Abstract
Uniconazole was a plant growth retardant with effect of regulating plant growth and development, however, there were very few studies on its application to mung bean. In this study, the leaves of mung bean were sprayed with uniconazole solution (30 mg·L−1) at V3 stage. Photosynthetic indicators, root distribution were measured at R5 and R6, and yield and components were measured at maturity. Uniconazole increased Gs (stomatal conductance) and Tr (transpiration rate) at R5 and R6, Pn (net photosynthesis rate) at R6, and SPAD value at R5. The SPAD value at R5 had the greatest correlation with yield with a correlation coefficient of 0.684. According to distribution pattern of decreasing root length density from top to bottom, large amounts of water absorbed by the roots was more likely to come from the upper soil layer, especially 0–20 cm soil layer. As the depth of soil layer increased, the proportion of root dry weight in different soil layers were 69, 14, 9, 5 and 3%, respectively. Uniconazole effectively reduced root proportion in 0–20 cm soil layer and increased root proportion in 20–60 cm soil layer. Root dry weight density in 20–40 cm soil layer and yield were significantly positively correlated (r = 0.938* at R5, r = 0.891* at R6). In addition, uniconazole increased hundred grain weight and yield, reduced pods number per plant and seeds number per pod. Based on the results, this study can provide guidance for mung bean production and high-yield breeding in the future.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Mung bean is one of the most ancient and extensively grown legumes with the characteristics of short-term growth, strong nitrogen fixation ability and barren tolerance (Muthu et al. 2018). The root of mung bean is tap root system, which contain nodule having the N2 fixing bacteria Rhizobium spp. (Khan et al. 2016). Agricultural researcher achieved the goal of high-yielding breeding by studying the relationship between root and yield. Ehdaie et al. (2012) reported that yield of bread wheat showed positive correlation with shallow and deep root dry weight under terminal drought. The study of Kanbar et al. (2009) revealed that root dry weight had the largest effect on grain yield of rice under well-watered condition. Kashiwagi et al. (2006) reported that root length density at 35 days after sowing showed a significant positive correlation with yield of chickpea in field trials. Izumi et al. (2004) showed that root length per unit area exhibited significantly positive correlation with yield in wheat but not in soybean. Karadavut and Sozen (2017) found a significant positive correlation between root weight and yield in chickpea, with a correlation coefficient of r = 0.671**. Mahdi (2013) found that there was a significant positive correlation between grain yield and root dry weight in mung bean.
Photosynthesis refers to the process in which plants absorb light energy and use carbon dioxide and water to synthesize organic matter while releasing oxygen (Pfannschmidt et al. 2010). Plants convert light energy into chemical energy while assimilating inorganic carbides and store it in the formed organic compounds (Losada et al. 1990). The light energy assimilated by photosynthesis is about 10 times more than the energy required by human beings every year. The chemical energy stored in organic matter is not only for plant itself and all heterotrophic organisms, but also the energy source for human nutrition and activities (Lucia et al. 2014). So it can be said that photosynthesis provides today’s main energy source. Photosynthesis is closely related to agricultural production (Jens et al. 1996). Exploring the correlation between photosynthetic indicators and yield is of great significance for improving crop yield (Pepó and Novák 2016). Wang et al. (2016) found that net photosynthetic rate, stomatal conductance, transpiration rate and SPAD value of leaves were significantly positively correlated with yield in Tartary buckwheat, respectively. The research results of Bort et al. (1998) showed that the grain yield of in field grown barley was positively correlated with SPAD value. Liu et al. (2012) found that net photosynthesis rate, transpiration rate, stomatal conductance, intercellular carbon dioxide concentration and SPAD value of leaves were positively correlated with yield in soybean.
