Abstract
Low temperature in later spring severely limits plant growth and causes considerable yield loss in wheat. In this study, the impacts of exogenous salicylic acid (SA) on plant growth, grain yield and key physiological parameters of wheat plants were investigated under field low-temperature conditions using a field air temperature control system (FATC). The results showed that low-temperature stress significantly decreased leaf net photosynthetic rate, plant height and biomass production of wheat plants at the jointing stage, resulting in a reduction in grain yield. Moreover, the growth period of wheat plants was prolonged by low-temperature stress. However, SA-treated plants significantly improved the photochemical efficiency of photosystem II, accumulation of osmo-protectants, activities of enzymatic antioxidants, and pool of non-enzymatic low molecular substances compared with non-SA-treated plants under low-temperature stress. Pretreatment with SA effectively alleviated low-temperature-induced reduction in leaf net photosynthetic rate, plant height, biomass production and grain yield as well as prolonging of growth period of wheat plants. However, SA-treated plants had no significant effects on the expression levels of cold-responsive genes compared with non-SA-treated plants under low-temperature stress. Our results demonstrated that exogenous application of SA is an appropriate strategy for wheat to resist late spring low-temperature stress under field conditions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Winter wheat acquires tolerance to freezing temperatures by cold acclimation. However, warm temperatures in the spring induce re-growth and development of cold acclimated plants and reduce freezing tolerance (Han et al. 2013). The growth and elongation of stem pushes the growing point above the insulating soil surface so that the growing point loses the protection of the soil and becomes more vulnerable to low temperatures (Zhong et al. 2008). Therefore, wheat plants at the jointing stage are highly sensitive to low temperature. The jointing stage is a critical period to determine the final grain yield due to the growing point switches to the production of reproductive primordia (Zhong et al. 2008). Ji et al. (2017) investigated the effects of different low-temperature intensities at the jointing stage on wheat grain yield and found that the grain yield was reduced by 4.6%–56.4% under different low-temperature intensities (2 °C/12 °C/7 °C− 6 °C/4 °C/–1 °C, Tmin/Tmax/Tmean, 2 d-6 d). The reduction of grain yield caused by low temperature is mainly due to the inhibition of plant growth and young spike development, which is associated with the accumulation of reactive oxygen species (ROS), dehydration of tissues and decrease of photosynthetic rate under low temperatures (Li et al. 2015a; Zhong et al. 2008).
The process of wheat growth and development has been accelerated by global warming, which leads to a significant advancement of the low-temperature sensitive stage of wheat and increasing the possibility of low-temperature injury (Wang et al. 2017). Li et al. (2015a) reported that winter warming exacerbated the negative effects of later spring freeze stress on wheat yield. In addition, unpredictable temperature fluctuations in the spring increase the frequency of low-temperature events (Xiao et al. 2018). Over the past decade, low temperatures in the spring have caused severe losses in wheat grain production worldwide including in Australia, Europe, and China (Ji et al. 2017). Therefore, exploration of cultural practices for improving low-temperature tolerance are vital to the security of wheat production.
Priming, defined as a temporally limited experience to an environmental stimulus, can enhance the plant’s level of resistance to a future environmental stress (Hilker et al. 2015). Li et al. (2014) reported that cold priming significantly increased tolerance to low temperature in wheat by improving antioxidant capacity and photosynthetic efficiency. However, this approach is difficult to popularize in practical production due to its poor operability and high cost. An alternative way of improving low-temperature tolerance is through the exogenous application of chemicals such as hormones, amino acid and polyamines (Janda et al. 1999; Nayyar and Chander 2004; Posmyk and Janas 2007), which could activate the plant resistance system similar to cold priming (Bruce et al. 2008). Exogenous application of chemical regulators is cheaper and easier to operate than cold priming and can be a feasible measure to increase wheat low-temperature tolerance under field conditions.
In our previous studies, we found that salicylic acid (SA) accumulated under cold priming treatment (unpublished) and exogenous application of SA could increase wheat seedlings tolerance to freezing stress (Wang et al. 2018; Wang et al. 2020). These findings suggested that exogenous SA plays a potential role in inducing wheat low-temperature tolerance in the field. SA is a naturally occurring phenolic compound in plants and plays an important role in plant response to biotic and abiotic stresses (Dong et al. 2014; Vlot et al. 2009). It has been documented that exogenous SA could improve plant low-temperature tolerance in many species (such as wheat, maize, and watermelon) through regulating the capacity of photosynthesis and antioxidants, accumulation of osmotic adjustment substances, and expression of cold-responsive genes (Cheng et al. 2016; Janda et al. 1999; Luo et al. 2014; Tasgin et al. 2003; Wang et al. 2006). However, most of these studies were conducted in the laboratory. The effects of SA on low-temperature tolerance in wheat under field conditions and its underlying mechanisms need further investigation.
In the present study, the responses of plant growth, grain yield and some important physiological attributes to SA and spring low-temperature stress were investigated using a field air temperature control system (FATC, Southeast Co. Ltd., Ningbo, China). Our main objectives were to (1) examine the effects of SA on low-temperature tolerance in wheat at the jointing stage under field conditions, (2) explore its underlying physiological mechanism. Our results indicated that SA effectively increased low-temperature tolerance in wheat under field conditions by improving photosynthetic efficiency, antioxidant capacity and osmotic adjustment.
Materials and Methods
Plant Material
This experiment was carried out at Tangquan Experimental Station (118°29′E, 32°07′N) of Nanjing Agricultural University, Nanjing, Jiangsu Province, China during the winter wheat growing season in 2016–2017. Before sowing, 120 kg N ha−1, 90 kg P2O5 ha−1 and 150 kg K2O ha−1 were applied as basal fertilizer and a further 120 kg N ha−1 was used as a topdressing at the returning green stage. The basal fertilizer and topdressing were broadcast spread by hand. Winter wheat (Triticum aestivum L. cv. Yangmai 16) was used in this experiment. The sowing date was 1 November 2016 with the seedling density of 240 m−2 and the row space of 25 cm. The seedlings were thinned out to maintain the same growth conditions at the two-leaf stage.
