Abstract
Nickel (Ni) element is strongly phytotoxic at high concentrations for several plants, but due to its dual behavior and complicated chemistry, it has received little attention in plant nutrition and relevant experimental data are limited. The current research was carried out to study the effect of Ni on maize (Zea mays L.) growth and phytoextraction potential with EDTA assistance, a process termed as chemical assisted phytoremediation. Treatments included two levels of EDTA (0 and 0.5 mM), two levels of Ni (0 and 40 µM) and their combination (EDTA+Ni) that were applied to maize plants grown in a pot experiment. Application of Ni alone or in combination with EDTA reduced maize root and shoot length by 7.8% to 13.3% and by 15.6% to 21.1%, respectively, compared with control, as well as root and shoot dry weight by 42.0% to 60.0% and by 29.8% to 46.6%, respectively. A similar declining trend was observed also for the content of photosynthetic pigments (chl-a, chl-b, total chlorophyll, and carotenoids) as well as total proteins. However, proline, total soluble sugars, and free amino acids showed an increasing trend with application of Ni and EDTA alone or in combination. These treatments significantly decreased P and Na content in maize roots, stems, leaves, and grains, while increased K content compared with control. Application of EDTA with Ni was the most effective treatment to enhance Ni accumulation in maize (50.23 mg per plant) compared with Ni alone (40.62 mg per plant), EDTA alone (27.75 mg per plant), and control (15.51 mg per plant). Application of EDTA in combination with Ni enhanced Ni accumulation by 4.9 folds in maize shoots and by 2.6 folds in roots over control. In conclusion, application of EDTA in suitable concentrations may enhance Ni uptake by maize providing an effective and economic phytoremediation method of Ni-contaminated soils.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Nickel (Ni) is an essential micronutrient for plants and plays a vital role in enzyme activity in biological systems. It exists in agricultural soils in the form of insoluble and soluble compounds such as oxides and sulfides, chlorides, and nitrates. In polluted soils, Ni concentration reached up to 26,000 mg kg−1, a value 20–30 folds higher than that of non-contaminated agricultural soils (Rinklebe and Shaheen 2017). Nickel is essential for normal plant growth and development. Although Ni is very important for plants, its toxicity may cause a variety of physiological disorders in plants and animals (Sarwar et al. 2017). Excess of Ni supply may negatively affect important physiological and metabolic processes in plants, i.e., nutrient uptake through roots, transpiration, stomata conductance, plant water relations, photosynthetic rate, nutrient metabolism, association of Rhizobium spp. with legume crops and N fixation, efficiency of plasma membrane-bound H+-ATPase, carbohydrates metabolism, and enzyme activity (Sheoran et al. 1990; Pandey and Sharma 2002; Gajewska and Skłodowska 2009; Yusuf et al. 2011; Negi et al. 2014; Zhou et al. 2019; Ahammed et al. 2020a,b,c).
In high concentrations, Ni may decrease water content and stomatal conductance which may provoke disruption of chloroplasts, inhibition of chlorophyll synthesis, disruption of electron transport, low activity of Calvin cycle enzymes, and a CO2 shortage due to stomata closing (Seregin and Ivanov 2001; Ouzounidou et al. 2006; Velikova et al. 2011; Hasan et al. 2019). Therefore, excessive Ni disturbs photosystem II and related metabolic activities (Amari et al. 2014). Nickel toxicity may also affect photosynthetic efficiency by inhibiting chlorophyll synthesis. All these impairments may interrupt the photosynthetic efficiency, i.e., light harvesting complex II, electron transport chain, and Hill’s reaction (Ghasemi et al. 2012) and also the efficiency of Calvin cycle (Dan et al. 2000). Visual toxicity symptoms of Ni appear first in the interveinal area of the younger leaves, including blotchy chlorosis (similar to iron, zinc, or copper deficiency) followed by suppressed leaf expansion, necrosis, and eventually plant death.
The use of green plants and related microbe population to reduce the toxicity of metal contaminants in the environment, termed as phytoremediation (Ali et al. 2013), is an effective approach to remove excessive Ni from contaminated soil (Sarwar et al. 2017). It is among the most economic and efficient approach for remediation of heavy metals and metalloids from soils than other methods, such as soil excavation, washing of soil, incineration, flushing, and solidification (Ali et al. 2013). Phytoextraction is considered to be the most reliable and important measure for remediation of metals from contaminated soils, waters, biosolids, and sediments (He et al. 2005; Seth 2012; Ali et al. 2013). Among all phytoremediation techniques phytoextraction is an appropriate approach and also suitable for commercial applications (Sun et al. 2011). Plants used for phytoextraction are selected based on the following criteria: (i) rapid growth rate, (ii) high biomass production, (iii) capacity for metals hyperaccumulation, (iv) broad distribution, (v) capacity for metal translocation from root to shoot, (vi) high tolerance against metal toxicity, (vii) tolerance to pests and pathogens, (viii) adaptability to dynamic climatic conditions, and (ix) easy cultivation and harvest (Tong et al. 2004; Shabani and Sayadi 2012; Ali et al. 2013). Suitability of plants for phytoextraction can be determined by shoot biomass and shoot metal concentration. Two different approaches of phytextraction have been used (i) hypperaccumulator plants with little biomass (ii) low metal-accumulating plants producing high biomass, such as Brassica juncea (Ali et al. 2013).