Uniconazole is a highly effective plant growth retardant, which has the effects of dwarfing crop plants, promoting root growth, preventing lodging, and improving crop resistance (Oshio et al. 1990; Fukuta et al. 2001). Uniconazole also has a bactericidal effect, and its biological activity is higher than that of paclobutrazol (Kohne and Sylvie 1989). The mechanism of uniconazole’s regulation on plants is that it can affect the activity of Ent-kaurene oxidase and inhibit the synthesis of GA precursors, thereby reducing the production of endogenous GA, while inhibiting the synthesis of endogenous IAA (Hisamatsu et al. 2004; Todoroki et al. 2009). After being absorbed by the surface of plant body, uniconazole would be transported to the top through the xylem, then inhibiting the synthesis of GA, resulting in the limitation of plant cell elongation and ultimately affecting plant morphology. In recent years, the experimental studies on the application of uniconazole in crops have increased. However, due to its activity is easily affected by the environment, the actual application of uniconazole in mung bean was still less.
Materials and Methods
Experimental Site
The experiment was carried out at outdoor test site in National Coarse Cereals Engineering Research Center, Daqing, China on June 5, 2016. The annual precipitation at the experimental site was 508.7 mm, the average annual temperature was 5.60 °C, the effective accumulated temperature was 2900–3000 °C, and the sunshine duration was 1158 h (Collected from Daqing Weather Station).
Experimental Devices
The experimental device was a cylindrical plastic barrel with a diameter of 30 cm and a height of 150 cm. In order to facilitate sampling, inside the vertical device was a plastic water belt of 30 cm in diameter and the soil was filled in the plastic water belt. The lower end of the plastic water belt was sealed and four round holes were cut using scissors (Fig. 1).
Soil Characteristics
The soil was chernozem, with physical and chemical properties characterized by a pH of 7.8, effective phosphorus of 13.69 mg·kg−1, alkali-hydrolyzed nitrogen of 134 mg·kg−1, available potassium of 204 mg·kg−1, and organic matter of 32.8 g·kg−1. The soil was screened before pouring into the devices to remove grass root, tree root and large granular clods and stones. Then the soil were filled into the device (1.15 × 102 kg·m−3 in density).
Experiment Design, Species and Seeding
Mung bean cultivar Jilv7 (drought resistance) and Gonglv2 (non-drought resistance) were planted at five seeds per device, separately. Two seedlings were retained, and grown with four replicates per growth stage. Uniconazole was evenly sprayed on the upper and lower surfaces of the leaves at V3 stage (30 mg·L−1). When solution was suspended but not dripping, stopping spraying.
Measurement of Leaf Photosynthesis
Photosynthetic indicators were measured at R5 (seed filling stage) and R6 stages (full seed stage). Selecting the inverted three fully expanded leaves with consistent growth status, and using CID340 photosynthesis instrument produced by the American CID company to determine stomatal conductance (Gs), transpiration rate (Tr), net photosynthesis rate (Pn) and intercellular carbon dioxide concentration (Ci). The SPAD-502 chlorophyll meter was used to determine SPAD value of the three leaves of inverted three leaves, and the mean value was calculated as final SPAD value.
Root Sample Collection
Root samples were collected from the devices at R5 (64th days after sowing) and R6 (77th days after sowing). The upper soil surface was taken as the starting point to obtain soil samples with root in 0–20, 20–40, 40–60, 60–80 and 80–100 cm soil layers, respectively. The plants were clipped at cotyledons by scissors before sampling. Soil samples containing root were soaked in a plastic bucket filled with water until the soil became soft and then filtered. The obtained root samples were washed with clean tap water and then placed in a plastic, sealable bag, and the bag was placed in a refrigerator for further use.
Data Collection
The harvested root samples were placed in a clear glass tray filled with water. The roots were washed to remove soil particles and other dirt that could hamper efficient scanning of root samples. The glass tray was placed on a scanner (Epson V700) and digital images were generated at 400 dpi. Digital image analysis of root samples was conducted using WinRHIZO (version 2014a, Reagent Instruments Inc., Quebec, Canada) to get data of root length, from which root length density (RLD) were estimated as follows:
where L is root length, V0 is soil volume, r is radius, and h is height.
