Abstract
Under light-limiting conditions, many ornamental greenhouse-grown plants show undesired morphological characteristics, such as plant elongation (hypocotyl and epicotyl length) and low dry mass, which reduce plant quality. Research has shown that use of plant growth regulators (PGRs) and changes in both light intensity and spectral composition can reduce these undesired characteristics. However, little is known about the role of the combined effects of supplemental lighting and PGRs on the production of ornamental seedlings. The objective of this study was to characterize the combined and independent effects of light intensity, spectral composition, and PGR applications on the greenhouse production of ornamental transplants. Petunia (Petunia × hybrida), geranium (Pelargonium × hortorum), pansy (Viola × wittrockiana) and dianthus (Dianthus chinensis) were grown for 32–42 days under three supplemental light (SL) treatments: 1) high-pressure sodium (HPS), 2) light-emitting diodes (LEDs) with a 6 blue (B):5 green (G):89 red (R) (percent photon flux ratio), and 3) LEDs with 19B:81R (100 μmol m−2 s−1, 18 h photoperiod for all treatments). A control (No SL) was also included. In addition, a portion of plants were also sprayed with the paclobutrazol PGR (PBZ and No PBZ). The synergistic effects of the combination of PBZ and supplemental lighting resulted in the most compact plants, caused by a reduction in plant height by PBZ and an increase in dry mass by SL. However, PBZ reduced shoot dry mass of most plant species and light combinations. Plant compactness was greater under the 6B:5G:89R LED composition for petunia and when combined with PBZ for geranium than for plants under HPS lighting. Root dry mass of petunia, geranium, and pansy plants increased in response to SL compared with no SL by 2.4–5.7-fold. Results from the two LED spectra were unexpected; plants under 6B:5G:89R were more compact (petunia, geranium), had higher anthocyanin concentrations (petunia), were shorter (petunia, pansy, dianthus) and had less leaf area (petunia, pansy, dianthus) than plants in the SL treatment with a higher B and lower G PF (19B:81R). Supplemental lighting and PBZ can be used in conjunction or independently to improve plant morphology. The increased light from SL provided the most benefits by improving dry mass, compactness, and leaf number for most plant species. However, when PBZ was used in combination with SL, plant compactness increased for some species. The spectral composition of SL had an impact on plant growth and morphology, warranting additional research on plant responses to small changes in the spectral composition of SL.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Plant growth regulators (PGRs) affect the balance of naturally synthesized plant hormones. Most common PGRs are exogenous hormones (naturally occurring or synthetic analogs) that inhibit the biosynthesis and translocation of endogenous hormones or block hormone receptors (Rademacher 2015). The main groups of endogenous plant hormones include auxins, gibberellins, cytokinins, abscisic acid, and ethylene. Among the different PGRs used in horticulture, gibberellin (GA) biosynthesis inhibitors represent one of the most important PGR groups in terms of area treated and economic value (Rademacher 2015). One of the nine main GA inhibitors (chlormequat chlorine, mepiquat chloride, ancymidol, flurprimidol, paclobutrazol, uniconazole-P, daminozide, trinexapac-ethyl-enecarboxylate, and prohexadione-calcium), paclobutrazol (PBZ) is often used in ornamental horticultural operations to control stem elongation and final plant height (Iftikhar et al. 2015; Whipker 2013). Paclobutrazol belongs to the group of active triazoles, and together with uniconazole, it is one of the most studied compounds (Davis et al. 1991). Paclobutrazol and other triazoles reduce shoot elongation through GA biosynthesis inhibition by blocking the P450 enzyme kaurene oxidase (Davis et al. 1991; Yokota 1999). In addition to their ability to reduce stem elongation, triazoles are also known to increase root growth (Berova and Zlatev 2000; Burrows et al. 1992; Moreno et al. 2011), increase stem thickness (Berova and Zlatev 2000; Tsegaw et al. 2005), increase photosynthetic activity (Abdul Jaleel et al. 2007; Berova and Zlatev 2000), lower water use (Iftikhar et al. 2013), and increase early fruit yields (Berova and Zlatev 2000). For the regulation of ornamental plant growth specifically, PBZ is often applied using different methods to increase plant compactness. For example, using PBZ as a liner dip (4–16 mg L−1), Blanchard and Runkle (2007) found a stem length inhibition range of 21–67% compared to that of nontreated plants of five different genera (Argyranthemum, Calibrachoa, Petunia, Scaevola, and Verbena). Using drench (0.45–1.12 mg active ingredient per pot (a.i. pot−1)) and spray (0.16–0.65 mg a.i. pot−1) PBZ applications, Bañón et al. (2002) showed Dianthus caryophyllus ‘Mondriaan’ height was 52–64% and 9–45% lower, respectively, compared to that of nontreated plants. Compared with untreated controls, geranium plants (Pelargonium × hortorum ‘Mustang’) to which PBZ was applied as a drench (0.6 mg a.i. pot−1) or spray (100 mg L−1) presented 47% and 57% lower plant heights, respectively (Cox 1991). Magnitskiy et al. (2006) treated pansy (Viola × wittrockiana ‘Bingo Yellow Blotch’) seeds with a 30 mg L−1 (seed soaking) PBZ solution for 5 min and found a 25% reduction in plant height 40–54 days after sowing compared to that of the untreated controls. Even though GA biosynthesis inhibitors such as PBZ often offer several positive outcomes when used in ornamental plant production, there are also several challenges associated with their use. For example, toxicity to aquatic invertebrates (Liu et al. 2016; MDAR. 2012) and undesired persistence of PBZ in the production system/environment (Adriansen and Odgaard 1997; Grimstad 1993; Ochoa et al. 2009; Sharma and Awasthi 2005). Paclobutrazol concentrations as low as 0.05 mg L−1 were found to be toxic to “water fleas” Daphnia magna embryos, suggesting that PBZ may cause acute and teratogenic effects on embryos and neonates of D. magna (Wang et al. (2011).
In most plant species, plant height and other morphological characteristics are frequently altered by variations in light intensity and spectral composition (Currey et al. 2012; Hernández and Kubota 2016; Lopez and Runkle 2017; Mitchell et al. 2015). A technique that can be used as an alternative or in conjunction with PGRs to manipulate plant growth is the use of supplemental electrical lighting. Light-emitting diodes (LEDs) and other supplemental lighting (SL) technology are commercially used to increase the cumulative amount of light (daily light integral: mol m−2 d−1) to increase plant growth, yield and cumulative light are linearly correlated (under not saturating light intensities) (Kubota et al. 2016; Marcelis et al. 2006). In addition, with LEDs, it is possible to customize the spectral output to manipulate plant morphology. For example, Poel and Runkle (2017) showed that snapdragon (Antirrhinum, ‘Montego Yellow’) seedlings grown under a supplemental LED spectral composition consisting of 45% blue (B) photon flux (PF) and 55% red (R) PF (45B:55R) presented approximately 25% lower plant height than did plants under 10B:90R. Hernández and Kubota (2015) grew cucumber (Cucumis sativus, ‘Cumlaude’) seedlings and found that, compared with plants under the 100B:0R treatment, plants under 0B:100R had 31% shorter hypocotyls. Currey and Lopez (2013) showed that petunia ‘Suncatcher Midnight Blue’ ornamental plants grown in a greenhouse under 0B:100R LED SL had 11% shorter stems than did plants grown under conventional high-pressure sodium (HPS) supplemental lighting. However, in the same study, Impatiens hawkeri (‘Celebrette Frost’) and geranium (‘Designer Bright Red’) showed no differences between spectral treatments. Even though the addition of SL to a production system often increases growth and improves plant morphology, its adoption often requires high capital investment and additional operational costs (electricity costs).
Research reports have separately described the impact of PBZ and SL on ornamental plant growth and development; however, to our knowledge, no studies have compared the growth and morphology of ornamental plants exposed to PGR application and different SL spectra. PBZ affect plant growth mainly by GA biosynthesis inhibition, while light can affect plant growth and morphology by the effect of light intensity, light spectrum, and their interaction (Kendrick and Kronenberg 1994). Light intensity or daily light integral mainly affects plant growth and indirectly morphology via photosynthesis. Furthermore, light quality perception which is accomplished be the differentiation of the number of photons of different discrete wavebands also affects plant morphology and indirectly growth via multiple receptors (UVR8, phytochrome, cryptochrome, phototropin, ZTL/FKF1/LKP2, and others). Therefore, the hypothesis of the present study was that light optimization and PBZ application will work synergistically or additive to improve plant quality (growth, compactness, morphology). Furthermore, the objective of this study was to compare the plant growth and morphology under different SL (light intensity) spectra (light quality) and the application of PBZ to quantify the benefits of each technique independently or together.