Traditional phytoremediation techniques have some limitations. For example, (i) a long period is required to clean up the soil due to low above ground biomass production, (ii) small amount of metal bioavailability, which varies due to factors like soil pH, organic matter, competitive cations, calcareousness, (iii) applicability of these methods to sites where the contamination level is low or moderate, (iv) a lack of knowledge about agronomic techniques, breeding potential, insect pest and disease spectrum, and (v) possibility for food chain contamination (Ali et al. 2013). The above limitations show that there is a need to modify traditional techniques of phytoremediation and guarantee a wide application range of phytoremediation. The application of soil amendments like metal chelates to soil enhance the metal bioavailability and increase heavy metals accumulation by plants (Maxted et al. 2007). Under normal conditions, high quantities of many metals remain attached to soil particles and there is no bioavailability (Garbisu and Alkorta 2001; Hasan et al. 2019). The availability of metals to plants can be maintained through reducing precipitation and sorption of metals by chemical chelates formation (Sarwar et al. 2017).
Nickel toxicity is reported to decrease significantly chlorophyll content in maize leaves as well as root dry weight, so the root accumulates large amounts of Ni and hence root-Ni content decreases (Baccouch et al. 1998). However, the effect of Ni on nutrient acquisition of maize has not been studied. Moreover, because of its high biomass production and metal accumulation capacity, maize is a good candidate for use in bioenergy production on contaminated soils (Tian et al. 2012). Nevertheless, phytoremediation studies with maize in Ni-contaminated soils do not exist in the literature, especially in combination with chelators, while little is known about whether these supplemental methods using chelates can assist plant species to remediate soils contaminated with Ni. Keeping in view the above-mentioned facts and considerations, a study was planned with a specific objective to investigate the physiological and biochemical changes in maize plants grown in a Ni-contaminated soil and to assess the influence of EDTA on the phytoremediation efficiency of maize in a Ni-contaminated soil.
Materials and Methods
Contamination and Preparation of Soil
A pot experiment was conducted at the Nuclear Institute of Agriculture and Biology (NIAB) in Faisalabad, Pakistan. Soil was collected from the research area of NIAB (0.15 cm) being irrigated with canal water. The collected soil was dried in open air, ground, sieved with the help of a 2-mm iron sieve, thoroughly mixed up, and analyzed for saturation paste EC (dS m−1), saturation paste pH, organic matter (OM), CaCO3 content, soil texture, and cation exchange capacity (CEC) according to Page (1982). Nickel concentration in the experimental soil was determined by using an atomic absorption spectrophotometer (Solar S-100, Thermo Electron Co-operation, USA). Pots were filled with prepared soil and selected treatments of EDTA and Ni were applied in the form of solution according to saturation percentage. The detailed analysis of the soil used in the experiment is given in Table 1. Maize seeds of variety Hybrid 6601 were sown in plastic pots of 28 cm height and 20 cm diameter, each filled with 8 kg of soil. Five maize seeds were sown in each pot at field capacity and let to germinate under environmental conditions. One plant per pot was established, while the remaining seedlings were gently uprooted, chopped, and placed in the same pot after one week of germination. Recommended doses of nitrogen (N), phosphorous (P) and potassium (K) were applied. The whole P, K, and half of the N were applied before sowing, while the remaining N was applied in two additional splits 35 and 65 days after emergence. After 60 days from emergence, leaf samples were collected for biochemical analysis. All other parameters were studied at maturity. Four treatments including control, EDTA 0.5 mM, Ni 40 µM and Ni+EDTA in combination were applied and arranged in a completely randomized design with five replications.
Biochemical Analyses
Nitrate reductase activity (NRA) was determined with the method of Sym (1984). One mL of fresh leaf extract and 0.5 mL of 0.02 M KNO3 were mixed in a 25-mL test tube and put in an incubator for 1 h at 32 °C. During incubation time, NO3 was converted into NO2 by NRA. One mL of the reaction mixture was mixed with 0.5 mL of sulphanilamide after 1 h of incubation, instantly followed by the addition of 0.5 mL of N-(1-naphthyl)-ethylene diamine dihydrochloride. Pink color was developed and the reaction mixture was diluted by the addition of 5 mL of water before reading absosrbance at 542 nm against set standards. A spectrophotometer (HITACHI U-2800) was used for reading samples.
Nitrite reductase activity (NiRA) was determined with the method of Ramarao et al. (1983). A N-(1-naphthyl)-ethylene diamine dihydrochloride solution was prepared by dissolving 0.02 g of N-(1-naphthyl)-ethylene diamine dihydrochloride in 100 mL of distilled water. A quantity of 0.5 g of chopped leaf was taken in 5 mL of phosphate buffer and 0.5 mL of 0.02 M NaNO2 was added. The tubes were evacuated with a pump for 2 min to release the vacuum, wrapped with aluminum foil, and incubated in water bath for 30 min at 30 °C with gentle shaking. Then, test tubes were transferred to boiling water for 2 min to terminate the reaction and were cooled at room temperature. After cooling these samples were read out in a spectrophotometer (HITACHI U-2800).
