Abstract
The purpose of current investigation was to explore the role of brassinosteroids (BRs) in Zea mays L. var. DKC 9106 seedlings subjected to salt stress. The seedlings were raised under controlled laboratory conditions and subjected to different concentrations of NaCl (0, 40, 60, 80, 100 mM) for 10 days. The impact of pre-sowing treatment of both 28-homobrassionolide (HBL) and 24-epibrassinolide (EBL) on defense system of Z. mays L. under salt stress was studied by analyzing Na+ and K+ ions, malondialdehyde content (MDA), antioxidative enzymes activities (peroxidase, POD; catalase, CAT; dehydroascorbate reductase, DHAR; monodehydroascorbate reductase, MDHAR), osmoprotectants (proline, glycine betaine, mannitol, and total osmolytes content), total phenolic content, total flavonoid content, and 1,1-diphenylpicrylhydrazyl (DPPH) free radical scavenging activity. The results of our finding showed that treatment of both HBL and EBL under high salt stress balanced the ionic status by decreasing the Na+ ions content by 21.23% and 38.94%, respectively, and enhancing the K+ ions content by 51.94% and 26.66%, respectively. Treatment of both BRs also overcome the oxidative damage induced due to salinity stress by reducing the MDA accumulation 19.50% and 45.0%, respectively, and enhancing the activities of antioxidative enzymes. The osmoprotectants: proline (50.08% and 17.03%), glycine betaine (35.57% and 28.16%), and mannitol content (2.80% and 20.98%) were markedly increased by the treatment of both HBL and EBL, respectively. Further, treatment of both HBL and EBL also increased the total phenolic content by 11.68% and 5.80%, total flavonoid content by 31.56 and 31.09% and DPPH free radical scavenging activity by 37.99% and 77.41%, respectively. Overall the treatment of BRs before seed sowing considerably conquer the salinity-induced damage by stimulating functional components of antioxidative defense system and ultimately reduced oxidative damage.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Salinity stress restraining the agricultural production and yield throughout the world. Almost 45 million hectare of irrigated land and 32 million of dry land agriculture were estimated to be salt affected (FAO 2016). The detrimental effects of salinity stress mainly involve the hyperosmotic stress, which reduces the water potential in roots and hyperionic stress, which causes the acquisition of Na+ and Cl− ions to a damaging level (Shabala and Cuin 2008; Marschner 2011). The other consequences of salt stress include the nutritional stress, oxidative stress, and reduction in photosynthetic performance (Tomescu et al. 2017; Tang and Luo 2018; Ahanger et al. 2019). In nutritional stress, Na+ ions replace the K+ ions and alter the normal metabolic processes regulated by potassium, such as stomatal regulation, osmoregulation, protein synthesis, and maintaining adequate membrane potential (Ahmad and Maathuis 2014; Chakraborty et al. 2018). Whereas in oxidative stress, enhanced fabrication of reactive oxygen species (ROS) occurs due to higher concentrations of salt. The most common ROS are singlet oxygen (1O2), hydrogen peroxide (H2O2), superoxide radical (O2·−), and hydroxyl radicals (·OH) (Abogadallah 2010; Bose et al. 2014; Guo et al. 2017). Once the production of ROS surpasses from the limits of defense system of the plants, oxidative stress generated in the cells leads to disruption of various cellular functions by reacting with other vital components (Tavakkoli et al. 2010; Mishra et al. 2011; Chen et al. 2017; Ahanger et al. 2017; Van Ruyskensvelde et al. 2018). Thus, to prevent the ROS-induced damage, plants develop antioxidant defense system which comprises enzymatic antioxidants: superoxide dismutase (SOD), guaiacol peroxidase (POD), catalase (CAT), ascorbate peroxidase (APOX), glutathione reductase (GR), monodehydroascorbate reductase (MDHAR), dehydroascorbate reductase (DHAR) and non-enzymatic antioxidants: glutathione, ascorbate, and tocopherol (Gill and Tuteja 2010; Ali et al. 2011). Other natural antioxidants or secondary metabolites, such as phenolics and flavonoids also play important role under stress conditions. Phenolics are one of the major groups of plant secondary metabolites which possess a wide range of biological functions in plants including protection from ROS (Sharma et al. 2019b). Phenolic compounds mainly attributed to the antioxidant effect of plant products (Souid et al. 2016; Sharma et al. 2016a, 2019b). Radical scavenging activity of flavonoids depends upon its structure and predominantly the hydroxyl position in the molecule (Wojdyło et al. 2007). Redox properties of phenolics permit them to act as hydrogen donors, metal chelators, and reducing agents which contribute towards their antioxidant potential (Canadanovic‐Brunet et al. 2005; Marimuthu et al. 2008).
Brassinosteroids are important category of plant hormones which are widely known for their involvement in stress alleviation and growth promotion (Bartwal et al. 2013; Sharma et al. 2018; Shahzad et al. 2018; Tanveer et al. 2018, 2019). BRs play important role in seed germination, plant growth and development, root and stem elongation, cell division and differentiation, cell signaling, photomorphogenesis, and organization of microtubules (Haubrick and Assmann 2006; Gudesblat and Russinova 2011; Sreeramulu et al. 2013; Wei and Li 2016). The stress ameliorating responses of BRs are concentration-dependent which may vary according to the type of species, developmental stages, and environmental conditions of the plants (Ahammed et al. 2015). BRs stimulated the growth and other related activities in plants and may act independently or in conjunction with other plant hormones. Several studies showed that BRs and signaling pathways of other hormones are often interconnected and their cross talk helps in regulating the various physiological and developmental processes (Choudhary et al. 2012; Hofmann 2015; Ahanger et al. 2018a; Peres et al. 2019). Thus, by keeping stress regulatory role of BRs in mind, current work was conducted to study the effect of two bioactive BRs i.e., 28-homobrassinolide and 24-epibrassinolide on physiological and biochemical responses of maize seedlings under salt stress.
