Abstract
Suaeda fruticosa and S. monoica are important halophytes for ecological rehabilitation of saline lands. We report differential physio-chemical, photosynthetic, and chlorophyll fluorescence responses in these halophytes under 100 mM sodium chloride (NaCl), 50% strength (16.25 ppt) of seawater (SW)-imposed salinity, and 10% polyethylene glycol 6000 imposed osmotic stress at 380 (ambient) and 1200 (elevated) µmol mol–1 CO2 concentrations. SW salinity enhanced the growth in both species; however, compared with S. fruticosa, the S. monoica exhibited comparatively better growth and biomass accumulation under saline conditions at elevated CO2. Results demonstrated better photosynthetic performances of S. monoica under stress conditions at both levels of CO2, and this resulted in higher accumulation of carbon, nitrogen, sugar, and starch contents. S. monoica exhibited improved antenna size, electron transfer at PSII donor side, and efficient working of photosynthetic machinery at elevated CO2, which might be due to efficient upstream utilization of reducing power to fix the CO2. The δ13C results supported the operation of C4 CO2 fixation in S. monoica and C3 or intermediate pathway of CO2 fixation in S. fruticosa. Lower accumulation of reactive oxygen species, reduced membrane damage, lowered solute potential, and higher accumulation of proline and polyphenol contents indicated elevated CO2-induced abiotic stress tolerance in Suaeda. Higher activity of antioxidant enzymes in both species at both levels of CO2 help plants to combat the oxidative stress. Upregulation of NADP-dependent malic enzyme and NADP-dependent malate dehydrogenase genes indicated their role in abiotic stress tolerance as well as photosynthetic carbon (C) sequestration. Operation of C4 type CO2 fixation in S. monoica and an intermediate CO2 fixation in S. fruticosa could be the possible reason for the superior photosynthetic efficiency of S. monoica under stress conditions at elevated CO2.
Similar content being viewed by others

Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Environmental stresses are the major threats to plant productivity (Ahammed et al. 2020a). Among various abiotic stresses, salinity and osmotic stress are the most common stresses and inter-related with each other in their physiological responses (Zhang et al. 2010). Salinity and osmotic stress negatively affect the plant development and result in lower productivity (Ahammed et al. 2018). Halophytes acquired excellent tolerance ability against salt and osmotic stress, and besides saline land reclamation, these can be exploited as cash crops and biomass producing crops (Rathore et al. 2016). Photosynthesis in plants is one of the major processes adversely affected by salinity and osmotic stress through reduction in CO2 fixation. CO2 is the key input for photosynthesis; therefore, varying CO2 concentrations have diverse effects on crop productivity (Sage and Coleman 2001; Ahammed et al. 2020b). The global climatic scenario is continuously changing and it is assumed that the level of CO2 will reach 1000 μmol mol−1 air by the end of this century, which will directly affect the photosynthesis (Li et al. 2019). Thus, the salinity and osmotic stress through reducing the soil fertility and the elevated CO2 (eCO2) with various effects on photosynthesis will become the major factors in determining the global crop productivity. One of the solutions under such conditions is the development of abiotic stress-tolerant crop having higher carbon (C) sequestration ability; however, it looks like a difficult solution. Another most promising approach is the use of halophytes that grow naturally under saline conditions and have better photosynthetic yield under abiotic stress conditions. Several halophytes find role as food, fodder, and in coastal protection, thus, possess potential for salty-land/wasteland reclamation (Giessler et al. 2009). The eCO2 up to certain levels improves the plant growth and productivity by increasing the CO2 fixation, and this enhances the tolerance against various abiotic stresses (Ainsworth and Rogers 2007; Pan et al. 2018). Thus, studying the effect of eCO2 in halophytes under abiotic conditions would be interesting and help to understand the mechanism involved in plant responses under interactive environment of eCO2 and abiotic stress.
The eCO2 increases the C assimilation, reduces stomatal conductance and N concentrations, and increases water-use efficiency (Ainsworth and Rogers 2007; Ellsworth et al. 2004; Morgan et al. 2011). The higher supply of CO2 in plants imparts certain level of stress tolerance through elevated photosynthesis (Zhang et al. 2020a, b). The eCO2 improves photosynthesis with strong stimulatory effect in C3 plants like Sorghum bicolor and Zea mays, whereas it has no effect on photosynthesis in C4 plants like Andropogon gerardii, Schizachyrium scoparium, and Sorghastrum nutans (Long et al. 2004). In Amaranthus and sugarcane, eCO2 alleviated the negative impact of mild water stress and had no effect under severe water stress (Ghannoum 2009). The improved survival of Aster tripolium L. at eCO2 under sodium chloride (NaCl) stress showed the positive impact of interactions between salinity and eCO2 on growth, photosynthesis, water relations, and chemical compositions in halophytes (Geissler et al. 2009, 2010). The higher CO2 leads to better water balance, reduction in stomatal conductance (gs), and improved osmotic potential in plants under saline stress; however, no significant biomass variations were recorded in Salicornia ramosissima, and this was attributed to the investment of higher energy for salinity stress defense mechanisms (Pérez-Romero et al. 2018). Atmospheric eCO2 enhanced the photosynthesis in C3 (Chenopodium quinoa) and C4 (Atriplex nummularia) halophyte; however, A. nummularia distinctly showed a higher level of salt resistance as compared to C. quinoa (Geissler et al. 2015). The CO2-concentrating mechanism in C4 plants supports their better survival under stress conditions through concentrating the CO2 around Rubisco, which minimizes the oxygenase activity and the resultant loss of carbon through photorespiration.
Suaeda fruticosa (L.) Forssk and S. monoica Forssk. ex J. F. Gmel are the two important halophytes exhibiting luxuriant growth under abiotic stress and possess potential of ecological rehabilitation of saline land. Suaeda genus possesses both C3 and C4 photosynthetic C fixation pathways with and without typical Kranz anatomy (Shomer-Ilan et al. 1975). The presence of Kranz anatomy was reported as an essential criterion for C4 photosynthetic operation; however, numerous literature reports demonstrated the functioning of C4 pathway in single-cell system or without typical Kranz anatomy (Park et al. 2009; Koteyeva et al. 2011; Shomer-Ilan et al. 1975). The C4 mode of CO2 fixation is comparatively efficient and advantageous over the C3 mode under hot, dry, and saline habitats. Salinity and osmotic stress primarily reduce the stomatal conductance and interfere with photosynthetic CO2-diffusion. The cost of photorespiration has been reported as the driving force behind the evolution of C4 photosynthesis (Sage 2004; Gowik and Westhoff 2011). Water stress severely affects C4 photosynthesis by reducing the intercellular CO2 concentrations; however, initially, the responses are not very harmful as the C4 pathway is capable of supplying CO2 through PEPC-medicated re-fixation of respiratory CO2 before escaping the bundle sheath. The abiotic stresses increase photorespiration as an adaptive response to maintain the availability of photosynthetic assimilates (Khatri and Rathore 2019).
The increasing atmospheric CO2, temperature, drought, and salinity strongly influence plant growth, productivity, habitat fragmentation, and C balance in a terrestrial ecosystem. The drought/osmotic and saline conditions are thought to suppress the photosynthetic activity in plants, and at the same time, eCO2 is expected to enhance the photosynthetic activity. Both C3 and C4 halophytes are well adapted to stress conditions; therefore, it would be interesting to evaluate the performance of S. fruticosa and S. monoica under abiotic stress at eCO2. Further, despite the progress in understanding the mechanism of plant responses to the eCO2, very few attempts have been made to study the effect of eCO2 in halophytes. Therefore, the focus of present study was to evaluate the interactive effect of salinity (NaCl and SW) and osmotic (polyethylene glycol-6000; PEG) stress with eCO2 (1200 µmol mol–1) on S. fruticosa and S. monoica to understand the physiological and photosynthetic responses involved in their survival during harsh conditions. The results would provide insights in understanding the mechanism involved in regulation of their growth, development, and biomass production in halophytes under abiotic stress conditions at eCO2. The results would help to choose the suitable species for biomass production through vegetation restoration in the degraded land.
Material and Methods
Plant Growth and Stress Treatment
Seeds of S. fruticosa and S. monoica were collected from mature plants growing along Bhavnagar seacoast (Gujarat, INDIA). Seeds were sterilized and germinated on sterile garden soil in 500 ml capacity plastic pots (each pot contained 350 g soil and made porous to avoid water-logging) at 30 ± 2 °C under diffused light conditions. The plants were irrigated with tap water. 7 days (d) old seedlings were transferred at 26 ± 1 °C for 12 h per day (hd–1) photoperiod of 1000 µmol m−2 s−1 photosynthetic photon flux density (PPFD) in a plant growth chamber (PGC-105, Percival, USA). The 6 weeks old and uniform plantlets were incubated at 380 [ambient (aCO2)] and 1200 (eCO2) µmol mol–1 CO2 concentrations in separate growth chambers and acclimatized for a week. In our previous observationks, at 1200 µmol mol–1 of CO2 in both species exhibited the highest photosynthesis rate (PN); therefore, this concentration was considered as eCO2. The plants (both at aCO2 and eCO2) were regularly irrigated with aqueous solution of 100 mM NaCl, 50% strength of SW salinity (16.25 ppt), and 10% PEG for 7 d to impose the salinity and osmotic stress. Tap water was used to irrigate the control set of plants. Fig. S1 depicted detailed experimental design. After 7 d, the photosynthetic gas exchange and chlorophyll fluorescence performances were recorded. The samples (from control and stress-treated plants) were harvested and stored at − 80 °C for subsequent physio-chemical analysis. A separate set of the experiment continued for 15 d to analyze the growth and morphological symptoms in plants.