Materials and Methods
Plant Material and Growing Conditions
Four ornamental plant species were investigated: Petunia × hybrida (petunia ‘Dreams Midnight’), Dianthus chinensis (dianthus ‘Super Parfait’ Mix), Pelargonium × hortorum (geranium ‘Maverick Red’), and Viola × wittrockiana (pansy ‘Delta Pure Red’) (Ball Horticultural, Co., West Chicago, IL, USA). Plants were germinated and grown (in the same tray) in a 156 m2 glass greenhouse at the Horticulture Field Laboratory of North Carolina State University (35°47′29"N, 078°41′54"W). The air exchange and temperature of the greenhouse were controlled by cooling pads, exhaust fans, and heaters managed by a climate control system (Priva, De Lier, The Netherlands). The greenhouse was divided into five growing areas: four areas for the different lighting treatments (see the lighting treatment section for more details) and one larger area for seed germination under a mist system and nonsupplemental lighting. The four plant species were evaluated simultaneously at two different times (replications one and two) following the same growing practices (Table 1). The greenhouse environment was maintained under similar conditions between the two replications, and differences between the two were minimal (∆T = 0.2 °C; ∆ daytime RH = 2.4%; ∆ night-time RH = 2.6%; ∆ solar DLI = 0.2 mol m−2 d−1). Seeds were germinated at 22.8 ± 2.6 and 22.4 ± 2.5 °C during replications one and two, respectively.
After germination (4–8 days), the trays were moved to one of the four lighting treatments. In each treatment, the air temperature was measured at 2–4 mm under the leaves with fine-wire thermocouples (type T, wire diameter of 0.13 mm, Omega, Inc., Stamford, CT, USA), and the position of the thermocouples was adjusted daily under the youngest expanded leaf. Photosynthetic photon flux density (PPFD) and relative humidity were measured in the middle of the greenhouse using an LI-190R/LI-1500 quantum sensor/logger (LI-COR, Inc., Lincoln, NE, USA) and a CS215L probe (Campbell Scientific, Inc., Logan, UT, USA) with a fan-aspirated shield, respectively (Fig. 1b and Table 2). The temperature and humidity sensors were connected to a data acquisition system (CR1000 logger, Campbell Scientific Inc.), and data were recorded each minute.
(a) Average photon flux density (PFD) per wavelength of sunlight in the greenhouse (GH), under HPS lighting, and under two LED fixtures. The average photosynthetic photon flux density (PPFD) (400–700 nm) of sunlight (83.4 µmol m−2 s−1) and the supplemental light treatments (100 µmol m−2 s−1) is displayed in the graph. (b) PPFD of sunlight throughout the day for the two replications; the horizontal dashed line indicates the average PPFD of the sunlight in both replications
A total of 1152 plug plants were grown per crop and replication and plant densities were maintained at 1887 plants m−2 using 288-cell trays (28 cm × 54.5 cm). The trays were filled with peat moss-based horticultural substrate (1P-RSI-Fafard, Sun Gro Horticulture, Agawam, MA, USA), and substrate moisture and fertilization were adjusted according to industry recommendations (Ballseed 2009, 2016). Substrate moisture levels were maintained during the first week using a mist system. After the first week, the moisture level was maintained for all the crops with a mist system and subirrigated with nutrient solution as needed (NS) (Table 1). The average pH of the tap water was 7.34, and the electrical conductivity (EC) was 0.24 dS m−1. However, the NS pH was 6.09, and the EC was 1.04 dS m−1 for a target of 100 mg L−1 nitrogen. The fertilizer source was Ultrasol™ 13 N-2P2O5-13K2O (SQM North America, Atlanta, GA, USA).
Light Treatments
Plants were grown under four light conditions: 1) sunlight without SL (No SL); 2) sunlight supplemented with high-pressure sodium (HPS, 400 W) lamp; 3) sunlight supplemented with a PF ratio of 6% B:5% green (G):89% R (6B:5G:89R) light provided by LEDs (Greenpower LED Top Lighting Deep Red/White, Philips, Eindhoven, NL); and 4) sunlight supplemented with 19% B and 89% R (19B:89R) light provided by LEDs (Greenpower LED Top Lighting Deep Red/Blue, Philips) (Table 3). The average supplemental PPFD for the three SL treatments was maintained at ~ 100 μmol m−2 s−1 at the canopy level by changing the height of the light fixtures. For each SL treatment, 20 PPFD measurements were taken at each growing area using a quantum meter (LI-1500 Sensor Logger and LI-190R Quantum Sensor, LI-COR, Lincoln, Nebraska, USA). Supplemental light fixtures were on daily from 0600 to 2200 MT (16 h). The solar photoperiod varied from 11.13 to 9.75 h (h), and the average solar photoperiod measured during the experiment was 10.7 h (0730 to 1810 MT). Details for light environment per each treatment are listed in Table 3 (Sager et al. 1988). Plants in the No SL treatment were only exposed to the solar photoperiod (10.7 h) in order to match commercial greenhouse conditions without SL. A 50% shade screen (Harmony Revolux XLS FR, Ludvig Svensson, Raleigh, NC, USA) was deployed in the greenhouse to better control the temperature and reduce the solar light intensity to simulate low solar radiation typical of winter/spring greenhouse plug production. In addition, supplemental light contamination between the four lighting treatments was prevented by hanging blackout curtains (Sunfilm®, Hawthorne Company, Vancouver, WA, CA) from a height above the light fixtures to the bench level.
Paclobutrazol Treatment
All the trays under the No SL, HPS light, and 6B:5G:89R treatments were divided into four Sects. (14 × 27.25 cm). Two sections were sprayed with PBZ (Piccolo®, Fine Americas, Inc., Walnut Creek, CA, USA) according to the information in Table 1, and two sections were not treated with PBZ and spray with tap water (No PBZ). The spray volume for all the crops was 0.2 L m−2, and the PBZ concentrations are described in Table 1. None of the plants under 19B:81R were treated with PBZ (No PBZ). However, the trays under 19B:81R were also divided into four groups for rotation purposes.
Chlorophyll and Anthocyanin Contents
For chlorophyll analysis, two fresh leaf samples (57 mm2 area) were collected from each plant (6 plants total–two largest leaves) during final data collection (39–50 days, Table 1) and extracted in 4 mL of N,N-dimethylformamide for 24 h in 4 °C. The absorbance of the extraction solution was subsequently measured by spectrophotometer (Genesys 10 s uv–vis, Thermo Fisher Scientific, Waltham, Massachusetts, USA) at 647 and 664 nm for determination of chlorophyll according to the methods of Moran and Porath (1980). Anthocyanin quantification was performed for plant species and tissues exhibiting purple coloration. Methods of Li and Kubota (2009) were followed. All the leaves and stems used for petunia, and all the leaves were used for geranium. Anthocyanin was measured on a per dry mass basis (mg g−1) for all leaves or the entire plant in order to quantify the impact on the majority of the shoot.
The samples were freeze dried and weighed immediately, and the results were expressed on a dry mass basis.
Biomass and Morphology Measurements
At the end of the experiment, plant height, number of leaves greater than 1 cm, and fresh mass of the shoots were measured. In addition, all leaves were scanned to determine the leaf area using ImageJ (NIH, Laboratory for Optical and Computational Instrumentation, Madison, WI, USA). The plant height was measured from the soil line to the top of the tallest leaf. Samples of the shoots and roots were dried in an oven at 70 °C for at least 4 days and then weighed. Root dry mass was not collected for dianthus since the roots were too small for accurate quantification. Shoot compactness was calculated as the ratio of shoot dry mass to plant height.