Experimental Design
To investigate the effects of SA on low-temperature tolerance in wheat, SA (100 μM, containing 0.1% ethanol and 0.05% tween 20) was sprayed on wheat leaves at the jointing stage (when the 1st node was detectable) three times, with an interval of 3 d between each application (17, 20 and 23 March 2017, respectively). The concentration of SA was based on the grain yield data from 2015–2016 (Table S1). Due to the low solubility of SA (Sigma-Aldrich, St. Louis, MO, USA) in water, 0.691 g SA was dissolved in 50 mL ethanol to prepare a stock solution (0.1 M). The stock solution was stored at 4 °C away from light and was diluted to the working solution (100 μM) by distilled water before using. Spraying treatment was conducted at dusk and plots were separated with plastic film to protect from drift. The spray volume per square meter per time was approximately 62.5 mL, i.e., about 0.863 mg SA was used per square meter per time. Plants sprayed with water (containing 0.1% ethanol and 0.05% tween 20) were used as a control. One day after SA pretreatment, half of both the SA-pretreated and non-SA-pretreated plants were subjected to a low-temperature stress for 6 d (from 24 to 30 March 2017). The other half continued to grow at ambient (control) temperature (Fig. 1a). Thus, four treatments were imposed: CC (non-SA pretreatment + non-low-temperature treatment), CL (non-SA pretreatment + low-temperature treatment), SC (SA pretreatment + non-low-temperature treatment) and SL (SA pretreatment + low-temperature treatment).
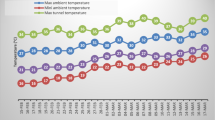
Low temperatures were generated with FATC as described by Li et al. (2015a). Each 4 m × 4 m plots were temporarily surrounded by 180-cm-high plastic film to create a more stable environment before the low-temperature treatment. The FATC mainly consists of control cabinet, compressor, blower, ducting and temperature sensor (Fig. S1). Air was cooled by a compressor, and then the cooled air was driven by an air blower into the contained plots through air ducting. The air ducting was covered with insulation film to mitigate temperature changes during FATC operation. Six temperature sensors were uniformly distributed in the plots to record the real-time temperature data. As shown in Fig. 1b, the mean daily canopy temperature in the low-temperature treatment was about 5.3 °C, which was about 12.4 °C in the control temperature treatment. During the temperature treatment, the plants of low-temperature treatment suffered a total of about 15 h of subzero temperatures and the lowest temperature recorded was − 7.0 °C, while the control temperature treatment plants were always above zero. The experiment was a completely randomized block design with four replicates for each treatment (Fig. S2).
To determine the effects of exogenous SA on wheat freezing tolerance, plant height and single stem dry weight in each treatment were measured at 0 d, 20 d and 60 d after low-temperature treatment, and the grain yield of each treatment were determined at 60 d after low-temperature treatment. To investigate the mechanism of SA-induced freezing tolerance, the latest fully expanded leaves of each treatment were sampled and immediately put into liquid nitrogen and stored at −80 °C for further analysis.
Plant Height, Dry Weight and Grain Yield
Ten labeled main stems from each treatment were randomly selected as one biological replicates for the measurement of plant height, which was measured from the base to the highest point of the plant. After measuring the plant height, the main stems were manually cut at the ground level using pruning-scissors and then killed at 105 °C for 30 min followed by oven-drying at 70 °C to a constant weight for measuring the main stem dry mass. Grain yield was determined by harvesting a fixed double row (1 m in length) in each plot, threshing the grain, and weighing.
Leaf Gas Exchange and Chlorophyll Fluorescence
Leaf gas exchange measurements were conducted on the latest fully expanded leaf at the end of low-temperature treatment using a portable photosynthesis system (LI-6400, LI-COR, NE, USA) as described by Wang et al. (2020). Chlorophyll fluorescence (Fv/Fm, the maximum photochemistry efficiency of photosystem II (PSII); ϕPSII, the actual photochemical efficiency of PSII) was determined on the same leaves measured for the leaf gas exchange at the end of low-temperature treatment using a PAM-2000 portable chlorophyll fluorometer (Heinz Walz GmbH, Effeltrich, Germany) according to Cui et al. (2015).
Lipid Peroxidation and Antioxidants Contents
The lipid peroxidation level was estimated by quantifying the malondialdehyde (MDA) content according to Hodges et al. (1999). The contents of reduced glutathione (GSH) and ascorbic acid (ASA) were measured by the method described by Jiang and Zhang (2001). Total phenolics content was measured using the Folin–Ciocalteu (FC) colorimetric method as described by Meyers et al. (2003).
Antioxidant Enzyme Extraction and Activity Assays
The extraction and activity measurement of superoxide dismutase (SOD; EC 1.15.1.1), catalase (CAT; EC 1.11.1.6), ascorbate peroxidase (APX; EC 1.11.1.11), glutathione reductase (GR; EC 1.6.4.2), guaiacol peroxidase (POD; EC 1.11.1.7) and glutathione S-transferase (GST; EC 2.5.1.18) were carried out as described by Wang et al. (2020). The phenylalanine ammonia-lyase (PAL; EC 4.3.1.5) was extracted by 5 mL100 mM sodium borate buffer (pH 8.8, containing 1 mM EDTA, 5 mM β-mercaptoethanol, and 0.1% polyvinylpyrrolidone), and its activity was measured according to the method of Kovacs et al. (2014). Soluble protein content was determined as described by Bradford (1976).
Total Soluble Sugars and Proline Content Measurements
Total soluble sugars content was determined as described by Sairam et al. (2002). Briefly, total soluble sugars in leaf samples (0.5 g) were extracted by 80% ethanol. About 0.2 mL of extract was mixed with 2 mL of anthrone reagent (0.2 g anthrone, 100 mL H2SO4, made by adding 500 mL concentrated H2SO4 to 200 mL of water), and incubated at 100 °C for 10 min and cooled to room temperature. The absorbance was measured at 620 nm, and the sugar content was determined using a sucrose standard. Free proline content was determined as described by Bates et al. (1973). Briefly, free proline in leaf samples (0.5 g) were extracted by 5 mL 3% sulfosalicylic acid. About 2 mL of extract was mixed with 2 mL of glacial acetic acid and 2 mL of ninhydrin reagent (2.5%), and heated at 100 °C for 30 min. After cooling the reaction mixture, 6 mL of toluene was added and mixed. After standing, the absorbance of the chromophore containing toluene was measured at 520 nm, and the proline content was determined using a proline standard.