Materials and Methods
Raising of Seedlings
Certified, disease-free and uniform sized seeds of Zea mays L. var. DKC 9106 were procured from the Department of Agriculture (Gurdaspur), Punjab, India. For pre-sowing treatments, two brassinosteroids (BRs), viz. HBL and EBL were taken. Stock solution of 10–3 M of each HBL and EBL was prepared in HPLC grade methanol. Different working concentrations of both HBL (0.0001, 0.01 and 1 µM) and EBL (0.0001, 0.01 and 1 µM) were prepared through serial dilutions of each stock solution and used for further experimentation. Seeds were soaked for 12 h in each concentration of HBL and EBL and raised in autoclaved petriplates lined with Whatman filter paper (grade 1). Seedlings were allowed to grow under controlled conditions of seed germinator (25 ± 0.5 °C, 16:8 h light:dark photoperiod) for ten days in different concentrations of salt (0, 40, 60, 80, 100 mM). Test solutions were supplied on alternative days up to 10 days. Then seedlings were harvested after ten days of sowing for further analysis.
MDA Content Estimation
MDA content was assessed according to method given by Heath and Packer (1968). One gram fresh seedlings were extracted with 3 ml of 0.1% (w/v) trichloroacetic acid. Supernatant from above extraction was mixed with trichloroacetic acid containing 0.5% (w/v) thiobarbituric acid. Mixture was warmed and then cooled instantly. The absorbance of the solution was taken spectophotometrically at 532 nm and 600 nm.
Estimation of Antioxidative Enzymes Activities
Homogenate for estimation of antioxidative enzymes activities was prepared by crushing 1 g shoot material with 3 ml of 100 mM potassium phosphate buffer (pH 7.0). Supernatant from above centrifuged homogenate was collected to estimate the enzymes activities of POD, CAT, DHAR, and MDHAR. Activity of POD was measured by the method of Putter (1974). For estimating the POD activity, 3 ml of phosphate buffer, 50 μl of guaiacol solution, 30 μl of H2O2 solution, and 100 μl of enzyme sample were taken in the cuvette and activity was determined spectrophotometrically at 436 nm. Activity of CAT enzyme was estimated according to the method of Aebi (1984). In a cuvette, 1.5 ml of phosphate buffer, 1.2 ml of hydrogen peroxide, and 300 μl of enzyme extract were taken and the rate of decomposition of H2O2 was estimated at 240 nm. DHAR and MDHAR activities were assessed according to Dalton et al. (1986) and Hossain et al. (1984) method. For DHAR activity, 50 mM of phosphate buffer, 0.1 mM ethylenediaminetetraacetic acid (EDTA), 1.5 mM glutathione reduced, 0.2 mM dehydroascorbate, and 400 μL enzyme extract were taken in the cuvette and measured spectrophotometrically at 265 nm, and for MDHAR activity, in the cuvette, 50 mM of phosphate buffer, 0.1 mM EDTA, 0.3 mM nicotinamide adenine dinucleotide (NADH), 0.25 units ascorbate oxidase, and 300 µl of enzyme extract were taken and absorbance was determined spectrophotometrically at 340 nm.
Estimation of Osmoprotectants
Proline content was determined according to Bates et al. (1973) and intensity of the reaction mixture was determined spectrophotometrically at 520 nm wavelength. The glycine betaine content and mannitol content were evaluated by Grieve and Grattan (1983) and Sanchez (1998) methods at 365 nm and at 412 nm wavelength accordingly. Total osmolytes content was analyzed using vapor pressure osmometer (VPO) (Vapro 5600). Leaves were thawed and sap was stored in − 20 °C. 10 µl of sap was pipetted out and used for the analysis. Instrument was calibrated using the standard solutions of NaCl of osmolalities 100, 290, 1000 Osm, and readings were taken at 25 °C.
Estimation of Total Phenolic Content, Flavonoid Content, and DPPH Radical Scavenging Activity
Total phenolic content was estimated spectrophotometrically at 760 nm following the method of Swain and Hills (1959). Total flavonoid content was estimated by Balbaa et al. (1974) method and DPPH assay was performed according to the method given by Blois (1958).
Statistical Analysis
Two-way analysis of variance (ANOVA) was performed using self-coded software (MS-excel 2010) and data were presented at significance of p ≤ 0.05. Each experiment was repeated for three times (three replicates).
Results
In the present study, accumulation of Na+ ions was found to increase with increasing salt concentration. Maximum accumulation of Na+ ions was observed in highest NaCl concentration. However, K+ ions were found to decrease under salt stress and maximum decrease in K+ ions was also found under highest salt treatment, as compared to control seedlings. Under stress conditions, pretreatment of HBL significantly lowered the Na+ ion accumulation. Under highest salt stress, HBL (0.01 µM) and EBL (0.0001 µM) application reduced Na+ content by 21.23% and 38.94%, respectively (Fig. 1a). Further pre-sowing treatment of HBL significantly increased the K+ ions content as compared to control. Application of HBL (0.01 µM) and EBL (0.0001 µM) under severe salt stress showed the recovery of K+ ions by 51.94% and 26.66%, respectively (Fig. 1b).
Effect of 28-homobrassinolide and 24-epibrassinolide on Na+ and K+ ions content in Zea mays seedlings subjected to salt stress. In figure, HBL-1, HBL-2, HBL-3 corresponds to 28-homobrassinolide (0.0001, 0.01 and 1 µM, respectively) concentrations and EBL-1, EBL-2, EBL-3 corresponds to 24-epibrassinolide (0.0001, 0.01 and 1 µM, respectively) concentrations. Data presented in mean ± SE and *Indicate statistically significant at p ≤ 0.05
MDA content was observed to increase with increasing salt concentrations. Maximum MDA content was observed at 100 mM NaCl concentration as compared to control seedlings. Seed pre-sowing treatment of both HBL and EBL to salt stressed seedlings resulted in lowering of MDA content. Under stress, maximum reduction of 19.5% in MDA content was observed with the pretreatment of 0.0001 µM HBL and 45% reduction in MDA content was observed with pretreatment of 1 µM EBL (Fig. 2).
Effect of 28-homobrassinolide and 24-epibrassinolide on MDA content in Zea mays seedlings under salt stress. In figure, HBL-1, HBL-2, HBL-3 corresponds to 28-homobrassinolide (0.0001, 0.01 and 1 µM, respectively) concentrations and EBL-1, EBL-2, EBL-3 corresponds to 24-epibrassinolide (0.0001, 0.01 and 1 µM, respectively) concentrations. Data presented in mean ± SE and *Indicate statistically significant at p ≤ 0.05
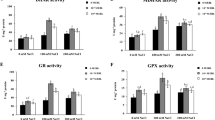
Antioxidative enzymes (POD and CAT) activities were found to enhance under salt stress to overcome the salinity-induced damage. Seedlings exposed to 80 mM and 60 mM NaCl concentrations showed the maximum increase in POD and CAT activities, respectively, as compared to control seedlings. Whereas under stress, seedlings raised from seeds pretreated with 0.0001 µM HBL and 0.01 µM EBL enhanced the POD activity by 20.97% and 22.01%, respectively (Fig. 3a). On the other hand, CAT activity was observed to increase 62.34% with the pretreatment of 1 µM HBL and 11.75% with 0.01 µM EBL treatment (Fig. 3b).