Histological Observations, Determination of Plant Growth, and Water Contents
Fresh leaf samples of both S. fruticosa and S. monoica growing under greenhouse conditions (27 ± 2 °C temperature, 50–60% relative humidity, and ambient light) were cut transversely. The field emission scanning electron microscope (SEM; JSM-7100F, JEOL USA) using Cryo-GATAN facility was used to document the anatomical features. Morphological symptoms and growth in plants were recorded on 0th, 7th, and 15th d of stress treatments at both levels of CO2. The fresh/dry weight biomass (FW/DW) and root growth were recorded after 15th d of stress treatments. Uniform plant tissues of approximately 100 mg (FW) from apical portions of control and stressed plants were dried at 80 °C for 48 h in a hot air oven, and DW was recorded. The water content (WC; ml g–1 FW) was calculated as (FW − DW/FW of sample) and subsequently converted as % of FW.
Elemental and δ13C Analysis in Suaeda Species
Fresh leaf samples from plants growing in natural habitat were harvested, dried, and powdered. The C and N elements were analyzed using an elemental analyzer (vario MICRO cube, Elementar, Germany). The leaf samples were also analyzed for C and its stable isotope ratios (δ13C) using elemental analyzer (vario MICRO cube, Elementar, Germany)—isotope ratio mass spectrometry (IsoPrime100, Isoprime Ltd., UK) following Chaudhary et al. (2018). The system was calibrated using international standards from the International Atomic Energy Agency. The Pee Dee Belemnite (PBD) was used as standard C and isotopic signals were expressed as δ notation as δ13C ‰ = [(Rsample − Rstandard)/Rstandard] × 103; where R = ratio 13C:12C of the samples.
Determination of Ion Contents and Energy-Dispersive X-Ray Analysis
Oven-dried and pre-weighed samples were acid [perchloric acid and nitric acid solution (3:1 v/v)] digested and heated to dryness. The residue was dissolved in deionized water, and the ion contents (Na+ and K+) were estimated by inductive coupled plasma optical emission spectrometer (Optima2000DV, PerkinElmer, Germany). Alternatively, energy-dispersive X-ray (EDX) mapping was performed for Na+ and K+ contents. For EDX analysis, leaf was cut into thin sections and mounted on a stub. Samples were loaded in a scanning electron microscope (SEM; JSM-7100F, JEOL USA) equipped with a quantitative EDX machine. The vacuum was applied to the samples for 30 min, and, subsequently, the mapping was performed following the instructions provided in the machine manual.
Determination of Electrolyte Leakage and MDA Content
The fresh leaves (uniform) harvested from stressed and unstressed Suaeda plants were washed with deionized water and immersed in 10 ml deionized water in a set of closed vials. These were incubated at 26 °C on a gyratory shaker for 24 h. The electrical conductivity (EC) of the solution (EC1) was measured using a conductivity meter (SevenEasy, Mettler Toledo AG 8603, Switzerland). These samples were autoclaved at 121 °C for 15 min, cooled up to 26 °C, and EC (EC2) was measured. The electrolyte leakage (%EL) was calculated as (EC1/EC2) × 100.
The lipid peroxidation was determined by estimating the malondialdehyde (MDA) concentration (nmol g−1 FW) following Hodges et al. (1999). The samples were extracted with 0.1% trichloroacetic acid (TCA), and 0.2 ml extract was reacted with 0.8 ml of thiobarbituric acid (TBA) reagent (0.5% TBA in 20% TCA) and subsequently boiled at 95 °C for 30 min. Samples were ice cooled and centrifuged at 10,000 × g for 5 min, and absorbance was read at 440, 532, and 600 nm.
Determination of ROS Accumulation and Activity of Antioxidant Enzymes
Accumulations of reactive oxygen species (ROS) namely hydrogen peroxide (H2O2) and superoxide (O2¯) were determined in vivo in fresh leaf samples from apical portion of the shoots. Accumulation of H2O2 was detected by immersing the samples in 3,3-diaminobenzidine (DAB) solution (1 mg ml–1 in 10 mM phosphate buffer; pH 3.8) at room temperature (RT) for 12 h in dark, thereafter exposing the samples to intense light until the brown spots showing accumulation of H2O2 appeared. Accumulation of O2¯ was detected by immersing the fresh samples in nitro-blue tetrazolium (NBT) solution (1 mg ml−1 in 10 mM phosphate buffer; pH 7.8) at RT for 6 h, thereafter, exposing to high irradiance for 12 h until the blue spots showing accumulation of O2¯ appeared. Before documentation, the chlorophyll contents were bleached by ethanol washing.
The samples were ground in liquid nitrogen, and the total protein was extracted in protein extraction buffer [Tris buffer: 50 mM (pH 7.0), 1 mM EDTA, 0.05% (w/v) triton x-100, and 5% (w/v) polyvinylpolypyrrolidone]. Total protein in the extract was determined following Bradford method (Bradford 1976). This extract was used for determination of the activity of catalase (CAT), superoxide dismutase (SOD), and guaiacol peroxidase (GPOX). The SOD activity was determined by monitoring the inhibition of nitro-blue tetrazolium (NBT) reduction following Beyer and Fridovich (1987). The NBT reduction was recorded at 560 nm, and the amount of enzyme required for 50% inhibition of NBT was considered as one-unit activity. The CAT activity was determined by monitoring the disappearance of H2O2 (Miyagawa et al. 2000) and taking 43.6 M−1 cm−1 as an extinction coefficient (∆e) at 240 nm (Patterson et al. 1984). The GPOX activity was determined following Jebara et al. (2005) by reading absorbance at 470 nm and considering 26.6 mM−1 cm−1 as ∆e.
Determination of Solute Potential and Photosynthetic Pigments
Fresh leaf tissues were frozen in liquid nitrogen, thawed, and centrifuged for 10 min at 10,000 × g to extract the sap. The ionic strength of the sap was measured using Vapro Pressure Osmometer (model-5600; Wescor, Logan UT, USA). Solute potential (Ψs) of sap was calculated as − nRT/V, where n represents numbers of solute molecules; R represents the universal gas constant; T represents temperature in K, and V is volume in liter.
Leaf samples (200 mg FW) were ground in liquid nitrogen and homogenized in 1 ml of N,N-dimethylformamide. After 30 min of incubation under dark conditions, the homogenate was centrifuged at 10,000 rpm for 15 min at 4 °C. The absorbance of the supernatant was recorded at 461, 647, 664, and 664.5 nm. The contents of photosynthetic pigments were calculated following Inskeep and Bloom (1985) and Chamovitz et al. (1993).
Photosynthetic Gas Exchange Measurement
The photosynthetic gas exchange was recorded using a conifer chamber (6400-05 LCF, Li-Cor) attached to an infrared gas analyzer (LI-6400XT; Li-Cor, Lincoln, NE, USA). The plants under natural habitat exhibited the highest rate of photosynthesis at 1000 µmol m–2 s–1 PPFD (data not given); therefore, gas exchange was recorded at this intensity. Data were recorded on plants (10 replicates per stress treatment and 10 readings per replicate, n = 100) acclimatized to conifer chamber conditions for 20–30 min. Reads were recorded at 1000 µmol m–2 s–1 PPFD, 380 or 1200 µmol mol–1 CO2, 26 ± 0.5 °C block temperature, and 60 ± 5% RH. The net photosynthesis rate (PN; µmol CO2 m–2 s–1), stomatal conductance (gs; mol H2O m–2 s–1), intercellular CO2 concentration (Ci; µmol CO2 mol air–1), transpiration rate (E; mmol H2O m–2 s–1), ratio of Ci to available CO2 concentration (Ci/CA), and vapor pressure deficit (VpdL) were determined. The water-use efficiency (WUE; µmol CO2 mmol–1 H2O) was calculated as the ratio of PN and E.