Total RNA Extraction and Gene Expression Analysis
The total RNA from leaves was isolated with RNAiso Plus (Takara, Dalian, China) following the manufacturer’s instruction. The cDNA was synthesized using the PrimeScript™ II 1st Strand cDNA Synthesis Kit (Takara, Dalian, China) and qRT-PCR was performed using Bio-Rad iCycler iQ5 fluorescence real-time PCR system (Bio-Rad, Hercules, CA, USA) and Power SYBR Green PCR Master Mix (Vazyme, Nanjing, China). Sequences and sources of the all specific primers are listed in Table S2. The relative expression levels of genes were calculated as described by Zhang et al. (2017b), using Actin as the reference gene.
Statistical Analysis
All the data were subjected to one-way analysis of variance (ANOVA) and mean values were compared by the Duncan’s multiple range test (Sigmaplot 10.0; Systat Software).
Results
Plant Growth and Grain Yield
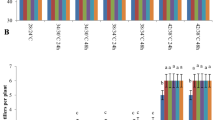
As shown in Fig. 2, SA treatment under control condition (SC) had no significant effects on plant height and single stem dry weight at 0 d, 20 d and 60 d after temperature treatment compared with control treatment (CC). Low-temperature treatment (CL) induced a significant reduction in plant height and single stem dry weight at 0 d and 20 d after temperature treatment, but not at 60 d compared to the CC treatment (Fig. 2). However, SA treatment under low temperature (SL) enhanced plant height at 0 d and 20 d after temperature treatment, and significantly increased single stem dry weight at 0 d, 20 d and 60 d after temperature treatment compared to the CC treatment (Fig. 2). Compared with the CL treatment, the plant height and single stem dry weight were increased by 5.7% and 9.1% under the SL treatment at 0 d after temperature treatment, by 3.4% and 16.5% at 20 d after temperature treatment, respectively. At 20 d after temperature treatment, the plants in the CC and SC treatments were in full-flowering stage, while the internode below the spike of plants in the CL treatment had not yet elongated (Fig. 3). This might be the cause of the higher plant height of the CC treatment than the CL treatment (Fig. 3). The internode below the spike of plants in the SL treatment began to elongate and the plants were in flowering at 20 d after temperature treatment (Fig. 3).
Effects of SA pretreatment on plant height and biomass accumulation of wheat under low-temperature stress. The measurements were taken at 0 d (30th Mar), 20 d (20th Apr) and 60 d (30th May) after temperature treatment. 0 d indicates the end of temperature treatment. CC refers to non-SA pretreatment + non-low-temperature treatment, SC refers to SA pretreatment + non-low-temperature treatment, CL refers to non-SA pretreatment + low-temperature treatment, SL refers to SA pretreatment + low-temperature treatment. Data are means ± SE of three biological replicates. Different letters atop bars indicate significant differences at P < 0.05 level under the same determination period
Phenotype of plants of each treatment at 20 d after temperature treatment (20th Apr). CC refers to non-SA pretreatment + non-low-temperature treatment, SC refers to SA pretreatment + non-low-temperature treatment, CL refers to non-SA pretreatment + low-temperature treatment, SL refers to SA pretreatment + low-temperature treatment. Red arrow indicates flowing plants of SL treatment
The SC treatment slightly increased grain yield and grain weight compared to the CC treatment (Table 1). Although the effects of low-temperature treatment on grain yield and yield components were not statistically significant, the CL treatment resulted in a reduction in grain yield (8.0%), kernels per spike (4.4%) and grain weight (3.9%) compared to the CC treatment (Table 1). However, the decrease in grain yield, kernels per spike and grain weight was lower in the SL treatment than the CL treatment (Table 1). The grain yield, kernels per spike and grain weight were increased by 7.9%, 4.9% and 3.3% under SL treatment compared to the CL treatment, respectively. There was no difference in spike number between CL and SL treatment, suggesting the improved kernels per spike and grain weight contributed to increased grain yield by SA treatment under low-temperature stress.
Photosynthetic Capacity and Osmotic Adjustment
Photosynthesis is one of the most sensitive processes affected by low temperature (Li et al. 2014). There were no significant differences in Fv/Fm, ϕPSII, Pn and gs between CC and SC treatment (Fig. 4). The CL treatment induced a significant reduction in Fv/Fm, ϕPSII, Pn and gs of wheat leaves compared to the CC treatment (Fig. 4). However, the SL treatment relatively enhanced Fv/Fm (4.9%), ϕPSII (5.9%), Pn (9.6%) and gs (10.7%) of wheat leaves compared to the CL treatment (Fig. 4).
Effects of SA pretreatment on net photosynthetic rate (Pn), stomatal conductance (gs), maximum quantum efficiency of PSII (Fv/Fm) and actual quantum efficiency of PSII (ϕPSII) of wheat leaves under low-temperature stress. The measurements were taken at the end of temperature treatment (30th Mar). CC refers to non-SA pretreatment + non-low-temperature treatment, SC refers to SA pretreatment + non-low-temperature treatment, CL refers to non-SA pretreatment + low-temperature treatment, SL refers to SA pretreatment + low-temperature treatment. Data are means ± SE of three biological replicates. Different letters atop bars indicate significant differences at P < 0.05 level
In this study, plants in low-temperature treatment were subjected to a subzero temperatures stress for 15 h during temperature treatment (Fig. 1b). It has been documented that subzero temperatures could induce extracellular ice crystal formation leading to the migration of water from intracellular space to extracellular space and causing cell dehydration and shrinkage (Ruellan et al. 2009). Soluble sugar and free proline play important roles in plant osmotic adjustment under stress conditions (Sairam et al. 2002). In this study, the SC treatment significantly increased the levels of total soluble sugar (9.8%) and free proline (50.0%) in wheat leaves compared to the CC treatment (Fig. 5). The CL treatment induced the accumulation of total soluble sugar and free proline in wheat leaves compared to the CC treatment (Fig. 5). However, the SL treatment showed significantly higher total soluble sugar (13.6%) and free proline (31.8%) contents than the CL treatment (Fig. 5).