After scanning, the roots were removed from glass tray and subsequently were placed in an oven at 105 °C for 2 h, then drying to constant weight in 75 °C oven. The dry weight of roots was obtained by analytical balance and the root dry weight density (RDWD) was estimated as:
where M is root dry weight.
Statistical Analysis
Difference between treatment and control was determined by LSD test. Pearson Correlation Analysis was used to evaluate the relationships between different traits by SPSS 22. Figure preparation was carried out by MicroCal Origin software 2017 (OriginLab).
Results
Effects of Uniconazole on Stomatal Conductance of Mung Bean Leaves
At R5, the stomatal conductance of S-Jilv7 was greater than that of CK-Jilv7; S-Gonglv2 also had a greater stomatal conductance than CK-Gonglv2. At R6, the stomatal conductance of S-Gonglv2 was greater than that of CK-Gonglv2; The stomatal conductance of S-JiLv7 was significantly higher than that of CK-Jilv7 by 71.05% (Fig. 2).
Effects of uniconazole on stomatal conductance of mung bean leaves at R5 and R6 stages. Gs (stomatal conductance), S-Jilv7 (the uniconazole treatment of mung bean cultivar Jilv7), CK-Jilv7 (the control of mung bean cultivar Jilv7), S-Gonglv2 (the uniconazole treatment of mung bean cultivar Gonglv2), CK-Gonglv2 (the control of mung bean cultivar Gonglv2); Data represent average ± standard error. Significant at the 0.05 probability level
Effects of Uniconazole on Transpiration Rate of Mung Bean Leaves
At R5, S-Jilv7 had a significantly greater transpiration rate than CK-Jilv7 by 34.22%, and the transpiration rate of S-Gonglv2 was significantly higher than that of CK-Gonglv2 by 228.46%. At R6, S-Jilv7 had a significantly greater transpiration rate than CK-Jilv7 by 51.08%; S-Gonglv2 had a greater transpiration rate than CK-Gonglv2 (Fig. 3).
Effects of uniconazole on transpiration rate of mung bean leaves at R5 and R6 stages. Tr (transpiration rate), S-Jilv7 (the uniconazole treatment of mung bean cultivar Jilv7), CK-Jilv7 (the control of mung bean cultivar Jilv7), S-Gonglv2 (the uniconazole treatment of mung bean cultivar Gonglv2), CK-Gonglv2 (the control of mung bean cultivar Gonglv2); Data represent average ± standard error. Significant at the 0.05 probability level
Effects of Uniconazole on Net Photosynthetic Rate of Mung Bean Leaves
At R5, S-Jilv7 had a greater net photosynthetic rate that CK-Jilv7, but the net photosynthetic rate of CK-Gonglv2 was greater than that of S- Gonglv2. At R6, the net photosynthetic rate of S-Jilv7 was greater than that of CK- Jilv7, and the net photosynthetic rate of S-Gonglv2 was greater than that of CK- Gonglv2 (Fig. 4).
Effects of uniconazole on net photosynthetic rate of mung bean leaves at R5 and R6 stages. Pn (net photosynthetic rate), S-Jilv7 (the uniconazole treatment of mung bean cultivar Jilv7), CK-Jilv7 (the control of mung bean cultivar Jilv7), S-Gonglv2 (the uniconazole treatment of mung bean cultivar Gonglv2), CK-Gonglv2 (the control of mung bean cultivar Gonglv2); Data represent average ± standard error. Significant at the 0.05 probability level
Effects of Uniconazole on Intercellular Carbon Dioxide Concentration of Mung Bean Leaves
CK-Gonglv2 had a greater intercellular carbon dioxide concentration than S-Gonglv2 at R5 and R6, but the intercellular carbon dioxide concentration of CK-Jilv7 was less than those of S-Jilv7, and the intercellular carbon dioxide concentration of S-Jilv7 was significantly higher than that of CK-Jilv7 by 32.05% at R5 (Fig. 5).