Proline determination followed the method of Bates et al. (1973). Fresh leaf material (0.5 g) was taken and ground in 3% sulfo-salicylic acid (10 mL). The sample material was properly filtered out through filter paper (Whatman No. 2). Filtrates (2 mL) were taken in a 25-mL test tube added with 2 mL of acid ninhydrin solution and 2.5 mL of glacial acetic acid. Test tubes were heated for 1 h at 100 °C. The reaction was ended in an ice-bath and the reaction mixture was removed with 10 mL of toluene which produced chromophore. Continuous air stream was passed forcefully for 1–2 min in the reaction mixture to disperse the aqueous phase from the chromophore containing toluene. Inaccessible colored phase was permitted to stand for 2–3 min at room temperature and its absorbance was recorded at 520 nm using a spectrophotometer (HITACHI U-2800). Toluene was also observed as a blank. The concentration of proline was determined by using a standard curve developed by Analar grade proline and calculated on fresh weight according to the formula below.
Chlorophyll contents were determined with the method of Davis (1957). Fresh leaves were chopped and dipped into 80% acetone overnight at 10 oC. Next day, the samples were centrifuged at 14,000 g for 15 min. Debris was removed and the upper layer was taken to read at 480, 645, and 663 nm in a spectrophotometer. On the basis of OD values observed in a spectrophotometer (HITACHI U-2800), chlorophyll a, b, total chlorophyll, and carotenoids were calculated by the following formulas.
Chl a = [12.7 (OD663) − 2.69 (OD645)] × V/1000 × W
Chl b = [22.9 (OD645) − 4.68 (OD663)] × V/1000 × W
Total Chl = [20.2 (OD645) − 8.02 (OD663)] × V/1000 × W
Total carotenoids (g mL−1) = Acar/Em100%
Acar = OD480 + 0.114 (OD663) − 0.638 (OD645)
where V = sample volume, W = sample (tissue) weight, Em100% = 2500.
Total soluble proteins (TSP) were estimated according to Lowry et al. (1951). Sampled leaves (0.5 g) were sliced and mixed with 10 mL phosphate buffer (0.2 M) of pH 7.0 which was ground to homogenize it. The ground material was centrifuged at 5000 g for 5 min and the debris was removed. The supernatant of the centrifugated extract was taken for protein determination. One mL of the leaf extract per treatment was taken in a test tube. The blank contained only 1 mL of phosphate buffer. Solution C (1 mL) was mixed to all test tubes. The reagents in the test tube were mixed thoroughly and allowed to stand for 10 min at room temperature. Then, Folin-phenol reagent (1:1 diluted) was added and the absorbance was read at 620 nm in a spectrophotometer (HITACHI U-2800) after incubation for 30 min. The protein concentration was calculated by using a standard curve developed by various concentrations of Bovine serum albumin (BSA).
Total free amino acids (TFA) were determined according to Van Slyke et al. (1941). After preparing all the required reagents, fresh leaves (0.5 g) were chopped and extracted with phosphate buffer (0.2 M) having pH 7.0. One mL of the extract was taken in a 50-mL volumetric flask. One mL of pyridine (10%) and 1 mL of ninhydrin (2%) solutions were added in the flask. The flasks with the sample mixture were heated in boiling water bath for about 30 min. The volume of each flask was made up to 50 mL with distilled water. A standard curve was drawn with Lucien and the OD was recorded at 570 nm using a spectrophotometer (HITACHI U-2800). Free amino acids were calculated using the formula given below.
Total free amino acids = Graph reading × volume of sample × dilution factor/weight of tissue (g) × 1000.
Total soluble sugars (TSS) were determined according to Riazi et al. (1985). Fresh plant material was ground well in a micromill and the material was sieved through a 1-mm sieve of a micromill. Plant material (0.5 g) was extracted with 5 mL of 80% ethanol solution for 6 h at 60 °C. Plant extract of 0.1 mL was taken in 25-mL test tubes and 6 mL anthrone reagent was added to each tube, heated in boiling water bath for 10 min. The test tubes were ice-cooled for 10 min and incubated for 20 min at room temperature (25 °C). Samples were read at 625 nm in a spectrophotometer (HITACHI U-2800). The concentration of soluble sugars was calculated from a standard curve developed by using different concentrations of glucose according to the above procedure.
Determination of Mineral Contents
Dried and ground plant material (0.5 g) was taken in a digestion flask and 5 mL of concentrated H2SO4 were added to each tube and incubated overnight at 25 °C. Then, 0.5 mL of H2O2 (35%) was added and heated at 350 °C in a digestion block for 30 min till fumes appeared. The digestion tubes were shifted from the block, cooled down and 0.5 mL of H2O2 was mixed. Then, the tubes were shifted back into the digestion block. This step was continued till the cooled digested material became colorless. The volume was maintained up to 50 mL in flasks. The extract was filtered and used for determining potassium (K) and sodium (Na) using a flamephotometer (JENWAY PFP-7). Phosphorus (P) was determined using a spectrophotometer (HITACHI U-2800), while Ni in different plant parts was determined by using an atomic absorption spectrophotometer (NovAA-400 Analytical Gena).
Total Ni accumulation (mg plant−1) by maize plants was calculated as follows:
Translocation factor (TF) was determined to evaluate the percentage of Ni translocated from roots to shoots according to the formula by Anamika et al. (2009) as below:
Data Analysis
All data regarding the above-mentioned parameters were statistically analyzed using Statistix 8.1 software. Analysis of variance (ANOVA) was applied to analyze the data. Data normality and homoscedasticity of each response variable were assessed before ANOVA. Means were separated using the least significant difference (LSD) test at P < 0.05.