Effect of 28-homobrassinolide and 24-epibrassinolide on activities of antioxidative enzymes (peroxidase, POD; catalase, CAT; dehydroascorbate reductase, DHAR; monodehydroascorbate reductase, MDHAR) in Zea mays seedlings under salt stress. In figure, HBL-1, HBL-2, HBL-3 corresponds to 28-homobrassinolide (0.0001, 0.01 and 1 µM, respectively) concentrations and EBL-1, EBL-2, EBL-3 corresponds to 24-epibrassinolide (0.0001, 0.01 and 1 µM, respectively) concentrations. Data presented in mean ± SE and *Indicate statistically significant at p ≤ 0.05
DHAR and MDHAR activities were also found to enhance under salt stress. A significant increase in activity of DHAR was observed under 80 mM NaCl concentration and MDHAR was observed under 60 mM NaCl concentration in contrast to control seedlings. Under stress conditions, 0.01 µM HBL and 1 µM EBL increase the DHAR enzyme activity by 17.82% and 27.87%, respectively (Fig. 3c), whereas MDHAR activity was found to increase with the pretreatment of 0.01 µM HBL and 0.01 µM EBL by 7.6% and 14.28%, respectively (Fig. 3d).
In the present study, osmolytes were found to enhance under salt stress. Maximum increase in proline content was observed under 80 mM NaCl concentration as compared to control seedlings. Further pretreatment of both HBL and EBL increased the production of osmolytes in salt stressed seedlings. Under stress, treatment of 0.0001 µM HBL significantly enhance the proline content by 50.08%, whereas 0.0001 µM EBL showed the maximum increase of 17.03% as compared to only 80 mM NaCl concentration (Fig. 4a).
Effect of 28-homobrassinolide and 24-epibrassinolide on osmolytes content (proline, glycine beatine, mannitol, and total osmolytes content) in Zea mays seedlings under salt stress. In figure, HBL-1, HBL-2, HBL-3 corresponds to 28-homobrassinolide (0.0001, 0.01 and 1 µM, respectively) concentrations and EBL-1, EBL-2, EBL-3 corresponds to 24-epibrassinolide (0.0001, 0.01 and 1 µM, respectively) concentrations. Data presented in mean ± SE and *Indicate statistically significant at p ≤ 0.05
Glycine betaine content at 80 mM NaCl and mannitol content at 100 mM NaCl concentration showed the higher increase in content. Pretreatment of 0.01 µM HBL and 0.01 µM EBL exhibited the maximum rise of glycine betaine content by 35.57% and 28.16%, respectively (Fig. 4b). Mannitol content was found to slightly increase by 2.8% with the treatment of 0.0001 µM HBL and 20.98% by the treatment of 0.0001 µM EBL (Fig. 4c). Pretreatments of HBL and EBL both also enhanced the total osmolytes content in stressed seedlings. Total osmolytes content were found to improve by 27.02% and 8.68% with the treatment of 0.01 µM HBL and 0.0001 µM EBL under 80 mM NaCl concentration (Fig. 4d).
In 10 days old seedlings, flavonoid content and phenolic content were reported to enhance with the severity of salinity stress. Maximum increase of flavonoid content was observed in 80 mM NaCl concentration in comparison to control. Pre-sowing treatment of both 0.01 µM HBL and 0.01 µM EBL under 80 mM salt stressed seedlings efficiently elevated the flavonoid content by 31.56% and 31.09% in contrast to only 80 mM NaCl concentration (Fig. 5a). Phenolic content showed maximum rise under 100 mM NaCl concentration as compared to control. Pre-sowing treatment of HBL and EBL significantly enhanced the total phenolic content in the seedlings. Under salt stressed seedlings, maximum increase of phenolic content was recorded 11.68% and 5.80% with the treatment of 0.0001 µM HBL and 0.01 µM EBL, respectively (Fig. 5b).
Effect of 28-homobrassinolide and 24-epibrassinolide on total flavonoid content and total phenolic content in Zea mays seedlings under salt stress. In figure, HBL-1, HBL-2, HBL-3 corresponds to 28-homobrassinolide (0.0001, 0.01 and 1 µM, respectively) concentrations and EBL-1, EBL-2, EBL-3 corresponds to 24-epibrassinolide (0.0001, 0.01 and 1 µM, respectively) concentrations. Data presented in mean ± SE and *Indicate statistically significant at p ≤ 0.05
Percentage inhibition of DPPH radical was found to decrease with increasing NaCl concentration. The extreme decrease was noticed at 100 mM NaCl treated seedlings as compared to control. Under stress, both of HBL and EBL enhanced the DPPH free radical scavenging activity. However, pretreatment of 1 µM HBL resulted in increment of DPPH free radical scavenging activity by 37.99% and pretreatment of 0.01 µM EBL enhanced the activity by 77.41% (Fig. 6).
Effect of 28-homobrassinolide and 24-epibrassinolide on 1,1-diphenylpicrylhydrazyl (DPPH) activity in Zea mays seedlings under salt stress. In figure, HBL-1, HBL-2, HBL-3 corresponds to 28-homobrassinolide (0.0001, 0.01, and 1 µM, respectively) concentrations and EBL-1, EBL-2, EBL-3 corresponds to 24-epibrassinolide (0.0001, 0.01, and 1 µM, respectively) concentrations. Data presented in mean ± SE and *Indicate statistically significant at p ≤ 0.05
Discussion
Salinity stress is a foremost issue of concern, reducing crop productivity and yield severely by affecting the various processes in plants. Among various sources of soil salinity, irrigation without suitable drainage is considered as most serious because it elevated the water table and permits the salty groundwater to come up on the upper soil layers (Zhu 2007; Chakraborty et al. 2018). Irrigation water contains mainly Ca2+, Mg2+, and Na+ ions, and among these, Ca2+ and Mg2+ ions often precipitates into carbonates with the evaporation of water and leaving behind the Na+ ions which causes ionic imbalance (Serrano et al. 1998). The ionic imbalance due to increase of Na+ ions impedes with other important ions, such as K+ ions, and affected the growth and even causes the death of plant cells (Craig Plett and Moller 2010; Cabot et al. 2014). In the present study, Na+ ions increased with the increase of salinity stress and K+ ions were found to decrease with the severity of stress. Similarly, increased Na+ ions accumulation and decreased K+ and Ca2+ ions uptake affected the growth, biomass and pigment synthesis in mung bean plants subjected to salt stress (Ahmad et al. 2019). Application of both HBL and EBL in the present study under salinity stress improved the ionic status by decreasing the Na+ ions and by restoring the K+ ions in the seedlings. The reduction in Na+ ions accumulation and increase of K+ ions content under salt stress with the treatment of EBL promoted the osmotic adjustment in perennial ryegrass by ameliorating the ion toxicity and nutritional imbalance (Sun et al. 2015). BRs help in maintaining the ionic balance by reducing the transportation of Na+ ions into the plants (Eleiwa et al. 2011). EBL application also alleviated the deleterious effects of salinity stress by improving the K+/Na+ ratio and nutrients content in Eucalyptus urophylla plants subjected to 250 mM NaCl stress (de Oliveira et al. 2019).