Effects of SA pretreatment on total soluble sugar and free proline contents of wheat leaves under low-temperature stress. The measurements were taken at the end of temperature treatment (30th Mar). CC refers to non-SA pretreatment + non-low-temperature treatment, SC refers to SA pretreatment + non-low-temperature treatment, CL refers to non-SA pretreatment + low-temperature treatment, SL refers to SA pretreatment + low-temperature treatment. Data are means ± SE of three biological replicates. Different letters atop bars indicate significant differences at P < 0.05 level
Antioxidant Capacity
In this study, the CL treatment significantly increased MDA content (34.5%) in wheat leaves compared to the CC treatment (Fig. 6), indicating low-temperature treatment induced oxidative injury. However, SL treatment effectively alleviated low-temperature-induced increase of MDA content compared to the CL treatment (Fig. 6). As shown in Table 2 and Fig. 6, the SC treatment significantly increased GSH content (9.9%) and POD activity (43.0%), slightly increased ASA content (14.8%) and CAT activity (7.9%), while having no effects on total phenolics content and activities of SOD, APX, GR, GST and PAL compared to the CC treatment. The CL treatment increased the levels of ASA, GSH and total phenolics, and decreased the activities of PAL, POD, CAT and GR, while having no effects on the activities of SOD, APX and GST as compared to the CC treatment (Table 2 and Fig. 6). However, the SL treatment significantly increased the levels of ASA (30.2%), GSH (7.2%) and total phenolics (13.1%) and the activities of POD (56.2%), CAT (11.8%), GR (25.6%) and PAL (19.3%), while having no effects on the activities of SOD, APX and GST compared to the CL treatment. These results indicated that SA could alleviate low-temperature-induced oxidative damage by increasing antioxidant capacity.
Effects of SA pretreatment on malondialdehyde (MDA), total phenolics, ascorbic acid (ASA) and reduced glutathione (GSH) contents of wheat leaves under low-temperature stress. The measurements were taken at the end of temperature treatment (30th Mar). CC refers to non-SA pretreatment + non-low-temperature treatment, SC refers to SA pretreatment + non-low-temperature treatment, CL refers to non-SA pretreatment + low-temperature treatment, SL refers to SA pretreatment + low-temperature treatment. Data are means ± SE of three biological replicates. Different letters atop bars indicate significant differences at P < 0.05 level
Cold-Responsive Gene Expression
To further investigate the underlying mechanism of SA inducing freezing tolerance in wheat, we analyzed the expression levels of six cold-responsive genes, including the WRKY-type transcription factor (WRKY19), ice recrystallization inhibition (IRI2), C-repeat binding factor (CBF3), cold-regulated (COR410), heat shock protein (HSP70) and alternative oxidase (AOX1a). As shown in Fig. 7, the SC treatment slightly upregulated the expression of CBF3, HSP70 and WRKY19, while having no effects on expression of COR410, AOX1a and HSP70. The CL treatment significantly upregulated the expression of COR410, AOX1a and IRI2, and slightly upregulated the expression of CBF3 and WRKY19, while having no effect on expression of HSP70 compared to the CC treatment (Fig. 7). The SL treatment slightly upregulated the expression of CBF3 and WRKY19, while having no effects on the expression of COR410, AOX1a, HSP70 and IRI2 compared to the CL treatment (Fig. 7).
Effects of SA pretreatment on the expression of cold-responsive genes of wheat leaves under low-temperature stress. The measurements were taken at the end of temperature treatment (30th Mar). CC refers to non-SA pretreatment + non-low-temperature treatment, SC refers to SA pretreatment + non-low-temperature treatment, CL refers to non-SA pretreatment + low-temperature treatment, SL refers to SA pretreatment + low-temperature treatment. Data are means ± SE of three biological replicates, and each biological replicate has three technical replicates. Different letters atop bars indicate significant differences at P < 0.05 level
Discussion
Low temperature causes multiple detrimental effects on crop physiological and metabolic processes, including inhibition of electron transfer in mitochondria and chloroplasts, inactivation of metabolic enzymes, and extracellular ice crystallization. These effects lead to the inhibition of plant growth and development, and ultimately grain yield loss (Ji et al. 2017; Li et al. 2014; Ruellan et al. 2009). In this study, low-temperature stress significantly inhibited plant growth and development (Figs. 2 and 3), which were well in accordance with previous findings (Li et al. 2015b; Zhong et al. 2008). It is worthy of noting that the mean daytime canopy temperature (7 a.m.–7 p.m.) in the low-temperature treatment was about 8.1 °C during temperature treatment (Fig. 1b), which had little adverse effect on wheat plants, while the mean nighttime canopy temperature (7 p.m.–7 a.m.) was about 2.4 °C (the total time of subzero temperature was about 15 h and the lowest temperature was − 7.0 °C). Therefore, the frigid temperature in the nighttime might be primarily responsible for the low-temperature treatment induced inhibition of wheat growth and development in this study. However, low-temperature stress insignificantly decreased grain yield and grain components in the present study (Table 1). Li et al. (2015b) reported that low temperature (8 °C lower temperature than ambient temperature, 5 d) at the jointing stage significantly decreased the grain yield of low-temperature sensitive wheat cultivar, but not low-temperature resistant cultivar. Ji et al. (2017) showed that low temperature (2 °C/12 °C/7 °C, Tmin/Tmax/Tmean, 6 d) at the jointing stage had no significant effect on grain yield of wheat plants, whereas the grain yield was significantly decreased under higher intensity of low-temperature stress (−2 °C/8 °C/3 °C, Tmin/Tmax/Tmean, 6 d). These findings indicated that the extent of grain yield reduction was determined by both low-temperature intensity and cultivar.