Effects of uniconazole on intercellular carbon dioxide concentration of mung bean leaves at R5 and R6 stages. Ci (intercellular carbon dioxide concentration), S-Jilv7 (the uniconazole treatment of mung bean cultivar Jilv7), CK-Jilv7 (the control of mung bean cultivar Jilv7), S-Gonglv2 (the uniconazole treatment of mung bean cultivar Gonglv2), CK-Gonglv2 (the control of mung bean cultivar Gonglv2); Data represent average ± standard error. Significant at the 0.05 probability level
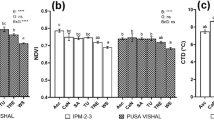
Effects of Uniconazole on SPAD Value of Mung Bean Leaves
At R5, S-Jilv7 and S-Gonglv2 had greater SPAD value than CK-Jilv7 and CK-Gonglv2, respectively. At R6, S-Jilv7 had a greater SPAD value than CK-Jilv7; But the SPAD value of CK-Gonglv2 was greater than that of S-Gonglv2 (Fig. 6).
Effects of uniconazole on SPAD value of mung bean leaves at R5 and R6 stages. S-Jilv7 (the uniconazole treatment of mung bean cultivar Jilv7), CK-Jilv7 (the control of mung bean cultivar Jilv7), S-Gonglv2 (the uniconazole treatment of mung bean cultivar Gonglv2), CK-Gonglv2 (the control of mung bean cultivar Gonglv2); Data represent average ± standard error. Significant at the 0.05 probability level
Correlation Between Different Photosynthetic Traits
The photosynthetic traits of mung bean leaves at R5 and R6 were significantly positively correlated with each other. SPAD values were positively correlated with all photosynthetic traits at R5 and R6; The SPAD value of mung bean leaves was significantly positively correlated with net photosynthetic rate at R6 (r = 0.722*) (Table 1).
Effects of Uniconazole on Root Length Density Distribution of Mung Bean
At R5, CK-Jilv7 had greater root length density than S-Jilv7 in 0–20 and 40–60 cm soil layers, and the root length density of CK-Jilv7 in 0–20 cm soil layer was significantly higher than that of S-Jilv7 by 16.18%; In 20–40, 60–80 and 80–100 cm soil layers, S-Jilv7 had greater root length density than CK-Jilv7. CK-Gonglv2 had greater root length density than S-Gonglv2 in each soil layer at R5.
At R6, S-Jilv7 had a greater root length density than CK-Jilv7 in 0–20 cm soil layer; In other soil layers, the root length density of CK-Jilv7 were greater than those of S-Jilv7. In 60–80 cm soil layer, S-Gonglv2 and CK-Gonglv2 had the same root length density; In 0–20, 20–40, 40–60 and 80–100 cm soil layers, the root length density of S-Gonglv2 were greater than those of CK-Gonglv2.
With the increase of soil layer depth, root length density showed a decreasing trend, and the greatest root length density was in 0–20 cm soil layer (Table 2).
Effects of Uniconazole on Root Dry Weight Density Distribution of Mung Bean
As the depth of soil layer increased, the ratio of root dry weight of mung bean control in different soil layers to total root dry weight were 69, 14, 9, 5 and 3%, respectively, and the ratio of root dry weight of mung bean treatment in different soil layers to total root dry weight were 66, 16, 10, 5 and 3%, respectively.
At R5, S-Jilv7 had greater root dry weight density than CK-Jilv7 at 20–40 and 40–60 cm soil layers; The root weight density of S-Jilv7 was significantly higher than that of CK-Jilv7 by 67.19% in 20–40 cm soil layer; In 0–20, 60–80 and 80–100 cm soil layers, CK-Jilv7 had greater root dry weight density than S-Jilv7. CK-Gonglv2 had greater root dry weight density than S-Gonglv2 in 0–20, 20–40 and 40–60 cm soil layers at R5; In other soil layers, the root dry weight density of S-Gonglv2 were greater than those of CK-Gonglv2.