Chlorophyll a Fluorescence Measurement
Chlorophyll a fluorescence was recorded using Handy Plant Efficiency Analyser (HPEA; Hansatech Instruments, UK). Measurements were recorded at RT on adaxial surface of the intact leaves through continuous excitation with high time resolution to investigate the rapid fluorescence induction (Oukarroum et al. 2015). Before measurement, the plants were dark adapted for 45 min using dark-adaptation facilities. Leaves were illuminated with saturating red light of 3000 µmol (photon) m–2 s–1 to close PSII reaction centers (RC) completely, and fluorescence signals were recorded for 1.0 s on a 4 mm diameter area of the dark-adapted leaves. The fluorescence detector used 730 ± 15 wavelength of 3000 µmol (photon) m–2 s–1 intensity. The instrument recorded prompt fluorescence (PF) when actinic light was on (light interval) and off (dark interval), respectively. The data were downloaded into PEA Plus software (version 1.10) and analyzed using its data analysis module. Based on chlorophyll a fluorescence transient various photosynthetic fluxes viz. plastoquinone pool size (area), minimal fluorescence (F0), maximum fluorescence (FM), variable fluorescence (FV), basal quantum yield of non-photochemical processes in photosystem II (F0/FM), maximum quantum efficiency of PSII (FV/FM), activity of the water-splitting complex (FV/F0), absorption per RC (ABS/RC), electron transport flux per PSII RC (ET0/RC), energy trapped in PSII RC (TR0/RC), energy dissipated from PSII (DI0/RC), and photochemical and non-photochemical de-excitation rate constant (kP and kN) were studied. The OJIP curve was plotted, and relative rise was interpreted as effects of the stress conditions on transfer of electron through PSII and PSI in plants under stress at aCO2 and eCO2. Amplitudes of I–P phase representing the ratio of PSII and PSI [δFIP = (FP − FI)] and electron transport (δVIP) around PSI to reduce final (i.e., ferredoxin and NADP) acceptors [δVIP = (Fm − F30ms)/(Fm − F0)] were determined following Schansker et al. (2005) and Khatri and Rathore (2019).
Determination of Proline, Sugar, Starch, and Polyphenol Contents
Proline contents from fresh leaf samples were extracted in 3.0% sulphosalicylic acid. The extract was reacted with ninhydrin reagent, and absorbance was read at 520 nm following Bates et al. (1973). Soluble sugar from samples was extracted repeatedly in 80% ethanol and estimated following anthrone-sulphuric acid method. The residual pellet left after extract of the soluble sugar was digested in 52% perchloric acid for starch estimation. The digest was diluted with milliQ water and processed as that of sugar estimation. Glucose was used as standard, and absorbance was read at 630 nm. For starch determination, the obtained values were multiplied by a factor of 0.9 to convert the glucose values into starch. Polyphenol contents were estimated following Chandler and Dodds (1983) by recording the absorbance at 650 nm against a standard curve prepared with catechol.
Determining the Expression of C4 Photosynthetic Pathway Genes
Total RNA was extracted from leaf samples following the GITC method (Chomczynski and Sacchi 1987). The cDNA was prepared using total RNA as template and a Superscript II RT first-strand cDNA synthesis kit (Promega, Madison, Wisconsin). Expression of the NADP-me (F5′-TGCCCATACCCCTTGAT-3′ and R5′-TTGGCAAAATCTTCGAACT-3′) and NADP-mdh (F5′-GCTTGCTTCTGGTGTGGT-3′ and R5′-CAATCAGAATGGCCCACT-3′) genes was determined using real-time PCR (RT-PCR). The tubulin (F5′-CACGCGCTGTATTCGTAGAT-3′ and R5`-TGACCACGAGCGAAGTTATTAG-3′) gene was used as an internal control. RT-PCR was performed using 1 × Sso Advanced SYBR green supermix (Bio-Rad). qRT-PCR conditions comprised denaturation at 94 °C for 5 min for 1 cycle; 40 cycles of each denaturation at 94 °C for 30 s (sec); annealing at 55 °C for 30 s; and extention at 72 °C for 30 s. Relative-fold expression was calculated using following Livak and Schmittgen (2001).
Statistical Analysis
The experiment was performed in a randomized block design with minimum 03 replicates (each replicate with 3 sub-replicates; n = 9) for physio-chemical estimations and with 10 replicates (each replicate with 10 sub-replicates; n = 100) for measurment of gas exchange and chlorophyll fluorescence. Data recorded were subjected to ANOVA for analysis of variance to determine the significance among mean values of the treated and control plants (Supplementary Table S1). Post-hoc multiple comparison of mean values was carried out following Tukey’s test at a significance level of p ≤ 0.05. To construct the radar plot (Fig. 9), the chlorophyll a fluorescence reads of control plants (under both levels of CO2) were considered as standard and the reads of stress-treated plants were calculated as relative values. The data are presented as mean ± standard error (SE) and significantly different mean values have been denoted by different lowercase letters.
Results
The eCO2 had differential effects on photosynthetic C capture potential, ion accumulation, physio-biochemical and growth responses in S. fruticosa and S. monoica under salt (100 mM NaCl and 50% strength of SW) and osmotic (10% PEG) stress. Leaf anatomy in both species showed differentiation of ground tissues as palisade and spongy mesophylls (Fig. 1a, b); however, none of these possess bundle sheath or Kranz anatomy (Fig. 1c, d).
Effect of eCO2 on Growth and Water Content in S. fruticosa and S. monoica
S. fruticosa exhibited improved growth under saline conditions and S. monoica exhibited improved growth under seawater salinity (Fig. S2) at aCO2. Similarly, both species showed improved growth at eCO2; however, the growth in S. monoica was comparatively better as compared to S. fruticosa as the eCO2 induced the epinastic symptoms in S. fruticosa leaves (Fig. S3). Similar to shoot growth, both species showed improved root growth under salinity stress at both levels of CO2. At eCO2, the SW salinity significantly improved the root growth in both species (Fig. S4). S. fruticosa and S. monoica yielded FW and DW in agreement with growth (Fig. S5a, b). At aCO2, the biomass accumulation in S. fruticosa was comparatively higher than S. monoica under saline conditions. The eCO2 improved the biomass yield in both species under SW salinity. Compared with aCO2 condition, both species exhibited lower WC under eCO2. Compared with control treatment, S. fruticosa maintained higher WC under stress conditions at eCO2, and S. monoica did not exhibit significant changes in WC (Fig. S5c). Both species exhibited poor growth attributes and WC under PEG stress. The interaction among Suaeda species, CO2 levels, and stress type significantly influenced the plant growth, fresh and dry biomass yield, WC, and root growth.
Effect of eCO2 on Carbon and Nitrogen Assimilation in Suaeda Species
Both species exhibited no significant variations in C contents under control and stress conditions at aCO2. The eCO2 reduced the C contents in S. fruticosa under stress conditions; however, S. monoica exhibited no significant variations (Fig. 2a). S. fruticosa showed comparatively lower accumulation of N contents, and S. monoica exhibited higher accumulation of N contents under saline conditions at eCO2 (Fig. 2b). The interaction among Suaeda species, CO2 levels, and stress treatments had significant influence over the accumulation of C and N contents. S. fruticosa and S. monoica exhibited the stable isotope ratio (δ13C) as − 21.44 and − 15.62, respectively.
Carbon (a), and nitrogen (b) contents in S. fruticosa and S. monoica under control and stress (100 mM NaCl, 50% seawater salinity and 10% PEG) treatments at ambient and elevated CO2 condition for 7 days. The values (mean ± SE, n = 3) followed by different lowercase letters as superscripts are significantly different by LSD (≥ 0.05%) at a particular CO2 level. In figure, SW represents seawater salinity, SF S. fruticosa, and SM S. monoica
Effect of eCO2 on Ion Accumulation and Solute Potential in Suaeda Species
Both species accumulated higher contents of Na+ and lower contents of K+ under stress conditions (Fig. 3b, c). The eCO2 significantly reduced accumulation of Na+ and K+ in both species under stress. The EDX-SEM observations confirmed the higher accumulation of Na+ and lower accumulation of K+ in leaf tissues and on root surface of both species under saline condition (Fig. S6). In agreement with ion accumulation, S. fruticosa and S. monoica exhibited significantly lower Ψs (more negative values) under stress condition at both levels of CO2 (Fig. 3a). The decrease in Ψs under stress condition was comparatively higher in both species at aCO2 than eCO2. The interactions among Suaeda species, CO2 levels, and stress type significantly influenced the Ψs and accumulation of ions.
Solute potential (a) and accumulation of sodium (b) and potassium (c) contents in leaves of S. fruticosa and S. monoica under control and stress (100 mM NaCl, 50% seawater salinity, and 10% PEG) treatments at ambient and elevated CO2 condition for 7 days. The values (mean ± SE, n = 3) followed by different lowercase letters as superscripts are significantly different by LSD (≥ 0.05%) at a particular CO2 level. In figure, SW represents seawater salinity, SF S. fruticosa, and SM S. monoica
Effect of eCO2 on ROS Accumulation and Membrane Properties in Suaeda Species
Stress induces accumulation of O2– and H2O2 in S. fruticosa and S. monoica at both levels of CO2. NaCl stress caused comparatively higher accumulation of O2– (Fig. 4a). S. fruticosa exhibited higher accumulation of H2O2 under stress at eCO2 while S. monoica exhibited higher accumulation at aCO2 (Fig. 4b). S. monoica exhibited higher accumulation of O2– and H2O2 under NaCl stress at both levels of CO2. At eCO2, S. monoica exhibited similar accumulation of ROS under control and saline condition. At eCO2, S. monoica under PEG stress exhibited higher accumulation of superoxide and lower accumulation of peroxide radicals.