In this study, exogenous application of SA effectively alleviated low-temperature-induced growth and development inhibition, as manifested by significantly higher plant height, stem dry weight and grain yield, and early flowering time in the SA-treated plants compared to the non-SA-treated plants under low-temperature stress (Table 1, Figs. 2 and 3). These results were consistent with findings in previous studies that SA could induce tolerance to low-temperature stress in wheat and maize seedlings grown in greenhouse (Tasgin et al. 2003; Farooq et al. 2008; Wang et al. 2018). SA could regulate cell growth by specifically affecting cell enlargement and division, protecting the cell structure (Kang et al. 2007; Vanacker et al. 2010), which might cause an increase in plant growth under low temperature.
Photosynthesis is the primary metabolic sink for plant growth and development (Ruellan et al. 2009). Low temperature suppresses photosynthetic rate through stomatal and non-stomatal limitation (Li et al. 2014). Fv/Fm and ϕPSII can reflect the photochemical activity of PSII (Cui et al. 2015). In this study, low-temperature treatment significantly decreased Pn of wheat leaves, accompanied by decreased gs, Fv/Fm and ϕPSII (Fig. 4). These results further suggested that the reduced photosynthetic rate under low temperature was due to both stomatal and non-stomatal factors. Previous studies showed that the effects of SA on the photosynthetic apparatus and photosynthetic activity are concentration-dependent under both optimal and stressful environmental conditions (Janda et al. 2014; Jayakannan et al. 2015). For instance, Poór et al. (2011) found that treatment of tomato plants with lower concentration SA (10–7 M) increased photosynthesis as compared with non-SA-treated plants under no stress condition, while the plants treated with higher concentration SA (10–4-10–3 M) showed relatively lower photosynthesis than non-SA-treated plants. However, the tomato plants pretreated with 10–4 M SA had a better photosynthetic performance than non-SA-treated plants under salt stress, while lower concentration SA (10–7 M) pretreatment had no positive effect on photosynthesis under salt stress. In this study, foliar application 100 μM SA had no negative effect on photosynthetic performance of wheat plants under control temperature, and significantly improved photosynthetic performance (higher Pn) under low-temperature stress as compared with control treatment (Fig. 4). This was consistent with enhanced plant growth under the SL treatment as compared with CL treatment (Fig. 2). In addition, the SL treatment significantly increased Fv/Fm and ϕPSII, and slightly increased gs of wheat leaves compared to the CL treatment (Fig. 4), suggesting that SA mitigated low-temperature-induced photoinhibition was dependent on stomata and photochemical.
Low temperatures induce accumulation of ROS in chloroplast by inhibiting thylakoid electron transport, reducing enzymatic activities such as Calvin cycle enzymes and ROS scavenging activities (Li et al. 2014; Ruellan et al. 2009). In addition, the sustained low-temperature stress can seriously affect the development of chloroplast, such as changing its shape and destroying its membrane structure, which aggravates the production of ROS in organelles (Peng et al. 2015). The accumulation of ROS can lead to peroxidation of plasma membrane and degradation of D1 proteins in PSII and stromal enzymes such as ribulose-1,5-bisphosphate carboxylase oxygenase (Rubisco), which results in more ROS production and further inhibition of photosynthesis (Ensminger et al. 2006; Ruellan et al. 2009). Thus, photosynthetic capacity of plants under low temperature is closely correlated to the generation and elimination of ROS in chloroplasts. ASA, GSH, APX and GR are important components of ASA-GSH cycle, which plays an important role in scavenging ROS in organelles, especially in chloroplasts (Huang and Shan 2018). The positive effects of exogenous SA on levels of ASA and GSH and activities of APX and GR under low-temperature stress had been well recorded (Wang et al. 2006; Duan et al. 2012). In this study, the SL treatment obviously increased the activity of GR and the content of ASA and GSH compared to the CL treatment, but had no significant effect on APX activity (Table 2 and Fig. 6). These results suggested that the SL treatment promoted elimination of ROS in chloroplast by improving ASA-GSH cycle under low-temperature stress. In addition, SA treatment could increase electron transport rate, and upregulate the expression of genes encoding carbonic anhydrase and Rubisco under low-temperature stress (Dong et al. 2014; Wang et al. 2018), indicating that SA could alleviate low temperature-induced an over-reduction of electron transport chain and ROS generation. All together, these findings suggested that SA could protect photosynthetic apparatus under low-temperature stress by increasing antioxidant contents, which might explain the higher photochemical efficiency and photosynthetic rate of SA-treated plants than non-SA-treated plants under low-temperature stress.
In this study, in addition to increasing the activity of GR and the content of ASA and GSH, the SL treatment significantly increased the activities of PAL, CAT, POD and the content of total phenolics compared to the CL treatment (Table 2). These results were consistent with lower MDA content in the SL treatment when compared with the CL treatment (Fig. 6), indicating that SA could alleviate low-temperature-induced oxidative damage by increasing the activities of enzymatic antioxidants and the pool of non-enzymatic low molecular substances in wheat under field conditions. Our study was in agreement with other studies showing that improving antioxidant capacity is an important mechanism of SA enhancing plant tolerance to abiotic stresses (Kang et al. 2013; Zhang et al. 2011). Siboza et al. (2014) reported that SA enhanced total phenolics contents by regulating phenolic metabolism, including increasing PAL activity and decreasing polyphenol oxidase activity. SA could upregulate the expression of genes encoding antioxidant enzymes such as CAT, GR and PAL (Chen et al. 2006, 2011; Ding et al. 2002), suggesting that SA could increase antioxidant enzymes activity by upregulating expression levels of their encoding genes.
Previous studies have shown that the levels of total soluble sugar and free proline were positively correlated with wheat low-temperature tolerance (Dörffling et al. 1990; Vágújfalvi et al. 1999). Exogenous SA could increase soluble sugar and free proline contents by regulating the activities of their metabolism-related enzymes such as sucrose phosphate synthase, sucrose synthase, pyrroline-5-carboxylate synthetase and proline dehydrogenase (Dong et al. 2011; Kaur and Asthir 2015). In this study, SA-treated plants significantly enhanced total soluble sugar and free proline contents under normal and low-temperature conditions (Fig. 5), which was consistent with the stronger tolerance to low temperature compared to their non-SA-treated counterparts. In addition to their role in osmotic regulation, soluble sugar and free proline could influence stress tolerance in multiple ways such as acting as an ROS scavenger and as nutrient and metabolic signals (Dong et al. 2011; Keunen et al. 2013; Szabados and Savourcb 2010). Intriguingly, the accumulated proline under stress can act as a substrate for mitochondrial respiration to supply energy for resumed growth after released from stress (Szabados and Savourcb 2010). This might the reason why the SL treatment increased the single stem dry weight by 9.1% at the end of temperature treatment but increased by 16.1% at 20 d after temperature treatment compared to the CL treatment (Fig. 2). Our results indicated that the accumulation of soluble sugar and free proline play important roles in SA-induced low-temperature tolerance in wheat under field conditions.