At R6, S-Jilv7 in each soil layer had a greater root dry weight density than CK-Jilv7; In 0–20 cm soil layer, the root dry weight density of S-Jilv7 was significantly higher than that of CK-Jilv7 by 37.19%. In 0–20, 20–40 and 40–60 cm soil layers, S-Gonglv2 had greater root dry weight density than CK-Gonglv2; In other soil layers, the root dry weight density of CK-Gonglv2 were greater than those of S-Gonglv2 (Table 3).
Effects of Uniconazole on Yield and Yield Components of Mung Bean
The hundred grain weight and yield per plant of S-Jilv7 were greater than those of CK-Jilv7, but CK-Jilv7 had greater pods number per plant and seeds number per pod than S-Jilv7. The hundred grain weight and yield per plant of S-Gonglv2 were greater than those CK-Gonglv2, but CK-Gonglv2 had greater pods number per plant and seeds number per pod than S-Gonglv2 (Table 4).
Correlation Between Photosynthetic Indicators and Yield
At R5, the net photosynthetic rate, stomatal conductance and SPAD value of mung bean were positively correlated with yield, and the SPAD value and yield had the greatest correlation coefficient. The intercellular carbon dioxide concentration and transpiration rate were negatively correlated with yield, respectively.
At R6, except stomatal conductance, the net photosynthetic rate, intercellular carbon dioxide concentration, SPAD value were positively correlated with yield of mung bean, and the transpiration rate and yield had the greatest correlation coefficient (Table 5).
Correlation Between Root and Yield
At R5, the root length density and root dry weight density of mung bean in different soil layers were positively correlated with yield; In 0–20 cm soil layer, root length density had the greatest correlation with yield; In 20–40 cm soil layer, root dry weight density and yield were significantly positively correlated (r = 0.938*).
At R6, both of the root length density and root dry weight density of mung bean in different soil layers were positively correlated with yield; The root dry weight density in 20–40 cm soil layer and yield were significantly positively correlated (r = 0.891*) (Table 6).
Discussion
Stomatal conductance was one of the parameters reflecting photosynthesis of plants (Miner et al. 2017). The greater stomatal conductance of plant leaves, the higher carbon dioxide content in the cells and the higher carbon dioxide content available for photosynthesis, resulting in an increase in photosynthetic rate. In this study, we found uniconazole increased stomatal conductance of two mung bean cultivars at R5 and R6. This was consistent with result of Yan et al. (2015) who found soybean with uniconazole raised stomatal conductance at R5. And we also found uniconazole had a regulatory effect on transpiration rate of mung bean leaves, in which the transpiration rate of mung bean leaves were improved at R5 and R6. But this result was contrary to the study of Duan et al. (2010) who found that uniconazole reduced the stomatal conductance of wheat leaves at seed filling stage (R5 is seed filling stage for legume) and maintained the transpiration rate. The reason may be that different crops have different levels of response to uniconazole, optimal preparations and application methods. The increase in transpiration rate was beneficial to reduce temperature of the leaves and avoid leaves from being burned by high temperature due to strong sunlight (Wuenscher and Kozlowski 2010). Transpiration was also a major driving force for absorption and transport of water by plants (Manzoni et al. 2013), especially tall plants. Meanwhile, since mineral salts (inorganic salts) had to be dissolved in water in order to be absorbed and operated by plants, then minerals were absorbed and distributed into the various parts of the plant body along with the absorption and flow of water. Thus, an increase in transpiration rate would help transport both substances through the plant.