In vivo detection of superoxide radials by NBT assay (a) and peroxide by DAB assay (b) in shoot apical portions of S. fruticosa and S. monoica grown under control and stress (100 mM NaCl, 50% seawater salinity, and 10% PEG) treatments at ambient and elevated CO2 conditions for 7 days. In figure, the C represents control, N NaCl, SW seawater salinity, and P PEG
S. monoica exhibited lower MDA contents under stress condition at aCO2. Stress increased the accumulation of MDA in S. fruticosa at both levels of CO2 except PEG stress at eCO2. S. monoica exhibited lower contents of MDA under stress condition at aCO2. In contrary to aCO2, the eCO2 significantly increased the MDA accumulation in S. monoica under 50% SW. The eCO2 increased MDA content in S. fruticosa and S. monoica under NaCl and SW stress, respectively (Fig. 5a). The eCO2 increased the EL in S. fruticosa under stress; however, S. monoica exhibited lower EL at both levels of CO2 under stress. NaCl stress caused higher EL than SW stress in both species at aCO2 (Fig. 5b). The eCO2 significantly reduced the EL in S. monoica under salinity stress compared with osmotic stress. The interaction among Suaeda species, CO2 levels, and stress type had a significant effect on MDA accumulation and EL.
MDA contents (a) and electrolyte leakage (b) in leaves of S. fruticosa and S. monoica under control and stress (100 mM NaCl, 50% seawater salinity, and 10% PEG) treatments grown at ambient and elevated CO2 condition for 7 days. The values (mean ± SE, n = 3) followed by different lowercase letters as superscripts are significantly different by LSD (≥ 0.05%) at a particular CO2 level. In figure, SW represents seawater salinity, SF S. fruticosa, and SM S. monoica
Effect of eCO2 on Expression of C4 Pathway Genes and Activity of Antioxidant Enzymes
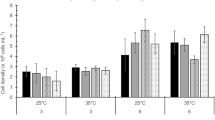
S. fruticosa and S. monoica exhibited up-regulation of NADP-me and NADP-mdh gene at both levels of CO2 under stress (Fig. 6). Both species showed the highest (more than sixfold changes) expression of NADP-me and NADP-mdh under SW salinity at eCO2. Stress induced the up-regulation of NADP-me and NADP-mdh gene in both species at both levels of CO2. Compared with aCO2, the eCO2-induced up-regulation of both genes in S. monoica under PEG stress.
Gene expression profiling of NADP-dependent malic enzyme (me) and malate dehydrogenase (mdh) in S. fruticosa (a) and S. monoica (b) under control and stress (100 mM NaCl, 50% seawater salinity, and 10% PEG) treatments at ambient and elevated CO2 condition for 7 days. The values (mean ± SE, n = 3) followed by different lowercase letters as superscripts are significantly different by LSD (≥ 0.05%) at a particular CO2 level. In figure, SW represent seawater salinity, SF S. fruticosa, SM S. monoica, ME malic enzyme, and MDH malate dehydrogenase
At aCO2, both species exhibited higher activity of SOD and CAT under stress; however, GPOX exhibited higher activity only under NaCl stress. Plants of both species under control treatment exhibited higher activity of SOD, CAT, and GPOX at eCO2 as compared to aCO2 (Fig. 7). Similarly, both species have higher antioxidant enzymes activity under stress condition at eCO2; however, S. fruticosa showed lower activity of CAT under stress condition. Difference in the activity of these enzymes varied with types of stress and plant species.
Activity of superoxide dismutase (a), catalase (b), and guaiacol peroxidase (c) in S. fruticosa and S. monoica under control and stress (100 mM NaCl, 50% seawater salinity and 10% PEG) treatments at ambient and elevated CO2 for 7 days. The values (mean ± SE, n = 3) followed by different lowercase letters as superscripts are significantly different by LSD (≥ 0.05%) at a particular CO2 level. In figure, SW represents seawater salinity, SF S. fruticosa, and SM S. monoica
Effect of eCO2 on Photosynthetic Pigments in Suaeda Species
S. fruticosa exhibited lower accumulation of chlorophyll and carotenoid pigments under stress condition except for NaCl stress at aCO2. The eCO2 improved the accumulation of photosynthetic pigments in S. fruticosa under stress condition (Fig. S7a, b); however, under NaCl stress, increase in the pigments was not statistically significant. The aCO2 and eCO2 have no significant effect on accumulation of total chlorophyll in S. monoica under stress condition. S. fruticosa showed higher accumulation of carotenoids under NaCl at aCO2 and under stress condition at eCO2 (Fig. S7b). S. monoica accumulated significantly higher content of carotenoids under PEG stress at aCO2 and under stress condition at eCO2 (Fig. S7b). Suaeda species, CO2 levels and stress type showed significant interaction for the accumulation of total chlorophyll and carotenoid contents.
Effect of eCO2 on Photosynthesis in Suaeda Species
The CO2 levels and stress types had differential effects on photosynthetic gas exchange in S. fruticosa and S. monoica. SW salinity improved the PN in S. fruticosa at both level of CO2; however, the increase was not significant. PEG and NaCl stress decreased the PN in S. fruticosa at both level of CO2. Salinity stress improved the PN in S. monoica at both levels of CO2; however, the improvement was only significant under SW salinity at eCO2. Compared with control plants, both species exhibited lower PN under PEG stress at both levels of CO2 (Fig. 8a); however, the reduction in PN was significant only at aCO2. S. fruticosa showed significantly higher gs under SW salinity and lower gs under PEG stress at aCO2. There were no significant changes in gs under stress at eCO2. S. monoica showed higher gs under stress conditions at aCO2 and lower gs under stress conditions at eCO2 except NaCl stress (Fig. 8b). S. fruticosa showed lower Ci under stress conditions at aCO2, while S. monoica showed lower Ci under stress conditions at eCO2 except NaCl stress. Compared with plants under control treatment, the S. fruticosa had higher Ci under stress condition at eCO2 and S. monoica had higher Ci under stress condition at aCO2 (Fig. 8c). S. fruticosa and S. monoica exhibited E in a trend as that of gs (Fig. 8d). S. fruticosa exhibited lower WUE under stress at both levels of CO2. S. monoica had lower WUE under stress at aCO2. S. monoica under stress showed WUE comparable to control or higher than control at eCO2. Both species exhibited Ci/CA in a trend as that of Ci. Both species showed VpdL almost comparable in plants under control and stress conditions at both levels of CO2 (Fig. S8a–c). Stress treatments and their interaction with CO2 levels had a significant effect on PN and WUE. Similarly, interaction among Suaeda species, CO2 levels, and stress treatments had a significant effect on gs, Ci, E, Ci/CA, and VpdL.
Net photosynthesis rate (a), stomatal conductance (b), intercellular co2 concentration (c) and transpiration (d) in leaves of S. fruticosa and S. monoica under control and stress (100 mM NaCl, 50% seawater salinity and 10% PEG) treatments at ambient and elevated CO2 for 7 days. The values represent mean ± SE (n = 3) and followed by different letters as superscripts are significantly different by LSD (≥ 0.05%) at a particular CO2 level. In figure, SW is seawater salinity, SF S. fruticosa, and SM S. monoica
Effect of eCO2 on Chlorophyll a Fluorescence in Suaeda Species
S. fruticosa and S. monoica showed variable chlorophyll a fluorescence transient, chlorophyll fluorescence parameter, and different photosynthetic flux under salinity and PEG stress at aCO2 and eCO2 (Fig. 9a–d). Under stress, S. fruticosa showed higher pool size of electron acceptors (QA) on reducing side of PSII (area) at aCO2 and lower at eCO2. In contrary, S. monoica showed reduced area at aCO2 and improved area at eCO2 under stress. Both species showed higher F0 under stress at both levels of CO2 except NaCl stress in S. fruticosa and SW salinity in S. monoica at aCO2. S. monoica exhibited lower FM and FV under stress at aCO2. S. fruticosa at both levels of CO2 and S. monoica at eCO2 exhibited higher FM and FV under stress. S. fruticosa exhibited lower F0/FM under stress at both levels of CO2. S. monoica had lower estimates of F0/FM under NaCl and PEG stress at eCO2. S. fruticosa at aCO2 and S. monoica at eCO2 yielded higher estimates of FV/FM and FV/F0 under stress. In contrary, S. monoica at aCO2 and S. fruticosa at eCO2 yielded lower estimates of FV/FM and FV/F0 under stress. S. fruticosa under stress showed higher FV/F0 at aCO2 and lower at eCO2. S. monoica yielded lower estimates of FV/FM under stress at aCO2 and under SW salinity at eCO2. Both plant species showed higher ABS/RC, DI0/RC, and TR0/RC under stress at both levels of CO2. S. fruticosa had higher ET0/RC under stress at both levels of CO2, while S. monoica at aCO2. Contrary to S. fruticosa, the S. monoica showed lower ET0/RC under stress at eCO2 level. S. fruticosa showed higher KP/ABS, and S. monoica showed lower KP/ABS under stress at eCO2.