Statistical Analysis
Analysis of variance (ANOVA) and mean separations via the Tukey–Kramer HSD test (alpha = 0.05) were computed using JMP Pro v12 (SAS Institute, Cary, NC, USA). The light (No SL, HPS, and 6B:5G:89R) and PBZ (PBZ and no PBZ) treatments were analyzed using a split-plot design where the whole-plot factor was the light, and the split-plot factor was the PBZ treatment. The whole plot was a full tray with a single plant species and individual lighting conditions. The split plots were the four sections within a tray (PBZ or No PBZ). Comparisons between the two LED treatments were performed t-test. In both cases, the analysis was performed by plant species. For greenhouse location error reduction, we rotated the lighting treatments daily as well as the location of the trays. We collected ten subsamples (individual plant within each tray section) for biomass/morphological determinations and six subsamples for chlorophyll and anthocyanin quantifications from each tray section. All samples were collected from the center of the tray to prevent any edge effects.
Results and Discussion
Effects of PGR and Supplemental Lighting
Plant Height
Plants treated with PBZ were shorter compared to plants without PBZ (No PBZ), except for pansy under SL (Tables 4,5,6,7; Fig. 2). Plant height was 20%, 35%, 23–43%, and 22% shorter in response to PBZ treatment for petunia, geranium, pansy (No SL), and dianthus, respectively. Shorter plants under PBZ were expected and the values were within the percentages presented in previous research for petunia (Blanchard and Runkle 2007), geranium (Cox 1991), pansy (Magnitskiy et al. 2006) and dianthus (Bañón et al. (2002). The plant height response to PBZ assures that the PGR application rates were adequate for the tested plant species.
Petunia plants in the 6B:5G:89R LED treatment were 64% and 46% shorter than the plants under the No SL and HPS light treatments, respectively (Table 4). Plant height was also affected by the interaction between SL and PBZ in pansy (Table 6). Pansy plants under 6B:5G:89R were 37% shorter than the control plants (no PBZ and No SL); however, when PBZ was applied, height was similar across all lighting treatments. Furthermore, the application of PBZ resulted in shorter plants under no SL; however, the application of PBZ did not reduce plant height in plants receiving SL (HPS and 6B:5G:89R). (Table 6). However, for geranium and dianthus, the light treatments did not have a significant effect on plant height (Tables 5, 7).
The effects of the light spectral composition on plant height are species specific (Currey and Lopez 2013; Hernández and Kubota 2016; Poel and Runkle 2017; Spalholz et al. 2020; Wollaeger and Runkle 2014). In the present experiment, the spectral composition of the LED treatment (6B:5G:89R) resulted in shorter petunia and pansy plants compared to plants in the HPS light treatment. Similar results were found for petunia when the plants were grown under HPS light vs. 100R LED light (Currey and Lopez 2013). The spectral composition of the SL in this experiment was 5B:54G:41R for the HPS light and 6B:5G:89R for the LED light (Table 3; Fig. 1). The increased percentage of G light is a possible explanation for the increase in plant height under HPS light compared to that of plants under 6B:5G:89R. Green light is known to activate the shade-avoidance syndrome (SAS) (Wang and Folta 2013; Zhang et al. 2011) and to reduce the expression of responses triggered by B light (reduced stem elongation) (Wang and Folta 2013; Wang et al. 2013). In the HPS light spectral composition, the amount of green light was 10.8 times greater than that in the LED treatment, which increased the SAS (increased stem length) and decreased cryptochrome-related responses (reduced stem elongation), resulting in taller plants. In addition to the increase in G light in the HPS light treatment, the 6B:5G:89R treatment had 2.2 times greater R PPFD and a higher red to far-red ratio (Table 3). Red light at the end of the day (high red to far-red ratio) triggers phytochrome-related responses by increasing the amount of active phytochrome (PFR). An increased amount of PFR before darkness will decrease plant height (Decoteau and Friend 1991; Kasperbauer and Peaslee 1973). It is plausible that plants under the 6B:5G:89R treatment had a greater amount of PFR at the end of the day than did plants under the HPS light treatment and, consequently, experienced greater inhibition of stem elongation.
Another possible explanation for the greater plant height under HPS light is to the increase in average leaf temperature by the greater infrared radiation emitted by the fixture. High-pressure sodium light fixtures emit radiation in the wavelength range of 800–2500 nm, and this radiation can increase leaf temperature by 0.5–2.0 °C in the greenhouse (Bergstrand and Schussler 2013; Dueck et al. 2012; Islam et al. 2012; Nelson and Bugbee 2015). Even though the air temperature measured at the leaf boundary layer in the HPS light treatment was only 0.5–0.7 °C higher than that in the LED treatments (Table 2), the actual leaf temperature was likely higher (not measured in this study). A higher leaf temperature has been associated with stem elongation (Gray et al. 1998; Hernández and Kubota 2015). Hernández and Kubota (2015) also found that, compared with those under supplemental LED light, cucumber seedlings under HPS light had a 0.5–0.7 °C higher leaf temperature, 36–50% greater epicotyl length and 46–61% greater hypocotyl length.
In addition to the greater average leaf temperature, temperature differential between day and night could have also contributed to the greater plant height in HPS. In the present experiment, a relatively warm leaf temperature during the day under the HPS lights could have impacted plant height because of the temperature differential (DIF: day and night differential) (Erwin et al. 1994). Plants under both SL treatments were subjected to the same night-time temperature (lights off), but plants in the HPS light treatment experienced higher daytime leaf temperatures. Consequently, the temperature differential between day and night was greater in plants under the HPS light treatment than under 6B:5G:89R LEDs, leading to increased stem elongation and reduced plant compactness. Erwin et al. (1994) grew Lilium longiflorum under different day and night-time temperature treatments and found an increase in internode length with an increase in daytime temperature under constant night-time temperature. Similarly, Erwin and Heins (1991) found that geranium stem elongation increased as the difference between the daytime and night-time temperature increased.
Shoot Dry Mass
For all the studied plant species, PBZ significantly decreased plant dry mass. For example, the average growth reduction by species was 10.2%, 13.5%, 17.4% and 21.4% for petunia, pansy, geranium, and dianthus, respectively (Tables 4,5,6,7). A reduction in growth by PBZ has been reported for ornamental seedling production (Getter 2015; Rezazadeh and Harkess 2015). For example, Getter (2015) showed a 41–47% lower geranium dry mass with the application of PBZ (30–45 mg L−1).
In our experiment, the SL treatments had 2.9 times greater DLI than did the No SL treatments (SL, 9.3 mol m−2 d−1; No SL, 3.2 mol m−2 d−1; Table 3); similarly, petunia plants had 2.9 times greater shoot dry mass than did those in the No SL treatment, and the geranium, pansy, and dianthus plants had 2.7 times greater shoot dry mass. The difference in spectral composition (HPS vs. 6B:5G:89R LED) had no significant effect on shoot dry mass (Tables 4,5,6,7). Interestingly, for geranium and dianthus, the depression in growth by PBZ was greater when SL lighting was used. For example, in geranium, the reduction in growth by PBZ was 13–21% in plants with SL (HPS and 6B:5G:89R LED) and only 7% in plants under No SL (Table 5). Similarly, in dianthus, the reduction in growth by PBZ was 20% in plants under HPS lighting and only 5.3% under No SL (Table 5).
The dry mass increase by SL was expected in this study, as a higher DLI is linearly correlated with increased plant growth (Kubota et al. 2016; Marcelis et al. 2006). The greater reduction in dry mass by PBZ for plants under SL can be explained by a reduction in leaf area (see leaf area section); therefore, the positive impact of SL on dry mass was not fully achieved in plants treated with PBZ.
Shoot Compactness
Increased shoot compactness, which is a ratio of shoot dry mass to plant height (g cm−1) provides an indication of plant “stockiness” or “sturdiness”. For all the plant species, PBZ significantly increased shoot compactness. For example, shoot compactness was 14%, 30%, 16.5%, and 7.5% greater with PBZ for petunia, geranium, pansy, and dianthus plants, respectively, than for plants without PBZ application (Tables 4,5,6,7).