Results
Maize Growth and Biomass Production
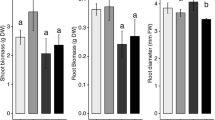
Root and shoot length decreased under various stress levels (Fig. 1). For both variables higher values were recorded in control plants (108 cm and 122 cm, respectively) than in plants grown under EDTA alone (104 cm and 113 cm, respectively) and Ni alone (100 cm and 103 cm, respectively). Plants exposed to Ni+EDTA showed a significant decrease in root fresh weight as compared with all other treatments (Fig. 2). Control plants maintained higher fresh root weight compared with treated plants, followed by EDTA alone and Ni alone. Similarly, shoot fresh weight exhibited a significant decrease. Higher fresh weight was maintained by control plants than plants grown under EDTA alone, Ni alone, and combination of Ni+EDTA. Root dry weight was significantly influenced by the different levels of Ni and EDTA (Fig. 3). Higher root dry weight was recorded in plants under control conditions than plants grown under EDTA alone and Ni alone. Ni+EDTA significantly decreased root dry weight compared with all other treatments. Similarly, shoot dry weight indicated a significant decrease when exposed to different applications of Ni alone, EDTA alone, and their combination (Fig. 3). Comparatively higher value was recorded in control plants than plants grown under EDTA alone and Ni alone. The lowest shoot dry weight was recorded in plants grown in the growth medium contaminated with Ni+EDTA.
Physiological Attributes
Contamination of EDTA and Ni in the growth medium significantly decreased chl-a contents (Fig. 4). Comparatively higher values were recorded in control plants than plants grown under EDTA alone, Ni alone, and Ni+EDTA. A similar trend was observed in chl-b content (Fig. 4). Nickel stress significantly decreased chl-b contents compared with control, while the lowest content of chl-b among treatments was observed in plants treated with Ni+EDTA. Total chlorophyll (chlt) content was also significantly affected under Ni and EDTA contamination (Fig. 4). The highest content of chlt was maintained by control plants followed by EDTA alone and Ni alone. Plants treated with Ni+EDTA showed the lowest content of chlt. Carotenoids content indicated a significant decrease in plants exposed to Ni, EDTA, and Ni+EDTA compared with control (Fig. 4). Plants under control environment maintained the highest carotenoids content followed by EDTA, Ni, and Ni+EDTA.
Nitrate reductase activity was significantly decreased due to exposure to EDTA, Ni, and Ni+EDTA (Fig. 5). Significantly higher NRA was recorded in control plants than plants grown under EDTA and Ni+EDTA. NiRA was significantly influenced by applications of EDTA alone, Ni alone, and their combination (Fig. 5). The higher value of NO2 indicated less NiRA which was observed in maize plants exposed to the above-mentioned treatments. Comparatively higher value of NO2 was maintained by Ni+EDTA than by Ni alone and EDTA alone. The lowest amount of NO2 was observed in control plants.
Proline accumulation significantly increased under Ni, EDTA, and Ni+EDTA (Fig. 6). Comparatively higher value of proline was recorded with Ni+EDTA than with Ni alone and EDTA alone. Control plants showed very low concentration of proline. Total leaf soluble proteins content was significantly decreased under different treatments of Ni and EDTA (Fig. 6). Comparatively higher concentration of proteins was noted in control plants than in plants grown under EDTA and Ni. The lowest concentration was recorded in plants treated with Ni+EDTA. Free amino acids accumulation significantly increased as the stress level increased (Fig. 6). The highest accumulation was recorded in Ni+EDTA, followed by Ni, EDTA, and control plants. Total soluble sugars significantly increased in plant exposed to different Ni and EDTA treatments (Fig. 6). Under Ni in combination with EDTA, plants showed the highest TSS content, followed by Ni and EDTA. Control plants showed low accumulation of TSS.
Mineral Acquisition
Potassium (K)
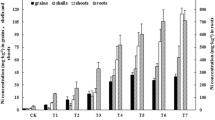
The maximum K+ content in root was found in plants grown in Ni+EDTA-contaminated medium, followed by Ni, EDTA, and control plants (Fig. 7). The highest stem-K+ content was recorded in plants exposed to Ni+EDTA followed by Ni and EDTA, while the lowest K+ content was recorded in control plants. A significant increase in leaf-K+ content was found in plants grown in medium contaminated with EDTA and Ni separately as well as in combination (Fig. 7). Plants grown in Ni+EDTA-contaminated soil maintained the highest concentration followed by Ni and EDTA, while the lowest leaf-K+ was recorded in control plants (Fig. 7). Plants exposed to EDTA and Ni in combination had the highest contents of grain-K+ followed by Ni and EDTA, while the lowest grain-K+ was recorded in plants grown under control conditions (Fig. 7).
Sodium (Na)
Exposure to Ni, EDTA, and Ni+EDTA affected root-Na+ significantly (Fig. 8). The maximum root-Na+ content was noted in plants grown in normal conditions, followed by EDTA, Ni, and Ni+EDTA. The highest stem-Na+ content was observed in plants grown in uncontaminated medium, followed by EDTA, Ni, and Ni+EDTA (Fig. 8). A significant decrease in leaf-Na+ content was noted under Ni, EDTA, and Ni+EDTA (Fig. 8). The maximum leaf-Na+ content was recorded in control plants as compared with the other treatments, followed by EDTA, Ni, and Ni+EDTA. Exposure of plants to different treatments of Ni, EDTA, and Ni+EDTA significantly decreased grain-Na+ content (Fig. 8). The maximum grain-Na+ content was recorded in grains of control plants, followed by EDTA, Ni, and Ni+EDTA.