Chlorophyll a fluorescence derived parameters and fluxes in S. fruticosa and S. monoica under control and stress (100 mM NaCl, 50% seawater salinity and 10% PEG) treatments at ambient (a, b) and elevated CO2 (c, d) for 7 days. The values represent mean ± SE (n = 10) and followed by different letters as superscripts are significantly different by LSD (≥ 0.05%) at a particular CO2 level. In figure, SW is seawater salinity. In picture, the area represents plastoquinone pool, F0—minimal fluorescence, FM—maximum capacity for photochemical quenching, FV—variable fluorescence, F0/FM—basal quantum yield of non-photochemical processes in PSII, FV/FM—maximum quantum efficiency of PSII, FV/F0—activity of the water-splitting complex, ABS/RC—absorption per RC, ET0/RC—electron transport flux per PSII RC, TR0/RC—energy trapped in PSII RC, DI0/RC—energy dissipated from PSII, and kP and kN—photochemical and non-photochemical de-excitation rate constant
OJIP transient curves based on PF data of S. fruticosa and S. monoica under stress at aCO2 and eCO2 are presented in Fig. S9. S. fruticosa showed lower relative rise in O to J phase under stress at aCO2 and exhibited the lowest rise in plants under PEG stress. S. monoica showed the highest relative rise in O to J phase under SW salinity stress at aCO2 and followed by unstressed plants. S. fruticosa showed the lowest relative rise in O to J phase under PEG stress at eCO2. S. monoica showed the highest relative rise in O to J phase under PEG stress at eCO2 and plants under SW and NaCl treatments followed this. S. fruticosa had higher δFIP under stress at aCO2. At eCO2, it showed significantly lower δFIP under stress except SW salinity as compared to control plants (Fig. S10a). S. monoica did not exhibit a significant change in δFIP under stress at aCO2, while at eCO2, it showed a significant increase in δFIP under saline condition. S. fruticosa showed a significantly lower δVIP under PEG stress at aCO2. S. monoica did not exhibit changes in δVIP under stress at aCO2. Both species exhibited significantly higher δVIP under SW salinity and PEG stress at eCO2 (Fig. S10b).
Effect of eCO2 on Accumulation of Sugar, Starch, Proline, and Polyphenol
Saline conditions induced accumulation of sugar in S. fruticosa at aCO2. The eCO2 lowered the accumulation of soluble sugar in S. fruticosa under stress except NaCl treatment. S. fruticosa showed higher accumulation of total sugar under saline conditions at aCO2; however, S. monoica exhibited lower accumulation under stress. S. monoica showed lower accumulation of soluble sugar under stress at eCO2 except SW salinity (Fig. 10a); however, reduction was not statistically significant. S. fruticosa accumulated higher contents of starch under stress at aCO2, while accumulation decreased at eCO2. S. monoica exhibited lower accumulation of starch under SW and PEG stress at aCO2, while it exhibited significantly higher accumulation under SW salinity at eCO2 (Fig. 10b). S. fruticosa exhibited higher accumulation of proline and polyphenol contents under stress at both levels of CO2 (Fig. 10c, d). The eCO2 increased the proline accumulation in S. monoica under saline condition at aCO2, and all the imposed strengths of stresses at eCO2. The polyphenol accumulation increased in S. monoica under saline condition at aCO2 and under SW and PEG stress at eCO2. The interaction among Suaeda species, CO2 levels, and stress type had a significant effect on the accumulation of total soluble sugar, starch, proline, and polyphenol contents.
Accumulation of soluble sugar (a), starch (b), proline (c), and polyphenol (d) contents in S. fruticosa and S. monoica under control and stress (100 mM NaCl, 50% seawater salinity and 10% PEG) treatments at ambient and elevated CO2 condition for 7 days. The values (mean ± SE, n = 3) followed by different lowercase letters as superscripts are significantly different by LSD (≥ 0.05%) at a particular CO2 level. In figure, SW represents seawater salinity, SF S. fruticosa, and SM S. monoica
Discussion
The eCO2 induced differential growth, physio-chemical, and photosynthetic responses in S. fruticosa and S. monoica under 100 mM NaCl/50% strength of SW/10% PEG-induced stress (Supplementary Table S2). The leaf anatomy clearly demonstrated the absence of typical Kranz anatomy in both species indicating it as a non-essential criterion in determining the type of C fixation in Suaeda species. The δ13C estimates supported the operation of C4 mode of CO2 fixation in S. monoica (Shomer-Ilan et al. 1975; Voznesenskaya et al. 2002) and C3 or intermediate pathway of CO2 fixation in S. fruticosa (Stutz et al. 2014). The δ13C estimates indicated the efficiency of CO2 fixation in Suaeda. The better growth, eco-physiological responses, and absence of the epinastic symptoms indicated higher photosynthetic efficiency in S. monoica at eCO2 and better utilization of CO2.
Stress induces accumulation of inorganic solutes and synthesis of different compatible solutes. The ionomic analysis clearly showed a higher accumulation of Na+ and lower accumulation of K+ under saline stress (Haque et al. 2017; Rathore et al. 2019). The accumulated inorganic solutes contribute to the osmotic adjustment after compartmentalization to avoid the cellular toxicity (Haque et al. 2017; Keisham et al. 2018). Lower solute potential helped plants to conserve and efficiently utilize the available water under physiological drought (Kumari et al. 2017; Rathore et al. 2019). The saline condition induced growth, and this indicated requirement of salts for optimum growth in Suaeda species (Haque et al. 2017; Rathore et al. 2019; Jacob et al. 2020). In consonance with present results, 100–300 mM NaCl salinity did not cause injuries in S. glauca and plants grew better (Jin et al. 2016). Similarly, the eCO2 improved the growth and biomass accumulation in both C3 and C4 species of Atriplex, Phaseolus, Xanthium, and Zea mays under saline condition (Schwarz and Gale 1983; Li et al. 2014). These results indicated the operation of an efficient mode of CO2 fixation in S. monoica as compared to S. fruticosa (Schwarz and Gale 1983), and this resulted in superior performance of S. monoica under stress at eCO2.
Stress caused oxidative damages through ROS accumulation adversely affects the physiology of plants and damages the photosynthetic machinery, thus, resulting in reduced growth (Ahammed et al. 2020c). The higher activity of antioxidant enzymes in both species under stress sequestered the ROS and combated the oxidative stress. Lower MDA accumulation and EL in S. monoica under stress condition at both levels of CO2 clearly indicated a lower degree of ROS-induced membrane damages, which indicated improved efficiency of the photosynthetic machinery. In consonance with present results, the eCO2 significantly curtailed the accumulation of ROS and MDA (Singh and Agrawal 2015). Lower accumulation of ROS might be a result of efficient utilization of light energy with available CO2 which otherwise generates free radicals and damage the membrane integrity, photosynthetic machinery, and PSII system. The eCO2-induced reduction in accumulation of ROS and MDA and the degree of EL are the indicators of efficient growth performances in S. monoica under stress. The present results clearly indicated role of eCO2 in effective management of ROS, better acclimatization, and efficient photosynthetic responses in S. monoica as compared to S. fruticosa under stress.
Photosynthetic pigments are visual indicators of photosynthetic performance in plants. Higher contents of photosynthetic pigments contribute efficient harvesting of light energy required for fixation of the available CO2 in photosynthesis (Ahammed et al. 2020c). The carotenoids sequester the free radicals (ROS) and acts as stress signaling molecule (the β-cyclocitral, an oxidation product of β-carotene) to induce the gene expression leading stress acclimation (Havaux et al. 2014). Compared with S. fruticosa, the S. monoica exhibited efficient photosynthetic CO2 fixation (PN) under stress indicating the operation of efficient CO2 fixation. The higher PN, lower Ci, and gs in S. monoica under stress indicated better C utilization (Ueno et al. 2006). The efficient CO2 fixation in S. monoica helped to maintain better PN under lower estimates of gs, Ci, and E at eCO2 under stress. Vice versa, results in S. fruticosa indicated the operation of an intermediate CO2 fixation pathway, which might not be as efficient as that of S. monoica. The eCO2 increased the WUE in S. monoica under SW and PEG stress, which helped in water conservation and adaptation under physiological drought. In consonance with present results, the eCO2 improved the photosynthesis in Aster tripolium and Suaeda species (Rozema et al. 1991; Yadav et al. 2018). Compared with S. fruticosa, the eCO2 helped in accumulation of higher contents of C in S. monoica; however, the N contents were lower. The accumulation of compatible solutes and their role in osmotic adjustment and sequestration of free radicals have been reported during stress tolerance in halophytes (Hong et al. 2000; Ashraf and Harris 2004; Moghaieb et al. 2004; Song et al. 2006; Ksouri et al. 2007; Lokhande et al. 2011; Haque et al. 2017; Rathore et al. 2019; Jacob et al. 2020). The higher content of starch in S. monoica at eCO2 indicated the availability of sugar as raw material for starch synthesis, and this might be due to eCO2-induced efficient photosynthesis. Up-regulations of SmNADP-me and SmNADP-mdh genes indicated operation of C4 pathway and involvement of these genes in stress tolerance probably through enhancing the photosynthetic assimilates (Schwarz and Gale 1983; Wheeler et al. 2005; Rondeau et al. 2005). Expression of PEPC and PPDK enzymes have already been reported higher in Suaeda species under abiotic stress and eCO2, respectively (Cheng et al. 2016; Yadav et al. 2018). Present results clearly indicated the role of eCO2 in betterment of photosynthetic responses in S. monoica under stress. Compared with S. fruticosa, photosynthetic gas exchange measurements clearly indicated the better photosynthetic C-sequestration potential in S. monoica under stress at both levels of CO2.