Petunia plants under HPS and 6B:5G:89R LEDs had 4.4 times and 7.3 times greater shoot compactness, respectively, than did those under No SL. Petunia plants under 6B:5G:89R LEDs had 66% greater shoot compactness than did those under HPS lighting. Geranium plants without PBZ and without SL (No PBZ/No SL) were less compact than plants treated with PBZ and with SL. Interestingly, geranium plants under 6B:5G:89R LEDs without PBZ were 15% more compact than were plants under HPS lighting without PBZ, and the compactness of these plants was similar to plants under HPS lighting with PBZ. In addition, geranium plants under 6B:5G:89R LEDs with PBZ presented the greatest shoot compactness of all treatments. For pansy and dianthus, SL increased shoot compactness by 3.3–3.5 and 2.1–2.4 times, respectively, compared to that under No SL.
In the present experiment, PBZ reduced both plant height and shoot dry mass for all the plant species. However, the effects on plant height were greater than those on dry mass, thereby increasing the overall compactness and plant quality. The addition of SL further increased plant compactness, mainly by increasing plant dry mass. Consequently, treatments with both SL and PBZ had the greatest shoot compactness. The synergistic effect of PBZ and SL can be explained by two different plant physiological responses. Paclobutrazol mainly increased compactness by reducing shoot elongation through GA biosynthesis inhibition by blocking the P450 enzyme kaurene oxidase (Davis et al. 1991; Yokota 1999), and SL increased compactness by increasing the rate of photosynthesis and accompanying growth rate associated with an increase in cumulative PPFD (increase in light), especially under the low natural-light conditions in this experiment (Table 3).
One unexpected result was the higher plant compactness under 6B:5G:89R LEDs than under HPS SL for geranium and petunia. A plausible explanation is the higher leaf temperature of plants under the HPS light spectral composition compared with the other treatments. Warmer leaf temperature can increase plant height (see plant height section) and plant growth (Adams et al. 2001; Lieth and Pasian 1990). In the present study, plants under HPS had significant greater plant height and higher dry mass (not significant) than plants in 6B:5G:89R LEDs; however, plant height increased at a higher degree than shoot dry mass. Therefore, the lower shoot compactness in HPS is mainly attributed to the impact on plant height.
Another plausible explanation for the difference in plant compactness could be due to the influence of the light spectral composition on plant dry mass and plant height discussed in the previous sections (the plant height and dry mass sections).
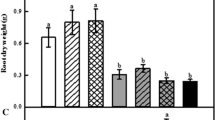
Root Dry Mass
Root dry mass was measured for petunia, geranium, and pansy (Tables 4,5,6). Paclobutrazol did not have a negative effect on root dry mass except for pansy plants grown under HPS light (Table 6). Compared with the No SL, HPS and 6B:5G:89R LED lighting increased root dry mass by 4.9–5.0, 2.4–2.5, and 5.5–5.7 times for the petunia, geranium, and pansy, respectively.
Previous studies have shown that PBZ can increase root mass (Berova and Zlatev 2000; Davis et al. 1991; Gao et al. 1988); however, in the present study, no benefit from PBZ on root growth was observed. Root dry mass is one of the most important characteristics of young plants since well-developed roots can improve plant survival and performance after transplanting (Soundy et al. 2005). In this study, the benefit of SL on increasing root dry mass can be explained by the increased growth rate due to the increased DLI, as described in the previous section (dry mass).
Interestingly, under HPS supplemental lighting, the root dry mass of pansy decreased by 16% in response to PBZ application. This response may be related to the overall reduction in growth caused by PBZ (reduced shoot dry mass, smaller leaf area). Nevertheless, this was not the case in the other plant species (petunia and geranium), whose shoot dry mass decreased in response to PBZ; however, no effect on root dry mass was observed.
Shoot Fresh Mass
For all the plant species, PBZ significantly decreased shoot fresh mass. For example, shoot fresh mass was 13%, 30%, 22%, and 22% greater without PBZ for petunia, geranium, pansy, and dianthus, respectively, compared with plants treated with PBZ (Tables 4,5,6,7).
The increase in fresh mass by SL can be explained by the increase in dry mass caused by greater DLI, since they are closely correlated. However, the impact of SL spectra on fresh mass was species specific. For example, petunia fresh mass under HPS lighting was on average 91% greater than that under 6B:5G:89R LEDs and No SL. Geranium plants under HPS-No PBZ had 80 and 120% greater fresh mass than under No SL with and without PBZ, respectively. Pansy plants under HPS lighting had 70% greater fresh mass than did plants under No SL. For dianthus, SL did not have a significant effect on fresh mass. Petunia plants are both high light tolerant and heat tolerant (USDA hardiness zones 8–11)(Gilman and Landrum 1999), and even though DLI and temperature in this experiment were relatively low, the combination of higher DLI and higher radiative heat from the HPS light treatment will likely have a greater impact on fresh mass and dry mass accumulation for this species than for species less acclimated to the increase in temperature, such as dianthus (hardiness zones 3–9) (Dansereau et al. 2007). Another plausible explanation for the increased fresh mass under HPS lighting compared to 6B:5G:89R LEDs (petunia and geranium) is the difference in spectral composition. Plants under HPS lighting were exposed to a greater percent G photon flux (54% under HPS lighting, 4.7% under 6B:5G:89R). Within the spectral composition, green light is known to trigger shade-avoidance responses such as stem elongation, which is achieved by greater turgor pressure (increased water in cell) and, consequently, increased cell wall extensibility (Sasidharan et al. 2008). Kim et al. (2004) showed that lettuce (Lactuca sativa, ‘Waldmann’s Green’) grown under a 15B:24G:61R spectral composition had 45% greater fresh mass than did lettuce grown under a 16B:84R spectral composition, and Hernández and Kubota (2015) found that cucumber transplants grown under HPS light had 28–31% greater fresh mass than did those grown under LED SL treatments.
Leaf Area and Leaf Number
For all the plant species, PBZ significantly decreased the leaf area. For example, leaf area was 20%, 43%, 21%, and 32% greater for petunia, geranium, pansy, and dianthus plants, respectively, not treated with PBZ compared with plants treated with PBZ (Tables 4,5,6,7). Applications of PBZ are known to reduce leaf area (Bahlebi et al. 2017; Burrows et al. 1992; Cox 1991); for example, Bahlebi et al. (2017) showed a 26–29% reduction in leaf area of potted geranium (Pelargonium spp.) plants in response to application of sprayed PBZ (100 mg L−1). Petunia leaf area under HPS lighting was 38% and 79% greater than that under No SL and 6B:5G:89R LEDs, respectively. The increase in leaf area in petunia under HPS lighting compared to No SL can be explained by the greater number of leaves observed under the HPS light treatment (Table 4). Supplemental lighting did not have a significant effect on leaf area for geranium, pansy, and dianthus.
PBZ significantly decreased the number of leaves by 7% and 6% for petunia and dianthus, respectively (Tables 4 and 7). However, PBZ did not have an effect on the leaf number of geranium and pansy plants. Petunia under HPS supplemental lighting had 39% more leaves compared with petunia under No SL. Similarly, geranium under both HPS and 6B:5G:89R LEDs had 22–28% more leaves compared with No SL. Pansy under HPS lighting had 32% and 8% more leaves compared with plants under No SL and 6B:5G:89R LEDs, respectively. In addition, pansy plants had 21% more leaves under 6B:5G:89R LEDs than under No SL. Dianthus had comparable leaf numbers in all SL treatments.
The increase in leaf number by SL can be explained by the increased growth rate caused by additional light, as described in a previous section (dry mass). The greater leaf number under the HPS light treatment compared to the 6B:5G:89R LED treatment can be explained by the greater leaf temperature under the HPS light treatment. Leaf initiation rates between a minimum (base) and optimum temperature for a crop are linearly correlated with an increase in temperature (Adams et al. 2001; Lieth and Pasian 1990; Miglietta 1989).