Phosphorus (P)
Growth medium contaminated with Ni, EDTA, and Ni+EDTA significantly affected P contents in plant root (Fig. 9). P contents decreased under different levels of EDTA and Ni, while was the maximum in control, followed by EDTA and Ni. The lowest content of P was recorded in root of plants treated with Ni+EDTA in combination. Phosphorus content in the stem was significantly decreased due to different treatments of Ni and EDTA in the growth medium (Fig. 9). The maximum P content in the stem was noted in control followed by EDTA. Exposure to Ni also decreased P content in the stem followed by minimum P in the stem under Ni+EDTA. The Ni and EDTA affected leaf-P content significantly, which was maximum in control, followed by EDTA and Ni (Fig. 9). Phosphorus content in plants grown in Ni-contaminated medium was very low. The exposure of plants to Ni alone, EDTA alone, and Ni+EDTA in combination affected grain-P significantly compared with control plants (Fig. 9). The highest P content was observed in plants grown under control conditions, followed by EDTA and the combination of Ni+EDTA. The lowest P content was recorded in Ni+EDTA in combination.
Nickel (Ni)
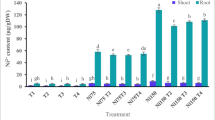
Root-Ni content at maturity significantly increased in plants grown in a contaminated growth medium compared with control plants (Fig. 10). Contamination with Ni+EDTA had the highest Ni content, followed by Ni alone and EDTA alone. In control plants, root-Ni content was the lowest. Contamination with Ni, EDTA, and Ni+EDTA significantly influenced stem-Ni contents (Fig. 10). Plants grown in soil contaminated with Ni+EDTA had highest stem-Ni content, followed by Ni. EDTA contamination in the growth medium significantly enhanced stem-Ni compared with control plants. The contamination of Ni+EDTA in combination increased Ni uptake in maize plants significantly. Nickel accumulation in the leaves of maize plants increased due to the presence of Ni, EDTA, and their combination compared with control plants (Fig. 10). However, the highest Ni accumulation was recorded in the leaves of plants treated with Ni+EDTA, followed by Ni alone and EDTA alone. Control plant leaves had the lowest Ni content. An increasing trend was observed in Ni content in grains of maize at maturity. Nickel contents significantly increased under EDTA, Ni, and their combination (Fig. 10). The highest Ni accumulation was recorded in plants grown in growth medium contaminated with Ni+EDTA, followed by Ni and EDTA. The minimum grain-Ni was found in control plants.
Discussion
This work assessed physiological and biochemical changes in maize plants grown in a Ni-contaminated soil and the effect of EDTA at 0.5 mM to enhance uptake of Ni from contaminated soil using maize. Phytoremediation studies with maize in Ni-contaminated soils do not exist in the literature, especially in combination with chelators, while little is known about the role of EDTA supplementation in a moderately sensitive maize crop to remediate Ni-contaminated soils.
Impact of EDTA-Assisted Phytoremediation on Maize Growth
In the present study, application of Ni alone and along with EDTA significantly decreased harvestable biomass indicating that maize was sensitive to Ni toxicity. However, plants receiving EDTA alone (without Ni) showed higher biomass production compared with the Ni treatments (Ni, Ni+EDTA), but low biomass production was observed with EDTA application compared with control. This response indicates a negative impact of EDTA on maize growth, namely, the adverse effects on plant growth were due to Ni toxicity. The higher reduction in biomass with Ni+EDTA compared with Ni alone might also be due to higher Ni uptake in the presence of chelate in soil. It may be inferred that reduction in biomass production could be the first visible indicator of Ni toxicity in metal-sensitive plants like maize. Ain et al. (2016) studied the effect of Ni on growth and physiology of wheat grown in saline soil in a pot experiment and inferred that exposure to Ni at a high dose (40 mg kg−1) significantly decreased above ground biomass possibly due to the toxic effects of Ni on wheat metabolism. Turgut et al. (2004) studied the effect of EDTA and citric acid application on the phytoremediation of a metal-contaminated soil using dwarf sunspot sunflower and teddy bear sunflower. They observed a significant decrease in above ground biomass production with application of 0.3 g kg−1 soil, i.e., 72.5% in sunspot and 48.3% in teddy bear plants. In the present study, the decrease in biomass production was 45.9% with Ni+EDTA compared with control. It may be inferred that exposure to excessive Ni may adversely affect plant growth and biomass production and these adverse effects might be further enhanced by the presence of EDTA in the growth medium.
Nickel toxicity generally decreased plant growth and development possibly due to decrease in the photosynthetic rate (Ci et al. 2010). This decrease in the photosynthetic rate was due to the declining contents of chlorophyll, including chl-a and chl-b, or destruction of important components of the photosystem II (Hasan et al. 2019; Ahammed et al. 2020a; Zhang et al. 2020). In the present investigation, total chlorophyll content significantly decreased in maize plants grown in the Ni-contaminated soil compared with control. This effect was further enhanced by the addition of EDTA in the Ni-contaminated medium. A similar trend was observed in chl-a and chl-b content. It may be inferred that Ni contamination at the rate of 40 µM caused destruction of chl-a and chl-b in affected plants possibly by altering chlorophyl structure, as Ni2+ may replace the central Mg2+ ion and may destroy the chlorophyll molecule. As the application of EDTA along with Ni may significantly enhance Ni uptake, the negative effect of Ni toxicity on chlorophyll content might be manifolded (Shafeeq et al. 2012). It was also observed that chl-a, chl-b, and total chl contents tended to decrease with an increasing Ni concentration in soil.