The SPAD value represented the relative content of chlorophyll in the leaves (Eszter et al. 2019). In this study, we found that uniconazole had different regulatory effects on SPAD value of different mung bean varieties. The result showed that uniconazole increased the SPAD value of Jilv7 at R5 and R6. But for Gonglv2, only SPAD value at R5 was promoted. As we knew, chlorophyll was one of the most important pigments related to photosynthesis (Bettini et al. 2016). As the reaction site of plant photosynthesis, chlorophyll provided an environment for electron transport and photophosphorylation (Herbst et al. 2018). Chlorophyll absorbed energy from light, which was then used to convert carbon dioxide into carbohydrates. The increase in chlorophyll content was very helpful to increase the light saturation point. Within a certain range, photosynthetic products can be increased. Based on the above important significance of chlorophyll for plants, the application of uniconazole can give Jilv7 a higher photosynthetic potential at R5 and R6. At the same time, we found that the SPAD value of mung bean at R5 had the greatest correlation with yield with a correlation coefficient of 0.684 compared with other photosynthetic indicators. This meant that this indicator was of great significance for increasing mung bean yield.
Root length density refered to the length of the roots per unit volume of soil at a specific depth, and it represented the proportion of the soil volume that supplied nutrients to root system (Moyassar et al. 2016). When root length density increased, the surface area for water absorption also increased, thereby shortening the distance of soil water transmission, which was beneficial to water absorption. In this study, the greatest root length density was found in 0–20 cm soil layer, and with the increase of soil layer depth, root length density decreased. This was consistent with the findings of Gao et al. (2010) in soybean. The distribution pattern of root length density indicated that water absorbed by mung bean roots mainly came from the upper soil layer, especially the 0–20 cm layer, in which the root length density was larger. Compared with upper soil layer, water supply from the deeper soil layer was likely to be auxiliary, such as the 80–100 cm soil layer in which root length density was only 0.015–0.060 cm·cm−3. However, this did not mean that roots from deeper soil had a weaker water absorption capacity than the roots from upper soil layer.
According to the distribution of root dry weight density, we found root dry weight density gradually decreased with the increase of soil layer depth. This was consistent with the distribution pattern that Benjamin and Nielsen (2006) found in soybeans. By spraying solution of uniconazole at V3, we found the proportion of root dry weight in 0–20 cm soil layer was reduced, while the root dry weight in 20–40 and 40–60 cm soil layers were increased, and no change happened in proportion of root dry weight in the soil layer below 60 cm. In addition, we also found root dry weight density of mung bean in 20–40 cm soil layer had a significantly positive correlation with yield (r = 0.938* at R5; r = 0.891* at R6). Although root dry weight density in 0–20 cm soil layer was greater than that in 20–40 cm soil layer, the degree of correlation between yield and root dry weight density in 20–40 cm soil layer was higher than that in 0–20 cm soil layer, which showed that the magnitude of root dry weight per unit soil volume couldn’t absolutely reflect the level of contribution to yield. The taprots occupied a considerable part in 0–20 cm soil layer. As the soil depth increased, the taprots gradually became thinner, and the lateral roots, whose diameter was much thinner than that of the taprots, gradually increased. Thinner roots leaded to a larger surface-to-volume ratio. By having a large surface area and low volume, it increased the efficiency of absorption of minerals and water. This may be the reason why the correlation between root dry weight density in 20–40 cm soil layer and yield was higher than that in 0–20 cm soil layer.
Conclusion
The application of uniconazole at V3 effectively improved the conditions required for photosynthesis to a certain extent and regulated the proportional distribution of root system in different soil layers, which promoted the absorption and transportation of water and inorganic salts. Based on the distribution pattern of decreasing root length density from top to bottom, the upper root had a potential to provide a large amount of water for mung bean growth and development.