Plants itself adopted various physiological mechanisms to minimize the damage triggered due to overproduction of ROS through the enhancement of activity of antioxidant enzymes, other secondary metabolites, and accumulation of various compatible solutes (Yan et al. 2013). One of the major consequences of salt stress includes the overproduction of ROS which leads to oxidative stress (Gupta and Huang 2014). Oxidative damage due to salinity stress in the present study was determined in terms of MDA content and it was found to increase in salt stressed maize seedlings. The increased MDA content leads to the generation of free radicals, resulted in disruption of cellular functioning by affecting lipids metabolism, physiochemical properties of cell membranes, alternations of ion transport, and metabolic processes (Ahanger and Agarwal 2017; Miller et al. 2010). On the other hand, treatment of HBL and EBL to salt stressed seedlings resulted in decline in lipid peroxidation as indicated by lowering of MDA content. BRs regulated MDA content under salt stress may involve the scavenging of ROS, and thus, reduced the membrane destruction caused due to peroxidation of lipids (Tanveer et al. 2018). The decline in MDA content with the BRs under high temperature stress reflects the positive role of BRs in stress amelioration (Jin et al. 2015).
One of the possible mechanisms that make a plant species tolerant to salinity stress is the presence of strong antioxidative defense system (Senadheera et al. 2012; AbdElgawad et al. 2016). However, to cope up with the oxidative damage, plants activates their antioxidative defense system. In our current study, salinity stress stimulated the activities of antioxidative enzymes (POD, CAT, DHAR, and MDAHR), which were further boosted with the treatment of both HBL and EBL in salt stressed maize seedlings. The increase in activity of POD with the application of BRs develops tolerance against salinity stress by overcoming the oxidative damage in wheat seedlings subjected to 120 mM NaCl stress (Dong et al. 2017). POD has been concerned in the synthesis of various phenolic polymers and its increased activity provides protection against the harmful concentrations of hydroperoxides (Verma et al. 2011). CAT is a ubiquitous enzyme that scavenges H2O2 by catalyzing its decomposition into molecular oxygen and water (Saraf 2013). Application of BRs significantly overcomes the salinity-induced damage in soybean plants by increasing the activity of CAT (Alam et al. 2019). Similarly BRs treatment also increased the activities of DHAR and MDHAR in Brassica juncea seedlings subjected to high temperature and salt stress, and thus, develop tolerance against stress. Finding of our study and above reports on increase in activities of antioxidative enzymes in response to salt stress support and strengthened the anti-stress role of brassinosteroids against salinity stress. BRs-mediated stress tolerance might involve a complex pathway, which regulates the plant defense system by activating BZR1/BES1 transcription factors (Anwar et al. 2018). BRs regulate thousands of genes under stress, which participate in induction of antioxidant systems and provide protection against the deleterious effects of ROS (Gruszka 2013).
Plants proficient defense system not only includes the antioxidative enzymes to combat the stress, but it also includes a variety of other scavengers to overcome the stress triggered ROS (Mittler et al. 2004; Gill et al. 2011). Plant secondary metabolites in response to salt stress play important role in maintaining the favorable cellular condition (Ramakrishna and Ravishankar 2011). The natural antioxidants such as phenolics play important role in stress tolerance (Gimenez et al. 2014). The redox properties of these phenolic compounds facilitate them to perform as antioxidants and detoxifying the ROS (Sharma et al. 2019b). In the present study, total phenolic content and flavonoid content was observed to increase under salt stress. Increased total phenolic content develop tolerance against saline stress in Salvia mirzayanii plants when subjected to moderate salinity stress (Valifard et al. 2014). Similarly Sarker et al. (2018) also reported the increased total polyphenolic, flavonoid content and total antioxidant activity under salt stress in Amaranthus tricolor leaves which provide protection against the damaging effects of salinity stress.
BRs application in the present study increased the total phenolic and flavonoid content under salt stress. Alam et al. (2019) reported the increase in total phenols and flavonoids with the treatment of EBL under salt stress in soybean plants. Similar observations were recorded by Xi et al. (2013), where EBL treatment could considerably promoted grape ripening and enhancing the phenolic content and antioxidant capacity in grape skins. Pre-sowing treatments of both HBL and EBL in present study also increased the DPPH activity under salt stress. Therefore, BRs energizes the antioxidative enzymes and secondary metabolites like phenolic and flavonoid compounds by promoting the scavenging of ROS and enable the plants to withstand under stress conditions by developing the tolerance against stress condition (Sharma et al. 2016b, 2019c; Gao et al. 2016).