Nickel toxicity may also affect the activity of some enzymes in maize under stress. Depending upon concentration, Ni may decrease or increase the activity of certain enzymes. In the present study, it was observed that the activity of nitrate reductase activity was significantly decreased in plants exposed to Ni and Ni+EDTA compared with control, with a more pronouced effect of EDTA than Ni alone. This decrease in enzyme activity may be due to decrease in the uptake of nitrate from soil responsible to trigger gene action for synthesis of nitrate reductase enzymes (Zhou et al. 2019; Ganie et al. 2020). Similar results were shown by Kevrešan et al. (1998). These authors observed a significant decrease in NRA in Beta vulgaris plants exposed to 1 mM Ni compared with control plants. On the other hand, proline accumulation in plants exposed to Ni significantly increased, confirming the role of proline as a stress responsive amino acid. Proline is produced in plants under stress and play a protective role against reactive oxygen species (ROS) and other stress metabolites potentially harmful to membranes and biomolecules like lipids and lipoproteins that are responsible for solute transport across membranes (Ahammed et al. 2020b,c). The results of the present study are in accordance with those of Gajewska et al. (2006). These authors inferred that proline accumulation depends upon concentration and time of Ni exposure. They observed an increasing trend in proline accumulation with an increasing Ni concentration in the growth medium and with time of Ni exposure. Maximum proline accumulation was observed at 200 µM Ni after 9 days.
The traditional phytoremediation approach to harvest Ni from contaminated soils was meant for the use of hyper-accumulator plants having ability to accumulate large quantities of metal in their above ground biomass with minimum decrease in biomass yield. However, in this approach certain limitations come across to harvest an optimum quantity of metal which could be economically feasible. Generally, hyper-accumulators are less adaptable to a variety of soil and climatic conditions, less biomass-producing, and slow-growing species (Sarwar et al. 2017). Chemical assisted phytoremediation of Ni using low accumulator plants with high biomass production, e.g., Z. mays, seems an attractive approach to overcome the above-mentioned demerits of hyper-accumulator plants.
Impact of EDTA-Assisted Phytoremediation on Mineral Acquisition
Nickel interacts with other nutrients particularly Na, K, and P in soil as well as in plant tissues. Strong antagonism existed between Na and K for their uptake and translocation in plants (Rus et al. 2001; Ain et al. 2016) as these cations share the same membrane transporters on the root surface for their uptake and translocation to aerial parts. This competition might be concentration dependent, as cation with higher concentration is more likely to be taken up (Rus et al. 2001; Zhang et al. 2019). High Ni concentration in soil might decrease Na+ uptake, as bivalent cations are more preferably taken up than monovalents. This effect might be due to damage to certain transport systems by increasing the concentration of Ni cations in the soil solution. However, in salt affected soils the scenario might be different as such soils contain higher Na concentration. In the present study, it was observed that an antagonistic relationship existed between Ni and Na in plant tissues (root, shoot, and grain), while the relationship was synergistic between Ni and K in all plant parts. Similar relationships were established by Ain et al. (2016) where increasing Ni application tended to decrease Na concentration in plants, while increased K concentration in normal soils. However, the opposite trend was observed in the case of salt affected soils. In the current study, the application of Ni alone and in combination with EDTA significantly decreased P concentration in plant tissues as compared with control. Furthermore, P concentration in root, shoot, and grain in plants exposed to Ni+EDTA was significantly lower compared with Ni treatment without EDTA. This decrease in P concentration might be due to poor root growth in plants affected by Ni toxicity because poor root development may lead to less P uptake by plants.
Previous studies in the field of metal contamination of agricultural soils indicated that phytoremediation is an interesting and suitable option to remediate contaminated sites, as it provides a green solution of the problem that is socially acceptable and economically feasible (Salt et al. 1995; Garbisu and Alkorta 2001; Rew 2007; Seth 2012; Ali et al. 2013). However, traditional phytoremediation approaches, i.e., use of hyper-accumulator plants to extract metals from contaminated soils posses certain limitations as discussed in detail in previous sections. Use of low accumulator plants with high biomass production potential, like maize assisted with suitable chelating agents, e.g., EDTA to enhance metal uptake from soil, might be an interesting and economical approach to harvest more metal in a relatively short time span (Sarwar et al. 2017). In the current study, addition of EDTA (0.5 mM) in the contaminated medium significantly enhanced Ni concentration in roots as well as in above ground plant parts as compared with all other treatments from the Ni-contaminated soil. This might be due to enhanced Ni uptake in the presence of EDTA, as EDTA application might enhance Ni bioavailablity by chelating Ni and minimizing its fixation in soil (Sarwar et al. 2017). A hydroponic experiment conducted to study the effect of EDTA and salicylic acid on Ni uptake at different time intervals by Lemna minor L. seedlings indicated that application of EDTA significantly increased Ni uptake at different time intervals (Kaur et al. 2015).
Conclusions
This study confirmed that addition of EDTA in Ni-contaminated medium resulted in higher accumulation of Ni in maize plants compared with control, while the uptake of essential nutrient P was decreased and K uptake was enhanced. The impact of Ni on biomass accumulation and root growth was also depressing and inhibitory; however, the increased uptake and per unit biomass concentration of Ni in the presence of EDTA shows the potential of using maize plants for bioremediation and phytoextraction of Ni from contaminated soils. The optimum concentration of EDTA, however, is needed to be standardized for the naturally Ni-contaminated soils and to find out the economic feasibility of the process. Moreover, the beneficial effects of the restricted Na and enhanced uptake of K and their relationship with Ni toxicity in terms of physiology and biochemical plant processes are required to be established with more extensive experimental and advanced analytical work.