References
Benjamin JG, Nielsen DC (2006) Water deficit effects on root distribution of soybean, field pea and chickpea. Field Crop Res 97:248–253. https://doi.org/10.1016/j.fcr.2005.10.005
Bettini PP, Marvasi M, Fani F, Lazzara L, Cosi E, Melani L (2016) Agrobacterium rhizogenes rolb gene affects photosynthesis and chlorophyll content in transgenic tomato (solanum lycopersicum l.) plants. J Plant Physiol 204:27–35. https://doi.org/10.1016/j.jplph.2016.07.010
Bort J, JoséLuis A, Hazzam H, Grando S, Ceccarelli S (1998) Relationships between early vigour, grain yield, leaf structure and stable isotope composition in field grown barley. Plant Physiol Biochem 36:889–897. https://doi.org/10.1016/S0981-9428(99)80007-2
Duan L, Guan C, Li J, Eneji AE, Zhai Z (2010) Compensative effects of chemical regulation with uniconazole on physiological damages caused by water deficiency during the grain filling stage of wheat. J Agron Crop Sci 194:9–14. https://doi.org/10.1111/j.1439-037X.2007.00284.x
Ehdaie B, Layne AP, Waines JG (2012) Root system plasticity to drought influences grain yield in bread wheat. Euphytica 186:219–232. https://doi.org/10.1007/s10681-011-0585-9
Eszter N, András N, András B, Zoltán P, Helyes L (2019) Physiological factors and their relationship with the productivity of processing tomato under different water supplies. Water 11:586. https://doi.org/10.3390/w11030586
Fukuta N, Arai M, Yukawa T, Matsumura O (2001) Effect of dwarfing induced by uniconazole-p on snow tolerance of the faba bean (vicia faba l.). Plant Prod Sci 4:189–195. https://doi.org/10.1626/pps.4.189
Gao Y, Duan A, Qiu X, Liu Z, Sun J, Zhang J (2010) Distribution of roots and root length density in a maize/soybean strip intercropping system. Agric Water Manage 98:199–212. https://doi.org/10.1016/j.agwat.2010.08.021
Herbst J, Girke A, Hajirezaei MR, Hanke G, Grimm B (2018) Potential roles of ycf54 and ferredoxin-nadph reductase for magnesium protoporphyrin monomethylester cyclase. The Plant J 94:485–496. https://doi.org/10.1111/tpj.13869
Hisamatsu T, Koshioka M, Mander LN (2004) Regulation of gibberellin biosynthesis and stem elongation by low temperature in eustoma grandiflorum. J Horticult Sci Biotechnol 79:354–359. https://doi.org/10.1080/14620316.2004.11511772
Izumi Y, Uchida K, Iijima M (2004) Crop production in successive wheat-soybean rotation with no-tillage practice in relation to the root system development. Plant Prod Sci 7:329–336. https://doi.org/10.1626/pps.7.329
Jens N, Wünsche LAN, Robinson TL, Lenz F, Denning SS (1996) The bases of productivity in apple production systems: the role of light interception by different shoot types. J Am Soc Hortic Sci 121:886–893
Kanbar A, Toorchi M, Shashidhar H (2009) Relationship between root and yield morphological characters in rainfed low land rice (Oryza sativa L.). Cereal Res Commun 37:261–268. https://doi.org/10.1556/CRC.37.2009.2.14
Karadavut U, Sozen O (2017) Pearson and canonical correlations between the root properties and some yield components of chickpea (Cicer Arietinum L.). Legume Res 40:890–895
Kashiwagi J, Krishnamurthy L, Crouch JH, Serraj R (2006) Variability of root length density and its contributions to seed yield in chickpea (Cicer arietinum L.) under terminal drought stress. Field Crop Res 95:171–181. https://doi.org/10.1016/j.fcr.2005.02.012
Khan MR, Mohidin FA, Khan U, Ahamad F (2016) Native Pseudomonas spp. suppressed the root-knot nematode in in vitro and in vivo, and promoted the nodulation and grain yield in the field grown mungbean. Biol Control 101:159–168. https://doi.org/10.1016/j.biocontrol.2016.06.012
Kohne JS, Sylvie K (1989) Comparison of growth regulators paclobutrazol and uniconazole on avocado. S Afr Avocado Grow Assoc Yearb 12:38–39
Liu G, Yang C, Xu K, Zhang Z, Li D, Wu Z (2012) Development of yield and some photosynthetic characteristics during 82 years of genetic improvement of soybean genotypes in northeast china. Aust J Crop Sci 6:1416–1422