The CO2 and stress induced variable changes in chlorophyll a fluorescence, photosynthetic fluxes, and OJIP transient in S. fruticosa and S. monoica. Under stress, eCO2 improved the pool size in S. monoica indicating the efficient electron transfer at PSII donor side. The reduced pool size in S. fruticosa at eCO2 indicated blockage of electron transfer from RC to quinone pool in S. fruticosa (Mehta et al. 2010; Khatri and Rathore 2019). Higher minimal fluorescence (F0) indicated damage to the PSII (Bussotti et al. 2011) and heat dissipation in an uncontrolled manner resulting over excitation of RC in both species under stress. The higher F0 and ABC/RC indicated improved antenna size with RCs, and this might be due to higher contents of photosynthetic pigments. The eCO2-induced increase in photochemical quenching (FM) indicated effective electron transport at PSII donor side and improved pool size of QA which supported the efficient utilization of accumulated P680+ (Govindjee 1995) in both species and resulted in lower non-photochemical quenching. The lower estimate of F0/FM in S. monoica under stress indicated eCO2-induced reduction in non-photochemical quenching (Ranjbarfordoei et al. 2006). These results indicated better abiotic stress tolerance in S. monoica at eCO2. The higher FV/FM indicated efficient working of the photosynthetic machinery in S. fruticosa at aCO2 and in S. monoica at eCO2 under stress conditions (Salvatori et al. 2014; Khatri and Rathore 2019). FV/FM indicated better photosynthetic functioning of PSII RCs in S. monoica at eCO2 under stress conditions. The water availability, pigment contents, and active and inactive RCs significantly influence the ABC/RC and F0 under which indicated the improved antenna size (Misra et al. 2001). A higher number of inactive RCs contribute dissipation of heat energy, thus, higher DI0/RC (Mathur et al. 2013). The eCO2 helps S. monoica to maintain higher number of active RCs. The higher TR0/RC indicated inefficiency of re-oxidation of reduced QA– to QB resulting in loss of energy as dissipation (Mathur et al. 2013). Besides higher estimates of TR0/RC and DI0/RC, in the present case, the higher ABC/RC corresponded efficient photosynthesis, and this clearly indicated efficient light harvest by RCs and its downstream utilization for CO2 fixation. Saline condition has been reported to have differential effects on electron transport (ET0/RC) flux (Mathur et al. 2013; Demetriou et al. 2007; Khatri and Rathore 2019). Stress-imposed inactivation of RCs might be a reason for lower ET0/RC in S. monoica under stress conditions at eCO2 (Mehta et al. 2010). The rise in O to J phase in OJIP transient curves clearly showed a better reduction of QA by PSII in both species at aCO2. The relative rise in O to J phase indicated better reduction of QA by PSII RCs in S. monoica at eCO2 and under 10% PEG, seawater and NaCl treatments. The δFIP in S. fruticosa at aCO2 and in S. monoica at eCO2 under stress conditions indicated better ratio of PSII and PSI (Schansker et al. 2005). The δVIP in S. monoica under stress at eCO2 indicated improved electron transport through PSI for reduction of final acceptors, i.e., ferredoxin and NADP (Schansker et al. 2005). Chlorophyll a fluorescence indicated comparatively reduced biophysical performances of photosynthetic system in S. fruticosa under stress, while S. monoica exhibited operation of an efficient photosynthetic system under stress conditions at both levels of CO2.
The present results clearly indicated that the photosynthetic C fixation under stress directly influences the stress tolerance. The eCO2 improved the growth and biomass accumulation in both species under stress; however, the performance of S. monoica was better as compared to S. fruticosa under eCO2. The eCO2-induced C and N assimilation in S. monoica under stress clearly indicated efficient functioning of photosynthetic machinery. The results demonstrated the efficient functioning of C concentration mode in S. monoica, which might be the major reason for eCO2-induced better physio-chemical and photosynthetic responses in S. monoica. The present results clearly indicated S. fruticosa and S. monoica as potential halophytes with differential photosynthetic and physio-chemical responses for reclamation of saline land through vegetation restoration for biomass production. Further S. monoica exhibited superior responses; thus, under increasing atmospheric CO2 condition, this would be the plant of choice. The study would be helpful in designing the management strategies to combat global climate changes in degraded land through vegetation restoration using halophytes.
Conclusion
S. fruticosa and S. monoica are important halophytes, and leaf histology does not differentiate CO2 fixation mode in these halophytes. The stable isotope ratio supported the operation of C3 or intermediate CO2 fixation pathway in S. fruticosa. S. monoica exhibited better photosynthetic gas exchange and a lower degree of ROS-induced damages under abiotic stress at eCO2. S. monoica under stress exhibited comparatively better photosynthetic pool size, maximum photosynthetic potential of PSII, water splitting, basal quantum yield of non-photochemical processes in PSII, light absorption, heat dissipation, and maximum electron transport flux at both levels of CO2. Assimilation of C and N supported efficient photosynthetic C-sequestration in S. monoica as compared to S. fruticosa. Expression analysis of C4 pathway genes under stress and at eCO2 suggested their involvement in stress tolerance. Overall, the eCO2 induced differential responses in these species under stress, and S. monoica responded comparatively better due to sufficient availability of photosynthetic assimilates because of its effective C-sequestration potential.
References
Ahammed GJ, Li Y, Li X, Han WY, Chen S (2018) Epigallocatechin-3-gallate alleviates salinity-retarded seed germination and oxidative stress in tomato. J Plant Growth Regul 37:1349–1356
Ahammed GJ, Li X, Liu A, Chen S (2020a) Brassinosteroids in plant tolerance to abiotic stress. J Plant Growth Regul 39:1451–1464
Ahammed GJ, Li X, Liu A, Chen S (2020b) Physiological and defense responses of tea plants to elevated CO2: a review. Front Plant Sci 11:305
Ahammed GJ, Li CX, Li X, Liu A, Chen S, Zhou J (2020c) Overexpression of tomato RING E3 ubiquitin ligase gene SlRING1 confers cadmium tolerance by attenuating cadmium accumulation and oxidative stress. Physiol Plant. https://doi.org/10.1111/ppl.13294
Ainsworth EA, Rogers A (2007) The response of photosynthesis and stomatal conductance to rising (CO2): mechanisms and environmental interactions. Plant Cell Environ 30:258–270
Ashraf M, Harris P (2004) Potential biochemical indicators of salinity tolerance in plants. Plant Sci 166:3–16
Bates LS, Waldren RP, Teare ID (1973) Rapid determination of free proline for water-stress studies. Plant Soil 39:205–207
Beyer WF Jr., Fridovich I (1987) Assaying for superoxide dismutase activity: some large consequences of minor changes in conditions. Ana Biochem 161:559–566
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Ana Biochem 72:248–254
Bussotti F, Desotgiu R, Cascio C, Pollastrini M, Gravano E, Gerosa G, Manes F (2011) Ozone stress in woody plants assessed with chlorophyll a fluorescence. A critical reassessment of existing data. Environ Exp Bot 73:19–30
Chamovitz D, Sandmann G, Hirschberg J (1993) Molecular and biochemical characterization of herbicide-resistant mutants of cyanobacteria reveals that phytoene desaturation is a rate-limiting step in carotenoid biosynthesis. J Biol Chem 268:17348–17353
Chandler SF, Dodds JH (1983) The effect of phosphate, nitrogen and sucrose on the production of phenolics and solasodine in callus cultures of Solanum laciniatum. Plant Cell Rep 2:205–208
Chaudhary DR, Seo J, Kang H, Rathore AP, Jha B (2018) Seasonal variation in natural abundance of δ13C and 15N in Salicornia brachiata Roxb. populations from a coastal area of India. Isotopes Environ Health Stud 54:209–224
Cheng G, Wang L, Lan H (2016) Cloning of PEPC-1 from a C4 halophyte Suaeda aralocaspica without Kranz anatomy and its recombinant enzymatic activity in responses to abiotic stresses. Enzyme Microb Technol 83:57–67
Chomczynski P, Sacchi N (1987) Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Ana Biochem 162:156–159
Demetriou G, Neonaki C, Navakoudis E, Kotzabasis K (2007) Salt stress impact on the molecular structure and function of the photosynthetic apparatus the protective role of polyamines. Biochim Biophys Acta 1767:272–280