References
Ahammed GJ, Li X, Yang Y, Liu C, Zhou G, Wan H, Cheng Y (2020a) Tomato WRKY81 acts as a negative regulator for drought tolerance by modulating guard cell H2O2-mediated stomatal closure. Environ Exp Bot 171:103960
Ahammed GJ, Wang Y, Mao Q, Wu M, Yan Y, Ren J, Wang X, Liu A, Chen S (2020b) Dopamine alleviates bisphenol A-induced phytotoxicity by enhancing antioxidant and detoxification potential in cucumber. Environ Pollut 259:113957
Ahammed GJ, Wu M, Wang Y, Yan Y, Mao Q, Ren J, Ma R, Liu A, Chen S (2020c) Melatonin alleviates iron stress by improving iron homeostasis, antioxidant defense and secondary metabolism in cucumber. Sci Hortic 265:109205
Ain Q, Akhtar J, Amjad M, Haq M, Saqib Z (2016) Effect of enhanced nickel levels on wheat plant growth and physiology under salt stress. Commun Soil Sci Plant Anal 47:2538–2546
Ali H, Khan E, Sajad MA (2013) Phytoremediation of heavy metals: concepts and applications. Chemosphere 91:869–881
Amari T, Ghnaya T, Debez A, Taamali M, Youssef NB, Lucchini G (2014) Comparative Ni tolerance and accumulation potentials between Mesembryanthemum crystallinum (halophyte) and Brassica juncea: metal accumulation, nutrient status and photosynthetic activity. J Plant Physiol 171:1634–1644
Anamika S, Eapen S, Fulekar MH (2009) Phytoremediation of cadmium, lead and zinc by Brassica juncea L. Czern Coss J Appl Biosci 13:726–736
Baccouch S, Chaoui A, El Ferjani E (1998) Nickel toxicity: effects on growth and metabolism of maize. J Plant Nutr 21:577–588
Bates L, Waldren R, Teare I (1973) Rapid determination of free proline for water-stress studies. Plant Soil 39:205–207
Ci D, Jiang D, Wollenweber B, Dai T, Jing Q, Cao W (2010) Genetic variance in cadmium tolerance and accumulation in wheat materials differing in ploidy and genome at seedling stage. J Agron Crop Sci 196:302–310
Dan TV, KrishnaRaj S, Saxena PK (2000) Metal tolerance of scented geranium (Pelargonium sp. ‘Frensham’): effects of cadmium and nickel on chlorophyll fluorescence kinetics. Int J Phytoremed 2:91–104
Davis P (1957) A method for the determination of chlorophyll in sea water. Marine CSIRO Aust. Div. Fish. Oceanogr. Rep. No. 7
Gajewska E, Skłodowska M (2009) Nickel-induced changes in nitrogen metabolism in wheat shoots. J Plant Physiol 166:1034–1044
Gajewska E, Skłodowska M, Słaba M, Mazur J (2006) Effect of nickel on antioxidative enzyme activities, proline and chlorophyll contents in wheat shoots. Biol Plant 50:653–659
Ganie SA, Ahammed GJ, Wani SH (2020) Vascular plant one zinc-finger (VOZ) transcription factors: novel regulators of abiotic stress tolerance in rice (Oryza sativa L.). Gen Res Crop Evol 67:799–807
Garbisu C, Alkorta I (2001) Phytoextraction: a cost-effective plant-based technology for the removal of metals from the environment. Biores Technol 77:229–236
Ghasemi F, Heidari R, Jameii R, Purakbar L (2012) Effects of Ni2+ toxicity on Hill reaction and membrane functionality in maize. J Stress Physiol Biochem 8:55–61
Hasan MK, Ahammed GJ, Sun S, Li M, Yin H, Zhou J (2019) Melatonin inhibits cadmium translocation and enhances plant tolerance by regulating sulfur uptake and assimilation in Solanum lycopersicum L. J Agric Food Chem 67:10563–10576
He ZL, Yang XE, Stoffella PJ (2005) Trace elements in agroecosystems and impacts on the environment. J Trace Elem Medic Biol 19:125–140
Kaur L, Gadgil K, Sharma S (2015) Comparative evaluation of salicylic acid and EDTA chelant induced phytoremediation of lead and nickel using Lemna minor L. Trop Plant Res 2:264–270
Kevrešan S, Petrović N, Popović M, Kandrač J (1998) Effect of heavy metals on nitrate and protein metabolism in sugar beet. Biol Plant 41:235–240
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Maxted A, Black C, West H, Crout N, McGrath S, Young S (2007) Phytoextraction of cadmium and zinc from arable soils amended with sewage sludge using Thlaspi caerulescens: development of a predictive model. Environ Pollut 150:363–372
Negi A, Singh HP, Batish DR, Kohli RK (2014) Ni+2-inhibited radicle growth in germinating wheat seeds involves alterations in sugar metabolism. Acta Physiol Plant 36:923–929
Ouzounidou G, Moustakas M, Symeonidis L, Karataglis S (2006) Response of wheat seedlings to Ni stress: effects of supplemental calcium. Arch Environ Contam Toxic 50:346–352
Page A (1982) Methods of soil analysis: chemical and microbiological properties. American Society of Agronomy, Madison, WI, USA
Pandey N, Sharma CP (2002) Effect of heavy metals Co2+, Ni2+ and Cd2+ on growth and metabolism of cabbage. Plant Sci 163:753–758