Another important strategy adopted by plants to grow under stress conditions, involves the accumulation of osmolytes which help in maintaining water balance between plant cell and environment under stress condition (Sharma et al. 2019a; Sadak et al. 2019). In our study under salt stress, osmolytes (proline, glycine betaine, mannitol) were observed to get accumulated in maize seedlings. The accumulation of these osmoprotectants enables the plants to grow under stress conditions. Increased accumulation of proline under salt stress has also been reported in rice due to overexpresssion of gene which is responsible for proline biosynthesis, and thus, accounts for tolerance against salt stress (Yang et al. 2012). Increase in glycine betaine content in Solanum lycopersicum subjected to 150 mM salt stress had been reported by Ahanger et al. (2018b). Mannitol accumulation develops salinity tolerance in Lemon balm subjected to salt stress by improving the growth, pigments and mineral content (Khalid and Cai 2011). BRs treatment increased the content of osmolytes in current study and help in maintaining the osmotic balance. EBL promoted the osmotic adjustment by enhancing the osmolytes, such as proline and glycine beatine content in both salt tolerant and salt sensitive genotypes of Pisum sativum plants supported the role of BRs in osmotic adjustment (Shahid et al. 2014). BRs mediated increase in proline accumulation, which involves the induction of genes expression responsible for the proline biosynthesis and helps in maintaining the osmotic balance (Ramakrishna and Rao 2015; Lalotra et al. 2017). According to metabolite profiling, BRs mediated increase of osmolytes accumulation, which might involve the overexpression of the BRL3 (vascular-enriched member of the BR receptor family) receptor, which triggers the production of an osmoprotectants in the plants (Fabregas et al. 2018).
Conclusion
From the above study, it is concluded that application of BRs assisted in maintaining the favorable cellular conditions in salinity stressed seedlings by regulating the ions metabolism, strengthening the antioxidative defense system, and enhancing the osmoprotectants accumulation, and overall help in overcoming the deleterious effects of salt stress.
References
AbdElgawad H, Zinta G, Hegab MM, Pandey R, Asard H, Abuelsoud W (2016) High salinity induces different oxidative stress and antioxidant responses in maize seedlings organs. Front Plant Sci 7:276. https://doi.org/10.3389/fpls.2016.00276
Abogadallah GM (2010) Antioxidative defense under salt stress. Plant Signal Behav 5:369–374. https://doi.org/10.4161/psb.5.4.10873
Aebi H (1984) Catalase in vitro. In: Packer L (ed) Methods in enzymology. Elsevier, Amsterdam, pp 121–126. https://doi.org/10.1016/S0076-6879(84)05016-3
Ahammed GJ, Xia XJ, Li X, Shi K, Yu JQ, Zhou YH (2015) Role of brassinosteroid in plant adaptation to abiotic stresses and its interplay with other hormones. Curr Protein Pept Sci 16:462–473. https://doi.org/10.2174/1389203716666150330141427
Ahanger MA, Agarwal RM (2017) Salinity stress induced alterations in antioxidant metabolism and nitrogen assimilation in wheat (Triticum aestivum L.) as influenced by potassium supplementation. Plant Physiol Biochem 115:449–460. https://doi.org/10.1016/j.plaphy.2017.04.017
Ahanger MA, Tomar NS, Tittal M, Argal S, Agarwal RM (2017) Plant growth under water/salt stress: ROS production; antioxidants and significance of added potassium under such conditions. Physiol Mol Biol Plants 23:731–744. https://doi.org/10.1007/s12298-017-0462-7
Ahanger MA, Ashraf M, Bajguz A, Ahmad P (2018a) Brassinosteroids regulate growth in plants under stressful environments and crosstalk with other potential phytohormones. J Plant Growth Regul 37:1007–1024. https://doi.org/10.1007/s00344-018-9855-2
Ahanger MA, Alyemeni MN, Wijaya L, Alamri SA, Alam P, Ashraf M, Ahmad P (2018b) Potential of exogenously sourced kinetin in protecting Solanum lycopersicum from NaCl-induced oxidative stress through up-regulation of the antioxidant system, ascorbate-glutathione cycle and glyoxalase system. PLoS ONE 13:e0202175. https://doi.org/10.1371/journal.pone.0202175
Ahanger MA, Qin C, Maodong Q, Dong XX, Ahmad P, Abd_Allah EF, Zhang L (2019) Spermine application alleviates salinity induced growth and photosynthetic inhibition in Solanum lycopersicum by modulating osmolyte and secondary metabolite accumulation and differentially regulating antioxidant metabolism. Plant Physiol Biochem 144:1–13. https://doi.org/10.1016/j.plaphy.2019.09.021
Ahmad I, Maathuis FJ (2014) Cellular and tissue distribution of potassium: physiological relevance, mechanisms and regulation. J Plant Physiol 171:708–714. https://doi.org/10.1016/j.jplph.2013.10.016
Ahmad P, Ahanger MA, Alam P, Alyemeni MN, Wijaya L, Ali S, Ashraf M (2019) Silicon (Si) supplementation alleviates NaCl toxicity in mung bean [Vigna radiata (L.) wilczek] through the modifications of physiobiochemical attributes and key antioxidant enzymes. J Plant Growth Regul 38:70–82. https://doi.org/10.1007/s00344-018-9810-2
Alam P, Albalawi TH, Altalayan FH, Bakht MA, Ahanger MA, Raja V, Ashraf M, Ahmad P (2019) 24-Epibrassinolide (EBR) confers tolerance against NaCl stress in soybean plants by up-regulating antioxidant system, ascorbate-glutathione cycle, and glyoxalase system. Biomolecules 9:640. https://doi.org/10.3390/biom9110640
Ali SG, Rab A, Khan NU, Nawab K (2011) Enhanced proline synthesis may determine resistance to salt stress in tomato cultivars. Pak J Bot 43:2707–2710
Anwar A, Liu Y, Dong R, Bai L, Yu X, Li Y (2018) The physiological and molecular mechanism of brassinosteroid in response to stress: a review. Biol Res 51:46. https://doi.org/10.1186/s40659-018-0195-2