Ellsworth DS, Reich PB, Naumburg ES, Koch GW, Kubiske ME, Smith SD (2004) Photosynthesis, carboxylation and leaf nitrogen responses of 16 species to elevated pCO2 across four free-air CO2 enrichment experiments in forest, grassland and desert. Global Change Biol 10:121–138
Geissler N, Hussin S, Koyro HW (2009) Interactive effects of NaCl salinity and elevated atmospheric CO2 concentration on growth, photosynthesis, water relations and chemical composition of the potential cash crop halophyte Aster tripolium L. Environ Exp Bot 65:220–231
Geissler N, Hussin S, Koyro HW (2010) Elevated atmospheric CO2 concentration enhances salinity tolerance in Aster tripolium L. Planta 231:583–594
Geissler N, Hussin S, El-Far MM, Koyro HW (2015) Elevated atmospheric CO2 concentration leads to different salt resistance mechanisms in a C3 (Chenopodium quinoa) and a C4 (Atriplex nummularia) halophyte. Environ Exp Bot 118:67–77
Ghannoum O (2009) C4 photosynthesis and water stress. Ann Bot 103:635–644
Govindjee (1995) Sixty-three years since Kautsky: Chlorophylla fluorescence. Aust J Plant Physiol 22:711–711
Gowik U, Westhoff P (2011) The path from C3 to C4 photosynthesis. Plant Physiol 155:56–63
Haque MI, Rathore MS, Gupta H, Jha B (2017) Inorganic solutes contribute more than organic solutes to the osmotic adjustment in Salicornia brachiata (Roxb.) under natural saline conditions. Aqu Bot 142:78–86
Havaux M (2014) Carotenoid oxidation products as stress signals in plants. Plant J 79:597–606
Hodges DM, DeLong JM, Forney CF, Prange RK (1999) Improving the thiobarbituric acid-reactive-substances assay for estimating lipid peroxidation in plant tissues containing anthocyanin and other interfering compounds. Planta 207:604–611
Hong Z, Lakkineni K, Zhang Z, Verma DPS (2000) Removal of feedback inhibition of Δ1-pyrroline-5-carboxylate synthetase results in increased proline accumulation and protection of plants from osmotic stress. Plant Physiol 122:1129–1136
Inskeep WP, Bloom PR (1985) Extinction coefficients of chlorophyll a and b in N, N-dimethylformamide and 80% acetone. Plant Physiol 77:483–485
Jacob PT, Siddiqui SA, Rathore MS (2020) Seed germination, seedling growth and seedling development associated physiochemical changes in Salicornia brachiata (Roxb.) under salinity and osmotic stress. Aquat Bot 1:166–103272
Jebara S, Jebara M, Limam F, Aouani ME (2005) Changes in ascorbate peroxidase, catalase, guaiacol peroxidase and superoxide dismutase activities in common bean (Phaseolus vulgaris) nodules under salt stress. J Plant Physiol 162:929–936
Jin H, Dong D, Yang Q, Zhu D (2016) Salt-responsive transcriptome profiling of Suaeda glauca via RNA sequencing. PLoS ONE 11:150504
Keisham M, Mukherjee S, Bhatla SC (2018) Mechanisms of sodium transport in plants progresses and challenges. Int J Mol Sci 19:647
Khatri K, Rathore MS (2019) Photosystem photochemistry, prompt and delayed fluorescence, photosynthetic responses and electron flow in tobacco under drought and salt stress. Photosynthetica 57:61–74
Koteyeva NK, Voznesenskaya EV, Berry JO, Chuong SD, Franceschi VR, Edwards GE (2011) Development of structural and biochemical characteristics of C4 photosynthesis in two types of Kranz anatomy in genus Suaeda (family Chenopodiaceae). J Exp Bot 62:3197–3212
Ksouri R, Megdiche W, Debez A, Falleh H, Grignon C, Abdelly C (2007) Salinity effects on polyphenol content and antioxidant activities in leaves of the halophyte Cakile maritima. Plant Physiol Biochem 45:244–249
Kumari J, Udawat P, Dubey AK, Haque MI, Rathore MS, Jha B (2017) Overexpression of SbSI-1, a nuclear protein from Salicornia brachiata (Roxb.) confers drought and salt tolerance in tobacco by curtailing oxidative damage and maintaining photosynthetic efficiency. Front Plant Sci 8:1215
Li T, Tao Q, Liang C, Yang X (2014) Elevated CO2 concentration increase the mobility of Cd and Zn in the rhizosphere of hyperaccumulator Sedum alfredii. Environ Sci Pollut Res 21:5899–5908
Li P, Li B, Seneweera S, Zong Y, Li FY, Han Y, Hao X (2019) Photosynthesis and yield response to elevated CO2, C4 plant foxtail millet behaves similarly to C3 species. Plant Sci 285:239–247
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2− ΔΔCT method. Methods 25:402–408
Lokhande VH, Srivastava AK, Srivastava S, Nikam TD, Suprasanna P (2011) Regulated alterations in redox and energetic status are the key mediators of salinity tolerance in the halophyte Sesuvium portulacastrum (L.) L. Plant Growth Regul 65:287
Long SP, Ainsworth EA, Rogers A, Ort DR (2004) Rising atmospheric carbon dioxide: plants FACE the future. Annu Rev Plant Biol 55:591–628
Mathur S, Mehta P, Jajoo A (2013) Effects of dual stress (high salt and high temperature) on the photochemical efficiency of wheat leaves (Triticum aestivum). Physiol Mol Biol Plants 19:179–188
Mehta P, Allakhverdiev SI, Jajoo A (2010) Characterization of photosystem II heterogeneity in response to high salt stress in wheat leaves (Triticum aestivum). Photosynth Res 105:249–255
Misra AN, Srivastava A, Strasser RJ (2001) Utilization of fast chlorophyll afluorescence technique in assessing the salt/ion sensitivity of mung bean and Brassica seedlings. J Plant Physiol 158:1173–1181
Miyagawa Y, Tamoi M, Shigeoka S (2000) Evaluation of the defense system in chloroplasts to photooxidative stress caused by paraquat using transgenic tobacco plants expressing catalase from Escherichia coli. Plant Cell Physiol 41:311–320
Moghaieb RE, Saneoka H, Fujita K (2004) Effect of salinity on osmotic adjustment, glycinebetaine accumulation and the betaine aldehyde dehydrogenase gene expression in two halophytic plants Salicornia Europaea and Suaeda Maritima. Plant Sci 166:1345–1349
Morgan JA, LeCain DR, Pendall E, Blumenthal DM, Kimball BA, Carrillo Y, Williams DG, Heisler-White J, Dijkstra FA, West M (2011) C4 grasses prosper as carbon dioxide eliminates desiccation in warmed semi-arid grassland. Nature 476:202
Oukarroum A, Bussotti F, Goltsev V, Kalaji HM (2015) Correlation between reactive oxygen species production and photochemistry of photosystems I and II in Lemna gibba L. plants under salt stress. Environ Exp Bot 109:80–88
Pan C, Ahammed GJ, Li X, Shi K (2018) Elevated CO2 improves photosynthesis under high temperature by attenuating the functional limitations to energy fluxes, electron transport and redox homeostasis in tomato leaves. Front Plant Sci 9:1739
Park J, Okita TW, Edwards GE (2009) Salt tolerant mechanisms in single-cell C4 species Bienertia sinuspersici and Suaeda aralocaspica (Chenopodiaceae). Plant Sci 176:616–626
Patterson BD, Payne LA, Chen YZ, Graham D (1984) An inhibitor of catalase induced by cold in chilling-sensitive plants. Plant Physiol 76:1014–1018
Pérez-Romero JA, Idaszkin YL, Barcia-Piedras JM, Duarte B, Redondo-Gómez S, Caçador I, Mateos-Naranjo E (2018) Disentangling the effect of atmospheric CO2 enrichment on the halophyte Salicornia ramosissima J. Woods physiological performance under optimal and suboptimal saline conditions. Plant Physiol Biochem 127:617–629
Ranjbarfordoei A, Samson R, Van DP (2006) Chlorophyll fluorescence performance of sweet almond [Prunus dulcis (Miller) D. Webb] in response to salinity stress induced by NaCl. Photosynthetica 44:513–522
Rathore AP, Chaudhary DR, Jha B (2016) Biomass production, nutrient cycling, and carbon fixation by Salicornia brachiata Roxb.: A promising halophyte for coastal saline soil rehabilitation. Int J Phytoremediation 18:801–811
Rathore MS, Balar N, Jha B (2019) Population structure and developmental stages associated eco-physiological responses in Salicornia brachiata. Ecol Res 34:644–658
Rondeau P, Rouch C, Besnard G (2005) NADP-malate dehydrogenase gene evolution in Andropogoneae (Poaceae): gene duplication followed by sub-functionalization. Ann Bot 96:1307–1314
Rozema J, Dorel F, Janissen R, Lenssen G, Broekman R, Arp W, Drake BG (1991) Effect of elevated atmospheric CO2 on growth, photosynthesis and water relations of salt marsh grass species. Aqu Bot 39:45–55
Sage RF (2004) The evolution of C4 photosynthesis. New Phytol 161:341–370
Sage RF, Coleman JR (2001) Effects of low atmospheric CO2 on plants: more than a thing of the past. Trends Plant Sci 6:1360–1385
Salvatori E, Fusaro L, Gottardini E, Pollastrini M, Goltsev V, Strasser RJ, Bussotti F (2014) Plant stress analysis: application of prompt, delayed chlorophyll fluorescence and 820 nm modulated reflectance. Insights from independent experiments. Plant Physiol Biochem 85:105–113