CBF transcription factors can bind to the promoters of CORs and activate their expression (Ruellan et al. 2009). COR410 is a dehydrin that is localized in the vicinity of the plasma membrane (Ganeshan et al. 2008). The regulation roles of SA on the expression of CBFs and CORs are controversial. Dong et al. (2014) reported that SA could remarkably upregulate the expression levels of CBF and COR genes in cucumber under low temperature. However, SA might play a role in negatively regulating CBF and COR genes expression in watermelon and Arabidopsis under low temperature (Kurepin et al. 2013; Cheng et al. 2016). AOX plays a crucial role in restraining ROS production in mitochondria and protecting photosynthetic apparatus under low temperature (Sugie et al. 2006; Zhang et al. 2012). The expression level of AOX is highly regulated by SA under both optimal and stressful environmental conditions (Rhoads et al. 1993; Lei et al. 2010; Poór 2020). HSP70 is an important molecular chaperone that is able to protect protein integrity under low temperature (Colinet et al. 2010). Previous studies indicated that exogenous SA could upregulate the expression level of HSP70 under environmental stresses (Wang et al. 2006; Wang et al. 2020). IRI2 is an antifreeze protein which participates in inhibiting extracellular ice recrystallization under freezing stress (Tremblay et al. 2005), and WRKY19 is involved in regulating antioxidant capacity and carbohydrate metabolism under low temperature (Niu et al. 2012). Our previous studies found that exogenous SA could significantly improve the expression levels of IR2 and WRKY19 under freezing stress in wheat plants (Wang et al. 2020). In this study, the CL treatment upregulated the expression of CBF3, COR410, AOX1a, IRI2 and WRKY19 compared to the CC treatment (Fig. 7), further indicating their important role in plant response to low temperature. However, there was no significant difference in expression of these genes between SA-treated and non-SA-treated plants under normal and low-temperature conditions (Fig. 7). Previous studies showed that SA pretreatment could upregulate the expression levels of CBF and COR genes within a 24 h low-temperature treatment (Wang et al. 2018; Zhang et al. 2017a), and upregulate expression levels of AOX1a, HSP70, IRI2 and WRKY19 within a 48 h low-temperature treatment (Wang et al. 2020). In the present study, the expression level of these genes was analyzed at 6 d of low-temperature treatment. It is likely that the regulation of SA on these genes’ expression was time dependent, but this requires further investigation.
In summary, low-temperature stress at the jointing stage induced severe oxidative damage and significantly suppressed photosynthesis of wheat plants, resulting in the inhibition of plant growth and the prolongation of growth period as well as the reduction of grain yield. Exogenous application of SA significantly improved the capacity of photosynthesis, antioxidants and osmotic adjustment under low temperature, hereby conferring tolerance to low temperature in wheat plants and effectively alleviating the adverse effects of low temperature on plant growth, development and grain yield. This study contributes to physiological understanding of SA-induced tolerance to low temperature and provides appropriate strategies to improve tolerance to low temperature in wheat plants under field conditions.
References
Bates LS, Waldren RP, Teare I (1973) Rapid determination of free proline for water-stress studies. Plant Soil 39:205–207
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Bruce TJA, Matthes MC, Napier JA, Pickett JA (2008) Stressful “memories” of plants: Evidence and possible mechanisms. Plant Sci 173:603–608
Chen JY, Wen PF, Kong WF, Pan QH, Zhan JC, Li JM, Wan SB, Huang WD (2006) Effect of salicylic acid on phenylpropanoids and phenylalanine ammonia-lyase in harvested grape berries. Postharvest Biol Tec 40:64–72
Chen S, Zime L, Cui J, Jiang D, Xia X, Liu D, Yu J (2011) Alleviation of chilling-induced oxidative damage by salicylic acid pretreatment and related gene expression in eggplant seedlings. Plant Growth Regul 65:101–108
Cheng F, Lu JY, Min G, Kai S, Kong Q, Huang Y, Bie Z (2016) Redox signaling and CBF-responsive pathway are involved in salicylic acid-improved photosynthesis and growth under chilling stress in watermelon. Front. Plant Sci. 7
Colinet H, Lee SF, Hoffmann A (2010) Temporal expression of heat shock genes during cold stress and recovery from chill coma in adult Drosophila melanogaster. FEBS J 277:174–185
Cui Y, Tian Z, Zhang X, Muhammad A, Han H, Jiang D, Cao W, Dai T (2015) Effect of water deficit during vegetative growth periods on post-anthesis photosynthetic capacity and grain yield in winter wheat (Triticum aestivum L.). Acta Physiol Plant 37:1–10
Dörffling K, Schulenburg S, Lesselich G, Dörffling H (1990) Abscisic acid and proline levels in cold hardened winter wheat leaves in relation to variety-specific differences in freezing resistance. J Agro Crop Sci 165:230–239
Ding CK, Wang C, Gross KC, Smith DL (2002) Jasmonate and salicylate induce the expression of pathogenesis-related-protein genes and increase resistance to chilling injury in tomato fruit. Planta 214:895–901
Dong C, Li L, Shang Q, Liu X, Zhang Z (2014) Endogenous salicylic acid accumulation is required for chilling tolerance in cucumber (Cucumis sativus L.) seedlings. Planta 240:687–700