Balbaa S, Zaki A, El-Shamy A (1974) Qualitative and quantitative study of the flavonoid content of the different organs of Sophora japonica at different stages of development. Planta Med 25:325–330. https://doi.org/10.1055/s-0028-1097951
Bartwal A, Mall R, Lohani P, Guru S, Arora S (2013) Role of secondary metabolites and brassinosteroids in plant defense against environmental stresses. J Plant Growth Regul 32:216–232. https://doi.org/10.1007/s00344-012-9272-x
Bates LS, Waldren RP, Teare I (1973) Rapid determination of free proline for water-stress studies. Plant Soil 39:205–207. https://doi.org/10.1007/BF00018060
Blois MS (1958) Antioxidant determinations by the use of a stable free radical. Nature 181:1199. https://doi.org/10.1038/1811199a0
Bose J, Rodrigo-Moreno A, Shabala S (2014) ROS homeostasis in halophytes in the context of salinity stress tolerance. J Exp Bot 65:1241–1257. https://doi.org/10.1093/jxb/ert430
Cabot C, Sibole JV, Barcelo J, Poschenrieder C (2014) Lessons from crop plants struggling with salinity. Plant Sci 226:2–13. https://doi.org/10.1016/j.plantsci.2014.04.013
Canadanovic-Brunet JM, Djilas SM, Cetkovic GS, Tumbas VT (2005) Free-radical scavenging activity of wormwood (Artemisia absinthium L.) extracts. J Sci Food and Agric 85:265–272. https://doi.org/10.1002/jsfa.1950
Chakraborty K, Basak N, Bhaduri D, Ray S, Vijayan J, Chattopadhyay K, Sarka KK (2018) Ionic basis of salt tolerance in plants: nutrient homeostasis and oxidative stress tolerance. In: Hasanuzzaman M, Fujita M, Oku H, Nahar K, Hawrylak-Nowak B (eds) Plant nutrients and abiotic stress tolerance. Springer, Singapore, pp 325–362
Chen X, Qiu L, Guo H, Wang Y, Yuan H, Yan D, Zheng B (2017) Spermidine induces physiological and biochemical changes in southern highbush blueberry under drought stress. Braz J Bot 40:841–851. https://doi.org/10.1007/s40415-017-0401-4
Choudhary SP, Yu JQ, Yamaguchi-Shinozaki K, Shinozaki K, Tran LS (2012) Benefits of brassinosteroid crosstalk. Trends in Plant Sci 17:594–605. https://doi.org/10.1016/j.tplants.2012.05.012
Craig Plett D, Moller IS (2010) Na(+) transport in glycophytic plants: what we know and would like to know. Plant Cell Environ 33:612–626. https://doi.org/10.1111/j.1365-3040.2009.02086.x
Dalton DA, Russell SA, Hanus FJ, Pascoe GA, Evans HJ (1986) Enzymatic reactions of ascorbate and glutathione that prevent peroxide damage in soybean root nodules. Proc Natl Acad Sci USA 83:3811–3815. https://doi.org/10.1073/pnas.83.11.3811
de Oliveira VP, Lima MDR, da Silva BRS, Batista BL, Lobato AKS (2019) Brassinosteroids confer tolerance to salt stress in Eucalyptus urophylla plants enhancing homeostasis, antioxidant metabolism and leaf anatomy. J Plant Growth Regul 38:557–573. https://doi.org/10.1007/s00344-018-9870-3
Dong YJ, Wang WW, Hu GQ, Chen WF, Zhuge YP, Wang ZL, He MR (2017) Role of exogenous 24-epibrassinolide in enhancing the salt tolerance of wheat seedlings. J Soil Sci Plant Nut 17:554–569. https://doi.org/10.4067/S0718-95162017000300001
Gupta B, Huang B (2014) Mechanism of salinity tolerance in plants: physiological, biochemical, and molecular characterization. Int J Genom 2014:1–18. https://doi.org/10.1155/2014/701596
Eleiwa ME, Bafeel SO, Ibrahim S (2011) Influence of brassinosteroids on wheat plant (Triticum aestivum L.) production under salinity stress conditions. I-Growth parameters and photosynthetic pigments. Aust J Basic Appl Sci 5:58–65
Fabregas N, Lozano-Elena F, Blasco-Escamez D, Tohge T, Martinez-Andujar C, Albacete A, Osorio S, Bustamante M, Riechmann JL, Nomura T, Yokota T, Conesa A, Alfocea FP, Fernie AR, Cano-Delgado AI (2018) Overexpression of the vascular brassinosteroid receptor BRL3 confers drought resistance without penalizing plant growth. Nat Commun 9:4680. https://doi.org/10.1038/s41467-018-06861-3
FAO (2016) Extent of salt-affected soils. The Food and Agriculture Organization of the United Nations. https://www.fao.org/soils-portal/soil-management/management-of-some-problem-soils/salt-affected-soils/more-information-on-salt-affected-soils/en/. Accessed 18 Aug 2019
Gao H, Zhang Z, Lv X, Cheng N, Peng B, Cao W (2016) Effect of 24-epibrassinolide on chilling injury of peach fruit in relation to phenolic and proline metabolisms. Postharvest Biol Technol 111:390–397. https://doi.org/10.1016/j.postharvbio.2015.07.031
Gill SS, Khan NA, Anjum NA, Tuteja N (2011) Amelioration of cadmium stress in crop plants by nutrients management: morphological, physiological and biochemical aspects. Plant Stress 5:1–23
Gill SS, Tuteja N (2010) Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol Biochem 48:909–930. https://doi.org/10.1016/j.plaphy.2010.08.016
Gimenez MJ, Valverde JM, Valero D, Guillen F, Martinez-Romero D, Serrano M, Castillo S (2014) Quality and antioxidant properties on sweet cherries as affected by preharvest salicylic and acetylsalicylic acids treatments. Food Chem 160:226–232. https://doi.org/10.1016/j.foodchem.2014.03.107
Grieve C, Grattan S (1983) Rapid assay for determination of water soluble quaternary ammonium compounds. Plant Soil 70:303–307. https://doi.org/10.1007/BF02374789
Gruszka D (2013) The brassinosteroid signaling pathway-new key players and interconnections with other signaling networks crucial for plant development and stress tolerance. Int J Mol Sci 14:8740–8774. https://doi.org/10.3390/ijms14058740
Gudesblat GE, Russinova E (2011) Plants grow on brassinosteroids. Curr Opin Plant Biol 14:530–537. https://doi.org/10.1016/j.pbi.2011.05.004
Guo H, Chen H, Hong C, Jiang D, Zheng B (2017) Exogenous malic acid alleviates cadmium toxicity in Miscanthus sacchariflorus through enhancing photosynthetic capacity and restraining ROS accumulation. Ecotoxicol Environ Saf 141:119–128. https://doi.org/10.1016/j.ecoenv.2017.03.018
