, a potential non-food feedstock in Southern China. Ind Crop Prod 122:107–113
Yang X, Zhao J, Zheng J, Leng PS, Li XL, Hu ZH, Liu JB, Meng X (2016) Analysis of floral scent emitted from Syringa plants. J Forestry Res 27:273–281
Zafari M, Ebadi A, Sedghi M, Jahanbakhsh S (2020) Alleviating effect of 24-epibrassinolide on seed oil content and fatty acid composition under drought stress in safflower. J Food Compos Anal 92:103544
Zhang Y, Liu AZ (2016) The correlation between soluble carbohydrate metabolism and lipid accumulation in castor seeds. Biotech Bullet 32:120–129
Zhang ZH, Wang XJ, Luo Y, Yu FY (2017) Carbon competition between fatty acids and starch during benzoin seeds maturation slows oil accumulation speed. Trees-Struct Funct 31:1025–1039
Zhang ZH, Luo Y, Wang XJ, Yu FY (2018a) Fruit spray of 24-epibrassinolide and fruit shade alter pericarp photosynthesis activity and seed lipid accumulation in Styrax tonkinensis. J Plant Growth Regul 37:1066–1084
Zhang ZH, Luo Y, Wang XJ, Yu FY (2018b) Quantitative spatiotemporal oil body ultrastructure helps to verify the distinct lipid deposition patterns in benzoin endosperm and embryo cells. Forests 9:265
Zonuz N, Akpinar AE, Akpinar N, Dag S, Akpinar MA (2020) Fatty acids of the seed oils of three Achillea species. Chem Nat Compd 56:115–118
Acknowledgements
This work was supported by National Natural Science Foundation of China (3197140894), Joint Research Project Based on Cooperative Program for Bachelor of Science in Forestry by Nanjing Forestry University and University of British Columbia, A Project Funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD), and Postgraduate Research & Practice Innovation Program of Jiangsu Province (SJKY19_0882).
Author information
Authors and Affiliations
Contributions
CC and FY designed the research. HC, CH and ZL helped collect the samples. CC and HC carried the experiment and analyzed the data. CC wrote the manuscript. FY and QW revised the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Handling Editor: Andrjez Bajguz.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Chen, C., Chen, H., Han, C. et al. 24-Epibrassinolide and Methyl Jasmonate Promoted Seed Development of Styrax tonkinensis and Affected Seed Chemical Compositions, Especially Seed Lipid Metabolism. J Plant Growth Regul 42, 2162–2175 (2023). https://doi.org/10.1007/s00344-022-10689-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00344-022-10689-z