Chlorophyll Concentration
Chlorophyll concentration increased by 12%, 27%, and 13% in response to PBZ application in petunia, geranium, and dianthus, respectively (Tables 4, 5 and 7). In addition, petunia had 52% and 66% greater chlorophyll concentrations in the HPS and 6B:5G:89R LED SL treatments, respectively than in the No SL treatment. Pansy plants under HPS lighting and treated with PBZ had 25–26% higher chlorophyll concentrations than plants under 6B:5G:89R LEDs with PBZ application and plants without SL (PBZ and no PBZ). Increased chlorophyll concentrations caused by the application of PBZ have been reported extensively in the literature. Berova and Zlatev (2000) found a 21–23% increase in chlorophyll concentration in tomato (Lycopersicon esculentum Mill, ‘Precador’) plants treated with foliar (25 mg L−1) and soil-based (1 mg L−1) applications of PBZ. The reason for the increase in chlorophyll concentration by PBZ has not been fully characterized; however, two possible explanations have been proposed. One possible explanation is the relatively high density of chloroplasts per leaf area resulting from the overall reduction in leaf area by PBZ (Balamani and Poovaiah 1985; Bandara and Tanino 1995; Barnes et al. 1989; Khalil 1995; Tekalign and Hammes 2004). The rationale being that PBZ does not affect cell division, just cell expansion; therefore, there are more cells (and possibly chloroplasts) concentrated into a smaller area. Another possible explanation is an increase in chloroplast differentiation and chlorophyll biosynthesis, as well as a reduction in chlorophyll degradation caused by PBZ stimulation of cytokinin biosynthesis (Berova and Zlatev 2000; Izumi et al. 1988; Khalil 1995; Tekalign and Hammes 2004).
Increased chlorophyll concentration in response to SL have been reported (Hao and Papadopoulos 1999; Hernández and Kubota 2014), and these increases were attributed to increases in light intensity and growth rate. An interesting result was observed in pansy under HPS with PBZ; these plants had a greater chlorophyll concentration than did plants under 6B:5G:89R LEDs with PBZ, granting further investigation.
Anthocyanin Concentration
The anthocyanin concentration in the shoots of petunia and in the leaves of geranium varied by plant species, PBZ application, and SL. Paclobutrazol had no effect on the anthocyanin concentration in petunia. Interestingly, petunia plants under 6B:5G:89R LEDs had 7.4–8.2 times more anthocyanins than did those under No SL or HPS lighting; this trend was similar but not significant in geranium plants. Geranium plants treated with PBZ had 175% greater anthocyanin concentrations than did those without PBZ. In addition, geranium plants under HPS lighting and 6B:5G:89R LEDs had 6.0–8.6 times greater anthocyanin concentrations than did plants under No SL.
Anthocyanins are known to accumulate in vegetative tissues under stress conditions, such as high light, low temperature, and nutrient deficiency (Albert et al. 2009; Chalker-Scott 1999; Dixon and Paiva 1995). In contrast, studies have also shown that the accumulation of anthocyanins does not have a negative effect on young plant growth or photosynthesis (Gould 2004; Hernández et al. 2016). The roles of anthocyanin accumulation in vegetative tissues are not yet fully understood; however, several explanations have been proposed. Studies have suggested that anthocyanins can protect leaf tissues from the absorption of excessive radiation and resulting photoinhibition, reducing the amount of light reaching the chloroplasts (Feild et al. 2001; Hughes et al. 2005; Merzlyak et al. 2008). Anthocyanins in vegetative tissues are also known to increase both light saturation and light compensation points (Albert et al. 2009).
The light spectral composition is also known to impact vegetative anthocyanin accumulation. For example, an increase in B PF has been shown to increase anthocyanin accumulation (Li and Kubota 2009; Owen and Lopez 2015; Spalholz et al. 2020). Owen and Lopez (2015) provided an end-of-production light treatment with high B PF to four lettuce cultivars and recommended a minimum of 5 days of exposure to 100 µmol m−2 s−1 of 50B:50R to increase red coloration (anthocyanins) of leaf tissue. However, the percent B PF is not likely impacting anthocyanin concentration in the present study since percent B photon flux was comparable between the HPS and 6B:5G:89R LED treatment (Table 3).
An increase in G PF is known to reduce the effects caused by B PF (Folta and Maruhnich 2007), including a reduction in anthocyanin accumulation. In the present study, plants under HPS lighting and 6B:5G:89R LEDs were exposed to similar percentages of B PF (5.3% B under HPS lighting; 6.1% B under 6B:5G:89R LEDs); however, plants under the HPS light had a greater percentage of G PF (40.5% B under HPS lighting; 4.7% B under 6B:5G:89R), which may have reduced vegetative anthocyanin accumulation under HPS lighting in petunia.
The effects of PBZ on increased anthocyanin concentrations have been reported previously. For example, Hajihashemi (2018) showed an increase in anthocyanins in Stevia rebaudiana with drench application of PBZ (10 mg L−1), and (Gopi et al. 2007) showed that Daucus carota had 24% and 51% greater anthocyanin concentrations in the leaves and taproot, respectively, with the soil drench application of PBZ (20 mg L−1 plant −1). Abscisic acid is known to initiate (Gagné et al. 2011) and increase (Jiang and Joyce 2003) anthocyanin production. Therefore, the increase in anthocyanins in response to PBZ can be explained by an increase in abscisic acid caused by triazole applications to plants (Asare-Boamah et al. 1986; Hsu and Kao 2005).
Effects of LED Spectra
Petunia, pansy, and dianthus plants under 19B:81R were 59%, 21% and 3% taller, respectively, than those under 6B:5G:89R (Table 8). For all plant species, there were no significant differences in shoot or root dry mas under either LED SL treatment. In petunia, those under 19B:81R were 12% less compact than plants under 6B:5G:89R (Table 8). Compared with those under 6B:5G:89R, petunia and pansy plants under 19B:81R had 75% and 20%, respectively, greater shoot fresh mas. Similarly, compared with those under 6B:5G:89R, petunia, pansy and dianthus plants under 19B:81R had 68%, 20%, and 4.4%, respectively, greater leaf area (Table 8). Compared with those under 6B:5G:89R, petunia and pansy plants under 19B:81R had 16% and 5% more leaves, respectively (Table 8). Compared with those under 19B:81R, petunia plants under 6B:5G:89R had a 4% greater chlorophyll concentration (Table 8) and 274% (3.7x) greater anthocyanin concentrations (Fig. 3) compared with those under 19B:81R.
Four ornamental species were grown in a greenhouse at a density of 1887 plants m−2 (288-cell tray) without supplemental light (No SL) or with supplemental light (HPS lighting, 6B:5B:81R via LEDs, 19B:81R via LEDs) and without paclobutrazol or with paclobutrazol (+ PBZ) application. SL was provided by a high-pressure sodium (HPS) light or by one of two LED fixtures, which produced spectral compositions of 6B:5G81R or 19B:81R. The LED treatments are labeled according to their percent photon flux density of blue (B), green (G), and red (R) light. The arrows indicate a 5-cm scale (colour figure online)
Unexpected results in terms of growth and morphology were observed between the two LED treatments. We expected no differences between the two LED spectra since the spectral differences were minimal under SL conditions (broad spectral composition similar to that of natural sunlight). Additionally, we expected higher plant compactness, lower plant height, higher anthocyanin concentration, and lower leaf area with an increase in B PF (19B:81R) through cryptochrome-regulated plant responses (Huché-Thélier et al. 2016). Instead, plants under a relatively lower B PF (6B:5G:89R) were generally more compact (lower plant height and lower leaf area) and had a higher anthocyanin concentration (petunia). We conducted a small follow-up experiment with increased background solar radiation (9.05 mol m−2 d−1) to determine whether plant responses to supplemental LED lighting varied based on the solar DLI (data not shown); however, results were consistent with the results of the current experiment (low solar radiation). One possible explanation is the presence of G light under 6B:5G:89R LEDs that suppressed plant growth compared to that of plants under 19B:81R, since plant height, fresh mass, and leaf area were lower under 6B:5G:89R than under 19B:81R for petunia, pansy, and dianthus (P = 0.076 for dianthus fresh mass). However, there were no significant differences between the two LED treatments in dry mass. Meng et al. (2020) grew lettuce under different LED spectra and found that lettuce plants grown under a spectral composition of 33B:33G:33R had less biomass than plants grown under 11B:89R. In that same study, plants grown under 11B:33G:55R had more biomass than plants grown under 33B:33G:33R, suggesting that small changes in the B:G ratio have an effect on overall growth.