Dong CJ, Wang XL, Shang QM (2011) Salicylic acid regulates sugar metabolism that confers tolerance to salinity stress in cucumber seedlings. Sci Hortic 129:629–636
Duan M, Feng HL, Wang LY, Li D, Meng QW (2012) Overexpression of thylakoidal ascorbate peroxidase shows enhanced resistance to chilling stress in tomato. J Plant Physiol 169:867–877
Ensminger I, Busch F, Huner NPA (2006) Photostasis and cold acclimation: sensing low temperature through photosynthesis. Physiol Plantarum 126:28–44
Farooq M, Aziz T, Basra SMA, Cheema MA, Rehman H (2008) Chilling tolerance in hybrid maize induced by seed priming with salicylic Acid. J Agron Crop Sci 194:161–168
Ganeshan S, Vitamvas P, Fowler DB, Chibbar RN (2008) Quantitative expression analysis of selected COR genes reveals their differential expression in leaf and crown tissues of wheat (Triticum aestivum L.) during an extended low temperature acclimation regimen. J Exp Bot 59:2393–2402
Han Q, Kang G, Guo T (2013) Proteomic analysis of spring freeze-stress responsive proteins in leaves of bread wheat (Triticum aestivum L.). Plant Physiol Biochem 63:236–244
Hilker M, Schwachtje J, Baier M, Balazadeh S, Bäurle I, Geiselhardt S, Hincha DK, Kunze R, Mueller-Roeber B, Rillig MC (2015) Priming and memory of stress responses in organisms lacking a nervous system. Biol Rev 1:393–406
Hodges DM, Delong JM, Forney CF, Prange RK (1999) Improving the thiobarbituric acid-reactive-substances assay for estimating lipid peroxidation in plant tissues containing anthocyanin and other interfering compounds. Planta 207:604–611
Huang G, Shan C (2018) Lanthanum improves the antioxidant capacity in chloroplast of tomato seedlings through ascorbate-glutathione cycle under salt stress. Sci Hortic 232:264–268
Janda T, Szalai G, Tari I, Paaldi E (1999) Hydroponic treatment with salicylic acid decreases the effects of chilling injury in maize (Zea mays L.) plants. Planta 208:175–180
Janda T, Gondor OK, Yordanova R, Szalai G, Pál M (2014) Salicylic acid and photosynthesis: signalling and effects. Acta Physiol Plant 36:2537–2546
Jayakannan M, Bose J, Babourina O, Rengel Z, Shabala S (2015) Salicylic acid in plant salinity stress signalling and tolerance. Plant Growth Regul 76:25–40
Ji H, Xiao L, Xia Y, Song H, Liu B, Tang L, Cao W, Zhu Y, Liu L (2017) Effects of jointing and booting low temperature stresses on grain yield and yield components in wheat. Agr Forest Meteorol 243:33–42
Jiang M, Zhang J (2001) Effect of abscisic acid on active oxygen species, antioxidative defence system and oxidative damage in leaves of maize seedlings. Plant Cell Physiol 42:1265–1273
Kang GZ, Li GZ, Lu GQ, Xu W, Peng XQ, Wang CY, Zhu YJ, Guo TC (2013) Exogenous salicylic acid enhances wheat drought tolerance by influence on the expression of genes related to ascorbate-glutathione cycle. Biol Plant 57:718–724
Kang GZ, Wang ZX, Xia KF, Sun GC (2007) Protection of ultrastructure in chilling-stressed banana leaves by salicylic acid. J Zhejiang Univ Sci 8:277–282
Kaur G, Asthir B (2015) Proline: a key player in plant abiotic stress tolerance. Biol Plant 59:609–619
Keunen E, Peshev D, Vangronsveld J, Van DEW, Cuypers A (2013) Plant sugars are crucial players in the oxidative challenge during abiotic stress: extending the traditional concept. Plant Cell Environ 36:1242–1255
Kovacs V, Gondor OK, Szalai G, Eva Darko IM, Janda T, Pál M (2014) Synthesis and role of salicylic acid in wheat varieties with different levels of cadmium tolerance. J Hazard Mater 280:12–19
Kurepin L, Dahal K, Savitch L, Singh J, Bode R, Ivanov A, Hurry V, Huener N (2013) Role of CBFs as integrators of chloroplast redox, phytochrome and plant hormone signaling during cold acclimation. Int J Mol Sci 14:12729–12763
Lei T, Feng H, Sun X, Dai QL, Zhang F, Liang HG, Lin HH (2010) The alternative pathway in cucumber seedlings under low temperature stress was enhanced by salicylic acid. Plant Growth Regul 60:35–42
Li X, Cai J, Liu F, Dai T, Cao W, Jiang D (2014) Cold priming drives the sub-cellular antioxidant systems to protect photosynthetic electron transport against subsequent low temperature stress in winter wheat. Plant Physiol Biochem 82:34–43
Li X, Cai J, Liu F, Dai T, Cao W, Jiang D (2015a) Spring freeze effect on wheat yield is modulated by winter temperature fluctuations: Evidence from meta-analysis and simulating experiment. J Agron Crop Sci 201:288–300
Li X, Pu H, Liu F, Zhou Q, Cai J, Dai T, Cao W, Jiang D (2015b) Winter wheat photosynthesis and grain yield responses to spring freeze. Agrono J 107:1002–1010
Luo Y, Su Z, Bi T, Cui X, Lan Q (2014) Salicylic acid improves chilling tolerance by affecting antioxidant enzymes and osmoregulators in sacha inchi (Plukenetia volubilis). Braz J Bot 37:357–363
Meyers KJ, Watkins CB, Pritts MP, Liu RH (2003) Antioxidant and antiproliferative activities of strawberries. J Agr Food Chem 51:6887–6892
Nayyar H, Chander S (2004) Protective effects of polyamines against oxidative stress induced by water and cold stress in chickpea. J Agron Crop Sci 190:355–365
Niu CF, Wei W, Zhou QY, Tian AG, Hao YJ, Zhang WK, Biao MA, Lin Q, Zhang ZB, Zhang JS (2012) Wheat WRKY genes TaWRKY2 and TaWRKY19 regulate abiotic stress tolerance in transgenic Arabidopsis plants. Plant Cell Environ 35:1156–1170
Peng X, Teng L, Yan X, Zhao M, Shen S (2015) The cold responsive mechanism of the paper mulberry: decreased photosynthesis capacity and increased starch accumulation. BMC genomics 16:898