Ramarao C, Patil V, Dhak B, Kadrekar S (1983) A simple in vivo method for the determination of nitrite reductase activity in rice roots. Z Pflanzenphysiol 109:81–85
Rew A (2007) Phytoremediation: an environmentally sound technology for pollution prevention, control and remediation in developing countries. Educ Res Rev 2:151–156
Riazi A, Matsuda K, Arslan A (1985) Water-stress induced changes in concentrations of proline and other solutes in growing regions of young barley leaves. J Exp Bot 36:1716–1725
Rinklebe J, Shaheen SM (2017) Redox chemistry of nickel in soils and sediments: a review. Chemosphere 179:265–278
Rus A, Estan M, Gisbert C, Garcia-Sogo B, Serrano R, Caro M (2001) Expressing the yeast HAL1 gene in tomato increases fruit yield and enhances K+/Na+ selectivity under salt stress. Plant Cell Environ 24:875–880
Salt DE, Blaylock M, Kumar NP, Dushenkov V, Ensley BD, Chet I (1995) Phytoremediation: a novel strategy for the removal of toxic metals from the environment using plants. Nat Biotechnol 13:468–474
Sarwar N, Imran M, Shaheen MR, Ishaq W, Kamran A, Matloob A (2017) Phytoremediation strategies for soils contaminated with heavy metals: modifications and future perspectives. Chemosphere 171:710–721
Seregin I, Ivanov V (2001) Physiological aspects of cadmium and lead toxic effects on higher plants. Russ J Plant Physiol 48:523–544
Seth CS (2012) A review on mechanisms of plant tolerance and role of transgenic plants in environmental clean-up. Bot Rev 78:32–62
Shabani N, Sayadi M (2012) Evaluation of heavy metals accumulation by two emergent macrophytes from the polluted soil: an experimental study. Environmentalist 32:91–98
Shafeeq A, Butt ZA, Muhammad S (2012) Response of nickel pollution on physiological and biochemical attributes of wheat (Triticum aestivum L.) var. Bhakar-02. Pak J Bot 44:111–116
Sheoran I, Singal H, Singh R (1990) Effect of cadmium and nickel on photosynthesis and the enzymes of the photosynthetic carbon reduction cycle in pigeonpea (Cajanus cajan L.). Photosynth Res 23:345–351
Sun Y, Zhou Q, Xu Y, Wang L, Liang X (2011) The role of EDTA on cadmium phytoextraction in a cadmium-hyperaccumulator Rorippa globosa. J Environ Chem Ecotox 3:45–51
Sym GJ (1984) Optimisation of the in-vivo assay conditions for nitrate reductase in barley (Hordeum vulgare L. cv. Igri). J Sci Food Agric 35:725–730
Tian Y, Zhang H, Guo W, Chen Z, Wei X, Zhang L, Han L, Dai L (2012) Assessment of the phytoremediation potential of bioenergy crop maize (Zea mays) in soil contaminated by cadmium: Morphology, photosynthesis and accumulation. Fresen Environ Bull 21:3575–3581
Tong Y-P, Kneer R, Zhu Y-G (2004) Vacuolar compartmentalization: a second-generation approach to engineering plants for phytoremediation. Trends Plant Sci 9:7–9
Turgut C, Pepe MK, Cutright TJ (2004) The effect of EDTA and citric acid on phytoremediation of Cd, Cr, and Ni from soil using Helianthus annuus. Environ Pollut 131:147–154
Van Slyke DD, Dillon RT, MacFadyen DA, Hamilton P (1941) Gasometric determination of carboxyl groups in free amino acids. J Biol Chem 141:627–669
Velikova V, Tsonev T, Loreto F, Centritto M (2011) Changes in photosynthesis, mesophyll conductance to CO2, and isoprenoid emissions in Populus nigra plants exposed to excess nickel. Environ Pollut 159:1058–1066
Yusuf M, Fariduddin Q, Hayat S, Ahmad A (2011) Nickel: an overview of uptake, essentiality and toxicity in plants. Bull Environ Contam Toxic 86:1–17
Zhang Y, Liang Y, Zhao X, Jin X, Hou L, Shi Y, Ahammed GJ (2019) Silicon compensates phosphorus deficit-induced growth inhibition by improving photosynthetic capacity, antioxidant potential, and nutrient homeostasis in tomato. Agronomy 9:733
Zhang Z, Wu P, Zhang W, Yang Z, Liu H, Ahammed GJ, Cui J (2020) Calcium is involved in exogenous NO-induced enhancement of photosynthesis in cucumber (Cucumis sativus L.) seedlings under low temperature. Sci Hortic 261:108953
Zhou Y, Guang Y, Li J, Wang F, Ahammed GJ, Yang Y (2019) The CYP74 gene family in watermelon: genome-wide identification and expression profiling under hormonal stress and root-knot nematode infection. Agronomy 9:872
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
Authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Tipu, M.I., Ashraf, M.Y., Sarwar, N. et al. Growth and Physiology of Maize (Zea mays L.) in a Nickel-Contaminated Soil and Phytoremediation Efficiency Using EDTA. J Plant Growth Regul 40, 774–786 (2021). https://doi.org/10.1007/s00344-020-10132-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00344-020-10132-1