Another plausible explanation is the effect of light quality during the photoperiodic hours without solar radiation. Research has shown the impact of light quality before and after the solar photoperiod. For example, (Chinchilla et al. 2018) provided blue, white, or red supplemental lighting at the end-of-day and/or pre-dawn for greenhouse lettuce (Lactuca sativa) and found that plants grown under end-of-day B had 18% greater shoot fresh mas than those under end-of-day and pre-dawn red. In this study, to achieve an 18 h photoperiod, plants were exposed to ~ 1.5 h and ~ 4 h of SL before sunrise and after sunset, respectively. The impact of the LED spectral composition on plant morphology and growth could have been determined during those hours. However, the responses were still unpredictable based on the SL spectral composition of the treatments.
In summary, the results of the present study suggest that small variations in the B:G ratio can have a significant effect on plant growth and morphology even under similar R PF and SL conditions. The spectral composition of SL should be carefully evaluated before adoption in large-scale greenhouses, and additional research is needed to characterize various plant-specific responses.
Conclusion
Supplemental lighting increased the growth (shoot and root biomass) and compactness of all the studied plant species. Paclobutrazol application, as expected, increased the compactness of all the plant species studied by restricting their height; however, plant growth (dry mass) also decreased. The application of PBZ also reduced leaf area and, consequently, the benefit of SL on plant growth (reduced light capture). Overall, the synergistic effects of the combination of PBZ and SL yielded the most compact plants because of a reduction in plant height and an increase in dry mass. The impact of SL quality was species specific; however, unexpected results were obtained when the effects of the two LED light spectra were compared. Plants under 6B:5G:89R were more compact (petunia, geranium), had higher anthocyanin concentrations (petunia), were shorter (petunia, pansy, dianthus) and had less leaf area (petunia, pansy, dianthus) than plants in the SL LED treatment with higher B PF (19B:81R). To our knowledge, the present study is the first report comparing the impact of both SL (different spectra) and PBZ on several ornamental plant species. Additional research on plant responses to SL quality, as well as on the impact of small changes in the spectral composition on plant growth and morphology is warranted.
References
Abdul Jaleel C, Manivannan P, Sankar B, Kishorekumar A, Sankari S, Panneerselvam R (2007) Paclobutrazol enhances photosynthesis and ajmalicine production in Catharanthus roseus. Process Biochem 42:1566–1570. https://doi.org/10.1016/j.procbio.2007.08.006
Adams SR, Cockshull KE, Cave CRJ (2001) Effect of temperature on the growth and development of tomato fruits. Ann Bot 88:869–877. https://doi.org/10.1006/anbo.2001.1524
Adriansen E, Odgaard P (1997) Residues of paclobutrazol and uniconazole in nutrient solutions from ebb and flood irrigation of pot plants. Sci Hortic 69:73–83. https://doi.org/10.1016/S0304-4238(96)00982-X
Albert NW, Lewis DH, Zhang H, Irving LJ, Jameson PE, Davies KM (2009) Light-induced vegetative anthocyanin pigmentation in Petunia. J Exp Bot 60:2191–2202. https://doi.org/10.1093/jxb/erp097
Asare-Boamah NK, Hofstra G, Fletcher RA, Dumbroff EB (1986) Triadimefon protects bean plants from water stress through its effects on abscisic acid. Plant Cell Physiol 27:383–390. https://doi.org/10.1093/oxfordjournals.pcp.a077114
Bahlebi KE, Ntombekhaya M, Viwe D, Kenias C (2017) Rose-scented geranium (Pelargonium spp.) plant growth, and essential oil yield and composition as affected by paclobutrazol application. HortScience 52:991–995. https://doi.org/10.21273/HORTSCI11452-16
Balamani V, Poovaiah BW (1985) Retardation of shoot growth and promotion of tuber growth of potato plants by paclobutrazol. Am Potato J 62:363–369. https://doi.org/10.1007/BF02855607
Ballseed (2009) Ornamental plants plug growing chart. Ball Horticultural Company. 1 https://www.ballseed.com/PDF/OrnamentalPlants-PlugGrowingChart.pdf. Accessed 29 November 2020
Ballseed (2016) 2017 seed product information guide. Ball Horticultural Company. 75 https://www.ballseed.com/PDF/15539_PIG_ENG_BSC_LR.pdf. Accesed 29 November 2020
Bandara PMS, Tanino KK (1995) Paclobutrazol enhances minituber production in Norland potatoes. J Plant Growth Regul 14:151. https://doi.org/10.1007/BF00210917
Bañón S, González A, Cano EA, Franco JA, Fernández JA (2002) Growth, development and colour response of potted Dianthus caryophyllus cv. Mondriaan to paclobutrazol treatment Sci Hortic 94:371–377. https://doi.org/10.1016/S0304-4238(02)00005-5
Barnes AM, Walser RH, Davis TD (1989) Anatomy of Zea mays and Glycine max seedlings treated with triazole plant growth regulators. Biol Plant 31:370–375. https://doi.org/10.1016/S0304-4238(02)00005-5
Bergstrand KJ, Schussler HK (2013) Growth, development and photosynthesis of some horticultural plants as affected by different dupplementary lighting technologies. Eur J Hortic Sci 78:119–125
Berova M, Zlatev Z (2000) Physiological response and yield of paclobutrazol treated tomato plants (Lycopersicon esculentum Mill.). Plant Growth Regul 30:117–123. https://doi.org/10.1023/A:1006300326975
Blanchard MG, Runkle E (2007) Dipping bedding plant liners in paclobutrazol or uniconazole inhibits subsequent stem extension. HortTechnology 17:178–182. https://doi.org/10.21273/HORTTECH.17.2.178
Burrows GE, Boag TS, Stewart WP (1992) Changes in leaf, stem, and root anatomy of Chrysanthemum cv. Lillian Hoek following paclobutrazol application. J Plant Growth Regul. https://doi.org/10.1007/BF02115476
Chalker-Scott L (1999) Environmental significance of anthocyanins in plant stress responses. Photochem Photobiol 70:1–9. https://doi.org/10.1111/j.1751-1097.1999.tb01944.x
Chinchilla S, Izzo LG, Van Santen E, Gómez C (2018) Growth and physiological responses of lettuce grown under pre-dawn or end-of-day sole-source light-quality treatments. Horticulturae 4:8. https://doi.org/10.3390/horticulturae4020008
Cox DA (1991) Gibberellic acid reverses effects of excess paclobutrazol on geranium. HortScience 26:39–40. https://doi.org/10.21273/HORTSCI.26.1.39
Currey CJ, Lopez RG (2013) Cuttings of impatiens, pelargonium, and petunia propagated under light-emitting diodes and high-pressure sodium lamps have comparable growth, morphology, gas exchange, and post-transplant performance. HortScience 48:428–434. https://doi.org/10.21273/HORTSCI.48.4.428
Currey CJ, Hutchinson VA, Lopez RG (2012) Growth, morphology, and quality of rooted cuttings of several herbaceous annual bedding plants are influenced by photosynthetic daily light integral during root development. HortScience 47:25–30. https://doi.org/10.21273/HORTSCI.47.1.25
Dansereau K, Kessler JR, Lu W (2007) Greenhouse production of dianthus-ANR-1313. Alabama cooperative extension system https://ssl.acesag.auburn.edu/pubs/docs/A/ANR-1313/ANR-1313-archive.pdf. Accessed 30 November 2020
Davis TD, Curry EA, Steffens GL (1991) Chemical regulation of vegetative growth. CRC Crit Rev Plant Sci 10:151–188. https://doi.org/10.1080/07352689109382310
Decoteau DR, Friend HH (1991) Growth and subsequent yield of tomatoes following end-of-day light treatment of transplants. HortScience 26:1528–1530
Dixon RA, Paiva NL (1995) Stress-induced phenylpropanoid metabolism. Plant Cell 7:1085. https://doi.org/10.1105/tpc.7.7.1085