Haubrick LL, Assmann SM (2006) Brassinosteroids and plant function: some clues, more puzzles. Plant Cell Environ 29:446–457. https://doi.org/10.1111/j.1365-3040.2005.01481.x
Heath RL, Packer L (1968) Photoperoxidation in isolated chloroplasts: I. Kinetics and stoichiometry of fatty acid peroxidation. Arch Biochem Biophysic 125:189–198. https://doi.org/10.1016/0003-9861(68)90654-1
Hofmann NR (2015) Taking hormone crosstalk to a new level: brassinosteroids regulate gibberellin biosynthesis. Plant Cell 27:2081. https://doi.org/10.1105/tpc.15.00700
Hossain MA, Nakano Y, Asada K (1984) Monodehydroascorbate reductase in spinach chloroplasts and its participation in regeneration of ascorbate for scavenging hydrogen peroxide. Plant Cell Physiol 25:385–395. https://doi.org/10.1093/oxfordjournals.pcp.a076726
Jin SH, Li XQ, Wang GG, Zhu XT (2015) Brassinosteroids alleviate high-temperature injury in Ficus concinna seedlings via maintaining higher antioxidant defence and glyoxalase systems. AoB Plants 7:plv009. https://doi.org/10.1093/aobpla/plv009
Khalid KA, Cai W (2011) The effects of mannitol and salinity stresses on growth and biochemical accumulations in lemon balm. Acta Ecol Sin 31:112–120. https://doi.org/10.1016/j.chnaes.2011.01.001
Lalotra S, Hemantaranjan A, Kumar S, Kant R (2017) Effect of brassinosteroid (brassinolide) on seedling traits, morphology and metabolism in mung bean under salinity stress. Annu Res Rev Biol 12:1–8. https://doi.org/10.9734/ARRB/2017/3223
Marimuthu P, Wu CL, Chang HT, Chang ST (2008) Antioxidant activity of the ethanolic extract from the bark of Chamaecyparis obtusa var. formosana. J Sci Food Agric 88:1400–1405. https://doi.org/10.1002/jsfa.3231
Marschner H (2011) Marschner's mineral nutrition of higher plants. Academic Press, Amsterdam
Miller G, Suzuki N, Ciftci-Yilmaz S, Mittler R (2010) Reactive oxygen species homeostasis and signalling during drought and salinity stresses. Plant Cell Environ 33:453–467. https://doi.org/10.1111/j.1365-3040.2009.02041.x
Mishra S, Jha AB, Dubey RS (2011) Arsenite treatment induces oxidative stress, upregulates antioxidant system, and causes phytochelatin synthesis in rice seedlings. Protoplasma 248:565–577. https://doi.org/10.1007/s00709-010-0210-0
Mittler R, Vanderauwera S, Gollery M, Van Breusegem F (2004) Reactive oxygen gene network of plants. Trends in Plant Sci 9:490–498. https://doi.org/10.1016/j.tplants.2004.08.009
Peres A, Soares JS, Tavares RG, Righetto G, Zullo MAT, Mandava NB, Menossi M (2019) Brassinosteroids, the sixth class of phytohormones: a molecular view from the discovery to hormonal interactions in plant development and stress adaptation. Int J Mol Sci 20:331. https://doi.org/10.3390/ijms20020331
Pütter J (1974) Peroxidases. In: Bergmeyer HU (ed) Methods of enzymatic analysis. Elsevier, Amsterdam, pp 685–690
Ramakrishna B, Rao SSR (2015) Foliar application of brassinosteroids alleviates adverse effects of zinc toxicity in radish (Raphanus sativus L.) plants. Protoplasma 252:665–677. https://doi.org/10.1007/s00709-014-0714-0
Ramakrishna A, Ravishankar GA (2011) Influence of abiotic stress signals on secondary metabolites in plants. Plant Signal Behav 6:1720–1731. https://doi.org/10.4161/psb.6.11.17613
Sadak MS, El-Bassiouny HMS, Dawood MG (2019) Role of trehalose on antioxidant defense system and some osmolytes of quinoa plants under water deficit. Bull Nat Res Centre 43:1–11. https://doi.org/10.1186/s42269-018-0039-9
Saraf N (2013) Enhancement of catalase activity under salt stress in germinating seeds of Vigna radiata. Asian J Biomed Pharma Sci 17:6–8
Sarker U, Islam MT, Oba S (2018) Salinity stress accelerates nutrients, dietary fiber, minerals, phytochemicals and antioxidant activity in Amaranthus tricolor leaves. PLoS ONE 13:e0206388. https://doi.org/10.1371/journal.pone.0206388
Sanchez J (1998) Colorimetric assay of alditols in complex biological samples. J Agric Food Chem 46:157–160. https://doi.org/10.1021/jf970619t
Senadheera P, Tirimanne S, Maathuis FJM (2012) Long term salinity stress reveals variety specific differences in root oxidative stress response. Rice Sci 19:36–43. https://doi.org/10.1016/S1672-6308(12)60018-3
Serrano R, Culiañz-Maciá FA, Moreno V (1998) Genetic engineering of salt and drought tolerance with yeast regulatory genes. Sci Hortic 78:261–269. https://doi.org/10.1016/S0304-4238(98)00196-4
Shabala S, Cuin TA (2008) Potassium transport and plant salt tolerance. Physiol Plant 133:651–669. https://doi.org/10.1111/j.1399-3054.2007.01008.x
Shahid MA, Balal RM, Pervez MA, Garcia-Sanchez F, Gimeno V, Abbas T, Mattson NS, Riaz A (2014) Treatment with 24-epibrassinolide mitigates NaCl-induced toxicity by enhancing carbohydrate metabolism, osmolyte accumulation, and antioxidant activity in Pisum sativum. Turk J Bot 38:511–525. https://doi.org/10.3906/bot-1304-45
Shahzad B, Tanveer M, Che Z, Rehman A, Cheema SA, Sharma A, Song H, Rehman S, Zhaorong D (2018) Role of 24-epibrassinolide (EBL) in mediating heavy metal and pesticide induced oxidative stress in plants: a review. Ecotoxicol Environ Saf 147:935–944. https://doi.org/10.1016/j.ecoenv.2017.09.066
Sharma A, Kumar V, Kumar R, Shahzad B, Thukral AK, Bhardwaj R (2018) Brassinosteroid-mediated pesticide detoxification in plants: a mini-review. Cogent Food Agric 4:1436212. https://doi.org/10.1080/23311932.2018.1436212
Sharma A, Kumar V, Thukral AK, Bhardwaj R (2016a) Epibrassinolide-imidacloprid interaction enhances non-enzymatic antioxidants in Brassica juncea L. Indian J Plant Physiol 21:70–75. https://doi.org/10.1007/s40502-016-0203-x