Schansker G, Tóth SZ, Strasser RJ (2005) Methylviologen and dibromothymoquinone treatments of pea leaves reveal the role of photosystem I in the Chl a fluorescence rise OJIP. BBA-Bioenergetics 1706:250–261
Schwarz M, Gale J (1983) The effect of heat and salinity stress on the carbon balance of Xanthium strumarium. Effects of stress on photosynthesis. Springer, Dordrecht, pp 325–333
Shomer-Ilan A, Beer S, Waisel Y (1975) Suaeda monoica, a C4 plant without typical bundle sheaths. Plant Physiol 56:676–679
Singh A, Agrawal M (2015) Effects of ambient and elevated CO2 on growth, chlorophyll fluorescence, photosynthetic pigments, antioxidants, and secondary metabolites of Catharanthus roseus (L.) G Don. grown under three different soil N levels. Environ Sci Pollut Res 22:3936–3946
Song J, Feng G, Tian CY, Zhang FS (2006) Osmotic adjustment traits of Suaeda physophora, Haloxylon ammodendron and Haloxylon persicum in field or controlled conditions. Plant Sci 170:113–119
Stutz SS, Edwards GE, Cousins AB (2014) Single-cell C4 photosynthesis: efficiency and acclimation of Bienertia sinuspersici to growth under low light. New Phytol 202:220–232
Ueno O, Kawano Y, Wakayama M, Takeda T (2006) Leaf vascular systems in C3 and C4 grasses: a two-dimensional analysis. Ann Bot 97:611–621
Voznesenskaya EV, Franceschi VR, Kiirats O, Artyusheva EG, Freitag H, Edwards GE (2002) Proof of C4 photosynthesis without Kranz anatomy in Bienertia cycloptera (Chenopodiaceae). Plant J 31:649–662
Wheeler MCG, Tronconi MA, Drincovich MF, Andreo CS, Flügge UI, Maurino VG (2005) A comprehensive analysis of the NADP-malic enzyme gene family of Arabidopsis. Plant Physiol 139:39–51
Yadav S, Mishra A, Jha B (2018) Elevated CO2 leads to carbon sequestration by modulating C4 photosynthesis pathway enzyme (PPDK) in Suaeda monoica and S. fruticosa. J Photochem Photobiol B 178:310–315
Zhang YH, Chen LJ, He JL, Qian LS, Wu LQ, Wang RF (2010) Characteristics of chlorophyll fluorescence and antioxidative system in super-hybrid rice and its parental cultivars under chilling stress. Biol Plant 54:164–168
Zhang Y, Wang Y, Wen W, Shi Z, Gu Q, Ahammed GJ, Cao K, Shah Jahan M, Shu S, Wang J, Sun J (2020a) Hydrogen peroxide mediates spermidine-induced autophagy to alleviate salt stress in cucumber. Autophagy 5:1–5
Zhang Y, Yao Q, Shi Y, Li X, Hou L, Xing G, Ahammed GJ (2020b) Elevated CO2 improves antioxidant capacity, ion homeostasis, and polyamine metabolism in tomato seedlings under Ca(NO3)2-induced salt stress. Sci Hortic 273:109644
Acknowledgements
CSIR-CSMCRI PRIS—113/2018. The authors are thankful to CSIR (Government of India), New Delhi for establishment of infrastructure facility and GSBTM, Govt. of Gujarat for financial support under GAP2080 (80G2DT) project. The authors also acknowledge the help of ADE&CIF division for SEM, elemental, and ICP analysis. Mr. IH and SAS thank UGC MANF and UGC for Junior/Senior Research Fellowship during their Ph.D. work. The authors are thankful to Dr. D.R. Chaudhary and Prof. Hojeong Kang, School of Civil and Environmental Engineering, Yonsei University, Seoul 03722, South Korea for IRMS analysis. Mr. IH and SAS thank MKSBU, Bhavnagar and AcSIR, Ghaziabad for registration in PhD program, respectively.
Author information
Authors and Affiliations
Contributions
Conceptualized and conceived the experiment: MSR/BJ; Designing the experiment: MSR and IH; Experimental execution and Data analysis: IH and SAS; Drafting/editing the manuscript: IH and SAS; Finalization of manuscript: MSR.
Corresponding author
Ethics declarations
Conflict of interest
Authors declare no conflict of interest.
Additional information
Handling editor: Golam jalal Ahammed.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
344_2021_10485_MOESM3_ESM.jpg
Fig. S1: Pictorial representation of experimental design to study the differential photosynthetic and physio-chemical responses in S. fruticosa and S. monoica under abiotic stress conditions at ambient and elevated CO2 conditions. Supplementary file3 (JPEG 691 KB)
344_2021_10485_MOESM4_ESM.jpg
Fig. S2: Growth in above ground portions of S. fruticosa and S. monoica grown under control and stress (100 mM NaCl, 50% seawater salinity and 10% PEG) treatments at ambient and elevated CO2 conditions for 7 (a) and 15 (b) days respectively. The values (mean±SE, n=3) followed by different lowercase letters as superscripts are significantly different by LSD (>0.05%) at a particular CO2 level. In figure SW represents seawater salinity, SF– S. fruticosa, and SM - S. monoica.Supplementary file4 (JPEG 446 KB)
344_2021_10485_MOESM5_ESM.jpg
Fig. S3: Pictotial documentations of growth/morphology in S. fruticosa and S. monoica grown under control and stress (100 mM NaCl, 50% seawater salinity and 10% PEG) treatments at ambient and elevated CO2 conditions for 0, 7 and 15 days. Supplementary file5 (JPEG 776 KB)
344_2021_10485_MOESM6_ESM.jpg
Fig. S4: Root growth in S. fruticosa and S. monoica grown under control and stress (100 mM NaCl, 50% seawater salinity and 10% PEG) treatments at ambient and elevated CO2 condition for 7 days. The values (mean±SE, n=3) followed by different lowercase letters as superscripts are significantly different by LSD (>0.05%) at a particular CO2 level. In figure C represent control, N- NaCl, SW- seawater salinity, P- PEG, SF- S. fruticosa, and SM- S. monoica. Supplementary file6 (JPEG 416 KB)
344_2021_10485_MOESM7_ESM.jpg
Fig. S5: Fresh and dry biomass yield (a-b) and water content (c) in S. fruticosa and S. monoica grown under control and stress (100 mM NaCl, 50% seawater salinity and 10% PEG) treatments at ambient and elevated CO2 condition for 7 days. The values (mean±SE, n=3) followed by different lowercase letters as superscripts are significantly different by LSD (>0.05%) at a particular CO2 level. In figure C represent control, N- NaCl, SW- seawater salinity, P- PEG, SF- S. fruticosa, and SM- S. monoica. Supplementary file7 (JPEG 473 KB)
344_2021_10485_MOESM8_ESM.jpg
Fig. S6: SEM-EDX mapping for Na and K contents in leaf tissues after 7 days growth (a) and root surface after 15 days growth (b) of S. fruticosa and S. monoica plants under control and stress (100 mM NaCl and 50% seawater salinity) treatments at ambient and elevated CO2 conditions. In figure the SW represents seawater salinity. Supplementary file8 (JPEG 831 KB)
344_2021_10485_MOESM9_ESM.jpg
Fig. S7: Total chlorophyll (a) and carotenoid (b) contents in S. fruticosa and S. monoica under control and stress (100 mM NaCl, 50% seawater salinity and 10% PEG) treatments at ambient and elevated CO2 condition for 7 days. The values (mean±SE, n=3) followed by different lowercase letters as superscripts are significantly different by LSD (>0.05%) at a particular CO2 level. In figure SW represent seawater salinity, SF- S. fruticosa, and SM- S. monoica. Supplementary file9 (JPEG 457 KB)
344_2021_10485_MOESM10_ESM.jpg
Fig. S8: Water use efficiency (a), Ci/CA (b) and vapor pressure deficit (c) in S. fruticosa and S. monoica under control and stress (100 mM NaCl, 50% seawater salinity and 10% PEG) treatments at ambient and elevated CO2 for 7 days. The values represent mean ± SE (n=3) and followed by different letters as superscripts are significantly different by LSD (>0.05%) at a particular CO2 level. In figure SW is seawater salinity, SF– S. fruticosa, and SM - S. monoica. Supplementary file10 (JPEG 487 KB)
344_2021_10485_MOESM11_ESM.jpg
Fig. S9: Chlorophyll a fluorescence derived OJIP curve in S. fruticosa and S. monoica under control and stress (100 mM NaCl, 50% seawater salinity and 10% PEG) treatments at ambient (a-b) and elevated CO2 (c-d) for 7 days. The values represent mean (n=10) of measurements at a particular CO2 level. Supplementary file11 (JPEG 541 KB)
344_2021_10485_MOESM12_ESM.jpg
Fig. S10: Chlorophyll a fluorescence derived δFIP (a) δVIP (b) in S. fruticosa and S. monoica under control and stress (100 mM NaCl, 50% seawater salinity and 10% PEG) treatments at ambient and elevated CO2 for 7 days. The values represent mean ± SE (n=10) and followed by different letters as superscripts are significantly different by LSD (>0.05%) at a particular CO2 level. In figure SW is seawater salinity, SF– S. fruticosa, and SM - S. monoica. Supplementary file12 (JPEG 408 KB)
Rights and permissions
About this article
Cite this article
Haque, M.I., Siddiqui, S.A., Jha, B. et al. Interactive Effects of Abiotic Stress and Elevated CO2 on Physio-Chemical and Photosynthetic Responses in Suaeda Species. J Plant Growth Regul 41, 2930–2948 (2022). https://doi.org/10.1007/s00344-021-10485-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00344-021-10485-1