Dueck TA, Janse J, Eveleens BA, Kempkes FLK, Marcelis LFM (2012) Growth of tomatoes under hybrid LED and HPS lighting. In: Kittas C, Katsoulas N, Bartzanas T (eds) International Symposium on Advanced Technologies and Management Towards Sustainable Greenhouse Ecosystems: Greensys 2011, vol 952. Int Soc Horticultural Science, Leuven, pp 335–342
Erwin J, Heins R (1991) Environmental effects on geranium development. Minnesota commercial flower growers association bulletin https://www.plantgrower.org/uploads/6/5/5/4/65545169/environmental_effects_on_geranium_development_mcfga_erwin_1992_ocr.pdf. Accessed 30 November 2020
Erwin J, Velguth P, Heins R (1994) Day/night temperature environment affects cell elongation but not division in Lilium longiflorum Thunb. J Exp Bot 45:1019–1025. https://doi.org/10.1093/jxb/45.7.1019
Feild TS, Lee DW, Holbrook NM (2001) Why leaves turn red in autumn. The role of anthocyanins in senescing leaves of red-osier dogwood. Plant Physiol. https://doi.org/10.1104/pp.010063
Folta KM, Maruhnich SA (2007) Green light: a signal to slow down or stop. J Exp Bot 58:3099–3111. https://doi.org/10.1093/jxb/erm130
Gagné S, Cluzet S, Mérillon J-M, Gény L (2011) ABA initiates anthocyanin production in grape cell cultures. J Plant Growth Regul 30:1–10. https://doi.org/10.1007/s00344-010-9165-9
Gao J, Hofstra G, Fletcher RA (1988) Anatomical changes induced by triazoles in wheat seedlings. Can J Bot 66:1178–1185. https://doi.org/10.1139/b88-168
Getter K, L. (2015) Average daily temperature effects on paclobutrazol efficacy of four floriculture crops. HortTechnology 25:742–746. https://doi.org/10.21273/HORTTECH.25.6.742
Gilman FG, Landrum L (1999) Ruellia caroliniensis - wild petunia - fact sheet FPS-514. University of Florida cooperative extension service http://hort.ufl.edu/database/documents/pdf/shrub_fact_sheets/ruecara.pdf. Accessed 30 November 2020
Gopi R, Jaleel CA, Sairam R, Lakshmanan GMA, Gomathinayagam M, Panneerselvam R (2007) Differential effects of hexaconazole and paclobutrazol on biomass, electrolyte leakage, lipid peroxidation and antioxidant potential of Daucus carota L. Colloids Surf B 60:180–186. https://doi.org/10.1016/j.colsurfb.2007.06.003
Gould KS (2004) Nature’s swiss army knife: the diverse protective roles of anthocyanins in leaves. J Biomed Biotechnol 2004:314–320. https://doi.org/10.1155/S1110724304406147
Gray WM, Ostin A, Sandberg G, Romano CP, Estelle M (1998) High temperature promotes auxin-mediated hypocotyl elongation in Arabidopsis. Proc Natl Acad Sci 95:7197–7202. https://doi.org/10.1073/pnas.95.12.7197
Grimstad SO (1993) Influence of paclobutrazol residues on greenhouse bench surfaces and the effect on growth and development of cucumber and tomato young plants. Gartenbauwissenschaft 58:59–63
Hajihashemi S (2018) Physiological, biochemical, antioxidant and growth characterizations of gibberellin and paclobutrazol-treated sweet leaf (Stevia rebaudiana B.) herb. J Plant Biochem Biot 27:237–240. https://doi.org/10.1007/s13562-017-0428-4
Hao X, Papadopoulos AP (1999) Effects of supplemental lighting and cover materials on growth, photosynthesis, biomass partitioning, early yield and quality of greenhouse cucumber. Sci Hortic 80:1–18. https://doi.org/10.1016/S0304-4238(98)00217-9
Hernández R, Kubota C (2014) Growth and morphological response of cucumber seedlings to supplemental red and blue photon flux ratios under varied solar daily light integrals. Sci Hortic 173C:92–99. https://doi.org/10.1016/j.scienta.2014.04.035
Hernández R, Kubota C (2015) Physiological, morphological, and energy-use efficiency comparisons of LED and HPS supplemental lighting for cucumber transplant production. HortScience 50:351–357. https://doi.org/10.21273/HORTSCI.50.3.351
Hernández R, Kubota C (2016) Physiological responses of cucumber seedlings under different blue and red photon flux ratios using LEDs. Environ Exp Bot 121:66–74. https://doi.org/10.1016/j.envexpbot.2015.04.001
Hernández R, Eguchi T, Deveci M, Kubota C (2016) Tomato seedling physiological responses under different percentages of blue and red photon flux ratios using LEDs and cool white fluorescent lamps. Sci Hortic 213:270–280. https://doi.org/10.1016/j.scienta.2016.11.005
Hsu YT, Kao CH (2005) Abscisic acid accumulation and cadmium tolerance in rice seedlings. Physiol Plant 124:71–80. https://doi.org/10.1111/j.1399-3054.2005.00490.x
Huché-Thélier L, Crespel L, Gourrierec JL, Morel P, Sakr S, Leduc N (2016) Light signaling and plant responses to blue and UV radiations—perspectives for applications in horticulture. Environ Exp Bot 121:22–38. https://doi.org/10.1016/j.envexpbot.2015.06.009
Hughes NM, Neufeld HS, Burkey KO (2005) Functional role of anthocyanins in high-light winter leaves of the evergreen herb Galax urceolata. New Phytol 168:575–587. https://doi.org/10.1111/j.1469-8137.2005.01546.x
Iftikhar A, Whipker BE, Dole MJ (2013) Paclobutrazol and ancymidol lower water use of potted ornamental plants and plugs. Eur J Hortic Sci. https://doi.org/10.21273/HORTSCI.50.9.1370
Iftikhar A, Brian EW, John MD (2015) Paclobutrazol or ancymidol effects on postharvest performance of potted ornamental plants and plugs. HortScience 50:1370–1374. https://doi.org/10.21273/HORTSCI.50.9.1370
Islam MA, Kuwar G, Clarke JL, Blystad DR, Gislerod HR, Olsen JE, Torre S (2012) Artificial light from light emitting diodes (LEDs) with a high portion of blue light results in shorter poinsettias compared to high pressure sodium (HPS) lamps. Sci Hortic 147:136–143. https://doi.org/10.1016/j.scienta.2012.08.034
Izumi K, Nakagawa S, Kobayashi M, Oshio H, Sakurai A, Takahashi N (1988) Levels of IAA, cytokinins, ABA and ethylene in rice plants as affected by a gibberellin biosynthesis inhibitor, Uniconazole-P. Plant Cell Physiol 29:97–104. https://doi.org/10.1093/oxfordjournals.pcp.a077480
Jiang Y, Joyce DC (2003) ABA effects on ethylene production, PAL activity, anthocyanin and phenolic contents of strawberry fruit. Plant Growth Regul 39:171–174. https://doi.org/10.1023/A:1022539901044
Kasperbauer MJ, Peaslee DE (1973) Morphology and photosynthetic efficiency of tobacco leaves that received end-of-day red or far red light during development. Plant Physiol 52:440–442. https://doi.org/10.1104/pp.52.5.440
Kendrick RE, Kronenberg GHM (1994) Photomorphogenesis in plants. Kluwer, Dordrecht, The Netherlands
Khalil IA (1995) Chlorophyll and carotenoid contents in cereals as affected by growth retardants of the triazole series. Cereal Res Commun 23:183–189
Kim H-H, Goins G, Wheeler R, Sager J (2004) Green-light supplementation for enhanced lettuce growth under red- and blue-light-emitting diodes. HortScience 39:1617–1622. https://doi.org/10.21273/HORTSCI.39.7.1617
Kubota C, Kroggel M, Both AJ, Whalen M (2016) Does supplemental lighting make sense for my crop?-empirical evaluations. Acta Hort. https://doi.org/10.17660/ActaHortic.2016.1134.52
Li Q, Kubota C (2009) Effects of supplemental light quality on growth and phytochemicals of baby leaf lettuce. Environ Exp Bot 67:59–64. https://doi.org/10.1016/j.envexpbot.2009.06.011
Lieth JH, Pasian CC (1990) A model for net photosynthesis of rose leaves as a function of photosynthetically active radiation, lef temperature, and leaf age. J Amer Soc Hort Sci 115:486–491
Liu Z, Wei X, Ren K, Zhu G, Zhang Z, Wang J, Du D (2016) Highly efficient detection of paclobutrazol in environmental water and soil samples by time-resolved fluoroimmunoassay. Sci Total Environ 569:1629–1634. https://doi.org/10.1016/j.scitotenv.2016.06.089