Losada M, Guerrero MG, Rosa MA, Serrano A, Hervás M, Ortega JM (1990) Sequential transduction of light into redox and acid—base energy in photosynthesis. J Electroanal Chem 23:105–128
Lucia B, Raghu C, Federico C, Christophe B (2014) The challenge of ecophysiological biodiversity for biotechnological applications of marine microalgae. Mar Drugs 12:1641–1675. https://doi.org/10.3390/md12031641
Mahdi Z (2013) Interrelationships among some agronomic traits in mungbean under drought stress and non-stress conditions. Euro J Exp Bio 3:511–515
Manzoni S, Vico G, Katul G, Palmroth S, Jackson RB, Porporato A (2013) Hydraulic limits on maximum plant transpiration and the emergence of the safety-efficiency trade-off. New Phytol 198:169–178. https://doi.org/10.1111/nph.12126
Miner GL, Bauerle WL, Baldocchi DD (2017) Estimating the sensitivity of stomatal conductance to photosynthesis: a review. Plant Cell Environ 40:1214–1238. https://doi.org/10.1111/pce.12871
Moyassar M, Aziz JA, Palta (2016) Five decades of selection for yield reduced root length density and increased nitrogen uptake per unit root length in Australian wheat varieties. Plant Soil 413:181–192. https://doi.org/10.1007/s11104-016-3059-y
Muthu MC, Sushree A, Srivastava R (2018) Influence of production factors on seed quality parameters of green gram (Vignaradiata) CV. KKM-3. Legume Res 41:891–894
Oshio H, Tanaka S, Izumi K (1990) Development of uniconazole for a new plant growth retardant and studies on its mechanism of action and the practical uses. Chem Regul Plants 25:8–18
Pepó P, Novák A (2016) Correlation between photosynthetic traits and yield in sunflower. Plant Soil Environ 62:335–340
Pfannschmidt T, Nilsson A, Tullberg A, Link G, Allen JF (2010) Direct transcriptional control of the chloroplast genes psba and psaab adjusts photosynthesis to light energy distribution in plants. IUBMB Life 48:271–276. https://doi.org/10.1080/713803507
Todoroki Y, Kobayashi K, Shirakura M, Aoyama H, Takatori K (2009) Abscinazole-f1, a conformationally restricted analogue of the plant growth retardant uniconazole and an inhibitor of aba 8′-hydroxylase cyp707a with no growth-retardant effect. Bioorg Med Chem 17:6620–6630. https://doi.org/10.1016/j.bmc.2009.07.070
Wang C, She HZ, Liu XB, Hu D, Yi ZL (2016) Effects of fertilization on leaf photosynthetic characteristics and grain yield in tartary buckwheat yunqiao1. Photosynthetica 55:1–8. https://doi.org/10.1007/s11099-016-0213-y
Wuenscher JE, Kozlowski TT (2010) The response of transpiration resistance to leaf temperature as a desiccation resistance mechanism in tree seedlings. Physiol Plant 24:254–259. https://doi.org/10.1111/j.1399-3054.1971.tb03488.x
Yan Y, WanY LW, Wang X, Yong T, Yang W (2015) Influence of seed treatment with uniconazole powder on soybean growth, photosynthesis, dry matter accumulation after flowering and yield in relay strip intercropping system. Plant Prod Sci 18:295–301. https://doi.org/10.1626/pps.18.295
Acknowledgements
The authors gratefully acknowledge the excellent technical support of Prof. Dianfeng Zheng and Prof. Naijie Feng, language modification of Seth Kutikoff from Kansas State University, and financial support provided by Guangdong Ocean University Innovation Project (230420006).
Author information
Authors and Affiliations
Contributions
HZ: collection of samples, data collection and analysis, and article writing; DZ, NF and XS: methods.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Handling Editor: Hinanit Koltai.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zhou, H., Zheng, D., Feng, N. et al. Effects of Uniconazole on Leaves Photosynthesis, Root Distribution and Yield of Mung Bean (Vigna radiata). J Plant Growth Regul 41, 2629–2637 (2022). https://doi.org/10.1007/s00344-021-10455-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00344-021-10455-7