Sharma A, Shahzad B, Kumar V, Kohli SK, Sidhu GPS, Bali AS, Handa N, Kapoor D, Bhardwaj R, Zheng B (2019a) Phytohormones regulate accumulation of osmolytes under abiotic stress. Biomolecules 9:285. https://doi.org/10.3390/biom9070285
Sharma A, Shahzad B, Rehman A, Bhardwaj R, Landi M, Zheng B (2019b) Response of phenylpropanoid pathway and the role of polyphenols in plants under abiotic stress. Molecules 24:2452. https://doi.org/10.3390/molecules24132452
Sharma A, Thakur S, Kumar V, Kanwar MK, Kesavan AK, Thukral AK, Bhardwaj R, Alam P, Ahmad P (2016b) Pre-sowing seed treatment with 24-epibrassinolide ameliorates pesticide stress in Brassica juncea L. through the modulation of stress markers. Front Plant Sci 7:1569. https://doi.org/10.3389/fpls.2016.01569
Sharma A, Yuan H, Kumar V, Ramakrishnan M, Kohli SK, Kaur R, Thukral AK, Bhardwaj R, Zheng B (2019c) Castasterone attenuates insecticide induced phytotoxicity in mustard. Ecotoxicol Environ Saf 179:50–61. https://doi.org/10.1016/j.ecoenv.2019.03.120
Sreeramulu S, Mostizky Y, Sunitha S, Shani E, Nahum H, Salomon D, Hayun LB, Gruetter C, Rauh D, Ori N, Sessa G (2013) BSKs are partially redundant positive regulators of brassinosteroid signaling in Arabidopsis. Plant J 74:905–919. https://doi.org/10.1111/tpj.12175
Souid A, Gabriele M, Longo V, Pucci L, Bellani L, Smaoui A, Abdelly C, Hamed KB (2016) Salt tolerance of the halophyte Limonium delicatulum is more associated with antioxidant enzyme activities than phenolic compounds. Funct Plant Biol 43:607–619. https://doi.org/10.1071/FP15284
Sun S, An M, Han L, Yin S (2015) Foliar application of 24-epibrassinolide improved salt stress tolerance of perennial ryegrass. HortScience 50:1518–1523. https://doi.org/10.21273/HORTSCI.50.10.1518
Swain T, Hillis W (1959) The phenolic constituents of Prunus domestica. I.—the quantitative analysis of phenolic constituents. J Sci Food Agric 10:63–68. https://doi.org/10.1002/jsfa.2740100110
Tang W, Luo C (2018) Overexpression of zinc finger transcription factor ZAT6 enhances salt tolerance. Open Life Sci 13:431–445. https://doi.org/10.1515/biol-2018-0052
Tanveer M, Shahzad B, Sharma A, Biju S, Bhardwaj R (2018) 24-Epibrassinolide; an active brassinolide and its role in salt stress tolerance in plants: a review. Plant Physiol Biochem 130:69–79. https://doi.org/10.1016/j.plaphy.2018.06.035
Tanveer M, Shahzad B, Sharma A, Khan EA (2019) 24-Epibrassinolide application in plants: an implication for improving drought stress tolerance in plants. Plant Physiol Biochem 135:295–303. https://doi.org/10.1016/j.plaphy.2018.12.013
Tavakkoli E, Rengasamy P, McDonald GK (2010) The response of barley to salinity stress differs between hydroponic and soil systems. Funct Plant Biol 37:621–633. https://doi.org/10.1071/FP09202
Tomescu D, Şumălan R, Copolovici L, Copolovici D (2017) The influence of soil salinity on volatile organic compounds emission and photosynthetic parameters of Solanum lycopersicum L. varieties. Open Life Sci 12:135–142. https://doi.org/10.1515/biol-2017-0016
Valifard M, Mohsenzadeh S, Kholdebarin B, Rowshan V (2014) Effects of salt stress on volatile compounds, total phenolic content and antioxidant activities of Salvia mirzayanii. S Afr J Bot 93:92–97. https://doi.org/10.1016/j.sajb.2014.04.002
Van Ruyskensvelde V, Van Breusegem F, Van Der Kelen K (2018) Post-transcriptional regulation of the oxidative stress response in plants. Free Radic Biol Med 122:181–192. https://doi.org/10.1016/j.freeradbiomed.2018.02.032
Verma A, Malik C, Gupta V (2011) In vitro effects of brassinosteroids on the growth and antioxidant enzyme activities in groundnut. ISRN Agron 2012:356485. https://doi.org/10.5402/2012/356485
Wei Z, Li J (2016) Brassinosteroids regulate root growth, development, and symbiosis. Mol Plant 9:86–100. https://doi.org/10.1016/j.molp.2015.12.003
Wojdyło A, Oszmiański J, Czemerys R (2007) Antioxidant activity and phenolic compounds in 32 selected herbs. Food Chem 105:940–949. https://doi.org/10.1016/j.foodchem.2007.04.038
Xi ZM, Zhang ZW, Huo SS, Luan LY, Gao X, Ma LN, Fang YL (2013) Regulating the secondary metabolism in grape berry using exogenous 24-epibrassinolide for enhanced phenolics content and antioxidant capacity. Food Chem 141:3056–3065. https://doi.org/10.1016/j.foodchem.2013.05.137
Yan K, Shao H, Shao C, Chen P, Zhao S, Brestic M, Chen X (2013) Physiological adaptive mechanisms of plants grown in saline soil and implications for sustainable saline agriculture in coastal zone. Acta Physiol Plant 35:2867–2878. https://doi.org/10.1016/j.foodchem.2007.04.038
Yang A, Dai X, Zhang WH (2012) A R2R3-type MYB gene, OsMYB2, is involved in salt, cold, and dehydration tolerance in rice. J Expt Bot 63:2541–2556. https://doi.org/10.1093/jxb/err431
Zhu JK (2007) Plant salt stress. In: O'Daly A (ed) Encyclopedia of life sciences. Wiley, Chichester. https://doi.org/10.1002/9780470015902.a0001300.pub2s
Acknowledgements
The authors are thankful to the Department of Botanical and Environmental Sciences, Guru Nanak Dev University, Amritsar, India and Hans Raj Mahila Maha Vidyalaya, Jalandhar, India for providing laboratory facilities.
Author information
Authors and Affiliations
Contributions
RB, AS and NK designed the experiment; AR and DK were involved in experimentation and writing original draft. AS and DK did editing and revision of original draft.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Rattan, A., Kapoor, D., Kapoor, N. et al. Brassinosteroids Regulate Functional Components of Antioxidative Defense System in Salt Stressed Maize Seedlings. J Plant Growth Regul 39, 1465–1475 (2020). https://doi.org/10.1007/s00344-020-10097-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00344-020-10097-1