Lopez R, Runkle E (2017) Light management in controlled environments Meister Media Worldwide. Willoughby, OH
Magnitskiy SV, Pasian CC, Bennett MA, Metzger JD (2006) Controlling plug height of verbena, celosia, and pansy by treating seeds with paclobutrazol. HortScience 41:158–161. https://doi.org/10.21273/HORTSCI.41.1.158
Marcelis LFM, Broekhuijsen AGM, Meinen E, Nijs EMFM, Raaphorst MGM (2006) Quantification of the growth response to light quantity of greenhouse grown crops. Acta Hortic. https://doi.org/10.17660/ActaHortic.2006.711.9
MDAR (2012) Paclobutrazol, review conducted by MDAR and MassDEP for use in sensitive areas or right-of-way in Massachusetts. Department of Agricultural Resources, Boston, MA
Meng Q, Boldt J, Runkle ES (2020) Blue radiation interacts with green radiation to influence growth and predominantly controls quality attributes of lettuce. J Amer Soc Hort Sci 145:75–87. https://doi.org/10.21273/JASHS04759-19
Merzlyak MN, Chivkunova OB, Solovchenko AE, Naqvi KR (2008) Light absorption by anthocyanins in juvenile, stressed, and senescing leaves. J Exp Bot 59:3903–3911. https://doi.org/10.1093/jxb/ern230
Miglietta F (1989) Effect of photoperiod and temperature on leaf initiation rates in wheat (Triticum spp.). Field Crops Res 21:121–130. https://doi.org/10.1016/0378-4290(89)90048-8
Mitchell CA et al (2015) Horticultural Reviews-Light-Emitting Diodes in Horticulture, vol 43. Horticultural Reviews, WILEY Blackwell
Moran R, Porath D (1980) Chlorophyll determination in intact tissues using N, N-Dimethylformamide. Plant Physiol 65:478–479. https://doi.org/10.1104/pp.65.3.478
Moreno D, Berli FJ, Piccoli PN, Bottini R (2011) Gibberellins and abscisic acid promote carbon allocation in roots and berries of grapevines. J Plant Growth Regul 30:220–228. https://doi.org/10.1007/s00344-010-9186-4
Nelson JA, Bugbee B (2015) Analysis of environmental effects on leaf temperature under sunlight, high pressure sodium and light emitting diodes. PLoS ONE. https://doi.org/10.1371/journal.pone.0138930
Ochoa J, Franco JA, Bañón S, Fernández JA, Fernández JA (2009) Distribution in plant, substrate and leachate of paclobutrazol following application to containerized Nerium oleander L. seedlings. Span J Agric Res. https://doi.org/10.5424/sjar/2009073-446
Owen WG, Lopez RG (2015) End-of-production supplemental lighting with red and blue light-emitting diodes (LEDs) influences red pigmentation of four lettuce varieties. HortScience 50:676–684. https://doi.org/10.21273/HORTSCI.50.5.676
Poel BR, Runkle ES (2017) Spectral effects of supplemental greenhouse radiation on growth and flowering of annual bedding plants and vegetable transplants. HortScience 52:1221–1228. https://doi.org/10.21273/hortsci12135-17
Rademacher W (2015) Plant growth regulators: backgrounds and uses in plant production. J Plant Growth Regul 34:845–872. https://doi.org/10.1007/s00344-015-9541-6
Rezazadeh A, Harkess RL (2015) Effects of pinching, number of cuttings per pot, and plant growth regulators on height control of purple firespike. HortTechnology 25:71–75. https://doi.org/10.21273/HORTTECH.25.1.71
Sager JC, Smith WO, Edwards JL, Cyr KL (1988) Photosynthetic efficiency and phytochrome photoequilibria determination using spectral data. Trans ASAE 31:1882–1889. https://doi.org/10.13031/2013.30952
Sasidharan R, Chinnappa CC, Voesenek LACJ, Pierik R (2008) The regulation of cell wall extensibility during shade avoidance: a study using two contrasting ecotypes of Stellaria longipes. Plant Physiol 148:1557. https://doi.org/10.1104/pp.108.125518
Sharma D, Awasthi MD (2005) Uptake of soil applied paclobutrazol in mango (Mangifera indica L.) and its persistence in fruit and soil. Chemosphere 60:164–169. https://doi.org/10.1016/j.chemosphere.2004.12.069
Soundy P, Cantliffe DJ, Hochmuth GJ, Stoffella PJ (2005) Management of nitrogen and irrigation in lettuce transplant production affects transplant root and shoot development and subsequent crop yields. HortScience 40:607–610. https://doi.org/10.21273/HORTSCI.40.3.607
Spalholz H, Perkins-Veazie P, Hernández R (2020) Impact of sun-simulated white light and varied blue:red spectrums on the growth, morphology, development, and phytochemical content of green- and red-leaf lettuce at different growth stages. Sci Hortic 264:109195. https://doi.org/10.1016/j.scienta.2020.109195
Tekalign T, Hammes PS (2004) Response of potato grown under non-inductive condition paclobutrazol: shoot growth, chlorophyll content, net photosynthesis, assimilate partitioning, tuber yield, quality, and dormancy. Plant Growth Regul 43:227–236. https://doi.org/10.1023/B:GROW.0000045992.98746.8d
Tsegaw T, Hammes S, Robbertse J (2005) Paclobutrazol-induced leaf, stem, and root anatomical modifications in potato. HortScience 40:1343–1346. https://doi.org/10.21273/HORTSCI.40.5.1343
Wang Y, Folta KM (2013) Contributions of green light to plant growth and development. Am J Bot 100:70–78. https://doi.org/10.3732/ajb.1200354
Wang K-S, Lu C-Y, Chang S-H (2011) Evaluation of acute toxicity and teratogenic effects of plant growth regulators by Daphnia magna embryo assay. J Hazard Mater 190:520–528. https://doi.org/10.1016/j.jhazmat.2011.03.068
Wang Y, Maruhnich S, Mageroy M, Justice J, Folta K (2013) Phototropin 1 and cryptochrome action in response to green light in combination with other wavelengths. Planta 237:225–237. https://doi.org/10.1007/s00425-012-1767-y
Whipker BE (2013) Plant growth regulator guide. Ball Publishing https://www.ballpublishing.com/pdf/PGR_GUIDE_2013-LowRez.pdf. Accessed 30 November 2020
Wollaeger HM, Runkle ES (2014) Growth of impatiens, petunia, salvia, and tomato seedlings under blue, green, and red light-emitting diodes. HortScience 49:734–740. https://doi.org/10.21273/HORTSCI.49.6.734
Yokota T (1999) Chapter 12–Brassinosteroids. In: Hooykaas PJJ, Hall MA, Libbenga KR (eds) New Comprehensive Biochemistry, vol 33. Elsevier, Amsterdam, pp 277–293
Zhang T, Maruhnich SA, Folta KM (2011) Green light induces shade avoidance symptoms. Plant Physiol 157:1528. https://doi.org/10.1104/pp.111.180661
Acknowledgments
This work was supported by the USDA National Institute of Food and Agriculture (Hatch: Improving Sustainability by Evaluating Novel Technologies and Techniques that Increase Energy Efficiency in Horticulture Production Systems; project 1007454). The authors would like to acknowledge Philips Horticulture for providing both the LED light fixtures and partial funding for the research. The authors would like to acknowledge Dr. Brian Whipker for his advice on the PBZ application dose and Austin Wrenn and Gregory Sheets for their help with site preparation and data collection.
Author information
Authors and Affiliations
Contributions
RH contribution: study conception and design; analysis and interpretation of data; drafting of manuscript; critical revision; funding acquisition. CC contribution: study conception and design; acquisition of data; analysis an interpretation of data; drafting of manuscript; critical revision; funding acquisition.
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Additional information
Handling Editor: Scott Finlayson.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Collado, C.E., Hernández, R. Effects of Light Intensity, Spectral Composition, and Paclobutrazol on the Morphology, Physiology, and Growth of Petunia, Geranium, Pansy, and Dianthus Ornamental Transplants. J Plant Growth Regul 41, 461–478 (2022). https://doi.org/10.1007/s00344-021-10306-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00344-021-10306-5