Posmyk M, Janas K (2007) Effects of seed hydropriming in presence of exogenous proline on chilling injury limitation in Vigna radiata L. seedlings. Acta Physiol Plant 29:509–517
Poór P (2020) Effects of salicylic acid on the metabolism of mitochondrial reactive oxygen species in plants. Biomolecules 10:341
Poór P, Gémes K, Horváth F, Szepesi Á, Simon ML, Tari I (2011) Salicylic acid treatment via the rooting medium interferes with stomatal response, CO2 fixation rate and carbohydrate metabolism in tomato, and decreases harmful effects of subsequent salt stress. Plant Biology 13:105–114
Rhoads DM, McIntosh L (1993) Cytochrome and alternative pathway respiration in tobacco (Effects of salicylic acid). Plant Physiol 103:877–883
Ruellan E, Vaultier M, Zachowski A, Hurry V (2009) Cold signalling and cold acclimation in plants. Adv Bot Res 49:35–150
Sairam RK, Rao KV, Srivastava G (2002) Differential response of wheat genotypes to long term salinity stress in relation to oxidative stress, antioxidant activity and osmolyte concentration. Plant Sci 163:1037–1046
Siboza XI, Bertling I, Odindo AO (2014) Salicylic acid and methyl jasmonate improve chilling tolerance in cold-stored lemon fruit (Citrus limon). J Plant Physiol 171:1722–1731
Sugie A, Naydenov N, Mizuno N, Nakamura C, Takumi AS (2006) Overexpression of wheat alternative oxidase gene Waox1a alters respiration capacity and response to reactive oxygen species under low temperature in transgenic Arabidopsis. Genes Genet Syst 81:349–354
Szabados LL, Savourcb A (2010) Proline: a multifunctional amino acid. Trends Plant Sci 15:89–97
Tasgin E, Atici O, Nalbantoglu B (2003) Effects of salicylic acid and cold on freezing tolerance in winter wheat leaves. Plant Growth Regul 41:231–236
Tremblay K, Ouellet F, Fournier J, Danyluk J, Sarhan F (2005) Molecular characterization and origin of novel bipartite cold-regulated ice recrystallization inhibition proteins from cereals. Plant Cell Physiol 46:884–891
Vágújfalvi A, Kerepesi I, Galiba G, Tischner T, Sutka J (1999) Frost hardiness depending on carbohydrate changes during cold acclimation in wheat. Plant Sci 144:85–92
Vanacker H, Lu H, Rate DN, Greenberg JT (2010) A role for salicylic acid and NPR1 in regulating cell growth in Arabidopsis. Plant J 28:209–216
Vlot AC, Dempsey MA, Klessig DF (2009) Salicylic acid, a multifaceted hormone to combat disease. Annu Rev Phytopathol 47:177–206
Wang L, Chen S, Kong W, Li S, Archbold DD (2006) Salicylic acid pretreatment alleviates chilling injury and affects the antioxidant system and heat shock proteins of peaches during cold storage. Postharvest Biol Tec 41:244–251
Wang S, Mo X, Liu Z, Baig MHA, Chi W (2017) Understanding long-term (1982–2013) patterns and trends in winter wheat spring green-up date over the North China Plain. Int J Appl Earth Obs 57:235–244
Wang W, Wang X, Huang M, Cai J, Zhou Q, Dai T, Cao W, Jiang D (2018) Hydrogen peroxide and abscisic acid mediate salicylic acid-induced freezing tolerance in wheat. Front. Plant Sci. 9
Wang W, Wang X, Zhang J, Huang M, Cai J, Zhou Q, Dai T, Jiang D (2020) Salicylic acid and cold priming induce late-spring freezing tolerance by maintaining cellular redox homeostasis and protecting photosynthetic apparatus in wheat. Plant Growth Regul 90:109–121
Xiao L, Liu L, Asseng S, Xia Y, Tang L, Liu B, Cao W, Zhu Y (2018) Estimating spring frost and its impact on yield across winter wheat in China. Agr Forest Meteorol 260:154–164
Zhang BB, Guo L, Song ZZ, Yu ML, Ma RJ (2017a) Effect of salicylic acid on freezing injury in peach floral organs and the expressions of CBF genes. Bio Plant 61:622–630
Zhang F, Zhang H, Xia Y, Wang G, Xu L, Shen Z (2011) Exogenous application of salicylic acid alleviates cadmium toxicity and reduces hydrogen peroxide accumulation in root apoplasts of Phaseolus aureus and Vicia sativa. Plant Cell Rep 30:1475–1483
Zhang J, Shi Y, Zhang X, Dub H, Xu B, Huang B (2017b) Melatonin suppression of heat-induced leaf senescence involves changes in abscisic acid and cytokinin biosynthesis and signaling pathways in perennial ryegrass (Lolium perenne L.). Environ Exp Bot 138:36–45
Zhang LT, Zhang ZS, Gao HY, Meng XL, Yang C, Liu JG, Meng QW (2012) The mitochondrial alternative oxidase pathway protects the photosynthetic apparatus against photodamage in Rumex K-1 leaves. BMC Plant Biol 12:40
Zhong X, Mei X, Li Y, Yoshida H, Zhao P, Wang X, Han L, Hu X, Huang S, Huang J (2008) Changes in frost resistance of wheat young ears with development during jointing stage. J Agron Crop Sci 194:343–349
Acknowledgements
This study was supported by the National Key Research and Development Program of China (2016YFD0300107), the Fundamental Research Funds for the Central Universities (KYZ201807), the National Natural Science Foundation of China (31401326, 31320020, and 31771693), the China Agriculture Research System (CARS-03) and Jiangsu Collaborative Innovation Center for Modern Crop Production (JCIC-MCP). The authors are thankful to Meifu Zheng and Ranping Chen (Southeast Co. Ltd., Ningbo, China) for providing technical support on machine maintenance and Allen Chen (Iowa State University, USA) for providing language help.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Wang, W., Wang, X., Huang, M. et al. Alleviation of Field Low-Temperature Stress in Winter Wheat by Exogenous Application of Salicylic Acid. J Plant Growth Regul 40, 811–823 (2021). https://doi.org/10.1007/s00344-020-10144-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00344-020-10144-x